Abstract
COPII vesicles mediate the export of secretory cargo from endoplasmic reticulum (ER) exit sites. However, of 60–90 nm diameter COPII vesicles are too small to accommodate secreted molecules such as the collagens. The ER exit site-located proteins TANGO1 and cTAGE5 are required for the transport of collagens and therefore provide a means to understand the export of big cargo and the mechanism of COPII carrier size regulation commensurate with cargo dimensions.
Keywords: collagen, COPII, cTAGE5, endoplasmic reticulum, TANGO1
Introduction
There are two distinct routes of protein secretion in eukaryotes: one, that requires the entry of the secretory cargo into the endoplasmic reticulum (ER) followed by its transfer to the Golgi and thence to the cell surface; the other, or the non-conventional pathway, which is independent of the ER-Golgi pathway (Pfeffer, 2007; Nickel and Rabouille, 2009). In the conventional protein secretion pathway, after translocation into the ER, the secreted cargo is collected at the ER exits sites, and then loaded into small membrane vesicles that are generated by a set of highly conserved proteins called the coat protein complex II (COPII) (Jensen and Schekman, 2011). But how can these small vesicles of 60–90 nm diameter transport bulky cargoes such as the abundantly secreted collagens some of which assemble in the ER into 300–400 nm rod-like structures? There are two possibilities: specific proteins may control the loading of big cargoes and regulate the size of COPII carriers accordingly. Alternatively, carriers distinct from the standard COPII vesicles may mediate the transport of big cargoes. Recent findings have revealed that the proteins TANGO1 and cTAGE5 assemble into a dimer at the ER exit sites and both are required for Collagen VII secretion. TANGO1 binds Collagen VII and both TANGO1 and cTAGE5 bind COPII coat proteins Sec23/Sec24 (Saito et al, 2009, 2011). TANGO1-null mice die at birth and are defective in the secretion of a number of different collagens (Wilson et al, 2011). These findings provide strong evidence that TANGO1 and cTAGE5 are required for the secretion of collagens in a COPII-dependent manner. These two new proteins thus provide a means to reveal the mechanism by which bulky cargoes such as the collagens are exported from the ER. In this article, we first summarize the events in the recruitment and assembly of COPII coats at the ER. We then discuss how TANGO1 and cTAGE5 facilitate collagen loading into COPII carriers and can potentially regulate the size of these carriers. Finally, we discuss our understanding of how these events could regulate the scission of COPII vesicles and the unresolved questions that must be addressed to provide a comprehensive understanding of cargo export at the ER.
The conserved components of the COPII vesicle biogenesis
COPII vesicles are essential for the export of secretory cargo at ER exit sites in all eukaryotes. The biogenesis of this class of transport carriers requires six highly conserved polypeptides. Briefly, the ER membrane protein Sec12 catalyses exchange of GTP for GDP on Sar1 and the latter is inserted into the ER membrane outer leaflet. The dimeric Sec23/Sec24 complex binds Sar1 by direct interaction of Sec23 with Sar1-GTP. This forms the first of two layers of coat proteins on the cytoplasmic surface of the ER (Bi et al, 2002). In this prebudding complex, Sec24 is thought to be key for the binding and concentration of transmembrane (TM) cargoes that contain di-hydrophobic, di-acidic and C-terminal aromatic motifs in their cytoplasmic domains, into COPII carriers (Wendeler et al, 2007). The Sec13/Sec31 dimer is then recruited to complete COPII assembly. Sec31 interacts directly with Sec23 through a proline-rich domain (PRD) (Bi et al, 2007). In addition to its function as a coat protein, Sec23 is also a GAP (GTPase activation protein) for Sar1 (Yoshihisa et al, 1993). The binding of the outer coat layer regulates the GAP activity of Sec23 on Sar1, more specifically by direct interaction with the PRD domain of Sec31. This regulation of Sar1 GTPase activity and the recruitment of the outer coat layer are suggested to time the completion of the COPII vesicle biogenesis and the uncoating of the transport carrier (Bi et al, 2007). The scission of COPII vesicles is independent of a dynamin-like GTPase and the purified COPII components are sufficient to generate small vesicles from synthetic liposomes (Matsuoka et al, 1998; Supek et al, 2002; Lee et al, 2004).
