Summary
Purpose
To facilitate studies of medications and sudden cardiac death, we developed and validated a computer case definition for these deaths. The study of community dwelling Tennessee Medicaid enrollees 30–74 years of age utilized a linked database with Medicaid inpatient/outpatient files, state death certificate files, and a state "all-payers" hospital discharge file.
Methods
The computerized case definition was developed from a retrospective cohort study of sudden cardiac deaths occurring between 1990 and 1993. Medical records for 926 potential cases had been adjudicated for this study to determine if they met the clinical definition for sudden cardiac death occurring in the community and were likely to be due to ventricular tachyarrhythmias. The computerized case definition included deaths with 1) no evidence of a terminal hospital admission/nursing home stay in any of the data sources; 2) an underlying cause of death code consistent with sudden cardiac death; and 3) no terminal procedures inconsistent with unresuscitated cardiac arrest. This definition was validated in an independent sample of 174 adjudicated deaths occurring between 1994 and 2005.
Results
The positive predictive value of the computer case definition was 86.0% in the development sample and 86.8% in the validation sample. The positive predictive value did not vary materially for deaths coded according to the IDC-9 (1994–1998, positive predictive value = 85.1%) or ICD-10 (1999–2005, 87.4%) systems.
Conclusion
A computerized Medicaid database, linked with death certificate files and a state hospital discharge database, can be used for a computer case definition of sudden cardiac death.
Introduction
Each year, there are as many as 400,000 sudden cardiac deaths in the U.S.1 An estimated 85–90% are caused by ventricular tachyarrhythmias,2 which in turn result from the interplay of host risk factors such as structural heart disease with transient factors such as drugs. 2–4 There is a long-standing concern that medications are an important risk factor because some have effects that can provoke lethal arrhythmias.5,6 Hence, an important strategy for prevention is identifying medications that increase the risk of sudden cardiac death in order to optimize pharmacotherapy.
Automated databases of medical care encounters for defined populations are a potentially valuable resource for the study of medications and sudden cardiac death. These databases include records of prescriptions written by clinicians, filled by patients or administered in institutions. These provide a reliable measure of drug exposure that would be difficult or very expensive to obtain in other ways.7 The prescription records permit tracking of changes in drug exposure on a day-to-day basis, which is important because many of the proposed mechanisms that may underlie medication-induced arrhythmias are acute. The quality of information from these automated records should be no different for deceased persons, thus overcoming one of the limitations of interview-based methods for study of decedents. Thus, such databases have been used extensively in the study of medications and sudden cardiac death.8–13
A major logistic challenge with automated databases is the identification of the cases of sudden cardiac death. One approach is to identify all deaths in the study cohorts from linked death certificates, exclude those highly unlikely to be sudden cardiac deaths, and review all available medical records for the remaining deaths. 8–10 However, this process is time-consuming and labor-intensive, particularly since most sudden cardiac deaths of interest in medication studies occur outside of the hospital (although many of these patients may present at the emergency department), the most convenient source of medical records. Because other types of records, such as those from emergency medical services, may be difficult to obtain or have limited information from which to determine case status, investigators may be unable to adjudicate a substantial proportion of potential cases, thus potentially introducing selection bias.
We thus utilized data from previous studies of sudden cardiac death to develop a definition of sudden cardiac death that is suitable for use in automated databases linked with computerized death certificates. We report here its validation in a Tennessee Medicaid population relative to a definition based upon review of medical records.
Methods
Design and Sources of Data
The computer case definition is based upon information from two large retrospective cohort studies of medications and sudden cardiac deaths. Our initial study, the development study, identified a broad spectrum of potential sudden cardiac deaths and sought to confirm each of these by medical record review. In order to more efficiently conduct a series of subsequent cohort studies of more recently marketed medications, we utilized data from the development study to construct a computer case definition for sudden cardiac death. The validation study utilized this definition to identify sudden cardiac deaths and included review of medical records for a sample of the deaths to confirm its positive predictive value.
