Abstract
Background
Atrial fibrillation (AF) is an important risk factor for stroke and overall mortality but information about the preventable burden of AF is lacking. The aim of this study was to determine what proportion of the burden of AF in African-Americans and whites could theoretically be avoided by the maintenance of an optimal risk profile.
Methods and Results
This study included 14,598 middle-aged, Atherosclerosis Risk in Communities Study cohort members. Previously established AF risk factors, namely high blood pressure, elevated body mass index, diabetes, cigarette smoking and prior cardiac disease were categorized into ‘optimal’, ‘borderline’ and ‘elevated’ levels. Based on their risk factor levels, individuals were classified into one of these three groups. The population attributable fraction of AF due to having a non-optimal risk profile was estimated separately for African-American and white men and women.
During a mean follow-up of 17.1 years, 1520 cases of incident AF were identified. The age-adjusted incidence rates were highest in white men and lowest in African-American women (7.45 and 3.67 per 1000 person-years, respectively). The overall prevalence of an optimal risk profile was 5.4% but this varied according to race and gender: 10% in white women versus 1.6% in African-American men. Overall, 56.5% of AF cases could be explained by having ≥ 1 elevated risk factors, of which elevated blood pressure was the most important contributor.
Conclusions
As with other forms of cardiovascular disease, more than half of the AF burden is potentially avoidable through the optimization of cardiovascular risk factors levels.
Keywords: arrhythmia, risk factors, epidemiology
INTRODUCTION
Atrial fibrillation (AF) is one of the most commonly diagnosed cardiac arrhythmias in clinical practice, affecting 2.3 million people in the United States (US) 1. Individuals with AF are at substantially increased risk of stroke, and have twice the mortality rate from cardiovascular disease (CVD) and overall compared with those with normal sinus rhythm 2,3. Moreover, AF is responsible for one-third of all hospitalizations for cardiac rhythm disturbances and consequently is associated with significant health care costs that in the US alone exceed $6 billion annually 4.
Aside from age, established risk factors for AF include prior cardiac disease and raised blood pressure (BP), and to a lesser extent, type-2 diabetes, obesity and cigarette smoking 5. Hence, because of the aging US population, combined with both improved survival rates after a coronary event and increasing mean population levels of diabetes and obesity, the number of AF cases is expected to increase to 6-12 million by 2050 1,6.
Compared with coronary heart disease (CHD), heart failure (HF) and stroke, where it has been estimated that 65 - 90% of events are potentially avoidable through maintaining “optimal” cardiovascular risk factors levels 7,8, it is presently unknown what fraction of AF is potentially avoidable by a similar optimization of pertinent risk factor levels. Moreover, given that the relationships between risk factors and cardiovascular events are largely continuous 9 and that the majority of the population, and hence events, tend to occur within individuals displaying “borderline” as opposed to “elevated” risk factor levels 10, consideration of the impact of having a borderline risk factor profile is also merited. The Atherosclerosis Risk in Communities (ARIC) Study provides an ideal opportunity to study the association between risk factor profile and the incidence of AF in both whites and African-Americans 11.
METHODS
Study design and subjects
The ARIC Study is a prospective cohort study of atherosclerotic diseases within four communities in the United States: Forsyth County, North Carolina; Jackson, Mississippi; Washington County, Maryland; and the northwest suburbs of Minneapolis, Minnesota. The recruitment of study participants is described in detail elsewhere 11. Briefly, the cohort comprised at baseline in 1987-89, 15 792 men and women aged 45 – 64 years who were selected by list of area probability sampling. The baseline home interview and clinic examination measured various risk factors and cardiovascular conditions. Three triennial study visits occurred subsequently with the last visit in 1996-98. Additionally, participants or their proxy were contacted annually by telephone to ascertain hospitalizations and death. The ARIC Study protocol was approved by the institutional review board of each participating university and informed consent was obtained from each study participant.
Definition of exposure categories
Based on previous evidence we categorized individuals as having an optimal AF risk factor profile if at baseline they met the following criteria 5,12-14: no history of cardiac disease (HF or CHD); systolic blood pressure (SBP) < 120 mmHg and diastolic BP (DBP) < 80 mmHg and no use of antihypertensive medication; body mass index (BMI) < 25 kg/m2; fasting serum glucose (FSG) < 100 mg/dL and no use of anti-diabetic medication and no history of physician-diagnosed diabetes; and never smoker. A borderline risk factor profile was defined as having any of the following criteria and no elevated risk factor profile characteristics (see below): SBP 120 - 139 mmHg and/or DBP 80 - 89 mmHg, and no use of antihypertensive medication; BMI 25 -< 30 kg/m2; FSG 100-125 mg/dL and no use of anti-diabetic medication and no history of physician-diagnosed diabetes; and former smoker. An elevated risk factor profile was defined as having any of the following criteria: history of cardiac disease (HF or CHD); SBP ≥ 140 mmHg or DBP ≥ 90 mmHg or use of antihypertensive medication; BMI ≥ 30 kg/m2; FSG ≥126 mg/dL or use of anti-diabetic medication or history of physician-diagnosed diabetes; or current smoker.
