Abstract
AIM: To investigate the expression of Toll-like receptor (TLR) 3, TLR4, TLR7 and TLR9 in esophageal squamous cell carcinoma (ESCC).
METHODS: Reverse transcription-polymerase chain reaction and immunohistochemistry were used to analyze the expression of TLR3, TLR4, TLR7 and TLR9 mRNA and protein in samples from 87 esophageal cancer patients consisting of both tumor and normal tissue.
RESULTS: A significant increase in TLR3, TLR4, TLR7 and TLR9 mRNA levels was detected in ESCC samples. Tumors exhibited high TLR protein expression, (70.1%, 72.4%, 66.7% and 78.2% for TLR3, TLR4, TLR7 and TLR9, respectively, P < 0.05). Nevertheless, a significant percentage of tumors also exhibited TLR4 expression in mononuclear inflammatory cells (48.3%) and TLR9 expression in fibroblast-like cells (60.9%). Tumors with high TLR3 expression in tumor cells or high TLR4 expression in mononuclear inflammatory cells were significantly associated with a higher probability of lymph node metastasis and increased depth of invasion. However, tumors with high TLR9 expression in fibroblast-like cells were associated with low probabilities of invasion and metastasis. There was no significant variation between the expression of TLR3, TLR4, TLR7 and TLR9 among different ethnic groups.
CONCLUSION: TLR3, TLR4, TLR7 and TLR9 expression appears important to the biological pathogenesis of ESCC. TLRs may represent therapeutic targets for ESCC.
Keywords: Esophageal squamous cell carcinoma, Invasion, Metastasis, Prognosis, Toll-like receptor
INTRODUCTION
Esophageal carcinoma (EC) remains a major threat to health worldwide, with a 5-year survival rate below 10%, and in China, EC is characterized by its distinct geographic distribution and differences in ethnic prevalence[1]. Xinjiang, in Western China, has one of the highest prevalences of esophageal squamous cell carcinoma (ESCC) in the world, and the ratio in ESCC incidence between different ethnic groups is as large as 13.4:1. ESCC has become the main cause of tumor-related deaths in the Kazak ethnic group in Xinjiang[2]. Despite advances in clinical treatment, ESCC prognosis remains poor due to its relapse and metastasis characteristics. For these reasons, prognostic factors are essential to improve the classic risk classification in ESCC.
Chronic infection and inflammation can induce cancer formation via cytokines and chemokines, which play vital roles in promoting angiogenesis and metastasis, the most important factors contributing to cancer development and growth. Toll-like receptors (TLRs) comprise an important family of pattern recognition receptors that allow immune cells to recognize pathogens and trigger inflammatory responses, as they are expressed not only in a variety of immune cells but also in non-immune cells such as fibroblasts and epithelial cells. These responses include the secretion of cytokines that increase the resistance of infected cells as well as the release of chemokines that recruit immune cells to necrotic cells. Chronic inflammation can promote carcinogenesis by inducing gene mutations, inhibiting apoptosis, or stimulating angiogenesis and cell proliferation. Research has demonstrated that basement membrane changes induced by chronic inflammation are correlated with the aberrant proliferation of esophageal epithelia[3]. The TLR signaling pathway activates several different signaling elements, including nuclear factor kappa B (NF-κB), extracellular signal regulated kinase, and Jun-NH-kinase/p38, which regulate many immunologically related proteins[4]. Several researchers also found that MyD88 (the TLR-mediated signaling adapter protein) plays an important role not only in the pathway of TLR-mediated inflammation but also in Ras-MAPK signaling, cell-cycle control, and cell transformation, which promote carcinogenesis[5,6]. Evidence indicates that TLR expression in tumor cells can promote inflammation and cell survival in the tumor microenvironment[7-9]. These results suggested that TLR stimulation could lead to tumor progression. These findings may be useful in elucidating potential prognostic markers.
The purpose of the present study was to investigate the expression of TLR3, TLR4, TLR7 and TLR9 in ESCC as well as their association with the clinicopathologic characteristics of ESCC. To this aim, we analyzed the protein levels of TLR3, TLR4, TLR7 and TLR9 by immunohistochemical techniques and their mRNA levels by reverse transcription-polymerase chain reaction (RT-PCR).
