Abstract
Background
The factors that contribute to the dual task (DT) changes in performance that occur when older adults walk while simultaneously performing other tasks are not well-known. We hypothesized that cognitive and motor reserve (e.g., executive function, EF, postural control, and walking abilities) and affect (e.g., anxiety, depressive symptoms) influence the DT decrements (DTDs) in gait.
Methods
228 community-living, healthy older adults (mean: 76.2±4.2 yrs; 59% women) walked with and without dual tasking, e.g., subtracting 7’s, phoneme monitoring. Mobility (e.g., the Dynamic Gait Index), cognitive function (e.g., memory, EF), and affect (e.g., Geriatric Depression Scale) were quantified. Bivariate and multivariate analyses identified factors associated with the DTD in gait speed (a general measure of locomotor function), swing time, (reflecting balance during gait), and swing time variability (a measure of stride-to-stride consistency).
Results
Gait speed and swing time decreased (p<0.001) and swing time variability increased (became worse) (p<0.001) during all DTs. The DTD in gait speed was correlated with comfortable-walking gait speed, but not with tests of mobility or cognitive function. The DTD in swing time variability was correlated with EF, mobility and affect (e.g., depressive symptoms). Much of the variance in the DTDs was unexplained.
Conclusions
Usual-walking abilities and cognitive function contribute to the DT effects on gait, but these relationships depend on specifics of the DT, the gait feature being studied, and the particulars of the cognitive domain. Meeting the everyday challenges of walking while dual tasking apparently relies on multiple factors including a consistent gait pattern and EF.
Keywords: dual task, cognitive function, executive function, gait, variability
INTRODUCTION
Despite an age-old neurological dogma that motor systems are anatomically and functionally quite separate from cognitive systems, it has become clear over the past two decades that such systems are inter-related at the level of the cerebrum. Thus, even seemingly automatic motor sequences, such as gait, which normally appear to operate completely independently from cognition, can be affected by cognitive tasks. Such interaction can be seen, for example, in the normal aging process, when the overall efficiency of the brain is reduced. Indeed, a growing number of studies have demonstrated that gait in older adults is not simply an automatic process (1,2). If it was automatic, the performance of attention-demanding, “dual tasks” during walking would not alter the gait pattern. Instead, many investigations have shown that dual tasking affects gait. For example, when older adults are asked to walk and simultaneously perform another task, gait speed is reduced (3–5). In certain impaired elderly populations, dual tasking may also increase the stride-to-stride variability of gait (4,6), a measure of the consistency of the gait pattern that has been associated with fall risk (4,7,8). The degree to which gait changes during the performance of another task has been related to the difficulty of the concurrent task and to the nature of the walk (4,6,9–11) as well as to fall risk and other disabling outcomes (12–14).
A range in the influence of dual task on gait has been observed in older adults (3,4,15,16), however, the factors that contribute to the gait changes in response to “dual tasking” (DT) among healthy older adults have not yet been fully elucidated. A priori, one could suggest that among a homogeneous group of healthy older adults with intact mobility and cognitive function, gait will be automatic and the dual task decrement (DTD) will be minimal. Therefore, any observed effects will merely be random or stochastic within group variations not attributable to subject-specific characteristics. Alternatively, age-associated changes may produce variability in dual task performance that is related to the spectrum of motor and cognitive abilities seen in healthy aging. Accordingly, subject-specific characteristics will explain the variations in the DTD in gait.
This second possibility is supported by several lines of reasoning. The DTD is generally larger in patient populations whose gait and mobility are more impaired compared to healthy controls (4,6,15,17,18). The DTD has also been related to cognitive function, in particular attention (capacity) and executive function (4,6,9,16). For example, patients with Alzheimer’s disease and patients with Parkinson’s disease who have more impaired executive function show a greater DTD (6,15,20). This suggests that “cognitive reserve”, i.e., the background cognitive capacity that a subject brings to a given task, may play an important role. Among healthy older adults, there is great heterogeneity in gait and mobility (19) as well as a wide range in cognitive abilities (21,22). This is in part the result of variability in age-associated changes in the structure and ability to recruit brain regions important to the executive function and attentional systems such as the prefrontal and frontal cortex (21,22). Therefore, it is not surprising that dual task abilities in general vary among older adults (4,6,9,16,23–25). As described by Colcombe et al. (26), some older individuals show performance on cognitive tasks that is “in the range of normal younger individuals, and others of the same cohort fall entirely out of the range of normal younger adults, even in the absence of obvious pathology.” Thus, it seems reasonable to suggest that the DTD among healthy older adults will be related to both mobility and cognitive function. For those elderly whose walking is more automated, the dual tasking influence will be minimal; if needed, executive function will compensate to reduce the effects of dual tasking.
Another potential source of variations in DTD performance is affect and emotional well-being. Both cognitive and motor performance may be influenced by anxiety, fear and depression (27). For example, patients who are depressed walk more slowly and take longer to respond to cognitive challenges (28,29) and fear of falling has been associated with an altered gait pattern (30–32). Given the prevalence of age-related changes in mental well-being among the elderly, it is possible that these factors may also play a role in any observed DTDs on gait.
The present study was designed to evaluate the factors that contribute to the DTD in healthy older adults. We hypothesized that even among relatively healthy older adults, the DTD in gait would be: 1) smaller in subjects with better cognitive function, especially attention and executive function; and 2) smaller in subjects with better postural control (i.e., balance and gait). Put differently, the present study tested the hypothesis that executive function and usual-walking abilities both contribute to the DTD in gait among community-living, healthy older adults, and further, evaluated how the DTD depends on affect, emotional well-being, the nature of the concurrent task, and specific gait features (e.g., gait speed vs. variability).
