Abstract
In everyday life our brain often receives information about events and objects in the real world via several sensory modalities, because natural objects often stimulate more than one sense. These different types of information are processed in our brain along different sensory-specific pathways, but are finally integrated into a unified percept. During the last years, studies provided compelling evidence that the neural basis of multisensory integration is not restricted to higher association areas of the cortex, but can already occur at low-level stages of sensory cortical processing and even in subcortical structures. In this article we will review the potential role of several thalamic structures in multisensory interplay and discuss their extensive anatomical connections with sensory-specific and multisensory cortical structures. We conclude that sensory-specific thalamic structures may act as a crucial processing node of multisensory interplay in addition to their traditional role as sensory relaying structure.
Key words: multisensory integration, cortex, fMRI, thalamus, anatomy
Several recent human functional imaging studies on multisensory integration provided converging evidence that sensory-specific or even primary cortical areas may be involved in the integration of multisensory stimulus attributes in addition to known integration hubs within higher association cortices, e.g., the superior temporal sulcus (STS),1,2 parts of the parietal cortex,3 or frontal regions.4,5 A large number of experiments using functional magnetic resonance imaging (fMRI) reported modulations of neuronal population response in sensory-specific areas due to multisensory stimulation,1,6–8 in accord with invasive animal studies.9–12 Moreover, studies using electroencephalography (EEG) in humans reported that earliest sensory-specific event-related potentials (ERP) were modulated by concurrent stimuli in a second modality,13–15 though others pointed at an alternative explanation for these early effects.16 However, the exact neural mechanisms of multisensory interplay causing modulations in sensory-specific cortices remain unclear. Recent reviews in reference 8, 17 and 18 suggested several possible neural mechanisms underlying multisensory modulations in sensory-specific cortices:
Feedback influences from multisensory convergence zones within higher association cortices to sensory-specific areas.
Feedback influences from multisensory convergence zones at the border of two sensory-specific cortices that have been identified just recently (i.e., secondary and tertiary “sensory-specific” cortices).
Direct cortico-cortical interconnections between low-level modality-specific areas (including primary sensory-specific areas).
Different sensory-specific information could be integrated at subcortical levels including sensory-specific thalamic nuclei and then fed forward to the sensory-specific cortices.
While the first three options have been discussed elsewhere (reviewed in ref. 8, 17 and 18), this paper will focus on the role of complex thalamo-cortical pathways in the integration of multisensory stimuli. In the first part of this review, we will discuss thalamic structures that show multisensory anatomical and functional characteristics in different species and in the second part we will highlight their possible functional role in multisensory interplay, i.e., how the neuronal processing of different sensory information within these nuclei might contribute to integration at the neuronal level, plus to the optimal behavioral responses in multisensory situations.
Anatomical Evidence
Several animal studies performed in various species identified thalamic nuclei which—based on their anatomical connections to structures of different sensory modalities and/or based on multisensory response characteristics of their neurons—might integrate multisensory information, sometimes even before the information has reached neocortical areas. One candidate is the medial geniculate body (MGB) which is a major structure of the sensory-specific auditory pathway and which consists of at least three subdivisions (e.g., cat19). Whereas the ventral division (MGBv) of the MGB is strongly involved in the processing of auditory information, the dorsal (MGBd) and the medial (MGBm) divisions might be rather regarded as multisensory (reviewed in ref. 17, 20 and 21). For instance, cells within the MGBm (rat22,23) and MGBd (rat;24 cat25) have been reported to respond to auditory but also to visual, vestibular and somato-sensory stimuli. Furthermore, several neuroanatomical studies report direct anatomical connections between the MGBm/ MGBd and auditory but also other sensory subcortical26,27 and cortical areas.26,28,29 These connections are well suited to mediate multisensory interplay at the level of the primary auditory cortex as observed, for instance, by means of invasive electrophysiological recordings in several animal species.9,10,12,30–32
Another important thalamic structure which is also linked with the processing of multimodal information is the pulvinar-posterior complex (pulvinar nucleus in primates). The pulvinar is usually associated with visual processes (e.g., macaque;33,34 rhesus monkey35), but several electrophysiological studies reported that neurons within the pulvinar can also be activated by other or more than one sensory modality.36,37 Moreover, the extensive connections between the pulvinar and different sensory-specific cortical areas might be instrumental in multisensory modulations of cortical activity within these areas.20,28,38–41 Most recently, Cappe et al.29 injected different retrograde neuronal tracers into the auditory, somatosensory and premotor cortex of the macaque monkey; they found that the pulvinar nucleus exhibited the most extensive overlap of differentially retrogradely labeled neurons and concluded that this nucleus may thus play a key role in multisensory and sensorimotor integration.