Baseline examination
Blood collection and processing techniques have been previously described. In brief, serum glucose was measured by a hexokinase/glucose-6-phosphate dehydrogenase method 15. Sitting BP was measured three times using a random-zero sphygmomanometer after 5 minutes of rest. The mean of the last two measurements were used for the analysis. Self-reported use of antihypertensive medications within the past two weeks was collected at baseline. Smoking status (current, former or never smokers) was derived from interviews. BMI (kg/m2) was computed from weight in a scrub suit and standing height. The primary analysis was conducted in all individuals without a history of AF and a secondary analysis was conducted after exclusion of individuals with prevalent CHD and heart failure at baseline. Prevalent CHD included individuals with a history of myocardial infarction (MI), MI adjudicated from the baseline ECG, or history of coronary bypass or angioplasty 15. Prevalent heart failure (HF) cases were identified if any of the two conditions as follows was true 16,17: a “yes” in response to the question “Were any of the medications you took during the last 2 weeks for heart failure?”; presence of stage 3 or ‘manifest HF’ as defined using the Gothenburg criteria 16. All current medications (taken within the preceding two weeks) were brought into the clinic and documented.
Outcome Ascertainment
Individuals with evidence of AF or atrial flutter on an electrocardiogram (ECG) at study baseline were excluded from this analysis. Diagnoses of incident AF and atrial flutter were obtained through the end of 2007 from three sources: ECGs done at study visits (visits 2 – 4), presence of an International Classification of Disease (ICD9) code for AF (427.31 or 427.32) in a hospital discharge, or AF listed as any cause of death on a death certificate. Hospitalizations with AF associated with open cardiac surgery were not considered events. Date of AF incidence was the earliest of any AF diagnosis. All ARIC examination ECGs were recorded using MAC PC Personal Cardiographs (Marquette Electronics, Inc, Milwaukee, WI). A standard supine 12-lead resting ECG was recorded at each clinic visit and was transmitted by modem to the ARIC ECG Reading Center for automatic reading and coding. All AF cases that were automatically detected from the study ECGs were visually rechecked by a cardiologist 15. Prior analysis within the ARIC cohort to determine the validity of hospital discharge diagnoses for AF reported 84% sensitivity and 98% specificity in the ascertainment of AF events 18.
Statistical analysis
Of the 15,792 initial ARIC participants, 1194 were excluded for the following reasons: non-white and non-African-American (n = 48); prevalent AF or atrial flutter (n = 37); no ECG or unreadable at baseline (n = 243); non-fasting blood sample (n = 592); missing covariates (n = 274). Means and standard deviations (SD) for the baseline continuous variables and percentages for the categorical variables were calculated separately for men and women and for whites and African-Americans.
Age- and gender-standardized prevalence of optimal, borderline and elevated risk factors were determined at study baseline (1987-89). The age- and gender-adjusted incidence of AF by levels of optimal risk factors were estimated separately in whites and African-Americans using Poisson regression. Person-years of follow-up were derived from the baseline examination until a first diagnosis of AF, loss to follow-up, death, or else until Dec 31, 2007. Associations of risk factor profile at baseline with the incidence of AF were estimated using Cox proportional hazards models that were adjusted for age, study site, education, income and height. Race and gender-specific analyses were conducted. The assumption of proportional hazards was examined by adding to the model an interaction term between follow-up time and exposure of interest, computing Schoenfeld residuals, and by inspection of the log(- log[survival function]) curves. A SAS macro developed by Zhang and colleagues 19 was used to estimate direct adjusted survival curves for each race/gender category, based on a stratified Cox model.
Population attributable fractions (PAFs) were calculated to determine the possible impact of altering risk profiles on AF occurrence. PAFs were computed according to the following formula 20:
where pdi is the proportion of cases falling into ith exposure level and RRi is the relative risk comparing ith exposure level with unexposed group (i=0). Poisson models were used to obtain the RR. To estimate the PAFs for each of the five risk factors, models were adjusted for age, study site, education, income, height and additionally for each of the other risk factors under investigation (i.e. SBP, BMI, diabetes and smoking). One thousand bootstrap samples were created to obtain the 95% confidence intervals for PAFs.
RESULTS
Prevalence of risk categories by race and gender
At baseline, the mean age (SD) of the 14 598 (55% female; 25% African-American) participants included in this analysis was 54.2 years (5.8). Table 1 shows both the race- and gender-specific prevalence of optimal, borderline and elevated levels of blood pressure, BMI, diabetes, smoking and prior cardiac disease at study baseline.. Overall, just over 5% of the cohort had optimal risk factor levels, just over one-quarter had one or more borderline risk factor levels and two-thirds of the cohort had one or more elevated risk factors at study baseline (Table 2). Over 80% of African-Americans had one or more elevated risk factors compared with approximately 60% in whites.
Table 1.