MATERIALS AND METHODS
Clinical samples
A total of 87 formalin-fixed and paraffin-embedded tissue blocks were obtained from esophageal carcinoma patients who had not received pre-operative radiotherapy or chemotherapy; all patients were treated at the Department of Thoracic Surgery of the First Affiliated Hospital in Medical University of Xinjiang from June 2007 to March 2009, and the borderline tumor tissues were used as controls. Patient ethnicity was as follows: Han, 30 patients; Uyghur, 25 patients; and Kazak, 32 patients. The mean age of the patients was 50.5 years; the youngest patient was 39 years old, and the oldest patient was 73 years old at the time of surgery. Each specimen was histologically examined, and the tumor was graded by at least two experienced pathologists. The main characteristics of ESCC patients, including tumor grade, stage, and lymph node status of the tumor, were categorized according to the TNM (American Joint Committee on Cancer, 4th edition) as follows: (1) 20 cases; (2) 34 cases; and (3) 33 cases. Among the 87 tumors were 30 well-differentiated tumors, 29 moderately differentiated tumors, and 28 poorly differentiated tumors. Fifty-three patients had lymph node metastases. In addition, 40 frozen biopsies that included 20 normal esophageal epithelia and 20 ESCC samples were subjected to RT-PCR for the detection of TLR3, TLR4, TLR7 and TLR9 mRNA expression. All patients were enrolled by written informed consent, and the study was approved by the Ethical Committee of the Medical University of Xinjiang.
Immunohistochemical studies
Sections of 3-μm-thick paraffin-embedded tissue were deparaffinized in xylene and then rehydrated in a graded ethyl alcohol series (100%, 95%, 80% and 70%). For increased specificity and sensitivity, tissues were microwave-treated at 95 °C for 15 min to retrieve the antigen. After cooling and rinsing in distilled water, endogenous peroxide activity was blocked with 3% H2O2 for 15 min, after which samples were rinsed in 0.01 mol/L phosphate-buffered saline [phosphate buffered saline (PBS), pH 7.4] for 10 min and then preincubated with a protein blocking solution for 10 min. Primary antibodies (mouse monoclonal anti-human TLR3, TLR4, TLR7 and TLR9 were obtained from Santa Cruz Biotechnology, Santa Cruz, CA, United States). Antibodies were diluted at 1:200 in PBS and applied at 4 °C overnight in a humid chamber. Slides were washed three times in PBS and then incubated with secondary biotinylated antibody for 15 min at room temperature. Antigen-antibody complexes were detected using the streptavidin-peroxidase method (15-min exposure) with diaminobenzidine [diaminobenzidine (DAB)] as the chromogen substrate (Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA, United States). The peroxidase signal was visualized by treatment with a DAB substrate-chromogen system for 8 min. Finally, the sections were stained lightly with hematoxylin, and PBS was used in place of the primary antibody as a negative control. All immunostained sections were coded and independently examined by two investigators using light microscopy. The results were scored on a scale from 0 to 4 for the percentage of positive cells and from 0 to 3 for the intensity of positive cells. The percentage of positive cells was scored as follows: ≤ 10%, 11%-25%, 26%-50%, 51%-75%, and ≥ 76%. The intensity of staining was scored as follows: absent, weak staining, moderate staining, and intense staining. The overall score (percentage of stained cells ×intensity of staining) was then used to identify the mean score by using an Excel spreadsheet [≥ mean score for positive (+); < mean score for negative (-)], in line with a previous study[10].
RNA extraction and RT-PCR
Total RNA was extracted from fresh frozen tissue using TRIzol (Invitrogen, Carlsbad, CA, United States) as described by the manufacturer. mRNA was reverse-transcribed with RevertAid (MBI Fermentas, Burlington, Ontario, Canada) at 42 °C for 60 min, and the synthesized cDNA (20 ng) was subjected to polymerase chain reaction (PCR) (95 °C for 1 min, 25 or 30 cycles of 95 °C for 3 s, 60 °C for 30 s, and 68 °C for 1 min, and a single extension at 68 °C for 10 min). PCR products were separated on a 4% agarose gel and visualized with ethidium bromide. Each analysis was repeated at least twice to ensure reproducibility. mRNA for β-actin was used as a normalization control in RT-PCR and as a loading control in conventional PCR. Forward and reverse primer pairs are listed in Table 1, and their products were 181 bp, 198 bp, 172 bp and 97 bp.