METHODS
Subjects
Subjects were older adult men and women who were participating in a prospective study designed to examine the relationship between gait and cognitive function. More specifically, the objectives of this longitudinal study include determination of how age-associated changes in cognitive function contribute to dual tasking deficits in gait; how gait, cognitive function and fall risk change over time; and how cognitive function changes contribute to fall risk among older adults. The results presented are based on the first year of the study. Subjects were recruited from local senior centers, via flyers, advertising, and word of mouth. After an initial screening by phone or interview, eligible subjects were invited to participate if they were between the ages of 70 and 90 years, were living in the community, were able to ambulate independently (without walking aids), and if they were free from disease likely to directly impact gait (e.g., vestibular, orthopedic, neurologic disease). Subjects were also excluded if they had acute illness, brain surgery, major depression, history of stroke or if they scored less then 25 on the Mini Mental State Exam (MMSE, Hebrew version) (33). The study was approved by the local human studies committee of the Tel-Aviv Sourasky Medical Center and informed written consent was obtained.
To characterize the study population, demographic information was obtained along with fall history and medical history using a structured interview, clinical exam and questionnaires. Height and weight were measured. The Charlson Comorbidity Index was used to quantify disease burden (higher scores indicate greater co-morbidity) (34). The Barthel Activities of Daily Living Index (35), the Frenchay Activities Index (36), the Physical Activity Scale for the Elderly (PASE) (37), and the relative components of the SF-36 (38) were used to characterize disability, lifestyle and functional independence, physical activity levels and self-report of general health, respectively. Higher scores on these four tests reflect better health and abilities.
Cognitive assessment
The Mindstreams® (NeuroTrax Corp., NJ) computerized neuropsychological test battery was used to quantify four domains of cognitive function: executive function, attention, memory, and visual spatial orientation (39,40). The battery included the Go-NoGo and the Stroop tests of executive function, tests of non-verbal memory, tests of visual-spatial function, and tests of finger tapping and hand-eye coordination. Age and education adjusted composite indices of each different cognitive domain were computed as previously established (6,39,40). The tests utilized were designed for and have been used in cognitively intact older adults as well as in older adults with Parkinson’s disease, dementia, mild cognitive impairment, Gaucher’s disease, and ADHD and have been significantly associated with traditional pen and paper based tests in a variety of populations (4,6,39,41,42).
Performance-based Measures of Balance and Mobility
The Berg Balance Test and the Dynamic Gait Index, widely used performance-based measures of balance and mobility, were used to evaluate these properties (43–45) (higher scores indicate better function). We used also the Timed Up and Go test (46), a simple, but commonly used measure of lower extremity function, functional mobility and fall risk (47).
Assessment of Affect
The Activities-specific Balance Confidence Scale was administered to assess fear of falling (higher scores indicate less fear and greater confidence) (48). The Geriatric Depression Scale (30 questions) measured depressive symptoms and emotional well-being (49). The State-Trait Anxiety Inventory (STAI) quantified anxiety (50).
Assessment of Gait and Dual Tasking
Gait was evaluated four times, during usual walking and under three dual task conditions: 1) phoneme-monitoring, 2) serial 3 subtractions, and 3) serial 7 subtractions. During phoneme-monitoring, subjects listened to a story (via headphones) while walking (knowing that they would be questioned about its contents) and counted the number of times two pre-specified words appeared in the text at random intervals. During the serial subtraction tasks, subjects walked while reciting out loud serial subtractions of seven or three, starting from a random 3-digit number. Before performing the task while walking, each of these tasks was conducted while sitting. A different text was used for phoneme-monitoring and different starting 3-digit numbers were used for the serial subtractions, but otherwise the tasks during sitting and walking were identical (e.g., same duration). Phoneme monitoring and serial subtractions were both applied to more broadly assess the DTD. These different constructs may tax distinct cognitive resources (51,52), and hence have differential effects on gait. The nature of the dual tasking also differs (e.g., the attention demands of phoneme monitoring are essentially uniform over time). Previous work in other populations supports the idea that while both phoneme monitoring and serial subtractions elicit a dual task effect on gait, the consequences are not the same (4,6) and, further, that even healthy young adults slow down when they perform these tasks (4,6). Another reason for using these two tasks is that aside from increasing reliability of findings, a priori, the two subtraction tasks provide distinct levels of difficulties.
Subjects were instructed to walk at their self-selected, normal (comfortable) pace under each of the four conditions (i.e., 4 walking trials). The instructions for the dual tasks conditions were to walk at a comfortable pace and to perform the additional, dual task simultaneously. No instruction for prioritization of one of the tasks (walking vs. cognitive task) was given. After a practice walk, the order of the tasks, including the “usual walking” condition, was randomized. Performance on the phoneme-monitoring task was evaluated using the total number of words counted correctly and the number of multiple choice questions correctly answered about the story. Evaluation of performance on the serial subtractions included the total number of subtractions and the number of mistakes.
Under each of the 4 conditions, subjects walked up and down a 25 meter-long, 2-meter wide hallway at their self-selected, usual walking speed for 2 minutes while wearing force-sensitive insoles. Average values and the coefficient of variation (CV) of the swing time of the leg with lowest variability during usual walking were determined using previously described methods that quantify balance during walking and the intrinsic dynamics of steady state walking (7,20,53). The CV assesses the variability, stride-to-stride consistency, and rhythmicity of gait (i.e., lower values reflect a more consistent gait pattern), a measure previously associated with fall risk (7,54,55). Swing time variability is an aspect of dynamic balance that is not influenced by gait speed (53); stance variability, a closely related measure, is associated with disability, independent of gait speed (56). Average gait speed was determined using a GaitRite mat (placed in the middle of the walkway).
