Abstract
Accurate chromosome segregation during mitosis is achieved by the kinetochore fibers (K-fibers) of the spindle apparatus. These fibers are bundles of microtubules (MTs) connected by non-motor bridges. We recently identified a TACC3/ch-TOG/clathrin complex that constitutes the shortest class of inter-MT bridge in K-fibers. TACC3 anchors the complex to MTs and this is dependent on phosphorylation by Aurora A kinase. Here we show that inhibition of Aurora A kinase using MLN8237 results in (1) loss of clathrin and TACC3 from spindles, (2) destabilization of K-fibers and (3) loss of inter-MT bridges. These results are similar to those in cells depleted of clathrin or TACC3; suggesting that TACC3/ch-TOG/clathrin bridges are the major class of bridge that is regulated by this kinase.
Key words: aurora A kinase, cancer, ch-TOG, clathrin, inter-microtubule bridge, microtubule, mitotic spindle, MLN8237, MLN8054, TACC3
The mitotic spindle is responsible for the accurate segregation of chromosomes that occurs during mitosis.1 The spindle apparatus is a dynamic assembly of microtubules (MTs), motors and non-motor proteins.2,3 Chromosome movements are performed by the kinetochore fibers (K-fibers) of the spindle. K-fibers are bundles of 20–40 MTs that run from the kinetochore on the chromosome to the spindle pole.4 The stability of these fibers is important for chromosome movement and successful mitosis.
In electron micrographs, the MTs in K-fibers are connected by electron-dense inter-MT bridges.5,6 These bridges are hypothesized to allow for the uniform transduction of forces throughout the MT bundle and to contribute to fiber stability. The inter-MT bridges in K-fibers vary in length from ∼16 to ∼60 nm5,7 and are thought to be comprised of non-motor proteins.2,3 In a recent paper, we described that the shortest type of bridges in K-fibers are comprised of a complex of transforming acidic coiled-coil protein 3 (TACC3), colonic, hepatic tumor overexpressed gene (ch-TOG) and clathrin.7 Depletion of clathrin heavy chain (CHC) by RNAi resulted in K-fibers that lacked the shortest type of inter-MT bridges. Similar results were found in TACC3-depleted cells. Longer bridges were still present, indicating that different proteins may constitute these other bridge classes. Finally, we showed labeling of inter-MT bridges with clathrin immunogold. Our results suggested that clathrin is the actual cross-bracing molecule with TACC3/ch-TOG acting as anchor points on adjacent micro-tubules. This interpretation accounts for our results7–10 and those of others.11–13
The activity of the mitotic kinase Aurora A was found to be crucial for the localization of the TACC3/ch-TOG/clathrin complex components on the spindle. We found that phosphorylation of TACC3, presumably at serine 558, by Aurora A kinase is required for TACC3 to bind to MTs and to subsequently recruit clathrin to the spindle.7 Acute inhibition of Aurora A kinase with the specific inhibitor MLN8237 (0.3 µM) resulted in a loss of TACC3 and clathrin from the spindle within 35 min. In addition, mutation of serine 558 to alanine completely blocked recruitment of TACC3 and clathrin to the spindle.7
A drawback of RNAi is that protein depletion is relatively prolonged compared to the length of the cell cycle. The mitotic spindle is constructed in the absence of the protein of interest and so compensatory mechanisms may complicate the interpretation of the experiment. The acute removal of clathrin and TACC3 from spindles by chemical inhibition of Aurora A kinase presents an opportunity to confirm that removal of these proteins results in loss of inter-MT bridges from spindles that have assembled normally.
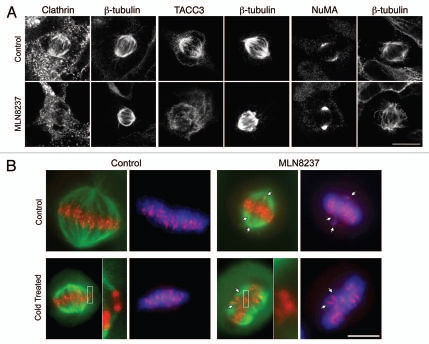
As reported previously in reference 7, treatment of cells near metaphase with MLN8237 (0.5 µM, 40 min) resulted in a loss of clathrin and TACC3 from mitotic spindles (Fig. 1A). Inhibition of Aurora A kinase did not result in a non-specific loss of all spindle proteins because NuMA, a protein that crosslinks MTs at the spindle pole,14 was still present. This indicated that the effect on TACC3 and clathrin was likely due to blocking phosphorylation of TACC3, a known Aurora A kinase substrate.15,16
Figure 1.