Thus, a set of five cytoplasmic proteins is recruited to ER exit sites through events initiated by membrane-bound Sec12. These six participants are highly conserved and required for the biogenesis of 60–90 nm, spherical COPII vesicles in all eukaryotes.
TANGO1 is required for loading collagens into COPII carriers
A genome-wide screen in Drosophila tissue culture cells led to the identification of new components that are required for protein secretion and Golgi organization. These proteins are nicknamed TANGO for Transport ANd Golgi Organization (Bard et al, 2006). One of these proteins, TANGO1, is required for the export of Collagen VII from the ER (Saito et al, 2009). TANGO1 of 1907 aa is localized specifically to ER exit sites in mammalian cells. The luminal portion of the TANGO1 protein is comprised of an SH3-like domain at its N-terminus followed by a coiled-coil domain; the cytoplasmic portion contains two coiled-coil domains and a PRD at the C-terminus. TANGO1 contains two closely spaced hydrophobic stretches: amino acids 1143–1165 and 1183–1205, respectively. It is suggested that one of these is a TM domain that spans the ER membrane, whereas the second is partially embedded in the outer or the inner leaflet. In this arrangement, the TM domain anchors TANGO1 in the ER and the partially inserted hydrophobic domain would act as a pivot to move the proximal domains perpendicular to the ER membrane (Saito et al, 2009).
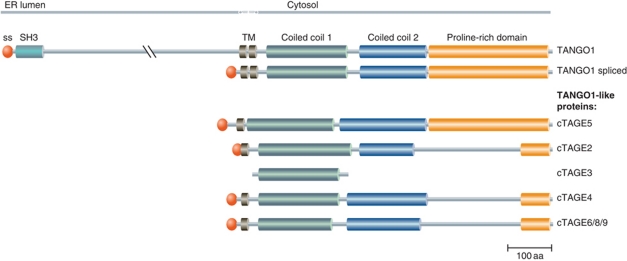
Human genome sequence analysis has revealed the presence of additional, TANGO1-like proteins. These include a widely expressed, short splice variant of TANGO1 and eight members of the cTAGE (cutaneous T-cell lymphoma-associated antigen) family of proteins (Usener et al, 2003). Sequence alignment of TANGO1, its short splice variant and members of the cTAGE family is shown in Figure 1. The family includes proteins comprised of a TM domain, two coiled-coil domains and a PRD, each of which is highly homologous to the C-terminal cytoplasmic portion of TANGO1. However, these proteins lack the N-terminal, lumenally oriented region of TANGO1.
Figure 1.
TANGO1 and TANGO1-like proteins. The amino-acid sequence of TANGO1, TANGO1 spliced, cTAGE5, cTAGE2, cTAGE3 (possible pseudogene), cTAGE4 and cTAGE6/7/8 (highly similar to cTAGE4, possible pseudogenes) were aligned and the domain structure indicated as shown in the UniProtKB database. ss, signal sequence; SH3, SRC homology 3 domain; TM, transmembrane domain.