Statistical Analysis
The DTD was defined as the difference between the single task and dual task performance for each of the gait parameters, i.e., single task minus dual task value. The results were generally similar if we analyzed % change or if the value under the dual task condition was considered to be the dependent measure (i.e., unadjusted). After applying ANOVA to determine the presence of any significant dual task effects, post-hoc paired Least Significant Differences (LSD) were applied to determine whether the usual-walking gait measure differed from the measures obtained during each of the 3 dual tasking conditions, for each of the 3 dependent gait measures (i.e., gait speed, swing time, swing time variability). Pearson’s correlation coefficients were used to quantify the bivariate associations between the DTD in gait speed, average swing time, and swing time variability and independent measures. To build a multivariate, parsimonious model of the factors associated with the DTDs, we used regression models (forward stepwise) to identify potential independent predictors within each domain (e.g., subject characteristics, cognitive function, affect; see Table 1). More specifically, any independent measure that was marginally associated (i.e., p<0.10) with a DTD was included in a multivariate model. Subsequently, the results of these tests were included in another model to establish a single multivariate model for each DTD in gait (i.e., gait speed, average swing time, and swing time variability). The resulting multivariate models are parsimonious descriptors of the independent predictors of the DTDs. P-values reported are based on two-tailed comparisons. Statistical analyses were performed using SPSS 14.0 for Windows.
Table 1.
Subject characteristics*
| Background measures | Mean±SD (or %) | |
| Age (yrs) | 76.2±4.2 | |
| Gender (% women) | 58.8 % | |
| Body-mass-index (kg/m2) | 26.6±3.7 | |
| Mini-Mental State Exam | 28.8±1.2 | |
| Education (yrs) | 13.6±3.8 | |
| Fell in past year (%) | 26.7 % | |
| SF-36 Health | 69.4±17 | |
| PASE | 111.6±67.4 | |
| Frenchay Activities Index | 31.2±5.4 | |
| Charlson Comorbidity Index | 0.8±1 | |
| Prescription medications (#) | 3.7±2.4 | |
| Cognitive Function | Executive function index | 99.2±10.7 |
| Attention index | 98.8±13.0 | |
| Memory index | 99.4±12.2 | |
| Visual-spatial function index | 97.1±15.9 | |
| Affect | Activities-specific Balance Confidence Scale | 92.2±9.7 |
| Geriatric Depression Scale | 4.9±4.5 | |
| Trait Anxiety Inventory | 33.4±8.6 | |
| State Anxiety Inventory | 31.5±9.9 | |
| Performance-based measures of Mobility | Dynamic Gait Index | 22.8±1.5 |
| Berg Balance Score | 54.1±2.3 | |
| Timed Up and Go (sec) | 9.5± 1.7 | |
| Usual-Walking Measures of Gait | Gait Speed (m/sec) | 1.3±0.2 |
| Average Swing Time (%) | 37.6±2.3 | |
| Swing Time Variability (%) | 2.2±1.0 |
All subjects were fully independent in activities of daily living (ADL’s) (a perfect score on the Barthel scale) and the Frenchay score indicated that subjects did not have major functional deficits or disabilities. Scores on the PASE reflected a level of physical activity consistent with that of healthy older adults. Average scores on the Dynamic Gait Index and the Berg Balance Scale were near perfect and the Timed Up and Go was also consistent with good mobility and low fall risk. Average values of the cognitive function indices were all close to 100.0, the mean value expected for a healthy cohort.
RESULTS
Subjects Characteristics
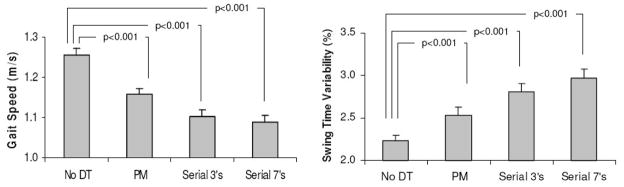
As summarized in Table 1, the 228 subjects who participated in this study were generally healthy, with intact mobility and postural control, and a low co-morbidity index. The effects of dual tasking on gait are summarized in Figure 1. ANOVA demonstrated significant dual tasks effects for each of the three dependent measures. Post-hoc pairwise analysis using the LSD method identified small, but significant effects on gait speed, average swing time, and swing time variability for all 3 dual tasks, compared to the baseline, usual walking values (p<0.0001). Gait speed and average swing time were reduced in response to phoneme-monitoring and reduced further in response to serial subtractions, while swing time variability increased. 88% of all subjects reduced their gait speed during phoneme-monitoring, and 97% and 93% slowed down during serial 3 and serial 7 subtractions, respectively. A dual task related increase in swing time variability was observed in 72%, 82% and 85% of the subjects during phoneme monitoring, serial 3 subtractions, and serial 7 subtractions, respectively. Compared to the usual-walking value, gait speed decreased on average by 0.10±0.12 m/sec, 0.15±0.12 and 0.17±0.16 m/sec during phoneme monitoring, serial 3 and serial 7 subtractions, respectively. Compared to the usual-walking value, swing time variability increased by 0.30±0.69 %, 0.58±1.01 % and 0.78±1.14 % during phoneme monitoring, serial 3 and serial 7 subtractions, respectively. For all results (e.g., Tables 2 and 3), the findings with respect to serial 3 and 7 subtractions were generally similar, therefore, henceforth, we only report the latter to conserve space.
Figure 1.
Gait speed and swing time variability during usual-walking (without any dual task, DT) and during the three dual tasks. Percentages shown in the columns are the % of subjects who showed a decrease in gait speed or an increase in swing time variability. For each dual task and each gait parameter, differences to the usual walking condition were highly significant (p<0.0001). PM: phoneme-monitoring.
Table 2.
