Abstract
Universal trafficking components within the cell can be recruited to coordinate and regulate the developmental signalling cascades. We will present ways in which the intracellular trafficking machinery is used to affect and modulate the outcome of signal transduction in developmental contexts, thus regulating multicellular development. Each of the signalling components must reach its proper intracellular destination, in a form that is properly folded and modified. In many instances, the ability to bring components together or segregate them into distinct compartments within the cell actually provides the switch mechanism to turn developmental signalling pathways on or off. The review will begin with a focus on the signal-sending cells, and the ways in which ligand trafficking can impinge on the signalling outcome, via processing, endocytosis and recycling. We will then turn to the signal-receiving cell, and discuss mechanisms by which endocytosis can affect the spatial features of the signal, and the compartmentalization of components downstream to the receptor.
Keywords: development, endocytosis, secretion, signal transduction, trafficking
Introduction
Development of multicellular organisms is guided by a handful of highly conserved signalling pathways. While every pathway is distinct in terms of the components involved and the signalling strategy it employs, all pathways share the same logic of transmitting information from the extracellular milieu, presented by ligands, into the cell, culminating in transcriptional activation in the nucleus. Each of these pathways is used numerous times and in different combinations with other pathways. For every signalling event, it is the cellular context that determines the final transcriptional outcome. In other words, the array of transcription factors already expressed by a given cell, will determine which target genes will be induced upon activation of the developmental signalling pathway. In many instances, the pathways dictate not only all-or-none switches, but actually determine distinct transcriptional responses, depending upon the level of activation.
After the components of each pathway are identified and their position within the signalling cascade determined, the focus shifts to characterization of the regulatory nodes in each pathway. How is the timing and position of activation determined for each pathway? How can the levels of signalling be modulated in the different biological scenarios in which each pathway operates? These questions pertain to both ends of the signalling cascade—the cell processing and sending the ligand, and the cell that receives the signal through transmembrane receptors, and relays it to the nucleus.
It is clear that such signalling events involve extensive intracellular trafficking, that is necessary to move molecules from one compartment to another, to facilitate their processing, modification and secretion, or conversely, their uptake and translocation to the nucleus. Work from a variety of laboratories over the past few years has demonstrated that components of the intracellular trafficking machinery do much more than just transfer these molecules from one compartment to another. It turns out that in many instances, the features of intracellular compartmentalization and trafficking are actually used as regulatory modalities, keeping components apart or bringing them together. In other cases, the biased choice between distinct trafficking routes can impinge on the efficiency of signalling, and hence on the spatial pattern of activation.
This review will focus on the mechanisms by which universal trafficking components within the cell can be recruited to coordinate and regulate the developmental signalling cascades. In other words, we will ask how the intracellular trafficking machinery converges with pathways that are dedicated to developmental signalling, thus regulating multicellular development. The review will begin with a focus on the signal-sending cells, and the ways in which ligand trafficking can impinge on the signalling outcome, via processing, endocytosis and recycling, or encounters with cellular chaperones. We will then switch to the signal-receiving cell, and discuss mechanisms by which endocytosis can affect the spatial features of the signal, and the compartmentalization of components downstream to the receptor. Specific trafficking of signalling components to cilia and endosomes will also be presented. Finally, while each of the signalling pathways functions as a distinct entity, interaction between pathways is often observed at the level of the regulatory sequences of target genes. However, there are also instances where intracellular trafficking serves as a means to coordinate between distinct signalling pathways.
This review is by no means a comprehensive description of the impact of intracellular trafficking on developmental signalling. Rather, we list a series of key examples of the trafficking-signalling connection, both in signal-sending and signal-receiving cells, with the intention of stressing the significance of this connection to processes of organism development and tissue differentiation. We do so by presenting some of the underlying molecular mechanisms, and putting forward ideas for the manner by which trafficking is used to affect and modulate the outcome of signal transduction in developmental contexts. We focus on trafficking within the exocytic and endocytic pathways, while trafficking into organelles including the nucleus or mitochondria is not covered. We hope the reader will emerge with an appreciation for the dynamic interface between developmental cascades, and the enormous complexity of the cells in which these signalling events take place.
Signal-sending cell
We describe and discuss three issues, which highlight the intricate involvement and regulatory capacity of intracellular trafficking in influencing the outcome and effectiveness of developmental signalling from signal-sending cells. These include mechanisms underlying the transport of ligands to the cell surface, and their activities once they reach this destination; utilization of differential compartmentalization as a means of segregating and uniting components of a given pathway; and the effects and regulation of key post-translational modifications. Prominent examples covering a variety of signalling pathways are presented and schematized in Figure 1.
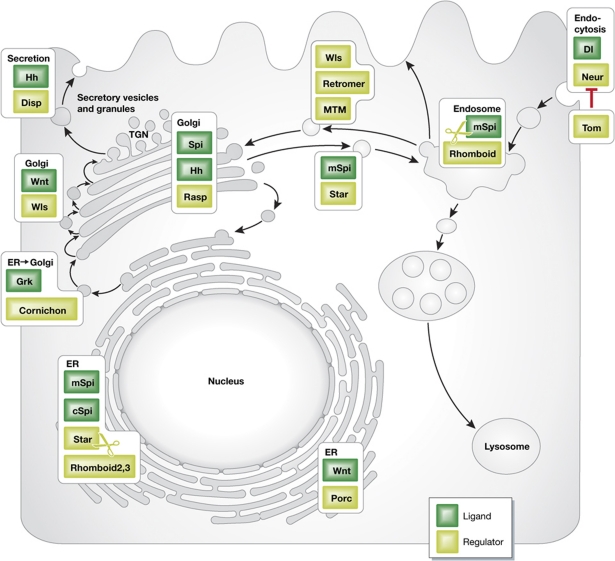
Figure 1.
The signal-sending cell—modulation of developmental signalling by intracellular trafficking. The ER is utilized for retention of the EGFR ligand Spi, and for localization of the intramembrane proteases Rhomboid-2 and Rhomboid-3 that attenuate signalling by compromising ligand trafficking from the ER. Productive cleavage by Rhomboids leading to secretion of active EGFR ligands takes place in the endosome. To circumvent processing in the ER of the EGFR ligand Grk, Cornichon facilitates its loading from the ER into secretory vesicles. Modulation of Wnt by the acyltransferase Porc also takes place in the ER. In the Golgi, modulation of Hh and Spi by Rasp is executed. Trafficking of Wnt from the Golgi to secretion is facilitated by Wls. Wls itself is recycled via endocytosis and trafficked by the retromer complex back to the Golgi. Hh secretion is facilitated by Disp, to overcome the immobility introduced by the addition of the cholesterol moiety. Finally, endocytosis of Dl from the cell surface, which is essential for its signalling capacity, is mediated by Neu. Inhibition of Neu activity by Tom restricts the domains within the embryo where effective Dl internalization will take place.
Regulated trafficking controls ligand transport to the cell surface
Wnt. Wnt ligands are lipid-modified, secreted glycoproteins, which mediate a broad array of developmental processes (Cadigan and Peifer, 2009; van Amerongen and Nusse, 2009). Modulation of Wnt ligand secretion by the seven-transmembrane domain protein Wntless (Wls)—also known as Evi and Sprinter—provides a prominent example for regulation by intracellular trafficking in the signal-producing cell, operating on the signalling molecule as well as on a key protein which directs its intracellular localization. The significance of these activities is underscored by the highly conserved requirement for Wls as a general and specific mediator of Wnt secretion, which has been demonstrated in diverse settings, including planaria, nematodes, flies, frogs, mice and human cell culture (Banziger et al, 2006; Bartscherer et al, 2006; Goodman et al, 2006; Fu et al, 2009; Kim et al, 2009). Wls physically associates with Wnt ligands, enabling their transport through the secretory pathway, from the Golgi network towards the plasma membrane. This interaction has recently been shown to require post-translational lipidation of the ligand, while disassociation of the Wls–Wnt complex appears to depend on the relatively acidic environment within post-Golgi secretory vesicles (Coombs et al, 2010). The molecular roles proposed for Wls in promoting Wnt ligand secretion, have mainly included activity as a chaperone, ensuring proper folding of Wnt proteins, and as a cargo receptor, mediating Wnt transport between secretory pathway compartments.
