Abstract
AIM: To investigate the effects of moxibustion on the morphology and function of mast cells (MC) at Tianshu (ST25) in rats with trinitro-benzene-sulfonic acid (TNBS)-induced colitis.
METHODS: A total of 53 male Sprague-Dawley rats were randomly divided into a normal group and experimental group. In the experimental group, a rat model of TNBS-induced colitis was established, and the rats were then randomly divided into a model group, moxibustion group, moxibustion plus disodium cromoglycate (M + DC) group and moxibustion plus normal saline (M + NS) group. Rats in the moxibustion group received suspended moxibustion at bilateral ST25 for 10 min, once a day for 7 d. Rats in the M + DC and M + NS groups were pretreated with disodium cromoglycate and normal saline at bilateral ST25, respectively, and were then concurrently subjected to the same treatment as rats in the moxibustion group. The hematoxylin-eosin staining method was used to observe histology of the colon and the toluidine blue-improved method was used to observe mast cells at ST25 acupoint areas.
RESULTS: An improvement in colonic injury in the moxibustion group was observed and the degranulation ratio of MC at ST25 acupoint was markedly higher in the moxibustion group than in the model group (45.91 ± 11.41 vs 32.58 ± 8.28, P < 0.05). After inhibition of degranulation of MC at ST25 by disodium cromoglycate, no improvement in colon tissue injury was observed.
CONCLUSION: Moxibustion exerted its effect on healing impaired colonic mucosa in rats with TNBS-induced colitis by increasing the degranulation ratio of local MC, but had little effect on the morphology of MC at ST25 acupoint.
Keywords: Disodium cromoglycate, Colitis, Mast cell, Moxibustion, ST25 acupoint
INTRODUCTION
Acupuncture, one of the alternative or complementary therapies, is receiving increasing acceptance in Western medicine for the treatment of certain medical conditions[1-6]. Moxibustion, involving warm stimulation by moxa combustion at acupoint areas, is one of the acupuncture-moxibustion therapies for treating certain disorders in the clinic[7-10]. It was shown that the effective mechanism of acupuncture stimulation is closely related to the activation of mast cells (MC) at acupoint areas[11]. Acupoints have complex structures and are composed of nerve endings, plexuses, blood vessels, lymphatic vessels and connective tissues[11-14]. However, the correlation between the effect of moxibustion and the response of MC at acupoint areas is still unclear and merits further study.
MC are common effector cells and are widespread in connective tissues, especially in subcutaneous and submucosal loose connective tissues. MC act in several ways including changes in degranulation and the release of various bioactive mediators [5-hydroxytryptamine (5-HT), P substance (SP), heparin, and leukotriene][15-17]. It was reported that acupuncture stimulation markedly increased the density of local MC and activated MC degranulation at needle acupoints, which led to downstream effects in activating certain cellular pathways[11-14,18]. Pretreating the acupuncture point with disodium cromoglycate not only counteracted degranulation, it also reduced the effect of acupuncture[18].
Previous studies by our research team have indicated that moxibustion has a beneficial effect on inflammatory bowel disease (IBD)[8-10]. Moreover, we found that moxibustion at ST25 can heal impaired colonic mucosa in a rat model of colitis created by an immunological method associated with local stimulation. ST25 is the primary large intestinal meridian point of hand Yangming, which regulates the function of the large intestine, spleen, and stomach. ST25 is an efficacious point in the clinical treatment of patients with IBD.
In this study, we established a colitis rat model induced by trinitro-benzene-sulfonic acid (TNBS). The hematoxylin-eosin staining method was adopted for histological assessment of colonic mucosal injuries after moxibustion intervention and the toluidine blue-improved method was used to observe morphology and degranulation of MC at ST25 acupoint areas.
MATERIALS AND METHODS
Animals
Fifty-three male Sprague-Dawley rats (SPF class), weighing 100-140 g, were supplied by the Experimental Animal Center of Shanghai University of Traditional Chinese Medicine (TCM), and randomly divided into a normal group (n = 11) and an experimental group (n = 42). All rats were housed at a constant temperature and humidity with free access to food and water. All studies were performed in accordance with the proposals of the Committee for Research and Ethical Issues of the International Association and approved by the Committee on the Use of Human and Animal Subjects in Teaching and Research, Shanghai University of TCM.