Bivariate correlations between the dual task decrements (DTD), cognitive function and affect*
| Average Swing Time DTD | Swing Time Variability DTD | ||||
|---|---|---|---|---|---|
| Outcome Measure | Phoneme Monitoring | Serial 7 Subtractions | Phoneme Monitoring | Serial 7 Subtractions | |
| Cognitive Function | Executive function index | 0.16 (0.016)† | 0.15 (0.027)† | −0.22 (0.0008)† | −0.20 (0.003)† |
| Attention index | 0.12 (0.072) | 0.13 (0.053) | −0.17 (0.009)† | −0.25 (0.0002)† | |
| Memory index | 0.13 (0.058) | 0.11 (0.126) | −0.14 (0.044)† | −0.10 (0.151) | |
| Visual-spatial function index | 0.02 (0.796) | −0.05 (0.505) | −0.06 (0.390) | −0.06 (0.385) | |
| Affect | ABC Scale | −0.01 (0.917) | −0.01 (0.915) | −0.04 (0.527) | −0.07 (0.304) |
| Geriatric Depression Scale | −0.13 (0.056) | −0.17 (0.016) | 0.07 (0.309) | 0.14 (0.050) | |
| Trait Anxiety Inventory | −0.10 (0.175) | −0.17 (0.017)† | 0.18 (0.011)† | 0.12 (0.095) | |
| State Anxiety Inventory | −0.122 (0.082) | −0.16 (0.028)† | 0.15 (0.029)† | 0.07 (0.299) | |
Numbers are Pearson’s correlation coefficients (p-value).
ABC: Activities-specific Balance Confidence. All correlations where p<0.05 are indicated with a †. Results were similar using Spearman’s correlation coefficients and if the DTD’s were first log transformed. The DTD in gait speed was not significantly correlated with any measure of cognitive function or affect.
Table 3.
Bivariate correlations between the dual task decrements (DTD), mobility and gait*
| Average Swing Time DTD | Swing Time Variability DTD | ||||
|---|---|---|---|---|---|
| Outcome Measure | Phoneme Monitoring | Serial 7 Subtractions | Phoneme Monitoring | Serial 7 Subtractions | |
| Performance- based measures of Mobility and Balance | Dynamic Gait Index | −0.11 (0.106) | −0.07 (0.309) | −0.26 (0.0001)† | −0.19 (0.005)† |
| Berg Balance Score | −0.05 (0.481) | −0.11 (0.124) | −0.19 (0.005)† | −0.19 (0.006)† | |
| Timed Up and Go | −0.10 (0.896) | 0.02 (0.726) | 0.19 (0.005)† | 0.20 (0.003)† | |
| Usual-Walking measures of Gait | Gait Speed | 0.03 (0.645) | −0.03 (0.643) | −0.26 (0.001)† | −0.23 (0.002)† |
| Average Swing Time | −0.06 (0.364) | −0.03 (0.611) | −0.15 (0.033)† | 0.07 (0.294) | |
| Swing Time Variability | 0.28 (0.001)† | −0.11 (0.116) | 0.21 (0.002)† | 0.14 (0.046)† | |
Numbers are Pearson’s correlation coefficients (p-value).
All correlations where p<0.05 are indicated with a †. Results were similar using Spearman’s correlation coefficients and if the DTD’s were first log transformed.
Which of the subject characteristics were related to the dual task decrements (DTDs)?
The DT decrements in gait speed were mildly correlated with usual-walking gait speed for phoneme-monitoring (r=0.27; p=0.001) and serial 7 subtractions (r=0.22; p=0.002), but not with functional measures of mobility, cognitive function or affect (p>0.10). The increases in swing time variability in response to dual tasking were correlated with executive function, attention, and performance-based measures of mobility (r values ranged from 0.14 to 0.26 for mobility, and from 0.14 to 0.25 for cognitive measures; see Tables 2 and 3).
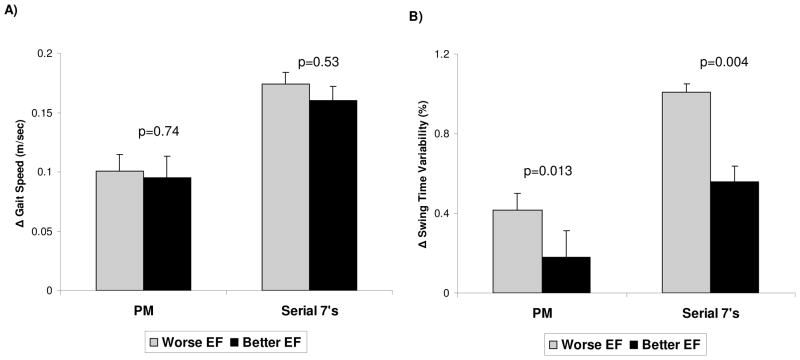
To visualize the modest associations between executive function and the dual task changes in gait, subjects were stratified based on their executive function scores into the 50% who had higher executive function (EF) indices (better; EF index score: 108.1 ± 5.4) and the 50% who had lower executive function indices (mean EF index score: 90.6 ± 6.8) using a median split based on EF index ranking. As shown in Figure 2, the DTD in swing time variability was lower (better) among subjects who had higher executive function scores. In contrast, the DTD in gait speed was not related to executive function when subjects were ranked this way. To a lesser degree, memory, depressive symptoms and anxiety were also correlated with the dual task increase in swing time variability (Table 2). As detailed further in Tables 2 and 3, average swing time shared properties of the other two gait measures.
Figure 2.
A) Changes in gait speed during phoneme-monitoring (PM) and serial 7 subtractions were not different in those with relatively lower or higher EF scores, as defined by a median split. B) The effects of PM and serial 7 subtractions on swing time variability were lower in subjects with higher EF indices. When dividing the subjects using a median split of EF scores, the mean EF index for subjects who did relatively worse was 90.6 ± 6.8, compared to 108.1 ± 5.4 in those who did relatively better (p<0.001). EF: executive function.
