Abstract
It is now clear that transport on microtubules by dynein and kinesin family motors has an important if not critical role in the replication and spread of many different viruses. Understanding how viruses hijack dynein and kinesin motors using a limited repertoire of proteins offers a great opportunity to determine the molecular basis of motor recruitment. In this review, we discuss the interactions of dynein and kinesin-1 with adenovirus, the α herpes viruses: herpes simplex virus (HSV1) and pseudorabies virus (PrV), human immunodeficiency virus type 1 (HIV-1) and vaccinia virus. We highlight where the molecular links to these opposite polarity motors have been defined and discuss the difficulties associated with identifying viral binding partners where the basis of motor recruitment remains to be established. Ultimately, studying microtubule-based motility of viruses promises to answer fundamental questions as to how the activity and recruitment of the dynein and kinesin-1 motors are coordinated and regulated during bi-directional transport.
Keywords: dynein, kinesin-1, microtubule transport, virus
Introduction
The transport of intracellular components is of central importance to all forms of eukaryotic life. Proteins, RNA, vesicles and even organelles must be moved from their site of production to locations appropriate for their function. Many of these cargoes are subsequently transported to alternative cellular locations to deliver signals, or undergo recycling and/or degradation. This constant flux of intracellular cargoes over micron distances is principally mediated by dynein and kinesin motors (Hirokawa et al, 2009; Kardon and Vale, 2009; Verhey and Hammond, 2009). These molecular motors are powered by the hydrolysis of ATP and transport their associated cargoes along microtubules in a polarized and coordinated manner (Gennerich and Vale, 2009). Phylogenetic analysis of the human and mouse genomes reveals they each encode some 45 kinesin motors that can be grouped into 14 different families (Hirokawa et al, 2009; Verhey and Hammond, 2009). With a few exceptions, kinesin motors transport cargoes in an anterograde manner towards the plus end of the microtubules, which are often located in the cell periphery (Hirokawa et al, 2009; Verhey and Hammond, 2009). Cytoplasmic dynein motors, on the other hand, transport cargoes in a retrograde manner towards the minus end of microtubules, which are frequently anchored at the microtubule-organizing centre (MTOC; Hook and Vallee, 2006; Kardon and Vale, 2009). There are many different dynein heavy chains, however, only dynein 1 (cytoplasmic dynein) and dynein 1B/dynein 2, which is involved in intraflagellar transport, are capable of transporting cargoes along microtubules (Pfister et al, 2005; Hook and Vallee, 2006; Kardon and Vale, 2009).
Unfortunately for the cell, many different viruses are capable of subverting the same efficient microtubule transport system to facilitate their replication and enhance their spread (Dohner et al, 2005; Greber and Way, 2006; Radtke et al, 2006; Brandenburg and Zhuang, 2007; Ward, 2011). The extent of use of the microtubule cytoskeleton and its associated motors depends upon the replication strategy of the particular virus. Several viruses use the microtubule transport system to move their nucleic acid/protein cores to intracellular replication sites immediately after they have gained entry to the cell. Others take advantage of the microtubule cytoskeleton to move newly assembled viral progeny to the plasma membrane to facilitate their spread into surrounding cells and tissues. Viruses also use the network to transport nucleic acid and protein components involved in virion assembly to specific cellular locations or to move partially assembled progeny at specific stages of their replication cycles.
Research conducted over the past 10 years has largely focused on determining how the microtubule cytoskeleton plays a role at various stages during viral replication and spread. Studies using drugs that disrupt microtubules have highlighted a requirement for the microtubule network during virus replication. A role for microtubules during viral infection is also often inferred from the speed and linear trajectories of fluorescently labelled virions or their constituent parts during live cell imaging (Seisenberger et al, 2001; Willard, 2002; Lakadamyali et al, 2003; Greber and Way, 2006; Brandenburg and Zhuang, 2007). In a few cases, simultaneous live cell imaging of virions and microtubules has provided direct evidence that viruses can move on microtubules (Suomalainen et al, 1999; Rietdorf et al, 2001; McDonald et al, 2002). Immunofluorescence analysis of infected cells has confirmed that many different viruses associate with microtubules at various stages of their replication cycles (Greber and Way, 2006; Radtke et al, 2006). In a number of cases, it has also been possible to detect viral-associated motor protein components (Sodeik et al, 1997; Rietdorf et al, 2001; Greber and Way, 2006; Bremner et al, 2009). Inhibition of particular motor complexes using dominant-negative or RNAi approaches has provided important functional data on the role of particular microtubule motors during viral transport processes (Suomalainen et al, 1999; Rietdorf et al, 2001; Dohner et al, 2002; Bremner et al, 2009; Salinas et al, 2009). The progress in highlighting a role for microtubule transport during viral infection has now opened up a new challenge—to determine how viruses recruit motors and regulate their activity.
Over the years, studies from a large number of groups have revealed that the two most common motor proteins involved in various aspects of viral transport are cytoplasmic dynein and kinesin-1 (Figure 1). Both motors are highly processive and can mediate long distance movement of cargoes towards opposite ends of microtubules. In this review, we focus on recent progress in determining the molecular basis of dynein and kinesin-1 recruitment to virus particles. We discuss viral systems where the molecular links to these two motors have been defined, highlight where important gaps still exist in our knowledge and examine some of the problems associated with identifying the key molecular link between the virus and the motor.
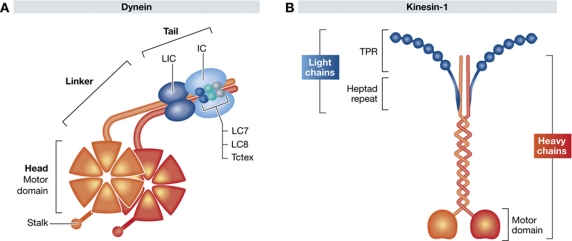
Figure 1.
Schematic representation of the subunit composition of dynein and kinesin-1. (A) The motor containing cytoplasmic dynein heavy chains are shown in orange and associated intermediate and light chains in shades of blue. The motor domain is composed of six AAA ATPase domains arranged in a hexameric ring from which a microtubule binding stalk projects. The N-terminal tail of the heavy chain mediates its dimerization and contains the binding sites for two intermediate chains (ICs) and two light intermediate chains (LICs). The two intermediate chains (ICs) also interact with three pairs of light chains: Tctex, LC7 and LC8. (B) Kinesin-1 is a heterotetramer composed of two motor containing heavy chains (orange) and two light chains (blue). The microtubule binding motor domain is found in the N-terminus of the heavy chain. The light chains associate with the heavy chains via heptad repeat regions in their N-terminus. The C-terminal half of the light chains is composed of six tetratricopeptide repeats (TPR), which represent cargo binding domains.
Composition of cytoplasmic dynein and viral transport
The cytoplasmic dynein motor is a large multimeric ∼1.5 MDa complex composed of two heavy chains (DHCs), two intermediate chains (ICs), two light intermediate chains (LICs) and several light chains (LCs) (Pfister et al, 2005; Kardon and Vale, 2009; Figure 1). The motor activity resides in the heavy chain, which is composed of a cargo binding N-terminus and C-terminal motor domain composed of six AAA ATPase domains arranged in a hexameric ring from which a microtubule binding stalk projects (Kardon and Vale, 2009; Carter et al, 2011; Kon et al, 2011). The N-terminus, which mediates dimerization of the heavy chain, also contains the LIC and IC interaction sites. The ICs contain binding sites for the LCs as well as for the regulatory, cargo and microtubule binding dynactin complex (Kardon and Vale, 2009). The dynactin complex, which itself is composed of 11 different subunits, has an important accessory role for virtually all the known cellular functions of dynein (Schroer, 2004; Kardon and Vale, 2009). Each dynein motor complex can contain up to three different LC dimers: Tctex/rp3 (DYNLT1/3), Roadblock/LC7 (DYNLRB1/2) and LC8 (DYNLL1/2) (Pfister et al, 2005; Kardon and Vale, 2009). Extensive biochemical analysis has revealed that the many different cargo binding and regulatory proteins, including Bicaudal-D, Lis1, NudE, NudC, NudEL and ZW10 are each capable of interacting with a specific set of subunits in the dynein motor complex (Kardon and Vale, 2009). It is thought that the potential combinatorial diversity and numerous interaction surfaces of the many different motor subunits and accessory proteins are central to the ability of dynein to transport such a diverse range of cargoes.
Many different viruses utilize cytoplasmic dynein to facilitate their directed movement towards the MTOC during the initial establishment of infection (Dohner et al, 2005; Greber and Way, 2006; Radtke et al, 2006; Ward, 2011). These include adenovirus, the α herpes viruses—herpes simplex virus (HSV1) and pseudorabies virus (PrV) and the retrovirus—human immunodeficiency virus type 1 (HIV-1). When examined collectively, these viruses nicely illustrate the variation in our knowledge of the molecular link between the virus and the dynein motor complex. They also highlight some of the issues that have arisen when attempting to determine these interactions. For adenovirus, an authentic link involving a direct interaction between motor subunits and the incoming viral capsid has been defined (Bremner et al, 2009). In contrast, for HSV1 exceptional biochemical evidence exists for the direct recruitment of dynein but as yet no specific viral receptor(s) has been identified (Radtke et al, 2010). In the case of HIV-1, inhibition of dynein activity abrogates viral transport towards the nucleus during the early stages of infection (McDonald et al, 2002). Nevertheless, it remains to be established whether the virus actually recruits dynein during the establishment of infection.
Hexon mediated recruitment of dynein to adenovirus
Currently, the most detailed picture for a dynein-virus link has emerged from studies on the role of microtubule transport during the establishment of adenovirus infection. Fluorescently labelled adenovirus particles undergo rapid microtubule-dependent bidirectional movements after entry into host cells before their replication in the nucleus (Suomalainen et al, 1999, 2001; Leopold et al, 2000; Salinas et al, 2009). The microtubule cytoskeleton is important to establish virus infection as its depolymerization with nocodazole results in a 90% reduction in nuclear targeting of adenovirus (Suomalainen et al, 1999; Leopold et al, 2000; Mabit et al, 2002; Engelke et al, 2011). Disruption of dynein activity by overexpression of dynactin subunit p50/dynamitin or the coiled-coil region of p150glued reduces the frequency of virus directed movements towards the MTOC (Suomalainen et al, 1999; Leopold et al, 2000; Engelke et al, 2011). Subsequent work showed that dynein is capable of mediating an interaction between adenovirus and microtubules in vitro (Kelkar et al, 2004).