Detailed analysis of Wls function in the Drosophila neuromuscular junction has provided further evidence for the multiple facets of activity of this protein (Korkut et al, 2009). Wls is associated with Wnt on the surface of the vesicles that are generated in the multivesicular body (MVB) and secreted from the presynaptic cell, and may facilitate Wnt presentation at the postsynaptic site. In addition, Wls is required in the postsynaptic cell for targeting a Wnt-receptor interacting protein termed dGRIP to the postsynaptic sites.
In addition to the mechanism by which Wls ensures progress of Wnt ligands through the secretory pathway, intracellular trafficking has been shown to have a prominent role in regulating availability of this novel element. The central feature of this regulatory pathway is recycling of Wls, which allows it to participate in multiple rounds of Wnt ligand secretion. Genetic analyses in C. elegans had initially demonstrated the importance of the retromer complex, an evolutionarily conserved retrograde trafficking protein machinery, in Wnt signalling (Coudreuse et al, 2006; Prasad and Clark, 2006). Further experiments demonstrated that the retromer complex is actually required for recycling and retrieval of endocytosed Wls (which is otherwise destined for lysosomal degradation), via trafficking from endosomes back to the trans-Golgi network (Belenkaya et al, 2008; Franch-Marro et al, 2008; Pan et al, 2008; Port et al, 2008; Yang et al, 2008).
The retromer complex is essential for Wls-mediated secretion of Wnt ligands in several systems. Recent work has implicated PI3P lipid phosphatases of the myotubularin (MTM) family (specifically a complex of MTM-6 and MTM-9) in retromer-mediated recycling of Wls. MTM phosphatase activity may exert this effect by releasing the sorting nexin SNX-3, a PI3P binding protein, from endosomes, thereby allowing it to associate with the retromer complex and enhance Wls transport (Silhankova et al, 2010). Importantly, overexpression of Wls can overcome the negative effects of disrupting both retromer complex and MTM function, underscoring the significance of retrograde trafficking in maintaining sufficient levels of active Wls in the proper compartment. The conserved functional contributions of Wls, the retromer complex and MTMs to Wnt signalling, and their apparent specificity to the Wnt pathway, identify this system as an important example of the regulatory influence of trafficking on generation of developmental signals.
Delta. The Notch signalling pathway is a prominent developmental cascade that is activated by membrane-bound ligands. The availability of ligands at the cell surface thus represents a critical regulatory junction in this context. While the Notch ligand Delta is initially subject to conventional trafficking through the secretory pathway to the plasma membrane, much attention has been recently focused on the role of endocytosis in generating an active ligand in this system. Long-standing observations demonstrate that endocytosis within the signal-sending cell is critical for Notch pathway function (Seugnet et al, 1997; Parks et al, 2000). These have since been ‘beefed up’ with considerable molecular detail, as fundamental elements of the endocytic pathway have been shown to influence Delta function. A key set of observations centres on the requirements for the E3 ubiquitin ligases Neuralized (Neur) and Mind Bomb, which are thought to mono-ubiquitinate Delta (as well as Serrate, a second membrane-bound Notch ligand), thereby priming the ligands for endocytosis (Deblandre et al, 2001; Lai et al, 2001; Pavlopoulos et al, 2001; Yeh et al, 2001; Itoh et al, 2003; Lai et al, 2005; Le Borgne et al, 2005; Pitsouli and Delidakis, 2005; Wang and Struhl, 2005; Commisso and Boulianne, 2007).
Two distinct, yet not necessarily exclusive models have been proposed to explain the involvement of endocytosis in potentiating Notch ligand function. The first of these centres on the notion that endocytosis provides an opportunity for recycling and transformation of the ligand into an active form. Such transformation could involve yet-to-be-characterized post-translational modifications by elements encountered within the recycling compartments. Alternatively, endocytosis may provide the means for trafficking and retargeting of the ligand to specific signalling domains. Two recent studies emphasize the significance of this latter mechanism, in the context of Notch-mediated determination of cell fates within the Drosophila sensory-organ lineage (Rajan et al, 2009; Benhra et al, 2010). Benhra et al demonstrated that Neur-dependent endocytosis leads to trafficking of Delta from the basolateral region of signal-sending cells to an apical membrane domain, where productive interaction with (apically restricted) Notch can proceed. This mechanism appears to be conserved, as a similar trafficking scenario is shown to operate in cultured mammalian polarized epithelia as well. The study from Rajan et al underscores the significance of apical presentation of Delta, possibly upon microvilli. Importantly, a specific microfilament-based structure was identified as the probable route which vesicles containing endocytosed Delta follow during basal-apical trafficking. These findings may explain the previously unresolved involvement of the Arp2/3-based actin polymerization machinery in sensory-organ development (Ben-Yaacov et al, 2001).
The second mechanism put forward to explain the requirement for ligand endocytosis, proposes that the relevant effect occurs only after initial association with the Notch receptor. According to this view, the force applied in order to internalize the ligand into the signal-sending cell results in ‘pulling’ of the receptor, thereby exposing a critical site of cleavage by an ADAM family protease (Parks et al, 2000; Nichols et al, 2007). Recent work favouring this scenario demonstrates that, in some developing tissues, Delta is internalized by endocytic pathways that do not employ established components of recycling pathways (Windler and Bilder, 2010; Banks et al, 2011).
Regardless of the nature of the mechanism employed, it appears that in certain biological scenarios, the actual availability of Neur to Delta is tightly regulated. This provides a method for utilizing the requirement for Delta endocytosis as a means for generating pattern. In the early Drosophila embryo, subdivision along the dorso-ventral axis gives rise to three distinct domains of zygotic gene expression: mesoderm, neuro-ectoderm and dorsal ectoderm. Snail is a transcriptional repressor that is exclusively expressed in the mesoderm. It represses, among many other genes, the expression of Twin-of-m4 (Tom), encoding a member of the Bearded protein family, which functions as a competitive inhibitor of Delta binding to Neur. Consequently, Delta will only be targeted to the membrane of the early embryo in the cells where Tom is not expressed, that is, the mesoderm. At the junction between the mesoderm and the neuro-ectoderm, an asymmetry is generated: the mesodermal cells present Delta to the adjacent neuro-ectoderm cells, while no ligand is presented by the neuro-ectoderm cells. This leads to local Notch activation in a single lateral cell row on each side of the embryo, and to induction of the mes-ectodermal cell fate, that will subsequently generate the midline glial cells (Bardin and Schweisguth, 2006; De Renzis et al, 2006).
Affecting signalling outcome by compartmentalization
The EGF receptor pathway in Drosophila triggers numerous developmental switches. Three out of the four ligands that activate this pathway are produced as inactive transmembrane precursors (Shilo, 2005). Cleavage and release of the extracellular domain by intramembrane proteases of the Rhomboid family generates the active secreted ligand (Urban et al, 2001). We will focus this discussion on the ligand that is used during most stages of development, termed Spitz (Spi). Interestingly, generation of active ligand is dictated by a dynamic expression pattern of the protease, Rhomboid. The ligand precursor itself is ubiquitously expressed, but retained in the ER through retrograde trafficking (Lee et al, 2001; Tsruya et al, 2002). This retention prevents the precursor from reaching the plasma membrane and encountering promiscuous metalloproteases that will release an active ligand inappropriately. Exit of the ligand precursor from the ER requires association with another transmembrane protein termed Star, which functions as a chaperone, and facilitates the trafficking of the ligand to a compartment that was identified as an Rab4- and Rab14-enriched endosomal compartment (Yogev et al, 2010). The protease Rhomboid-1 resides within the same compartment, and productive cleavage and secretion of the ligand takes place. In addition, it was shown that the Star protein is also a substrate for cleavage by Rhomboid (Tsruya et al, 2007).