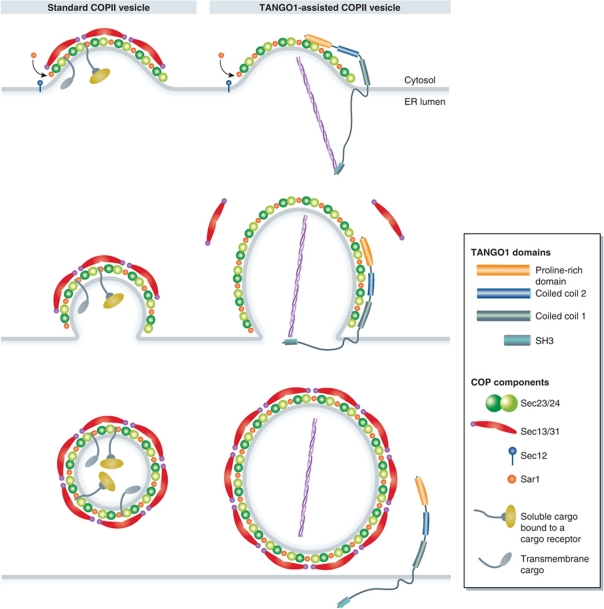
Depletion of TANGO1 in HeLa cells by RNAi did not have any appreciable effect on the organization of ER exit sites or on general protein secretion. However, ER export of Collagen VII was blocked upon TANGO1 depletion. In the same experiments, Collagen I secretion was not perturbed (Saito et al, 2009). The SH3 domain of TANGO1 binds to Collagen VII, while the PRD binds Sec23A and Sec24C and is required for TANGO1 localization at ER exit sites. Based on these findings, it has been proposed that TANGO1 facilitates the loading of Collagen VII into COPII-coated carriers at ER exit sites (Saito et al, 2009). TANGO1, itself, is not packed into the COPII-coated carriers during Collagen VII export. A model depicting TANGO1-dependent collagen loading compared with standard (non-collagen containing) COPII vesicle biogenesis is shown in Figure 2. Binding of Collagen VII to the SH3 domain of TANGO1 in the lumen of the ER is proposed to change TANGO1's conformation, triggering the binding of its PRD to Sec23/Sec24, which would inhibit PRD-dependent binding of Sec31 to Sec23. This delays Sar1-GTP hydrolysis and thus promotes the growth of COPII carriers to accommodate large cargoes. Once Collagen VII is encapsulated into a COPII carrier of the right size (mega carrier), the collagen molecule would dissociate from TANGO1, a process that would be coupled with the dissociation of TANGO1's PRD from Sec23/Sec24. Sec13/Sec31 would then be recruited to the exposed Sec23/Sec24 and trigger the separation of the Collagen VII containing mega carrier from the ER (Saito et al, 2009). We suggest that TANGO1 may act as a kinetic timer to load cargo while blocking scission of the COPII carrier.
Figure 2.
Comparison of normal and TANGO1-assisted formation of COPII carriers. TANGO1 is not required for general protein secretion and, therefore, has no role in the biogenesis of a generic COPII vesicle of 60–90 nm average diameter. TANGO1 binds Collagen VII through its SH3 domain and Sec23/Sec24 through its PRD. In this mode, the Sec13/Sec31 dimer cannot bind Sec23/Sec24 and the completion of Collagen VII containing COPII carrier is delayed. The carrier thus continues to grow in size. Release of Collagen VII triggers separation of TANGO1's PRD from the Sec23/Sec24. Sec13/Sec31 can then bind Sec23/Sec24 to generate a mega COPII carrier.
TANGO1 dimerizes with cTAGE5 at the ER exit site
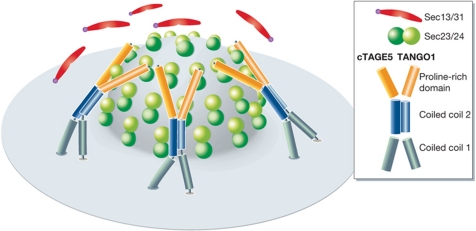
cTAGE5 localizes to ER exit sites in HeLa cells, binds TANGO1 via its second coiled-coil domain and Sec23/Sec24 via its PRD and is also required for Collagen VII export (Saito et al, 2011). The dimerization of TANGO1 and cTAGE5 at ER exit sites is thought to be needed for the efficient Collagen VII packaging. In this model, TANGO1's SH3 domain binds collagen, the PRD's of TANGO1 and cTAGE5 bind Sec23/Sec24 and are thus more effective in preventing the binding of Sec13/Sec31 to Sec23/Sec24 (Saito et al, 2011; Figure 3). The intracellular location and function of the other TANGO1-like proteins is not known. Perhaps TANGO1 assembles into a larger complex that contains other TANGO1-like molecules, in addition to cTAGE5. A multi-component TANGO1 complex, or a transient coat, would increase the number of PRD's available for binding Sec23/Sec24, to more effectively inhibit or delay the recruitment of Sec13/Sec31. Simply put, TANGO1 binds the cargo; the cytoplasmic coiled-coil domains bind other TANGO1-like molecules, and the respective PRD's bind Sec23/Sec24. The main purpose of this complex of TANGO1 and its associates is to delay the Sar1-GTP hydrolysis by retarding the recruitment of Sec13/Sec31 to Sec23/Sec24.