Given the substantial evidence for a role of dynein during the establishment of adenovirus infection, it is surprising that the motor has only recently been detected on incoming virus particles (Bremner et al, 2009). The same study also finally provided the identity of the viral and motor components responsible for dynein recruitment. Bremner et al found that the hexon capsid subunit of adenovirus interacts directly with both the dynein IC and LIC1 subunits. Interestingly, these interactions are dependent upon hexon being exposed to low pH. This suggests that only viruses that have passed through an endocytic compartment during entry are capable of recruiting dynein (Bremner et al, 2009). Various perturbations of the dynein–hexon interaction, including microinjection of dynein IC or hexon antibodies, knockdown of dynein or overexpression of hexon disrupt accumulation of the virus at the centrosome/nucleus (Bremner et al, 2009). Live cell imaging demonstrated that this reduced accumulation is due to a decrease in run length rather than the velocity of the virus. Dynactin, which is also recruited to the incoming virus is not required for recruitment of dynein but does have an essential role in promoting nuclear accumulation of the virus. Consistent with this, dynactin was not found in association with dynein IC or LICs in hexon pull-down experiments. The dynein accessory proteins, NudE, NudEL, LIS and ZW10 were found to be associated with incoming virions to varying extents. However, neither dominant-negative inhibition of NudE, NudEL and LIS nor siRNA depletion of ZW10 affected dynein recruitment or virus transport (Bremner et al, 2009). Collectively, these data offer a relatively simple model for motor recruitment in which the hexon trimer in the viral capsid couples directly to dynein via its IC and LIC subunits. This suggestion is consistent with a recent computational model of bi-directional transport of adenovirus, which was based on live cell imaging (Gazzola et al, 2009). It may be that the region of hexon that binds IC and/or LIC is a structural mimic of a cellular adaptor that normally links cargoes to dynein. Indeed, it has recently been shown that LIC mediate the direct recruitment of the dynein motor to lysosomes and late endosomes (Tan et al, 2011).
Herpes virus tegument proteins interact with dynein
During the initial establishment of infection, non-enveloped cytosolic HSV1 and PrV capsids undergo bidirectional microtubule-dependent movements that ultimately result in a net retrograde motility towards the nucleus, where the virus can establish a latent infection (Sodeik et al, 1997; Dohner et al, 2002; Smith et al, 2004; Diefenbach et al, 2008; Lyman and Enquist, 2009; Antinone and Smith, 2010; Figure 2). The activity of the dynein–dynactin motor complex, which is recruited by these incoming capsids, is required to establish infection (Sodeik et al, 1997; Dohner et al, 2002; Mabit et al, 2002). HSV1 capsids purified from extracellular virions can also bind and traffic along microtubules in a dynactin-dependent manner in vitro, but only in the presence of cytosol and energy (Wolfstein et al, 2006). Consistent with this, dynein and dynactin can bind these purified viral capsids but not those derived from the nucleus (Wolfstein et al, 2006; Radtke et al, 2010).
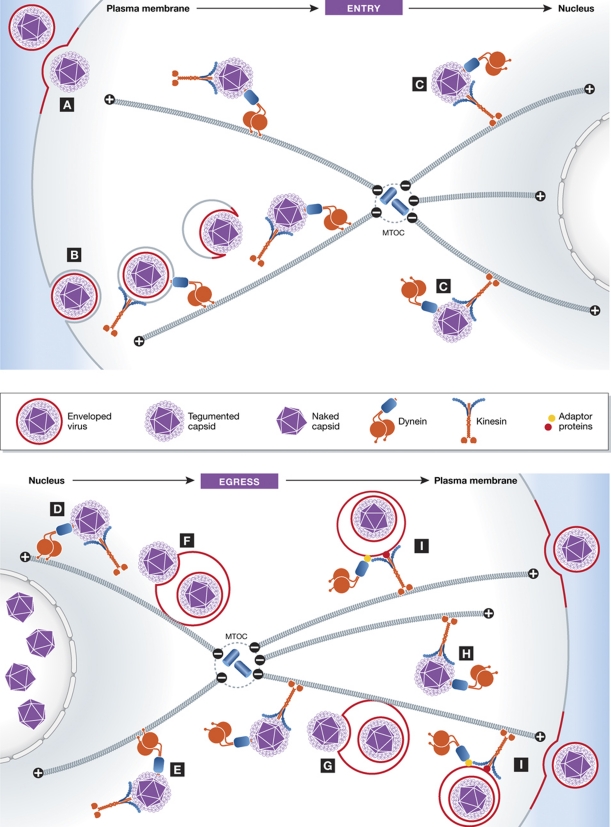
Figure 2.
Transport of herpes virus during entry and egress. ENTRY: Depending on the cell type, viruses enter by (A) directly fusing with the plasma membrane or (B) via an endocytic-based mechanism. Regardless of the mode of entry, viruses within endosomes or more usually as non-enveloped capsids probably recruit both kinesin and dynein even though they move in net retrograde (minus end) direction along microtubules towards the nucleus. Depending on the position MTOC and organization of the microtubule cytoskeleton relative to the nucleus, it is also possible that kinesin-1-dependent plus-end directed movement along microtubules (C) may be required for the virus to reach the nuclear envelope. EGRESS: Following their exit from the nucleus, the tegument of non-enveloped capsids recruits kinesin-1 and dynein to facilitate their bidirectional transport on microtubules (D, E). Viruses will move along microtubules until they encounter membrane compartments into which they can bud to form enveloped virions (F, G). The location of these membrane compartments will vary depending on the position of the MTOC and organization of the microtubule cytoskeleton, which will be cell type dependent. Consequently, viral movements towards these membrane compartment may require a net minus-end (dynein) or plus-end (kinesin-1) driven transport depending on the site of envelopment (F, G) with respect to the MTOC. Some viruses, however, may never encounter the right membrane compartment and will continue to move as non-enveloped capsids throughout the cell (E, H). After envelopment, viruses within vesicular compartments (I) will be transported in a net anterograde manner, possibly by kinesin-1, towards the plasma membrane, where they fuse and are released.
Identification of the link between dynein and adenovirus was greatly facilitated by the relatively few candidate proteins that remain associated with virions during their transit to the nucleus. This is not the case for α herpes viruses (Diefenbach et al, 2008; Radtke et al, 2010). For example, HSV1 consists of 8 capsid and 26 potential tegument proteins that are associated with the capsid (Radtke et al, 2010). Identification of potential HSV1 and PrV dynein binding proteins has relied on a combination of live imaging of fluorescently labelled viruses as well as biochemical approaches using purified virions. Imaging recombinant viruses encoding various combinations of RFP- and GFP-tagged viral proteins reveals that while VP1/2 (UL36) and UL37 can be detected, the majority of tegument proteins are lost from capsids undergoing retrograde transport to the nucleus (Luxton et al, 2005; Antinone and Smith, 2010), consistent with previous extensive ultrastructural studies, for example, see (Granzow et al, 2005; Maurer et al, 2008). Deletion analysis has confirmed that the majority of tegument proteins are not required for nuclear directed capsid transport (Antinone et al, 2006). As observed with adenovirus, recruitment of dynein to purified capsids is not dependent on dynactin (Radtke et al, 2010). Interestingly, purified capsids can also bind directly to dynactin, although this interaction does not appear to enhance dynein motor recruitment (Radtke et al, 2010). Biochemical analysis, however, reveals that the interaction between viral capsids and dynein is enhanced when they are treated with a high salt buffer, which removes the outer tegument proteins. Based on the differential extraction properties of the tegument proteins, it now appears that an inner tegument component contains the principle receptor(s) for dynein interaction (Wolfstein et al, 2006; Radtke et al, 2010). This elegant biochemical analysis, which is consistent with results obtained from live imaging, has considerably narrowed the list of potential dynein interacting proteins (Radtke et al, 2010). Based on the available data, VP1/2 (UL36) and UL37 are currently the strongest candidates to recruit dynein, although there is still no evidence that either interacts with any motor subunit (Radtke et al, 2010). Consistent with this suggestion, loss of UL37 significantly delays translocation of PrV to the nucleus (Krautwald et al, 2009).
Yeast two hybrid experiments combined with in vitro binding assays have revealed that the HSV1 proteins pUL9, pUL34 and VP26 (pUL35) can interact with different subunits of the dynein motor complex (Ye et al, 2000; Martinez-Moreno et al, 2003; Douglas et al, 2004). The viral helicase pUL9 and pUL34 interact with dynein LCs and ICs, respectively (Ye et al, 2000; Martinez-Moreno et al, 2003). However, the functional significance of these interactions in the retrograde transport of HSV1 is unclear as neither protein is a capsid or tegument component (Diefenbach et al, 2008). The small capsid protein VP26 (pUL35) can bind directly to the dynein LCs Tctex (DYNLT1) and RP3 (DYNLT3) (Douglas et al, 2004). Microinjected capsids lacking VP26 assembled using a baculovirus expression system do not accumulate at the nuclear envelope (Douglas et al, 2004). However, subsequent studies in which cells were infected with a recombinant virus lacking the pUL35 gene revealed that capsids were still capable of undergoing dynein-dependent retrograde transport in the absence of VP26 (Desai et al, 1998; Antinone et al, 2006; Dohner et al, 2006). Viral capsids lacking VP26 were also still capable of binding to dynein (Wolfstein et al, 2006; Radtke et al, 2010). In addition, HSV1 capsids isolated from the nucleus cannot bind dynein even though they contain VP26 (Wolfstein et al, 2006; Radtke et al, 2010). Thus, while VP26 may contribute to and/or stabilize dynein recruitment via a LC interaction, the current data all points to the inner tegument components as forming the primary interface (Radtke et al, 2010). It may be difficult to pin dynein recruitment down to a single viral protein, as it is likely that inner tegument proteins adopt higher order assemblies that will result in new interaction surfaces. Such assemblies may also lead to cooperativity/avidity effects that promote the recruitment of the dynein–dynactin complex.
Retrograde transport of HIV-1
Higher order protein assemblies or complexes, which can be difficult to recapitulate from purified proteins in vitro, may also explain the difficulty experienced in identifying the HIV-1 components mediating dynein recruitment. Incoming HIV-1 cores/reverse transcription complexes associate with the microtubule network after fusion of the viral envelope with the plasma membrane. They then move in a microtubule- and dynein-dependent manner towards the MTOC (McDonald et al, 2002). The HIV-1 core is composed of a relatively limited number of components—the viral RNA, nucleocapsid and capsid proteins, as well as the reverse transcriptase and integrase enzymes. Why then should identifying the link to dynein prove so elusive? The difficulty may arise from the metastable nature of the HIV-1 core, which undergoes structural and compositional changes after cell entry including the reverse transcription of viral RNA into DNA. The precise nature of these changes and in particular the timing and scale of loss of the capsid protein from the core remains controversial (Arhel, 2010). The case of HIV-1 highlights how virus transport processes may not be completely independent of capsid maturation processes and those required for nuclear import of the viral genomes.