Interestingly, two other members of the Rhomboid family, Rhomboid-2 and -3, carry out the processing of ligand in the germ line and eye disc, respectively. These proteases display a more elaborate intracellular distribution: In addition to the Rab4/14 compartment, they are also localized to the ER (Yogev et al, 2008). If we consider the intracellular journey of the ligand precursor and its chaperone, they would first encounter Rhomboids in the ER. This initial encounter has a dramatic effect on the signalling profile. Cleavage of the ligand in the ER generates a form that is retained in the ER, and therefore does not contribute to signalling (Schlesinger et al, 2004). In parallel, cleavage of the chaperone Star leads to its inactivation even before it facilitates a single round of ligand translocation. Thus, the amount of ligand precursor that will be productively translocated to the Rab4/14 compartment is significantly lower, resulting in a correspondingly lower level of secreted ligand and subsequent signalling (Yogev et al, 2008). This attenuation by ER-localized Rhomboid is utilized in the eye disc, where the EGFR pathway is activated multiple times during the formation of each ommatidium, and the signalling by ligand must be highly restricted and spatially confined at each stage.
The ER localization of Rhomboid, Star and Spitz was shown to have an additional role in the course of eye development. Once photoreceptor cells are induced within the disc epithelium, they send long projections that will contact the outer (lamina) layer of the brain. The same ligand that was utilized for induction of neighbouring cells following apical secretion within the disc epithelium, is also utilized for induction of neuronal fates in the lamina. How is the ligand transferred along the axon? It turns out that ER structures are detected throughout the entire axon. The ER localization of all three components of the ligand-processing machinery is essential for the ability of the axon to induce EGFR activation in the lamina. Thus, the ER is used as a conduit to efficiently translocate the processing machinery across the axon. Once they reach the axon terminus, these components exit the ER and are translocated to the Rab4/14 compartment, where they will induce productive signalling (Yogev et al, 2010). In conclusion, the ER localization of Rhomboid-3 serves to attenuate signalling at the apical side, within the neuronal cell body, but in contrast facilitates signalling at the basal (axonal) end of the cell.
While the ER localization of Rhomboid-3 is effectively utilized in eye development, a different scenario unfolds in the germ line, where Rhomboid-2 is expressed. Like Rhomboid-3, it also displays the dual localization to the ER and the late compartment. During oogenesis, the gene encoding the EGFR ligand Gurken is transcribed by the nurse cells, and the processed gurken mRNA is transported to the oocyte, where it is anchored around the nucleus. Stochastic migration of the nucleus to one of the corners of the oocyte, therefore, marks the future dorsal side of the egg and embryo, by determining the site of gurken mRNA translation and Gurken protein processing and activity. Gurken is, however, ‘in danger’ of encountering Rhomboid-2 already in the ER, resulting in premature cleavage, diffusion throughout the ER and loss of polarity. Cornichon is a multi-transmembrane domain protein functioning as an ER cargo receptor that is conserved from yeast to humans. Cornichon was shown to be essential for efficient export of Gurken from the ER to COPII secretory vesicles, thus circumventing its cleavage in the ER by Rhomboid-2 (Bokel et al, 2006a).
Post-translational modification/lipidation
Transport through the various compartments that comprise the secretory pathway provides ample opportunities to influence and regulate the structure and properties of developmental signalling ligands through post-translational modification. Ligand lipidation via acyltransferases has emerged as a prominent example in this context.
Wnt-family ligands undergo two major forms of lipidation: palmitoylation of a conserved cysteine (C77 of mouse Wnt3a; Willert et al, 2003) and addition of palmitoleic acid to a conserved serine (S209 of Wnt3a; Takada et al, 2006). The multipass transmembrane protein Porcupine (Porc), a conserved, ER-resident member of the membrane bound O-acyltransferase (MBOAT) family, has been identified as a key mediator of Wnt lipidation (van den Heuvel et al, 1993; Kadowaki et al, 1996; Zhai et al, 2004; Galli et al, 2007). Lipid modification and Porc function affect the ability of Wnt ligands to traffic out of the ER, as well as ligand activity levels, and the eventual range of signalling, although many details still need to be ‘ironed out’ by additional experimentation.
A second MBOAT family member, the Golgi element Rasp (also known as Sightless and Skinny Hedgehog), has been shown to modify both Hedgehog (Hh) ligands as well as the major Drosophila EGFR ligand Spitz (Spi), by mediating addition of palmitate moieties (Amanai and Jiang, 2001; Chamoun et al, 2001; Lee and Treisman, 2001; Micchelli et al, 2002; Miura et al, 2006). Rasp-mediated lipid modification enhances the signalling capacity of both these ligands, but via distinct mechanisms. While palmitoylation increases the signalling range and receptor activation capacity of Hh ligands, in a manner reminiscent of the effects of lipidation on Wnts, similar modification of Spi actually restricts the range of ligand diffusion, leading to an increase in local concentration.
Lipid modification commonly serves to potentiate ligand activity, but may also impose restrictions on trafficking that need to be overcome. Hh ligands, which in addition to palmitoylation are also lipid modified by an attached cholesterol molecule, serve as a case in point. Secretion of Hh proteins through the membrane requires the multitransmembrane protein Dispatched (Disp; Burke et al, 1999; Kawakami et al, 2002; Ma et al, 2002; Tian et al, 2005). A variety of observations in Drosophila and mice imply that Disp is required to release Hh from the cells where it is produced, but not for its capacity to trigger the responding cells. In the Drosophila wing imaginal disc, cell clones deficient for disp in the posterior compartment where Hh is produced and released, but not in the anterior compartment where its downstream pathway is triggered, gave rise to mutant phenotypes (Burke et al, 1999). In mice, conditional knockout of Disp1 using a Cre driver that is expressed in the pattern of the ligand Shh, phenocopied the Shh phenotypes (Tian et al, 2005). Importantly, the Disp1 defects could mostly be rescued by expressing the N-terminal part of Hh in a form that is not cholesterol modified, substantiating the role of Disp as a factor required for ligand release imposed by lipid modification.
Signal-receiving cell
Depending on the pathway that is used and the signalling context, two basic modes of signalling are known. One invokes an ‘all or none’ response providing essentially a binary switch. Regulation of signalling levels in this case would affect the range of signalling from the source, that is, the number of cells that will respond to a signal. Alternatively, signalling by the ligand may form a gradient, where different levels of the signal would be interpreted in a distinct manner by the receiving cells. Any effect on the efficiency or duration of signalling may thus influence the range and shape of the resulting pattern of gene expression. We, therefore, discuss and provide below examples (schematized in Figure 2) for the way in which trafficking impinges on the efficiency of signal reception, and consequently on the spatial pattern of the response.
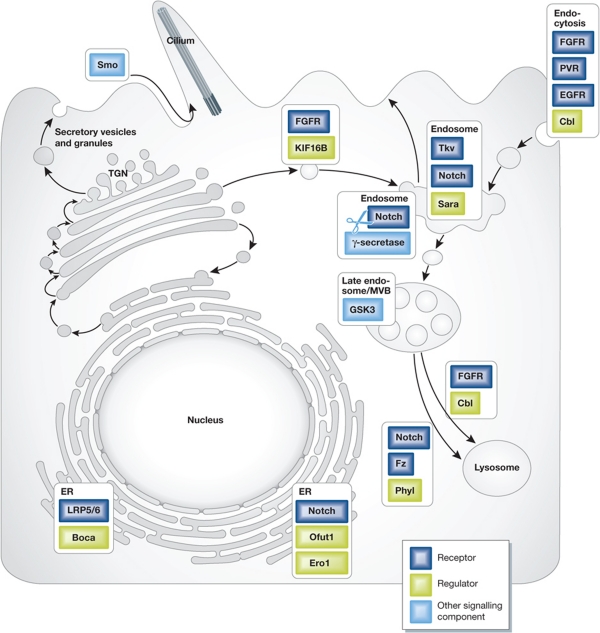
Figure 2.
