Abstract
Being deeply connected to signalling, cell dynamics, growth, regulation, and defence, endocytic processes are linked to almost all aspects of cell life and disease. In this review, we focus on endosomes in the classical endocytic pathway, and on the programme of changes that lead to the formation and maturation of late endosomes/multivesicular bodies. The maturation programme entails a dramatic transformation of these dynamic organelles disconnecting them functionally and spatially from early endosomes and preparing them for their unidirectional role as a feeder pathway to lysosomes.
Keywords: degradation, endocytosis, intralumenal vesicle (ILV), multivesicular body (MVB), sorting
Introduction
Endocytosis is the general term for internalization of fluid, solutes, macromolecules, plasma membrane components, and particles by the invagination of the plasma membrane and the formation of vesicles and vacuoles through membrane fission. In metazoan cells, endocytosed cargo includes a spectrum of nutrients and their carriers, receptor–ligand complexes, fluid, solutes, lipids, membrane proteins, extracellular–matrix components, cell-debris, bacteria, viruses, etc. By sorting, processing, recycling, storing, activating, silencing, and degrading incoming substances and receptors, endosomes are responsible for regulation and fine-tuning of numerous pathways in the cell.
Having left endosome research after the early discovery period in the 1980s, and returning to it only recently, one of us (AH) has been impressed by the amount of information that has become available in the meantime on most aspects of endocytosis. Also, it is evident that the central role of endocytosis in cell life and pathogenesis is now much more fully appreciated. The reason for concentrating again on this topic is our interest in host cell entry of animal viruses, the majority of which enter cells by endocytosis and exploit endosomes and the endocytic pathways for penetration into the cytosol (Marsh and Helenius, 1989; Mercer et al, 2010). While virus particles themselves are relative simple and in many cases well characterized, the challenge is to understand the cellular processes used by them, and the responses of the cell to the invasion. Deeper knowledge of endocytosis is urgently required. Incoming viral particles also provide a tool to learn more about the endocytic machinery.
In this review, we focus on a relatively narrow topic; the formation and maturation of late endosomes (LEs) in mammalian cells. They are also known as multivesicular bodies, because—although heterogeneous and variable in size and composition—most LEs have a multivesicular morphology, that is, they contain intralumenal vesicles (ILVs). There are many excellent reviews to recommend dealing with endosomes and different aspects of LE maturation. These include the following (Mellman, 1996; Zerial and McBride, 2001; Maxfield and McGraw, 2004; Piper and Katzmann, 2007; Luzio et al, 2007b; van Meel and Klumperman, 2008; Woodman and Futter, 2008; Saftig and Klumperman, 2009; Jovic et al, 2010; Von Bartheld and Altick, 2011).
The logistics of the endosome system
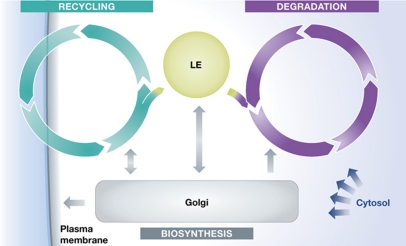
In a basic, ‘stripped-down’ representation, the classical endocytic pathway has only a few elements (Figure 1). The elements include a recycling circuit for plasma membrane components and their ligands, a degradative system for digestion of macromolecules, and a connecting, unidirectional feeder pathway for transport of fluid and selected membrane components from the recycling circuit to the degradative system. The feeder function is mediated by LEs. LEs also function as a system for mediating transport of lysosomal components from the trans-Golgi network (TGN) to lysosomes. This allows maintenance, diversification, and expansion of the recycling and degradative systems. Finally, the cytosol must be included among the essential elements, because it provides a spectrum of transiently associated, peripheral membrane components that support, regulate, and define the pathway (Figure 1).
Figure 1.
The basic elements of the endocytic machinery. The membrane organelles involve a recycling circuit (the plasma membrane (PM), the EEs, the recycling endosomes, and a variety vesicular carriers), a degradation cycle (lysosomes), and a connecting ‘feeder’ pathway (LEs) from the recycling circuit to the degradative system. The main interacting partner in the Golgi providing lysosomal components is the TGN, which communicates with the PM, EEs and LEs. The recycling circuit has functions independent of the degradative cycle. The degradative cycle is, in turn, a shared ‘facility’ for degradation in the cell and is not only used for substrates delivered via endosomes. The cytosol has a central role by providing peripheral proteins to all the membrane compartments. These proteins define functions such as molecular sorting, membrane fusion and fission, compartment identity, and organelle motility.
Of the cargo internalized by ongoing endocytosis in mammalian cells, the majority is recycled back to the plasma membrane via early endosomes (EEs) (Figure 2). In typical mammalian cells, the equivalent of 50–180% of the surface area of the plasma membrane is cycled in and out of the cell every hour (Steinman et al, 1983). The amount of fluid internalized by macrophages corresponds to some 30% of cell volume per hour of which about two-thirds are returned to the extracellular space within about 10–15 min (Steinman et al, 1976, 1983; Besterman and Low, 1983).
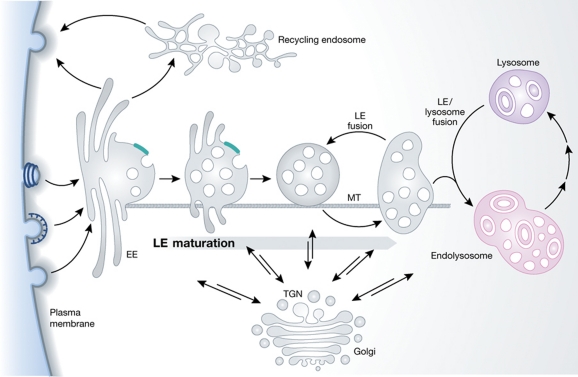
Figure 2.
The endosome/lysosome system. The primary endocytic vesicles deliver their contents and their membrane to EEs in the peripheral cytoplasm. After a period of about 8–15 min during which the EEs accumulate cargo and support recycling to the plasma membrane (directly or via recycling endosomes in the perinuclear region), conversion of the EEs to LE takes place. Thus, as the endosomes are moving towards the perinuclear space along microtubules (MT), the nascent LE are formed inheriting the vacuolar domains of the EE network. They carry a selected subset of endocytosed cargo from the EE, which they combine en route with newly synthesized lysosomal hydrolases and membrane components from the secretory pathway. They undergo homotypic fusion reactions, grow in size, and acquire more ILVs. Their role as feeder system is to deliver this mixture of endocytic and secretory components to lysosomes. To be able to do it, they continue to undergo a maturation process that prepares them for the encounter with lysosomes. The fusion of an endosome with a lysosome generates a transient hybrid organelle, the endolysosome, in which active degradation takes place. What follows is another maturation process; the endolysosome is converted to a classical dense lysosome, which constitutes a storage organelle for lysosomal hydrolases and membrane components.
One of the consequences of the active recycling is that transport to lysosomes via LEs is a side pathway limited to a relatively small fraction of internalized fluid and especially membrane components. To enter this side pathway, membrane components undergo stringent selection so that only a specific cohort is transported to lysosomes and degraded. The majority of large particles (such as viruses and ILVs) are also targeted to LEs. The bulk fluid and solutes diverted into this side pathway are not specifically sorted.
In addition to ferrying cargo for degradation, LEs transport new lysosomal hydrolases and membrane proteins to lysosomes for the maintenance and amplification of the degradative compartment. Lysosomes depend on the influx of new components, because without incoming endosomal traffic, they loose their intactness, acidity, and perinuclear localization (Bucci et al, 2000).
It is evident that the magnitude of the side pathway from EEs to lysosomes is under regulation through some of the cargo. Thus, formation of LEs and inward vesiculation to form ILVs has been shown to increase upon signalling via growth factor receptors. This suggests that the cell adjusts the use of this side pathway according to need (White et al, 2006). In doing so, it probably also adjusts the size of the degradative compartment. How such adjustment is achieved and which factors influence it, is an interesting question that deserves careful study.
Unlike the secretory pathway, the endocytic pathway has the advantage that the starting compartment, the extracellular space, is open and accessible. This means that a variety of ligands, fluid, solutes, and particles can be added to cells, and their fate after endocytosis followed in different ways. In mammalian tissue culture cells, the most commonly used cargo markers today are transferrin (Tf) and its receptor (TfR), which faithfully follow the recycling pathway, and epidermal growth factor (EGF) and its receptor (EGFR), which after ubiquitination of the receptor's cytosolic domain and inclusion in ILVs take the pathway to lysosomes for inactivation and degradation. Fluid uptake is usually followed using fluorescently-labeled dextran and other fluorescent solutes that do not adsorb to the cell surface. The membrane as such can be followed using fluorescent lipid markers (Maier et al, 2002). Viruses and bacterial toxins are also useful tools.
In addition to tissue culture cells, the most valuable system for endocytosis studies has been yeast, which seems to have endosomal compartments comparable to animal cells (Lewis et al, 2000; Pelham, 2002). More recently, filamentous fungi such as Aspergillus nidulans, have proven to be excellent systems to study endosomes and their maturation (Penalva, 2010). Caenorhabditis elegans and Drosophila melanogaster also have an impact given the background of strong genetics and the possibility of in situ studies in a multicellular organism (Grant and Sato, 2006; Michelet et al, 2010; Poteryaev et al, 2010). Endocytosis in plant cells is actually quite active (Irani and Russinova, 2009). The most important difference is the apparent lack of an independent EE compartment. The functions of EEs are carried out by an organelle that combines EEs and the TGN (Dettmer et al, 2006; Niemes et al, 2010). Conceptually, the situation in plants and fungi implies that by participating actively in secretory functions, endosomes can be viewed as an extension of the TGN.
Early endosomes
EEs provide the starting point for LE maturation. Defined initially as the compartment that first receives incoming cargo and fluid (Helenius et al, 1983), EEs are now recognized as the main sorting station in the endocytic pathway. Exactly how EEs arise is not entirely clear, but the membrane and volume is mainly derived from primary endocytic vesicles that fuse with each other. EEs receive endocytic cargo not only through the clathrin-mediated pathway but several other pathways including caveolar-, GEEC-, and ARF6-dependent pathways (Mayor and Pagano, 2007). Typically, an EE accepts incoming vesicles for about 10 min during which time membrane and fluid is rapidly recycled away, while some of the incoming cargo is retained and accumulates over the lifetime of the EE to be included in the LEs (Maxfield and McGraw, 2004).
Association of proteins from the cytosol to the cytosolic surface of the EE membrane defines many of its functional attributes. Rab5 is a key component together with its effector VPS34/p150, a phosphatidylinositol 3-kinase (PI(3)K) complex that generates the phosphoinositide (PI) PtdIns(3)P and thus helps to manifest the identity of the organelle (Christoforidis et al, 1999; Zerial and McBride, 2001; Behnia and Munro, 2005). Rab5 follows the endocytic membrane from the beginning through various stages of EE maturation, and is later the main regulator of the conversion to LEs. EEs communicate with the TGN through bidirectional vesicle exchange. The arrival of hydrolases gives them an initial degradative identity further strengthened during maturation of LEs.
EEs are heterogenous in terms of morphology, localization, composition, and function (Miaczynska et al, 2004; Lakadamyali et al, 2006; van Meel and Klumperman, 2008). Most of them are relatively small and patrol the peripheral cytoplasm close to the plasma membrane through saltatory movement along microtubules (Nielsen et al, 1999; Hoepfner et al, 2005). The overall distribution of EEs is cell-type dependent.
Individual EEs have a complex structure with tubular and vacuolar domains (Figure 3A). Most of the membrane surface area is in the tubules, and much of the volume in the vacuoles. The limiting membrane contains a mosaic of subdomains that differ in composition and function (Zerial and McBride, 2001). They include domains enriched in Rab5, Rab4, Rab11, Arf1/COPI, retromer, and caveolae (Vonderheit and Helenius, 2005; Rojas et al, 2008; Hayer et al, 2010). Many of the domains are located in the tubular extensions where they provide for molecular sorting and generate vesicle carriers targeted to distinct organelles, including the plasma membrane, the recycling endosomes, and the TGN (Bonifacino and Rojas, 2006) (Figure 2).
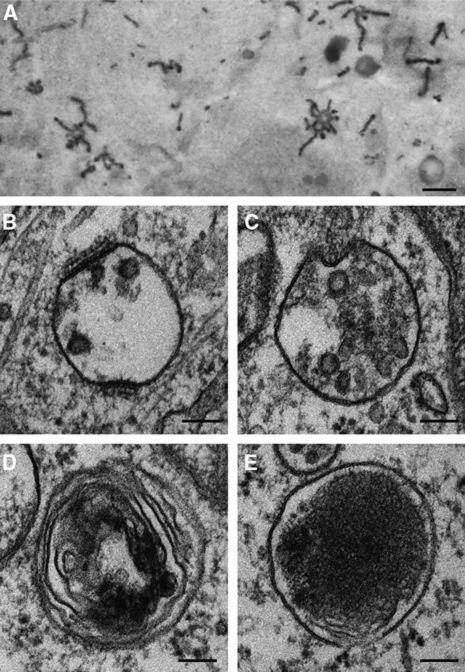
Figure 3.
Morphologies of endosomes and lysosomes at the ultrastructural level. (A) Electron micrographs of peripherally located EEs containing HRP-conjugated Tf. They contain vacuolar and tubular domains. Courtesy of Tooze and Hollinshead (1992). Electron micrographs of (B) EE with clathrin lattices and a few ILVs; (C) LE, containing numerous ILVs; (D) endolysosome, with partial electron dense areas; and (E) lysosomes, with electron dense lumen. Images are all from HeLa cells that had been processed for thin section EM. Scale bars in (A): 500 nm and (B–E): 100 nm. Figure 3A is reproduced with kind permission from Rockefeller University Press;©2009 Rockefeller University Press. Originally published in J Cell Biol 118: 813–830. doi: 10.1083/jcb.118.4.813.
The formation of ILVs begins already in EEs. For this the cytosolic surface of the EE membrane has characteristic ‘plaques’ containing clathrin and components of the endosomal sorting complex required for transport (ESCRT), machinery responsible for sorting of ubiquitinated membrane proteins into ILVs (Raiborg et al, 2002; Sachse et al, 2002) (Figure 3B). The lumen of the vacuolar EE domains often contains several ILVs; in HepG2 cells there are 1–8 ILVs (van Meel and Klumperman, 2008). EEs are weakly acidic (pH 6.8–5.9) (Maxfield and Yamashiro, 1987), and contain a relatively low Ca2+ concentration (Gerasimenko et al, 1998).
The traffic between endosomes and the TGN is a continuously ongoing process that has been extensively studied. It is responsible for the delivery of lysosomal and removal of endosomal components during endosome maturation. It occurs at the level of EEs, maturing LEs, and possibly for some time after the fusion of LEs with lysosomes. At the endosome level, the sorting and vesicle formation for transport to the TGN depends on factors such as Rab7, Rab9, and the retromer complex (Bonifacino and Hurley, 2008; Pfeffer, 2009). The retromer is a multimeric complex composed of sorting nexins and other proteins recruited to the cytosolic surface of EEs and maturing LEs.
LEs and lysosomes
Mature LEs are typically round or oval and have a diameter of 250–1000 nm (Figure 3C). They have a low bouyant density, and a high negative surface charge (Bayer et al, 1998; Falguieres et al, 2008). The limiting membrane contains lysosomal membrane proteins such as LAMP1 and the lumen contains a complement of acid hydrolases. The lumen also has numerous ILVs (often up to ⩾30) with a diameter of about 50–100 nm. The pH ranges between pH 6.0–4.9 (Maxfield and Yamashiro, 1987).