Effect of inhibition of Aurora A kinase on TACC3/clathrin localization and K-fiber stability. (A) Inhibition of Aurora A kinase resulted in loss of clathrin and TACC3 from mitotic spindles. HEK293 cells near metaphase were treated with MLN8237 (0.5 µM, 40 min) prior to fixation and staining with the indicated antibodies. All experimental details are as described previously in reference 7, anti-NuMA (#3888, Cell Signaling). Bar, 10 µm. (B) Inhibition of Aurora A kinase resulted in destabilization of kinetochore fibers. HeLa cells near metaphase were treated with no drug (Control) or MLN8237 (0.3 µM, 40 min) and then incubated for 6 min in warm (control) or cold (cold treated) media to depolymerize any non-stable MTs. Cells were fixed and stained for βtubulin (green), CENP-B (red) and DNA (DAPI, blue). Bar, 10 µm. Note the misaligned chromosomes (arrows) and the “orphan” centromeres in the inset. Similar observations were reported in clathrin-depleted cells.9,10
We next examined kinetochore fiber stability using a qualitative immunofluorescence assay. Previously, depletion of CHC was shown to result in misaligned chromosomes in metaphase-like cells.9 These cells had K-fibers that were not stably attached and could be depolymerized by cold treatment. We tested whether or not cells treated with MLN8237 (0.3 µM) for 40 min had a similar mitotic defect. Figure 1B shows that control HeLa cells at metaphase had good alignment of the metaphase plate and most kinetochores had cold-stable K-fiber attachments. In cells where Aurora A kinase had been inhibited, misaligned chromosomes were evident and several “orphan” kinetochores could be found. Orphan kinetochores could be found at the metaphase plate suggesting that they had congressed normally but had lost their stable attachment to the spindle (Fig. 1B). The effect of inhibiting Aurora A kinase during metaphase is therefore similar to depletion of CHC by RNAi.9
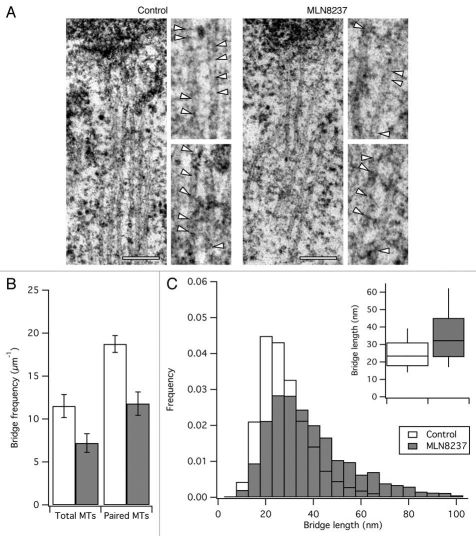
In a previous study, we found that depletion of either CHC or TACC3 resulted in a loss of inter-MT bridges from K-fibers. The missing bridges were the shortest form of inter-MT bridge and the longer types of bridges were found to be slightly upregulated.7 We analyzed by electron microscopy the inter-MT bridges in K-fibers of cells close to metaphase where Aurora A kinase was inhibited using MLN8237 (Fig. 2A). The frequency of bridges per unit length of total MT or of parallel MT was reduced by ∼37% (Fig. 2B). The frequency distribution of bridge lengths was also altered with median bridge lengths of 23.4 and 32.2 nm in controls versus MLN8237-treated cells (Fig. 2C). Again, these results are broadly similar to the data from clathrin-depleted and TACC3-depleted cells.7 This suggests that these defects arise from the removal of clathrin/TACC3 from K-fibers.
Figure 2.
Effect of inhibition of Aurora A kinase on inter-MT bridges. Inhibition of Aurora A kinase resulted in loss of inter-MT bridges. MLN8237-treated HeLa cells near metaphase were processed for EM. (A) Representative sections to show the morphology of K-fibers. Zoomed regions show bridges indicated by arrowheads. Scale bar, 200 nm. (B and C) Quantification of inter-MT bridges in K-fibers from control or MLN-treated cells. (B) Bar chart to show the average frequency of bridges per unit length of total or paired MTs. Student's t-test, p = 0.066 and p = 0.014; Ncell = 3; Bars, mean ± SEM. (C) Frequency histogram to show the length distribution of inter-MT bridges. Inset: Tukey plots of the length distribution data.
MLN8237 is a specific inhibitor of Aurora A kinase and so off-target effects are minimized.17 However, there are many other Aurora A substrates18 whose localization or function at the spindle is likely to depend on the activity of the kinase. The observed changes in inter-MT bridges following brief incubation with low concentrations of MLN8237 represent a very specific change in spindle structure. This suggests that TACC3/ch-TOG/clathrin bridges are the main class of bridge whose localization is dependent of Aurora A kinase. However, the antiproliferative property of this class of inhibitors19 is more likely to result from the global inhibition of Aurora A phosphorylations rather than solely on the specific effect of inter-MT bridge removal.
Rapid and specific removal of spindle proteins is likely to be a major advance over long-term depletion studies. For example, in clathrin-depleted cells, loss of short inter-MT bridges appeared to precede loss of MTs in K-fibers.7 It may be possible to separate temporally bridge loss and MT loss using chemical inhibition. Future studies will therefore concentrate on methods to rapidly remove spindle proteins specifically.
Acknowledgements
We thank Bill Earnshaw for the gift of anti-CENP-B. This work was supported by a Career Establishment Award from Cancer Research UK (C25425/A8722). L.P.C. and D.G.B. are recipients of Wellcome Trust Prize Studentships and I.A.P. is a Royal Society University Research Fellow.