Both studies utilized computerized files from the Tennessee Medicaid program,14 including an enrollment file as well as files recording prescriptions filled at a pharmacy, hospital admissions, outpatient visits, and long-term care residence. The Medicaid files have been augmented by linkage with computerized death certificates15 and, since 1998, with the State Hospital Discharge File, an "all payers" database of hospital discharges and emergency department visits, which provides information occasionally missing from Medicaid files. These files permitted identification of study populations, tracking of current use of study medications, classification of subjects according to cardiovascular risk, and ascertainment of potential sudden cardiac deaths.7,14
Cohorts
Development study
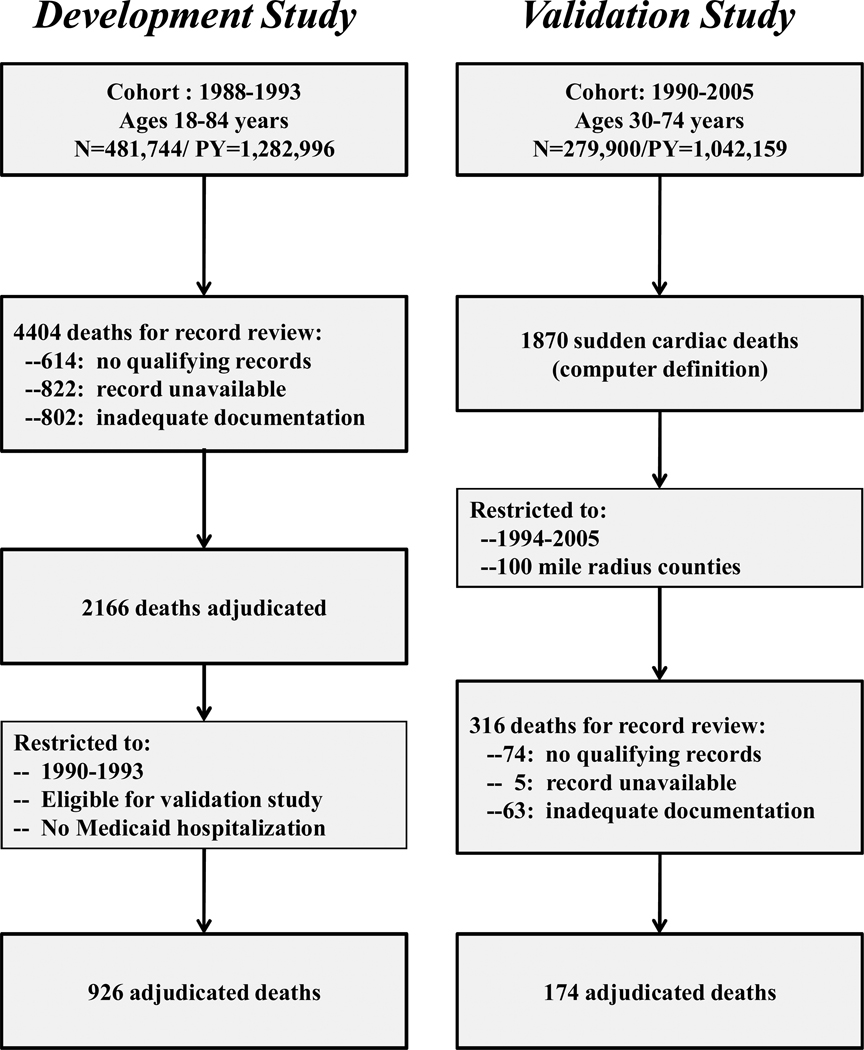
The development study cohort was assembled to study several commonly used medications suspected of increasing the risk of sudden cardiac death.8–10 It included all qualifying Medicaid enrollees between 1988 and 1993 who were 18–84 years of age, had ≥365 days of continuous Medicaid enrollment, were not in a nursing home in the past 365 days, and had no evidence of a life-threatening, non-cardiac illness (chronic renal failure, chronic liver disease, metastatic or other cancer with very poor prognosis, severe chronic obstructive pulmonary disease, or HIV infection). Study followup began on the first day the criteria for cohort membership were met and ended on the first of: 12/31/1993, the date of death, or whenever the criteria for cohort membership no longer were met. Person-time during hospitalization and the 30 days following hospital discharge was not included in the follow-up, because in-hospital deaths were not considered study endpoints and Medicaid files do not record medications dispensed in the hospital. The cohort ultimately included 481,744 persons with 1,282,996 person-years of followup (Figure 1).
Figure 1.
Identification of potential sudden cardiac deaths that were subsequently adjudicated by medical record review and used both to develop and validate a computer case definition for sudden cardiac death. This includes both the development study, the deaths from which were used to construct the computer case definition, and the validation study, the deaths from which were used to validate the definition derived from the development study
Validation study
The validation study cohort was assembled to study antipsychotics and sudden cardiac death and thus consisted of antipsychotic users and matched nonuser controls.11 The cohort included qualifying Medicaid enrollees between 1990 and 2005. It was restricted to persons 30 to 74 years of age because for younger persons sudden cardiac death is very rare and may have a different etiology,16 and for older persons we found death certificates to be less reliable for identifying sudden cardiac deaths (unpublished data). Cohort membership required 730 days of prior enrollment (gaps < 7 days allowed) with full pharmacy benefits and evidence of regular use of medical care, defined as at least one filled prescription and outpatient encounter in each of the two preceding 365 day periods. The cohort excluded patients: in the nursing home (except for stays <30 days following hospital discharge), hospitalized in the past 30 days, with a serious non-cardiovascular illnesses (cancer other than non-melanoma skin cancer, HIV, renal failure, liver disease, respiratory failure, organ transplantation, multiple sclerosis, home oxygen excluding CPAP, or hospice care) or with diagnosed recreational drug dependency. Followup extended through the first of: the end of the study, death, loss of Medicaid enrollment, or whenever the cohort eligibility criteria were no longer met. Followup person-time excluded hospitalization and the subsequent 30 days. The cohort thus included 93,300 baseline antipsychotic users and 186,600 nonuser controls with 1,042,159 person-years of cohort followup (Figure 1).