Table 1.
Polymerase chain reaction primers used for the detection of Toll-like receptors
| TLR | Forward | Reverse |
| TLR3 | AGTGCCGTCTATTTGCCACACA | AACAGTGCACTTGGTGGTGGAG |
| TLR4 | TCTTCAACCAGACCTCTACATTCCA | GGAACATCCAGAGTGACATCACAG |
| TLR7 | CCGTGACAATTACCTGGCCTTC | CAGGGCCTTCAGCTGGTTTC |
| TLR9 | AGGATGATGCCAGGATGATGTC | TCAGGTCCAGGTTCTTGGTTGAG |
| β-actin | GGCACCCAGCACAATGAAG | CCGATCCACACGGAGTACTTG |
TLR: Toll-like receptor.
Statistical analysis
All statistical analyses were performed with the SPSS statistical software package (version 15.0). The chi-square test was used to compare the differences in cumulative TLR3, TLR4, TLR7 and TLR9 expression between normal and ESCC groups, and to determine whether the clinicopathologic variables were associated with the levels of TLR3, TLR4, TLR7 and TLR9 as well as compare the mRNA expression in fresh frozen ESCC tissues with that of normal samples as determined by RT-PCR. P values < 0.05 were considered statistically significant.
RESULTS
TLR protein expression in ESCC and their association with ESCC clinicopathologic characteristics
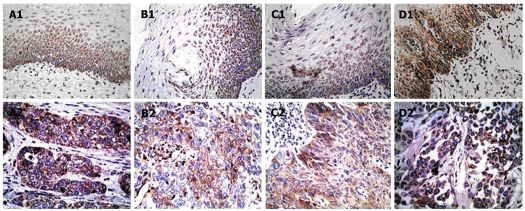
Immunohistochemistry (IHC) staining of 87 primary ESCC lesions and normal esophageal tissues was performed using anti-TLRs antibodies (Table 2). Representative staining patterns for TLRs are shown in Figure 1. IHC staining demonstrated that TLRs were localized in the cytoplasm, but TLR3 was also expressed in the cell membrane. Positive staining for TLR4 and TLR9 was generally observed within normal esophageal surface epithelium, but weak or no TLR4 and TLR9 staining was detected in stromal cells. However, in ESCC, TLRs were strongly expressed not only in cancer cells, but also in some stromal cells, such as fibroblast-like cells and mononuclear inflammatory cells. The positive rates of TLR3, TLR4, TLR7 and TLR9 expression in the normal esophageal surface epithelium were 8.0%, 5.7%, 9.2% and 4.6%, respectively. These values sharply increased to 70.1%, 66.7%, 72.4% and 78.2%, respectively, in ESCC lesions (P < 0.05 compared with the positive rate in healthy tissue). Nevertheless, a significant percentage of tumors also exhibited TLR4 expression in mononuclear inflammatory cells (48.3%) and TLR9 expression in fibroblast-like cells (60.9%). Table 3 summarizes the percentages of TLR staining in each cellular type. TLR3 and TLR7 were mainly expressed in esophageal tumor cells, and there was a statistically significant difference compared with the expression in the control group.
Table 2.