Establishment of the colitis rat model
An experimental colitis rat model was established according to the TNBS-induced method reported by Morris[19]. After weighing and administering anesthesia (1% sodium pentobarbital; i.p., 45 mg/kg), the 42 experimental rats were injected with TNBS/ethanol (100 mg/kg TNBS + 50% ethanol 0.25 mL) into the anus via a rubber tube; the solution was retained in the gut cavity at a depth of 6 cm-8 cm. Rats in the normal control group were given an enema with 0.9% NaCl of the same volume as given to the experimental rats. The rats were subsequently held upside down before removing the enema apparatus, and were kept in this position for 1 min to prevent the solution from flowing out.
After colitis was induced, one rat from the normal group and two rats from the model group were dissected to remove colon tissue. The tissue was stained with hematoxylin-eosin to confirm the establishment of the experimental colitis model. The remaining rats in the experimental group were then randomly divided into four groups: a model group, moxibustion group, moxibustion plus pretreated disodium cromoglycate (M + DC) group and moxibustion plus pretreated normal saline (M + NS) group.
Treatment
Location of ST25 acupoints in the rats were based on an anatomic method referenced in the “Map of Animal Acupoints” from Shi Yan Zhen Jiu Xue written by Lin WZ. In the moxibustion group, moxibustion was administered at bilateral ST25 acupoints using a fine moxa stick with the smoldering end 2 cm away from the acupoints for 10 min once daily for 7 d in total. In the M + DC group, bilateral ST25 acupoints were injected with disodium cromoglycate (55 mg/kg 0.2 mL; 0.1 mL for each acupoint) before moxibustion. In the M + NS group, bilateral ST25 acupoints were injected with normal saline (0.2 mL; 0.1 mL for each acupoint) before moxibustion.
Observation of colonic mucosa by hematoxylin eosine method
Following sacrifice of the animal and laparotomy, the inflamed segment of colon approximately 8 cm from the anus was removed, washed with iced saline, fixed in 10% formalin, embedded in paraffin, sectioned, stained with hematoxylin and eosin, dehydrated in 95%, 90% and 70% ethanol, cleared in xylene, mounted in Permount or Histoclad, and observed under a microscope.
Observation of MC at ST25 acupoints by the toluidine blue-improved method
Sequential paraffin slices 4-μm thick were prepared after 48 h of fixation at 4 °C in fixing solution (10% formalin). The subcutaneous tissue samples were stained with 0.5% toluidine blue. The numbers of MC per microscopic view (0.16 mm2 at × 200 magnification) were counted at 4 areas per slice and then averaged. Mast cells with more than 3 granules outside the cell shape or with empty cavities in the cytoplasm were considered to be degranulated. The ratios of degranulated to total MC were calculated. Representative photomicrographs were obtained at a magnification of × 400 for morphological evaluation.
Statistical analysis
Experimental data were expressed as mean ± SD. Statistical analyses were performed using SPSS 13.0 (SPSS Inc., United States). Differences in mean were compared by one way ANOVA. P < 0.05 was considered statistically significant.