The signal-receiving cell—modulation of developmental signalling by intracellular trafficking. The ER serves as the compartment for folding of the Wnt LRP5/6 receptor by Boca, and modification and folding of Notch by Ofut1 and Ero1. Lateral trafficking of Smo to the cilium upon activation by Hh facilitates activation of the intracellular signalling pathway. Recruitment to the early endosome of receptor tyrosine kinases including FGFR, PVR and EGFR by Cbl can serve to attenuate or facilitate their signalling and redistribution. An endosomal compartment that contains Tkv, Notch and Sara, controls their distribution between daughter cells upon cell division. FGFR is trafficked from endosomes to the plasma membrane by the kinesin motor KIF16B. The endosome is also the compartment where productive cleavage of Notch by γ secretase takes place, to trigger signalling. The late endosome/MVB provides a site to trap GSK3, thus modulating Wnt signalling. Trafficking from the late endosome/MVB to the lysosome for degradation serves as a critical point of control, where trafficking of FGFR is facilitated by Cbl, and trafficking of Notch and Fz is facilitated by Phyl.
Trafficking to the cell surface
An obvious yet essential prerequisite for developmental signalling is proper trafficking and display of the receptor and associated components on the surface of the signal-receiving cells. In this section, we discuss two aspects of this process, which have been shown to affect developmental signalling pathways: (I) the role of dedicated ER chaperones in ensuring the structural integrity of receptors; (II) the diversity of trafficking pathways utilized in targeting of receptors to specific cell-surface domains.
(I) The complex structure of different receptors often requires elaborate folding schemes within the ER, to enable trafficking and incorporation into the plasma membrane. Utilization of dedicated chaperones that mediate receptor folding constitutes a common solution. Prominent examples are Boca/Mesd proteins, which act in the Wnt signalling pathway in both flies and mice (Culi and Mann, 2003; Hsieh et al, 2003), and the Notch pathway element O-fucosyltransferase1 (Ofut1). Boca/Mesd is required for folding and subsequent ER exit of several cell-surface proteins of the low-density lipoprotein receptor (LDLR) family, including LRP5/6, which act together with Frizzled proteins as conserved co-receptors for Wnt ligands. Boca/Mesd is specifically required for maturation of modules composed of a six-bladed β-propeller domain followed by an EGF repeat (Culi et al, 2004), which are characteristic of LDLRs.
The Ofut1 ‘story’ presents an added layer of complexity, as this ER protein performs two separate roles leading to production of an active Notch receptor: receptor fucosylation, a post-translational modification that is required for further glycosylation by Fringe; and chaperone activity, ensuring proper folding of EGF repeats within the Notch extracellular domain (Okajima et al, 2005, 2008). While fucosylation is relevant only to the Fringe-dependent subset, the chaperone function of Ofut is a general mediator of Notch-related processes. Additional proteins that affect correct folding of Notch, impinge on its proper targeting. Ero1 is an ER-resident thiol oxidase required for formation of disulphide bonds in the Notch extracellular domain, and in its absence high levels of Notch accumulate in the ER and induce the unfolded protein response (Tien et al, 2008).
(II) The cellular and molecular mechanisms employed in transporting receptors and associated elements to the plasma membrane, particularly for polarized epithelia, are well described (Mellman and Nelson, 2008; Weisz and Rodriguez-Boulan, 2009). Such mechanisms involve coordination between vesicular trafficking machineries, the cytoskeleton and intrinsic features within the receptor protein sequences. Receptor presentation on the cell surface is commonly achieved by trafficking of secretory vesicles from the Golgi. These vesicles, in which receptors are embedded via their transmembrane domains, are either trafficked directly to the plasma membrane, or following a ‘detour’ through an endosomal compartment.
Such an alternative to the direct route has been recently described in considerable molecular detail for the final stages of fibroblast growth factor (FGF) receptor trafficking to the cell surface of early mouse embryos. FGFRs mediate diverse developmental decisions in all multicellular organisms. In the early postimplantation mouse embryo, FGF signalling induces cells of the primitive endoderm to secrete a basement membrane, which is critical for proper establishment and differentiation of the epiblast, the primary source of embryonic tissues (Li et al, 2004; Chazaud et al, 2006). Impairment of FGFR function, therefore, results in arrest of embryogenesis at early postimplantation stages (Arman et al, 1998; Li et al, 2001). Trafficking of FGFRs in this context has now been shown to employ a Golgi-to-endosome route of vesicle exocytosis (Ueno et al, 2011). FGFR-containing vesicles produced at the Golgi are associated with Rab14. Conversion of Rab14 to the GTP-bound form mediates interaction of these vesicles with the kinesin-3 family motor protein KIF16B, thus promoting vesicle trafficking towards the plus end of microtubules. This form of transport leads to accumulation of FGFR-containing vesicles within an endosomal compartment adjacent to the plasma membrane, from where the final steps of exocytosis are carried out. Disruption of KIF16B function, as well as overexpression of GDP-bound Rab14, give rise to early embryonic phenotypes highly similar to those observed following loss of FGFR2, highlighting the role of the KIF16B/Rab14 motor in trafficking FGF receptors to their required cell-surface destination.
While targeting of receptors towards distinct plasma membrane regions commonly takes place at the trans-Golgi network or via recycling endosomes, lateral transport to the final (functional) destination following initial cell-surface expression is also possible. This route has been recently shown to be operating in the context of the Hedgehog (Hh) signalling pathway (Milenkovic et al, 2009). Transduction of the Hh signal involves an interplay between two cell-surface, multipass transmembrane proteins, Patched (the Hh receptor) and Smoothened. In vertebrates, this and most other main events of Hh signal reception take places at the primary cilium (Wong and Reiter, 2008; Goetz and Anderson, 2010). Smoothened, however, is initially displayed throughout the plasma membrane. Binding of Hh ligands to the receptor Patched at the primary cilium allows Smoothened to localize to this specialized cell surface organelle, and activate downstream elements of the pathway. Trafficking of Smoothened to the primary cilium does not involve an internalization step, but rather is achieved by lateral transport, mediated by cAMP-Protein kinase A activity (Milenkovic et al, 2009). Full details of this intriguing mechanism, which necessitates overcoming a ‘diffusion barrier’ at the base of the primary cilium (Vieira et al, 2006), are yet to be elucidated.
Endocytosis as a means to modify the signal
Receptors bound to their ligand are targeted for endocytosis. In the case of RTKs, this targeting is facilitated by the Cbl protein, which has a dual function. One aspect of Cbl function is to serve as an adaptor, bridging the bound receptors to the endocytic machinery (Soubeyran et al, 2002; Huang et al, 2007). In addition, by virtue of its ubiquitin ligase activity, Cbl ubiquitinates the bound receptor, thus targeting it to lysosomal degradation (Haugsten et al, 2008). A dominant-negative Cbl construct can selectively inhibit the ubiquitination step, while maintaining the adaptor function. This form was used to follow the dynamics of FGF8 gradient interpretation in the zebrafish embryo (Nowak et al, 2011). By live colocalization studies in the embryo, it was possible to assess changes in the distribution of the active receptor. This manipulation decreased the efficiency of lysosomal targeting, thus increasing the fraction of active endocytosed receptor. As a result, the cells were more sensitive to the ligand and exhibited a broader response. Since the manipulation did not affect the initial endocytosis step, the shape of the extracellular FGF gradient was unaltered. This experiment highlights a paradigm where the ‘state’ of the receiving cell can affect its responsiveness to the extracellular signal. In the case of Cbl, this state can be altered by phosphorylation following RTK activation, thus providing a dynamic alteration of responsiveness to the ligand.
It is also possible to envisage other scenarios, where the responsiveness of a tissue would be genetically predetermined, according to the expression of signalling or trafficking modifiers. EGFR signalling was recently shown to be modulated by cytohesin proteins, which promote dimerization of the phosphorylated receptors (Bill et al, 2010). Differences in cytohesin expression between tissues may thus create a distinct range of signalling for the same set of stimuli.