Sudden Cardiac Death
Clinical definition
The study endpoint was sudden cardiac death occurring in a community setting.17–20 This was defined as a sudden pulseless condition (arrest) that was immediately fatal (or rarely resuscitated with death in 48 hours) and was consistent with a ventricular tachyarrhythmia occurring in the absence of a known noncardiac condition as the proximate cause of the death.19 Sudden cardiac deaths were classified as either probable or possible. Probable sudden cardiac deaths included a witnessed, sudden collapse with no pulse and respiration (or agonal), or an unwitnessed collapse in a person known to be alive within the previous hour, or ventricular fibrillation/tachycardia prior to start of cardiopulmonary resuscitation, or autopsy findings excluding causes other than a ventricular tachyarrhythmia. Possible sudden cardiac deaths were those in which no arrest was witnessed and the person was found moribund or dead, but with evidence that the subject had been alive in the preceding 24 hours. Both definitions excluded deaths from arrests that occurred in a hospital or other institutional setting (this did not exclude patients for whom the arrest occurred in the community and were pronounced dead in or died shortly after arrival to the emergency department), that were not sudden, or with documentation suggesting an underlying noncardiac cause (e.g., substance overdose or pneumonia) or a different cardiac etiology (e.g., heart failure or bradyarrhythmia).
Development study: medical record review sample
In the development study, potential cases of sudden cardiac death were identified from deaths with a terminal diagnosis code consistent with sudden cardiac death. 8–10 These were hypertensive heart disease, excluding malignant hypertension (ICD-9 codes 401–405, excluding 401.0,402.0, 403.0), ischemic heart disease, excluding aneurysms (410–414, excluding 414.1), cardiomyopathy (425), conduction disorders (426), dysrrhythmias (427), myocarditis or cardiomegaly (429.0,429.1,429.3), atherosclerotic cardiovascular disease (429.2), acute, but ill defined, cerebrovascular disease (436), heart disease, not otherwise specified (429.9), atherosclerosis not otherwise specified (440.9), sudden death (798.0–798.9), unknown cause of death (799.9), diabetes, uncomplicated (250.0), and heart failure (428). The terminal diagnosis could be either an underlying cause of death code or the primary diagnosis from a terminal Medicaid hospital stay of no more than one day or an emergency department visit (diagnoses for the latter did not include diabetes or heart failure). For hospital/emergency department diagnoses, there could be no secondary diagnoses indicating a non-cardiac cause of death.
Development study: record retrieval and adjudication
Study nurses sought to abstract the records of medical care encounters (hospital, emergency department, emergency medical services) around the time of death as well as autopsy reports when available. Of 4404 potential cases of sudden cardiac death identified during followup, informative medical records were obtained for 2166 (Figure 1). The medical record abstracts were adjudicated by a study physician, masked to medication use; questionable cases were reviewed by a similarly masked cardiac electrophysiologist.
Development study: sample for computer case definition
The computer case definition was based upon the adjudicated deaths from the development study. Our ultimate objective was to use this definition in the cohort from which the validation sample was drawn. Thus, prior to developing the computer case definition, the deaths for the development study sample were restricted to those that occurred during the time period for the validation study (1990–1993, Figure 1) and were for persons eligible for that study. We also excluded deaths with a terminal Medicaid hospital admission record (does not exclude emergency department visits), because these deaths, even for short stays, nearly always were in-hospital deaths that did not meet our case definition. After applying these exclusions, the development sample had 926 adjudicated deaths.
Computer case definition
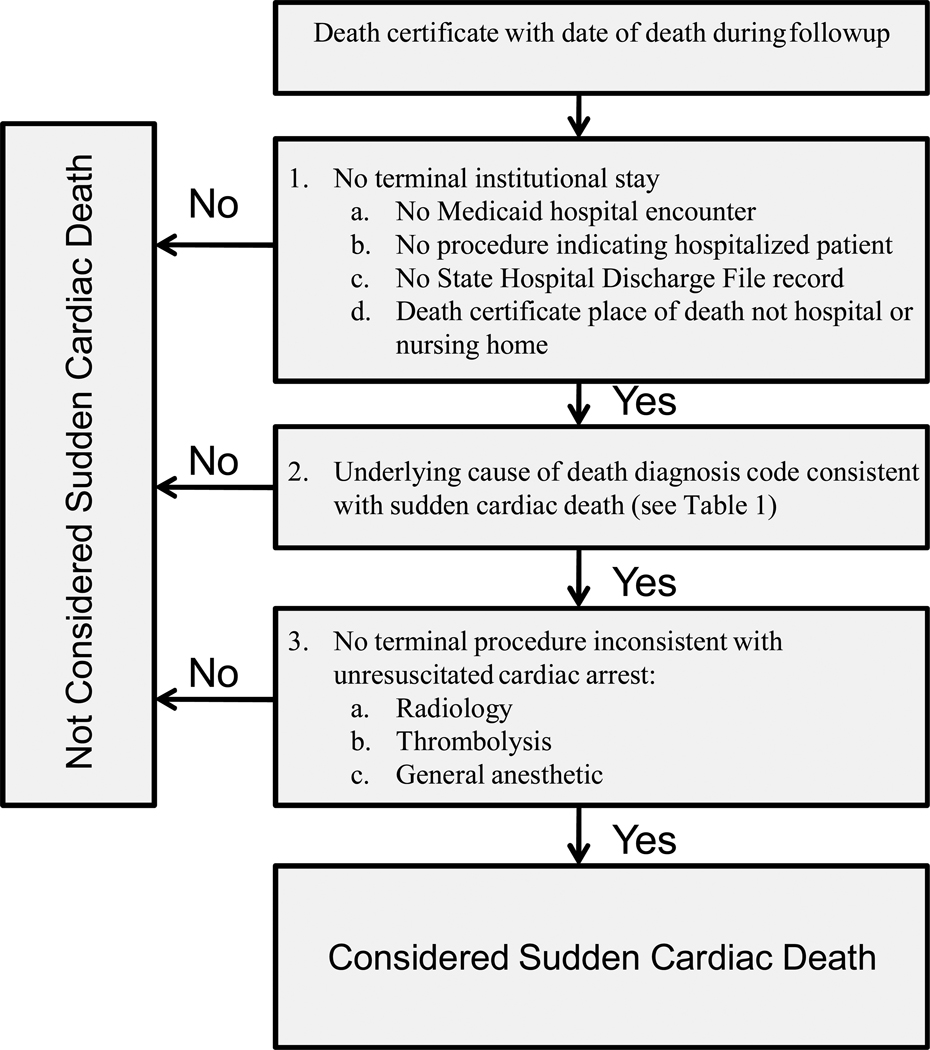
Review of the adjudicated deaths in the development sample led to the following computer case definition for a sudden cardiac death (Figure 2):
No evidence of a terminal institutional stay. In addition to excluding deaths with a terminal Medicaid hospital admission record, this criterion also excluded deaths with other evidence of a terminal hospital admission or nursing home stay: a Medicaid procedure code indicating hospital admission, a State Hospital Discharge File record, or a death certificate place of death of hospital or nursing home.