Distribution (%) of individual risk factors by race and gender in the Atherosclerosis Risk in Communities (ARIC) study at baseline (1987-1989)
| White (n = 10933) |
African-American (n = 3665) |
||||
|---|---|---|---|---|---|
| Total | Women (n=5788) |
Men (n=5145) |
Women (n=2266) |
Men (n=1399) |
|
| Age, mean years (SD) | 54.2 (5.8) | 54.0 (5.7) | 54.8 (5.7) | 53.3 (5.7) | 53.9 (6.0) |
| Blood pressure (%) | |||||
| Optimal | 38.5 | 47.2 | 41.1 | 21.3 | 21.2 |
| Borderline | 22.7 | 20.0 | 26.4 | 20.3 | 24.1 |
| Elevated | 38.7 | 32.8 | 32.4 | 58.4 | 54.7 |
| BMI (%) | |||||
| Optimal | 33.5 | 46.3 | 27.0 | 17.5 | 30.2 |
| Borderline | 39.5 | 30.5 | 51.0 | 34.7 | 42.5 |
| Elevated | 27.0 | 23.2 | 22.0 | 47.8 | 27.2 |
| Diabetes (%) | |||||
| Optimal | 51.8 | 61.7 | 43.6 | 48.7 | 46.1 |
| Borderline | 37.7 | 30.5 | 46.6 | 34.5 | 39.3 |
| Elevated | 10.5 | 7.76 | 9.74 | 16.8 | 14.6 |
| Smoking (%) | |||||
| Optimal | 41.6 | 50.8 | 28.0 | 57.6 | 28.2 |
| Borderline | 32.7 | 24.5 | 48.2 | 17.4 | 34.2 |
| Elevated | 25.7 | 24.7 | 23.9 | 25.0 | 37.6 |
| Hist. Cardiac Disease (%) | |||||
| Optimal | 91.8 | 94.2 | 89.7 | 90.4 | 92.0 |
| Elevated | 8.22 | 5.84 | 10.3 | 9.62 | 8.01 |
Hist. cardiac disease = history of cardiac disease (heart failure or coronary disease): optimal = no history, elevated = history of either. Blood pressure: optimal = Systolic blood pressure (SBP) <120 mmHg and diastolic blood pressure (DBP) < 80 mmHg, borderline = SBP 120-139 mmHg or DBP 80-90 mmHg (pre-hypertension), elevated = SBP≥140 mmHg or DBP ≥ 90 mmHg and/or treatment for hypertension. Body mass index (BMI): optimal = <25 kg/m2, borderline = 25- <30 kg/m2 and elevated+ ≥ 30 kg/m2. Diabetes: optimal = fasting serum glucose (FSG) < 100 mg/dl and no history of diabetes, borderline = FSG 100 – 125 mg/dl and no history of diabetes, elevated = FSG ≥ 126 mg/dl or diabetic. Smoking: optimal = never smoker, borderline = former smoker, elevated = current smoker.
Table 2.
Prevalence (%) Baseline Risk Factors by Race and Gender in the Atherosclerosis Risk in Communities (ARIC), 1987-1989
| Risk Factor Category |
No. of Elevated Risk Factors |
No. of Borderline Risk Factors |
Total (n=14598) |
Women (White) (n=5788) |
Men (White) (n=5145) |
Women (African- American) (n=2266) |
Men (African- American) (n=1399) |
|---|---|---|---|---|---|---|---|
| All risk factor levels optimal |
0 | 0 | 5.44 | 9.99 | 2.72 | 2.34 | 1.64 |
| 0 | 1 | 10.3 | 14.9 | 8.71 | 5.43 | 4.72 | |
| 0 | 2 | 9.99 | 9.83 | 12.7 | 6.09 | 7.15 | |