In instances of developmental cell migration, the ability to detect a spatial bias in the signal guiding migration is crucial. When migration is carried out over a long distance such a bias may be subtle, and thus enhancement of an initial asymmetry in the detected signal may contribute to a robust and reproducible directionality of migration. This scenario is observed in the case of border cell migration in the Drosophila egg chamber. This group of 8–10 cells detaches from the anterior follicle cells that surround the germ cells, and migrates as a cluster between the membranes of the nurse cells, until reaching the oocyte (Rorth, 2002; Montell, 2003). Signals emanating from the oocyte activate PVR and EGFR, two RTKs expressed by the border cells (Duchek and Rorth, 2001; Duchek et al, 2001; McDonald et al, 2003, 2006). In view of the considerable initial distance between the oocyte and the border cells, and the large size of the oocyte which is the source for the ligands, it is assumed that any ligand gradient that will be formed would be shallow.
The effect on border cell migration of a variety of signalling manipulations was examined (Jekely et al, 2005). The overall capacity to receive the signal by the border cells within a reasonable range was modulated, for example, by increasing receptor expression levels or decreasing their targeting to degradation. While overall signalling levels, as assayed by phospho-tyrosine staining, were significantly elevated, directionality of migration was not perturbed, indicating that the cells are sensing local differences in signalling levels, rather than the level of signalling per se.
Interestingly, mutations in Cbl perturbed directionality of migration, without affecting the overall level of signalling. Cbl was shown to associate with the phosphorylated PVR and EGFR receptors, and target them for endocytosis. Compromising endocytosis by other means, for example, by mutating Dynamin, also affected migration. An attractive hypothesis is that in these cells the endocytosed receptors are not targeted for degradation, but are rather recycled to the membrane. If recycling could be biased towards regions within the membrane where the RTKs are more highly activated, this would further enhance localized activation within the cell and provide a more robust response to the guidance cues. Such amplification of asymmetry could be dynamic and adjusted to changes in the domain of highest signalling on the membrane. This kind of bias is relevant in a situation where a cell is not monitoring its overall level of activation, but rather a spatially restricted activation. Since RTK signalling is thought to direct migration by local activation of actin nucleation at the cell cortex, biasing the distribution of receptors on the membrane may be an effective strategy. The activity of Cbl in these cells may contribute to the biased deposition of recycled receptors. The putative mechanism for detecting a local signalling bias on the membrane and targeting endocytosed receptors to this domain is not known. In line with this hypothesis, it was recently demonstrated that trafficking through the early and recycling endosome of the border cells is essential for polarized RTK activity (Assaker et al, 2010).
Compartmentalization
A key segment of our discussion of trafficking in the signal-sending cell focused on the versatility and regulatory capacity provided by utilization of distinct trafficking compartments, which serve to separate or bring together elements of specific signalling pathways. Compartmentalization can have similar roles in signal-receiving cells, and we present examples for its effect on (I) the strength and duration of signalling; (II) regulation of signalling by segregation of pathway elements; (III) distribution of signalling components during cell division.
(I) Signalling in the Notch pathway culminates in proteolytic cleavage of the Notch receptor by the γ-secretase complex, releasing the cytoplasmic, C-terminal end of Notch, which is now free to directly enter the nucleus, and affect transcription of pathway target genes. Control of the final proteolytic step is therefore critical, as it produces the transcription-mediating element of the pathway. Studies in mammalian cell culture established that endocytic activity and the hallmark mono-ubiquitin modification were required for γ-secretase cleavage of Notch (Gupta-Rossi et al, 2004). The ability to arrest endocytosis at distinct steps using mutants available in Drosophila provides a matching in vivo setting. This approach has demonstrated that Notch signalling following γ-secretase cleavage is strongly reduced when trafficking to endosomes is blocked (Vaccari et al, 2008), and that signalling is elevated if traffic out of endosomes is impaired by disruption of ESCRT complex activity (Vaccari and Bilder, 2005). These studies suggest, therefore, that endosomal compartments serve as the meeting ground between the Drosophila Notch receptor and the activating protease (although this has been recently challenged by mammalian cell-culture studies; Sorensen and Conner, 2010). Furthermore, they imply that trafficking of the receptors (even those that have not encountered ligand) through the endosomal compartment must be rapid and efficient, as retention (and therefore, prolonged exposure to proteolysis) leads to deleterious effects as a result of sustained signalling (Vaccari et al, 2008).
(II) Compartments within the signal-receiving cell can be used not only as arenas in which pathway elements are brought together for activation purposes, but also as means for sequestration of critical components. A case in point is the sorting mechanism recently shown to mediate Wnt signalling in Xenopus embryos (Taelman et al, 2010). It is well established that β-catenin serves as the key transcriptional activator of target genes in the canonical Wnt pathway (Moon, 2005). In the absence of Wnt signal transduction in receiving cells, β-catenin is efficiently targeted for proteosomal degradation by a cytoplasmic ‘destruction complex’. An important component of this protein complex is glycogen synthase kinase 3 (GSK3), which phosphorylates β-catenin on several sites, prolonging its association with the complex, and the resulting susceptibility to ubiquitylation and degradation (Wu and Pan, 2010). Binding of Wnt ligands to their receptors leads to formation of a cell-surface complex that recruits GSK3 (Zeng et al, 2008). Taelman et al show that internalization of this complex via endocytosis results in its sequestration within MVBs, effectively blocking contact between GSK3 and β-catenin, which can now accumulate in the cytoplasm and travel to the nucleus. The significance of sequestration of GSK3 in MVBs is underscored by the observation that GSK3 enzymatic activity per se is not affected by Wnt signalling. The protective effect of Wnt signalling turns out to be extensive in nature, since GSK3 is a promiscuous kinase that phosphorylates many substrates (Taelman et al, 2010). Endosomal compartmentalization within the Wnt signal-receiving cell, therefore, provides a global mechanism for promoting protein stability.
Sequestration of β-catenin itself has been recently proposed as a strategy for modulating the efficiency of Wnt signalling. Remarkably, the primary cilium serves as the sequestering compartment in this instance (Lancaster et al, 2011). The context-dependent Wnt pathway regulator Jouberin becomes localized, via intraflagellar transport, to the cilium basal body in ciliated cells. Under circumstances where such cells respond to a Wnt signal, Jouberin associates with and diverts β-catenin to the cilium and away from the nucleus, resulting in a dampening of the Wnt response.
(III) A particularly ‘sophisticated’ use of compartmentalization within receiving cells has been described for distribution of signalling elements during cell division. The Decapentaplegic (Dpp) signal transduction pathway is an important mediator of Drosophila imaginal wing disc development. Dpp, a TGFβ/BMP-like ligand, is produced in a stripe of cells at the centre of the disc, from which it emanates to form a patterning gradient, within the proliferating field of disc cells (Lecuit et al, 1996; Nellen et al, 1996). The adaptor protein Sara (Smad anchor for receptor activation), which can simultaneously associate with Dpp/TGFβ receptors, pathway transcriptional activators of the Smad family and membrane phospholipids, ensures that the receptors and activators are endocytosed into specialized endosomes. During mitosis, these so-called ‘Sara endosomes’ come to be positioned at the midzone of the mitotic spindle (‘central spindle’), leading to their equal distribution between the pair of daughter cells following cytokinesis (Bokel et al, 2006b). The endosomal compartment, therefore, serves in this case as a means of ensuring that the capacity to transduce signals remains uniform within a dividing field of cells.
In a further twist to this intriguing story, asymmetric segregation of Sara endosomes during mitosis has been shown to influence a second developmental signalling process, in which the Notch pathway directs acquisition of distinct cell fates within the Drosophila peripheral nervous system. In this case, pIIa and pIIb, the pair of progeny cells derived from the sensory-organ precursor (SOP) cell, communicate with each other via Delta-Notch signalling to establish their distinct fates. Several mechanisms are at play to ensure that signalling is asymmetric (with Notch being active only in pIIa), although both progeny express both the Delta ligand and the Notch receptor (Gonczy, 2008). One of these employs Sara endosomes that form in the SOP before its division and contain both proteins (Coumailleau et al, 2009). Sara endosomes in the SOP respond to cues controlling asymmetry as this cell enters mitosis, so that their association with the (evenly positioned) central spindle is biased towards the posterior side of the cell, which will give rise to pIIa. Endosomal compartmentalization, therefore, establishes an immediate bias in distribution of the Notch signalling protein within the SOP cell lineage.