Statistical analysis of Toll-like receptor expression and clinicopathologic factors in esophageal carcinoma
| Characteristics | TLR3 | P | TLR4 | P | TLR7 | P | TLR9 | P |
| Positive (%) | Positive (%) | Positive (%) | Positive (%) | |||||
| Normal control (n = 87) | 7 (8) | 5 (5.7) | 8 (9.2) | 4 (4.6) | ||||
| Tumor differentiation | 61 (70.1) | 63 (72.4) | 58 (66.7) | 68 (78.2) | ||||
| W (n = 30) | 22 (75.3) | 0.889 | 22 (75.3) | 0.539 | 14 (46.7) | 0.003 | 18 (60.0) | 0.004 |
| M (n = 29) | 20 (69.1) | 19 (65.6) | 19 (65.6) | 23 (79.3) | ||||
| P (n = 28) | 19 (67.9) | 22 (78.6) | 25 (89.3) | 27 (96.4) | ||||
| Depth of invasion | ||||||||
| ≤ muscularis (n = 37) | 18 (48.6) | < 0.001 | 27 (73.1) | 0.92 | 23 (62.2) | 0.002 | 27 (73.1) | 0.314 |
| ≥ adventitia (n = 50) | 43 (86.0) | 36 (72.0) | 35 (66.0) | 41 (82.0) | ||||
| LN metastasis | ||||||||
| Negative (n = 34) | 18 (52.9) | 0.005 | 20 (58.8) | 0.023 | 26 (76.5) | 0.12 | 29 (85.3) | 0.197 |
| Positive (n = 53) | 43 (81.1) | 43 (81.1) | 32 (60.3) | 39 (73.6) | ||||
| Ethnic groups | ||||||||
| Han (n = 30) | 23 (76.7) | > 0.05 | 22 (73.3) | > 0.05 | 17 (56.7) | > 0.05 | 23 (76.7) | > 0.05 |
| Uyghur (n = 25) | 18 (72.0) | 20 (80.0) | 16 (64.0) | 20 (80.0) | ||||
| Kazak (n = 32) | 20 (62.5) | 21 (65.6) | 25 (78.1) | 25 (78.1) |
All values are presented as the number of cases, with percentages in parentheses. W: Well differentiated; M: Moderately differentiated; P: Poorly differentiated; LN: Lymph node; TLR: Toll-like receptor.
Figure 1.
Immunohistochemistry staining of esophageal lesions with Toll-like receptor-specific mAbs. A1 to D1 show the expression of Toll-like receptor (TLR) 3, TLR4, TLR7 and TLR9 in normal esophageal epithelium, respectively. A2 and C2 show positive staining for TLR3 and TLR7 in esophageal squamous cell carcinoma cells. B2 shows positive TLR4 staining in tumor cells and mononuclear inflammatory cells, and D2 shows positive TLR9 staining in tumor cells and fibroblast-like cells (Original magnification, × 400).
Table 3.
The percentage expression of Toll-like receptors in each cellular type within esophageal squamous cell carcinoma tissues
|
Tumor cells |
Fibroblast |
MICs |
|
| Factors | Positive cases (%) | Positive cases (%) | Positive cases (%) |
| TLR3 | 61 (70.1) | 3 (3.4) | 4 (4.6) |
| TLR4 | 63 (72.4) | 8 (9.2) | 47 (48.3) |
| TLR7 | 58 (66.7) | 5 (5.7) | 2 (2.3) |
| TLR9 | 68 (78.2) | 53 (60.9) | 12 (13.8) |
All values are presented as the number of cases, with percentages in parentheses. MICs: Mononuclear inflammatory cells; TLR: Toll-like receptor.
We also evaluated the possible relationship between the expression of TLRs in tumor cells and the clinicopathologic characteristics of ESCC including tumor stage, histological grade, lymph node metastasis, and depth of invasion. TLR3 expression in tumor cells was significantly associated with depth of invasion and lymph node metastasis. TLR4 expression in tumor cells was significantly associated with lymph node metastasis. TLR7 expression in tumor cells was significantly associated with tumor grade. TLR9 expression was found to gradually increase with worsening histopathological grade (P < 0.005, Table 2). However, the TLR9 IHC staining scores did not correlate with the depth of invasion and lymph node metastasis.
We analyzed the association between the expression of TLR4 and TLR9 in tumor stromal cells and poor prognostic indicators because a significant percentage of tumors also exhibited TLR4 expression in mononuclear inflammatory cells and TLR9 expression in fibroblast-like cells. We found that carcinoma patients with higher TLR4 expression in the stromal compartment had a significantly higher risk of disease progression. TLR4 expression in mononuclear inflammatory cells (48.3%) was significantly associated with the depth of invasion and lymph node metastasis. Conversely, TLR9 expression in fibroblast-like cells (60.9%) was significantly associated with reduced depth of invasion and lymph node metastasis (Table 4). We also observed variations between the expression of TLR3, TLR4, TLR7 and TLR9 in tumor cells among different ethnic groups in Xinjiang, although the differences were not statistically significant.
Table 4.