| Borderline risk factor levels only |
0 | 3 | 5.92 | 4.15 | 9.93 | 2.25 | 4.43 |
| 0 | 4 | 1.64 | 0.52 | 3.48 | 0.53 | 1.36 | |
| Total | 27.8 | 29.4 | 34.8 | 14.3 | 17.7 | ||
| 1 | Any | 35.7 | 36.3 | 36.4 | 32.0 | 37.4 | |
| Elevated risk factor level |
2 | Any | 20.8 | 16.5 | 18.2 | 32.7 | 29.2 |
| 3 | Any | 8.10 | 6.39 | 6.36 | 14.9 | 10.6 | |
| 4 | Any | 1.86 | 1.30 | 1.55 | 3.31 | 3.00 | |
| 5 | Any | 0.21 | 0.16 | 0.10 | 0.44 | 0.50 | |
| Total | 66.7 | 60.6 | 62.6 | 83.4 | 80.7 | ||
Incidence of atrial fibrillation
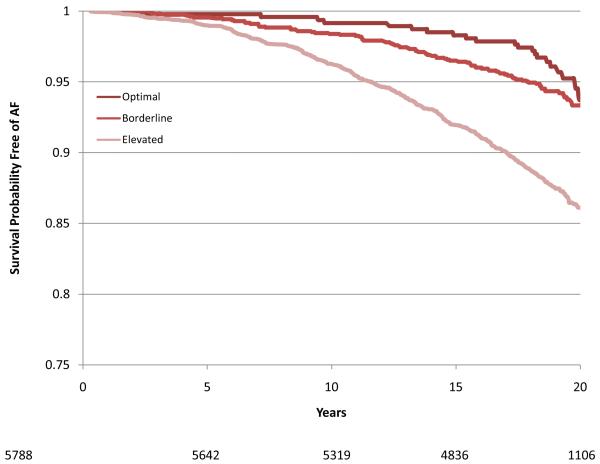
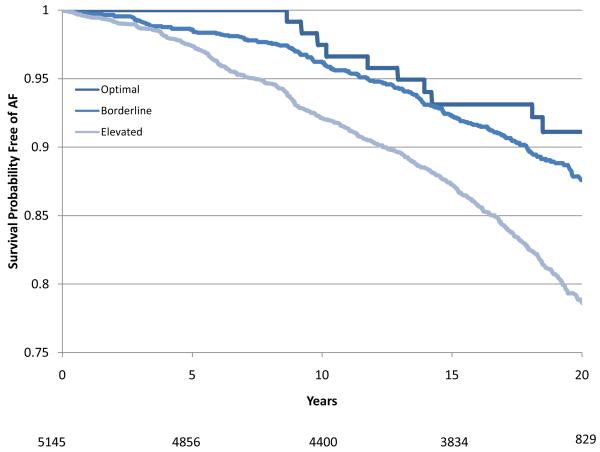
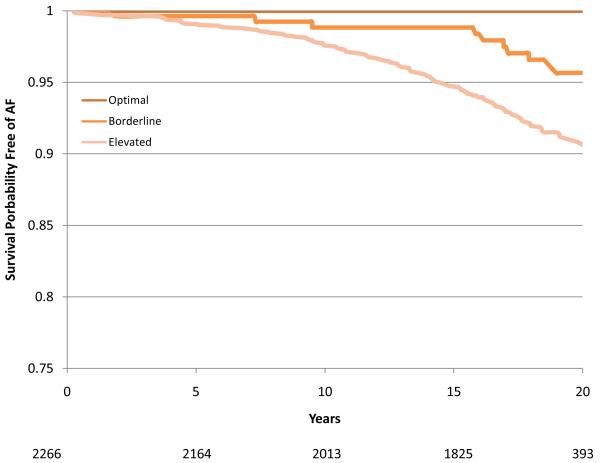
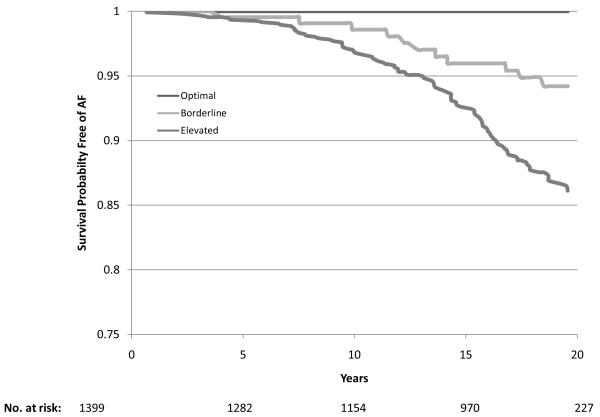
During a mean follow-up of 17.1 years there were 1520 cases of incident AF. Of these, 98.8% were identified from hospitalizations, 7.8% from study ECGs, and 5.5% from death certificates (some cases were identified by more than one method). The age-adjusted incidence rates were 7.45, 4.59, 5.27 and 3.67 per 1000 person-years in white men, white women, African-American men and African-American women, respectively. Compared with those with no risk factors, the age-adjusted incidence rates were three times higher in those with one or more elevated risk factors: 2.19 versus 6.59 per 1000 person years, respectively (Table 3). Individuals with one or more borderline risk factor levels had intermediate incidence rates. For any category of risk factor levels, white men had the highest rates and African-American women the lowest (Table 3). Overall, among individuals exhibiting an optimal risk factor profile the multiple-adjusted relative hazard (RH) (95% CI) of AF was 0.33 (0.23 – 0.47) and in those with one or more borderline risk factors it was 0.50 (0.44 – 0.57) compared with those with one or more elevated risk factors. These estimates were comparable across the four race and gender groups (Table 3). The gender and race-specific survival curves for the time spent free from AF according to risk factor profile are shown in Figure 1 (a-d). In a sensitivity analysis that included only those individuals who had their AF diagnosed at a study visit (n = 119), 25 cases of AF occurred among those with a borderline risk factor profile and 94 occurred in those with an elevated risk factor profile (no events occurred in the optimal risk profile group). The relative hazard (95% CI) for having a borderline risk factor profile compared with having an elevated risk profile was 0.56 (0.36 to 0.87).
Table 3.