If HIV-1 recruitment of dynein were to mirror that of adenovirus and HSV1, namely a direct interaction between a viral component and the motor complex, then the p24 capsid protein would be the most likely candidate. The capsid protein assembles into hexameric rings that oligomerize to form the distinctive conical shell, which is delivered into the cytoplasm after membrane fusion. It would appear to be in an ideal position on the surface of the virion to interact with cytoplasmic dynein. Some reports, however, suggest that the core is highly unstable and that the capsid is rapidly disassembled (Fassati and Goff, 2001). Nevertheless, capsid was shown to be associated with microtubule bound cores (McDonald et al, 2002). More recently, apparently intact HIV-1 cores were detected close to the nuclear membrane (Arhel et al, 2007). Despite capsid protein being a prime candidate, no interaction with any dynein component has been reported. This may be a common theme for capsid interacting proteins, given the observation that a direct interaction between capsid and the restriction factor, Trim5α could only be demonstrated on higher ordered capsid assemblies (Stremlau et al, 2006; Ganser-Pornillos et al, 2011). It is tempting to speculate that a similar higher order assembly is required to bind dynein or other cellular proteins that subsequently recruit the motor complex. Interestingly, the adenovirus hexon preparations that interact with dynein IC and LICs are also in their native trimeric form (Bremner et al, 2009).
An alternative mechanism to the ‘direct recruitment’ would be that the incoming virion ‘piggy backs’ on an existing retrograde trafficking pathway involving dynein. One possible cellular trafficking pathway may involve importins, which can bind dynein and target components to the nuclear pore complex to facilitate their nuclear import (Hanz et al, 2003; Yudin et al, 2008; Wagstaff and Jans, 2009). HIV-1 integrase interacts with the importins 7 and α3 (Fassati et al, 2003; Hearps and Jans, 2006; Ao et al, 2007, 2010; Zaitseva et al, 2009). Intriguingly, when expressed in yeast, HIV-1 integrase traffics to the MTOC in a microtubule- and dynein-dependent manner before nuclear import (Desfarges et al, 2009). An alternative ‘piggy back’ mechanism may be provided by the pathway used by the cell for dealing with aggregated proteins (Dohner et al, 2005; Wileman, 2007). In this pathway, aggregates are transported in a dynein-dependent manner towards the MTOC where they undergo proteosomal or autophagosomal degradation (Garcia-Mata et al, 2002; Johnston et al, 2002; Wileman, 2007). It is conceivable that incoming virus particles could be transported via such a mechanism. This, however, would necessitate two important additional steps. The first would be the identification and marking of the virus for transport and degradation either by post-translational modifications such as ubiquitination or by association of additional proteins that are already destined for degradation. There is no evidence that incoming HIV-1 components are themselves highly ubiquitinated. Curiously, the best candidates to mediate an aggresome-like transport mechanism for HIV-1 may be the very factors that target incoming virions for degradation. Trim5α, which associates with incoming virions, like other family members, is an E3 ubiquitin ligase that can undergo autoubiquitination (Meroni and Diez-Roux, 2005; Campbell et al, 2008; Langelier et al, 2008; Pertel et al, 2011). In addition, Trim5α is transported on microtubules and, when overexpressed, forms aggregates that are degraded by autophagy (Diaz-Griffero et al, 2006; Campbell et al, 2007). However, it seems unlikely that Trim5α is responsible for mediating retrograde transport of HIV, given that its ability to bind to capsid correlates with its antiviral activity (Sebastian and Luban, 2005; Stremlau et al, 2006). Nonetheless, the Trim protein family is very large and the function of most members remains unknown. It is also intriguing that several family members appear to promote or enhance retroviral replication (Uchil et al, 2008). If such a mechanism exists, then the second essential step after transport to the MTOC would be a Houdini-like escape of the viral nucleic acid from the cellular degradation machinery before the viral genome is destroyed. It is conceivable that viral uncoating would also remove any degradative complex to allow nuclear import of the genome and associated proteins. Such a mechanism would mean there will be a fine balance between degradation and progression of virus replication. Interestingly, inhibition of the proteosome enhances retroviral replication in permissive and can rescue reverse transcription in restrictive cells (Wei et al, 2005; Wu et al, 2006).
Viral coupling to dynein LCs and transport
The example of HSV and HIV-1 underlines the difficulties in establishing which viral protein(s) bind which dynein subunits to recruit the motor for the purposes of transport. Of all the dynein motor subunits, the LC8 family members DYNLL1/2 stand out above all others in binding viral proteins from a number of different viruses including African swine fever, rabies, ebola, human foamy viruses (Jacob et al, 2000; Raux et al, 2000; Alonso et al, 2001; Poisson et al, 2001; Rodriguez-Crespo et al, 2001; Petit et al, 2003; Kubota et al, 2009). Despite evidence for dynein-mediated retrograde transport for several of these viruses, in no case has biochemical evidence of a virus-LC8 link to the motor itself been demonstrated. LC8 also independently associates with Myosin V, the transcription factor Trps1, as well as the yeast nucleoporin Nup159 where five LC8 molecules cooperate to promote dimerization of the protein (Espindola et al, 2000; Kaiser et al, 2003; Stelter et al, 2007). It has been proposed that LC8 primarily acts as a hub to promote dimerization of its interacting partners, and that its role as a dynein cargo adaptor is perhaps secondary (Barbar, 2008). Dimerization of LC8 generates two interfaces that bind two TKQTQTT motifs in the dimeric dynein ICs (Benison et al, 2006, 2007). To act as a cargo adaptor, one of these LC8 interfaces would have to interact with the IC and the other with a viral cargo. The stability of the LC8 dimer and nature of its interactions with DIC would make such a scenario very unlikely (Barbar, 2008; Radnai et al, 2010). Therefore, to demonstrate it forms a functional dynein-virus link, it is imperative to show that LC8 associated with viruses can also interact with other dynein subunits. Indeed, studies with rabies suggest that the LC8 interaction is probably not involved in viral transport. A virus lacking its LC8 binding motif was still capable of transport from the peripheral to the central nervous system but did have a significant reduction in the transcriptional activity of its viral polymerase (Tan et al, 2007). The LC8 binding sequences in the rabies phosphoprotein P also have a role in its nuclear import (Moseley et al, 2007). Thus, while LC8 appears to participate in the rabies replication cycle it seems unlikely that it is functionally connected to dynein-mediated viral transport.
As with LC8, there is again controversy over the potential cargo binding function of the Tctex family LCs. Structural studies have suggested that Tctex is unlikely to interact simultaneously with both IC and cargo proteins (Williams et al, 2007). Another study has, however, suggested that cargo and IC binding sites on Tctex might be independent (Wu et al, 2005). Data from Human Papilloma Virus would support the latter of these two hypotheses (Florin et al, 2006; Schneider et al, 2011). Association of the virus with microtubules requires the C-terminal 40 amino acids of L2, a minor capsid protein (Florin et al, 2006). The same sequences were also required to associate with the dynein IC (Florin et al, 2006). More recent studies demonstrate that L2 interacts directly with the Tctex LCs (DYNLT1/3), which both associate with virions (Schneider et al, 2011). This implies that Tctex might bridge IC and L2. RNAi-mediated depletion of either LC results in a loss in infectivity and an accumulation of L2 in the cytoplasm. A structural understanding of the L2–Tctex interaction should help resolve the outstanding issues over the cargo binding function of Tctex.
Composition of kinesin-1, the first plus-end motor
Multiple members of the kinesin superfamily will undoubtedly contribute to various transport steps during viral infection but here we will only focus on kinesin-1, which is also known as conventional kinesin or Kif5 (Figure 1). Kinesin-1, which was the first plus-end directed microtubule motor to be identified, is involved in a wide range of cellular processes by virtue of its ability to interact with many different types of cargo (Hirokawa et al, 2009; Verhey and Hammond, 2009). Kinesin-1 is a heterotetramer composed of two heavy and two light chains (Figure 1). The microtubule binding motor domain is found in the N-terminus of the heavy chain, which can be encoded by three different genes (Kif5A, Kif5B and Kif5C). Kif5A and Kif5C are only expressed in neuronal cells, whereas Kif5B is ubiquitous (Hirokawa et al, 2009; Verhey and Hammond, 2009). Each heavy chain dimer associates with two copies of KLC1 or KLC2, which are expressed in most cell types. A single kinesin-1 tetramer always contains two identical heavy and light chains (Gyoeva et al, 2004). The LCs associate with the heavy chains via heptad repeat regions near their N-terminus. Cargoes can bind directly to specific sites on the kinesin-1 heavy chain. However, they are also capable of interacting with the motor via the tetratricopeptide repeats (TPRs) in the C-terminal half of the kinesin LC (Gindhart, 2006; Hammond et al, 2008). The expression of multiple KLC1 splice variants, each with a different C-terminus is also thought to contribute to cargo binding specificity (Gyoeva et al, 2000; McCart et al, 2003; Wozniak and Allan, 2006). Like dynein, our understanding of kinesin-1 recruitment to viral cargoes varies significantly depending upon the virus studied (Dohner et al, 2005; Greber and Way, 2006; Radtke et al, 2006; Ward, 2011). Here, we will only discuss the interaction of kinesin-1 with vaccinia and herpes viruses, which currently represent the most extensively studied systems.