Trafficking as a means to mediate communication between pathways
In the Drosophila eye imaginal disc, a highly ordered array of cell–cell interactions leads to the formation of ∼750 ommatidia, each composed of photoreceptor cells, cone and pigment cells. The major signalling pathways converge to determine the intricate array of photoreceptors within each ommatidium, in a manner that is highly orchestrated, both temporally and spatially. This is perhaps the most critical junction where coordination between pathways is required. While typically most coordination events take place by convergence of the signals at the level of individual promoter/enhancer sequences in the relevant target genes, in this instance cross talk between pathways was identified at the level of the signalling components per se.
Phyllopod (Phyl) is a novel adaptor protein whose cytoplasmic partners are not known. In the absence of phyl, alterations in photoreceptor cell fate were observed, that are consistent with an increase in Notch and Wg signalling. Indeed, higher levels of Delta, Notch and the Wg receptor Frizzled (Fz) were observed in the leading photoreceptor rows of phyl mutants. The accumulation was mapped to endocytic vesicles, and the outcome indicated increased signalling by both pathways (Nagaraj and Banerjee, 2009). Thus, the compartments in which Notch/Delta and Wg/Fz are stabilized, can still promote active signalling. Normally, restriction of the time these molecules spend in the endocytic compartment, following expression of Phyl, leads to attenuation of Notch and Wg signalling. Interestingly, expression of Phyl is induced by activation of the EGFR pathway. Thus, activation of EGFR leads to a concomitant reduction in Notch and Wg signalling. Successive induction of EGFR is responsible for the reiterated recruitment of photoreceptor, cone and pigment cells to the developing ommatidium. It now appears that this activation also serves to restrict the activity of Notch and Wg, by inducing the expression of Phyl.
Conclusion
We can expect in the future a continuation of the fruitful convergence between the studies of developmental signalling pathways and cellular trafficking. Analyses at the cellular and subcellular level should reveal the intricate ways in which modulation of intracellular trafficking serves to regulate developmental signalling at the level of the whole organism. In parallel, the capacity to identify organismal phenotypes of various mutations that can be linked to one or more developmental signalling pathways, may reveal new modalities and regulatory networks of cellular trafficking.
Acknowledgments
This work was supported by a grant from the Minerva foundation to BS and ES. BS is an incumbent of the Hilda and Cecil Lewis Chair in Molecular Genetics.
Footnotes
The authors declare that they have no conflict of interest.
References
- Amanai K, Jiang J (2001) Distinct roles of Central missing and Dispatched in sending the Hedgehog signal. Development 128: 5119–5127 [DOI] [PubMed] [Google Scholar]
- Arman E, Haffner-Krausz R, Chen Y, Heath JK, Lonai P (1998) Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc Natl Acad Sci U S A 95: 5082–5087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaker G, Ramel D, Wculek SK, Gonzalez-Gaitan M, Emery G (2010) Spatial restriction of receptor tyrosine kinase activity through a polarized endocytic cycle controls border cell migration. Proc Natl Acad Sci U S A 107: 22558–22563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks SM, Cho B, Eun SH, Lee JH, Windler SL, Xie X, Bilder D, Fischer JA (2011) The functions of auxilin and rab11 in Drosophila suggest that the fundamental role of ligand endocytosis in notch signaling cells is not recycling. PLoS One 6: e18259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K (2006) Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 125: 509–522 [DOI] [PubMed] [Google Scholar]
- Bardin AJ, Schweisguth F (2006) Bearded family members inhibit Neuralized-mediated endocytosis and signaling activity of Delta in Drosophila. Dev Cell 10: 245–255 [DOI] [PubMed] [Google Scholar]
- Bartscherer K, Pelte N, Ingelfinger D, Boutros M (2006) Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell 125: 523–533 [DOI] [PubMed] [Google Scholar]
- Belenkaya TY, Wu Y, Tang X, Zhou B, Cheng L, Sharma YV, Yan D, Selva EM, Lin X (2008) The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev Cell 14: 120–131 [DOI] [PubMed] [Google Scholar]
- Benhra N, Vignaux F, Dussert A, Schweisguth F, Le Borgne R (2010) Neuralized promotes basal to apical transcytosis of delta in epithelial cells. Mol Biol Cell 21: 2078–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yaacov S, Le Borgne R, Abramson I, Schweisguth F, Schejter ED (2001) Wasp, the Drosophila Wiskott-Aldrich syndrome gene homologue, is required for cell fate decisions mediated by Notch signaling. J Cell Biol 152: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bill A, Schmitz A, Albertoni B, Song JN, Heukamp LC, Walrafen D, Thorwirth F, Verveer PJ, Zimmer S, Meffert L, Schreiber A, Chatterjee S, Thomas RK, Ullrich RT, Lang T, Famulok M (2010) Cytohesins are cytoplasmic ErbB receptor activators. Cell 143: 201–211 [DOI] [PubMed] [Google Scholar]
- Bokel C, Dass S, Wilsch-Brauninger M, Roth S (2006a) Drosophila Cornichon acts as cargo receptor for ER export of the TGFalpha-like growth factor Gurken. Development 133: 459–470 [DOI] [PubMed] [Google Scholar]
- Bokel C, Schwabedissen A, Entchev E, Renaud O, Gonzalez-Gaitan M (2006b) Sara endosomes and the maintenance of Dpp signaling levels across mitosis. Science 314: 1135–1139 [DOI] [PubMed] [Google Scholar]
- Burke R, Nellen D, Bellotto M, Hafen E, Senti KA, Dickson BJ, Basler K (1999) Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell 99: 803–815 [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Peifer M (2009) Wnt signaling from development to disease: insights from model systems. Cold Spring Harb Perspect Biol 1: a002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamoun Z, Mann RK, Nellen D, von Kessler DP, Bellotto M, Beachy PA, Basler K (2001) Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science 293: 2080–2084 [DOI] [PubMed] [Google Scholar]
- Chazaud C, Yamanaka Y, Pawson T, Rossant J (2006) Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev Cell 10: 615–624 [DOI] [PubMed] [Google Scholar]
- Commisso C, Boulianne GL (2007) The NHR1 domain of Neuralized binds Delta and mediates Delta trafficking and Notch signaling. Mol Biol Cell 18: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs GS, Yu J, Canning CA, Veltri CA, Covey TM, Cheong JK, Utomo V, Banerjee N, Zhang ZH, Jadulco RC, Concepcion GP, Bugni TS, Harper MK, Mihalek I, Jones CM, Ireland CM, Virshup DM (2010) WLS-dependent secretion of WNT3A requires Ser209 acylation and vacuolar acidification. J Cell Sci 123: 3357–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudreuse DY, Roel G, Betist MC, Destree O, Korswagen HC (2006) Wnt gradient formation requires retromer function in Wnt-producing cells. Science 312: 921–924 [DOI] [PubMed] [Google Scholar]
- Coumailleau F, Furthauer M, Knoblich JA, Gonzalez-Gaitan M (2009) Directional Delta and Notch trafficking in Sara endosomes during asymmetric cell division. Nature 458: 1051–1055 [DOI] [PubMed] [Google Scholar]
- Culi J, Mann RS (2003) Boca, an endoplasmic reticulum protein required for wingless signaling and trafficking of LDL receptor family members in Drosophila. Cell 112: 343–354 [DOI] [PubMed] [Google Scholar]
- Culi J, Springer TA, Mann RS (2004) Boca-dependent maturation of beta-propeller/EGF modules in low-density lipoprotein receptor proteins. EMBO J 23: 1372–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblandre GA, Lai EC, Kintner C (2001) Xenopus neuralized is a ubiquitin ligase that interacts with XDelta1 and regulates Notch signaling. Dev Cell 1: 795–806 [DOI] [PubMed] [Google Scholar]