Incidence rate, relative hazard (95% confidence intervals) and population attributable fraction for atrial fibrillation by race and gender in the Atherosclerosis Risk in Communities (ARIC) study, 1987-2007
| Risk Profile | No. at Risk |
No. of incident AF |
Incidence Rate* |
RH (95% CI) † | PAF % | 95% CI |
|---|---|---|---|---|---|---|
| Total | ||||||
| Optimal RF | 794 | 31 | 2.19 | 0.33 (0.23-0.47) | 0.00 | - |
| Borderline RF only | 4064 | 288 | 3.68 | 0.50 (0.44-0.57) | 6.53 | 1.28 to 11.3 |
| Elevated RF | 9740 | 1201 | 6.59 | 1 [Ref] | 50.0 | 37.5 to 58.5 |
| White Female | ||||||
| Optimal RF | 578 | 21 | 2.02 | 0.33 (0.21-0.52) | 0.00 | - |
| Borderline RF only | 1704 | 89 | 2.68 | 0.45 (0.36-0.56) | 4.29 | −2.73 to 10.1 |
| Elevated RF | 3506 | 415 | 6.04 | 1 [Ref] | 50.0 | 34.4 to 61.1 |
| White Male | ||||||
| Optimal RF | 140 | 10 | 3.95 | 0.40 (0.21-0.75) | 0.00 | - |
| Borderline RF only | 1789 | 179 | 5.17 | 0.55 (0.46-0.65) | 5.85 | −7.40 to 16.8 |
| Elevated RF | 3216 | 538 | 9.07 | 1 [Ref] | 38.2 | 12.7 to 57.5 |
| African-American | ||||||
| Female** | ||||||
| Optimal | 53 | 0 | 0.00 | --- | 0.00 | - |
| Borderline RF | 324 | 9 | 1.69 | 0.42 (0.21-0.83) | 1.57 | −0.72 to 4.50 |
| Elevated RF | 1889 | 141 | 4.10 | 1 [Ref] | 59.5 | 44.2 to 73.5 |
| African-American Male‡ | ||||||
| Optimal | 23 | 0 | 0.00 | --- | 0.00 | - |
| Borderline RF | 247 | 11 | 2.63 | 0.41 (0.22-0.76) | 2.63 | −2.07 to 7.47 |
| Elevated RF | 1129 | 107 | 6.04 | 1 [Ref] | 49.4 | 21.9 to 73.3 |
Indicates the incidence rate of AF per 1000 person-years adjusted for age (mean age=54.2 years).
Adjusted for age, study site, education, income and height.
African-American females and males did not have any incident cases of AF in the optimal group, so optimal and borderline were combined and PAF from the whole population was applied to obtain the PAF. PAF = population attributable fraction; RH = relative hazard; CI = 95% confidence intervals; RF = risk factors.
Figure 1.
Survival curves adjusted for age, study center, education and height showing time free from atrial fibrillation according to risk factor group (Optimal, Borderline or Elevated) in White women (1A), White men (1B), African-American women (1C) and African-American men (1D). The number of subjects at risk throughout the duration of study follow-up are shown on the x-axis.
Overall, the PAF estimates indicated that having one or more elevated risk factor levels could explain 50% (95% CI 37.5 to 58.5%) of AF events (Table 3). In whites, the PAF estimates were comparable in women and men: 50% and 38.2%, respectively. In African-Americans, the PAF estimates associated with having one or more elevated risk factors were 94% and 91% in women and men, respectively. However, this PAF estimate is subject to considerable variability due to small numbers in the optimal risk factor category (n = 83), and an alternative estimate based on the RR in the total population of whites and African-Americans and the risk factor prevalence in African-Americans yielded a PAF of 49.4% in African-American men and 59.5% in women (Table 3). Borderline levels of risk factors explained an additional 6.5% of all AF cases. Thus, the proportion of all AF cases that could be explained by having one or more borderline or elevated risk factor levels ranged from 44% in white men to 61% in African-American women (Table 3). After exclusion of those 1412 individuals with a prior history of cardiac disease at baseline, the overall percentage of AF incident cases that could be attributed to one or more elevated and borderline risk factors was reduced to 59.8%, 46.6%, 49.7% and 36.6% in African-American women, African-American men, white women and white men, respectively.
Examination of the PAFs associated with each of the five risk factors in turn indicated that elevated blood pressure, which affected 38.7% of the entire cohort, was the most important contributor accounting for 21.6% (95% CI: 16.8 to 26.7%) of incident AF cases. This rose to 24.5% if borderline levels of blood pressure, which affected a further 22.7% of the cohort, were also included (Table 4). Obesity and overweight explained 17.9% of all AF cases and diabetes and impaired glucose tolerance combined, accounted for the smallest fraction of the AF burden in this cohort (3.9%; Table 4).
Table 4.
Incidence rate, relative hazard (95% confidence intervals) and population attributable fractions for atrial fibrillation for risk factors in the Atherosclerosis Risk in Communities (ARIC) study, 1987 – 2007
| No. at Risk |
No. Incident AF |
IR* | RH (95% CI) † | PAF % | 95% CI | |
|---|---|---|---|---|---|---|
| History of Cardiac Disease (%) |
||||||
| Optimal | 13398 | 1259 | 5.00 | 0.54 (0.46-0.62) | 0.00 | - |
| Elevated | 1200 | 261 | 12.17 | 1 [Ref] | 5.35 | 3.32 to 7.45 |
| Blood pressure (%) | ||||||
| Optimal | 5626 | 381 | 3.93 | 0.55 (0.48-0.63) | 0.00 | - |
| Borderline | 3317 | 304 | 4.72 | 0.65 (0.56-0.74) | 2.89 | −0.11 to 5.64 |
| Elevated | 5655 | 835 | 7.65 | 1 [Ref] | 21.6 | 16.8 to 26.7 |
| BMI (%) | ||||||
| Optimal | 4889 | 389 | 4.27 | 0.65 (0.56-0.74) | 0.00 | - |
| Borderline | 5767 | 591 | 5.28 | 0.70 (0.62-0.79) | 5.16 | 0.93 to 9.26 |
| Elevated | 3942 | 531 | 7.36 | 1 [Ref] | 12.7 | 9.30 to 16.3 |
| Diabetes (%) | ||||||
| Optimal | 7558 | 645 | 4.68 | 0.67 (0.58-0.78) | 0.00 | - |
| Borderline | 5491 | 617 | 5.83 | 0.71 (0.61-0.82) | 0.78 | −3.52 to 4.84 |
| Elevated | 1533 | 253 | 8.77 | 1 [Ref] | 3.08 | 0.91 to 5.30 |
| Smoking (%) | ||||||
| Optimal | 6077 | 510 | 4.23 | 0.55 (0.48-0.62) | 0.00 | - |
| Borderline | 4769 | 550 | 5.76 | 0.60 (0.52-0.68) | 2.06 | −2.05 to 6.05 |
| Elevated | 3752 | 460 | 7.45 | 1 [Ref] | 9.78 | 6.74 to 12.9 |
IR = Incidence Rate of AF per 1000 person-years adjusted for age (mean age = 54.2 years);
Adjusted for age, gender, race, study site, education, income and height and each of the other risk factors.