References
- 1.Scholey JM, Brust-Mascher I, Mogilner A. Cell division. Nature. 2003;422:746–752. doi: 10.1038/nature01599. [DOI] [PubMed] [Google Scholar]
- 2.Peterman EJ, Scholey JM. Mitotic microtubule cross-linkers: insights from mechanistic studies. Curr Biol. 2009;19:1089–1094. doi: 10.1016/j.cub.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 3.Manning AL, Compton DA. Structural and regulatory roles of nonmotor spindle proteins. Curr Opin Cell Biol. 2008;20:101–106. doi: 10.1016/j.ceb.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rieder CL. Kinetochore fiber formation in animal somatic cells: dueling mechanisms come to a draw. Chromosoma. 2005;114:310–318. doi: 10.1007/s00412-005-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hepler PK, McIntosh JR, Cleland S. Intermicrotubule bridges in mitotic spindle apparatus. J Cell Biol. 1970;45:438–444. doi: 10.1083/jcb.45.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witt PL, Ris H, Borisy GG. Structure of kinetochore fibers: microtubule continuity and inter-microtubule bridges. Chromosoma. 1981;83:523–540. doi: 10.1007/BF00328277. [DOI] [PubMed] [Google Scholar]
- 7.Booth DG, Hood FE, Prior IA, Royle SJ. A TACC3/ ch-TOG/clathrin complex stabilizes kinetochore fibres by inter-microtubule bridging. EMBO J. 2011;30:906–919. doi: 10.1038/emboj.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hood FE, Royle SJ. Functional equivalence of the clathrin heavy chains CHC17 and CHC22 in endocytosis and mitosis. J Cell Sci. 2009;122:2185–2190. doi: 10.1242/jcs.046177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Royle SJ, Bright NA, Lagnado L. Clathrin is required for the function of the mitotic spindle. Nature. 2005;434:1152–1157. doi: 10.1038/nature03502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Royle SJ, Lagnado L. Trimerisation is important for the function of clathrin at the mitotic spindle. J Cell Sci. 2006;119:4071–4078. doi: 10.1242/jcs.03192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu W, Tao W, Zheng P, Fu J, Bian M, Jiang Q, et al. Clathrin recruits phosphorylated TACC3 to spindle poles for bipolar spindle assembly and chromosome alignment. J Cell Sci. 2010;123:3645–3651. doi: 10.1242/jcs.075911. [DOI] [PubMed] [Google Scholar]
- 12.Hubner NC, Bird AW, Cox J, Splettstoesser B, Bandilla P, Poser I, et al. Quantitative proteomics combined with BAC TransgeneOmics reveals in vivo protein interactions. J Cell Biol. 2010;189:739–754. doi: 10.1083/jcb.200911091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin CH, Hu CK, Shih HM. Clathrin heavy chain mediates TACC3 targeting to mitotic spindles to ensure spindle stability. J Cell Biol. 2010;189:1097–1105. doi: 10.1083/jcb.200911120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaglio T, Saredi A, Compton DA. NuMA is required for the organization of microtubules into aster-like mitotic arrays. J Cell Biol. 1995;131:693–708. doi: 10.1083/jcb.131.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinoshita K, Noetzel TL, Pelletier L, Mechtler K, Drechsel DN, Schwager A, et al. Aurora A phosphorylation of TACC3/maskin is required for centrosome-dependent microtubule assembly in mitosis. J Cell Biol. 2005;170:1047–1055. doi: 10.1083/jcb.200503023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LeRoy PJ, Hunter JJ, Hoar KM, Burke KE, Shinde V, Ruan J, et al. Localization of human TACC3 to mitotic spindles is mediated by phosphorylation on Ser558 by Aurora A: a novel pharmacodynamic method for measuring Aurora A activity. Cancer Res. 2007;67:5362–5370. doi: 10.1158/0008-5472.CAN-07-0122. [DOI] [PubMed] [Google Scholar]
- 17.Sloane DA, Trikic MZ, Chu ML, Lamers MB, Mason CS, Mueller I, et al. Drug-resistant aurora A mutants for cellular target validation of the small molecule kinase inhibitors MLN8054 and MLN8237. ACS Chem Biol. 2010;5:563–576. doi: 10.1021/cb100053q. [DOI] [PubMed] [Google Scholar]
- 18.Sardon T, Pache RA, Stein A, Molina H, Vernos I, Aloy P. Uncovering new substrates for Aurora A kinase. EMBO Rep. 2010;11:977–984. doi: 10.1038/embor.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manfredi MG, Ecsedy JA, Meetze KA, Balani SK, Burenkova O, Chen W, et al. Antitumor activity of MLN8054, an orally active small-molecule inhibitor of Aurora A kinase. Proc Natl Acad Sci USA. 2007;104:4106–4111. doi: 10.1073/pnas.0608798104. [DOI] [PMC free article] [PubMed] [Google Scholar]