Recruitment of kinesin-1 to vaccinia virus
Kinesin-1 appears to have several important roles during the replication cycle of vaccinia virus (Schepis et al, 2007). Nevertheless, so far the only stage where progress has been made in identifying a viral kinesin-1 binding partner is in the egress of the intracellular enveloped virus (IEV) from their perinuclear site of formation to the plasma membrane (Roberts and Smith, 2008; Ward, 2011). IEV are formed when infectious intracellular mature virions become ‘wrapped’ by a double membrane cisternae derived from trans-Golgi or endosomal membranes (Smith et al, 2002; Roberts and Smith, 2008). The mechanistic basis for this wrapping step is poorly understood but involves several integral viral membrane proteins as well as the viral proteins, E2 and F12 (Smith et al, 2002; Domi et al, 2008; Dodding et al, 2009). The resulting ‘wrapped’ IEV is able to recruit kinesin-1 and undergo anterograde microtubule-dependent transport to the cell periphery, where its outer membrane fuses with the plasma membrane to release an infectious virion into the extracellular environment (Geada et al, 2001; Hollinshead et al, 2001; Rietdorf et al, 2001; Ward and Moss, 2001a, 2001b; Dodding et al, 2009; Morgan et al, 2010; Figure 3). The recruitment of kinesin-1 to IEV is dependent on A36, an integral viral membrane protein (Rietdorf et al, 2001; Ward and Moss, 2004). In the absence of A36, IEV remains largely restricted to their perinuclear site of assembly, as they are unable to spread to the cell periphery (Rietdorf et al, 2001; Ward and Moss, 2001a; Ward et al, 2003; Dodding and Way, 2009). Loss of A36 consequently results in a small plaque phenotype, due to the dramatic impact on the cell-to-cell spread of the virus (Ward and Moss, 2001a; Smith et al, 2002; Doceul et al, 2010). Residues 81–111 of A36 are actually capable of interacting directly with the cargo binding TPRs of KLC (Ward and Moss, 2004). The interaction between A36 and KLC has recently been confirmed in infected cells using fluorescence resonance energy transfer approaches (Jeshtadi et al, 2010). Curiously, residues 81–111 of A36 contain a short WD motif that is similar to the KLC binding site in Calsyntenin-1 (Konecna et al, 2006; Araki et al, 2007; Dodding and Way, 2009; Morgan et al, 2010). A related WE sequence that is similar to the functional KLC binding motif in Caytaxin and γ-BAR (also known as Gadkin) (Aoyama et al, 2009; Schmidt et al, 2009) is also present at residues 64–65 of A36 (Morgan et al, 2010; Dodding et al, in preparation). Recent data using overexpression approaches, however, suggest that the WD and WE motifs in A36 are not required for vaccinia to reach the plasma membrane and induce actin tails (Morgan et al, 2010). In contrast, we have found using recombinant viruses that these two motifs are required for kinesin-1 recruitment and viral transport to the plasma membrane (Dodding et al, in preparation).
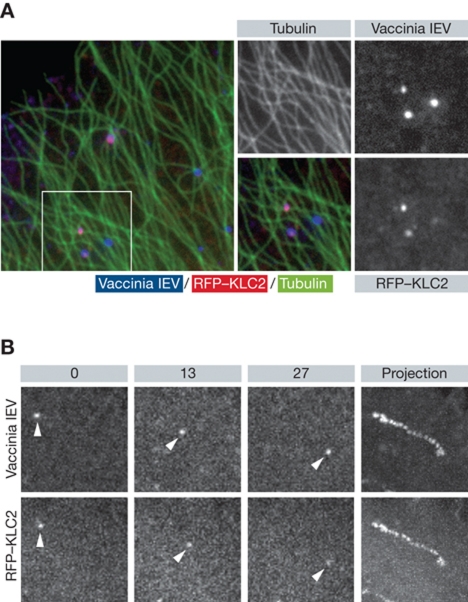
Figure 3.
Vaccinia IEV recruit kinesin-1 and move on microtubules. (A) Immunofluorescence images showing recruitment of kinesin-1 (RFP–KLC, red) to vaccinia IEV (blue) associated with microtubules (green). (B) Stills taken from live cell imaging of the movement of YFP-tagged vaccinia IEV in cells expressing RFP-tagged kinesin light chain 2. The time in seconds is indicated and the right panel shows a maximum intensity projection to highlight the path taken by the virus.
In addition to A36, it has also been proposed that F12 has a direct role in the microtubule-dependent transport of IEV to the plasma membrane (van Eijl et al, 2002; Morgan et al, 2010). Consistent with this, F12 is associated with IEV moving on microtubules (van Eijl et al, 2002; Dodding et al, 2009). Moreover, loss of F12 leads to a perinuclear accumulation of IEV and a dramatic reduction in actin tail formation (Zhang et al, 2000; van Eijl et al, 2002; Dodding et al, 2009; Morgan et al, 2010). Live cell imaging, however, shows that some IEV are still capable of moving in a linear manner, presumably along microtubules, at ∼1 μm/s even in the absence of F12 (Dodding et al, 2009). This would suggest that while F12 may be involved in IEV transport, it is not essential. Based on its primary sequence and structural predictions, which suggest the protein contains 14 TPR-like motifs, it has been suggested that F12 may represent a viral KLC-like mimic to recruit kinesin-1 to IEV (Morgan et al, 2010). TPRs, which form protein interaction surfaces in a wide range of proteins, are composed of two small antiparallel α helices joined by a short linker (D’Andrea and Regan, 2003). If F12 is a viral KLC mimic, then it must bind the kinesin-1 heavy chain in a manner that is fundamentally different to KLC as it lacks heptad repeats (Figure 1). Moreover, there is still no published data indicating that F12 can bind directly to the kinesin-1 heavy chain. However, F12 can interact directly with A36 and E2, the latter of which is also predicted to contain 15 TPR-like repeats (Johnston and Ward, 2008; Dodding et al, 2009; Morgan et al, 2010). The binding site of F12 on A36 overlaps with that of KLC, raising the intriguing possibility that F12, together with E2, functions to inhibit kinesin-1 recruitment until IEV formation is complete (Ward and Moss, 2004; Johnston and Ward, 2008). Such a mechanism would prevent premature transport of incompletely wrapped IEV. However, this does not explain why F12 and E2 remain associated with IEV moving on microtubules, if their primary function is to inhibit A36-mediated KLC recruitment. While the role of F12–E2 complex during IEV formation and transport remains to be determined, current data clearly demonstrate that A36 provides a direct link between the vaccinia and kinesin-1.
Herpes virus tegument also interacts with kinesin-1
HSV1 and PrV undergo anterograde transport during bidirectional movements towards the nucleus as well as later during infection when new viral progeny egress along the axon towards the plasma membrane (Smith et al, 2001, 2004; Diefenbach et al, 2008; Lyman and Enquist, 2009; Antinone and Smith, 2010; Antinone et al, 2010; Ward, 2011; Figure 2). It is likely that one or more kinesin family members drive these plus-end directed motilities. Understanding the mechanism of kinesin recruitment during egress, however, is complicated by the fact that both enveloped and non-enveloped forms of the virus are transported on microtubules (Antinone and Smith, 2006; Antinone et al, 2010; Wisner et al, 2011). Currently, as for dynein, most progress has been made in understanding kinesin recruitment for the non-enveloped cytoplasmic form of the virus. Kinesin-1 and kinesin-2 can bind directly to purified HSV1 capsids (Radtke et al, 2010). Whether their recruitment is mediated by the motor heavy chains, KLC or KAP in the case of kinesin-2 remains to be established. It also remains to be established whether these two motors are actually recruited to viral capsids moving in infected cells. Once again, the inner tegument proteins are implicated in motor binding, as capsids purified from the nucleus, which lack tegument proteins cannot bind either kinesin. As observed for dynein, treatment of purified capsids with 0.5 M salt enhanced kinesin-1 or 2 binding. In contrast to dynein, however, kinesin-1 binding was lost in very high salt conditions (1 M), suggesting that the motors bind distinct features on the HSV1 virion. The only HSV1 tegument protein so far implicated in interaction with kinesin-1 is US11, which interacts with the heptad repeat binding region of the motor heavy chain (Diefenbach et al, 2002). HSV1 virions that lack US11 were, however, still capable of binding kinesin-1 (Radtke et al, 2010). It is possible that even if US11, which also associates with the KLC-related protein PAT1 (Benboudjema et al, 2003) does not directly couple the virus and motor, it may still have a role in virus transport by regulating motor activity. More recently, live cell imaging has suggested that the inner tegument protein VP1/2 (pUL36) may be a good candidate to bind kinesin-1, as new viral progeny lacking the UL36 gene were unable to undergo microtubule directed transport during their egress from the nucleus to the plasma membrane (Luxton et al, 2006). Curiously, HSV1 UL36 contains a number of WD/E motifs similar to those found in A36 of vaccinia and other cellular KLC binding proteins (Dodding and Way, 2009; Morgan et al, 2010; Dodding et al, in preparation). A number of these are also conserved in UL36 from PrV. Interestingly, UL37 from PrV but not HSV1 also contains several WD/E motifs that may represent potential kinesin-1 recruitment sites (M Schären and H Favoreel, personal communication). If these WD/E motifs are involved in kinesin-1 recruitment then their selective disruption would provide important insights into the precise role of kinesin-1 in both entry and egress of HSV1 and PrV.
HSV1 capsids purified from extracellular virions can clearly bind kinesin-1 (Radtke et al, 2010). Nevertheless, it has long been a matter of controversy whether it is these non-enveloped particles or the enveloped form of the virus that undergoes axonal transport (Diefenbach et al, 2008; Curanovic and Enquist, 2009; Miranda-Saksena et al, 2009; Wisner et al, 2011). Until recently, all studies addressing this important question were based on fixed images of HSV1-infected cells, for example, see Saksena et al (2006) and Snyder et al (2006). However, live cell imaging has now revealed that the majority (∼70%) of HSV1 undergoing anterograde axonal transport in rat or chicken dorsal root ganglia sensory neurons are enveloped (Antinone et al, 2010). These findings are consistent with previous observations using PrV (Antinone and Smith, 2006; Feierbach et al, 2007). Moreover, the enveloped HSV1 virions were found to be in close association with markers of the neuronal secretory pathway and to traffic with APP-positive vesicles during egress (Antinone et al, 2010; Cheng et al, 2011). Detailed ultrastructural analysis of HSV1- and PrV-infected primary rat neurons also came to a similar conclusion, finding that the majority of virions were enveloped and found within vesicles (Maresch et al, 2010; Negatsch et al, 2010; Huang et al, 2011). In vitro motility assays have also established that HSV within TGN46-positive organelles are capable of undergoing energy-dependent kinesin-based transport along microtubules (Lee et al, 2006).
After years of debate, it now appears a clearer picture of α herpes virus egress is emerging (Figure 2). Following their exit from the nucleus, non-enveloped capsids recruit kinesin-1 and dynein to undergo transport to their site of envelopment, which may vary depending on the cell type. The ability to recruit both motors and move bidirectionally will help ensure that non-enveloped capsids can reach this site, regardless of its location with respect to the MTOC or microtubule polarity (Figure 2). It seems likely that the non-enveloped capsids engaging in anterograde axonal transport represent virions that are yet to come into contact with the right membrane compartment to undergo envelopment. Ultrastructural analysis suggests that envelopment involves budding into the lumen of a vesicular compartment, which is then transported to the plasma membrane. This immediately raises the question, whether it is a viral or cellular protein that is responsible for recruiting dynein and kinesin-1 to these enveloped capsids. Finally, studies using dominant-negative approaches also point to a role for Myosin Va in promoting release of enveloped virions at the plasma membrane (Roberts and Baines, 2010).