- De Renzis S, Yu J, Zinzen R, Wieschaus E (2006) Dorsal-ventral pattern of Delta trafficking is established by a Snail-Tom-Neuralized pathway. Dev Cell 10: 257–264 [DOI] [PubMed] [Google Scholar]
- Duchek P, Rorth P (2001) Guidance of cell migration by EGF receptor signaling during Drosophila oogenesis. Science 291: 131–133 [DOI] [PubMed] [Google Scholar]
- Duchek P, Somogyi K, Jekely G, Beccari S, Rorth P (2001) Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell 107: 17–26 [DOI] [PubMed] [Google Scholar]
- Franch-Marro X, Wendler F, Guidato S, Griffith J, Baena-Lopez A, Itasaki N, Maurice MM, Vincent JP (2008) Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol 10: 170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Jiang M, Mirando AJ, Yu HM, Hsu W (2009) Reciprocal regulation of Wnt and Gpr177/mouse Wntless is required for embryonic axis formation. Proc Natl Acad Sci U S A 106: 18598–18603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli LM, Barnes TL, Secrest SS, Kadowaki T, Burrus LW (2007) Porcupine-mediated lipid-modification regulates the activity and distribution of Wnt proteins in the chick neural tube. Development 134: 3339–3348 [DOI] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV (2010) The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet 11: 331–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonczy P (2008) Mechanisms of asymmetric cell division: flies and worms pave the way. Nature reviews 9: 355–366 [DOI] [PubMed] [Google Scholar]
- Goodman RM, Thombre S, Firtina Z, Gray D, Betts D, Roebuck J, Spana EP, Selva EM (2006) Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development 133: 4901–4911 [DOI] [PubMed] [Google Scholar]
- Gupta-Rossi N, Six E, LeBail O, Logeat F, Chastagner P, Olry A, Israel A, Brou C (2004) Monoubiquitination and endocytosis direct gamma-secretase cleavage of activated Notch receptor. J Cell Biol 166: 73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugsten EM, Malecki J, Bjorklund SM, Olsnes S, Wesche J (2008) Ubiquitination of fibroblast growth factor receptor 1 is required for its intracellular sorting but not for its endocytosis. Mol Biol Cell 19: 3390–3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh JC, Lee L, Zhang L, Wefer S, Brown K, DeRossi C, Wines ME, Rosenquist T, Holdener BC (2003) Mesd encodes an LRP5/6 chaperone essential for specification of mouse embryonic polarity. Cell 112: 355–367 [DOI] [PubMed] [Google Scholar]
- Huang F, Goh LK, Sorkin A (2007) EGF receptor ubiquitination is not necessary for its internalization. Proc Natl Acad Sci U S A 104: 16904–16909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Kim CH, Palardy G, Oda T, Jiang YJ, Maust D, Yeo SY, Lorick K, Wright GJ, Ariza-McNaughton L, Weissman AM, Lewis J, Chandrasekharappa SC, Chitnis AB (2003) Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev Cell 4: 67–82 [DOI] [PubMed] [Google Scholar]
- Jekely G, Sung HH, Luque CM, Rorth P (2005) Regulators of endocytosis maintain localized receptor tyrosine kinase signaling in guided migration. Dev Cell 9: 197–207 [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Wilder E, Klingensmith J, Zachary K, Perrimon N (1996) The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes Dev 10: 3116–3128 [DOI] [PubMed] [Google Scholar]
- Kawakami T, Kawcak T, Li YJ, Zhang W, Hu Y, Chuang PT (2002) Mouse dispatched mutants fail to distribute hedgehog proteins and are defective in hedgehog signaling. Development 129: 5753–5765 [DOI] [PubMed] [Google Scholar]
- Kim H, Cheong SM, Ryu J, Jung HJ, Jho EH, Han JK (2009) Xenopus Wntless and the retromer complex cooperate to regulate XWnt4 secretion. Mol Cell Biol 29: 2118–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, Budnik V (2009) Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell 139: 393–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai EC, Deblandre GA, Kintner C, Rubin GM (2001) Drosophila neuralized is a ubiquitin ligase that promotes the internalization and degradation of delta. Dev Cell 1: 783–794 [DOI] [PubMed] [Google Scholar]
- Lai EC, Roegiers F, Qin X, Jan YN, Rubin GM (2005) The ubiquitin ligase Drosophila Mind bomb promotes Notch signaling by regulating the localization and activity of Serrate and Delta. Development 132: 2319–2332 [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Schroth J, Gleeson JG (2011) Subcellular spatial regulation of canonical Wnt signalling at the primary cilium. Nat Cell Biol 13: 702–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne R, Remaud S, Hamel S, Schweisguth F (2005) Two distinct E3 ubiquitin ligases have complementary functions in the regulation of delta and serrate signaling in Drosophila. PLoS Biol 3: e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit T, Brook WJ, Ng M, Calleja M, Sun H, Cohen SM (1996) Two distinct mechanisms for long-range patterning by Decapentaplegic in the Drosophila wing. Nature 381: 387–393 [DOI] [PubMed] [Google Scholar]
- Lee JD, Treisman JE (2001) Sightless has homology to transmembrane acyltransferases and is required to generate active Hedgehog protein. Curr Biol 11: 1147–1152 [DOI] [PubMed] [Google Scholar]
- Lee JR, Urban S, Garvey CF, Freeman M (2001) Regulated intracellular ligand transport and proteolysis control EGF signal activation in Drosophila. Cell 107: 161–171 [DOI] [PubMed] [Google Scholar]
- Li L, Arman E, Ekblom P, Edgar D, Murray P, Lonai P (2004) Distinct GATA6- and laminin-dependent mechanisms regulate endodermal and ectodermal embryonic stem cell fates. Development 131: 5277–5286 [DOI] [PubMed] [Google Scholar]
- Li X, Chen Y, Scheele S, Arman E, Haffner-Krausz R, Ekblom P, Lonai P (2001) Fibroblast growth factor signaling and basement membrane assembly are connected during epithelial morphogenesis of the embryoid body. J Cell Biol 153: 811–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Erkner A, Gong R, Yao S, Taipale J, Basler K, Beachy PA (2002) Hedgehog-mediated patterning of the mammalian embryo requires transporter-like function of dispatched. Cell 111: 63–75 [DOI] [PubMed] [Google Scholar]
- McDonald JA, Pinheiro EM, Kadlec L, Schupbach T, Montell DJ (2006) Multiple EGFR ligands participate in guiding migrating border cells. Dev Biol 296: 94–103 [DOI] [PubMed] [Google Scholar]
- McDonald JA, Pinheiro EM, Montell DJ (2003) PVF1, a PDGF/VEGF homolog, is sufficient to guide border cells and interacts genetically with Taiman. Development 130: 3469–3478 [DOI] [PubMed] [Google Scholar]
- Mellman I, Nelson WJ (2008) Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev 9: 833–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micchelli CA, The I, Selva E, Mogila V, Perrimon N (2002) Rasp, a putative transmembrane acyltransferase, is required for Hedgehog signaling. Development 129: 843–851 [DOI] [PubMed] [Google Scholar]
- Milenkovic L, Scott MP, Rohatgi R (2009) Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. J Cell Biol 187: 365–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura GI, Buglino J, Alvarado D, Lemmon MA, Resh MD, Treisman JE (2006) Palmitoylation of the EGFR ligand Spitz by Rasp increases Spitz activity by restricting its diffusion. Dev Cell 10: 167–176 [DOI] [PubMed] [Google Scholar]
- Montell DJ (2003) Border-cell migration: the race is on. Nat Rev 4: 13–24 [DOI] [PubMed] [Google Scholar]
- Moon RT (2005) Wnt/beta-catenin pathway. Sci STKE 2005 (271): cm1. [DOI] [PubMed] [Google Scholar]
- Nagaraj R, Banerjee U (2009) Regulation of Notch and Wingless signalling by phyllopod, a transcriptional target of the EGFR pathway. EMBO J 28: 337–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellen D, Burke R, Struhl G, Basler K (1996) Direct and long-range action of a DPP morphogen gradient. Cell 85: 357–368 [DOI] [PubMed] [Google Scholar]
- Nichols JT, Miyamoto A, Olsen SL, D′Souza B, Yao C, Weinmaster G (2007) DSL ligand endocytosis physically dissociates Notch1 heterodimers before activating proteolysis can occur. J Cell Biol 176: 445–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak M, Machate A, Yu SR, Gupta M, Brand M (2011) Interpretation of the FGF8 morphogen gradient is regulated by endocytic trafficking. Nat Cell Biol 13: 153–158 [DOI] [PubMed] [Google Scholar]