Analysis of the relationship between the expression of Toll-like receptors in each cellular type with the clinicopathologic characteristics of esophageal squamous cell carcinoma n (%)
| Factors |
Tumor Grade |
Depth of invasion |
LN metastasis |
||||
| W | M | P | ≤ muscularis | ≥ adventitia | Negative | Positive | |
| Number of cases | 30 | 29 | 28 | 37 | 50 | 34 | 53 |
| TLR4 MICs (+) | 19 (63.3) | 16 (55.2) | 12(42.9) | 12(32.4) | 35(70) | 14(41.2) | 33(62.3) |
| P | 0.245 | 0.001 | 0.018 | ||||
| TLR9 fibroblast (+) | 20 (66.7) | 18 (62.1) | 17(60.7) | 30(81.1) | 23(43.4) | 29(85.3) | 24(45.3) |
| P | 0.885 | 0.001 | 0.006 | ||||
All values are presented as the number of cases, with percentages in parentheses. TLR: Toll-like receptor; LN: Lymph node; MICs: Mononuclear inflammatory cells; W: Well differentiated; M: Moderately differentiated; P: Poorly differentiated.
TLR mRNA expression in ESCC and normal controls
To confirm the IHC results, TLR3, TLR4, TLR7 and TLR9 mRNA expression in esophageal biopsies was detected by RT-PCR (Figure 2). Similar to the IHC results, TLR3, TLR4, TLR7 and TLR9 mRNA expression was increased in ESCC tissues. TLR3 and TLR7 gene expression was quantified in 15 ESCC and 3 normal esophageal tissues. TLR4 mRNA expression was higher in ESCC samples than in normal controls after normalization to β-actin expression. The percentages of TLR9 mRNA positivity in ESCC and normal tissues were 55% and 15%, respectively. Although the sample size was limited, the differences in TLR3, TLR4, TLR7 and TLR9 mRNA expression levels between normal esophageal epithelia and ESCC were statistically significant (P < 0.05).
Figure 2.
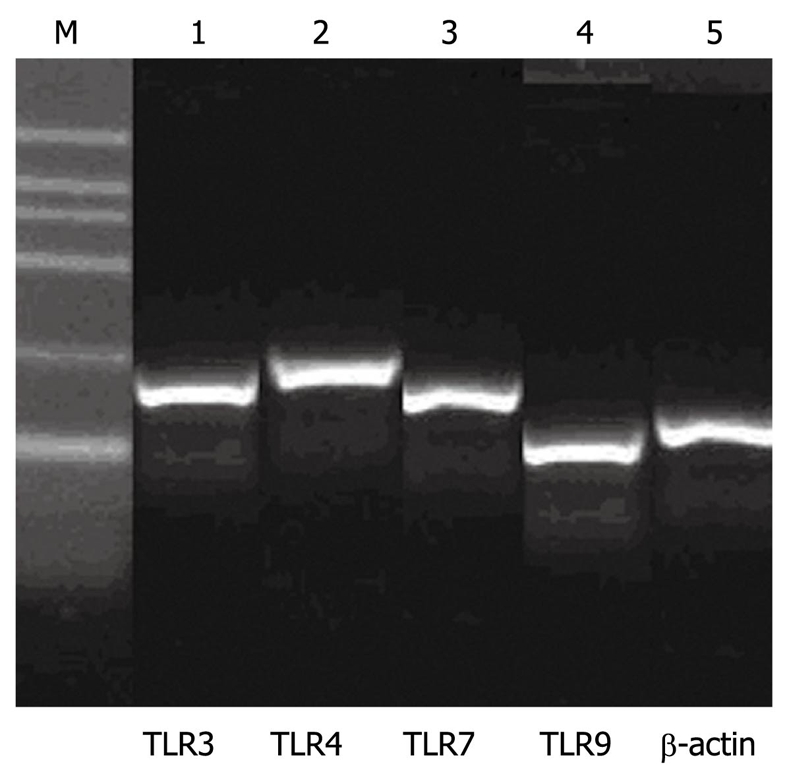
mRNA expression patterns of Toll-like receptor 3, Toll-like receptor 4, Toll-like receptor 7 and Toll-like receptor 9. M: 100-600 bp marker ladder. Lanes 1 to 4 show the expression of Toll-like receptors, and lane 5 shows the expression of β-actin.
DISCUSSION
This study demonstrated that samples of recurrent EC exhibited significantly higher mRNA levels of TLR3, TLR4, TLR7 and TLR9 than normal tissue. ESCC tumors exhibited high TLR protein expression in cancer cells. Nevertheless, a significant percentage of tumors also exhibited TLR4 expression in mononuclear inflammatory cells and TLR9 expression in fibroblast-like cells. Tumors with high TLR3 expression in tumor cells or high TLR4 expression in mononuclear inflammatory cells were significantly associated with poor prognosis. However, tumors with high TLR9 expression in fibroblast-like cells were associated with a low probability of metastasis.