Underlying cause of death code consistent with sudden cardiac death. The diagnosis codes utilized (Table 1) were development sample codes with at least 10 deaths and a positive predictive value of 70% or greater (after excluding cases with any evidence of terminal hospital admission) or codes with fewer than 10 deaths that plausibly indicated sudden cardiac death.
No terminal procedures inconsistent with unresuscitated cardiac arrest. This criterion excluded deaths with radiology or other procedures (thrombolysis, general anesthetic) generally not performed on patients in cardiac arrest.
Figure 2.
Computer case definition for sudden cardiac death.
Table 1.
Underlying cause of death diagnosis codes for computer sudden cardiac death case definition. NOS denotes "not otherwise specified"
| ICD9 | ICD10 | ||
|---|---|---|---|
| 401.9 | Essential hypertension, NOS | I10 | Essential hypertension |
| 402 | Hypertensive heart disease, NOS | I11.9 | Hypertensive heart disease w/o heart failure |
| 410 | Acute myocardial infarction | I21 | Acute myocardial infarction |
| I22 | Subsequent myocardial infarction | ||
| I23 | Certain current complications following AMI | ||
| 411 | Other acute/subacute ischemic heart disease | I24 | Other acute ischemic heart disease |
| 412 | Old myocardial infarction | I25.2 | Old myocardial infarction |
| 413 | Angina pectoris | I20 | Angina pectoris |
| 414 | Other forms of chronic ischaemic heart disease | I25 | Chronic ischemic heart disease |
| 425.4 | Primary cardiomyopathy, other/NOS | I42.9, I42.8 | Cardiomyopathy, NOS |
| 427.5 | Cardiac arrest | I46 | Cardiac arrest |
| I47.0 | Reentry ventricular arrhythmia | ||
| 427.1 | Paroxysmal ventricular tachycardia | I47.2 | Ventricular tachycardia |
| 427.4 | Ventricular fibrillation and flutter | I49.0 | Ventricular fibrillation and flutter |
| 427.8 | Arrhythmia, other but not specified | I49.8 | Oth.specified cardiac arrhythmias |
| 427.9 | Arrhythmia (cardiac), NOS | I49.9 | Cardiac arrhythmia, unspecified |
| 429.2 | Cardiovascular disease, unspecified | I51.6 | Cardiovascular disease, unspecified |
| 429.9 | Heart disease, unspecified | I51.9 | Heart disease, unspecified |
| 440.9 | Arteriosclerosis, NOS | I70.9 | Atherosclerosis, NOS |
| 798.2 | Death in <24 hours | R96.1 | Death in <24 hours |
| 798.9 | Unattended death | R98 | Unattended death |
Validation sample
The validation study cohort had 1870 deaths that met the computer case definition for sudden cardiac death (Figure 1). To avoid overlap with the development sample, the validation sample was restricted to deaths that occurred between 1994 and 2005. To reduce the time and expense of record retrieval, the sample was further restricted to 316 deaths in counties within a 100 mile radius of Nashville. Of these, records were not sought for 74 (23.4%) because they had no institutional (hospital, emergency department, or hospital-based clinic) or emergency medical services encounters in the year preceding death. Our past experience suggested that absent these types of encounters, information on the circumstances of death would be insufficient to permit adjudication. Medical records were sought for the remaining 242 deaths; access was refused for 5 (2.0%) and the records had inadequate documentation for 63 (26.0%). This left 174 deaths for which medical records (redacted to remove personal identifiers and medication history) were adjudicated (Figure 1) by both a study physician and a cardiac electrophysiologist.
Results
Development Sample
The 926 adjudicated deaths in the development sample were the basis for constructing the computer case definition for sudden cardiac death. Of these deaths, 706 were classified as cases of sudden cardiac death (49% probable, 51% possible), a positive predictive value of 76.2% (Table 2).