DISCUSSION
Overall, in this US cohort of middle-aged adults who had been followed prospectively for, on average, 17 years, 57% of incident AF (95% confidence interval 38% - 70%) could be attributed to elevated or borderline levels of risk factors for AF namely: elevated blood pressure, overweight/obesity, diabetes, smoking and prior cardiac disease. The PAF estimates were broadly consistent across the race and gender groups although they were slightly higher for African-Americans than for whites, in part, reflecting the greater prevalence of underlying risk factors in African-Americans. It is also possible that the PAF in African-Americans was underestimated. As explained previously, if the race-specific estimates had been used, then all of the AF burden in African-Americans would have been explained by having one or more borderline or elevated risk factors. But given that there were no events within those African-Americans with an optimal risk profile the variability around this estimate was considered to be too substantial to be meaningful. Hence, it was considered more appropriate to use risk estimates from the total-population, as opposed to race-specific risk estimates.
This is only the second study to attempt to quantify the burden of AF due to major and modifiable risk factors. The previous study was based on the Framingham cohort which reported that combined, cigarette smoking, diabetes, hypertension and prevalent CHD explained 44% of the burden in men and 58% in women.13 These estimates are broadly comparable with those of the current study, which also sought to quantify the burden of AF due to having borderline, rather than elevated, levels of risk factors. This is an important consideration given that a significant proportion of the population has suboptimal levels of BMI, blood pressure and blood glucose. In addition, we provide estimates for both whites and African-Americans.
Elevated blood pressure was the most important contributor to the burden of AF. It explained more than one-fifth of all AF cases and nearly one-quarter if borderline blood pressure was also included. In comparison, only 3% of AF cases were attributable to diabetes, which in part reflects the much higher prevalence of elevated blood pressure in the study cohort (38.7%) compared with diabetes (10.1%). The current finding that elevated levels of risk factors explained 50% of the overall incidence of AF in the ARIC cohort is lower than previously reported ARIC estimates of the PAF for heart failure and CHD and stroke (63.6% and 70.2% due to non-optimal risk factor levels, respectively) 7,8 suggests that other factors, possibly genetic, may have a greater role in the etiology of AF. Indeed, studies have indicated that the heritability component of AF is larger than it is for either heart failure or coronary artery disease: 62% versus 28% versus 50%, respectively 21-23. It is also possible that the PAFs estimated in the current study may actually have underestimated the contribution of individual risk factors, such as obesity to the incidence of AF. Obesity may increase the risk of AF through several different physiological pathways, for example, by increasing the risk of diabetes or hypertension that in turn increase the risk of AF. However, the model used in the current study only estimated the direct, and independent, contribution of obesity on subsequent risk of AF and did not take into account the possible indirect pathways by which obesity may impact on future risk of AF.
Potential for primary prevention
From a public health perspective our data highlight the substantial potential for AF risk reduction through primary prevention strategies that target modification and improvement in behavioral (e.g. cigarette smoking, sedentary lifestyle) and dietary risk factors (e.g. salt intake, excess calorie consumption). Moreover, as improvement in these behaviors would also impact favorably on other AF risk factors such as diabetes and impaired glucose tolerance, the reduction in incidence of AF would be even greater than that expected through blood pressure lowering alone. However, it should be noted that the current PAF estimates refer only to the avoidance, rather than the reversal, of suboptimal levels of risk factors in individuals with, for example, a history of obesity, hypertension or diabetes. In such individuals, it is possible that long-term exposure to the effects of these morbidities may cause irreversible damage to the atrium. Consequently, the risk of AF may remain elevated in such individuals, even if levels of risk factors were to be normalized.
Possible explanations for racial disparity in incidence rates of AF
Individuals exhibiting an optimal risk profile had one-third the incidence rate of AF compared with those with elevated risk factors. The rates at each risk profile were markedly lower in African-Americans than in whites, especially men. The reasons for this racial difference are unknown but unlikely to be explained by differences in risk factor levels, particularly as levels of hypertension and obesity were higher in African-Americans compared with Whites. As discussed in a previous publication, the racial difference is also unlikely to be due to lower case ascertainment or poorer access to medical care in African-Americans compared with whites 18. Hence, these data imply that among otherwise healthy White individuals there are unknown risk factors, possibly genetic or other yet unknown factors, which are important determinants of AF risk 21,24.