LEs formed in the peripheral cytoplasm move to the perinuclear area of the cell where they fuse with each other to form larger bodies and to undergo transient (‘kiss-and-run’) fusions and eventually full fusions with lysosomes and pre-existing hybrid organelles between endosomes and lysosomes (Luzio et al, 2007b) (Figure 2). By fusing with lysosomes, the LEs follow what is essentially a unidirectional, dead-end pathway. Whereas most of the components of LEs are degraded in the lysosomal environment, others are longer lived and contribute, as already mentioned, to the maintenance and generation of lysosomes. It has been suggested that a few components such as mannose-6-phosphate receptors, tetraspanins, and SNAREs may escape degradation through vesicle trafficking after LE fusion with lysosomes, but this pathway is not well characterized.
To determine whether a multivesicular organelle is a LE, or a fusion product between LEs and lysosomes is not always straightforward because the hybrid organelles contain components from both fusion partners (Figure 3D and E). We will call these hybrid organelles endolysosomes, to distinguish them from classical dense, primary lysosomes and from lysosomes generated through fusion with phagosomes, macropinosomes, and autophagosomes. It is in the endolysosomes that most of the hydrolysis of endocytosed cargo takes place.
The lysosomal compartment as a whole consists of a collection of vacuoles of heterogeneous composition, morphology, location, and density. The heterogeneity is due to the diversity of cargo and the existence of several feeder pathways of which the classical endosome pathway is a major one. Heterogeneity is also generated by the varying degree of cargo degradation within individual vacuoles, and by fusion events between the various vacuoles. The classical lysosomes of high buoyant density and high hydrolase content correspond to the end point of the degradation process. They serve in part as storage vacuoles for lysosomal components ready to be redeployed. These components include the hydrolases, the limiting membrane protected by LAMPs, and other substances resistant to degradation. They also contain slowly degraded lumenal lipids often present as multilamellar membrane whirls.
Taken together, the EEs, LEs, endolysosomes, and lysosomes provide a dynamic and adaptable continuum (Von Bartheld and Altick, 2011). The pathway is elusive because the organelles are scattered, and undergoing continuous maturation, transformation, fusion, and fission. Specific protein and lipid components are only partially useful as molecular markers because the majority is either transiently associated with the organelles or follow the organelles through several steps of transformation. In the pathway, events occur, moreover, non-synchronously. A cohort of cargo molecules simultaneously internalized from the plasma membrane may, for example, arrive at a certain acidic pH value in a time window spread over hours (Kielian et al, 1986). The ambiguity, heterogeneity, and lack of synchrony in the endocytic system may, in part, explain a certain lack of common, universally agreed concepts and models for endosome function and maturation.
Formation of LEs
LEs are derived from the vacuolar domains of EEs. By itself this constitutes molecular sorting because in addition to a large fraction of the fluid, the vacuolar domains of EEs have a different composition than the tubules. In the lumen, they contain ligands dissociated from their receptors as well as proteins and solutes internalized as components of the bulk fluid. The ILVs, and other large particles such as incoming viruses, are also present in the vacuolar elements of EEs. The membrane probably contains most of the cholesterol and sphingolipid-rich lipid rafts, membrane protein aggregates, V-ATPases, clathrin, ESCRT complexes, and a selected spectrum of membrane proteins destined for degradation (Ukkonen et al, 1986; Mukherjee et al, 1999; Mukherjee and Maxfield, 2004).
The formation of a new LE is preceded by the generation of a Rab7 domain (Rink et al, 2005; Vonderheit and Helenius, 2005). This leads to the transient formation of a hybrid Rab5/Rab7 endosome. As discussed below, Rab7 is recruited to the EE by Rab5-GTP. How the tubules and the rest of the EE domains are lost during the formation of an LE is not entirely clear, but there are two possibilities. Evidence obtained by analysing large, spherical, juxtanuclear Rab5-positive endosomes supports a mechanism in which Rab5 after recruiting Rab7 is converted to the GDP-bound form and dissociates together with its effectors. A process like this was observed by live microscopy (Rink et al, 2005). Rab5 was lost within a few minutes, and replaced with Rab7.
Another mechanism envisages a fission event that separates parts of the hybrid endosome containing the nascent Rab7 domain from the rest. Gruenberg and Stenmark (2004) call the newly formed LEs endosomal carrier vesicles (ECVs), and suggest that these serve as transporters of cargo to a stable LE compartment before delivery to lysosomes. While following viruses after endocytosis in small peripheral endosomes by live microscopy, we found support for such splitting of the endosomes. We observed microtubule-dependent events in which the virus and the Rab7 domain separated from the rest of the endosome containing Rab5, Rab4, and Arf1 (Vonderheit and Helenius, 2005). Consistent with this, it has been shown that dynein-mediated pulling forces are critical for the separation of endosomal elements containing the recycling ligand Tf from the lysosome-targeted ligand EGF (Driskell et al, 2007). Similar observations were recently made by a group that proposed a role for dynamin in fission of LEs from EEs (Mesaki et al, 2011). It is possible that different mechanisms exist for the peripheral and perinuclear populations of EEs, with gradual maturation occurring in perinuclear endosomes and a fission-based mechanism in peripherally located, relatively small and motile endosomes.
Maturation of LEs
Regardless of the mechanism of initial formation, the newly formed LEs continue to undergo a multitude of changes (Table I). As a result, by the time they fuse with lysosomes some 10–40 min later, they have completed a remarkable transformation leaving them with few similarities to EEs. The maturation process involves exchange of membrane components, movement to the perinuclear area, a shift in choice of fusion partners, formation of additional ILVs, a drop in lumenal pH, acquisition of lysosomal components, and a change in morphology (Table I). The programme is closely coordinated and regulated by factors recruited to the surface of the limiting membrane from the cytosol.
Table 1. The endosome maturation programme.
| Rab switch. Rab5 is exchanged for Rab7. This switch reprograms the association of effector proteins from the cytosol and redefines many of the properties of the endosomes. Other Rabs, such as Rab4, Rab11, are Rab22 also lost, while Rab9 is added. |
| Formation of ILVs. Ubiquitinated cargo recruits machinery from the cytosol (ESCRT and other factors) that induce inward-budding of the limiting membrane, and thus the formation of ILVs containing membrane proteins and lipids targeted for lysosomal degradation. Capacitation of microphagocytosis-like mechanisms for the inclusion of cytoplasmic proteins and RNAs, of ILV backfusion with the limiting membrane, and exosome release. |
| Acidification. The lumenal pH drops from values above pH 6 to pH 6.0–4.9. |
| PI conversion. PtdIns(3)P is converted to PtdIns(3,5)P(2), and some of the PtdIns(3)P is sorted into the ILVs. |
| Change in size and morphology. The tubular extensions present on EEs are lost and the endosomes acquire a round or oval shape and grow in size. |
| Loss of recycling with the plasma membrane. Recycling receptors are lost from the organelle and recycling of membrane and fluid to the cell surface stops. |
| Gain of lysosomal hydrolases and membrane proteins. These lysosomal components are transported mainly from the TGN. Some of them are active already in the maturing endosomes. |
| Switch in fusion specificity. The endosomes can no longer fuse with EEs. Instead, they acquire the necessary tethering complexes and SNAREs to fuse with each other, with lysosomes, and with autophagosomes. The conversion of CORVET to HOPS complex on membranes. The HOPS/CORVET complexes are involved in a number of processes, including membrane tethering, the Rab5/7 switch, and mediating SNARE assembly. |
| A switch in cytoplasmic motility. The endosomes associate with a new set of microtubule-dependent motors that allow them to move into the perinuclear region of the cell. |
| Changes in lumenal ionic environment. In addition to the drop in pH, there is an increase in Cl−, and changes in Ca2+, Na+, K+ concentrations. |
| Change in temperature sensitivity. Unlike earlier steps in endocytosis, LE formation or their fusion with lysosomes is blocked at temperatures below 19–20°C. |
| Decrease in buoyant density and increase in negative surface charge. These properties are used to isolate LEs. |
Before considering the conversion in more detail, it is important to ask why LEs are subject to such a dramatic transformation. What is the reason for this complex maturation programme? The general answer is that the programme is in place to close down recycling and other functions of EEs and to allow the union of LEs with the degradative compartment. Similar maturation using some of the same factors occurs in phagosomes, autophagosomes, and probably macropinosomes before they fuse with LEs and lysosomes (Eskelinen, 2008; Kinchen and Ravichandran, 2008; Kerr and Teasdale, 2009).
Since lysosomes constitute a point-of-no-return for most macromolecules and lipids, the cargo contents of LEs must be narrowed down to molecules and particles that need to be degraded, and to cargo that the LEs and lysosomes require for their functionality. Some of the membrane-bound cargo, moreover, needs to be presented in a form easily digested by hydrolases, which may explain in part the formation and the composition of ILVs. The limiting membrane of the LEs must, on the other hand, be rendered resistant to the hydrolases by inclusion of lysosomal glycoproteins such as LAMPs. Moreover, interactions with the cytoskeleton have to be altered so that the LEs can move to the region of the cytoplasm where the lysosomes are localized. LEs also need new tethering factors and SNAREs to be able to fuse with each other, with lysosomes, and perhaps with macropinosomes and autophagosomes.
In the following sections, we will focus on some of the events and factors involved in endosome maturation. These include the Rab GTPase switch, phosphatydylinositide conversion, Arf1/COPI association, ILV biogenesis, acidification, and LE motility. Finally, we will discuss some of the roles that LEs have beyond the degradative function.
The Rab switch
Rab GTPases, especially Rab5 and Rab7 (Vps21p and Ypt7p in yeast, respectively), provide the most important organelle identity markers and master regulators in the endocytic pathway. Rab5 itself, its guanine-nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs), GDP dissociation inhibitors (GDIs), and GDI displacement factors (GDFs) as well as its effectors determine the functions of EEs (Chavrier et al, 1990; Ullrich et al, 1994; Rink et al, 2005; Jovic et al, 2010). Rab7 and its corresponding factors have a similar role in LEs and lysosomes (Chavrier et al, 1990; Meresse et al, 1995; Tjelle et al, 1996).
Endosome maturation involves a conversion from Rab5 to Rab7 (Rink et al, 2005; Vonderheit and Helenius, 2005; Poteryaev et al, 2010). The conversion can be blocked by expressing a constitutively active mutant of Rab5 (Q79L), and by depletion of VPS39, a subunit of the HOPS complex (Rink et al, 2005). This results in the formation of hybrid endosomal compartments with markers for both EEs and LEs. These abnormal hybrid compartments seem to arise from homotypic fusions of EEs and heterotypic fusions with LEs or lysosomes, and are found to accumulate ILVs (Rosenfeld et al, 2001; Hirota et al, 2007; Wegner et al, 2010). Expression of the Rab5(Q79L) mutant results in sorting defects of both recycling and degradative cargo. Lysosome biogenesis is also affected as the amount of lysosomes in dense Percoll gradient fractions is greatly reduced (Rosenfeld et al, 2001).
Initially, Rabex-5, a GEF for Rab5, is recruited to EEs where it activates Rab5 (Horiuchi et al, 1997; Barr and Lambright, 2010). Interestingly, in addition to its GEF activity, Rabex-5 also possesses ubiquitin (Ub) E3 ligase activity and can bind to ubiquitinated proteins, which is indeed required for its association with EE membranes (Mattera and Bonifacino, 2008). In the cytosol Rabex-5 itself is monoubiquitinated and it is thought that a cycle of Ub binding and monoubiquitination regulates its recruitment. The GEF activity of Rabex-5 is promoted by Rabaptin-5, a Rab5 effector with which it forms a complex (Horiuchi et al, 1997) (Figure 4). This complex is required to establish a feedback loop, whereby Rab5-GTP promotes further Rab5 binding (Lippe et al, 2001). This causes a rapid recruitment of numerous Rab5 effectors including the VPS34/p150 complex that produces PtdIns(3)P (Christoforidis et al, 1999; Jovic et al, 2010), which in turn promotes the binding of a spectrum of proteins with specific PI-binding domains.
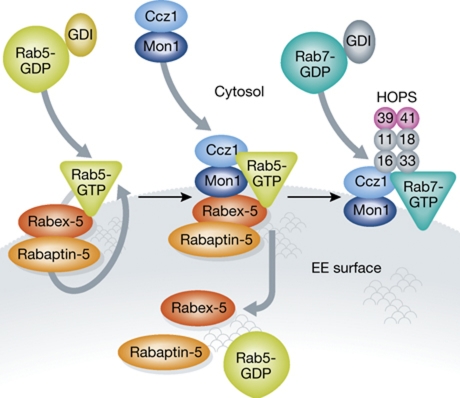
Figure 4.
The Rab5/Rab7 switch during endosome maturation. Normally, Rab5-GDP and Rab7-GDP reside in the cytosol bound to its GDI. Rab5 is activated to its GTP-bound and membrane-associated form by the GEF Rabex-5 on EE membranes. The Rab5 effector Rabaptin-5 binds to Rabex-5 and promotes the activation of Rab5, thus forming a positive feedback loop in which more Rab5 molecules are activated and recruited. To initiate the Rab switch, Mon1/SAND-1 complexed with Ccz1 binds to Rab5, PtdIns(3)P, and Rabex-5, causing disassociation of Rabex-5 from the membrane. This in turn terminates the feedback loop, resulting in Rab5 inactivation and disassociation. The Mon1/SAND-1–Ccz1 complex promotes (directly or indirectly) the recruitment and activation of Rab7. Members of the HOPS complex (Vps11, Vps16, Vps18, Vps33, Vps39, and Vps41) are able to bind both Rab7 and the Mon1/SAND-1–Ccz1 complex. The HOPS complex mediates membrane tethering, needed for fusion with other LEs and lysosomes. Adapted from Cabrera and Ungermann (2010).
Removal of Rab5 and its replacement with Rab7 is an essential step in LE formation and in the transport of cargo to lysosomes. Rab5 removal requires both inhibition of the feedback loop for Rab5 binding as well as a GAP activity to activate Rab5's GTP hydrolysis activity. On the basis of theoretical and experimental approaches, Del Conte-Zerial and colleagues have proposed a ‘cutoff switch’ model to explain the paradox of rapid initial growth of Rab5 domains and their sudden disassembly during Rab conversion (Del Conte-Zerial et al, 2008). In this model, Rab5 activates Rab7, and Rab7 displays autoactivation and suppresses Rab5. In this way, Rab5 orchestrates its own removal from the endosomes and at the same time initiates the takeover by Rab7.
Additional key players in the Rab5/Rab7 conversion are starting to emerge. According to recent data, these include SAND-1/Mon1 and Ccz1, two factors recruited from the cytosol to endosomal membranes. They were first identified as factors required for vacuole fusion in yeast, and in transport of yolk proteins from EEs to LEs in C. elegans (Wang et al, 2003; Poteryaev et al, 2007). Recent work focusing on phagosomes in C. elegans have provided evidence that SAND-1/Mon1 and Ccz1 are involved in the Rab conversion in endosomes and phagosomes (Kinchen and Ravichandran, 2010; Poteryaev et al, 2010).
Taken together, the results indicate that SAND-1/Mon1 (or the SAND-1/Mon1 complexed with Ccz1) can (1) bind to Rab5-GTP; (2) recruit Rab7 (and bind Rab7 when both SAND-1/Mon and Ccz1 are present); (3) bind to PtdInd(3)P; (4) interact with components of the HOPS complex; and (5) displace Rabex-5 from the endosomal membrane. In this way, SAND-1/Mon1 could (together with Ccz1) promote Rab7 binding by potentially displacing the GDI from Rab7 and/or by activating the VPS39 subunit of the HOPS complex, which in yeast has been proposed to act as a GEF for Ypt7p (Rab7) (Wurmser et al, 2000). Moreover, by interacting with Rabex-5 and displacing it from the membrane, it could interrupt the positive feedback loop that enhances Rab5 binding to EEs (Poteryaev et al, 2010). As a result, SAND-1/Mon1 (or the SAND-1/Mon1 complexed with Ccz1) would turn off Rab5 at the same time as it would turn on Rab7 (Figure 4).