Table 2. Development study sample.
Adjudication by medical record review of potential cases of sudden cardiac death. The positive predictive values (PPVs) and false negative (FN) proportions are shown for the entire development sample and for subsets of that sample identified by successively applying the three criteria of the computer case definition for sudden cardiac death (see Figure 2).
| Deaths adjudicated |
Confirmed cases sudden cardiac death |
PPV,% | FN | FN,% | |
|---|---|---|---|---|---|
| Entire development study sample | 926 | 706 | 76.2 | 0 | 0 |
| Additional restrictions in computer case definition | |||||
| 1. No terminal institutional stay* | 800 | 637 | 79.6 | 69 | 9.8 |
| 2. Underlying cause of death consistent with sudden cardiac death | 644 | 548 | 85.1 | 158 | 22.4 |
| 3. No terminal procedure inconsistent with sudden cardiac death | 616 | 530 | 86.0 | 176 | 24.9 |
Did not include the State Hospital Discharge File, which was unavailable at the time the development study was performed.
We assessed the effect of the successive application of each of the three criteria in the computer case definition intended to reduce misclassification by restricting deaths to those more likely to represent true sudden cardiac deaths (Figure 2). Restriction to deaths with no evidence of a terminal institutional stay (Figure 2, criterion 1, 800 deaths) increased the positive predictive value to 79.6% (Table 2). Restriction to those with an underlying cause of death diagnosis code more compatible with sudden cardiac death (Table 1 and Figure 2, criterion 2, 644 deaths) increased the positive predictive value to 85.1%. Finally, excluding those deaths where terminal medical care was inconsistent with cardiac arrest (Figure 2, criterion 3, 616 deaths) increased the positive predictive value to 86.0%. These further restrictions led to misclassification of 176 deaths adjudicated as sudden cardiac deaths, a false negative proportion of 24.9%.
We assessed the performance of the individual cause of death codes used to identify the deaths in the development sample (Table 3). This assessment utilized the 800 cases in the development sample with no evidence of a terminal hospitalization and included both codes ultimately used and not used for the computer case definition. For the codes used in the computer case definition, the most frequently recorded were myocardial infarction (285 deaths, positive predictive value of 82.1%) and cardiovascular arteriosclerosis (112 deaths, positive predictive value of 86.6%). For the cases with codes not used in the case definition, the most frequently recorded causes of death were diabetes (38 deaths, positive predictive value of 65.8%) or unknown cause (39 deaths, positive predictive value of 64.1%). There were 59 deaths identified from emergency department visit diagnoses that indicated sudden cardiac death when the death certificate cause of death did not; the positive predictive value for these deaths was 44.1%.
Table 3. Development study sample.
Positive predictive value (PPV) of individual underlying cause of death diagnosis codes used to identify potential sudden cardiac deaths, according to whether or not these were used in the computer case definition for sudden cardiac death. NOS denotes "not otherwise specified"
| ICD9 Code | Diagnosis | Deaths | Confirmed | PPV |
|---|---|---|---|---|
| All | 800 | 637 | 79.6% | |
| Codes used in the computer case definition for sudden cardiac death | ||||
| 401.9 | Essential hypertension, NOS | 8 | 7 | 87.5% |
| 402.9 | Hypertensive heart disease, NOS | 49 | 46 | 93.9% |
| 410 | Myocardial infarction | 285 | 234 | 82.1% |
| 411 | Other acute/subacute ischemic heart disease | 1 | 0 | 0.0% |
| 414.0 | Coronary atherosclerosis | 94 | 86 | 91.5% |
| 414.8 | Chronic ischemic heart disease, other | 2 | 2 | 100.0% |
| 414.9 | Chronic ischemic heart disease, unspecified | 40 | 30 | 75.0% |
| 425.4 | Primary cardiomyopathy, NOS | 11 | 10 | 90.9% |
| 427.1 | Paroxysmal ventricular tachycardia | 1 | 1 | 100.0% |
| 427.4 | Ventricular fibrillation and flutter | 2 | 2 | 100.0% |
| 427.5 | Cardiac arrest | 23 | 18 | 78.3% |
| 427.9 | Cardiac dysrhythmia, NOS | 9 | 8 | 88.9% |
| 429.2 | Cardiovascular arteriosclerosis | 112 | 97 | 86.6% |
| 429.9 | Cardiovascular disease, NOS | 2 | 2 | 100.0% |
| 440.9 | Atherosclerosis, generalized and unspecified | 4 | 4 | 100.0% |
| 798.9 | Unattended death | 1 | 1 | 100.0% |
| Codes not used in the computer case definition for sudden cardiac death | ||||
| 250.0 | Diabetes, NOS | 38 | 25 | 65.8% |
| 404.9 | Hypertensive heart and renal disease, NOS | 1 | 0 | 0.0% |
| 425.1 | Hypertrophic obstructive cardiomyopathy | 1 | 1 | 100.0% |
| 428 | Heart failure | 4 | 3 | 75.0% |
| 429.0 | Myocarditis, NOS | 2 | 1 | 50.0% |
| 436 | Acute, but ill-defined, cerebrovascular disease | 12 | 8 | 66.7% |
| 799.9 | Unknown cause of death | 39 | 25 | 64.1% |
| Other* | 59 | 26 | 44.1% | |
Terminal emergency department visit present for which primary diagnosis code indicates potential sudden cardiac death (codes for diabetes (250.0) and heart failure (428) not used) even though the underlying cause of death code does not.