Strengths and Limitations
In addition to the 20-year follow-up, other strengths of the study include information on a wide range of variables to allow for adjustment, although as with any observational study it is not possible to fully exclude the possibility of residual confounding. There are however, some important limitations. First, we were unable to differentiate subtypes of AF and, hence, are assuming that the associations between risk factors and outcome are consistent across AF subtypes. This assumption is not unreasonable given that some studies, which have been able to differentiate AF subtypes have not reported any difference in the magnitude of the associations. For example, Dublin and colleagues observed no significant difference in the positive relationship between diabetes and AF according to whether it was paroxysmal, persistent or permanent 25. Second, as discussed in a previous ARIC publication 18 as cases of AF were mainly ascertained through hospital discharge codes, this may have led to under-ascertainment of cases that perhaps, were not severe enough to warrant hospitalization. The third major limitation relates to possible misclassification of study participants over the course of the follow-up period. For example, in an extreme case scenario, a participant with AF may have had an optimal risk profile at baseline but over time, may have become obese, hypertensive and diabetic, resulting in an elevated risk factor profile. However, as this individual would have been classified as having an optimal profile (at baseline) this would have resulted in an underestimation of the impact of these risk factors on subsequent risk of AF. Third, as is common in this type of analysis we assumed censoring to be ignorable, that is, we took into account the fact that some individuals may have died from CHD before developing AF, by adopting the common solution to this problem, which was to adjust for all factors that are likely causes of both AF and CHD namely hypertension, diabetes, smoking, obesity, and heart failure, as well as age, gender and race. For this reason, we believe our assumption that censoring is ignorable is correct to a reasonable approximation, although it is also possible that we did not adjust for all possible factors.
Finally, as more than 98% of AF cases were diagnosed through hospital discharge forms, the PAF’s may be more indicative of only the severest forms of AF that require hospitalization. For example, individuals with hypertension and diabetes require more frequent medical contact, and hence, may be more likely to have their AF detected compared with those without these conditions and who may be asymptomatic for AF. However, in a sensitivity analysis that included only those cases of AF diagnosed by ECG at a study visit, the results were highly comparable with the result from the overall population. Further evidence to support the generalizability of the study findings comes from a comparison of AF incidence rates in ARIC with those from other population-based cohorts that were less reliant on hospitalized records for diagnosis of AF. For example, in Framingham, the incidence rates for AF per 1000 person-years in men and women aged 55-64 years were 6.2 and 3.8, respectively, which are compatible with the incidence rates reported here.13 Moreover, and importantly, the population diversity in ARIC is greater than in previous cohorts comprising as it does individuals from four states in the United States and a significant proportion of African-Americans.
In summary, findings from this well-characterized cohort of middle-aged men and women indicate that maintaining an optimal risk profile would theoretically avoid more than half of the overall burden of AF. This study further reinforces the need for successful primary prevention strategies that enable individuals to adopt and maintain healthy diet and behavioral patterns as a means of reducing future cardiovascular risk.
CLINICAL PERSPECTIVE.
This study, which is based on more than 17 years follow-up in over 14,500 men and women, represents an important contribution to the literature on the major and modifiable causes of atrial fibrillation (AF). AF is an important risk factor for stroke and overall mortality and affects between 0.4 to 1.0% of the US population AF. Unlike other forms of cardiovascular disease, information about the preventable burden of AF is lacking. The aim of this study therefore was to determine what proportion of the burden of AF in African-Americans and whites could theoretically be avoided by the maintenance of an optimal risk profile. Previously established modifiable AF risk factors, namely high blood pressure, elevated body mass index, diabetes, cigarette smoking and prior cardiac disease were categorized into ‘optimal’, ‘borderline’ and ‘elevated’ levels. Based on their risk factor levels, individuals were classified into one of these three groups. Overall, 57% of AF cases could be explained by having one or more elevated risk factors, of which sub-optimal blood pressure was the most important contributor accounting for one-quarter of the burden of AF. In comparison, only 3% of AF cases were attributable to diabetes. These findings illustrate that, as with other forms of cardiovascular disease, more than half of the AF burden is potentially avoidable through the maintenance of optimal levels of classical cardiovascular risk factors, further reinforcing the need for effective primary prevention strategies that enable individuals to adopt and maintain healthy diet and behavioral patterns.
ACKNOWLEDGEMENTS
The authors thank the staff and participants of the ARIC study for their important contributions. RH had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Grants:
Lloyd E. Chambless wchambless@unc.edu N01 HC055015 Atherosclerosis Risk in Communities (ARIC) Study
Aaron R. Folsom folso001@umn.edu N01 HC055019 The Atherosclerosis Risk in Communities Study
Kenneth K. Wu kenneth.k.wu@uth.tmc.edu N01 HC055022 Central Hemostatis Laboratory for CCSP
Christie M. Ballantyne cmb@bcm.edu N01 HC055016 Community and Cohort Surveillance Programs
Moyses Szklo mszklo@jhsph.edu N01 HC055020 CCSP Field Center
Richard G. Hutchinson nihms-hd@ncbi.nlm.nih.gov N01 HC055021 FIELD CENTER [CCSP-FIELD CENTER]
Alvaro Alonso alonso@umn.edu RC1 HL099452-02 Epidemiologic Study of Risk Factors and Biomarkers of Atrial Fibrillation
Funding Sources: The Atherosclerosis Risk in Communities Study is funded by NHLBI contracts N01-HC- 55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, N01-HC-55022. This study was additionally funded by NHLBI grant RC1-HL099452 and American Heart Association grant 09SDG2280087.