The timing of SAND-1/Mon1 association with EEs is critical because the Rab conversion should not occur until the endosome has accumulated cargo for degradation. In this context, it was found that SAND-1/Mon1 recognizes PtdIns(3)P, and that the PI(3)K inhibitor wortmannin prevented SAND-1/Mon1 binding to endosomes and thus Rab5 inactivation (Vieira et al, 2003; Poteryaev et al, 2010). This suggests that the concentration of PtdIns(3)P on endosomes could be a determinant defining the timing of the Rab conversion.
Many molecular details in this interesting model remain unclear. For example, it has been proposed that the HOPS complex component VPS39 serves as a GEF for Rab7 (Rink et al, 2005) because the yeast orthologue Vps39p/Vam6p possesses GEF activity towards Ypt7p in yeast (Wurmser et al, 2000). However, it was recently reported that in mammalian cells, VPS39 does not possess GEF activity towards Rab7 (Peralta et al, 2010). The GEF might, instead, be the Mon1–Ccz1 complex itself as recently shown in yeast (Nordmann et al, 2010). Another question is whether SAND-1/Mon1 directly interacts with the GDI of Rab7 or whether the reported effects of SAND-1/Mon1 on the disassociation of the GDI from Rab7 are indirect (Cabrera and Ungermann, 2010). In mammals, there are two SAND-1 orthologues, Mon1a and Mon1b. It is unclear what their differences are and how redundantly they function.
To terminate Rab5 activation on endosomes, a GAP has to activate Rab5-mediated hydrolysis of bound GTP into GDP. Several GAPs have been assigned to Rab5, including RN-Tre and RAB GAP-5 (Lanzetti et al, 2000; Haas et al, 2005). However, the target of RN-Tre may actually be Rab41 (Haas et al, 2005). A recent addition to the list of Rab5 GAPs is TBC-2, which in C. elegans was shown to be required both during endosome and phagosome maturation (Li et al, 2009; Chotard et al, 2010). It is recruited to endosomes only when Rab7 is already present, thereby allowing coordination of Rab5 inactivation with Rab7 activation. Confusion is caused by the observation that the closest human homologue of TBC-2, Armus, possesses GAP activity towards Rab7 (Frasa et al, 2010). Earlier studies have recognized a role for the yeast Gyp7p and its human orthologue TBC1D15 as Ypt7p/Rab7 GAPs (Vollmer et al, 1999; Zhang et al, 2005; Peralta et al, 2010).
Once established as a domain in the hybrid endosome, Rab7-GTP recruits its own effectors. These include factors such as RILP, a protein that connects LEs to dynein motors; components of the retromer complex that support vesicle traffic to the TGN; components of the HOPS complex serving as a tether for LE fusion; and Rabring7, a RING-type E3 ligase (Zhang et al, 2009). In contrast to these, Rubicon and PLEKHM1, recently identified Rab7 effectors, act as negative regulators of endosome maturation (Sun et al, 2010; Tabata et al, 2010).
One crucial role of the Rab5/Rab7 conversion is to exchange the fusion machinery on the endosomal membrane so that LEs can only fuse with other LEs, lysosomes, and possibly autophagosomes. Two complexes that are thought to regulate the fusion machinery are the class C VPS CORVET and HOPS complexes, for EEs and LEs, respectively (Nickerson et al, 2009). They both have a core of four proteins (Vps11, Vps16, Vps18, and Vps33), but differ in accessory proteins; CORVET is associated with Vps3 and Vps8 and HOPS with Vps39 and Vps41. The composition and function of these complexes have mainly been studied in yeast, where they were first identified. Differences to mammalian cells may exist; it is still uncertain whether for instance VPS39 occurs in both CORVET and HOPS complexes in mammalians. Nevertheless, as Rab5/Vps21p can interact with the CORVET and Rab7/Ypt7p conversely with the HOPS complex, the current model suggests that the Rab5/Rab7 and the CORVET/HOPS conversion are interlinked and coordinately regulated.
Early studies in yeast showed that the Rab7 orthologue, Ypt7p, was required for delivery of degradative cargo to the vacuole, that is, to the yeast lysosome (Wichmann et al, 1992; Schimmoller and Riezman, 1993). Subsequent studies in mammalian cells demonstrated that expression of dominant negative mutants of Rab7 results in dispersal of endosomes, a block in cargo trafficking to the lysosomes, and the deterioration of lysosome acidification and functionality (Bucci et al, 2000). By recruiting a variety of effector proteins on LEs, Rab7/Ypt7p coordinates various maturation processes, and renders the organelle capable of fusion with other LEs as well as lysosomes (or the vacuole in yeast). In addition, in yeast, Ypt7p is known to be directly involved in the assembly of the fusion machinery at both the LEs and the vacuole membrane (Haas et al, 1995; Ostrowicz et al, 2008).
In this context, it is interesting that Ypt7p has been found among ubiquitinated proteins in vacuoles and may be a substrate for proteasomal degradation during vacuole fusion (Kleijnen et al, 2007). A role for the proteasome in the degradative pathway has also been suggested on several occasions in mammalian cells (van Kerkhof and Strous, 2001; Longva et al, 2002; Khor et al, 2003; Geetha and Wooten, 2008), although the functions and molecular details remain elusive. Interestingly, in mammalian cells, Rab7 interacts with a subunit of the proteasome (Dong et al, 2004). However, no evidence for the proteasomal degradation of Rab7 in mammalian cells was found. One proposed function for the proteasome at endosomes is regulation of cargo deubiquitination and sorting into ILVs (Longva et al, 2002; Alwan et al, 2003; Geetha and Wooten, 2008). Interactions between a proteasome accessory protein, Ecm29 and LE resident proteins, including components of ESCRT, retromer and motor proteins have also been reported (Gorbea et al, 2010), suggesting that proteasomes have perhaps multiple functions at LEs.
The PI conversion
Like the Rab GTPase switch, the PI conversion plays an indisputable role in endosome maturation. The two important PIs are PtdIns(3)P and PtdIns(3,5)P(2), two lipids that contribute to the identity of EE and LE membranes. As mentioned earlier, PIs recruit a number of effector proteins with PI-binding domains including FYVE, PH, PX, and GRAM. The PI species are synthesized locally in organelle membranes by the action of specific kinases and phosphatases allowing tight control of compartmentalization (Vicinanza et al, 2008).
PtdIns(3)P is mostly found on the cytosolic leaflet of EE membranes, generated mainly by a class III PI(3)K, VPS34 (Schu et al, 1993), which is recruited by Rab5-GTP through direct interaction with its partner, p150 (Christoforidis et al, 1999; Murray et al, 2002). Inhibition of VPS34 activity causes enlarged LEs with fewer ILVs, and EGFR accumulates in the limiting membrane (Futter et al, 2001). Dephosphorylation of PtdIns(3)P is executed by the myotubularin family of 3-phosphatases (Robinson and Dixon, 2006).
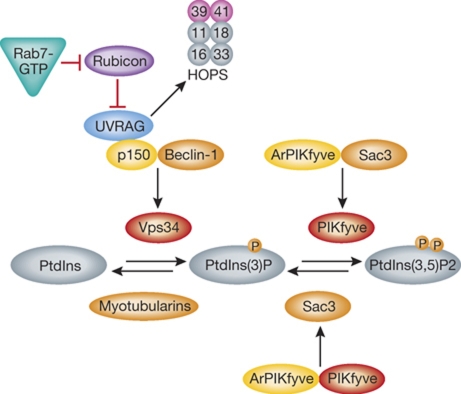
VPS34 acts in concert with other proteins that regulate its kinase activity; p150 (Vps15 in yeast) and Beclin-1. Together, these form a core complex (Funderburk et al, 2010), which on endosomes associates with a protein called UVRAG (Itakura et al, 2008), an activator of the HOPS complex. Recent findings indicate that a Rab7 effector, Rubicon, acts as a negative regulator of UVRAG by sequestering it away from the HOPS complex (Sun et al, 2010). Upon activation, Rab7 competes for binding with Rubicon, causes the release of UVRAG, and allows it to associate with the HOPS complex (Figure 5).
Figure 5.
Phosphatidylinositide regulation on endosomes. On EEs, PtdIns(3)P is synthesized by the kinase VPS34, which forms a core complex together with p150 and Beclin-1. The complex binds on endosomes to UVRAG (Itakura et al, 2008), which is normally inhibited by a Rab7 effector, Rubicon (Sun et al, 2010). Once activated, Rab7 sequesters Rubicon from UVRAG, allowing it to activate the HOPS complex. Dephosphorylation of PtdIns(3)P is catalyzed by members of the Myotubularin family. The kinase responsible for conversion of PtdIns(3)P to PtdIns(3,5)P(2) is PIKfyve (Fab1p) (Gary et al, 1998; Ikonomov et al, 2002). It forms an active complex with its activator ArPIKfyve (Vac14p) and the phosphatase Sac3 (Fig4p). This complex is required for both the kinase and the phosphatase activities (Sbrissa et al, 2008; Ikonomov et al, 2009).
PtdIns(3,5)P(2) is important later in the degradative pathway. It is generated by a phosphatidylinositol 3-phosphate 5-kinase, PIKfyve (Fab1p in yeast). Originally characterized in yeast (Gary et al, 1998), PIKfyve/Fab1p was later found to play an essential role in the endolysosomal system of C. elegans, D. melanogaster, and mammalian cells. This is highlighted by the finding that PtdIns(3,5)P(2) depletion leads to highly vacuolated phenotypes (Ikonomov et al, 2001; Nicot et al, 2006; Rusten et al, 2006; Jefferies et al, 2008).
Membrane localization of PIKfyve/Fab1p is brought about by its PtdIns(3)P binding FYVE domain, which links the production of PtdIns(3,5)P(2) to existing membranes rich in PtdIns(3)P. PIKfyve activity is regulated by its activator ArPIKfyve/Vac14p and the PtdIns(3,5)P(2) phosphatase Sac3/Fig4p (Shisheva, 2008), with which it forms a stable complex critical for both lipid kinase and phosphatase activities (Sbrissa et al, 2008; Ikonomov et al, 2009) (Figure 5). PIKfyve is also able to contribute to the production of PtdIns(5)P (Sbrissa et al, 2002). The functional significance of this in the endocytic system is, however, unclear. In addition to its lipid substrates, PIKfyve has been found to possess kinase activity towards proteins, exemplified by p40, an effector of Rab9 (Ikonomov et al, 2003b). This activity might be partially coupled to PIKfyve's proposed role in LE transport to the TGN, as perturbation of PIKfyve activity results in a block of endosome–TGN transport of both retromer-dependent and -independent cargo (de Lartigue et al, 2009).
Open questions still remain regarding the various functions of PIKfyve/Fab1p (Shisheva, 2008; Dove et al, 2009). For instance, acidification of LEs and lysosomes/vacuoles is dependent on PIKfyve/Fab1p activity in lower organisms, such as yeast, C. elegans and D. melanogaster (Gary et al, 1998; Nicot et al, 2006; Rusten et al, 2006), but in mammalian cells this is not entirely clear (Ikonomov et al, 2003a; Jefferies et al, 2008; de Lartigue et al, 2009). Enlarged vacuoles induced by inhibition of PIKfyve are not acidic in mammalian cells. However, in the same cells a fraction of endosomes is found to accumulate dyes that mark acidic LE or lysosomal compartments. How acidification is controlled by PIKfyve/Fab1p is not understood.
PIKfyve has also been linked to the regulation of ion channels, mostly by controlling their localization at the plasma membrane by exocytosis (Shisheva, 2008). In addition, PIKfyve may directly regulate the activity of channels in LEs. The calcium permeable cation channel TRPML1 was recently demonstrated to be directly activated by the presence of PtdIns(3,5)P(2) enabling the efflux of Ca2+ (Dong et al, 2010). Ca2+ is known to have an important regulatory function in homotypic and heterotypic fusions of LEs and lysosomes/vacuoles as well as in reformation of lysosomes from endolysosomes (Luzio et al, 2007a). The localized production of PtdIns(3,5)P(2) on endosomal membranes thus allows the control of channel activity in confined regions and may enable regulation of LE fusion/fission in a spatio-temporal manner. It is plausible that the highly vacuolated phenotype of PIKfyve/Fab1p mutants is partially the result of osmotic swelling or deregulated fusion/fission processes induced by the misregulation of endosomal ion channels (Shisheva, 2008).
PI conversion seems to be tightly linked to other endosome maturation steps. Parts of the ESCRT machinery responsible for ILV formation are recruited by both PtdIns(3)P and PtdIns(3,5)P(2) (Katzmann et al, 2003; Whitley et al, 2003; Teo et al, 2006). This probably allows ILV formation and cargo sorting to be coordinated with the rest of the maturation programme. Likewise, interactions occur between RabGTPases and the PI converting machinery: VPS34 is known to interact with both Rab5 and Rab7. The difference is the activation state of the Rab: Rab5 recruits VPS34 more prominently in the GTP-bound form (Christoforidis et al, 1999), while interaction with Rab7 is more prominent when Rab7 is in its inactive GDP-bound form (Stein et al, 2003). Therefore, activation of Rab7 reduces interaction of VPS34 on membranes providing a feedback control to PtdIns(3)P synthesis.
COPI and Arf1
A role for the coatomer COPI in endosome maturation was first suggested by the effects of brefeldin A, a drug that prevents Arf1 activation and thus COPI binding to membranes (Hunziker et al, 1992). It was found to cause massive tubulation and redistribution of endosomes as well as loss of ILVs (Lippincott-Schwartz et al, 1991; Tooze and Hollinshead, 1992). COPI and Arf1 are both present on EEs where they form a domain (Aniento et al, 1996).
The most convincing evidence for a role of COPI in endosome maturation comes from the analysis of a temperature-sensitive mutant of CHO cells called ldlF, which looses COPI subunit epsilon at non-permissive temperature (Guo et al, 1996). In addition to defects in the secretory pathway, ldlF cells are impaired in the endocytic pathway (Daro et al, 1997; Gu et al, 1997): EEs take the form of tubular clusters without vacuolar domains. Formation of ILVs is inhibited, and fluid phase accumulation is reduced. Sorting of EGF and lysosomal proteins to lysosomes is inefficient, and the sensitivity to acid-activated bacterial toxins and viruses is lost (Daro et al, 1997; Abrami et al, 2004).
A model to explain these effects has been proposed by Gruenberg and coworkers, who suggest that Arf1 binding to EEs is regulated by an unidentified membrane protein activated by the low lumenal pH (Clague et al, 1994; Aniento et al, 1996; Gu and Gruenberg, 1999). Arf1 in turn recruits the COPI subunits, which promote molecular sorting of membrane proteins, the formation of ILVs, and the ECVs. Now when it is clear that the main mediator for ILV formation is the ESCRT complex and that formation of LEs is driven by other mechanisms, it would seem important to revisit the role of Arf1 and COPI in endosome maturation.
The formation of ILVs
One of the most important processes in LE biogenesis is the formation of ILVs. It is critical for the selective sorting of membrane-associated cargo for degradation in lysosomes, and for the maturation and fate of LEs. There are several reasons for ILV formation. One is that by inclusion in ILVs, signalling receptors are inactivated because they are deprived of contact with the cytosol (Figure 7A). Lipids and membrane proteins are, moreover, delivered to lysosomes in a form easily accessible to the hydrolases. Unlike the limiting membrane, which is covered by a coat of highly glycosylated proteins, such as LAMPs, the ILV membrane is devoid of a protective glycocalyx. In addition, ILVs in late compartments contain a phospholipid bis(monoacylglycero)phosphate/lysobisphoshatidic acid (BMP/LBPA), which is thought to promote lipid hydrolysis (Kobayashi et al, 1998). BMP/LBPA is negatively charged and therefore contributes to the recruitment of hydrolases that are positively charged in an acidic milieu. An example is acid sphingomyelinase, which converts sphingomyelin in ILVs to ceramide (Kolter and Sandhoff, 2010). That yeast does not possess LAMPs or LBPA/BMP implies that there are other mechanisms to ensure coordinated degradation of ILVs and their contents.