Other thalamic nuclei, some of them surrounding the MGB and pulvinar, which show also diverse multisensory responses and which have multiple connections with subcortical and cortical areas of various sensory modalities are, for instance, the suprageniculate (SG), posterior intralaminar (PIN), laterodorsal (LD), lateral and ventral posterior (LP, VP) and posterior thalamic nucleus (Po).22,26,28,29,42–44 They may have similar, but yet uncovered functions within the multisensory thalamo-cortical network similar to the medial geniculate and pulvinar nuclei (see also ref. 45 and 46 for additional discussion of specific thalamic cell types that may also subserve multisensory integration).
Functional Role of Thalamic Structures in Multisensory Integration
The functional role of thalamic structures and in particular their relevance for multisensory integration is still debated. Nonetheless, some studies provide evidence of thalamic influence on multisensory information processes in rats47 and humans48 and others link modulations of neuronal activity in subcortical structures with behavioral consequences like audiovisual speech processing,49 audiovisual stimulus onset asynchrony detection50 and multisensory attention tasks.51 Kreifelts et al. reported in humans an enhanced classification accuracy of audiovisual emotional stimuli (relative to unimodal presentation) and linked this increase in perceptual performance to enhanced fMRI-signals in multisensory convergence zones of the cortex (STS) and thalamus.
In another recent human fMRI study we tested how co-occurring sounds modulate the subjects' perceptual sensitivity and the neural responses to visual stimuli of higher or lower intensity.53
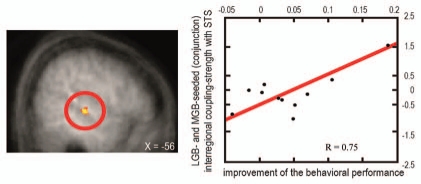
We found that a task-irrelevant auditory stimulus increases the sensitivity to low-intensity but not to high-intensity visual targets and that this perceptual enhancement relates to fMRI-signal increases in sensory-specific and multisensory cortical brain regions (reviewed in ref. 1, 9 and 11). Most importantly, modulations of the fMRI-signals were also observed in the sensory-specific visual (lateral geniculate body, LGB) and auditory (MGB) thalamus (Fig. 1). Furthermore, LGB and MGB showed a stronger interregional coupling (psychophysiological interaction54) with the STS, and the strength of these functional connections scaled with the subjects' behavioral performance (Fig. 2). These results provide evidence in humans that sensory-specific thalamic structures are involved in multisensory integration processes and resulting behavior performances, here psychophysical detection sensitivity.
Figure 1.
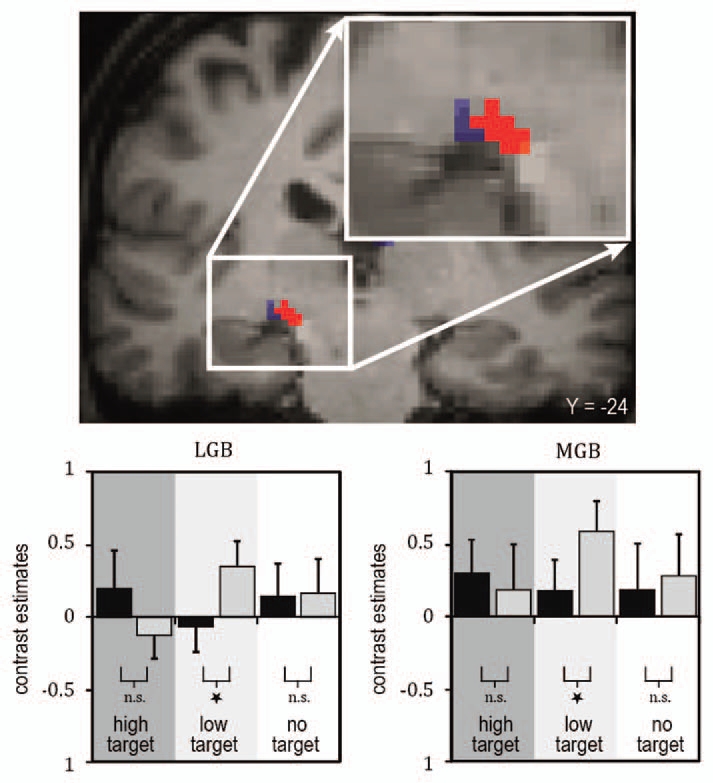
Illustration of fMRI BOLD (blood oxygen level dependent) responses in subject-specific visual and auditory thalamus (adapted from Noesselt et al.53). Top: Brain section depicts visual (LGB, blue) and auditory (MGB, red) thalamus for one illustrative individual subject (for more details see Noesselt et al.53). The bar graphs below of the brain sections depict the height of the fMRI-signal for the experimental conditions deduced from subject-specific ROIs (region of interest; see bar graphs, with grey bars for sound conditions and black for no-sound for the three visual stimuli: high intensity, low intensity, no visual target). An enhanced fMRI-signal was found when a sound was added to a lower-intensity visual target, but no significant change in response when the same sound was added to a higher-intensity visual target in accord with the behavioral findings.