Figure 3.
A dimer of TANGO1 and cTAGE5 is more effective in Collagen VII loading into COPII carriers. A view from the top of newly forming COPII carrier. In the TANGO1–cTAGE5 dimer, TANGO 1 binds Collagen VII; both, however, provide a PRD for interaction with the Sec23/Sec24. The stoichiometry of TANGO1–cTAGE5 dimer at the ER exit site is not known but the dimeric PRD is likely to be more effective in inhibiting Sec13/Sec31 binding, to promote COPII carrier growth.
TANGO1 and MIA proteins
MIA or melanoma inhibitory activity was identified as a 12-kDa protein expressed and secreted by malignant melanomas (Blesch et al, 1994). The structural analysis of MIA or cartilage-derived retinoic acid-sensitive protein (CD-RAP) revealed an SH3-like domain with terminal extensions of 20 aa at both the N and the C-termini (Lougheed et al, 2001). MIA is expressed predominantly in the chondrocytes and is reported to have a role in cartilage development and maintenance. It inhibits the adhesion of melanoma cells to the extracellular matrix composed solely of fibronectin and laminin and could therefore promote metastasis by inhibiting the attachment of melanoma cells to the ECM (Bosserhoff and Buettner, 2002). Gene searches have revealed paralogs of MIA and called OTOR (128 aa, expressed specifically in cochlea and eye (Robertson et al, 2000)) and TANGO130. Bosserhoff et al (2003, 2004) have identified two additional homologues of MIA. These proteins are called MIA2 (522 aa, expressed specifically in hepatocytes) and TANGO (11.9 kDa, expressed ubiquitously in the mouse is the same as TANGO130). These proteins: MIA, OTOR, MIA2 TANGO/TANGO130 and TANGO1 share extensive homology in the SH3 domain but apart from the SH3 domain TANGO1/MIA3 has no similarities with MIA, OTOR, MIA2 or TANGO130/TANGO. There are no reports on the involvement of MIA, OTOR, MIA2 and TANGO130/TANGO in protein secretion and MIA1-null mice are viable (Moser et al, 2002). TANGO1/MIA3 is a resident of the ER exit sites, whereas MIA, OTOR, MIA2 and TANGO130/TANGO are secreted from the cell. TANGO1/MIA3, therefore, should not be confused with MIA, OTOR, MIA2 and TANGO130/TANGO.
TANGO1-null mice are defective in collagen secretion
Do TANGO1 and cTAGE5 have a role in Collagen VII secretion in vivo? Do these two proteins form a specific receptor for the trafficking of Collagen VII? The answer to these questions has come from mouse knockout of the TANGO1/MIA3 gene (Wilson et al, 2011). TANGO1 knockout mice show defects in extracellular matrix composition, development of chondrocytes and in bone mineralization, which leads to dwarfism and neonatal lethality. The defect in mice lacking TANGO1 has been mapped to a block in ER export of collagen in chondrocytes, fibroblasts, endothelial cells and mural cells. The authors report a role for TANGO1 in the export of a large number but not all collagens (Wilson et al, 2011). There are >30 collagens and collagen-related proteins and thus far only Collagen VII is known to bind TANGO1 directly. The SH3 domain of TANGO1 binds Collagen VII; however, the binding site in Collagen VII is not known. It has been suggested that unlike the conventional SH3 domains, MIA does not recognize polyproline helices (Lougheed et al, 2002).It is therefore not obvious whether the SH3 domain of TANGO1 binds polyproline helices of the collagens or whether TANGO1 binds all collagens and participates directly in their ER export. While the precise specificity of TANGO1 for collagen secretion awaits further analysis, TANGO1 is clearly an important component of the ER export machinery and represents a novel player that contributes to the secretion of collagen.