Among the main players in ILV biogenesis are the ESCRT complexes (ESCRT-0, -I, II, -III) and a number of accessory proteins such as the AAA-type ATPase VPS4 and Alix. These are individually recruited from the cytosol to the surface of endosomes. Since the ESCRT machinery and its functions has been reviewed on many occasions (Babst, 2005; Eden et al, 2009; Raiborg and Stenmark, 2009; Woodman, 2009; Hurley, 2010; Hurley and Hanson, 2010; Roxrud et al, 2010), we will only discuss some of the general aspects.
The role of the ESCRT machinery is to sort out among the cargo that enter EEs, a cohort of pre-tagged membrane proteins and their ligands, to segregate them into domains within the limiting membrane, and to generate inward-budding vesicles. Sorting is based on the presence of Ub-tags in the cytosolic domains of membrane proteins recognized by components of the ESCRT machinery through their various Ub-binding domains. Parts of the ESCRT machinery bind in addition to clathrin and PIs, which help to recruit the complexes to endosomal membranes. Ub-tagged cargo molecules include a variety of activated growth factor and other signalling receptors, adhesion molecules such as integrins, ion channels, and other proteins such as unassembled caveolin-1 oligomers (Staub et al, 1997; Vaccari and Bilder, 2005; Sorkin and Goh, 2008; Hayer et al, 2010; Lobert et al, 2010). Before vesicle closure, the Ub-tag is removed by deubiquitinating enzymes (DUBs) (Clague and Urbe, 2006).
Depletion of components of the ESCRT machinery results in fewer ILVs, and accumulation of cargo in endosomes with abnormal morphology (Rieder et al, 1996; Doyotte et al, 2005; Razi and Futter, 2006). EGF-induced formation of LEs is strongly reduced by the depletion of TSG101 (ESCRT-I), whereas the effect of Hrs (ESCRT-0) depletion is milder (Razi and Futter, 2006). In TSG101-depleted cells, unlike Vps24 (ESCRT-III)-depleted cells, the acidification of EGF-containing endosomes is perturbed (Bache et al, 2006). In mammalian cells and in yeast, the loss of TSG101/Vps23p induces formation of multicisternal endosomes, the so-called class E compartments (Rieder et al, 1996; Doyotte et al, 2005) Altogether, it is evident that among the ESCRT components TSG101 has a central role in the biogenesis of LEs.
Although in yeast the ESCRT machinery is essential for ILV biogenesis (Odorizzi et al, 1998), in higher organisms, ESCRT-independent mechanisms also exist. Depletion of factors of all four ESCRTs simultaneously causes a reduction in ILV filled LEs (Stuffers et al, 2009). However, this only affects the EGF-induced population, an EGF-independent population of LEs is largely unaffected. An example of ESCRT-independent ILV cargo is the melanosomal protein Pmel17, which relies on a luminal domain that enables its sorting and inclusion into ILVs, without cargo ubiquitination (Theos et al, 2006). Likewise, major histocompatibility complex class II (MHC II) molecules in activated dendritic cells (DCs) are inserted into ILVs independently of ubiquitination (Buschow et al, 2009).
Factors proposed to be involved in ESCRT-independent ILV biogenesis include the lipids BMP/LBPA and ceramide, both of which have been demonstrated to spontaneously induce formation of internal vesicles in liposomes in vitro in the absence of any proteins (Matsuo et al, 2004; Trajkovic et al, 2008). For this, BMP/LBPA requires an acidic environment. Although not belonging to the ESCRT machinery, GGA proteins and the Tom1–Tollip complex have been implicated in the sorting of ubiquitinated cargo into LEs (Katoh et al, 2004; Puertollano and Bonifacino, 2004; Berger et al, 2007; Lauwers et al, 2009). However, due to similarity in structure to the ESCRT-0 component Hrs, and their capacity to interact with the ESCRT-I protein TSG101 (Puertollano and Bonifacino, 2004), it is likely that they act in a manner redundant with Hrs or that their role is upstream of the ESCRT machinery (Puertollano and Bonifacino, 2004).
ILV biogenesis is only partially dependent on factors that drive other events during endosome maturation. Lack of acidification does not seem to perturb ILV biogenesis, as in the absence of V-ATPase activity LEs are found to accumulate high numbers of ILVs (Vaccari et al, 2010) (our unpublished observations). The increased number of ILVs, in this case, is probably due in part to decreased degradation. As mentioned earlier, overexpression of the constitutively active Rab5(Q79L) mutant does not block ILV formation or sorting of cargo into ILVs (Wegner et al, 2010). Similarly, Rab7 activity is not required as Rab7-depleted cells tend to have LEs with high numbers of ILVs (Vanlandingham and Ceresa, 2009).
In contrast, the absence of RILP, a Rab7 effector, causes a dramatic decrease in ILV formation. There is evidence that RILP interacts with ESCRT-II, but the molecular consequences are inadequately understood (Progida et al, 2006, 2007; Wang and Hong, 2006). Although more prominently known for its role in establishing contact with dynactin/dynein motors and thereby regulating LE motility (Jordens et al, 2001; Johansson et al, 2007), RILP may have additional Rab7-independent functions. Possibly, it coordinates ILV biogenesis with endosome motility (Progida et al, 2007).
Deletion of the PI kinases VPS34 and PIKfyve/Fab1p both result in enlarged early/late hybrid endosomes with few ILVs (Odorizzi et al, 1998; Futter et al, 2001; Ikonomov et al, 2003a). Recruitment of some of the ESCRT proteins to endosomal membranes is mediated in part by their PtdIns(3)P and PtdIns(3,5)P(2) binding domains (Raiborg et al, 2001; Whitley et al, 2003; Slagsvold et al, 2005). However, the highly enlarged endosomes observed especially in PIKfyve mutant cells are not similar to those seen after depletion of the ESCRT machinery. For instance, depletion of a component of ESCRT-III, VPS24, which binds to PtdIns(3,5)P(2), causes a decrease in the size of LEs (Bache et al, 2006). Therefore, it is likely that the effects of PtdIns(3)P and PtdIns(3,5)P(2) depletion on ILV biogenesis extend beyond ESCRT function.
The lipids of ILVs are different from the limiting membrane. ILVs contain more cholesterol, sphingolipids, PtdIns(3)P, and BMP/LBPA. Cholesterol and sphingolipids are especially enriched in ILVs at early stages, whereas ILVs in older LEs and lysosomes contain high concentrations of BMP/LBPA and ceramide (Mobius et al, 2003). The decrease in cholesterol is due to extraction and transport to the ER through the action of NPC1 and NPC2, mutant forms of which cause Niemann-Pick disease, a lysosomal storage disorder in which cholesterol accumulates in LEs and endolysosomes (Karten et al, 2009). NPC2 is a soluble protein that extracts cholesterol from ILVs, while NPC1 resides in the limiting membrane and transfers cholesterol across.
Cholesterol extraction from ILVs is also dependent on sphingolipid degradation and formation of ceramide (Kolter and Sandhoff, 2010). Therefore, enrichment of sphingolipids also leads to cholesterol accumulation. The amount of BMP/LBPA correlates with the total level of cholesterol in cells, presumably due to the role of BMP/LBPA in controlling endosomal storage of cholesterol (Chevallier et al, 2008). The accumulation of cholesterol and sphingolipids causes severe disturbances in the endolysosomal pathway (Lebrand et al, 2002; Sobo et al, 2007; Vitner et al, 2010).
PtdIns(3)P in ILVs disappears during later phases most probably due to degradation or modification by kinases/phosphatases (Gillooly et al, 2000; Wurmser and Emr, 1998). ILVs that contain EGFR and PtdIns(3)P occur in LEs distinct from those that have BMP/LBPA (Gillooly et al, 2000; Kobayashi et al, 2002). This suggests that different populations of LEs exist, supported by the observation that in the absence of ESCRT machinery, a population of LEs still contains ILVs, devoid of EGFR (Stuffers et al, 2009).
Acidification
The lumenal pH of endocytic organelles and lysosomes is acidic, and acidification and its regulation constitute an important part of endosome maturation. EEs have a pH in the 6.8–6.1 range, LEs in the 6.0–4.8 range, and in lysosomes the pH can drop to values around 4.5 (Maxfield and Yamashiro, 1987). The low pH not only provides a better environment for hydrolytic reactions, but it is also essential for membrane trafficking, for the sorting and routing of cargo, for the inactivation of internalized pathogens, etc. The difference in extracellular and EE pH (or between the TGN and LEs) provides the asymmetry needed to allow receptors to bind ligands in one compartment and release them in the other. Thus, the progressively decreasing pH in the endocytic pathway can provide incoming cargo a ‘sense’ as to their location within the pathway. This is most clearly illustrated by viruses and bacterial toxins that have a relative sharp pH threshold adjusted so that they are specifically activated when they arrive in EEs, in LEs, or in lysosomes (Mercer et al, 2010).
The V-ATPases are large, complex proton pumps with a membrane-associated V0 complex that serves as a transmembrane pore for protons, and a soluble cytosolic V1 complex that binds and hydrolyzes the ATP (Forgac, 2007; Marshansky and Futai, 2008). The regulation of lumenal pH involves adjustment of the V-ATPase concentration in the membrane, selection of V-ATPase isoforms, as well as the association and dissociation of the V0 and V1 complexes (Trombetta et al, 2003; Kane, 2006; Lafourcade et al, 2008). Since V-ATPases generate a positive inside membrane potential, acidification is also affected by a variety of independent factors such as Na+/K+ ATPase, and channels for the influx of counter ions such as Cl− or efflux of cations such as Ca2+, Na+, and K+ (Huynh and Grinstein, 2007; Jentsch, 2008; Marshansky and Futai, 2008). Since V-ATPase partition into detergent resistant membrane domains, its location is sensitive to the concentration of cholesterol (Marshansky and Futai, 2008).
The pH drops rapidly by about 0.5 pH units after LE formation and keeps decreasing until fusion occurs with a lysosome. It is possible that one reason for the initial drop is that V-ATPases are predominantly localized in the vacuolar domains of EEs and therefore become concentrated in LEs. There is also evidence that the V1 complexes interact more efficiently with V0 in LEs generating a larger fraction of functional pumps (Lafourcade et al, 2008).
Three classes of inhibitors are frequently used to elevate the pH in endosomes and other acidic organelles. Lysosomotropic weak bases (NH4Cl, chloroquine, methylamine, etc.) elevate the pH by entering the acid compartment in their unprotonated form and titrating the pH upwards (Ohkuma and Poole, 1978). Carboxylic ionophores, such as monensin and nigericin, cause electroneutral exchange of protons across the membrane against other monovalent cations (Reijngoud et al, 1976). They do not affect the membrane potential, but like lysomotropic weak bases, they cause vacuolization. Finally, bafilomycins and concanamycins are potent inhibitors of the V-ATPase (Bowman et al, 1988). Like the other agents, they rapidly elevate the pH in acidic organelles of the cell.
In addition to elevating the lumenal pH, these agents have other effects, which renders interpretation of results often complicated (Weisz, 2003). Although causing tubulation of EEs, bafilomycin A usually has no dramatic effect on the recycling pathway through EEs unless recycling depends on acid-mediated dissociation of receptor/ligand complexes (Baravalle et al, 2005). However, transfer of cargo from EEs to LEs is blocked in most cases indicating a defect in endosome maturation (Clague et al, 1994; D’Arrigo et al, 1997; Bayer et al, 1998; Baravalle et al, 2005). This effect may be somewhat cell-type dependent because one study concluded that the block occurs in LE to lysosome fusion stage (van Weert et al, 1995).
Gruenberg and colleagues have proposed that the detachment of LEs (or ECVs) from EEs requires low lumenal pH. The suggested mechanism involves regulated binding of Arf1, which in turn mediates binding of COPI to the cytosolic surface of endosomes (Aniento et al, 1996; Gu and Gruenberg, 1999). There is independent evidence that Arf1 binding to microsomes in vitro increases when the lumenal pH is reduced (Zeuzem et al, 1992). Moreover, as already mentioned, it was recently reported that isolated endosomes can form ILVs in vitro, a process reduced by both bafilomycin A and monensin (Falguieres et al, 2008).
It remains unclear, however, how the pH in the lumen can be sensed on the cytosolic surface of the endosome. The presence of a trans-membrane pH-sensor has been proposed. One possible sensor is the V-ATPase itself. It is involved in recruiting ARNO (a GEF for Arf6 GTPase) to the cytosolic surface of endosomes (Hurtado-Lorenzo et al, 2006). It is interesting in this context that bafilomycin A, which inhibits the V-ATPase directly, blocks formation of LEs. Lysosomotropic weak bases and carboxylic ionophores have a similar effect on lumenal pH, but generally do not have dramatic effects on the generation of LEs or on the transport of cargo to lysosomes (Ascoli, 1984; Ukkonen et al, 1986; Clague et al, 1994).
In addition to acidification, the ionic environment in endosomes undergoes other changes that may be important for maturation and function. These have been recently reviewed (Scott and Gruenberg, 2011). The calcium concentration drops initially, but seems to recover late in the pathway. There is evidence that a calcium channel and the efflux of calcium may be important both for fusion with lysosomes and for reformation of lysosomes from endolysosomes (Pryor et al, 2000; Luzio et al, 2007a). The sodium concentration drops while potassium increases. Serving as a counter ion during acidification, chloride ions increase during maturation. For a detailed review of organelle acidification and related topics, the reader is referred to Weisz (2003).
Endosome motility
Endosomes are motile, and their movements tightly linked to their stage of maturation and function. In addition, it is clear from recent data that location in the cytoplasm, measured as distance from the nucleus, is a key parameter that influences number, size, and cargo contents of endosomes consistent with the spatio-temporal progression of endosome maturation (Collinet et al, 2010). Many small endosomes in the periphery are replaced later by fewer, larger endosomes close to the nucleus. During formation in the cell periphery, EEs are subject to slow, short-range back and forth movements, but as they mature, many of them undergo rapid long-range saltatory oscillations with a net movement towards the perinuclear region, where the majority of lysosomes are localized. The centripetal movement is mediated by both kinesin and dynein motor proteins along microtubules that radiate out from the microtubule organizing centre. Conversion to LEs can occur in the periphery, in transit, or in the perinuclear region (Nielsen et al, 1999; Bananis et al, 2000; Hoepfner et al, 2005; Soldati and Schliwa, 2006; Driskell et al, 2007; Loubery et al, 2008).
Disruption of microtubules causes the dispersal of LEs and lysosomes throughout the cytoplasm (Bayer et al, 1998). At the same time, maturation of endosomes is delayed, as Rab5 persists on endosomes together with Rab7 (Vonderheit and Helenius, 2005). Inhibiting dynein has a similar effect; Tf and EFG separation and loss of Rab5 is delayed (Driskell et al, 2007). Interestingly, although endosome acidification to ∼pH 6.0 has been reported to occur mainly at the perinuclear area after microtubule-mediated transport (Lakadamyali et al, 2003), disruption of microtubules by nocodazole treatment does not seem to inhibit initial acidification of endosomes (∼pH 6.0) (Mesaki et al, 2011). However, it does prevent cargo from reaching compartments with lower pH (∼pH 5.0) found in mature LEs and lysosomes (Bayer et al, 1998). Consistent with this, nocodazole treatment delays cargo degradation (Mesaki et al, 2011).
Motility of endosomes depends on dynein and kinesin motors, providing opposing forces that move endosomes in opposite directions (Murray et al, 2000; Soppina et al, 2009) (Figure 6A). Why do endosomes undergo such oscillatory motion on microtubules? Studies indicate that kinesin and dynein motor activity is required for fission of EEs and sorting of cargo into degradative and recycling compartments (Bananis et al, 2000; Hoepfner et al, 2005; Driskell et al, 2007). Evidence also points to a role for these motor proteins in fusion of endosomes with each other during different stages of maturation (Bomsel et al, 1990; Aniento et al, 1993; Driskell et al, 2007).