Table 4 summarizes the results of the multivariate analyses which examined whether or not any of the subject characteristics and other independent measures (e.g., those shown in Table 1) modified the relationships shown in Tables 2 and 3, and identified the independent factors that were associated with the three dependent gait measures (i.e., the DTD in gait speed, swing time, and swing time variability). In multivariate analyses, the DTD in gait speed was associated with usual-walking gait speed and, for serial 7 subtractions, with a measure of functional independence (see Table 4). The combination of mobility and executive function was correlated with the DTD in swing time variability in multivariate analysis. Similar results were obtained if the EF index was replaced with the attention index. The factors that were associated with the DTD in swing time variability were similar, but not identical, during phoneme monitoring and serial subtractions (Table 4), suggesting a differential effect.
Table 4.
Multivariate regression models of the dual task decrement (DTD) in gait. Entries are β, the standardized regression coefficient, and the associated p-values (in parentheses) from the results of step wise regression analyses from the 6 different models.
| Dependent Measure | Independent Factors | ||||||
|---|---|---|---|---|---|---|---|
| Executive Function Index | Attention Index | Frenchay Activities Index | Dynamic Gait Index | Timed Up & Go | Usual Gait Speed | Usual Swing Time Variability | |
| Gait Speed under Phoneme Monitoring DTD | ns | ns | ns | ns | ns | 0.27 (0.001) | ns |
| Gait Speed under Serial 7 DTD | ns | ns | −0.17 (0.021) | ns | ns | 0.23 (0.001) | ns |
| Average Swing Time under Phoneme Monitoring DTD | ns | ns | ns | ns | ns | ns | 0.28 (0.001) |
| Average Swing Time under Serial 7 DTD | 0.19 (0.011) | ns | ns | ns | ns | ns | ns |
| Swing Time Variability & Phoneme Monitoring DTD | −0.16 (0.032) | ns | ns | −0.28 (0.001) | ns | ns | ns |
| Swing Time Variability under Serial 7 DTD | ns | −0.18 (0.029) | ns | ns | 0.18 (0.022) | ns | ns |
Potential independent measures included in the step wise analyses were factors listed in Table 1 (e.g., age, gender, BMI, MMSE, SF-36, fall history, executive function, etc) and any other potential covariates that were marginally related to the DTD (p<0.10) in bivariate analyses (recall Tables 2 and 3). Only variables which made significant contributions in the step wise regression analyses are shown. ns (not significant) indicate that these factors were not significantly associated with the specific dependent variable in the multivariate analyses.
A subject’s ability to perform the cognitive task by itself might affect the DTD in gait. For example, the DTD might be lower for those who demonstrated greater mastery of phoneme monitoring when it was performed as a single task in the seated position. Adjustment for sitting task performance in the multivariate regressions generally did not change the results. For example, inclusion of measures of phoneme-monitoring performance did not change the final model for average swing time or for swing time variability; for gait speed, the final model included usual-walking gait speed (β=0.33; p<0.001) and the number of correct answers to content recall (β=−0.19; p<0.012).
DISCUSSION
Participants in this study were relatively healthy older adults whose scores on tests of balance and mobility were near the maximum and who also had good scores on a cognitive test battery. Nonetheless, these subjects altered their gait pattern in response to dual tasking. Most subjects reduced their gait speed, as seen in other studies (3–6), spent less time in swing, and increased their stride-to-stride variability. These changes were generally small. For example, the DT increase (delta) in swing time variability during serial 7’s was 0.7% in the present study (recall Figure 1), compared to a change of 1.5% and 1.1% in patients with Parkinson’s disease and elderly fallers, respectively (4,6). Still the response to dual tasking was highly consistent, demonstrating that even passive listening (i.e., phoneme monitoring) elicits a non-zero DTD in healthy older adults. This finding stands in contrast to previous reports in healthy older adults where significant effects were not observed (4,6). This difference could be explained by the large sample size and/or the heterogeneity of the cohort studied in the present sample. Thus, the current results seem more likely to reflect the spectrum seen in healthy aging.
The present findings are consistent with previous reports which observed that executive function is associated with the DT effects on gait (4,6,9,10,16). We find that even among healthy older adults with intact cognitive function, the DTD is associated with executive function, at least with respect to certain aspects of gait. In addition, motor abilities (e.g., gait speed), mobility and, to a lesser degree, memory are also associated with DT changes. Affect and mental well-being also appeared to play a role in the DTD, however, the multivariate findings suggest that these factors may not have had an independent contribution. In general, the results suggest that gait under dual task is a multidimensional task.
Dual tasking gait performance was not always related to usual-walking abilities or to the single-task performance of the cognitive task, especially in multivariate analyses (e.g., recall Table 4). The results also underscore the fact that the effect on gait is related to the nature of the dual task and, equally important, to the specific aspect of gait under study. Different aspects of gait have different DT dependencies. As noted in Table 4, the usual-walking gait speed was included among the multivariate predictors of the DTD in gait speed, but it did not predict the DTD in swing time variability. It is also important to keep in mind that during steady state, obstacle free walking, the observed correlations are relatively small and the combination of mobility and cognitive function still does not fully explain the observed changes in the walking pattern in response to dual tasking (note the beta values in Table 4). That being said, it is interesting to note the non-zero influence of executive function on the DTD in a relatively homogenous group of healthy older adults, even after adjusting for potential confounds. Perhaps when gait and/or executive function becomes impaired as a result of disease, the modest association between executive function and gait, especially during dual tasking, may take on a more prominent role, as seen in other studies where the DTD in gait speed was related to executive function (9,16).