RESULTS
Improvement of colonic ulceration in rats treated with moxibustion
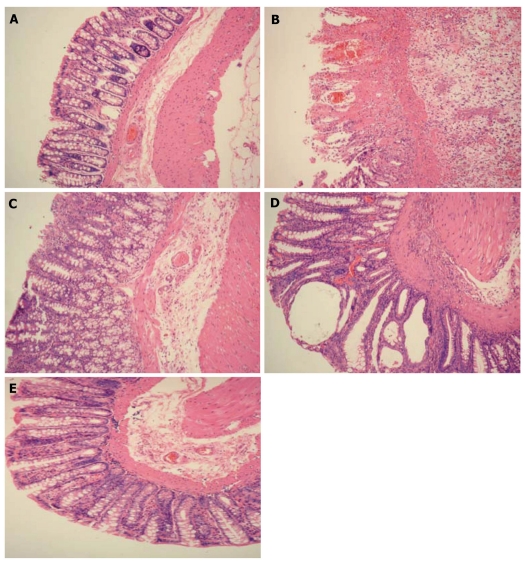
In the stained colon tissue slices, colonic glands and caliciform cells were observed by light microscopy (Figure 1). In the normal group, the colonic mucosa epithelium was complete and the colonic gland was regularly arranged, with no apparent inflammatory cell infiltration (Figure 1A). In the model group, damage to colonic mucosa was observed, mucosal villi were damaged or missing, there was congestion and edema in the submucosa, the gland was damaged or missing, caliciform cells were reduced, monocytes or mast cells were present, a large number of infiltrating inflammatory cells was present in the mucosa or submucosa, and ulceration was noted (Figure 1B). In the moxibustion group, the colonic gland was regularly arranged, ulceration was covered by regenerated epithelium, submucosal edema was found, and a small number of infiltrating inflammatory cells was observed (Figure 1C). In the M + DC group, the colonic gland was distended and irregularly arranged, slight congestion of colonic mucosa and fibroplasia of the submucosa were found, and a large number of infiltrating inflammatory cells was noted, however, this was not as serious as in the model group (Figure 1D). In the M + NS group, the colonic gland was regularly arranged, there was slight congestion of the colonic mucosa, and edema and inflammatory cells had infiltrated the submucosa (Figure 1E).
Figure 1.
Results of hematoxylin eosine staining of rat colonic tissue (× 100). A: Normal group: the colonic mucosa was complete and the colonic gland was regularly arranged, with no apparent inflammatory cell infiltration; B: Model group: damage to colonic mucosa was found and there were monocytes and a large number of inflammatory cells infiltrating the mucosa or submucosa; C: Moxibustion group: the colonic gland was regularly arranged compared with the model group and ulceration was covered by regenerated epithelium; D: Moxibustion plus disodium cromoglycate group: slight congestion of colonic mucosa and fibroplasia of submucosa were found and a large number of infiltrating inflammatory cells, however, this was not as serious as in the model group; E: Moxibustion plus normal saline group: the colonic gland was regularly arranged and inflammatory cell infiltration of the submucosa was noted.
Effects of moxibustion on the morphology and function of MC
There were no significant differences among the groups in the average diameter and size of MC at ST25 acupoint areas (Table 1). The number of degranulated MC and the ratio of MC in the normal group were lower than those in the other groups (P < 0.05); the degranulation ratio of MC in the moxibustion group was greater than those in the model group and the M + DC group (P < 0.05); no significant difference was found in the degranulation ratio of MC between the moxibustion group and the M + NS group (Table 2, Figure 2).
Table 1.
Average diameter and size of mast cells at ST25 acupoint areas (mean ± SD)
| Group | n | MC average diameter | MC average size |
| Normal | 10 | 14.0266 ± 2.1240 | 163.9111 ± 51.3831 |
| Model | 10 | 14.6944 ± 2.8082 | 182.1338 ± 79.6975 |
| Moxibustion | 10 | 12.8357 ± 3.5726 | 148.2694 ± 88.3540 |
| M + DC | 10 | 15.2929 ± 1.5578 | 194.111 ± 35.6967 |
| M + NS | 10 | 14.2835 ± 1.5379 | 162.4508 ± 28.2383 |
MC: Mast cells; M + DC: Moxibustion plus disodium cromoglycate; M + NS: Moxibustion plus normal saline.
Table 2.