- Okajima T, Reddy B, Matsuda T, Irvine KD (2008) Contributions of chaperone and glycosyltransferase activities of O-fucosyltransferase 1 to Notch signaling. BMC Biol 6: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima T, Xu A, Lei L, Irvine KD (2005) Chaperone activity of protein O-fucosyltransferase 1 promotes notch receptor folding. Science 307: 1599–1603 [DOI] [PubMed] [Google Scholar]
- Pan CL, Baum PD, Gu M, Jorgensen EM, Clark SG, Garriga G (2008) C. elegans AP-2 and retromer control Wnt signaling by regulating mig-14/Wntless. Dev Cell 14: 132–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks AL, Klueg KM, Stout JR, Muskavitch MA (2000) Ligand endocytosis drives receptor dissociation and activation in the Notch pathway. Development 127: 1373–1385 [DOI] [PubMed] [Google Scholar]
- Pavlopoulos E, Pitsouli C, Klueg KM, Muskavitch MA, Moschonas NK, Delidakis C (2001) Neuralized Encodes a peripheral membrane protein involved in delta signaling and endocytosis. Dev Cell 1: 807–816 [DOI] [PubMed] [Google Scholar]
- Pitsouli C, Delidakis C (2005) The interplay between DSL proteins and ubiquitin ligases in Notch signaling. Development 132: 4041–4050 [DOI] [PubMed] [Google Scholar]
- Port F, Kuster M, Herr P, Furger E, Banziger C, Hausmann G, Basler K (2008) Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat Cell Biol 10: 178–185 [DOI] [PubMed] [Google Scholar]
- Prasad BC, Clark SG (2006) Wnt signaling establishes anteroposterior neuronal polarity and requires retromer in C. elegans. Development 133: 1757–1766 [DOI] [PubMed] [Google Scholar]
- Rajan A, Tien AC, Haueter CM, Schulze KL, Bellen HJ (2009) The Arp2/3 complex and WASp are required for apical trafficking of Delta into microvilli during cell fate specification of sensory organ precursors. Nat Cell Biol 11: 815–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorth P (2002) Initiating and guiding migration: lessons from border cells. Trends Cell Biol 12: 325–331 [DOI] [PubMed] [Google Scholar]
- Schlesinger A, Kiger A, Perrimon N, Shilo BZ (2004) Small wing PLCγ is required for ER retention of cleaved Spitz during eye development in Drosophila. Dev Cell 7: 535–545 [DOI] [PubMed] [Google Scholar]
- Seugnet L, Simpson P, Haenlin M (1997) Requirement for dynamin during Notch signaling in Drosophila neurogenesis. Dev Biol 192: 585–598 [DOI] [PubMed] [Google Scholar]
- Shilo BZ (2005) Regulating the dynamics of EGF receptor signaling in space and time. Development 132: 4017–4027 [DOI] [PubMed] [Google Scholar]
- Silhankova M, Port F, Harterink M, Basler K, Korswagen HC (2010) Wnt signalling requires MTM-6 and MTM-9 myotubularin lipid-phosphatase function in Wnt-producing cells. EMBO J 29: 4094–4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen EB, Conner SD (2010) Gamma-secretase-dependent cleavage initiates notch signaling from the plasma membrane. Traffic 1: 1234–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubeyran P, Kowanetz K, Szymkiewicz I, Langdon WY, Dikic I (2002) Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature 416: 183–187 [DOI] [PubMed] [Google Scholar]
- Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, Vorwald PP, Gumper I, Sabatini DD, De Robertis EM (2010) Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell 143: 1136–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S (2006) Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell 11: 791–801 [DOI] [PubMed] [Google Scholar]
- Tian H, Jeong J, Harfe BD, Tabin CJ, McMahon AP (2005) Mouse Disp1 is required in sonic hedgehog-expressing cells for paracrine activity of the cholesterol-modified ligand. Development 132: 133–142 [DOI] [PubMed] [Google Scholar]
- Tien AC, Rajan A, Schulze KL, Ryoo HD, Acar M, Steller H, Bellen HJ (2008) Ero1L, a thiol oxidase, is required for Notch signaling through cysteine bridge formation of the Lin12-Notch repeats in Drosophila melanogaster. J Cell Biol 182: 1113–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsruya R, Schlesinger A, Reich A, Gabay L, Sapir A, Shilo BZ (2002) Intracellular trafficking by Star regulates cleavage of the Drosophila EGF receptor ligand Spitz. Genes Dev 16: 222–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsruya R, Wojtalla A, Carmon S, Yogev S, Reich A, Bibi E, Merdes G, Schejter E, Shilo BZ (2007) Rhomboid cleaves Star to regulate the levels of secreted Spitz. EMBO J 26: 1211–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H, Huang X, Tanaka Y, Hirokawa N (2011) KIF16B/Rab14 molecular motor complex is critical for early embryonic development by transporting FGF receptor. Dev Cell 20: 60–71 [DOI] [PubMed] [Google Scholar]
- Urban S, Lee JR, Freeman M (2001) Drosophila rhomboid-1 defines a family of putative intramembrane serine proteases. Cell 107: 173–182 [DOI] [PubMed] [Google Scholar]
- Vaccari T, Bilder D (2005) The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev Cell 9: 687–698 [DOI] [PubMed] [Google Scholar]
- Vaccari T, Lu H, Kanwar R, Fortini ME, Bilder D (2008) Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J Cell Biol 180: 755–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R, Nusse R (2009) Towards an integrated view of Wnt signaling in development. Development 136: 3205–3214 [DOI] [PubMed] [Google Scholar]
- van den Heuvel M, Harryman-Samos C, Klingensmith J, Perrimon N, Nusse R (1993) Mutations in the segment polarity genes wingless and porcupine impair secretion of the wingless protein. EMBO J 12: 5293–5302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WL, Simons K (2006) FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc Natl Acad Sci U S A 103: 18556–18561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Struhl G (2005) Distinct roles for Mind bomb, Neuralized and Epsin in mediating DSL endocytosis and signaling in Drosophila. Development 132: 2883–2894 [DOI] [PubMed] [Google Scholar]
- Weisz OA, Rodriguez-Boulan E (2009) Apical trafficking in epithelial cells: signals, clusters and motors. J Cell Sci 122: 4253–4266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR 3rd, Nusse R (2003) Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423: 448–452 [DOI] [PubMed] [Google Scholar]
- Windler SL, Bilder D (2010) Endocytic internalization routes required for delta/notch signaling. Curr Biol 20: 538–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SY, Reiter JF (2008) The primary cilium at the crossroads of mammalian hedgehog signaling. Curr Top Dev Biol 85: 225–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Pan W (2010) GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci 35: 161–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PT, Lorenowicz MJ, Silhankova M, Coudreuse DY, Betist MC, Korswagen HC (2008) Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev Cell 14: 140–147 [DOI] [PubMed] [Google Scholar]
- Yeh E, Dermer M, Commisso C, Zhou L, McGlade CJ, Boulianne GL (2001) Neuralized functions as an E3 ubiquitin ligase during Drosophila development. Curr Biol 11: 1675–1679 [DOI] [PubMed] [Google Scholar]
- Yogev S, Schejter ED, Shilo BZ (2008) Drosophila EGFR signalling is modulated by differential compartmentalization of Rhomboid intramembrane proteases. EMBO J 27: 1219–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev S, Schejter ED, Shilo BZ (2010) Polarized secretion of Drosophila EGFR ligand from photoreceptor neurons is controlled by ER localization of the ligand-processing machinery. PLoS Biol 8: e1000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, Almeida K, Wang J, Doble B, Woodgett J, Wynshaw-Boris A, Hsieh JC, He X (2008) Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development 135: 367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai L, Chaturvedi D, Cumberledge S (2004) Drosophila Wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine. J Biol Chem 279: 33220–33227 [DOI] [PubMed] [Google Scholar]