Validation Sample
Of the 174 adjudicated deaths in the validation sample, 151 were classified after medical record review as sudden cardiac deaths, a positive predictive value of 86.8% (Table 4). Of these, 94 were probable and 57 possible cases. The deaths that did not meet the medical record review criteria for sudden cardiac death included 10 cases (5.7%) of pulseless electrical activity. Although these cases met the definition for sudden death, such deaths are generally considered to be inconsistent with drug-induced ventricular tachyarrhythmias21 and thus were adjudicated as false positives. The positive predictive value for deaths occurring between 1994–1998 (ICD-9 coding) was 85.1%; that for deaths between 1999–2005 (ICD-10 coding) was 87.4%.
Table 4. Validation study sample.
Performance of the computer case definition for sudden cardiac death.
| N | % | |
|---|---|---|
| Adjudicated cases | 174 | 100.0 |
| Not sudden death | 13 | 7.5 |
| Sudden death | 161 | 92.5 |
| Pulseless electrical activity | 10 | 5.7 |
| Sudden cardiac death | 151 | 86.8 |
| Probable | 94 | 54.0 |
| Possible | 57 | 32.8 |
Review of the adjudicated deaths revealed two potential refinements to the computer case definition. One death had a possible thrombolysis procedure that had not been excluded as specified by the definition (Figure 2, criterion 3) because the code used was ICD-9-CM rather than the expected CPT4. A second death had a terminal emergency department visit with a non-primary diagnosis indicating suicide. We did not exclude this death because the death certificate cause of death did not indicate a probable or possible suicide. Neither of these deaths were classified as sudden cardiac deaths according to medical record review. Exclusion of either of these deaths led to a positive predictive value of 87.2% (151/173); exclusion of both to a positive predictive value of 87.8% (151/172).
Because the validation sample came from a cohort of antipsychotic users and matched nonuser controls, it was not a random sample of deaths among Tennessee Medicaid enrollees. We thus assessed whether the performance of the computer case definition varied according to antipsychotic use status on the date of cohort entry. For baseline antipsychotic users, the positive predictive value for deaths meeting the computer definition for sudden cardiac death was 86.4%, nearly identical to that of 87.4% for such deaths among nonuser controls.
Discussion
We developed and validated a computer case definition for sudden cardiac death for use in automated databases linked with death certificates. Our motivation was to improve the efficiency and quality of studies of medications and sudden cardiac death by reducing the time and expense required for medical record review and avoiding bias due to the exclusion of cases for whom medical records are either unavailable or non-informative. In the development sample, the definition had a positive predictive value of 86.0%; in the validation sample the positive predictive value was 86.8%. This suggests the computer case definition will be useful for studies of medications and sudden cardiac death.
Our study positive predictive value is higher than that from five previous studies that assessed the performance of death-certificate based definitions of sudden cardiac death,22–26 which reported values of 19%,22 27%,23 32%,24 77%,25 and 85%.26 We believe these differences resulted from three separate factors: the computer case definition for sudden cardiac death, the population studied, and the gold-standard definition used.
The previous studies used only the death certificates to identify potential sudden cardiac deaths. In contrast, we utilized three distinct sources of information: the death certificates, a state hospital discharge database, and records of medical encounters from the Medicaid program. The additional Tennessee data sources were essential for exclusion of deaths unlikely to meet the case definition. For example, in the Minnesota study, of 1409 deaths considered for medical record review, 285 (20.2%) actually occurred following hospital admission,22 despite a death certificate indicating out-of-hospital death. In that study, these deaths were false positives, whereas, in our study, such deaths would have been excluded.
Our study also was selective with regard to the specific cause-of-death diagnostic codes considered. For example, it did not include underlying cause of death codes for heart failure or stroke, which would have been included in some of the other definitions.
We excluded persons 75 years of age or older and nursing home residents because our experience indicated that the positive predictive value would be lower for these populations. This exclusion also may have increased the predictive value of the Tennessee definition relative to that of the other studies. This exclusion is an important limitation as the patient groups account for a substantial fraction of sudden cardiac deaths. If our definition is to be used in these populations, further validation studies would be necessary.
Our definition included both probable and possible cases of sudden cardiac death. Some of the prior studies were restricted to probable sudden cardiac deaths;22 had we done this the positive predictive value would have been 54%.
A limitation of the validation study was that 45% of deaths in the sample (142/316) were not adjudicated, primarily for two reasons. First, for 74 deaths (23%) the augmented Medicaid files did not have either autopsy reports or records for the year preceding death from sources we have found to be most informative and accessible: hospital emergency departments, hospital-based clinics, and emergency medical services. This proportion decreased with calendar year, with 34% of deaths not adjudicated for this reason prior to 2000 as opposed to 17% subsequently. However, the PPV actually increased slightly for the later period (85% prior to 2000 versus 88% subsequently), suggesting this factor had little effect on our findings. Second, the records retrieved for 63 deaths (20%) did not have sufficient information for adjudication, primarily because the record did not contain observer reports describing the arrest or the patient's prior health. Further studies with higher proportions of adjudicated deaths would be useful, although these might need to go beyond medical record review and either seek to interview decedents' contacts or use prospective methods.23
The validation sample only included deaths that met the computer case definition and thus cannot be used to estimate sensitivity. However, in the development sample, we reviewed a broader range of deaths, following a strategy similar to that in the Minnesota study.22 For that sample, we found that the computer case definition misclassified 25% of confirmed sudden cardiac deaths. This suggests that the sensitivity is less than 75%, given that a review of all deaths (for example, including those coded as due to cancer, excluded from several of the studies22) would identify even more confirmed cases. Thus, the computer case definition may be more useful for etiologic investigations than for studies of the absolute incidence of sudden cardiac death.