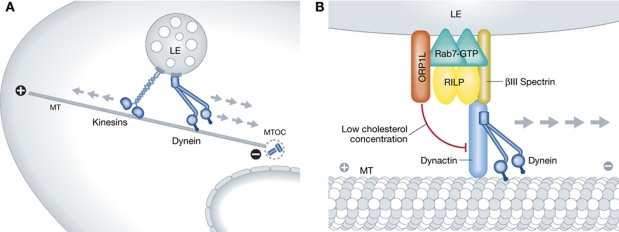
Figure 6.
LE motility. (A) LEs are transported on microtubules (MT) in a bidirectional manner, by the help of both kinesin and dynein motor proteins. Net movement is towards the microtubule organizing centre (MTOC), located in the perinuclear region of the cell, where most of the LEs and lysosomes localize. (B) Schematic of LE attachment to dynein motor through RILP. Activated Rab7 binds to its effectors RILP and ORP1L. Homodimeric RILP then recruits dynein–dynactin motor complex through the p150(Glued) subunit. The complex has to additionally interact with the dynein membrane receptor βIII spectrin (Johansson et al, 2007). This allows transport of cargo towards the MTOC. In the presence of low cholesterol on LE membranes, sensed by ORP1L, the complex is disassembled due to a conformational change of ORP1L, and resulting in inhibition of motility towards the MTOC (Rocha et al, 2009).
Kinesins constitute a large family of mainly plus-end-directed motor proteins (Hirokawa et al, 2009). Some of them have been implicated in EE and LE motility. KIF16B is a kinesin-3 motor protein responsible for plus-end-directed movement of EEs (Hoepfner et al, 2005). It attaches to EEs through its PtdIns(3)P binding PX domain when Rab5 is activated. KIF16B seems to alter the balance between degradation and recycling of cargo; depletion leads to a block of cargo recycling and an increase in cargo degradation whereas overexpression has the opposite effect. The kinesin-2 motor protein subunit KIF3A has been implicated in LE trafficking (Brown et al, 2005). Impairment of its activity results in clustering of LEs in the perinuclear region.
LE motility that brings the cargo to the vicinity of lysosomes in the perinuclear area is thought to be mainly dependent on minus-end-directed, dynein-dependent transport (Aniento et al, 1993; Bananis et al, 2004). In contrast to the large number of different kinesin motors, dynein is the only member of its family (Kardon and Vale, 2009). Organelles bind to dynein motors either directly or through adaptor protein complexes. Direct binding is mediated by the intermediate, light intermediate, and light chains of dynein. Indirect binding can be accomplished through the dynactin multiprotein complex. Attachment of LEs through dynactin is dependent on Rab7 activity (Jordens et al, 2001). Upon activation, Rab7-GTP binds its effectors RILP and the oxysterol-binding protein ORP1L that in turn recruit the dynactin subunit p150(Glued) through the dynactin receptor βIII spectrin (Johansson et al, 2007) (Figure 6B). This interaction mediates the minus-end-directed movement of LEs. Another Rab7 effector, FYCO1, has been reported to have the opposite function and to counteract the effects of RILP by mediating plus-end-directed LE and autophagosome motility (Pankiv et al, 2010). The authors propose that FYCO1 links Rab7-positive vesicles to kinesin motors, although direct interaction with kinesins is yet to be shown.
Recent findings indicate a dynactin independent linkage between LEs and dynein through a protein called Snapin, originally identified as a neuronal SNARE-binding protein and implicated in synaptic vesicle fusion (Ilardi et al, 1999; Pan et al, 2009). Snapin links LE cargo by binding directly to dynein intermediate chains. It has a crucial role in controlling LE retrograde transport and maturation in neurons (Cai et al, 2010). Deletion of Snapin in neurons results in accumulation of immature LEs, further highlighting the role of motility in coordinating endosomal maturation. The relationship between Snapin- and the dynactin/RILP-mediated LE transport remains unclear.
Cholesterol has been shown to have a role in the regulation of LE motility (Lebrand et al, 2002; Sugii et al, 2006). Accumulation of cholesterol, as seen in cells from Niemann-Pick C patients, impairs the bidirectional movement of LEs, and leads to their concentration in the perinuclear area of cells. This is partially explained by the cholesterol sensing ability of ORP1L, a LE-associated protein that dictates LE binding to the dynactin complex (Rocha et al, 2009).
A recent study contradicts the view of dynein as the major motor protein in transport of LEs towards the minus end of microtubules (Loubery et al, 2008). The authors argue for an indirect role in regulating the actin cytoskeleton. The actin cytoskeleton, well known for its various roles in endocytosis (Kaksonen et al, 2006), seems indeed to have a role in the endosomal degradative pathway. It has been long known that disruption of the actin cytoskeleton results in a block of cargo transport from EEs to LEs (Gruenberg et al, 1989); however, the actual function of actin has remained unclear. A series of recent studies have, however, shed some light on to the matter by indicating that Arp2/3-driven actin nucleation occurs on EEs and is required for a number of membrane fission processes (Derivery et al, 2009; Gomez and Billadeau, 2009; Morel et al, 2009; Duleh and Welch, 2010). These include sorting and vesicle fission in the recycling pathway, and cargo transport to the TGN and lysosomes. Members of the annexin family of proteins, such as annexins A2 and A8, link actin to endosome membranes (Goebeler et al, 2008; Morel et al, 2009). Annexin A2 was shown to have a role in LE biogenesis, perhaps by regulating fission from EEs.
There is also evidence in both yeast and mammalian cells that actin plays a role in fusion processes that occur between LEs and lysosomes/vacuoles, phagosomes and lysosomes, as well as between autophagosomes and lysosomes (van Deurs et al, 1995; Jahraus et al, 2001; Eitzen et al, 2002; Kjeken et al, 2004; Lee et al, 2010). In yeast, where this process has been studied in more detail, it has been demonstrated that actin nucleation initiated during tethering/docking and persisting through membrane fusion, is induced by the Rho GTPases Rho1p and Cdc42p, which themselves are activated by the yeast Rab7 homologue, Ypt7p (Eitzen et al, 2001).
Microautophagy, expulsion of exosomes, and backfusion
The inwards budding of the endosomal membrane and the formation of ILVs not only detaches membrane components from the limiting membrane (Figure 7A), but also traps a small volume of cytosol. Is this used for targeting cytosolic components to the lysosome? Recent studies indicate that cells have indeed evolved different mechanisms for selective uptake of cytosolic proteins and nucleic acids into ILVs during LE formation.
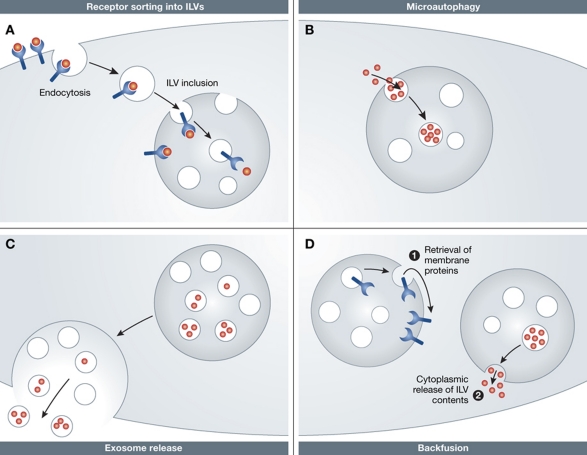
Figure 7.
Function of LEs. (A) Cargo that needs to degraded is endocytosed and sorted into ILVs. This can be also used to silence signalling from receptors, normally exposed to the cytosol. (B) Microautophagy is used to bring cytosolic contents into LEs. (C) ILVs can be secreted as exosomes to the extracellular environment upon fusion of LEs with the plasma membrane. (D) ‘Backfusion’ of ILVs with the limiting membrane of LEs allows retrieval of membrane cargo or release of ILV contents into the cytosol.
Microautophagy is a process in which cytosolic components are taken up through invaginations of LEs, in both a selective and unselective manner (Figure 7B). Selective microautophagy involves the cytosolic chaperone hsc70 and the ESCRT-I and ESCRT-III complexes, which together allow uptake of cytosolic proteins that bear a KFERQ sequence motif (Sahu et al, 2011). This is thought to rely on the electrostatic association of hsc70 with phosphatidylserine in the cytosolic leaflet of the LE membrane. A similar process has, interestingly, been proposed for the inclusion of certain RNAs into ILVs (Valadi et al, 2007; Gibbings and Voinnet, 2010).
Exosomes are vesicles found in various body fluids that have their origin in LEs (Figure 7C). They are small (40–100 nm in diameter), and contain a single membrane. They are released when LEs fuse with the plasma membrane (Simons and Raposo, 2009; Von Bartheld and Altick, 2011). Their composition reflects that of ILVs, and their biogenesis is linked to endosome maturation. They have received considerable attention in recent years because there is increasing evidence that they may have a role in intercellular communication.
The formation of exosomes depends on ESCRT function, although it is apparent that ESCRT-independent mechanisms may also exist. In immature DCs, MHC II is ubiquitinated, enters ILVs, and is targeted for degradation, while in mature DCs the MHC II containing ILVs are secreted as exosomes (Buschow et al, 2009). How the LEs fuse with the plasma membrane has been the focus of recent studies. In HeLa cells, Rab27a, Rab27b and their effectors Slp4 and Slac2b were found to promote fusion (Ostrowski et al, 2010). In oligodendrocytes, Rab35 and its GAPs, TBC1D10A-C have been implicated (Hsu et al, 2010).
There is also evidence, although still indirect, that ILVs can fuse with the limiting membrane of the LEs (Figure 7D). As a result of such ‘backfusion’, the membrane proteins in an ILV may become part of the limiting membrane of an endosome or lysosome from which they can recycle to the TGN or the plasma membrane and escape lysosomal degradation (van der Goot and Gruenberg, 2006). This phenomenon has been proposed for the recycling of MHC II in DCs, mannose-6-phosphate receptors, and tetraspannin proteins, all of which are found to be enriched in ILVs (Kobayashi et al, 1998, 2002; Kleijmeer et al, 2001; Trombetta and Mellman, 2005). Backfusion has also been suggested as mechanism for delaying the release of anthrax toxin and incoming vesicular stomatitis virus capsids into the cytosol (van der Goot and Gruenberg, 2006). The fusion of ILVs with the limiting membrane of the LE or endolysosome is thought to be enhanced by BMP/LBPA and the ESCRT accessory protein Alix (Kobayashi et al, 2002; Abrami et al, 2004; Le Blanc et al, 2005).
In addition to regulating cargo trafficking, storage, and degradation, endosomes are used as signalling compartments (Sorkin and von Zastrow, 2009; Platta and Stenmark, 2011). Signalling occurs in early as well as late compartments. Organelle segregation allows spatio-temporal regulation, and endosome maturation is used for silencing and activation. K-Ras/MAPK and mTORC1-dependent signalling cascades are examples that occur on LEs (Teis et al, 2002; Nada et al, 2009; Flinn et al, 2010). Some signalling molecules undergo proteolytic activation in the degradative environment of LEs. For example, TLR9 requires cathepsin-mediated cleavage of its ectodomain to initiate signalling (Ewald et al, 2008). To what extent such signalling activities affect endosome maturation is receiving increasing attention (Sorkin and von Zastrow, 2009; Collinet et al, 2010). For instance, MAPK signalling on LEs has been found to regulate LE dynamics and cargo degradation (Teis et al, 2006).
Perspectives
Endosomes have proven infinitely more complex than ever imagined in the early 1980s when their existence as a universal, pre-lysosomal compartment network was first demonstrated. Based on a wealth of studies in many different cell types and organisms, one can now appreciate these multifunctional organelles as a dynamic, highly adaptable system deeply embedded within the core fabric of cell life. As research in the field moves forward, it will be important to define the common mechanisms and concepts underlying endosome function and regulation. The maturation programme discussed above is coordinated so that many of the various changes are interdependent and cooperative. These and other central aspects of the endosome machinery can now be approached at the molecular as well as at a systems level in different organisms.
More work will be required to map the fate of incoming cargo including the activation and inactivation of receptors and signalling proteins. The endosomal system is vital as an extension of the plasma membrane in signalling and cell regulation. Endosomes play a fundamental role in numerous diseases and pathogenic states, and are therefore of critical medical significance. To us, the analysis of viruses and toxins that traverse the pathway is especially interesting, because these exploit the properties of the pathway in many elaborate ways. By the use of perturbants to modify endosome behaviour, it may possible to prevent infection and intoxication. Furthermore, the endosomes provide a much needed entry port for the introduction of membrane impermeant drugs, proteins, and nucleic acids into cells, a function that is increasingly important in gene therapy and medicine.