Regulation of kinesin-1 activity and bidirectional transport
During the establishment of their infectious cycles, adenovirus, HSV1 and PrV undergo both retrograde and anterograde directed transport with frequent changes in direction (Suomalainen et al, 1999; Leopold et al, 2000; Smith et al, 2004; Salinas et al, 2009; Antinone and Smith, 2010; Engelke et al, 2011). Similar bidirectional transport is also observed for HSV1 and PrV during their egress (Smith et al, 2001, 2004). This bidirectional transport may help viruses to avoid obstacles on microtubules such as other cargoes and/or ensure they end up at the right cellular location, as suggested for cellular cargoes (Welte, 2004). The existence of bidirectional transport, however, immediately raises the question as to how the net direction of viral transport towards the MTOC or the cell periphery is controlled during entry and egress? Three possible scenarios have been put forward to explain how changes in directionality of cellular cargoes might arise (Welte, 2004; Akhmanova and Hammer, 2010; Verhey et al, 2011). In the first, the cargo would selectively and sequentially bind and/or release either retrograde or anterograde motors. In the second, the cargo simultaneously engages retrograde and anterograde motors that compete in a tug-of-war, with the net direction of travel being defined by the winner. In the third scenario, both motors would be engaged simultaneously but their activity would be coordinated such that competition does not occur.
Several studies have provided evidence that the motility of vesicles both in vitro and in vivo is consistent with a tug-of-war between dynein and kinesin motors, where the competing activities and number of motors define the direction of travel (Muller et al, 2008; Shubeita et al, 2008; Soppina et al, 2009; Akhmanova and Hammer, 2010; Hendricks et al, 2010; Verhey et al, 2011). Other observations have demonstrated that motors of opposite polarity regulate the activity of each other as they are mechanically and/or biochemically coupled (Ally et al, 2009; Gennerich and Vale, 2009; Akhmanova and Hammer, 2010; Encalada et al, 2011). Understanding the nature of this coupling and how the communication between the different motors is regulated is not an easy task. This is in part because most cellular cargoes are relatively heterogeneous and ill defined with respect to their composition as well as the number and activity of their associated motors. Working with viruses may help to overcome some of these difficulties. Viruses have relatively well-defined compositions, many can be genetically manipulated to remove potential motor binding sites and/or introduce fluorescent tags. Virus transport is amenable to quantitative live cell imaging (see references cited throughout review). Viruses can also often be purified to homogeneity to use in motor binding studies and in vitro motility assays (Wolfstein et al, 2006; Bremner et al, 2009; Radtke et al, 2010). As discussed for HSV and PrV, it is, however, essential that the right viral components and cellular markers be used to ensure the unambiguous identification of the viral entity being imaged or purified. In contrast to the majority of cellular cargoes, it has also been possible to detect motors associated with viruses and in the case of vaccinia, to even detect kinesin-1 on moving virus in live cells (Greber and Way, 2006; Figure 3). The ability to image motors associated with viruses raises the possibility to use quantitative imaging approaches to correlate the number of motors with speed and direction of viral transport as well as their rate of exchange with the cytoplasmic pool, as has recently been done for the vaccinia actin tail nucleating complex (Weisswange et al, 2009).
Another important issue that needs to be resolved if we are to understand motor coordination during bidirectional transport is not only the number but also their activity status and arrangement on the cargo during anterograde and retrograde transport (Erickson et al, 2011). The highly localized viral recruitment of motors provides an ideal model system to both develop and employ biosensors that report the activity status of motors to address this fundamental transport question (Figure 3). It also remains to be established whether viruses recruit motors in an inactive or activated state. This issue is important as motors such as kinesin-1 are thought to exist in an inactive closed conformation when they are not associated with cargo (Verhey and Hammond, 2009; Akhmanova and Hammer, 2010; Verhey et al, 2011). Binding of cargo is thought to overcome this autoinhibition, although there is some evidence to suggest that the ability to recruit and activate kinesin-1 may not always reside in a single protein (Blasius et al, 2007; Verhey and Hammond, 2009; Akhmanova and Hammer, 2010). It is possible that some viruses are capable of recruiting inactive motors, which are then activated by virally encoded regulatory proteins that are not directly involved in coupling the virus to the motor. Possible viral activators of kinesin-1 might include US11 and F12 from HSV1 and vaccinia, respectively (Diefenbach et al, 2002; Morgan et al, 2010).
Viral infection frequently results in the dramatic modulation of signalling networks from the moment the virus touches the cell, until new progeny leave. It is highly likely that changes in the activity of signalling networks will impact on virus transport as phosphorylation of kinesin-1 light chain negatively regulates association of the motor with cellular cargoes (Morfini et al, 2002; Du et al, 2010; Amato et al, 2011; Vagnoni et al, 2011). Activation of JNK signalling pathways also promotes the dissociation of kinesin-1 from the light chain binding protein JIP1 (Horiuchi et al, 2007; Verhey and Hammond, 2009; Akhmanova and Hammer, 2010). Phosphorylation of the kinesin-1 heavy chain is also known to inhibit its association with microtubules (Morfini et al, 2009). Phosphorylation of the motor at particular stages during viral replication may represent an efficient way to inhibit kinesin-1 recruitment, modulate its activity and/or promote its release. Consistent with this, adenovirus-mediated activation of c-AMP-dependent protein kinase A and p38/MAPK stimulates nuclear targeting of the virus by enhancing the frequency of retrograde transport possibly by reducing the activity of a plus-end directed motor such as kinesin-1 (Suomalainen et al, 2001). In addition, Src-mediated phosphorylation of A36 has been shown to promote release of kinesin-1 from vaccinia at the plasma membrane (Newsome et al, 2004).
Concluding remarks
The examples we have discussed illustrate the progress in our understanding of how viruses recruit dynein and kinesin-1. They also highlight where our knowledge of motor recruitment is at best patchy, and thus where important future insights are likely to be made. This will include how viral modulation of signalling pathways and accessory proteins regulates both motor recruitment and activity. Live cell imaging of viruses and their associated motors will continue to provide important quantitative information. However, a full molecular understanding will require the development of in vitro motility assays together with detailed biochemical, biophysical and structural analyses of viral proteins/complexes bound to their respective motors. Studying the virus–motor interface promises to provide important insights into the general mechanisms of motor recruitment and regulation by cellular cargoes. Ultimately, it may also help facilitate the development of therapeutic tools that specifically inhibit viral transport rather than those occurring between cellular cargoes and their motors.
Acknowledgments
We would like to thank Greg Smith (Northwestern University, Chicago, USA) and Urs Greber (University of Zurich, Switzerland) as well as Giampietro Schiavo, Helen Walden and the Way laboratory (London Research Institute, Cancer Research UK) for comments and advice. We would also like to thank Herman Favoreel (University of Ghent, Belgium) for discussion on WD/E motifs in PrV.
Footnotes
The authors declare that they have no conflict of interest.
References
- Akhmanova A, Hammer JA III (2010) Linking molecular motors to membrane cargo. Curr Opin Cell Biol 22: 479–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally S, Larson AG, Barlan K, Rice SE, Gelfand VI (2009) Opposite-polarity motors activate one another to trigger cargo transport in live cells. J Cell Biol 187: 1071–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso C, Miskin J, Hernaez B, Fernandez-Zapatero P, Soto L, Canto C, Rodriguez-Crespo I, Dixon L, Escribano JM (2001) African swine fever virus protein p54 interacts with the microtubular motor complex through direct binding to light-chain dynein. J Virol 75: 9819–9827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato S, Liu X, Zheng B, Cantley L, Rakic P, Man HY (2011) AMP-activated protein kinase regulates neuronal polarization by interfering with PI 3-kinase localization. Science 332: 247–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinone SE, Shubeita GT, Coller KE, Lee JI, Haverlock-Moyns S, Gross SP, Smith GA (2006) The Herpesvirus capsid surface protein, VP26, and the majority of the tegument proteins are dispensable for capsid transport toward the nucleus. J Virol 80: 5494–5498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinone SE, Smith GA (2006) Two modes of herpesvirus trafficking in neurons: membrane acquisition directs motion. J Virol 80: 11235–11240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinone SE, Smith GA (2010) Retrograde axon transport of herpes simplex virus and pseudorabies virus: a live-cell comparative analysis. J Virol 84: 1504–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinone SE, Zaichick SV, Smith GA (2010) Resolving the assembly state of herpes simplex virus during axon transport by live-cell imaging. J Virol 84: 13019–13030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao Z, Danappa Jayappa K, Wang B, Zheng Y, Kung S, Rassart E, Depping R, Kohler M, Cohen EA, Yao X (2010) Importin alpha3 interacts with HIV-1 integrase and contributes to HIV-1 nuclear import and replication. J Virol 84: 8650–8663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao Z, Huang G, Yao H, Xu Z, Labine M, Cochrane AW, Yao X (2007) Interaction of human immunodeficiency virus type 1 integrase with cellular nuclear import receptor importin 7 and its impact on viral replication. J Biol Chem 282: 13456–13467 [DOI] [PubMed] [Google Scholar]
- Aoyama T, Hata S, Nakao T, Tanigawa Y, Oka C, Kawaichi M (2009) Cayman ataxia protein caytaxin is transported by kinesin along neurites through binding to kinesin light chains. J Cell Sci 122: 4177–4185 [DOI] [PubMed] [Google Scholar]