Many sudden cardiac deaths were identified from underlying cause of death codes for acute myocardial infarctions and coronary atherosclerosis. Standard definitions of sudden cardiac death19 include myocardial infarctions, so long as they are rapidly fatal and thus meet the "sudden" criteria. In susceptible patients, an ischemic event such as a myocardial infarction is often, but not always the trigger of ventricular tachyarrhthmias – the most common cause of sudden cardiac death.2 If the patient has a genetic predisposition or is taking a proarrhythmic drug, an infarction may be more likely provoke a lethal arrhythmia than in a comparable patient without these factors. When cardiac arrest occurs outside of the hospital and the patient dies quickly, there often is very limited medical investigation of the death. Thus, the appropriate codes on the death certificate are those that indicate a death related to coronary artery disease, such as myocardial infarction or atherosclerosis.
Both the development and validation samples for the computer case definition consisted of Tennessee Medicaid enrollees. Thus, its performance may be different in other populations. However, performance of computer case definitions has been broadly similar in Medicaid and non-Medicaid populations for other diseases, including gastroduodenal ulcers,27 stroke,28 and myocardial infarction.29
An important limitation of the computer case definition is that approximately 13% of cases identified will be false positives that could have been excluded by medical record review. If this misclassification is non-differential, it will bias to the null, thus modestly reducing the power of studies to detect medication effects. Of greater concern is differential misclassification according to drug exposure status, which would bias medication studies. However, our findings provide some evidence that this does not occur, as the positive predictive value in the validation sample was the same for both baseline users and nonusers of antipsychotics.
In conclusion, we developed and validated a computer case definition for sudden cardiac death for use in automated databases linked with death certificates. The definition had a positive predictive value of 86.8% in the validation sample, which should make it a useful tool for pharmacoepidemiologists.
Key Points.
There is considerable interest in effects of medications on the risk of sudden cardiac death. Automated databases with prescription records and linked to computerized death certificates have potential for such studies. However, medical record review for possible sudden deaths poses formidable logistic difficulties. We developed and validated by medical record review a sudden cardiac death computer case definition in the linked Tennessee Medicaid database. The positive predictive value was 87%, making this definition a useful tool for pharmacoepidemiologists.
Acknowledgments
Supported by grants from the NHLBI (# HL081707) and the Vanderbilt Center for Education and Research on Therapeutics (CERTs, cooperative agreement # HS1-0384)
We gratefully acknowledge the Tennessee Bureau of TennCare and the Department of Health, which provided study data.
Reference List
- 1.State-specific mortality from sudden cardiac death- United States, 1999. MMWR. 2002;51:123–126. [PubMed] [Google Scholar]
- 2.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 3.Antezano ES, Hong M. Sudden cardiac death. J Intensive Care Med. 2003;18:313–329. doi: 10.1177/0885066603258140. [DOI] [PubMed] [Google Scholar]
- 4.Myerburg RJ, Kessler KM, Castellanos A. Sudden cardiac death: epidemiology, transient risk, and intervention assessment. Ann Intern Med. 1993;119:1187–1197. doi: 10.7326/0003-4819-119-12-199312150-00006. [DOI] [PubMed] [Google Scholar]
- 5.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 6.Owens RC. QT prolongation with antimicrobial agents: understanding the significance. Drugs. 2004;64:1091–1124. doi: 10.2165/00003495-200464100-00005. [DOI] [PubMed] [Google Scholar]
- 7.Ray WA. Population-based studies of adverse drug effects. N Engl J Med. 2003;349:1592–1594. doi: 10.1056/NEJMp038145. [DOI] [PubMed] [Google Scholar]
- 8.Ray WA, Meredith S, Thapa PB, Meador KG, Hall K, Murray KT. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001;58:1161–1167. doi: 10.1001/archpsyc.58.12.1161. [DOI] [PubMed] [Google Scholar]