Acknowledgments
We are grateful to Renate Fuchs and Anne Spang for critical reading and helpful comments of the manuscript. We thank John Tooze for allowing us to use the image in Figure 3A and Sarah Stauffer for providing us with the images for Figure 3B–E. This work was supported by grants from the European Research Council, from SystemsX (LipidX, InfectX), and ETH Zurich. We apologize to authors for not citing all original publications due to reasons of space.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abrami L, Lindsay M, Parton RG, Leppla SH, van der Goot FG (2004) Membrane insertion of anthrax protective antigen and cytoplasmic delivery of lethal factor occur at different stages of the endocytic pathway. J Cell Biol 166: 645–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwan HA, van Zoelen EJ, van Leeuwen JE (2003) Ligand-induced lysosomal epidermal growth factor receptor (EGFR) degradation is preceded by proteasome-dependent EGFR de-ubiquitination. J Biol Chem 278: 35781–35790 [DOI] [PubMed] [Google Scholar]
- Aniento F, Emans N, Griffiths G, Gruenberg J (1993) Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J Cell Biol 123: 1373–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniento F, Gu F, Parton RG, Gruenberg J (1996) An endosomal beta COP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J Cell Biol 133: 29–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli M (1984) Lysosomal accumulation of the hormone-receptor complex during receptor-mediated endocytosis of human choriogonadotropin. J Cell Biol 99: 1242–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst M (2005) A protein's final ESCRT. Traffic 6: 2–9 [DOI] [PubMed] [Google Scholar]
- Bache KG, Stuffers S, Malerod L, Slagsvold T, Raiborg C, Lechardeur D, Walchli S, Lukacs GL, Brech A, Stenmark H (2006) The ESCRT-III subunit hVps24 is required for degradation but not silencing of the epidermal growth factor receptor. Mol Biol Cell 17: 2513–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bananis E, Murray JW, Stockert RJ, Satir P, Wolkoff AW (2000) Microtubule and motor-dependent endocytic vesicle sorting in vitro. J Cell Biol 151: 179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bananis E, Nath S, Gordon K, Satir P, Stockert RJ, Murray JW, Wolkoff AW (2004) Microtubule-dependent movement of late endocytic vesicles in vitro: requirements for Dynein and Kinesin. Mol Biol Cell 15: 3688–3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baravalle G, Schober D, Huber M, Bayer N, Murphy RF, Fuchs R (2005) Transferrin recycling and dextran transport to lysosomes is differentially affected by bafilomycin, nocodazole, and low temperature. Cell Tissue Res 320: 99–113 [DOI] [PubMed] [Google Scholar]
- Barr F, Lambright DG (2010) Rab GEFs and GAPs. Curr Opin Cell Biol 22: 461–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer N, Schober D, Prchla E, Murphy RF, Blaas D, Fuchs R (1998) Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implications for viral uncoating and infection. J Virol 72: 9645–9655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnia R, Munro S (2005) Organelle identity and the signposts for membrane traffic. Nature 438: 597–604 [DOI] [PubMed] [Google Scholar]
- Berger AC, Salazar G, Styers ML, Newell-Litwa KA, Werner E, Maue RA, Corbett AH, Faundez V (2007) The subcellular localization of the Niemann-Pick Type C proteins depends on the adaptor complex AP-3. J Cell Sci 120: 3640–3652 [DOI] [PubMed] [Google Scholar]
- Besterman JM, Low RB (1983) Endocytosis: a review of mechanisms and plasma membrane dynamics. Biochem J 210: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsel M, Parton R, Kuznetsov SA, Schroer TA, Gruenberg J (1990) Microtubule- and motor-dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell 62: 719–731 [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Hurley JH (2008) Retromer. Curr Opin Cell Biol 20: 427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Rojas R (2006) Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol 7: 568–579 [DOI] [PubMed] [Google Scholar]
- Bowman EJ, Siebers A, Altendorf K (1988) Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA 85: 7972–7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CL, Maier KC, Stauber T, Ginkel LM, Wordeman L, Vernos I, Schroer TA (2005) Kinesin-2 is a motor for late endosomes and lysosomes. Traffic 6: 1114–1124 [DOI] [PubMed] [Google Scholar]
- Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B (2000) Rab7: a key to lysosome biogenesis. Mol Biol Cell 11: 467–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschow SI, Nolte-’t Hoen EN, van Niel G, Pols MS, ten Broeke T, Lauwen M, Ossendorp F, Melief CJ, Raposo G, Wubbolts R, Wauben MH, Stoorvogel W (2009) MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic 10: 1528–1542 [DOI] [PubMed] [Google Scholar]
- Cabrera M, Ungermann C (2010) Guiding endosomal maturation. Cell 141: 404–406 [DOI] [PubMed] [Google Scholar]
- Cai Q, Lu L, Tian JH, Zhu YB, Qiao H, Sheng ZH (2010) Snapin-regulated late endosomal transport is critical for efficient autophagy-lysosomal function in neurons. Neuron 68: 73–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M (1990) Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell 62: 317–329 [DOI] [PubMed] [Google Scholar]
- Chevallier J, Chamoun Z, Jiang G, Prestwich G, Sakai N, Matile S, Parton RG, Gruenberg J (2008) Lysobisphosphatidic acid controls endosomal cholesterol levels. J Biol Chem 283: 27871–27880 [DOI] [PubMed] [Google Scholar]
- Chotard L, Mishra AK, Sylvain MA, Tuck S, Lambright DG, Rocheleau CE (2010) TBC-2 regulates RAB-5/RAB-7-mediated endosomal trafficking in Caenorhabditis elegans. Mol Biol Cell 21: 2285–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis S, Miaczynska M, Ashman K, Wilm M, Zhao L, Yip SC, Waterfield MD, Backer JM, Zerial M (1999) Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat Cell Biol 1: 249–252 [DOI] [PubMed] [Google Scholar]
- Clague MJ, Urbe S (2006) Endocytosis: the DUB version. Trends Cell Biol 16: 551–559 [DOI] [PubMed] [Google Scholar]
- Clague MJ, Urbe S, Aniento F, Gruenberg J (1994) Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J Biol Chem 269: 21–24 [PubMed] [Google Scholar]
- Collinet C, Stoter M, Bradshaw CR, Samusik N, Rink JC, Kenski D, Habermann B, Buchholz F, Henschel R, Mueller MS, Nagel WE, Fava E, Kalaidzidis Y, Zerial M (2010) Systems survey of endocytosis by multiparametric image analysis. Nature 464: 243–249 [DOI] [PubMed] [Google Scholar]
- D’Arrigo A, Bucci C, Toh BH, Stenmark H (1997) Microtubules are involved in bafilomycin A1-induced tubulation and Rab5-dependent vacuolation of early endosomes. Eur J Cell Biol 72: 95–103 [PubMed] [Google Scholar]
- Daro E, Sheff D, Gomez M, Kreis T, Mellman I (1997) Inhibition of endosome function in CHO cells bearing a temperature-sensitive defect in the coatomer (COPI) component epsilon-COP. J Cell Biol 139: 1747–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lartigue J, Polson H, Feldman M, Shokat K, Tooze SA, Urbe S, Clague MJ (2009) PIKfyve regulation of endosome-linked pathways. Traffic 10: 883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Conte-Zerial P, Brusch L, Rink JC, Collinet C, Kalaidzidis Y, Zerial M, Deutsch A (2008) Membrane identity and GTPase cascades regulated by toggle and cut-out switches. Mol Syst Biol 4: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derivery E, Sousa C, Gautier JJ, Lombard B, Loew D, Gautreau A (2009) The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev Cell 17: 712–723 [DOI] [PubMed] [Google Scholar]
- Dettmer J, Hong-Hermesdorf A, Stierhof YD, Schumacher K (2006) Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 18: 715–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Chen W, Welford A, Wandinger-Ness A (2004) The proteasome alpha-subunit XAPC7 interacts specifically with Rab7 and late endosomes. J Biol Chem 279: 21334–21342 [DOI] [PubMed] [Google Scholar]
- Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, Cheng X, Zhang Y, Weisman LS, Delling M, Xu H (2010) PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat Commun 1: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove SK, Dong K, Kobayashi T, Williams FK, Michell RH (2009) Phosphatidylinositol 3,5-bisphosphate and Fab1p/PIKfyve underPPIn endo-lysosome function. Biochem J 419: 1–13 [DOI] [PubMed] [Google Scholar]
- Doyotte A, Russell MR, Hopkins CR, Woodman PG (2005) Depletion of TSG101 forms a mammalian ‘Class E’ compartment: a multicisternal early endosome with multiple sorting defects. J Cell Sci 118: 3003–3017 [DOI] [PubMed] [Google Scholar]
- Driskell OJ, Mironov A, Allan VJ, Woodman PG (2007) Dynein is required for receptor sorting and the morphogenesis of early endosomes. Nat Cell Biol 9: 113–120 [DOI] [PubMed] [Google Scholar]
- Duleh SN, Welch MD (2010) WASH and the Arp2/3 complex regulate endosome shape and trafficking. Cytoskeleton (Hoboken) 67: 193–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden ER, White IJ, Futter CE (2009) Down-regulation of epidermal growth factor receptor signalling within multivesicular bodies. Biochem Soc Trans 37: 173–177 [DOI] [PubMed] [Google Scholar]
- Eitzen G, Thorngren N, Wickner W (2001) Rho1p and Cdc42p act after Ypt7p to regulate vacuole docking. EMBO J 20: 5650–5656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitzen G, Wang L, Thorngren N, Wickner W (2002) Remodeling of organelle-bound actin is required for yeast vacuole fusion. J Cell Biol 158: 669–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelinen EL (2008) New insights into the mechanisms of macroautophagy in mammalian cells. Int Rev Cell Mol Biol 266: 207–247 [DOI] [PubMed] [Google Scholar]
- Ewald SE, Lee BL, Lau L, Wickliffe KE, Shi GP, Chapman HA, Barton GM (2008) The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature 456: 658–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falguieres T, Luyet PP, Bissig C, Scott CC, Velluz MC, Gruenberg J (2008) In vitro budding of intralumenal vesicles into late endosomes is regulated by Alix and Tsg101. Mol Biol Cell 19: 4942–4955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinn RJ, Yan Y, Goswami S, Parker PJ, Backer JM (2010) The late endosome is essential for mTORC1 signaling. Mol Biol Cell 21: 833–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgac M (2007) Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol 8: 917–929 [DOI] [PubMed] [Google Scholar]
- Frasa MA, Maximiano FC, Smolarczyk K, Francis RE, Betson ME, Lozano E, Goldenring J, Seabra MC, Rak A, Ahmadian MR, Braga VM (2010) Armus is a Rac1 effector that inactivates Rab7 and regulates E-cadherin degradation. Curr Biol 20: 198–208 [DOI] [PubMed] [Google Scholar]
- Funderburk SF, Wang QJ, Yue Z (2010) The Beclin 1-VPS34 complex—at the crossroads of autophagy and beyond. Trends Cell Biol 20: 355–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter CE, Collinson LM, Backer JM, Hopkins CR (2001) Human VPS34 is required for internal vesicle formation within multivesicular endosomes. J Cell Biol 155: 1251–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary JD, Wurmser AE, Bonangelino CJ, Weisman LS, Emr SD (1998) Fab1p is essential for PtdIns(3)P 5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. J Cell Biol 143: 65–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha T, Wooten MW (2008) TrkA receptor endolysosomal degradation is both ubiquitin and proteasome dependent. Traffic 9: 1146–1156 [DOI] [PubMed] [Google Scholar]
- Gerasimenko JV, Tepikin AV, Petersen OH, Gerasimenko OV (1998) Calcium uptake via endocytosis with rapid release from acidifying endosomes. Curr Biol 8: 1335–1338 [DOI] [PubMed] [Google Scholar]
- Gibbings D, Voinnet O (2010) Control of RNA silencing and localization by endolysosomes. Trends Cell Biol 20: 491–501 [DOI] [PubMed] [Google Scholar]
- Gillooly DJ, Morrow IC, Lindsay M, Gould R, Bryant NJ, Gaullier JM, Parton RG, Stenmark H (2000) Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J 19: 4577–4588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebeler V, Poeter M, Zeuschner D, Gerke V, Rescher U (2008) Annexin A8 regulates late endosome organization and function. Mol Biol Cell 19: 5267–5278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez TS, Billadeau DD (2009) A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev Cell 17: 699–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbea C, Pratt G, Ustrell V, Bell R, Sahasrabudhe S, Hughes RE, Rechsteiner M (2010) A protein interaction network for Ecm29 links the 26 S proteasome to molecular motors and endosomal components. J Biol Chem 285: 31616–31633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BD, Sato M (2006) Intracellular trafficking. In WormBook, pp 1–9; doi:10.1895/wormbook.1.77.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J, Griffiths G, Howell KE (1989) Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J Cell Biol 108: 1301–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J, Stenmark H (2004) The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol 5: 317–323 [DOI] [PubMed] [Google Scholar]
- Gu F, Aniento F, Parton RG, Gruenberg J (1997) Functional dissection of COP-I subunits in the biogenesis of multivesicular endosomes. J Cell Biol 139: 1183–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F, Gruenberg J (1999) Biogenesis of transport intermediates in the endocytic pathway. FEBS Lett 452: 61–66 [DOI] [PubMed] [Google Scholar]
- Guo Q, Penman M, Trigatti BL, Krieger M (1996) A single point mutation in epsilon-COP results in temperature-sensitive, lethal defects in membrane transport in a Chinese hamster ovary cell mutant. J Biol Chem 271: 11191–11196 [DOI] [PubMed] [Google Scholar]
- Haas A, Scheglmann D, Lazar T, Gallwitz D, Wickner W (1995) The GTPase Ypt7p of Saccharomyces cerevisiae is required on both partner vacuoles for the homotypic fusion step of vacuole inheritance. EMBO J 14: 5258–5270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas AK, Fuchs E, Kopajtich R, Barr FA (2005) A GTPase-activating protein controls Rab5 function in endocytic trafficking. Nat Cell Biol 7: 887–893 [DOI] [PubMed] [Google Scholar]
- Hayer A, Stoeber M, Ritz D, Engel S, Meyer HH, Helenius A (2010) Caveolin-1 is ubiquitinated and targeted to intralumenal vesicles in endolysosomes for degradation. J Cell Biol 191: 615–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A, Mellman I, Wall D, Hubbard A (1983) Endosomes. Trends Biochem Sci 8: 245–250 [Google Scholar]
- Hirokawa N, Noda Y, Tanaka Y, Niwa S (2009) Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol 10: 682–696 [DOI] [PubMed] [Google Scholar]
- Hirota Y, Kuronita T, Fujita H, Tanaka Y (2007) A role for Rab5 activity in the biogenesis of endosomal and lysosomal compartments. Biochem Biophys Res Commun 364: 40–47 [DOI] [PubMed] [Google Scholar]
- Hoepfner S, Severin F, Cabezas A, Habermann B, Runge A, Gillooly D, Stenmark H, Zerial M (2005) Modulation of receptor recycling and degradation by the endosomal kinesin KIF16B. Cell 121: 437–450 [DOI] [PubMed] [Google Scholar]
- Horiuchi H, Lippe R, McBride HM, Rubino M, Woodman P, Stenmark H, Rybin V, Wilm M, Ashman K, Mann M, Zerial M (1997) A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell 90: 1149–1159 [DOI] [PubMed] [Google Scholar]
- Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Gronborg M, Mobius W, Rhee J, Barr FA, Simons M (2010) Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol 189: 223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W, Whitney JA, Mellman I (1992) Brefeldin A and the endocytic pathway. Possible implications for membrane traffic and sorting. FEBS Lett 307: 93–96 [DOI] [PubMed] [Google Scholar]
- Hurley JH (2010) The ESCRT complexes. Crit Rev Biochem Mol Biol 45: 463–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley JH, Hanson PI (2010) Membrane budding and scission by the ESCRT machinery: it's all in the neck. Nat Rev Mol Cell Biol 11: 556–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado-Lorenzo A, Skinner M, El Annan J, Futai M, Sun-Wada GH, Bourgoin S, Casanova J, Wildeman A, Bechoua S, Ausiello DA, Brown D, Marshansky V (2006) V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat Cell Biol 8: 124–136 [DOI] [PubMed] [Google Scholar]