Figure 2.
Brain-behavior relations for coupling of thalamic structures with higher association cortices as a function of behavioral performance. Right: The scatter plot depicts the relation between the size of the behavioral interaction pattern (i.e., the difference in subject's visual detection hit rate for sound minus no-sound conditions being more pronounced for lower-than higher-intensity visual targets; along the y-axis) and the significant changes in LGB- and MGB-seeded (conjunction) interregional coupling-strength (PPI, along the x-axis) with the remote region STS (shown on the left side). This analysis highlights stronger coupling of both LGB and MGB with multisensory STS for subjects with higher behavior benefit than for those with lower behavioral benefit (adapted from Noesselt et al.53).
Due to the known limitations in temporal resolution of the used method (fMRI), conclusions about the functional direction or type of connections (feed-forward or feed-back) could not be made. However, one possible mechanism underlying the detected multisensory integration effects was suggested by Schroeder/Lakatos and colleagues on the basis of experiments on time-frequency relationships of multisensory inputs into the primate cortex.12,55–57 Lakatos et al.12 reported enhanced neuronal responses in the auditory cortex of macaques during simultaneous auditory and tactile stimulation which was accompanied by a phase reset of neural oscillations in the auditory cortex. The authors suggested that this phase reset may be mediated by fast feed-forward projections from thalamic structures. Such phase resettings might also help to increase the signal-to-noise ratio between concurrent sounds and low-intensity visual targets (cf. Lakatos et al.55 for attention-related phase resetting of visual cortex) and may reflect one role of the thalamus in coupling of “functionally distant” cortical regions.
Taken together, there is now converging evidence that not only sensory non-specific (i.e., nonlemniscal) nuclei but also sensory-specific thalamic nuclei may integrate different sensory stimuli and may influence cortical multisensory processing by means of thalamo-cortical feed-forward connections. Modulations of connection strength of these sensory-specific thalamic nuclei with cortical regions are directly linked to behavioral performance and strongly suggest that a neurobiologically plausible theory of multisensory integration needs to take subcortical and especially thalamic influences into account.
Acknowledgments
This work was funded by Deutsche Forschungsgemeinschaft Grants DFG-SFB-TR31/TPA8 and TP13.