Paralogs of COPII coat components and their roles in cargo selection
Mammalian cells contain two Sar1 paralogs, Sar1A and Sar1B; two Sec23 paralogs, Sec23A and Sec23B; four Sec24 paralogs, Sec24A, Sec24B, Sec24C and Sec24D; a single Sec13; and two Sec31 paralogs, Sec31A and Sec31B (Fromme et al, 2008; Hughes and Stephens, 2008). Mutations in Sar1B have been linked with chylomicron retention disease and Anderson disease (Jones et al, 2003; Shoulders et al, 2004). Individuals with these fat-malabsorption diseases have low blood cholesterol levels and show a lack of chylomicrons in their blood. Chylomicrons are 75–450 nm in diameter, produced in the ER and secreted via the conventional ER-Golgi secretory pathway. These findings suggest the possibility that Sar1B might be dedicated to the secretion of bulky cargo that is too big to fit into a standard COPII vesicle. Mutations in Sec24D in the zebrafish are characterized by embryonic lethality, skeletal dysmorphology and reduced extracellular matrix (Sarmah et al, 2010). The absence of a functional Sec24D in fish chondrocytes leads to a distended ER filled with Collagen 2α1. Mutations in Sec23A and Sec24B are also linked to transport of specific cargoes (Bianchi et al, 2009; Routledge et al, 2010). How these paralogs affect COPII coat assembly in a cargo-dependent manner and whether they differ in their affinity for TANGO1/TANGO1-like proteins is not yet known. It is tempting to propose that TANGO1–cTAGE5 interact with a subset of Sec23/Sec24 dimers that are utilized for export of large cargoes.
Membrane fission to separate COPII-coated transport carriers at the ER exit site
The separation (scission) of clathrin-coated vesicles from the plasma membrane requires the dynamin GTPase. How dynamin cuts the neck of a clathrin-coated vesicle is not clear; however, it is well established that dynamin is recruited to the membrane in a GTP-bound form and nucleotide hydrolysis parallels events leading to separation of clathrin-coated vesicles from the plasma membrane (Doherty and McMahon, 2009). Surprisingly, the formation of clathrin-coated vesicles at the plasma membrane in Saccharomyces cerevisiae and at the TGN in all eukaryotes is dynamin independent. In these latter cases, actin is thought to perform the function of dynamin (Galletta and Cooper, 2009). Dynamin and actin are very different proteins and it is hard to imagine how they can replace each other's function. Nevertheless, these proteins are important for fission of clathrin-coated vesicles. Since neither dynamin nor actin is required for COPII vesicle biogenesis, is scission mediated entirely by the two layers of proteins that comprise COPII coats? Is Sar1 assembly the key to changes in membrane curvature and fission? Sar1p-GTP inserts into the outer leaflet of the ER through an amphipathic helix at its N-terminus (Barlowe et al, 1993). The N-terminal 9 aa are essential for anchoring Sar1-GTP to synthetic liposomes. A mutant form that lacks these N-terminal residues is functionally inactive and cannot replace endogenous Sar1 in SAR1-deleted yeast (Bielli et al, 2005). Indeed, a single change (F5D) in Sarl's N-terminus dramatically reduces Sar1 binding to isolated ER and Sec23 recruitment (Huang et al, 2001). These findings highlight the significance of Sar1's N-terminus in its anchoring at ER exit sites, the subsequent recruitment of Sec23 and the resulting membrane curvature needed to induce tubulation. Moreover, oligomerization of membrane-bound Sar1 is reportedly sufficient to break tubules by membrane fission (Lee et al, 2005; Long et al, 2010). The net effect of Sar1 insertion into the membrane, followed by recruitment of the first and second layer COPII coat components, might be sufficient to bend the membrane and cut COPII-coated transport carriers from the ER.