Degranulation ratio of mast cells at ST25 acupoint areas (mean ± SD)
| Group | n | MC total number | MC degranulation number | Degranulation ratio (%) |
| Normal | 10 | 7.16 ± 1.27 | 1.22 ± 0.29 | 17.01 ± 4.11 |
| Model | 10 | 10.38 ± 2.52 | 3.44 ± 1.27a | 32.58 ± 8.28a |
| Moxibustion | 10 | 13.22 ± 4.40a | 5.78 ± 1.97ab | 45.91 ± 11.41ab |
| M + DC | 10 | 10.14 ± 4.26 | 3.36 ± 1.64ac | 33.41 ± 9.56ac |
| M + NS | 10 | 12.24 ± 4.34a | 5.12 ± 2.26ab | 42.41 ± 7.71ab |
MC: Mast cells; M + DC: Moxibustion plus disodium cromoglycate; M + NS: Moxibustion plus normal saline..
P < 0.05 vs normal group;
P < 0.05 vs model group;
P < 0.05 vs moxibution group.
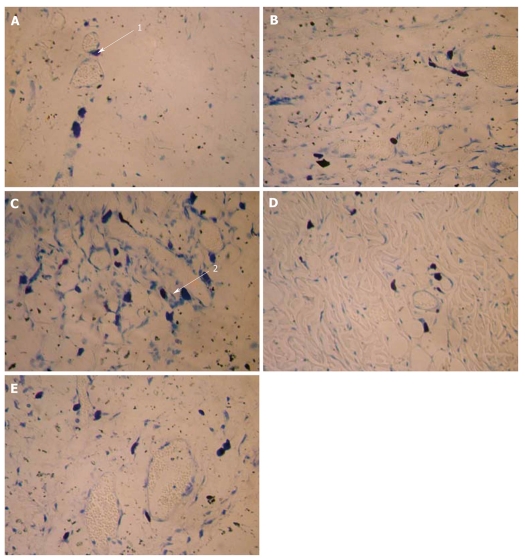
Figure 2.
Results of the mast cells toluidine blue-improved method at ST25 (× 400). Normal group (A), model group (B), moxibustion group (C), moxibustion plus disodium cromoglycate group (D) and moxibustion plus normal saline group (E). Arrow 1: Intact mast cells (MC); Arrow 2: Degranulated MC. MC plasma stains purple, and nucleus is shown as dark blue, scattering in subcutaneous loose connective tissues, or gathering in a group or lining up; cell shape appears round, oval, shuttle-like, erose; small cells had little plasma and a clear shape, large cells had more plasma and an unclear shape.
DISCUSSION
It has been suggested that MC could play a primary role in the effective mechanism of moxibustion at acupoint areas. An improvement in colonic injury in the moxibustion group was demonstrated and the degranulation ratio of MC at ST25 acupoint areas was remarkably higher in the moxibustion group than in the model group, which indicated a correlation between MC and moxibustion effects. Following inhibition of the degranulation of MC at ST25 acupoints by injection of the MC stabilizer, disodium cromoglycate, we found that not only did this treatment counteract degranulation of MC, but there was no improvement in colon tissue injury after moxibustion. In addition, it was found that moxibustion had no obvious influence on the size of MC at ST25 acupoints, which further suggested that the degranulation of MC at ST25 acupoints could participate in the mechanisms of moxibustion therapy.
The degranulation of MC at acupoint areas has been confirmed to participate in the analgesic activity of acupuncture[20-22]. These studies revealed that there are many more MC degranulated at acupoints following acupuncture intervention. The shapes of the degranulated MC were irregular with a vague boundary and the granules in the cytoplasm were scattered and small in size, and some granules released by MC spread over the entire tissue space. It has been shown that the analgesic effect of acupuncture could be significantly attenuated by repression of the degranulation of MC at acupoint areas using sodium cromoglycate[23]. Kimura found that the region of moxibustion treatment instantly received a large number of immunocyte infiltrations, which consisted of lymphocytes, monocytes, some granulocytes and MC[24]. Menjo showed that there was immediate degeneration of the epidermal cell layer and increased amounts of MC were observed after moxibustion treatment[25]. In this study, we also demonstrated a correlation between the activation of MC at moxibustion acupoint areas and the effects of moxibustion in TNBS-induced colitis rats.