References
- 1.Noesselt T, Rieger JW, Schoenfeld MA, Kanowski M, Hinrichs H, Heinze HJ, et al. Audiovisual temporal correspondence modulates human multisensory superior temporal sulcus plus primary sensory cortices. J Neurosci. 2007;27:11431–11441. doi: 10.1523/JNEUROSCI.2252-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Werner S, Noppeney U. Superadditive responses in superior temporal sulcus predict audiovisual benefits in object categorization. Cereb Cortex. 2010;20:1829–1842. doi: 10.1093/cercor/bhp248. [DOI] [PubMed] [Google Scholar]
- 3.Bremmer F, Schlack A, Shah NJ, Zafiris O, Kubischik M, Hoffmann K, et al. Polymodal motion processing in posterior parietal and premotor cortex: a human fMRI study strongly implies equivalencies between humans and monkeys. Neuron. 2001;29:287–296. doi: 10.1016/s0896-6273(01)00198-2. [DOI] [PubMed] [Google Scholar]
- 4.Calvert GA. Crossmodal processing in the human brain: insights from functional neuroimaging studies. Cereb Cortex. 2001;11:1110–1123. doi: 10.1093/cercor/11.12.1110. [DOI] [PubMed] [Google Scholar]
- 5.Miller LM, D'Esposito M. Perceptual fusion and stimulus coincidence in the cross-modal integration of speech. J Neurosci. 2005;25:5884–5893. doi: 10.1523/JNEUROSCI.0896-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calvert GA, Brammer MJ, Bullmore ET, Campbell R, Iversen SD, David AS. Response amplification in sensory-specific cortices during crossmodal binding. Neuroreport. 1999;10:2619–2623. doi: 10.1097/00001756-199908200-00033. [DOI] [PubMed] [Google Scholar]
- 7.von Kriegstein K, Kleinschmidt A, Sterzer P, Giraud AL. Interaction of face and voice areas during speaker recognition. J Cogn Neurosci. 2005;17:367–376. doi: 10.1162/0898929053279577. [DOI] [PubMed] [Google Scholar]
- 8.Driver J, Noesselt T. Multisensory interplay reveals crossmodal influences on “sensory-specific” brain regions, neural responses and judgments. Neuron. 2008;57:11–23. doi: 10.1016/j.neuron.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brosch M, Selezneva E, Scheich H. Nonauditory events of a behavioral procedure activate auditory cortex of highly trained monkeys. J Neurosci. 2005;25:6797–6806. doi: 10.1523/JNEUROSCI.1571-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghazanfar AA, Maier JX, Hoffman KL, Logothetis NK. Multisensory integration of dynamic faces and voices in rhesus monkey auditory cortex. J Neurosci. 2005;25:5004–5012. doi: 10.1523/JNEUROSCI.0799-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kayser C, Petkov CI, Logothetis NK. Visual modulation of neurons in auditory cortex. Cereb Cortex. 2008;18:1560–1574. doi: 10.1093/cercor/bhm187. [DOI] [PubMed] [Google Scholar]
- 12.Lakatos P, Chen CM, O'Connell MN, Mills A, Schroeder CE. Neuronal oscillations and multisensory interaction in primary auditory cortex. Neuron. 2007;53:279–292. doi: 10.1016/j.neuron.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foxe JJ, Morocz IA, Murray MM, Higgins BA, Javitt DC, Schroeder CE. Multisensory auditory-somatosensory interactions in early cortical processing revealed by high-density electrical mapping. Brain Res Cogn Brain Res. 2000;10:77–83. doi: 10.1016/s0926-6410(00)00024-0. [DOI] [PubMed] [Google Scholar]
- 14.Giard MH, Peronnet F. Auditory-visual integration during multimodal object recognition in humans: a behavioral and electrophysiological study. J Cogn Neurosci. 1999;11:473–90. doi: 10.1162/089892999563544. [DOI] [PubMed] [Google Scholar]
- 15.Molholm S, Ritter W, Murray MM, Javitt DC, Schroeder CE, Foxe JJ. Multisensory auditory-visual interactions during early sensory processing in humans: a high-density electrical mapping study. Brain Res Cogn Brain Res. 2002;14:115–128. doi: 10.1016/s0926-6410(02)00066-6. [DOI] [PubMed] [Google Scholar]
- 16.Teder-Salejarvi WA, McDonald JJ, Di Russo F, Hillyard SA. An analysis of audio-visual crossmodal integration by means of event-related potential (ERP) recordings. Brain Res Cogn Brain Res. 2002;14:106–114. doi: 10.1016/s0926-6410(02)00065-4. [DOI] [PubMed] [Google Scholar]