- Huynh KK, Grinstein S (2007) Regulation of vacuolar pH and its modulation by some microbial species. Microbiol Mol Biol Rev 71: 452–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomov OC, Sbrissa D, Fenner H, Shisheva A (2009) PIKfyve-ArPIKfyve-Sac3 core complex: contact sites and their consequence for Sac3 phosphatase activity and endocytic membrane homeostasis. J Biol Chem 284: 35794–35806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomov OC, Sbrissa D, Foti M, Carpentier JL, Shisheva A (2003a) PIKfyve controls fluid phase endocytosis but not recycling/degradation of endocytosed receptors or sorting of procathepsin D by regulating multivesicular body morphogenesis. Mol Biol Cell 14: 4581–4591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomov OC, Sbrissa D, Mlak K, Deeb R, Fligger J, Soans A, Finley RL Jr, Shisheva A (2003b) Active PIKfyve associates with and promotes the membrane attachment of the late endosome-to-trans-Golgi network transport factor Rab9 effector p40. J Biol Chem 278: 50863–50871 [DOI] [PubMed] [Google Scholar]
- Ikonomov OC, Sbrissa D, Mlak K, Kanzaki M, Pessin J, Shisheva A (2002) Functional dissection of lipid and protein kinase signals of PIKfyve reveals the role of PtdIns 3,5-P2 production for endomembrane integrity. J Biol Chem 277: 9206–9211 [DOI] [PubMed] [Google Scholar]
- Ikonomov OC, Sbrissa D, Shisheva A (2001) Mammalian cell morphology and endocytic membrane homeostasis require enzymatically active phosphoinositide 5-kinase PIKfyve. J Biol Chem 276: 26141–26147 [DOI] [PubMed] [Google Scholar]
- Ilardi JM, Mochida S, Sheng ZH (1999) Snapin: a SNARE-associated protein implicated in synaptic transmission. Nat Neurosci 2: 119–124 [DOI] [PubMed] [Google Scholar]
- Irani NG, Russinova E (2009) Receptor endocytosis and signaling in plants. Curr Opin Plant Biol 12: 653–659 [DOI] [PubMed] [Google Scholar]
- Itakura E, Kishi C, Inoue K, Mizushima N (2008) Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell 19: 5360–5372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahraus A, Egeberg M, Hinner B, Habermann A, Sackman E, Pralle A, Faulstich H, Rybin V, Defacque H, Griffiths G (2001) ATP-dependent membrane assembly of F-actin facilitates membrane fusion. Mol Biol Cell 12: 155–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies HB, Cooke FT, Jat P, Boucheron C, Koizumi T, Hayakawa M, Kaizawa H, Ohishi T, Workman P, Waterfield MD, Parker PJ (2008) A selective PIKfyve inhibitor blocks PtdIns(3,5)P(2) production and disrupts endomembrane transport and retroviral budding. EMBO Rep 9: 164–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ (2008) CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit Rev Biochem Mol Biol 43: 3–36 [DOI] [PubMed] [Google Scholar]
- Johansson M, Rocha N, Zwart W, Jordens I, Janssen L, Kuijl C, Olkkonen VM, Neefjes J (2007) Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin. J Cell Biol 176: 459–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordens I, Fernandez-Borja M, Marsman M, Dusseljee S, Janssen L, Calafat J, Janssen H, Wubbolts R, Neefjes J (2001) The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr Biol 11: 1680–1685 [DOI] [PubMed] [Google Scholar]
- Jovic M, Sharma M, Rahajeng J, Caplan S (2010) The early endosome: a busy sorting station for proteins at the crossroads. Histol Histopathol 25: 99–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaksonen M, Toret CP, Drubin DG (2006) Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 7: 404–414 [DOI] [PubMed] [Google Scholar]
- Kane PM (2006) The where, when, and how of organelle acidification by the yeast vacuolar H+-ATPase. Microbiol Mol Biol Rev 70: 177–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardon JR, Vale RD (2009) Regulators of the cytoplasmic dynein motor. Nat Rev Mol Cell Biol 10: 854–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karten B, Peake KB, Vance JE (2009) Mechanisms and consequences of impaired lipid trafficking in Niemann-Pick type C1-deficient mammalian cells. Biochim Biophys Acta 1791: 659–670 [DOI] [PubMed] [Google Scholar]
- Katoh Y, Shiba Y, Mitsuhashi H, Yanagida Y, Takatsu H, Nakayama K (2004) Tollip and Tom1 form a complex and recruit ubiquitin-conjugated proteins onto early endosomes. J Biol Chem 279: 24435–24443 [DOI] [PubMed] [Google Scholar]
- Katzmann DJ, Stefan CJ, Babst M, Emr SD (2003) Vps27 recruits ESCRT machinery to endosomes during MVB sorting. J Cell Biol 162: 413–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr MC, Teasdale RD (2009) Defining macropinocytosis. Traffic 10: 364–371 [DOI] [PubMed] [Google Scholar]
- Khor R, McElroy LJ, Whittaker GR (2003) The ubiquitin-vacuolar protein sorting system is selectively required during entry of influenza virus into host cells. Traffic 4: 857–868 [DOI] [PubMed] [Google Scholar]
- Kielian MC, Marsh M, Helenius A (1986) Kinetics of endosome acidification detected by mutant and wild-type Semliki Forest virus. EMBO J 5: 3103–3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchen JM, Ravichandran KS (2008) Phagosome maturation: going through the acid test. Nat Rev Mol Cell Biol 9: 781–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchen JM, Ravichandran KS (2010) Identification of two evolutionarily conserved genes regulating processing of engulfed apoptotic cells. Nature 464: 778–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjeken R, Egeberg M, Habermann A, Kuehnel M, Peyron P, Floetenmeyer M, Walther P, Jahraus A, Defacque H, Kuznetsov SA, Griffiths G (2004) Fusion between phagosomes, early and late endosomes: a role for actin in fusion between late, but not early endocytic organelles. Mol Biol Cell 15: 345–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijmeer M, Ramm G, Schuurhuis D, Griffith J, Rescigno M, Ricciardi-Castagnoli P, Rudensky AY, Ossendorp F, Melief CJ, Stoorvogel W, Geuze HJ (2001) Reorganization of multivesicular bodies regulates MHC class II antigen presentation by dendritic cells. J Cell Biol 155: 53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijnen MF, Kirkpatrick DS, Gygi SP (2007) The ubiquitin-proteasome system regulates membrane fusion of yeast vacuoles. EMBO J 26: 275–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Beuchat MH, Chevallier J, Makino A, Mayran N, Escola JM, Lebrand C, Cosson P, Gruenberg J (2002) Separation and characterization of late endosomal membrane domains. J Biol Chem 277: 32157–32164 [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Stang E, Fang KS, de Moerloose P, Parton RG, Gruenberg J (1998) A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature 392: 193–197 [DOI] [PubMed] [Google Scholar]
- Kolter T, Sandhoff K (2010) Lysosomal degradation of membrane lipids. FEBS Lett 584: 1700–1712 [DOI] [PubMed] [Google Scholar]
- Lafourcade C, Sobo K, Kieffer-Jaquinod S, Garin J, van der Goot FG (2008) Regulation of the V-ATPase along the endocytic pathway occurs through reversible subunit association and membrane localization. PLoS One 3: e2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakadamyali M, Rust MJ, Babcock HP, Zhuang X (2003) Visualizing infection of individual influenza viruses. Proc Natl Acad Sci USA 100: 9280–9285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakadamyali M, Rust MJ, Zhuang X (2006) Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell 124: 997–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzetti L, Rybin V, Malabarba MG, Christoforidis S, Scita G, Zerial M, Di Fiore PP (2000) The Eps8 protein coordinates EGF receptor signalling through Rac and trafficking through Rab5. Nature 408: 374–377 [DOI] [PubMed] [Google Scholar]
- Lauwers E, Jacob C, Andre B (2009) K63-linked ubiquitin chains as a specific signal for protein sorting into the multivesicular body pathway. J Cell Biol 185: 493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc I, Luyet PP, Pons V, Ferguson C, Emans N, Petiot A, Mayran N, Demaurex N, Faure J, Sadoul R, Parton RG, Gruenberg J (2005) Endosome-to-cytosol transport of viral nucleocapsids. Nat Cell Biol 7: 653–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrand C, Corti M, Goodson H, Cosson P, Cavalli V, Mayran N, Faure J, Gruenberg J (2002) Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J 21: 1289–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Koga H, Kawaguchi Y, Tang W, Wong E, Gao YS, Pandey UB, Kaushik S, Tresse E, Lu J, Taylor JP, Cuervo AM, Yao TP (2010) HDAC6 controls autophagosome maturation essential for ubiquitin-selective quality-control autophagy. EMBO J 29: 969–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MJ, Nichols BJ, Prescianotto-Baschong C, Riezman H, Pelham HR (2000) Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol Biol Cell 11: 23–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zou W, Zhao D, Yan J, Zhu Z, Lu J, Wang X (2009) C. elegans Rab GTPase activating protein TBC-2 promotes cell corpse degradation by regulating the small GTPase RAB-5. Development 136: 2445–2455 [DOI] [PubMed] [Google Scholar]
- Lippe R, Miaczynska M, Rybin V, Runge A, Zerial M (2001) Functional synergy between Rab5 effector Rabaptin-5 and exchange factor Rabex-5 when physically associated in a complex. Mol Biol Cell 12: 2219–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan L, Tipper C, Amherdt M, Orci L, Klausner RD (1991) Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell 67: 601–616 [DOI] [PubMed] [Google Scholar]
- Lobert VH, Brech A, Pedersen NM, Wesche J, Oppelt A, Malerod L, Stenmark H (2010) Ubiquitination of alpha 5 beta 1 integrin controls fibroblast migration through lysosomal degradation of fibronectin-integrin complexes. Dev Cell 19: 148–159 [DOI] [PubMed] [Google Scholar]
- Longva KE, Blystad FD, Stang E, Larsen AM, Johannessen LE, Madshus IH (2002) Ubiquitination and proteasomal activity is required for transport of the EGF receptor to inner membranes of multivesicular bodies. J Cell Biol 156: 843–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loubery S, Wilhelm C, Hurbain I, Neveu S, Louvard D, Coudrier E (2008) Different microtubule motors move early and late endocytic compartments. Traffic 9: 492–509 [DOI] [PubMed] [Google Scholar]
- Luzio JP, Bright NA, Pryor PR (2007a) The role of calcium and other ions in sorting and delivery in the late endocytic pathway. Biochem Soc Trans 35: 1088–1091 [DOI] [PubMed] [Google Scholar]
- Luzio JP, Pryor PR, Bright NA (2007b) Lysosomes: fusion and function. Nat Rev Mol Cell Biol 8: 622–632 [DOI] [PubMed] [Google Scholar]
- Maier O, Oberle V, Hoekstra D (2002) Fluorescent lipid probes: some properties and applications (a review). Chem Phys Lipids 116: 3–18 [DOI] [PubMed] [Google Scholar]
- Marsh M, Helenius A (1989) Virus entry into animal cells. Adv Virus Res 36: 107–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshansky V, Futai M (2008) The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function. Curr Opin Cell Biol 20: 415–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo H, Chevallier J, Mayran N, Le Blanc I, Ferguson C, Faure J, Blanc NS, Matile S, Dubochet J, Sadoul R, Parton RG, Vilbois F, Gruenberg J (2004) Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science 303: 531–534 [DOI] [PubMed] [Google Scholar]
- Mattera R, Bonifacino JS (2008) Ubiquitin binding and conjugation regulate the recruitment of Rabex-5 to early endosomes. EMBO J 27: 2484–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield FR, McGraw TE (2004) Endocytic recycling. Nat Rev Mol Cell Biol 5: 121–132 [DOI] [PubMed] [Google Scholar]
- Maxfield FR, Yamashiro DJ (1987) Endosome acidification and the pathways of receptor-mediated endocytosis. Adv Exp Med Biol 225: 189–198 [DOI] [PubMed] [Google Scholar]
- Mayor S, Pagano RE (2007) Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol 8: 603–612 [DOI] [PubMed] [Google Scholar]
- Mellman I (1996) Endocytosis and molecular sorting. Annu Rev Cell Dev Biol 12: 575–625 [DOI] [PubMed] [Google Scholar]
- Mercer J, Schelhaas M, Helenius A (2010) Virus entry by endocytosis. Annu Rev Biochem 79: 803–833 [DOI] [PubMed] [Google Scholar]
- Meresse S, Gorvel JP, Chavrier P (1995) The rab7 GTPase resides on a vesicular compartment connected to lysosomes. J Cell Sci 108 (Pt 11): 3349–3358 [DOI] [PubMed] [Google Scholar]
- Mesaki K, Tanabe K, Obayashi M, Oe N, Takei K (2011) Fission of tubular endosomes triggers endosomal acidification and movement. PLoS One 6: e19764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaczynska M, Christoforidis S, Giner A, Shevchenko A, Uttenweiler-Joseph S, Habermann B, Wilm M, Parton RG, Zerial M (2004) APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell 116: 445–456 [DOI] [PubMed] [Google Scholar]
- Michelet X, Djeddi A, Legouis R (2010) Developmental and cellular functions of the ESCRT machinery in pluricellular organisms. Biol Cell 102: 191–202 [DOI] [PubMed] [Google Scholar]
- Mobius W, van Donselaar E, Ohno-Iwashita Y, Shimada Y, Heijnen HF, Slot JW, Geuze HJ (2003) Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic 4: 222–231 [DOI] [PubMed] [Google Scholar]
- Morel E, Parton RG, Gruenberg J (2009) Annexin A2-dependent polymerization of actin mediates endosome biogenesis. Dev Cell 16: 445–457 [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Maxfield FR (2004) Membrane domains. Annu Rev Cell Dev Biol 20: 839–866 [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Soe TT, Maxfield FR (1999) Endocytic sorting of lipid analogues differing solely in the chemistry of their hydrophobic tails. J Cell Biol 144: 1271–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JT, Panaretou C, Stenmark H, Miaczynska M, Backer JM (2002) Role of Rab5 in the recruitment of hVps34/p150 to the early endosome. Traffic 3: 416–427 [DOI] [PubMed] [Google Scholar]
- Murray JW, Bananis E, Wolkoff AW (2000) Reconstitution of ATP-dependent movement of endocytic vesicles along microtubules in vitro: an oscillatory bidirectional process. Mol Biol Cell 11: 419–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada S, Hondo A, Kasai A, Koike M, Saito K, Uchiyama Y, Okada M (2009) The novel lipid raft adaptor p18 controls endosome dynamics by anchoring the MEK-ERK pathway to late endosomes. EMBO J 28: 477–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson DP, Brett CL, Merz AJ (2009) Vps-C complexes: gatekeepers of endolysosomal traffic. Curr Opin Cell Biol 21: 543–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicot AS, Fares H, Payrastre B, Chisholm AD, Labouesse M, Laporte J (2006) The phosphoinositide kinase PIKfyve/Fab1p regulates terminal lysosome maturation in Caenorhabditis elegans. Mol Biol Cell 17: 3062–3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen E, Severin F, Backer JM, Hyman AA, Zerial M (1999) Rab5 regulates motility of early endosomes on microtubules. Nat Cell Biol 1: 376–382 [DOI] [PubMed] [Google Scholar]
- Niemes S, Langhans M, Viotti C, Scheuring D, San Wan Yan M, Jiang L, Hillmer S, Robinson DG, Pimpl P (2010) Retromer recycles vacuolar sorting receptors from the trans-Golgi network. Plant J 61: 107–121 [DOI] [PubMed] [Google Scholar]
- Nordmann M, Cabrera M, Perz A, Brocker C, Ostrowicz C, Engelbrecht-Vandre S, Ungermann C (2010) The Mon1-Ccz1 complex is the GEF of the late endosomal Rab7 homolog Ypt7. Curr Biol 20: 1654–1659 [DOI] [PubMed] [Google Scholar]
- Odorizzi G, Babst M, Emr SD (1998) Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell 95: 847–858 [DOI] [PubMed] [Google Scholar]
- Ohkuma S, Poole B (1978) Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci USA 75: 3327–3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowicz CW, Meiringer CT, Ungermann C (2008) Yeast vacuole fusion: a model system for eukaryotic endomembrane dynamics. Autophagy 4: 5–19 [DOI] [PubMed] [Google Scholar]
- Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C (2010) Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 12: 19–30; sup pp 11–13 [DOI] [PubMed] [Google Scholar]
- Pan PY, Tian JH, Sheng ZH (2009) Snapin facilitates the synchronization of synaptic vesicle fusion. Neuron 61: 412–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankiv S, Alemu EA, Brech A, Bruun JA, Lamark T, Overvatn A, Bjorkoy G, Johansen T (2010) FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end-directed vesicle transport. J Cell Biol 188: 253–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham HR (2002) Insights from yeast endosomes. Curr Opin Cell Biol 14: 454–462 [DOI] [PubMed] [Google Scholar]
- Penalva MA (2010) Endocytosis in filamentous fungi: Cinderella gets her reward. Curr Opin Microbiol 13: 684–692 [DOI] [PubMed] [Google Scholar]
- Peralta ER, Martin BC, Edinger AL (2010) Differential effects of TBC1D15 and mammalian Vps39 on Rab7 activation state, lysosomal morphology, and growth factor dependence. J Biol Chem 285: 16814–16821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR (2009) Multiple routes of protein transport from endosomes to the trans Golgi network. FEBS Lett 583: 3811–3816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Katzmann DJ (2007) Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol 23: 519–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platta HW, Stenmark H (2011) Endocytosis and signaling. Curr Opin Cell Biol 23: 393–403 [DOI] [PubMed] [Google Scholar]