- 9.Ray WA, Meredith S, Thapa PB, Meador KG, Hall K, Murray KT. Cyclic antidepressants and the risk of sudden cardiac death. Clin Pharmacol Ther. 2003;75:234–241. doi: 10.1016/j.clpt.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Ray WA, Murray KT, Meredith S, Narasimhulu SS, Hall K, Stein CM. Oral erythromycin use and the risk of sudden cardiac death. N Engl J Med. 2004;351:1089–1096. doi: 10.1056/NEJMoa040582. [DOI] [PubMed] [Google Scholar]
- 11.Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360:235. doi: 10.1056/NEJMoa0806994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Straus SMJM, Bleumink GS, Dieleman JP, et al. Antipsychotics and the risk of sudden cardiac death. Arch Intern Med. 2004;164:1293–1297. doi: 10.1001/archinte.164.12.1293. [DOI] [PubMed] [Google Scholar]
- 13.Straus SMJM, Sturkenboom MCJM, Bleumink GS, et al. Non-cardiac QTc-prolonging drugs and the risk of sudden cardiac death. Eur Heart J. 2005;26:2007–2012. doi: 10.1093/eurheartj/ehi312. [DOI] [PubMed] [Google Scholar]
- 14.Ray WA, Griffin MR. Use of Medicaid data for pharmacoepidemiology. Am J Epidemiol. 1989;129:837–849. doi: 10.1093/oxfordjournals.aje.a115198. [DOI] [PubMed] [Google Scholar]
- 15.Piper JM, Ray WA, Griffin MR, Fought R, Daugherty JR, Mitchel E., Jr Methodological issues in evaluating expanded Medicaid coverage for pregnant women. Am J Epidemiol. 1990;132:561–571. doi: 10.1093/oxfordjournals.aje.a115692. [DOI] [PubMed] [Google Scholar]
- 16.Liberthson RR. Sudden death from cardiac causes in children and young adults. N Engl J Med. 1996;334:1039–1044. doi: 10.1056/NEJM199604183341607. [DOI] [PubMed] [Google Scholar]
- 17.Marcus FI, Cobb LA, Edwards JE, et al. Mechanism of death and prevalence of myocardial ischemic symptoms in the terminal event after acute myocardial infarction. Am J Cardiol. 1988;61:8–15. doi: 10.1016/0002-9149(88)91295-7. [DOI] [PubMed] [Google Scholar]
- 18.Hinkle LE, Thaler HT. Clinical classification of cardiac deaths. Circulation. 1982;65:457–464. doi: 10.1161/01.cir.65.3.457. [DOI] [PubMed] [Google Scholar]
- 19.Siscovick DS, Raghunathan TE, Psaty BM, et al. Diuretic therapy for hypertension and the risk of primary cardiac arrest. N Engl J Med. 1994;330:1852–1857. doi: 10.1056/NEJM199406303302603. [DOI] [PubMed] [Google Scholar]
- 20.Albert CM, Hennekens CH, O'Donnell CJ, et al. Fish consumption and risk of sudden cardiac death. JAMA. 1998;279:23–28. doi: 10.1001/jama.279.1.23. [DOI] [PubMed] [Google Scholar]
- 21.Desbiens N. Simplifying the diagnosis and management of pulseless electrical activity in adults: A qualitative review. N Engl J Med. 2008;36:391–396. doi: 10.1097/CCM.0b013e318161f504. [DOI] [PubMed] [Google Scholar]
- 22.Iribarren C, Crow RS, Hannan PJ, Jacobs DR, Luepker RV. Validation of death certificate diagnosis of out-of-hospital sudden cardiac death. Am J Cardiol. 1998;82:50–53. doi: 10.1016/s0002-9149(98)00240-9. [DOI] [PubMed] [Google Scholar]
- 23.Chugh SS, Jui J, Gunson K, et al. Current burden of sudden cardiac death: Multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Col Cardiology. 2004;44:1268–1275. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 24.Fox CS, Evans JC, Larson MG, et al. A comparison of death certificate out-of-hospital coronary heart disease death with physician-adjudicated sudden cardiac death. Am J Cardiol. 2005;95:856–859. doi: 10.1016/j.amjcard.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Goraya TY, Jacobsen SJ, Belau PG, Weston SA, Kottke TE, Roger VL. Validation of death certificate diagnosis of out-of-hospital coronary heart disease deaths in Olmsted County, Minnesota. Mayo Clin Proc. 2000;75:681–687. doi: 10.4065/75.7.681. [DOI] [PubMed] [Google Scholar]
- 26.Every NR, Parsons L, 't Hart BJ, et al. Use and accuracy of state death certificates for classification of sudden cardiac deaths in high-risk populations. Am Heart J. 1997;134:1129–1132. doi: 10.1016/s0002-8703(97)70035-8. [DOI] [PubMed] [Google Scholar]
- 27.Ray WA, Stein M, Daugherty JR, Hall K, Arbogast P, Griffin MR. COX-2 selective non-steroidal anti-inflammatory drugs and risk of serious coronary heart disease. Lancet. 2002;360:1071–1073. doi: 10.1016/S0140-6736(02)11131-7. [DOI] [PubMed] [Google Scholar]
- 28.Roumie CL, Mitchel E, Gideon PS, Varas-Lorenzo C, Castellsague J, Griffin MR. Validation of ICD-9 codes with a high positive predictive value for incident strokes resulting in hospitalization using Medicaid health data. Pharmaco Drug Safety. 2008;17:20–26. doi: 10.1002/pds.1518. [DOI] [PubMed] [Google Scholar]
- 29.Ray WA, Chung CP, Stein CM, Smalley WE, Arbogast PG, Griffin MR. Risk of peptic ulcer hospitalizations in users of NSAIDs with gastroprotective cotherapy versus coxibs. Gastroenterol. 2007;133:790–798. doi: 10.1053/j.gastro.2007.06.058. [DOI] [PubMed] [Google Scholar]