- 17.Budinger E, Scheich H. Anatomical connections suitable for the direct processing of neuronal information of different modalities via the rodent primary auditory cortex. Hear Res. 2009;258:16–27. doi: 10.1016/j.heares.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 18.Ghazanfar AA, Schroeder CE. Is neocortex essentially multisensory? Trends Cogn Sci. 2006;10:278–285. doi: 10.1016/j.tics.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Winer JA. The medial geniculate body of the cat. Adv Anat Embryol Cell Biol. 1985;86:1–97. [PubMed] [Google Scholar]
- 20.Cappe C, Rouiller EM, Barone P. Multisensory anatomical pathways. Hear Res. 2009;258:28–36. doi: 10.1016/j.heares.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Smiley JF, Falchier A. Multisensory connections of monkey auditory cerebral cortex. Hear Res. 2009;258:37–46. doi: 10.1016/j.heares.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bordi F, LeDoux JE. Response properties of single units in areas of rat auditory thalamus that project to the amygdala. I. Acoustic discharge patterns and frequency receptive fields. Exp Brain Res. 1994;98:261–274. doi: 10.1007/BF00228414. [DOI] [PubMed] [Google Scholar]
- 23.Wepsic JG. Multimodal sensory activation of cells in the magnocellular medial geniculate nucleus. Exp Neurol. 1966;15:299–318. doi: 10.1016/0014-4886(66)90053-7. [DOI] [PubMed] [Google Scholar]
- 24.Ledoux JE, Ruggiero DA, Forest R, Stornetta R, Reis DJ. Topographic organization of convergent projections to the thalamus from the inferior colliculus and spinal cord in the rat. J Comp Neurol. 1987;264:123–146. doi: 10.1002/cne.902640110. [DOI] [PubMed] [Google Scholar]
- 25.Calford MB, Aitkin LM. Ascending projections to the medial geniculate body of the cat: evidence for multiple, parallel auditory pathways through thalamus. J Neurosci. 1983;3:2365–2380. doi: 10.1523/JNEUROSCI.03-11-02365.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campi KL, Bales KL, Grunewald R, Krubitzer L. Connections of auditory and visual cortex in the prairie vole (Microtus ochrogaster): evidence for multisensory processing in primary sensory areas. Cereb Cortex. 2010;20:89–108. doi: 10.1093/cercor/bhp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LeDoux JE, Ruggiero DA, Reis DJ. Projections to the subcortical forebrain from anatomically defined regions of the medial geniculate body in the rat. J Comp Neurol. 1985;242:182–213. doi: 10.1002/cne.902420204. [DOI] [PubMed] [Google Scholar]
- 28.Budinger E, Heil P, Hess A, Scheich H. Multisensory processing via early cortical stages: Connections of the primary auditory cortical field with other sensory systems. Neuroscience. 2006;143:1065–1083. doi: 10.1016/j.neuroscience.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 29.Cappe C, Morel A, Barone P, Rouiller EM. The thalamocortical projection systems in primate: an anatomical support for multisensory and sensorimotor interplay. Cereb Cortex. 2009;19:2025–2037. doi: 10.1093/cercor/bhn228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu S, Fan S, Chen L. Event-related potentials reveal involuntary processing of orientation changes in the visual modality. Psychophysiology. 2003;40:770–775. doi: 10.1111/1469-8986.00077. [DOI] [PubMed] [Google Scholar]
- 31.Kayser C, Petkov CI, Augath M, Logothetis NK. Integration of touch and sound in auditory cortex. Neuron. 2005;48:373–384. doi: 10.1016/j.neuron.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 32.Wallace MT, Ramachandran R, Stein BE. A revised view of sensory cortical parcellation. Proc Natl Acad Sci USA. 2004;101:2167–2172. doi: 10.1073/pnas.0305697101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bender DB. Retinotopic organization of macaque pulvinar. J Neurophysiol. 1981;46:672–693. doi: 10.1152/jn.1981.46.3.672. [DOI] [PubMed] [Google Scholar]
- 34.Benevento LA, Miller J. Visual responses of single neurons in the caudal lateral pulvinar of the macaque monkey. J Neurosci. 1981;1:1268–1278. doi: 10.1523/JNEUROSCI.01-11-01268.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen SE, Robinson DL, Keys W. Pulvinar nuclei of the behaving rhesus monkey: visual responses and their modulation. J Neurophysiol. 1985;54:867–886. doi: 10.1152/jn.1985.54.4.867. [DOI] [PubMed] [Google Scholar]
- 36.Avanzini G, Broggi G, Franceschetti S, Spreafico R. Multisensory convergence and interaction in the pulvinar-lateralis posterior complex of the cat's thalamus. Neurosci Lett. 1980;19:27–32. doi: 10.1016/0304-3940(80)90250-5. [DOI] [PubMed] [Google Scholar]