In summary, GTP hydrolysis by Sar1 is key to the events leading to changes in membrane curvature and cutting. Hydrolysis is regulated by the combined action of Sec 23 and Sec31. Therefore, a mechanism that delays the binding of these two proteins would influence GTP hydrolysis, and in principle, promote growth of a COPII carrier by delaying the Sar1-dependent membrane fission event. The PRD domains of TANGO1 and its binding partners are therefore excellent candidates for factors that could delay Sar1-GTP hydrolysis in a cargo-dependent manner, by binding Sec23/Sec24 and delaying recruitment of the Sec13/Sec31 complex. In this manner, TANGOs can promote extended growth of COPII carriers.
What else is involved in the biogenesis of COPII carriers?
The highly conserved six polypeptides that comprise the COPII machinery represent the nuts and bolts that generate small vesicles for cargo export at the ER. A number of new proteins have been identified that are required to load specific cargoes and may also potentially regulate the size of the COPII carriers. TANGO1 links collagens in the lumen with the COPII machinery in the cytoplasm. We suggest that TANGO1 and its interactor cTAGE5, act as a kinetic timer to promote mega carrier biogenesis in a cargo-dependent manner. An understanding of the cargo that is directly exported by TANGO1, assembly of TANGO1 and the related molecules (cTAGE5, etc.) at the ER exit sites, their role in the regulation of the Sar1 GTPase activity, could reveal the mechanism of packing big cargo and potentially the size regulation mechanisms of the COPII carriers. We also look forward to an understanding of the molecular composition of the ER exit site that makes them unique to recruit the peripheral membrane protein Sec16 and anchor TANGO1. Sec16 interacts with Sec31 and Sec13 but surprisingly does not have a direct role in COPII biogenesis (Watson et al, 2006; Bhattacharyya and Glick, 2007). What then is the role of Sec16 in the ER to Golgi transport? In addition, phospholipase D and phosphatidylinositol 4-phosphate affect cargo export at the ER, suggesting the involvement of modified lipids in COPII-mediated cargo export (Pathre et al, 2003; Blumental-Perry et al, 2006). The molecular analysis of this new collection of proteins will reveal heretofore unknown details regarding the mechanism by which eukaryotic cells regulate the, size, shape and number of COPII carriers, to commensurate with secretory cargo at ER exit sites.
Acknowledgments
We thank Drs Pfeffer and Fromme for valuable discussions, Anne-Marie Alleaume and the whole Malhotra Lab for help with figures and corrections. V Malhotra is an Institució Catalana de Recerca i Estudis Avançats (ICREA) professor at the Center for Genomic Regulation.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bard F, Casano L, Mallabiabarrena A, Wallace E, Saito K, Kitayama H, Guizzunti G, Hu Y, Wendler F, Dasgupta R, Perrimon N, Malhotra V (2006) Functional genomics reveals genes involved in protein secretion and Golgi organization. Nature 439: 604–607 [DOI] [PubMed] [Google Scholar]
- Barlowe C, d’ Enfert C, Schekman R (1993) Purification and characterization of SAR1p, a small GTP-binding protein required for transport vesicle formation from the endoplasmic reticulum. J Biol Chem 268: 873–879 [PubMed] [Google Scholar]
- Bhattacharyya D, Glick BS (2007) Two mammalian Sec16 homologues have nonredundant functions in endoplasmic reticulum (ER) export and transitional ER organization. Mol Biol Cell 18: 839–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X, Corpina RA, Goldberg J (2002) Structure of the Sec23/24-Sar1 pre-budding complex of the COPII vesicle coat. Nature 419: 271–277 [DOI] [PubMed] [Google Scholar]