MC, as resident cells in human loose connective tissue, are usually found gathered around small vessels and collaterals, and are particularly rich at nerve endings and nerve plexuses, forming a complex system of intercellular communications[26-30]. The cytoplasts of MC are filled with metachromatic basophilic granules in which various bioactive mediators are resident. Under acupuncture and moxibustion stimulation, large quantities of these bioactive mediators are released by activated MC to interact with surrounding tissues,producing the original so-called Qi sensation[20,22,30-32]. These bioactive substances (histamine, SP and 5-HT) in the granules penetrate into the tissue spaces, and on the one hand, transmit to other MC through the tissue fluid in a direction flowing along the meridian line, which induces further degranulation of MC[33]; on the other hand, these bioactive substances directly stimulate peripheral nerve receptors or nerve endings causing neuraxial reflection, releasing substance P, which can induce MC degranulation, stimulating the adjacent nerve endings further. Moxibustion signals are integrated and modification occurs in different stages from acupoint areas to the center and target organs, by which the moxibustion effect is achieved and target organs adjust.
It has also been reported that there was a significantly different effect between acupuncture and moxibustion on the quantities of degranulated cells and the distribution of MC at acupoint areas. Furthermore, the effect of moxibustion is stronger than that of acupuncture, and this may be attributed to heat radiation and some chemical substances released from the burning of moxa, which possibly stimulates the MC at acupoint areas by the penetrating effect of moxibustion heat.
In conclusion, moxibustion stimulation may exert its effect on TNBS-induced colitis rats by triggering the degranulation of local MC at ST25 acupoints.
COMMENTS
Background
Previous studies on the effective mechanism of acupuncture stimulation show that it is closely related to the degranulation of mast cells (MC) at acupoint areas. The research has indicated that moxibustion stimulation has a beneficial effect on inflammatory bowel disease, and ST25 is an efficacious point in the clinical treatment of patients with inflammatory bowel disease. However, the correlation between the effect of moxibustion and the response of MC at acupoint areas is still unclear.
Research frontiers
With further study on the mechanism of moxibustion, more and more data show that MC at acupoints play an important role in bridging acupoint areas and target organs, which had become a the hot topic of study.
Innovations and breakthroughs
The results of the authors’ study have proved that moxibustion is effective in TNBS-induced colitis rats. Moxibustion therapy exerts its effect on healing impaired colonic mucosa by triggering degranulation of local MC at ST25 acupoints.
Applications
The experimental data has important clinical significance and can be used in the further study of moxibustion therapy in the treatment of inflammatory bowel disease.
Peer review
This study investigated the relationship between the mast cell degranulation at the Tianshu (ST25) acupoint of moxibustion and the development of trinitro-benzene-sulfonic acid-induced colitis in rats. This is a study supported by The National Basic Research Program of China (973 program), etc, attempting to explore the mechanism of moxibustion. This kind of research would have important clinical significance and should certainly be encouraged. In general, this paper was well written.
Footnotes