- 37.Gattass R, Oswaldo-Cruz E, Sousa AP. Visuotopic organization of the cebus pulvinar: a double representation the contralateral hemifield. Brain Res. 1978;152:1–16. doi: 10.1016/0006-8993(78)90130-0. [DOI] [PubMed] [Google Scholar]
- 38.de la Mothe LA, Blumell S, Kajikawa Y, Hackett TA. Thalamic connections of the auditory cortex in marmoset monkeys: core and medial belt regions. J Comp Neurol. 2006;496:72–96. doi: 10.1002/cne.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hackett TA, De La Mothe LA, Ulbert I, Karmos G, Smiley J, Schroeder CE. Multisensory convergence in auditory cortex, II. Thalamocortical connections of the caudal superior temporal plane. J Comp Neurol. 2007;502:924–952. doi: 10.1002/cne.21326. [DOI] [PubMed] [Google Scholar]
- 40.Morel A, Garraghty PE, Kaas JH. Tonotopic organization, architectonic fields and connections of auditory cortex in macaque monkeys. J Comp Neurol. 1993;335:437–459. doi: 10.1002/cne.903350312. [DOI] [PubMed] [Google Scholar]
- 41.Yeterian EH, Pandya DN. Thalamic connections of the cortex of the superior temporal sulcus in the rhesus monkey. J Comp Neurol. 1989;282:80–97. doi: 10.1002/cne.902820107. [DOI] [PubMed] [Google Scholar]
- 42.Benedek G, Pereny J, Kovacs G, Fischer-Szatmari L, Katoh YY. Visual, somatosensory, auditory and nociceptive modality properties in the feline suprageniculate nucleus. Neuroscience. 1997;78:179–189. doi: 10.1016/s0306-4522(96)00562-3. [DOI] [PubMed] [Google Scholar]
- 43.Linke R, Braune G, Schwegler H. Differential projection of the posterior paralaminar thalamic nuclei to the amygdaloid complex in the rat. Exp Brain Res. 2000;134:520–532. doi: 10.1007/s002210000475. [DOI] [PubMed] [Google Scholar]
- 44.Phillips DP, Irvine DR. Acoustic input to single neurons in pulvinar-posterior complex of cat thalamus. J Neurophysiol. 1979;42:123–136. doi: 10.1152/jn.1979.42.1.123. [DOI] [PubMed] [Google Scholar]
- 45.Jones EG. A new view of specific and nonspecific thalamocortical connections. Adv Neurol. 1998;77:49–71. [PubMed] [Google Scholar]
- 46.Macchi G, Bentivoglio M. Is the “nonspecific” thalamus still “nonspecific”? Arch Ital Biol. 1999;137:201–226. [PubMed] [Google Scholar]
- 47.Komura Y, Tamura R, Uwano T, Nishijo H, Ono T. Auditory thalamus integrates visual inputs into behavioral gains. Nat Neurosci. 2005;8:1203–1209. doi: 10.1038/nn1528. [DOI] [PubMed] [Google Scholar]
- 48.Baier B, Kleinschmidt A, Muller NG. Cross-modal processing in early visual and auditory cortices depends on expected statistical relationship of multisensory information. J Neurosci. 2006;26:12260–12265. doi: 10.1523/JNEUROSCI.1457-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Musacchia G, Sams M, Nicol T, Kraus N. Seeing speech affects acoustic information processing in the human brainstem. Exp Brain Res. 2006;168:1–10. doi: 10.1007/s00221-005-0071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bushara KO, Grafman J, Hallett M. Neural correlates of auditory-visual stimulus onset asynchrony detection. J Neurosci. 2001;21:300–304. doi: 10.1523/JNEUROSCI.21-01-00300.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vohn R, Fimm B, Weber J, Schnitker R, Thron A, Spijkers W, et al. Management of attentional resources in within-modal and cross-modal divided attention tasks: an fMRI study. Hum Brain Mapp. 2007;28:1267–1275. doi: 10.1002/hbm.20350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kreifelts B, Ethofer T, Grodd W, Erb M, Wildgruber D. Audiovisual integration of emotional signals in voice and face: an event-related fMRI study. Neuroimage. 2007;37:1445–1456. doi: 10.1016/j.neuroimage.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 53.Noesselt T, Tyll S, Boehler CN, Budinger E, Heinze HJ, Driver J. Sound-induced enhancement of low-intensity vision: multisensory influences on human sensory-specific cortices and thalamic bodies relate to perceptual enhancement of visual detection sensitivity. J Neurosci. 2010;30:13609–13623. doi: 10.1523/JNEUROSCI.4524-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 55.Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- 56.Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schroeder CE, Lakatos P, Kajikawa Y, Partan S, Puce A. Neuronal oscillations and visual amplification of speech. Trends Cogn Sci. 2008;12:106–113. doi: 10.1016/j.tics.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]