- Poteryaev D, Datta S, Ackema K, Zerial M, Spang A (2010) Identification of the switch in early-to-late endosome transition. Cell 141: 497–508 [DOI] [PubMed] [Google Scholar]
- Poteryaev D, Fares H, Bowerman B, Spang A (2007) Caenorhabditis elegans SAND-1 is essential for RAB-7 function in endosomal traffic. EMBO J 26: 301–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Progida C, Malerod L, Stuffers S, Brech A, Bucci C, Stenmark H (2007) RILP is required for the proper morphology and function of late endosomes. J Cell Sci 120: 3729–3737 [DOI] [PubMed] [Google Scholar]
- Progida C, Spinosa MR, De Luca A, Bucci C (2006) RILP interacts with the VPS22 component of the ESCRT-II complex. Biochem Biophys Res Commun 347: 1074–1079 [DOI] [PubMed] [Google Scholar]
- Pryor PR, Mullock BM, Bright NA, Gray SR, Luzio JP (2000) The role of intraorganellar Ca(2+) in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J Cell Biol 149: 1053–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puertollano R, Bonifacino JS (2004) Interactions of GGA3 with the ubiquitin sorting machinery. Nat Cell Biol 6: 244–251 [DOI] [PubMed] [Google Scholar]
- Raiborg C, Bache KG, Gillooly DJ, Madshus IH, Stang E, Stenmark H (2002) Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat Cell Biol 4: 394–398 [DOI] [PubMed] [Google Scholar]
- Raiborg C, Bremnes B, Mehlum A, Gillooly DJ, D’Arrigo A, Stang E, Stenmark H (2001) FYVE and coiled-coil domains determine the specific localisation of Hrs to early endosomes. J Cell Sci 114: 2255–2263 [DOI] [PubMed] [Google Scholar]
- Raiborg C, Stenmark H (2009) The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 458: 445–452 [DOI] [PubMed] [Google Scholar]
- Razi M, Futter CE (2006) Distinct roles for Tsg101 and Hrs in multivesicular body formation and inward vesiculation. Mol Biol Cell 17: 3469–3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijngoud DJ, Oud PS, Tager JM (1976) Effect of ionophores on intralysosomal pH. Biochim Biophys Acta 448: 303–313 [DOI] [PubMed] [Google Scholar]
- Rieder SE, Banta LM, Kohrer K, McCaffery JM, Emr SD (1996) Multilamellar endosome-like compartment accumulates in the yeast vps28 vacuolar protein sorting mutant. Mol Biol Cell 7: 985–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink J, Ghigo E, Kalaidzidis Y, Zerial M (2005) Rab conversion as a mechanism of progression from early to late endosomes. Cell 122: 735–749 [DOI] [PubMed] [Google Scholar]
- Robinson FL, Dixon JE (2006) Myotubularin phosphatases: policing 3-phosphoinositides. Trends Cell Biol 16: 403–412 [DOI] [PubMed] [Google Scholar]
- Rocha N, Kuijl C, van der Kant R, Janssen L, Houben D, Janssen H, Zwart W, Neefjes J (2009) Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 Glued and late endosome positioning. J Cell Biol 185: 1209–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas R, van Vlijmen T, Mardones GA, Prabhu Y, Rojas AL, Mohammed S, Heck AJ, Raposo G, van der Sluijs P, Bonifacino JS (2008) Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J Cell Biol 183: 513–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld JL, Moore RH, Zimmer KP, Alpizar-Foster E, Dai W, Zarka MN, Knoll BJ (2001) Lysosome proteins are redistributed during expression of a GTP-hydrolysis-defective rab5a. J Cell Sci 114: 4499–4508 [DOI] [PubMed] [Google Scholar]
- Roxrud I, Stenmark H, Malerod L (2010) ESCRT & Co. Biol Cell 102: 293–318 [DOI] [PubMed] [Google Scholar]
- Rusten TE, Rodahl LM, Pattni K, Englund C, Samakovlis C, Dove S, Brech A, Stenmark H (2006) Fab1 phosphatidylinositol 3-phosphate 5-kinase controls trafficking but not silencing of endocytosed receptors. Mol Biol Cell 17: 3989–4001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachse M, Urbe S, Oorschot V, Strous GJ, Klumperman J (2002) Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting toward lysosomes. Mol Biol Cell 13: 1313–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saftig P, Klumperman J (2009) Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol 10: 623–635 [DOI] [PubMed] [Google Scholar]
- Sahu R, Kaushik S, Clement CC, Cannizzo ES, Scharf B, Follenzi A, Potolicchio I, Nieves E, Cuervo AM, Santambrogio L (2011) Microautophagy of cytosolic proteins by late endosomes. Dev Cell 20: 131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbrissa D, Ikonomov OC, Deeb R, Shisheva A (2002) Phosphatidylinositol 5-phosphate biosynthesis is linked to PIKfyve and is involved in osmotic response pathway in mammalian cells. J Biol Chem 277: 47276–47284 [DOI] [PubMed] [Google Scholar]
- Sbrissa D, Ikonomov OC, Fenner H, Shisheva A (2008) ArPIKfyve homomeric and heteromeric interactions scaffold PIKfyve and Sac3 in a complex to promote PIKfyve activity and functionality. J Mol Biol 384: 766–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmoller F, Riezman H (1993) Involvement of Ypt7p, a small GTPase, in traffic from late endosome to the vacuole in yeast. J Cell Sci 106(Pt 3): 823–830 [DOI] [PubMed] [Google Scholar]
- Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD (1993) Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science 260: 88–91 [DOI] [PubMed] [Google Scholar]
- Scott CC, Gruenberg J (2011) Ion flux and the function of endosomes and lysosomes: pH is just the start: the flux of ions across endosomal membranes influences endosome function not only through regulation of the luminal pH. Bioessays 33: 103–110 [DOI] [PubMed] [Google Scholar]
- Shisheva A (2008) PIKfyve: partners, significance, debates and paradoxes. Cell Biol Int 32: 591–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Raposo G (2009) Exosomes—vesicular carriers for intercellular communication. Curr Opin Cell Biol 21: 575–581 [DOI] [PubMed] [Google Scholar]
- Slagsvold T, Aasland R, Hirano S, Bache KG, Raiborg C, Trambaiolo D, Wakatsuki S, Stenmark H (2005) Eap45 in mammalian ESCRT-II binds ubiquitin via a phosphoinositide-interacting GLUE domain. J Biol Chem 280: 19600–19606 [DOI] [PubMed] [Google Scholar]
- Sobo K, Le Blanc I, Luyet PP, Fivaz M, Ferguson C, Parton RG, Gruenberg J, van der Goot FG (2007) Late endosomal cholesterol accumulation leads to impaired intra-endosomal trafficking. PLoS One 2: e851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldati T, Schliwa M (2006) Powering membrane traffic in endocytosis and recycling. Nat Rev Mol Cell Biol 7: 897–908 [DOI] [PubMed] [Google Scholar]
- Soppina V, Rai AK, Ramaiya AJ, Barak P, Mallik R (2009) Tug-of-war between dissimilar teams of microtubule motors regulates transport and fission of endosomes. Proc Natl Acad Sci USA 106: 19381–19386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A, Goh LK (2008) Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res 314: 3093–3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A, von Zastrow M (2009) Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol 10: 609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub O, Gautschi I, Ishikawa T, Breitschopf K, Ciechanover A, Schild L, Rotin D (1997) Regulation of stability and function of the epithelial Na+ channel (ENaC) by ubiquitination. EMBO J 16: 6325–6336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MP, Feng Y, Cooper KL, Welford AM, Wandinger-Ness A (2003) Human VPS34 and p150 are Rab7 interacting partners. Traffic 4: 754–771 [DOI] [PubMed] [Google Scholar]
- Steinman RM, Brodie SE, Cohn ZA (1976) Membrane flow during pinocytosis. A stereologic analysis. J Cell Biol 68: 665–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Mellman IS, Muller WA, Cohn ZA (1983) Endocytosis and the recycling of plasma membrane. J Cell Biol 96: 1–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuffers S, Sem Wegner C, Stenmark H, Brech A (2009) Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 10: 925–937 [DOI] [PubMed] [Google Scholar]
- Sugii S, Lin S, Ohgami N, Ohashi M, Chang CC, Chang TY (2006) Roles of endogenously synthesized sterols in the endocytic pathway. J Biol Chem 281: 23191–23206 [DOI] [PubMed] [Google Scholar]
- Sun Q, Westphal W, Wong KN, Tan I, Zhong Q (2010) Rubicon controls endosome maturation as a Rab7 effector. Proc Natl Acad Sci USA 107: 19338–19343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata K, Matsunaga K, Sakane A, Sasaki T, Noda T, Yoshimori T (2010) Rubicon and PLEKHM1 negatively regulate the endocytic/autophagic pathway via a novel Rab7-binding domain. Mol Cell Biol 21: 4162–4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teis D, Taub N, Kurzbauer R, Hilber D, de Araujo ME, Erlacher M, Offterdinger M, Villunger A, Geley S, Bohn G, Klein C, Hess MW, Huber LA (2006) p14-MP1-MEK1 signaling regulates endosomal traffic and cellular proliferation during tissue homeostasis. J Cell Biol 175: 861–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teis D, Wunderlich W, Huber LA (2002) Localization of the MP1-MAPK scaffold complex to endosomes is mediated by p14 and required for signal transduction. Dev Cell 3: 803–814 [DOI] [PubMed] [Google Scholar]
- Teo H, Gill DJ, Sun J, Perisic O, Veprintsev DB, Vallis Y, Emr SD, Williams RL (2006) ESCRT-I core and ESCRT-II GLUE domain structures reveal role for GLUE in linking to ESCRT-I and membranes. Cell 125: 99–111 [DOI] [PubMed] [Google Scholar]
- Theos AC, Truschel ST, Tenza D, Hurbain I, Harper DC, Berson JF, Thomas PC, Raposo G, Marks MS (2006) A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev Cell 10: 343–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjelle TE, Brech A, Juvet LK, Griffiths G, Berg T (1996) Isolation and characterization of early endosomes, late endosomes and terminal lysosomes: their role in protein degradation. J Cell Sci 109(Pt 12): 2905–2914 [DOI] [PubMed] [Google Scholar]
- Tooze J, Hollinshead M (1992) In AtT20 and HeLa cells brefeldin A induces the fusion of tubular endosomes and changes their distribution and some of their endocytic properties. J Cell Biol 118: 813–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319: 1244–1247 [DOI] [PubMed] [Google Scholar]
- Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I (2003) Activation of lysosomal function during dendritic cell maturation. Science 299: 1400–1403 [DOI] [PubMed] [Google Scholar]
- Trombetta ES, Mellman I (2005) Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol 23: 975–1028 [DOI] [PubMed] [Google Scholar]
- Ukkonen P, Lewis V, Marsh M, Helenius A, Mellman I (1986) Transport of macrophage Fc receptors and Fc receptor-bound ligands to lysosomes. J Exp Med 163: 952–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich O, Horiuchi H, Bucci C, Zerial M (1994) Membrane association of Rab5 mediated by GDP-dissociation inhibitor and accompanied by GDP/GTP exchange. Nature 368: 157–160 [DOI] [PubMed] [Google Scholar]
- Vaccari T, Bilder D (2005) The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev Cell 9: 687–698 [DOI] [PubMed] [Google Scholar]
- Vaccari T, Duchi S, Cortese K, Tacchetti C, Bilder D (2010) The vacuolar ATPase is required for physiological as well as pathological activation of the Notch receptor. Development 137: 1825–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654–659 [DOI] [PubMed] [Google Scholar]
- van der Goot FG, Gruenberg J (2006) Intra-endosomal membrane traffic. Trends Cell Biol 16: 514–521 [DOI] [PubMed] [Google Scholar]
- van Deurs B, Holm PK, Kayser L, Sandvig K (1995) Delivery to lysosomes in the human carcinoma cell line HEp-2 involves an actin filament-facilitated fusion between mature endosomes and preexisting lysosomes. Eur J Cell Biol 66: 309–323 [PubMed] [Google Scholar]
- van Kerkhof P, Strous GJ (2001) The ubiquitin-proteasome pathway regulates lysosomal degradation of the growth hormone receptor and its ligand. Biochem Soc Trans 29: 488–493 [DOI] [PubMed] [Google Scholar]
- van Meel E, Klumperman J (2008) Imaging and imagination: understanding the endo-lysosomal system. Histochem Cell Biol 129: 253–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weert AW, Dunn KW, Gueze HJ, Maxfield FR, Stoorvogel W (1995) Transport from late endosomes to lysosomes, but not sorting of integral membrane proteins in endosomes, depends on the vacuolar proton pump. J Cell Biol 130: 821–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlandingham PA, Ceresa BP (2009) Rab7 regulates late endocytic trafficking downstream of multivesicular body biogenesis and cargo sequestration. J Biol Chem 284: 12110–12124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicinanza M, D’Angelo G, Di Campli A, De Matteis MA (2008) Function and dysfunction of the PI system in membrane trafficking. EMBO J 27: 2457–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira OV, Bucci C, Harrison RE, Trimble WS, Lanzetti L, Gruenberg J, Schreiber AD, Stahl PD, Grinstein S (2003) Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase. Mol Cell Biol 23: 2501–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitner EB, Platt FM, Futerman AH (2010) Common and uncommon pathogenic cascades in lysosomal storage diseases. J Biol Chem 285: 20423–20427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer P, Will E, Scheglmann D, Strom M, Gallwitz D (1999) Primary structure and biochemical characterization of yeast GTPase-activating proteins with substrate preference for the transport GTPase Ypt7p. Eur J Biochem 260: 284–290 [DOI] [PubMed] [Google Scholar]
- Von Bartheld CS, Altick AL (2011) Multivesicular bodies in neurons: distribution, protein content, and trafficking functions. Prog Neurobiol 93: 313–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderheit A, Helenius A (2005) Rab7 associates with early endosomes to mediate sorting and transport of Semliki forest virus to late endosomes. PLoS Biol 3: e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CW, Stromhaug PE, Kauffman EJ, Weisman LS, Klionsky DJ (2003) Yeast homotypic vacuole fusion requires the Ccz1-Mon1 complex during the tethering/docking stage. J Cell Biol 163: 973–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Hong W (2006) RILP interacts with VPS22 and VPS36 of ESCRT-II and regulates their membrane recruitment. Biochem Biophys Res Commun 350: 413–423 [DOI] [PubMed] [Google Scholar]
- Wegner CS, Malerod L, Pedersen NM, Progida C, Bakke O, Stenmark H, Brech A (2010) Ultrastructural characterization of giant endosomes induced by GTPase-deficient Rab5. Histochem Cell Biol 133: 41–55 [DOI] [PubMed] [Google Scholar]
- Weisz OA (2003) Acidification and protein traffic. Int Rev Cytol 226: 259–319 [DOI] [PubMed] [Google Scholar]
- White IJ, Bailey LM, Aghakhani MR, Moss SE, Futter CE (2006) EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J 25: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley P, Reaves BJ, Hashimoto M, Riley AM, Potter BV, Holman GD (2003) Identification of mammalian Vps24p as an effector of phosphatidylinositol 3,5-bisphosphate-dependent endosome compartmentalization. J Biol Chem 278: 38786–38795 [DOI] [PubMed] [Google Scholar]
- Wichmann H, Hengst L, Gallwitz D (1992) Endocytosis in yeast: evidence for the involvement of a small GTP-binding protein (Ypt7p). Cell 71: 1131–1142 [DOI] [PubMed] [Google Scholar]
- Woodman P (2009) ESCRT proteins, endosome organization and mitogenic receptor down-regulation. Biochem Soc Trans 37: 146–150 [DOI] [PubMed] [Google Scholar]
- Woodman PG, Futter CE (2008) Multivesicular bodies: co-ordinated progression to maturity. Curr Opin Cell Biol 20: 408–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurmser AE, Emr SD (1998) Phosphoinositide signaling and turnover: PtdIns(3)P, a regulator of membrane traffic, is transported to the vacuole and degraded by a process that requires lumenal vacuolar hydrolase activities. EMBO J 17: 4930–4942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurmser AE, Sato TK, Emr SD (2000) New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion. J Cell Biol 151: 551–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M, McBride H (2001) Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2: 107–117 [DOI] [PubMed] [Google Scholar]
- Zeuzem S, Feick P, Zimmermann P, Haase W, Kahn RA, Schulz I (1992) Intravesicular acidification correlates with binding of ADP-ribosylation factor to microsomal membranes. Proc Natl Acad Sci USA 89: 6619–6623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Chen L, Wang S, Wang T (2009) Rab7: roles in membrane trafficking and disease. Biosci Rep 29: 193–209 [DOI] [PubMed] [Google Scholar]
- Zhang XM, Walsh B, Mitchell CA, Rowe T (2005) TBC domain family, member 15 is a novel mammalian Rab GTPase-activating protein with substrate preference for Rab7. Biochem Biophys Res Commun 335: 154–161 [DOI] [PubMed] [Google Scholar]