Supported by National Basic Research Program of China (973 program), No. 2009CB522900; National Natural Science Foundation of China, No. 30973785; Shanghai Leading Academic Discipline Project, No. S30304; Shanghai Rising-Star Program, No. 10QA1406100
Peer reviewer: Xiaofa Qin, MD, PhD, Department of Surgery, UMDNJ-New Jersey Medical School, 185 South Orange Avenue, Newark, NJ 07103, United States
S- Editor Tian L L- Editor Webster JR E- Editor Zhang DN
References
- 1.Zhang D, Ding GH, Shen XY, Yao W, Zhang ZY, Zhang YQ, Lin JY. [Influence of mast cell function on the analgesic effect of acupuncture of “Zusanli” (ST 36) in rats] Zhen Ci Yan Jiu. 2007;32:147–152. [PubMed] [Google Scholar]
- 2.Lin J, Huang H, Ding GH, Zhang D. [Relationship between the function of mast cells and acupuncture analgesia in adjuvant arthritis rats] Zhen Ci Yan Jiu. 2007;32:16–19. [PubMed] [Google Scholar]
- 3.Yu XJ, Zhan R, Huang H, Ding GH. [Analysis on the difference of afferent mechanism of analgesic signals from manual acupuncture and electroacupuncture of “Zusanli” (ST 36)] Zhen Ci Yan Jiu. 2008;33:310–315. [PubMed] [Google Scholar]
- 4.Yu XJ, Ding GH, Yao W, Zhan R, Huang M. [The role of collagen fiber in “Zusanli” (ST 36) in acupuncture analgesia in the rat] Zhongguo Zhen Jiu. 2008;28:207–213. [PubMed] [Google Scholar]
- 5.Huang H, Zhan R, Yu XJ, Zhang D, Li WM, Ding GH. [Effects of acupoint-nerve block on mast cell activity, manual acupuncture- and electroacupuncture-induced analgesia in adjuvant arthritis rats] Zhen Ci Yan Jiu. 2009;34:31–35, 56. [PubMed] [Google Scholar]
- 6.Cheng K, Shen XY, Ding GH, Wu F. [Relationship between laser acupuncture analgesia and the function of mast cells] Zhongguo Zhen Jiu. 2009;29:478–483. [PubMed] [Google Scholar]
- 7.Wu HG, Liu HR, Zhang ZA, Zhou EH, Wang XM, Jiang B, Shi Z, Zhou CL, Qi L, Ma XP. Electro-acupuncture relieves visceral sensitivity and decreases hypothalamic corticotropin-releasing hormone levels in a rat model of irritable bowel syndrome. Neurosci Lett. 2009;465:235–237. doi: 10.1016/j.neulet.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Wu HG, Liu HR, Tan LY, Gong YJ, Shi Y, Zhao TP, Yi Y, Yang Y. Electroacupuncture and moxibustion promote neutrophil apoptosis and improve ulcerative colitis in rats. Dig Dis Sci. 2007;52:379–384. doi: 10.1007/s10620-006-9561-y. [DOI] [PubMed] [Google Scholar]
- 9.Wu HG, Gong X, Yao LQ, Zhang W, Shi Y, Liu HR, Gong YJ, Zhou LB, Zhu Y. Mechanisms of acupuncture and moxibustion in regulation of epithelial cell apoptosis in rat ulcerative colitis. World J Gastroenterol. 2004;10:682–688. doi: 10.3748/wjg.v10.i5.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu HG, Zhou LB, Pan YY, Huang C, Chen HP, Shi Z, Hua XG. Study of the mechanisms of acupuncture and moxibustion treatment for ulcerative colitis rats in view of the gene expression of cytokines. World J Gastroenterol. 1999;5:515–517. doi: 10.3748/wjg.v5.i6.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langevin HM, Yandow JA. Relationship of acupuncture points and meridians to connective tissue planes. Anat Rec. 2002;269:257–265. doi: 10.1002/ar.10185. [DOI] [PubMed] [Google Scholar]
- 12.Langevin HM, Churchill DL, Cipolla MJ. Mechanical signaling through connective tissue: a mechanism for the therapeutic effect of acupuncture. FASEB J. 2001;15:2275–2282. doi: 10.1096/fj.01-0015hyp. [DOI] [PubMed] [Google Scholar]
- 13.Langevin HM, Churchill DL, Fox JR, Badger GJ, Garra BS, Krag MH. Biomechanical response to acupuncture needling in humans. J Appl Physiol. 2001;91:2471–2478. doi: 10.1152/jappl.2001.91.6.2471. [DOI] [PubMed] [Google Scholar]
- 14.Zhang D, Ding GH, Shen XY, Wang L, Liu C. Development of researches on the connective tissues and acupuncture. Zhen Ci Yan Jiu. 2004;29:77–81. [Google Scholar]