In this study, high TLR3 expression in esophageal cancer cells was associated with a high probability of lymph node metastasis. Similar observations were made in different cancer types. Studies on breast and prostate carcinomas demonstrated that high TLR3 expression was significantly associated with higher probabilities of metastasis and biochemical recurrence[10,11], which is in agreement with previous studies indicating that TLR3 expression is related to tumor aggressiveness[12,13]. Although the precise effect of increased TLR3 expression requires further investigation, our work suggests that TLR3 plays a vital role in esophageal carcinogenesis. Therefore, TLR3 may represent a good therapeutic target in esophageal cancer.
This study also demonstrated that the expression level of TLR4 in tumor cells was significantly associated with depth of invasion. TLR7 and TLR9 expression was positively associated with tumor grade in ESCC. Moreover, studies on TLR4 and TLR7 expression in gastric and lung cancer cells have suggested that high TLR expression results in increased tumor progression[14,15] and stimulation with TLR7 agonists lead to NF-κB activation, upregulated expression of the antiapoptotic protein Bcl-2, increased tumor cell survival, and chemoresistance[16,17]. It has also been reported that TLR9 expression gradually increased during the progression from normal cervical squamous epithelial tissues to cervical intraepithelial neoplasia and invasive cervical cancer[18]. These findings indicated that increased TLR protein expression may interfere with normal TLR signaling pathways and function and may represent useful markers of the malignant transformation of cancer cells. In addition, cancer cells activated by TLR signals may release cytokines and chemokines that in turn recruit and stimulate immune cells to release additional cytokines and chemokines. This process results in immune tolerance, cancer progression, and propagation of the tumor microenvironment.
In this study, we also observed the expression of TLR3, TLR4, TLR7 and TLR9 in tumor stromal cells as well as their association with the clinicopathologic characteristics of ESCC. Tumor stromal cells such as fibroblast-like cells, mononuclear inflammatory cells, and numerous intracellular mediators comprise the tumor microenvironment. These factors actively participate in tumor progression and infiltration, where the tumor microenvironment not only responds to and supports carcinogenesis but also contributes to tumor initiation, progression, and metastasis. The interaction between transformed cells and the microenvironment determines the fate of the tumor. Another interesting finding was TLR4 expression in mononuclear inflammatory cells and TLR9 expression in fibroblast-like cells, which are associated with ESCC prognostic factors. TLR4 expression in mononuclear inflammatory cells was associated with an increased incidence of lymph node metastasis and depth of invasion, and TLR9 expression in fibroblast-like cells was associated with a low rate of lymph node metastasis. These findings are supported by research that demonstrated the importance of tumor stromal cells in tumor behavior through the release of various growth factors, proteases, and extracellular matrix proteins, which induce gene mutations, inhibit apoptosis, and stimulate angiogenesis and cell proliferation[19-21].
Metastatic relapse attributable to the presence of tumor cells within lymph nodes is the most frequent cause of cancer-related death in patients with esophageal tumors[22]. In the current study, the high expression of TLR4 by mononuclear inflammatory cells was associated with an increased incidence of lymph node metastasis and depth of invasion, suggesting that the regulation of the immune response within the tumor microenvironment might be another consequence of TLR activation. TLR9 expression by fibroblast-like cells was associated with good patient prognosis, suggesting that the surrounding connective tissue of the tumor is important for preventing tumor spread. Therefore, our results also suggest the existence of different phenotypes of stromal cells that influence prognosis depending upon the expression pattern of TLRs. In this study, TLR3, TLR4, TLR7 and TLR9 expression appeared important to the biological pathogenesis of esophageal cancer. However, different phenotypes of TLR expression in stromal cells can lead to different results and as a series of candidate prognostic factors, the function of these markers in ESCC should be further investigated.
COMMENTS
Background
The prognosis of esophageal carcinoma remains poor due to its relapse and metastasis characteristics. Toll-like receptors (TLRs) comprise an important family of pattern recognition receptors, and the TLR signaling pathway activates several different signaling elements, including nuclear factor kappa B, extracellular signal regulated kinase, and Jun-NH-kinase/p38, which regulate many immunologically related proteins, alter the microenvironment of tumors, and promote tumor progression and metastasis. Esophageal squamous cell carcinoma (ESCC) progression is associated with TLR stimulation, a crucial event in immune escape and metastasis.