- Bi X, Mancias JD, Goldberg J (2007) Insights into COPII coat nucleation from the structure of Sec23.Sar1 complexed with the active fragment of Sec31. Dev Cell 13: 635–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi P, Fermo E, Vercellati C, Boschetti C, Barcellini W, Iurlo A, Marcello AP, Righetti PG, Zanella A (2009) Congenital dyserythropoietic anemia type II (CDAII) is caused by mutations in the SEC23B gene. Hum Mutat 30: 1292–1298 [DOI] [PubMed] [Google Scholar]
- Bielli A, Haney CJ, Gabreski G, Watkins SC, Bannykh SI, Aridor M (2005) Regulation of Sar1 NH2 terminus by GTP binding and hydrolysis promotes membrane deformation to control COPII vesicle fission. J Cell Biol 171: 919–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesch A, Bosserhoff AK, Apfel R, Behl C, Hessdoerfer B, Schmitt A, Jachimczak P, Lottspeich F, Buettner R, Bogdahn U (1994) Cloning of a novel malignant melanoma-derived growth-regulatory protein, MIA. Cancer Res 54: 5695–5701 [PubMed] [Google Scholar]
- Blumental-Perry A, Haney CJ, Weixel KM, Watkins SC, Weisz OA, Aridor M (2006) Phosphatidylinositol 4-phosphate formation at ER exit sites regulates ER export. Dev Cell 11: 671–682 [DOI] [PubMed] [Google Scholar]
- Bosserhoff AK, Buettner R (2002) Expression, function and clinical relevance of MIA (melanoma inhibitory activity). Histol Histopathol 17: 289–300 [DOI] [PubMed] [Google Scholar]
- Bosserhoff AK, Moser M, Buettner R (2004) Characterization and expression pattern of the novel MIA homolog TANGO. Gene Expr Patterns 4: 473–479 [DOI] [PubMed] [Google Scholar]
- Bosserhoff AK, Moser M, Schölmerich J, Buettner R, Hellerbrand C (2003) Specific expression and regulation of the new melanoma inhibitory activity-related gene MIA2 in hepatocytes. J Biol Chem 278: 15225–15231 [DOI] [PubMed] [Google Scholar]
- Doherty GJ, McMahon HT (2009) Mechanisms of endocytosis. Annu Rev Biochem 78: 857–902 [DOI] [PubMed] [Google Scholar]
- Fromme JC, Orci L, Schekman R (2008) Coordination of COPII vesicle trafficking by Sec23. Trends Cell Biol 18: 330–336 [DOI] [PubMed] [Google Scholar]
- Galletta BJ, Cooper JA (2009) Actin and endocytosis: mechanisms and phylogeny. Curr Opin Cell Biol 21: 20–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Weissman JT, Beraud-Dufour S, Luan P, Wang C, Chen W, Aridor M, Wilson IA, Balch WE (2001) Crystal structure of Sar1-GDP at 1.7 A resolution and the role of the NH2 terminus in ER export. J Cell Biol 155: 937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes H, Stephens DJ (2008) Assembly, organization, and function of the COPII coat. Histochem Cell Biol 129: 129–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen D, Schekman R (2011) COPII-mediated vesicle formation at a glance. J Cell Sci 124: 1–4 [DOI] [PubMed] [Google Scholar]
- Jones B, Jones EL, Bonney SA, Patel HN, Mensenkamp AR, Eichenbaum-Voline S, Rudling M, Myrdal U, Annesi G, Naik S, Meadows N, Quattrone A, Islam SA, Naoumova RP, Angelin B, Infante R, Levy E, Roy CC, Freemont PS, Scott J et al. (2003) Mutations in a Sar1 GTPase of COPII vesicles are associated with lipid absorption disorders. Nat Genet 34: 29–31 [DOI] [PubMed] [Google Scholar]
- Lee MCS, Miller EA, Goldberg J, Orci L, Schekman R (2004) Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol 20: 87–123 [DOI] [PubMed] [Google Scholar]
- Lee MCS, Orci L, Hamamoto S, Futai E, Ravazzola M, Schekman R (2005) Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell 122: 605–617 [DOI] [PubMed] [Google Scholar]
- Long KR, Yamamoto Y, Baker AL, Watkins SC, Coyne CB, Conway JF, Aridor M (2010) Sar1 assembly regulates membrane constriction and ER export. J Cell Biol 190: 115–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lougheed JC, Holton JM, Alber T, Bazan JF, Handel TM (2001) Structure of melanoma inhibitory activity protein, a member of a recently identified family of secreted proteins. Proc Natl Acad Sci USA 98: 5515–5520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lougheed JC, Domaille PJ, Handel TM (2002) Solution structure and dynamics of melanoma inhibitory activity protein. J Biomol NMR 22: 211–223 [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Orci L, Amherdt M, Bednarek SY, Hamamoto S, Schekman R, Yeung T (1998) COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell 93: 263–275 [DOI] [PubMed] [Google Scholar]