Footnotes
Disclosures None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics–2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 4.Coyne KS, Paramore C, Grandy S, Mercader M, Reynolds MR, Zimetbaum P. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006;9:348–356. doi: 10.1111/j.1524-4733.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, Calkins H, Connolly SJ, Curtis AB, Darbar D, Ellinor PT, Go AS, Goldschlager NF, Heckbert SR, Jalife J, Kerr CR, Levy D, Lloyd-Jones DM, Massie BM, Nattel S, Olgin JE, Packer DL, Po SS, Tsang TS, Van Wagoner DR, Waldo AL, Wyse DG. Prevention of atrial fibrillation: report from a National Heart, Lung, and Blood Institute Workshop. Circulation. 2009;119:606–18. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 7.Folsom AR, Yamagishi K, Hozawa A, Chambless LE. Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail. 2009;2:11–17. doi: 10.1161/CIRCHEARTFAILURE.108.794933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hozawa A, Folsom AR, Sharrett R, Chambless LE. Absolute and attributable risks of cardiovascular disease incidence in relation to optimal and borderline risk factors. Arch Intern Med. 2007;167:573–579. doi: 10.1001/archinte.167.6.573. [DOI] [PubMed] [Google Scholar]
- 9.Law MR, Wald NJ. Risk factor thresholds: their existence under scrutiny. BMJ. 2002;324:1570–1576. doi: 10.1136/bmj.324.7353.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawes CM, Vander Hoorn S, Rodgers A, International Society of Hypertension Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–8. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 11.ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 12.Heeringa J, Kors JA, Hofman A, van Rooij FJ, Witteman JC. Cigarette smoking and risk of atrial fibrillation: the Rotterdam Study. Am Heart J. 2008;156:1163–9. doi: 10.1016/j.ahj.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–4. [PubMed] [Google Scholar]
- 14.Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98:476–84. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 15. [Accessed July 15, 2010];The Atherosclerosis Risk In Communities (ARIC) manuals, forms and data collection. http://www.cscc.unc.edu/aric/displaydatatree.php.
- 16.Eriksson H, Caidahl K, Larsson B, Ohlson LO, Welin L, Wilhelmsen L, Svärdsudd K. Cardiac and pulmonary causes of dyspnoea - validation of a scoring test for clinical-epidemiological use: the Study of Men Born in 1913. Eur Heart J. 1987;8:1007–1014. doi: 10.1093/oxfordjournals.eurheartj.a062365. [DOI] [PubMed] [Google Scholar]
- 17.Loehr LR, Rosamund WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities Study) Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 18.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African-Americans: The Atherosclerosis Risk in Communities (ARIC) study. American Heart Journal. 2009;158:111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Loberiza FR, Klein JP, Zhang MJ. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88:95–101. doi: 10.1016/j.cmpb.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Publ Health. 1998;88:15–19. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christophersen IE, Ravn LS, Budtz-Joergensen E, Skytthe A, Haunsoe S, Svendsen JH, Christensen K. Familial aggregation of atrial fibrillation: a study in Danish twins. Circ Arrythm Electrophysiol. 2009;2:378–83. doi: 10.1161/CIRCEP.108.786665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Velagaleti RS, O’Connell CJ. Genomics of heart failure. Heart Fail Clin. 2010;6:115–24. doi: 10.1016/j.hfc.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer M, Broeckel U, Holmer S, Baessler A, Hengstenberg C, Mayer B, Erdmann J, Klein G, Riegger G, Jacob HJ, Schunkert H. Distinct heritable patterns of angiographic coronary artery disease in families with myocardial infarction. Circulation. 2005;111:855–62. doi: 10.1161/01.CIR.0000155611.41961.BB. [DOI] [PubMed] [Google Scholar]
- 24.Marcus GM, Alonso A, Peralta CA, Lettre G, Vittinghoff E, Lubitz SA, Fox ER, Levitzky YS, Mehra R, Kerr KF, Deo R, Sotoodehnia N, Akylbekova M, Ellinor PT, Paltoo DN, Soliman EZ, Benjamin EJ, Heckbert SR. European ancestry as a risk factor for atrial fibrillation in African Americans. Candidate-Gene Association Resource (CARe) Study. Circulation. 2010;122:2009–15. doi: 10.1161/CIRCULATIONAHA.110.958306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dublin S, Glazer NL, Smith NL, Psaty BM, Lumley T, Wiggins KL, Page RL, Heckbert SR. Diabetes mellitus, glycemic control, and risk of atrial fibrillation. J Gen Intern Med. 2010;25:853–8. doi: 10.1007/s11606-010-1340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]