Several theories have been put forth to explain why gait is altered in response to dual tasking including the bottleneck theory and the capacity sharing theory (2,57–59). Some have argued that when serial subtractions are performed out loud, the motor act of articulation brings about competition for limited, overlapping resources and leads to the DTD, perhaps because this requires coordination between articulatory, phonatory, and respiratory processes (51,52). In the present study of healthy older adults, even passive listening (i.e., phoneme-monitoring) had a significant effect on three aspects of gait (i.e., gait speed, swing time and swing time variability), lending support to the capacity sharing model, over the bottleneck theory. Still, if the capacity sharing theory is correct, one has to wonder why a relatively simple task like phoneme-monitoring exceeds capacity and causes a DTD in gait in healthy older adults, both with respect to gait speed and gait variability. It is somewhat surprising that healthy older adults whose gait and balance were largely intact were not able to perform another task without altering their gait pattern.
A possible explanation for the observed decline in walking performance relates to the concept of prioritization. When asked to walk and perform another task, certain subject groups may give inappropriate prioritization to the concurrent task, sacrificing attention resources needed for gait by using a “posture second” strategy (17). In the present study, subjects were not given explicit instructions regarding which task to prioritize (similar to what happens during normal activities of daily living), yet gait was clearly affected by all of the dual tasks. In the future, it would be interesting to examine whether explicit instructions regarding prioritization alter these changes in healthy older adults, as it does in a more impaired cohort (60). Nonetheless, the present results suggest that under normal conditions, healthy older adults do not give full priority to gait during dual task situations.
The present study has several limitations. A ceiling effect likely contributed to the low magnitude of the correlations observed. One can speculate that in a more heterogeneous cohort, the magnitude of the correlations would be higher. On the other hand, the observation of non-zero correlations between the DTD and other factors, even though only subjects with good mobility and cognitive function were included, is an important finding. Our results demonstrate that among healthy older adults such associations exist and, thus, may give us added insight into aging and age-associated changes in gait and dual tasking. Another limitation is that we did not explicitly adjust for the multiple comparisons or control for the timing of the dual tasks with respect to the gait cycle. The loading of attention during phoneme monitoring was relatively constant, however, we did not examine whether subjects timed their serial subtractions to specific events in the gait cycle, possibly minimizing the dual task effects. Perhaps this contributed to the modest nature of the observed associations. Studies that contrast the present findings to those of young adults would also be helpful to shed further light on these issues and on aging of the processes that are involved in the DTD.
Prospective studies that directly examine the clinical relevance of the observed DTDs would also be of interest. Nonetheless, the results from the present study shed some light on this important question. A previous study reported that swing time variability increased by 1.15 % (delta with respect to usual walking performance) during serial 7 subtractions in elderly fallers, while it essentially did not change in elderly non-fallers (4). In the present study, the increase in swing time variability among the subjects who had relatively poor executive function (recall Figure 2) was similar to that previously reported among elderly fallers. This finding suggests that for these subjects the observed changes are likely be associated with an increased risk of falls and supports the idea that to meet the everyday challenges of dual tasking, a consistent gait pattern is influenced, to some degree, by executive function as well as motor abilities.
In conclusion, the present findings provide additional evidence to support the idea that concurrent performance of other tasks affects the gait of healthy older adults. Different factors apparently mediate the changes in gait speed and gait variability in response to the simultaneous performance of another task and both motor and cognitive function apparently influence the DTD in healthy older adults, along with other factors that have yet to be identified. This may explain why the effects of dual tasking are even more pronounced among patients who have impaired mobility and reduced executive function. Furthermore, these findings provide insight into the factors that contribute to the association between dual task performance and fall risk.
Acknowledgments
This work was support in part by the National Institute on Aging (AG14100) and by the Israel Ministry of Absorption. The authors are indebted to the participants and staff of the “Holchim Rachok” project for their invaluable contribution to this project especially Marina Brozgol, Noit Inbar-Borovsky, Leon Maryasin, Aner Weiss and Leor Gruendlinger.
Footnotes
This work was presented in part at the meeting of the International Society of Posture and Gait Research, July, 2007 and at the American Geriatrics Society meeting, Seattle, May, 2007.