- Araki Y, Kawano T, Taru H, Saito Y, Wada S, Miyamoto K, Kobayashi H, Ishikawa HO, Ohsugi Y, Yamamoto T, Matsuno K, Kinjo M, Suzuki T (2007) The novel cargo Alcadein induces vesicle association of kinesin-1 motor components and activates axonal transport. EMBO J 26: 1475–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arhel N (2010) Revisiting HIV-1 uncoating. Retrovirology 7: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arhel NJ, Souquere-Besse S, Munier S, Souque P, Guadagnini S, Rutherford S, Prevost MC, Allen TD, Charneau P (2007) HIV-1 DNA Flap formation promotes uncoating of the pre-integration complex at the nuclear pore. EMBO J 26: 3025–3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbar E (2008) Dynein light chain LC8 is a dimerization hub essential in diverse protein networks. Biochemistry 47: 503–508 [DOI] [PubMed] [Google Scholar]
- Benboudjema L, Mulvey M, Gao Y, Pimplikar SW, Mohr I (2003) Association of the herpes simplex virus type 1 Us11 gene product with the cellular kinesin light-chain-related protein PAT1 results in the redistribution of both polypeptides. J Virol 77: 9192–9203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benison G, Karplus PA, Barbar E (2007) Structure and dynamics of LC8 complexes with KXTQT-motif peptides: swallow and dynein intermediate chain compete for a common site. J Mol Biol 371: 457–468 [DOI] [PubMed] [Google Scholar]
- Benison G, Nyarko A, Barbar E (2006) Heteronuclear NMR identifies a nascent helix in intrinsically disordered dynein intermediate chain: implications for folding and dimerization. J Mol Biol 362: 1082–1093 [DOI] [PubMed] [Google Scholar]
- Blasius TL, Cai D, Jih GT, Toret CP, Verhey KJ (2007) Two binding partners cooperate to activate the molecular motor Kinesin-1. J Cell Biol 176: 11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenburg B, Zhuang X (2007) Virus trafficking—learning from single-virus tracking. Nat Rev Microbiol 5: 197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner KH, Scherer J, Yi J, Vershinin M, Gross SP, Vallee RB (2009) Adenovirus transport via direct interaction of cytoplasmic dynein with the viral capsid hexon subunit. Cell Host Microbe 6: 523–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EM, Dodding MP, Yap MW, Wu X, Gallois-Montbrun S, Malim MH, Stoye JP, Hope TJ (2007) TRIM5 alpha cytoplasmic bodies are highly dynamic structures. Mol Biol Cell 18: 2102–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EM, Perez O, Anderson JL, Hope TJ (2008) Visualization of a proteasome-independent intermediate during restriction of HIV-1 by rhesus TRIM5alpha. J Cell Biol 180: 549–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AP, Cho C, Jin L, Vale RD (2011) Crystal structure of the dynein motor domain. Science 331: 1159–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SB, Ferland P, Webster P, Bearer EL (2011) Herpes simplex virus dances with amyloid precursor protein while exiting the cell. PLoS One 6: e17966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curanovic D, Enquist L (2009) Directional transneuronal spread of alpha-herpesvirus infection. Future Virol 4: 591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Andrea LD, Regan L (2003) TPR proteins: the versatile helix. Trends Biochem Sci 28: 655–662 [DOI] [PubMed] [Google Scholar]
- Desai P, DeLuca NA, Person S (1998) Herpes simplex virus type 1 VP26 is not essential for replication in cell culture but influences production of infectious virus in the nervous system of infected mice. Virology 247: 115–124 [DOI] [PubMed] [Google Scholar]
- Desfarges S, Salin B, Calmels C, Andreola ML, Parissi V, Fournier M (2009) HIV-1 integrase trafficking in S. cerevisiae: a useful model to dissect the microtubule network involvement of viral protein nuclear import. Yeast 26: 39–54 [DOI] [PubMed] [Google Scholar]
- Diaz-Griffero F, Li X, Javanbakht H, Song B, Welikala S, Stremlau M, Sodroski J (2006) Rapid turnover and polyubiquitylation of the retroviral restriction factor TRIM5. Virology 349: 300–315 [DOI] [PubMed] [Google Scholar]
- Diefenbach RJ, Miranda-Saksena M, Diefenbach E, Holland DJ, Boadle RA, Armati PJ, Cunningham AL (2002) Herpes simplex virus tegument protein US11 interacts with conventional kinesin heavy chain. J Virol 76: 3282–3291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbach RJ, Miranda-Saksena M, Douglas MW, Cunningham AL (2008) Transport and egress of herpes simplex virus in neurons. Rev Med Virol 18: 35–51 [DOI] [PubMed] [Google Scholar]
- Doceul V, Hollinshead M, van der Linden L, Smith GL (2010) Repulsion of superinfecting virions: a mechanism for rapid virus spread. Science 327: 873–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodding MP, Newsome TP, Collinson LM, Edwards C, Way M (2009) An E2-F12 complex is required for IEV morphogenesis during vaccinia infection. Cell Microbiol 11: 808–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodding MP, Way M (2009) Nck- and N-WASP-dependent actin-based motility is conserved in divergent vertebrate poxviruses. Cell Host Microbe 6: 536–550 [DOI] [PubMed] [Google Scholar]
- Dohner K, Nagel CH, Sodeik B (2005) Viral stop-and-go along microtubules: taking a ride with dynein and kinesins. Trends Microbiol 13: 320–327 [DOI] [PubMed] [Google Scholar]
- Dohner K, Radtke K, Schmidt S, Sodeik B (2006) Eclipse phase of herpes simplex virus type 1 infection: Efficient dynein-mediated capsid transport without the small capsid protein VP26. J Virol 80: 8211–8224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohner K, Wolfstein A, Prank U, Echeverri C, Dujardin D, Vallee R, Sodeik B (2002) Function of dynein and dynactin in herpes simplex virus capsid transport. Mol Biol Cell 13: 2795–2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domi A, Weisberg AS, Moss B (2008) Vaccinia virus E2L null mutants exhibit a major reduction in extracellular virion formation and virus spread. J Virol 82: 4215–4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas MW, Diefenbach RJ, Homa FL, Miranda-Saksena M, Rixon FJ, Vittone V, Byth K, Cunningham AL (2004) Herpes simplex virus type 1 capsid protein VP26 interacts with dynein light chains RP3 and Tctex1 and plays a role in retrograde cellular transport. J Biol Chem 279: 28522–28530 [DOI] [PubMed] [Google Scholar]
- Du J, Wei Y, Liu L, Wang Y, Khairova R, Blumenthal R, Tragon T, Hunsberger JG, Machado-Vieira R, Drevets W, Wang YT, Manji HK (2010) A kinesin signaling complex mediates the ability of GSK-3beta to affect mood-associated behaviors. Proc Natl Acad Sci USA 107: 11573–11578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encalada SE, Szpankowski L, Xia CH, Goldstein LS (2011) Stable kinesin and dynein assemblies drive the axonal transport of Mammalian prion protein vesicles. Cell 144: 551–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelke MF, Burckhardt CJ, Morf AK, Greber UF (2011) The dynactin complex enhances the speed of microtubule-dependent motions of adenovirus both towards and away from the nucleus. Viruses 3: 233–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson RP, Jia Z, Gross SP, Yu CC (2011) How molecular motors are arranged on a cargo is important for vesicular transport. PLoS Comput Biol 7: e1002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espindola FS, Suter DM, Partata LB, Cao T, Wolenski JS, Cheney RE, King SM, Mooseker MS (2000) The light chain composition of chicken brain myosin-Va: calmodulin, myosin-II essential light chains, and 8-kDa dynein light chain/PIN. Cell Motil Cytoskeleton 47: 269–281 [DOI] [PubMed] [Google Scholar]
- Fassati A, Goff SP (2001) Characterization of intracellular reverse transcription complexes of human immunodeficiency virus type 1. J Virol 75: 3626–3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassati A, Gorlich D, Harrison I, Zaytseva L, Mingot JM (2003) Nuclear import of HIV-1 intracellular reverse transcription complexes is mediated by importin 7. EMBO J 22: 3675–3685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierbach B, Bisher M, Goodhouse J, Enquist LW (2007) In vitro analysis of transneuronal spread of an alpha herpesvirus infection in peripheral nervous system neurons. J Virol 81: 6846–6857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin L, Becker KA, Lambert C, Nowak T, Sapp C, Strand D, Streeck RE, Sapp M (2006) Identification of a dynein interacting domain in the papillomavirus minor capsid protein l2. J Virol 80: 6691–6696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganser-Pornillos BK, Chandrasekaran V, Pornillos O, Sodroski JG, Sundquist WI, Yeager M (2011) Hexagonal assembly of a restricting TRIM5alpha protein. Proc Natl Acad Sci USA 108: 534–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata R, Gao YS, Sztul E (2002) Hassles with taking out the garbage: aggravating aggresomes. Traffic 3: 388–396 [DOI] [PubMed] [Google Scholar]
- Gazzola M, Burckhardt CJ, Bayati B, Engelke M, Greber UF, Koumoutsakos P (2009) A stochastic model for microtubule motors describes the in vivo cytoplasmic transport of human adenovirus. PLoS Comput Biol 5: e1000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geada MM, Galindo I, Lorenzo MM, Perdiguero B, Blasco R (2001) Movements of vaccinia virus intracellular enveloped virions with GFP tagged to the F13L envelope protein. J Gen Virol 82: 2747–2760 [DOI] [PubMed] [Google Scholar]
- Gennerich A, Vale RD (2009) Walking the walk: how kinesin and dynein coordinate their steps. Curr Opin Cell Biol 21: 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gindhart JG (2006) Towards an understanding of kinesin-1 dependent transport pathways through the study of protein-protein interactions. Brief Funct Genomic Proteomic 5: 74–86 [DOI] [PubMed] [Google Scholar]
- Granzow H, Klupp BG, Mettenleiter TC (2005) Entry of pseudorabies virus: an immunogold-labeling study. J Virol 79: 3200–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber UF, Way M (2006) A superhighway to virus infection. Cell 124: 741–754 [DOI] [PubMed] [Google Scholar]
- Gyoeva FK, Bybikova EM, Minin AA (2000) An isoform of kinesin light chain specific for the Golgi complex. J Cell Sci 113: 2047–2054 [DOI] [PubMed] [Google Scholar]
- Gyoeva FK, Sarkisov DV, Khodjakov AL, Minin AA (2004) The tetrameric molecule of conventional kinesin contains identical light chains. Biochemistry 43: 13525–13531 [DOI] [PubMed] [Google Scholar]
- Hammond JW, Griffin K, Jih GT, Stuckey J, Verhey KJ (2008) Co-operative versus independent transport of different cargoes by Kinesin-1. Traffic 9: 725–741 [DOI] [PubMed] [Google Scholar]
- Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J, Twiss JL, Fainzilber M (2003) Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron 40: 1095–1104 [DOI] [PubMed] [Google Scholar]