Research frontiers
TLR expression is a common event in several cancers; this can promote carcinogenesis by inducing gene mutations, inhibiting apoptosis, or stimulating angiogenesis and cell proliferation. However, TLR expression has not been reported in esophageal carcinoma. In this study, the authors demonstrated that the overexpression of TLR3, TLR4, TLR7 and TLR9 in ESCC appears to be important in the biological pathogenesis of ESCC.
Innovations and breakthroughs
This is the first study to report that TLR3, TLR4, TLR7 and TLR9 overexpression appears important in the biological pathogenesis of ESCC. Furthermore, high TLR4 expression in mononuclear inflammatory cells was significantly associated with a higher probability of lymph node metastasis and depth of invasion, and high TLR9 expression in fibroblast-like cells was associated with low probabilities of invasion and metastasis.
Applications
TLR3, TLR4, TLR7 and TLR9 expression may represent potential prognostic markers and therapeutic targets for ESCC.
Terminology
TLRs, a family of pattern recognition receptors expressed in immune and non-immune cells, play a crucial role in the innate immune response and the subsequent induction of adaptive immune responses against microbial infection or tissue injury. Furthermore, TLR expression in cancer cells is associated with tumor proliferation and invasion.
Peer review
This manuscript by Dr. Sheyhidin et al investigated the expression of TLR3, TLR4, TLR7 and TLR9 in ESCC tissues from a good number of patients. The resulted data confirmed upregulation of TLRs in ESCC and its association with clinic pathological characteristics. This study is clinical significance because it included a good number of patients including patients from various ethnic groups. Nevertheless, the data generated from this study are confirmative in nature.
Footnotes
Supported by Doctoral Program of Higher Specialized Research Fund project (20106517110003)
Peer reviewer: Xian-Ming Chen, MD, Associate Professor, Department of Medical Microbiology and Immunology, Creighton University, 2500 California Plaza, Omaha NE 68178, United States
S- Editor Tian L L- Editor Webster JR E- Editor Li JY
References
- 1.He J, Gu D, Wu X, Reynolds K, Duan X, Yao C, Wang J, Chen CS, Chen J, Wildman RP, et al. Major causes of death among men and women in China. N Engl J Med. 2005;353:1124–1134. doi: 10.1056/NEJMsa050467. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y. The distribution of esophageal cancer in Xinjiang. Journal of xinjiang medical university. 1988;11:139–144. [Google Scholar]
- 3.Zhang GH, Su M, Tian DP. Effect of chronic inflammation-induced basement membrane changes on esophageal carcinogenesis. Ai Zheng. 2005;24:1071–1075. [PubMed] [Google Scholar]
- 4.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 5.Coste I, Le Corf K, Kfoury A, Hmitou I, Druillennec S, Hainaut P, Eychene A, Lebecque S, Renno T. Dual function of MyD88 in RAS signaling and inflammation, leading to mouse and human cell transformation. J Clin Invest. 2010;120:3663–3667. doi: 10.1172/JCI42771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 7.Yu L, Chen S. Toll-like receptors expressed in tumor cells: targets for therapy. Cancer Immunol Immunother. 2008;57:1271–1278. doi: 10.1007/s00262-008-0459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith MF, Mitchell A, Li G, Ding S, Fitzmaurice AM, Ryan K, Crowe S, Goldberg JB. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-kappa B activation and chemokine expression by epithelial cells. J Biol Chem. 2003;278:32552–32560. doi: 10.1074/jbc.M305536200. [DOI] [PubMed] [Google Scholar]
- 9.Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, Visintin I, Rutherford T, Mor G. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006;66:3859–3868. doi: 10.1158/0008-5472.CAN-05-3948. [DOI] [PubMed] [Google Scholar]