References
- 1.Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- 2.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23:329–342. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pajala S, Era P, Koskenvuo M, et al. Contribution of genetic and environmental factors to individual differences in maximal walking speed with and without second task in older women. J Gerontol A Biol Sci Med Sci. 2005;60:1299–1303. doi: 10.1093/gerona/60.10.1299. [DOI] [PubMed] [Google Scholar]
- 4.Springer S, Giladi N, Peretz C, Yogev G, Simon ES, Hausdorff JM. Dual-tasking effects on gait variability: the role of aging, falls, and executive function. Mov Disord. 2006;21:950–957. doi: 10.1002/mds.20848. [DOI] [PubMed] [Google Scholar]
- 5.Shkuratova N, Morris ME, Huxham F. Effects of age on balance control during walking. Arch Phys Med Rehabil. 2004;85:582–588. doi: 10.1016/j.apmr.2003.06.021. [DOI] [PubMed] [Google Scholar]
- 6.Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM. Dual tasking, gait rhythmicity, and Parkinson’s disease: Which aspects of gait are attention demanding? Eur J Neurosci. 2005;22:1248–1256. doi: 10.1111/j.1460-9568.2005.04298.x. [DOI] [PubMed] [Google Scholar]
- 7.Hausdorff JM, Rios D, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82:1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- 8.Barak Y, Wagenaar RC, Holt KG. Gait characteristics of elderly people with a history of falls: a dynamic approach. Phys Ther. 2006;86:1501–1510. doi: 10.2522/ptj.20050387. [DOI] [PubMed] [Google Scholar]
- 9.Ble A, Volpato S, Zuliani G, et al. Executive function correlates with walking speed in older persons: The InCHIANTI study. J Am Geriatr Soc. 2005;53:410–415. doi: 10.1111/j.1532-5415.2005.53157.x. [DOI] [PubMed] [Google Scholar]
- 10.Persad CC, Giordani B, Chen HC, et al. Neuropsychological predictors of complex obstacle avoidance in healthy older adults. J Gerontol B Psychol Sci Soc Sci. 1995;50:272–277. doi: 10.1093/geronb/50b.5.p272. [DOI] [PubMed] [Google Scholar]
- 11.Shumway-Cook A, Guralnik JM, Phillips CL, et al. Age-associated declines in complex walking task performance: the Walking InCHIANTI toolkit. J Am Geriatr Soc. 2007;55:58–65. doi: 10.1111/j.1532-5415.2006.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lundin-Olsson L, Nyberg L, Gustafson Y. “Stops walking when talking” as a predictor of falls in elderly people. Lancet. 1997;349:617. doi: 10.1016/S0140-6736(97)24009-2. [DOI] [PubMed] [Google Scholar]
- 13.Verghese J, Buschke H, Viola L, et al. Validity of divided attention tasks in predicting falls in older individuals: a preliminary study. J Am Geriatr Soc. 2002;50:1572–1576. doi: 10.1046/j.1532-5415.2002.50415.x. [DOI] [PubMed] [Google Scholar]
- 14.Faulkner KA, Redfern MS, Cauley JA, et al. Multitasking: association between poorer performance and a history of recurrent falls. J Am Geriatr Soc. 2007;55:570–576. doi: 10.1111/j.1532-5415.2007.01147.x. [DOI] [PubMed] [Google Scholar]
- 15.Camicioli R, Howieson D, Lehman S, Kaye J. Talking while walking: the effect of a dual task in aging and Alzheimer’s disease. Neurology. 1997;48:955–958. doi: 10.1212/wnl.48.4.955. [DOI] [PubMed] [Google Scholar]
- 16.Coppin AK, Shumway-Cook A, Saczynski JS, et al. Association of executive function and performance of dual-task physical tests among older adults: analyses from the InChianti study. Age Ageing. 2006;35:619–624. doi: 10.1093/ageing/afl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bloem BR, Grimbergen YA, van Dijk JG, Munneke M. The “posture second” strategy: a review of wrong priorities in Parkinson’s disease. J Neurol Sci. 2006;248:196–204. doi: 10.1016/j.jns.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 18.O’Shea S, Morris ME, Iansek R. Dual task interference during gait in people with Parkinson disease: effects of motor versus cognitive secondary tasks. Phys Ther. 2002;82:888–897. [PubMed] [Google Scholar]
- 19.Alexander NB. Gait disorders in older adults. J Am Geriatr Soc. 1996;44:434–451. doi: 10.1111/j.1532-5415.1996.tb06417.x. [DOI] [PubMed] [Google Scholar]
- 20.Sheridan PL, Solomont J, Kowall N, Hausdorff JM. Influence of executive function on locomotor function: divided attention increases gait variability in Alzheimer’s disease. J Am Geriatr Soc. 2003;51:1633–1637. doi: 10.1046/j.1532-5415.2003.51516.x. [DOI] [PubMed] [Google Scholar]
- 21.Charlton RA, Barrick TR, McIntyre DJ, et al. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66:217–222. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- 22.Raz N. Aging of the brain and its impact on cognitive performance: integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. Mahwah, NJ: Lawrence Erlbaum Associates, Inc; 2000. [Google Scholar]
- 23.Holtzer R, Stern Y, Rakitin BC. Age-related differences in executive control of working memory. Mem Cognit. 2004;32:1333–1345. doi: 10.3758/bf03206324. [DOI] [PubMed] [Google Scholar]
- 24.Holtzer R, Stern Y, Rakitin BC. Predicting age-related dual-task effects with individual differences on neuropsychological tests. Neuropsychology. 2005;19:18–27. doi: 10.1037/0894-4105.19.1.18. [DOI] [PubMed] [Google Scholar]
- 25.Verhaeghen P, Cerella J. Aging, executive control, and attention: a review of meta-analyses. Neurosci Biobehav Rev. 2002;26:849–857. doi: 10.1016/s0149-7634(02)00071-4. [DOI] [PubMed] [Google Scholar]
- 26.Colcombe SJ, Kramer AF, Erickson KI, Scalf P. The implications of cortical recruitment and brain morphology for individual differences in inhibitory function in aging humans. Psychol Aging. 2005;20:363–375. doi: 10.1037/0882-7974.20.3.363. [DOI] [PubMed] [Google Scholar]
- 27.Atkinson HH, Rosano C, Simonsick EM, et al. Cognitive function, gait speed decline, and comorbidities: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2007;62:844–850. doi: 10.1093/gerona/62.8.844. [DOI] [PubMed] [Google Scholar]