- Hearps AC, Jans DA (2006) HIV-1 integrase is capable of targeting DNA to the nucleus via an importin alpha/beta-dependent mechanism. Biochem J 398: 475–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks AG, Perlson E, Ross JL, Schroeder HW III, Tokito M, Holzbaur EL (2010) Motor coordination via a Tug-of-War mechanism drives bidirectional vesicle transport. Curr Biol 20: 697–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Noda Y, Tanaka Y, Niwa S (2009) Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol 10: 682–696 [DOI] [PubMed] [Google Scholar]
- Hollinshead M, Rodger G, Van Eijl H, Law M, Hollinshead R, Vaux DJ, Smith GL (2001) Vaccinia virus utilizes microtubules for movement to the cell surface. J Cell Biol 154: 389–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook P, Vallee RB (2006) The dynein family at a glance. J Cell Sci 119: 4369–4371 [DOI] [PubMed] [Google Scholar]
- Horiuchi D, Collins CA, Bhat P, Barkus RV, Diantonio A, Saxton WM (2007) Control of a kinesin-cargo linkage mechanism by JNK pathway kinases. Curr Biol 17: 1313–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Lazear HM, Friedman HM (2011) Completely assembled virus particles detected by transmission electron microscopy in proximal and mid-axons of neurons infected with herpes simplex virus type 1, herpes simplex virus type 2 and pseudorabies virus. Virology 409: 12–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob Y, Badrane H, Ceccaldi PE, Tordo N (2000) Cytoplasmic dynein LC8 interacts with lyssavirus phosphoprotein. J Virol 74: 10217–10222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeshtadi A, Burgos P, Stubbs CD, Parker AW, King LA, Skinner MA, Botchway SW (2010) Interaction of poxvirus intracellular mature virion proteins with the TPR domain of kinesin light chain in live infected cells revealed by two-photon-induced fluorescence resonance energy transfer fluorescence lifetime imaging microscopy. J Virol 84: 12886–12894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JA, Illing ME, Kopito RR (2002) Cytoplasmic dynein/dynactin mediates the assembly of aggresomes. Cell Motil Cytoskeleton 53: 26–38 [DOI] [PubMed] [Google Scholar]
- Johnston SC, Ward BM (2008) The Vaccinia virus protein F12 associates with IEV through an interaction with A36. J Virol 83: 1708–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser FJ, Tavassoli K, Van den Bemd GJ, Chang GT, Horsthemke B, Moroy T, Ludecke HJ (2003) Nuclear interaction of the dynein light chain LC8a with the TRPS1 transcription factor suppresses the transcriptional repression activity of TRPS1. Hum Mol Genet 12: 1349–1358 [DOI] [PubMed] [Google Scholar]
- Kardon JR, Vale RD (2009) Regulators of the cytoplasmic dynein motor. Nat Rev Mol Cell Biol 10: 854–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelkar SA, Pfister KK, Crystal RG, Leopold PL (2004) Cytoplasmic dynein mediates adenovirus binding to microtubules. J Virol 78: 10122–10132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon T, Sutoh K, Kurisu G (2011) X-ray structure of a functional full-length dynein motor domain. Nat Struct Mol Biol 18: 638–642 [DOI] [PubMed] [Google Scholar]
- Konecna A, Frischknecht R, Kinter J, Ludwig A, Steuble M, Meskenaite V, Indermuhle M, Engel M, Cen C, Mateos JM, Streit P, Sonderegger P (2006) Calsyntenin-1 docks vesicular cargo to kinesin-1. Mol Biol Cell 17: 3651–3663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krautwald M, Fuchs W, Klupp BG, Mettenleiter TC (2009) Translocation of incoming pseudorabies virus capsids to the cell nucleus is delayed in the absence of tegument protein pUL37. J Virol 83: 3389–3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T, Matsuoka M, Chang TH, Bray M, Jones S, Tashiro M, Kato A, Ozato K (2009) Ebolavirus VP35 interacts with the cytoplasmic dynein light chain 8. J Virol 83: 6952–6956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakadamyali M, Rust MJ, Babcock HP, Zhuang X (2003) Visualizing infection of individual influenza viruses. Proc Natl Acad Sci USA 100: 9280–9285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langelier CR, Sandrin V, Eckert DM, Christensen DE, Chandrasekaran V, Alam SL, Aiken C, Olsen JC, Kar AK, Sodroski JG, Sundquist WI (2008) Biochemical characterization of a recombinant TRIM5alpha protein that restricts human immunodeficiency virus type 1 replication. J Virol 82: 11682–11694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GE, Murray JW, Wolkoff AW, Wilson DW (2006) Reconstitution of herpes simplex virus microtubule-dependent trafficking in vitro. J Virol 80: 4264–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold PL, Kreitzer G, Miyazawa N, Rempel S, Pfister KK, Rodriguez-Boulan E, Crystal RG (2000) Dynein- and microtubule-mediated translocation of adenovirus serotype 5 occurs after endosomal lysis. Hum Gene Ther 11: 151–165 [DOI] [PubMed] [Google Scholar]
- Luxton GW, Haverlock S, Coller KE, Antinone SE, Pincetic A, Smith GA (2005) Targeting of herpesvirus capsid transport in axons is coupled to association with specific sets of tegument proteins. Proc Natl Acad Sci USA 102: 5832–5837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxton GW, Lee JI, Haverlock-Moyns S, Schober JM, Smith GA (2006) The pseudorabies virus VP1/2 tegument protein is required for intracellular capsid transport. J Virol 80: 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman MG, Enquist LW (2009) Herpesvirus interactions with the host cytoskeleton. J Virol 83: 2058–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabit H, Nakano MY, Prank U, Saam B, Dohner K, Sodeik B, Greber UF (2002) Intact microtubules support adenovirus and herpes simplex virus infections. J Virol 76: 9962–9971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresch C, Granzow H, Negatsch A, Klupp BG, Fuchs W, Teifke JP, Mettenleiter TC (2010) Ultrastructural analysis of virion formation and anterograde intraaxonal transport of the alphaherpesvirus pseudorabies virus in primary neurons. J Virol 84: 5528–5539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Moreno M, Navarro-Lerida I, Roncal F, Albar JP, Alonso C, Gavilanes F, Rodriguez-Crespo I (2003) Recognition of novel viral sequences that associate with the dynein light chain LC8 identified through a pepscan technique. FEBS Lett 544: 262–267 [DOI] [PubMed] [Google Scholar]
- Maurer UE, Sodeik B, Grunewald K (2008) Native 3D intermediates of membrane fusion in herpes simplex virus 1 entry. Proc Natl Acad Sci USA 105: 10559–10564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCart AE, Mahony D, Rothnagel JA (2003) Alternatively spliced products of the human kinesin light chain 1 (KNS2) gene. Traffic 4: 576–580 [DOI] [PubMed] [Google Scholar]
- McDonald D, Vodicka MA, Lucero G, Svitkina TM, Borisy GG, Emerman M, Hope TJ (2002) Visualization of the intracellular behavior of HIV in living cells. J Cell Biol 159: 441–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meroni G, Diez-Roux G (2005) TRIM/RBCC, a novel class of ‘single protein RING finger’ E3 ubiquitin ligases. Bioessays 27: 1147–1157 [DOI] [PubMed] [Google Scholar]
- Miranda-Saksena M, Boadle RA, Aggarwal A, Tijono B, Rixon FJ, Diefenbach RJ, Cunningham AL (2009) Herpes simplex virus utilizes the large secretory vesicle pathway for anterograde transport of tegument and envelope proteins and for viral exocytosis from growth cones of human fetal axons. J Virol 83: 3187–3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G, Szebenyi G, Elluru R, Ratner N, Brady ST (2002) Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. EMBO J 21: 281–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini GA, You YM, Pollema SL, Kaminska A, Liu K, Yoshioka K, Bjorkblom B, Coffey ET, Bagnato C, Han D, Huang CF, Banker G, Pigino G, Brady ST (2009) Pathogenic huntingtin inhibits fast axonal transport by activating JNK3 and phosphorylating kinesin. Nat Neurosci 12: 864–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan GW, Hollinshead M, Ferguson BJ, Murphy BJ, Carpentier DC, Smith GL (2010) Vaccinia protein F12 has structural similarity to kinesin light chain and contains a motor binding motif required for virion export. PLoS Pathog 6: e1000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley GW, Roth DM, DeJesus MA, Leyton DL, Filmer RP, Pouton CW, Jans DA (2007) Dynein light chain association sequences can facilitate nuclear protein import. Mol Biol Cell 18: 3204–3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MJ, Klumpp S, Lipowsky R (2008) Tug-of-war as a cooperative mechanism for bidirectional cargo transport by molecular motors. Proc Natl Acad Sci USA 105: 4609–4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negatsch A, Granzow H, Maresch C, Klupp BG, Fuchs W, Teifke JP, Mettenleiter TC (2010) Ultrastructural analysis of virion formation and intraaxonal transport of herpes simplex virus type 1 in primary rat neurons. J Virol 84: 13031–13035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome TP, Scaplehorn N, Way M (2004) SRC mediates a switch from microtubule- to actin-based motility of vaccinia virus. Science 306: 124–129 [DOI] [PubMed] [Google Scholar]
- Pertel T, Hausmann S, Morger D, Zuger S, Guerra J, Lascano J, Reinhard C, Santoni FA, Uchil PD, Chatel L, Bisiaux A, Albert ML, Strambio-De-Castillia C, Mothes W, Pizzato M, Grutter MG, Luban J (2011) TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature 472: 361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit C, Giron ML, Tobaly-Tapiero J, Bittoun P, Real E, Jacob Y, Tordo N, De The H, Saib A (2003) Targeting of incoming retroviral Gag to the centrosome involves a direct interaction with the dynein light chain 8. J Cell Sci 116: 3433–3442 [DOI] [PubMed] [Google Scholar]
- Pfister KK, Fisher EM, Gibbons IR, Hays TS, Holzbaur EL, McIntosh JR, Porter ME, Schroer TA, Vaughan KT, Witman GB, King SM, Vallee RB (2005) Cytoplasmic dynein nomenclature. J Cell Biol 171: 411–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisson N, Real E, Gaudin Y, Vaney MC, King S, Jacob Y, Tordo N, Blondel D (2001) Molecular basis for the interaction between rabies virus phosphoprotein P and the dynein light chain LC8: dissociation of dynein-binding properties and transcriptional functionality of P. J Gen Virol 82: 2691–2696 [DOI] [PubMed] [Google Scholar]
- Radnai L, Rapali P, Hodi Z, Suveges D, Molnar T, Kiss B, Becsi B, Erdodi F, Buday L, Kardos J, Kovacs M, Nyitray L (2010) Affinity, avidity, and kinetics of target sequence binding to LC8 dynein light chain isoforms. J Biol Chem 285: 38649–38657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke K, Dohner K, Sodeik B (2006) Viral interactions with the cytoskeleton: a hitchhiker's guide to the cell. Cell Microbiol 8: 387–400 [DOI] [PubMed] [Google Scholar]
- Radtke K, Kieneke D, Wolfstein A, Michael K, Steffen W, Scholz T, Karger A, Sodeik B (2010) Plus- and minus-end directed microtubule motors bind simultaneously to herpes simplex virus capsids using different inner tegument structures. PLoS Pathog 6: e1000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raux H, Flamand A, Blondel D (2000) Interaction of the rabies virus P protein with the LC8 dynein light chain. J Virol 74: 10212–10216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietdorf J, Ploubidou A, Reckmann I, Holmstrom A, Frischknecht F, Zettl M, Zimmermann T, Way M (2001) Kinesin-dependent movement on microtubules precedes actin-based motility of vaccinia virus. Nat Cell Biol 3: 992–1000 [DOI] [PubMed] [Google Scholar]