- Moser M, Bosserhoff A-K, Hunziker EB, Sandell L, Fässler R, Buettner R (2002) Ultrastructural cartilage abnormalities in MIA/CD-RAP-deficient mice. Mol Cell Biol 22: 1438–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel W, Rabouille C (2009) Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol 10: 148–155 [DOI] [PubMed] [Google Scholar]
- Pathre P, Shome K, Blumental-Perry A, Bielli A, Haney CJ, Alber S, Watkins SC, Romero G, Aridor M (2003) Activation of phospholipase D by the small GTPase Sar1p is required to support COPII assembly and ER export. EMBO J 22: 4059–4069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR (2007) Unsolved mysteries in membrane traffic. Annu Rev Biochem 76: 629–645 [DOI] [PubMed] [Google Scholar]
- Robertson NG, Heller S, Lin JS, Resendes BL, Weremowicz S, Denis CS, Bell AM, Hudspeth AJ, Morton CC (2000) A novel conserved cochlear gene, OTOR: identification, expression analysis, and chromosomal mapping. Genomics 66: 242–248 [DOI] [PubMed] [Google Scholar]
- Routledge KE, Gupta V, Balch WE (2010) Emergent properties of proteostasis-COPII coupled systems in human health and disease. Mol Membr Biol 27: 385–397 [DOI] [PubMed] [Google Scholar]
- Saito K, Yamashiro K, Ichikawa Y, Erlmann P, Kontani K, Malhotra V, Katada T (2011) cTAGE5 mediates collagen secretion through interaction with TANGO1 at endoplasmic reticulum exit sites. Mol Biol Cell, Available at: http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-02-0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Chen M, Bard F, Chen S, Zhou H, Woodley D, Polischuk R, Schekman R, Malhotra V (2009) TANGO1 facilitates cargo loading at endoplasmic reticulum exit sites. Cell 136: 891–902 [DOI] [PubMed] [Google Scholar]
- Sarmah S, Barrallo-Gimeno A, Melville DB, Topczewski J, Solnica-Krezel L, Knapik EW (2010) Sec24D-dependent transport of extracellular matrix proteins is required for zebrafish skeletal morphogenesis. PLoS ONE 5: e10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoulders CC, Stephens DJ, Jones B (2004) The intracellular transport of chylomicrons requires the small GTPase, Sar1b. Curr Opin Lipidol 15: 191–197 [DOI] [PubMed] [Google Scholar]
- Supek F, Madden DT, Hamamoto S, Orci L, Schekman R (2002) Sec16p potentiates the action of COPII proteins to bud transport vesicles. J Cell Biol 158: 1029–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usener D, Schadendorf D, Koch J, Dübel S, Eichmüller S (2003) cTAGE: a cutaneous T cell lymphoma associated antigen family with tumor-specific splicing. J Invest Dermatol 121: 198–206 [DOI] [PubMed] [Google Scholar]
- Watson P, Townley AK, Koka P, Palmer KJ, Stephens DJ (2006) Sec16 defines endoplasmic reticulum exit sites and is required for secretory cargo export in mammalian cells. Traffic 7: 1678–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendeler MW, Paccaud J-P, Hauri H-P (2007) Role of Sec24 isoforms in selective export of membrane proteins from the endoplasmic reticulum. EMBO Rep 8: 258–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D, Phamluong K, Li L, Sun M, Cao T, Liu P, Modrusan Z, Sandoval W, Rangell L, Carano R, Peterson A, Solloway M (2011) Global defects in collagen secretion in a Mia3/TANGO1 knockout mouse. J Cell Biol 193: 935–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihisa T, Barlowe C, Schekman R (1993) Requirement for a GTPase-activating protein in vesicle budding from the endoplasmic reticulum. Science 259: 1466–1468 [DOI] [PubMed] [Google Scholar]