- 28.Hausdorff JM, Peng CK, Goldberger AL, Stoll AL. Gait unsteadiness and fall risk in two affective disorders: a preliminary study. BMC Psychiatry. 2004;4:39. doi: 10.1186/1471-244X-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemke MR, Wendorff T, Mieth B, Buhl K, Linnemann M. Spatiotemporal gait patterns during over ground locomotion in major depression compared with healthy controls. J Psychiatr Res. 2000;34:277–283. doi: 10.1016/s0022-3956(00)00017-0. [DOI] [PubMed] [Google Scholar]
- 30.Herman T, Giladi N, Gurevich T, Hausdorff JM. Gait instability and fractal dynamics of older adults with a “cautious” gait: why do certain older adults walk fearfully? Gait Posture. 2005;21:178–185. doi: 10.1016/j.gaitpost.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 31.Menz HB, Lord SR, Fitzpatrick RC. Acceleration patterns of the head and pelvis when walking are associated with risk of falling in community-dwelling older people. J Gerontol A Biol Sci Med Sci. 2003;58:M446–M452. doi: 10.1093/gerona/58.5.m446. [DOI] [PubMed] [Google Scholar]
- 32.Menz HB, Lord SR, Fitzpatrick RC. A structural equation model relating impaired sensorimotor function, fear of falling and gait patterns in older people. Gait Posture. 2007;25:243–249. doi: 10.1016/j.gaitpost.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 34.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 35.Wade DT, Collin C. The Barthel ADL Index: a standard measure of physical disability? Int Disabil Stud. 1988;10:64–67. doi: 10.3109/09638288809164105. [DOI] [PubMed] [Google Scholar]
- 36.Turnbull JC, Kersten P, Habib M, McLellan L, Mullee MA, George S. Validation of the Frenchay Activities Index in a general population aged 16 years and older. Arch Phys Med Rehabil. 2000;81:1034–1038. doi: 10.1053/apmr.2000.7162. [DOI] [PubMed] [Google Scholar]
- 37.Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52:643–651. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 38.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Dwolatzky T, Whitehead V, Doniger GM, et al. Validity of a novel computerized cognitive battery for mild cognitive impairment. BMC Geriatr. 2003;3:4. doi: 10.1186/1471-2318-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schweiger A, Doniger GM, Dwolatzky T, Jaffe D, Simon ES. Reliability of a novel computerized neuropsychological battery for mild cognitive impairment. Acta Neuropsychologica. 2003;1:407–413. [Google Scholar]
- 41.Schweiger A, Abramovitch A, Doniger GM, Simon ES. A clinical construct validity study of a novel computerized battery for the diagnosis of ADHD in young adults. J Clin Exp Neuropsychol. 2007;29:100–111. doi: 10.1080/13803390500519738. [DOI] [PubMed] [Google Scholar]
- 42.Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Exp Brain Res. 2005;164:541–548. doi: 10.1007/s00221-005-2280-3. [DOI] [PubMed] [Google Scholar]
- 43.Berg K, Wood-Dauphinee S, Williams JI. The Balance Scale: reliability assessment with elderly residents and patients with an acute stroke. Scand J Rehabil Med. 1995;27:27–36. [PubMed] [Google Scholar]
- 44.Chiu YP, Fritz SL, Light KE, Velozo CA. Use of item response analysis to investigate measurement properties and clinical validity of data for the dynamic gait index. Phys Ther. 2006;86:778–787. [PubMed] [Google Scholar]
- 45.Whitney SL, Hudak MT, Marchetti GF. The dynamic gait index relates to self-reported fall history in individuals with vestibular dysfunction. J Vestib Res. 2000;10:99–105. [PubMed] [Google Scholar]
- 46.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 47.AGS Guidelines. Guideline for the prevention of falls in older persons. American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. J Am Geriatr Soc. 2001;49:664–672. [PubMed] [Google Scholar]
- 48.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A:M28–M34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 49.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 50.Spielberger C, et al. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: 1983. State-Trait Anxiety Inventory. Self-evaluation questionnaire (form Y) [Google Scholar]
- 51.Dault MC, Yardley L, Frank JS. Does articulation contribute to modifications of postural control during dual-task paradigms? Brain Res Cogn Brain Res. 2003;16:434–440. doi: 10.1016/s0926-6410(03)00058-2. [DOI] [PubMed] [Google Scholar]
- 52.Yardley L, Gardner M, Leadbetter A, Lavie N. Effect of articulatory and mental tasks on postural control. Neuroreport. 1999;10:215–219. doi: 10.1097/00001756-199902050-00003. [DOI] [PubMed] [Google Scholar]
- 53.Frenkel-Toledo S, Giladi N, Peretz C, Herman T, Gruendlinger L, Hausdorff JM. Effect of gait speed on gait rhythmicity in Parkinson’s disease: variability of stride time and swing time respond differently. J NeuroEng Rehabil. 2005;2 doi: 10.1186/1743-0003-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. J Am Geriatr Soc. 1997;45:313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 55.Schaafsma JD, Giladi N, Balash Y, Bartels AL, Gurevich T, Hausdorff JM. Gait dynamics in Parkinson’s disease: relationship to Parkinsonian features, falls and response to levodopa. J Neurol Sci. 2003;212:47–53. doi: 10.1016/s0022-510x(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 56.Brach JS, Studenski SA, Perera S, VanSwearingen JM, Newman AB. Gait variability and the risk of incident mobility disability in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007 doi: 10.1093/gerona/62.9.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tombu M, Jolicoeur P. A central capacity sharing model of dual-task performance. J Exp Psychol Hum Percept Perform. 2003;29:3–18. doi: 10.1037//0096-1523.29.1.3. [DOI] [PubMed] [Google Scholar]
- 58.Posner MI, Sheese BE, Odludas Y, Tang Y. Analyzing and shaping human attentional networks. Neural Netw. 2006;19:1422–1429. doi: 10.1016/j.neunet.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 59.Ruthruff E, Pashler HE, Klaassen A. Processing bottlenecks in dual-task performance: structural limitation or strategic postponement? Psychon Bull Rev. 2001;8:73–80. doi: 10.3758/bf03196141. [DOI] [PubMed] [Google Scholar]
- 60.Verghese J, Kuslansky G, Holtzer R, et al. Walking while talking: effect of task prioritization in the elderly. Arch Phys Med Rehabil. 2007;88:50–53. doi: 10.1016/j.apmr.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]