- Roberts KL, Baines JD (2010) Myosin Va enhances secretion of herpes simplex virus 1 virions and cell surface expression of viral glycoproteins. J Virol 84: 9889–9896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts KL, Smith GL (2008) Vaccinia virus morphogenesis and dissemination. Trends Microbiol 10: 472–479 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Crespo I, Yelamos B, Roncal F, Albar JP, Ortiz de Montellano PR, Gavilanes F (2001) Identification of novel cellular proteins that bind to the LC8 dynein light chain using a pepscan technique. FEBS Lett 503: 135–141 [DOI] [PubMed] [Google Scholar]
- Saksena MM, Wakisaka H, Tijono B, Boadle RA, Rixon F, Takahashi H, Cunningham AL (2006) Herpes simplex virus type 1 accumulation, envelopment, and exit in growth cones and varicosities in mid-distal regions of axons. J Virol 80: 3592–3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas S, Bilsland LG, Henaff D, Weston AE, Keriel A, Schiavo G, Kremer EJ (2009) CAR-associated vesicular transport of an adenovirus in motor neuron axons. PLoS Pathog 5: e1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepis A, Stauber T, Krijnse Locker J (2007) Kinesin-1 plays multiple roles during the vaccinia virus life cycle. Cell Microbiol 9: 1960–1973 [DOI] [PubMed] [Google Scholar]
- Schmidt MR, Maritzen T, Kukhtina V, Higman VA, Doglio L, Barak NN, Strauss H, Oschkinat H, Dotti CG, Haucke V (2009) Regulation of endosomal membrane traffic by a Gadkin/AP-1/kinesin KIF5 complex. Proc Natl Acad Sci USA 106: 15344–15349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MA, Spoden GA, Florin L, Lambert C (2011) Identification of the dynein light chains required for human papillomavirus infection. Cell Microbiol 13: 32–46 [DOI] [PubMed] [Google Scholar]
- Schroer TA (2004) Dynactin. Annu Rev Cell Dev Biol 20: 759–779 [DOI] [PubMed] [Google Scholar]
- Sebastian S, Luban J (2005) TRIM5alpha selectively binds a restriction-sensitive retroviral capsid. Retrovirology 2: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seisenberger G, Ried MU, Endress T, Buning H, Hallek M, Brauchle C (2001) Real-time single-molecule imaging of the infection pathway of an adeno-associated virus. Science 294: 1929–1932 [DOI] [PubMed] [Google Scholar]
- Shubeita GT, Tran SL, Xu J, Vershinin M, Cermelli S, Cotton SL, Welte MA, Gross SP (2008) Consequences of motor copy number on the intracellular transport of kinesin-1-driven lipid droplets. Cell 135: 1098–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GA, Gross SP, Enquist LW (2001) Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc Natl Acad Sci USA 98: 3466–3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GA, Pomeranz L, Gross SP, Enquist LW (2004) Local modulation of plus-end transport targets herpesvirus entry and egress in sensory axons. Proc Natl Acad Sci USA 101: 16034–16039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GL, Vanderplasschen A, Law M (2002) The formation and function of extracellular enveloped vaccinia virus. J Gen Virol 83: 2915–2931 [DOI] [PubMed] [Google Scholar]
- Snyder A, Wisner TW, Johnson DC (2006) Herpes simplex virus capsids are transported in neuronal axons without an envelope containing the viral glycoproteins. J Virol 80: 11165–11177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodeik B, Ebersold MW, Helenius A (1997) Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J Cell Biol 136: 1007–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soppina V, Rai AK, Ramaiya AJ, Barak P, Mallik R (2009) Tug-of-war between dissimilar teams of microtubule motors regulates transport and fission of endosomes. Proc Natl Acad Sci USA 106: 19381–19386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelter P, Kunze R, Flemming D, Hopfner D, Diepholz M, Philippsen P, Bottcher B, Hurt E (2007) Molecular basis for the functional interaction of dynein light chain with the nuclear-pore complex. Nat Cell Biol 9: 788–796 [DOI] [PubMed] [Google Scholar]
- Stremlau M, Perron M, Lee M, Li Y, Song B, Javanbakht H, Diaz-Griffero F, Anderson DJ, Sundquist WI, Sodroski J (2006) Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5alpha restriction factor. Proc Natl Acad Sci USA 103: 5514–5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen M, Nakano MY, Boucke K, Keller S, Greber UF (2001) Adenovirus-activated PKA and p38/MAPK pathways boost microtubule-mediated nuclear targeting of virus. EMBO J 20: 1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen M, Nakano MY, Keller S, Boucke K, Stidwill RP, Greber UF (1999) Microtubule-dependent plus- and minus end-directed motilities are competing processes for nuclear targeting of adenovirus. J Cell Biol 144: 657–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan GS, Preuss MA, Williams JC, Schnell MJ (2007) The dynein light chain 8 binding motif of rabies virus phosphoprotein promotes efficient viral transcription. Proc Natl Acad Sci USA 104: 7229–7234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SC, Scherer J, Vallee RB (2011) Recruitment of dynein to late endosomes and lysosomes through light intermediate chains. Mol Biol Cell 22: 467–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchil PD, Quinlan BD, Chan WT, Luna JM, Mothes W (2008) TRIM E3 ligases interfere with early and late stages of the retroviral life cycle. PLoS Pathog 4: e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnoni A, Rodriguez L, Manser C, De Vos KJ, Miller CC (2011) Phosphorylation of kinesin light chain 1 at serine 460 modulates binding and trafficking of calsyntenin-1. J Cell Sci 124: 1032–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eijl H, Hollinshead M, Rodger G, Zhang WH, Smith GL (2002) The vaccinia virus F12L protein is associated with intracellular enveloped virus particles and is required for their egress to the cell surface. J Gen Virol 83: 195–207 [DOI] [PubMed] [Google Scholar]
- Verhey KJ, Hammond JW (2009) Traffic control: regulation of kinesin motors. Nat Rev Mol Cell Biol 10: 765–777 [DOI] [PubMed] [Google Scholar]
- Verhey KJ, Kaul N, Soppina V (2011) Kinesin assembly and movement in cells. Annu Rev Biophys 40: 267–288 [DOI] [PubMed] [Google Scholar]
- Wagstaff KM, Jans DA (2009) Importins and beyond: non-conventional nuclear transport mechanisms. Traffic 10: 1188–1198 [DOI] [PubMed] [Google Scholar]
- Ward BM (2011) The taking of the cytoskeleton one two three: how viruses utilize the cytoskeleton during egress. Virology 411: 244–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BM, Moss B (2001a) Vaccinia virus intracellular movement is associated with microtubules and independent of actin tails. J Virol 75: 11651–11663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BM, Moss B (2001b) Visualization of intracellular movement of vaccinia virus virions containing a green fluorescent protein-B5R membrane protein chimera. J Virol 75: 4802–4813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BM, Moss B (2004) Vaccinia virus A36R membrane protein provides a direct link between intracellular enveloped virions and the microtubule motor kinesin. J Virol 78: 2486–2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BM, Weisberg AS, Moss B (2003) Mapping and functional analysis of interaction sites within the cytoplasmic domains of the vaccinia virus A33R and A36R envelope proteins. J Virol 77: 4113–4126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei BL, Denton PW, O’Neill E, Luo T, Foster JL, Garcia JV (2005) Inhibition of lysosome and proteasome function enhances human immunodeficiency virus type 1 infection. J Virol 79: 5705–5712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisswange I, Newsome TP, Schleich S, Way M (2009) The rate of N-WASP exchange limits the extent of ARP2/3-complex-dependent actin-based motility. Nature 458: 87–91 [DOI] [PubMed] [Google Scholar]
- Welte MA (2004) Bidirectional transport along microtubules. Curr Biol 14: R525–R537 [DOI] [PubMed] [Google Scholar]
- Wileman T (2007) Aggresomes and pericentriolar sites of virus assembly: cellular defense or viral design? Annu Rev Microbiol 61: 149–167 [DOI] [PubMed] [Google Scholar]
- Willard M (2002) Rapid directional translocations in virus replication. J Virol 76: 5220–5232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JC, Roulhac PL, Roy AG, Vallee RB, Fitzgerald MC, Hendrickson WA (2007) Structural and thermodynamic characterization of a cytoplasmic dynein light chain-intermediate chain complex. Proc Natl Acad Sci USA 104: 10028–10033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner TW, Sugimoto K, Howard PW, Kawaguchi Y, Johnson DC (2011) Anterograde transport of herpes simplex virus capsids in neurons by both Separate and Married mechanisms. J Virol 85: 5919–5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfstein A, Nagel CH, Radtke K, Dohner K, Allan VJ, Sodeik B (2006) The inner tegument promotes herpes simplex virus capsid motility along microtubules in vitro. Traffic 7: 227–237 [DOI] [PubMed] [Google Scholar]
- Wozniak MJ, Allan VJ (2006) Cargo selection by specific kinesin light chain 1 isoforms. EMBO J 25: 5457–5468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Maciejewski MW, Takebe S, King SM (2005) Solution structure of the Tctex1 dimer reveals a mechanism for dynein-cargo interactions. Structure 13: 213–223 [DOI] [PubMed] [Google Scholar]
- Wu X, Anderson JL, Campbell EM, Joseph AM, Hope TJ (2006) Proteasome inhibitors uncouple rhesus TRIM5alpha restriction of HIV-1 reverse transcription and infection. Proc Natl Acad Sci USA 103: 7465–7470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye GJ, Vaughan KT, Vallee RB, Roizman B (2000) The herpes simplex virus 1 U(L)34 protein interacts with a cytoplasmic dynein intermediate chain and targets nuclear membrane. J Virol 74: 1355–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudin D, Hanz S, Yoo S, Iavnilovitch E, Willis D, Gradus T, Vuppalanchi D, Segal-Ruder Y, Ben-Yaakov K, Hieda M, Yoneda Y, Twiss JL, Fainzilber M (2008) Localized regulation of axonal RanGTPase controls retrograde injury signaling in peripheral nerve. Neuron 59: 241–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitseva L, Cherepanov P, Leyens L, Wilson SJ, Rasaiyaah J, Fassati A (2009) HIV-1 exploits importin 7 to maximize nuclear import of its DNA genome. Retrovirology 6: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WH, Wilcock D, Smith GL (2000) Vaccinia virus F12L protein is required for actin tail formation, normal plaque size, and virulence. J Virol 74: 11654–11662 [DOI] [PMC free article] [PubMed] [Google Scholar]