- 10.González-Reyes S, Fernández JM, González LO, Aguirre A, Suárez A, González JM, Escaff S, Vizoso FJ. Study of TLR3, TLR4, and TLR9 in prostate carcinomas and their association with biochemical recurrence. Cancer Immunol Immunother. 2011;60:217–226. doi: 10.1007/s00262-010-0931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.González-Reyes S, Marín L, González L, González LO, del Casar JM, Lamelas ML, González-Quintana JM, Vizoso FJ. Study of TLR3, TLR4 and TLR9 in breast carcinomas and their association with metastasis. BMC Cancer. 2010;10:665. doi: 10.1186/1471-2407-10-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shojaei H, Oberg HH, Juricke M, Marischen L, Kunz M, Mundhenke C, Gieseler F, Kabelitz D, Wesch D. Toll-like receptors 3 and 7 agonists enhance tumor cell lysis by human gammadelta T cells. Cancer Res. 2009;69:8710–8717. doi: 10.1158/0008-5472.CAN-09-1602. [DOI] [PubMed] [Google Scholar]
- 13.Scarlett UK, Cubillos-Ruiz JR, Nesbeth YC, Martinez DG, Engle X, Gewirtz AT, Ahonen CL, Conejo-Garcia JR. In situ stimulation of CD40 and Toll-like receptor 3 transforms ovarian cancer-infiltrating dendritic cells from immunosuppressive to immunostimulatory cells. Cancer Res. 2009;69:7329–7337. doi: 10.1158/0008-5472.CAN-09-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmausser B, Andrulis M, Endrich S, Müller-Hermelink HK, Eck M. Toll-like receptors TLR4, TLR5 and TLR9 on gastric carcinoma cells: an implication for interaction with Helicobacter pylori. Int J Med Microbiol. 2005;295:179–185. doi: 10.1016/j.ijmm.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 15.He W, Liu Q, Wang L, Chen W, Li N, Cao X. TLR4 signaling promotes immune escape of human lung cancer cells by inducing immunosuppressive cytokines and apoptosis resistance. Mol Immunol. 2007;44:2850–2859. doi: 10.1016/j.molimm.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Cherfils-Vicini J, Platonova S, Gillard M, Laurans L, Validire P, Caliandro R, Magdeleinat P, Mami-Chouaib F, Dieu-Nosjean MC, Fridman WH, et al. Triggering of TLR7 and TLR8 expressed by human lung cancer cells induces cell survival and chemoresistance. J Clin Invest. 2010;120:1285–1297. doi: 10.1172/JCI36551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherfils-Vicini J, Damotte D, Fridman WH, Sautès-Fridman C, Cremer I. Human lung cancer: role of TLR7 and TLR8 in cell survival and chemoresistance. Med Sci (Paris) 2010;26:435–437. doi: 10.1051/medsci/2010264435. [DOI] [PubMed] [Google Scholar]
- 18.Lee JW, Choi JJ, Seo ES, Kim MJ, Kim WY, Choi CH, Kim TJ, Kim BG, Song SY, Bae DS. Increased toll-like receptor 9 expression in cervical neoplasia. Mol Carcinog. 2007;46:941–947. doi: 10.1002/mc.20325. [DOI] [PubMed] [Google Scholar]
- 19.Flores-Reséndiz D, Castellanos-Juárez E, Benítez-Bribiesca L. Proteases in cancer progression. Gac Med Mex. 2009;145:131–142. [PubMed] [Google Scholar]
- 20.Noël A, Emonard H, Polette M, Birembaut P, Foidart JM. Role of matrix, fibroblasts and type IV collagenases in tumor progression and invasion. Pathol Res Pract. 1994;190:934–941. doi: 10.1016/S0344-0338(11)80999-4. [DOI] [PubMed] [Google Scholar]
- 21.González LO, Pidal I, Junquera S, Corte MD, Vázquez J, Rodríguez JC, Lamelas ML, Merino AM, García-Muñiz JL, Vizoso FJ. Overexpression of matrix metalloproteinases and their inhibitors in mononuclear inflammatory cells in breast cancer correlates with metastasis-relapse. Br J Cancer. 2007;97:957–963. doi: 10.1038/sj.bjc.6603963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izbicki JR, Hosch SB, Pichlmeier U, Rehders A, Busch C, Niendorf A, Passlick B, Broelsch CE, Pantel K. Prognostic value of immunohistochemically identifiable tumor cells in lymph nodes of patients with completely resected esophageal cancer. N Engl J Med. 1997;337:1188–1194. doi: 10.1056/NEJM199710233371702. [DOI] [PubMed] [Google Scholar]