- 15.Theoharides TC, Bielory L. Mast cells and mast cell mediators as targets of dietary supplements. Ann Allergy Asthma Immunol. 2004;93:S24–S34. doi: 10.1016/s1081-1206(10)61484-6. [DOI] [PubMed] [Google Scholar]
- 16.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 17.Theoharides TC, Singh LK, Boucher W, Pang X, Letourneau R, Webster E, Chrousos G. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its proinflammatory effects. Endocrinology. 1998;139:403–413. doi: 10.1210/endo.139.1.5660. [DOI] [PubMed] [Google Scholar]
- 18.Zhang D, Ding G, Shen X, Yao W, Zhang Z, Zhang Y, Lin J, Gu Q. Role of mast cells in acupuncture effect: a pilot study. Explore (NY) 2008;4:170–177. doi: 10.1016/j.explore.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- 20.Li M, Shi J, Liu XC, Wang LN, Zhang J, Li LL, Guan XM. Effects of electroacupuncture on the number of subcutaneous mast cells in and beside the acupoint and the inflammatory pain focus in the rat. Zhongguo Zhen Jiu. 2003;23:597–601. [Google Scholar]
- 21.Yang YM, Wang PP. Preliminary study on the effect of acupuncture on experimental cardiac infarction. Journal of J Tradit Chin Med. 1980;5:79–80. [Google Scholar]
- 22.Ming CR, Cai S, Cai YW, Ma TM. Observation of MC in the deep aponeurosis under the fluorescent microscope by electric needling in “Zusanli”. Zhen Ci Yan Jiu. 2000;25:51–53. [Google Scholar]
- 23.He JN, Luo MF. [Progress in the study on the relationship between effects of acu-moxibustion and mast cells in acupoints] Zhen Ci Yan Jiu. 2007;32:214–216. [PubMed] [Google Scholar]
- 24.Kimura M, Mastrogiovanni F, Toda S, Kuroiwa K, Tohya K, Sugata R, Ohnishi M. An electron microscopic study of the acupuncture or moxibustion stimulated regional skin and lymph node in experimental animals. Am J Chin Med. 1988;16:159–167. doi: 10.1142/S0192415X88000236. [DOI] [PubMed] [Google Scholar]
- 25.Menjo Y, Kobayashi M, Hayashi A, Nakayama H, Kobayashi K. [Ultrastructural changes of collagen fibrils in mouse dermal connective tissue after moxibustion treatment] Kaibogaku Zasshi. 2002;77:7–15. [PubMed] [Google Scholar]
- 26.Piotrowski W, Devoy MA, Jordan CC, Foreman JC. The substance P receptor on rat mast cells and in human skin. Agents Actions. 1984;14:420–424. doi: 10.1007/BF01973842. [DOI] [PubMed] [Google Scholar]
- 27.Zhang BZ, Wang JM. Discovery of the neuromastocytic junctions on the meridian line in human skin II. The afferent neuromastocytic junction and the Schwann cells accompanying efferent axons. Shenjing Jiepouxue Zazhi. 1985;1:107–111. [Google Scholar]
- 28.Wang JM, Zhang BZ. Light and electron microscopic study of the relationship of the substance P and vasoactive intestinal polypeptide immunoreactive nerve fibres with mast cells on meridian line in human skin. Shenjing Jiepouxue Zazhi. 1986;2:79–84. [Google Scholar]
- 29.Chen LW, Zhang BZ. Discovery of the neuromastocytic junction on the modelled meridian line in mouse skin - a study by light and electron microscopical immunohistochemistry. Shenjing Jiepouxue Zazhi. 1987;3:253–258. [Google Scholar]
- 30.Deng Y, Zeng T, Zhou Y, Guan X. [The influence of electroacupuncture on the mast cells in the acupoints of the stomach meridian] Zhen Ci Yan Jiu. 1996;21:68–70. [PubMed] [Google Scholar]
- 31.Yang YM, Waug PP. [Morphological observation on the effect of acupuncture on mast cells at the zusanli point] Zhen Ci Yan Jiu. 1986;11:298–302. [PubMed] [Google Scholar]
- 32.Shen DK. A study on the morphological structure for inducing needling sensations in acupiont. Zhonghua Zhongyiyao Zazhi. 1989;4:57–64. [Google Scholar]
- 33.Ding GH, Shen XY, Yao W, Dang RS, Yang J, Chen EY. Dynamic mechanism of tissue fluid directional flowing and meridian phenomenon of human body. Prog Nat Sci. 2005;15:61–70. [Google Scholar]