Abstract
Interactions between extracellular matrix (ECM) proteins and transmembrane receptors mediate changes in cell shape during cell migration, adhesion, differentiation and polarization. Cytokinesis is the final step in cell division as cells employ a contractile ring composed of actin and myosin to partition one cell into two. During the partition process, an invagination in nascent membrane forms a new extracellular space called the cleavage furrow. Despite the dramatic changes in cell shape during cytokinesis, existing models include no role for the ECM. In a recent paper, we show that hemicentins assemble in the cleavage furrow of C. elegans germ cells and mouse embryo blastomeres. Hemicentin depletion results in membrane destabilization, cleavage furrow retraction and cytokinesis failure. The data suggest that hemicentins and other ECM proteins stabilize the cleavage furrow during cytokinesis of multiple cell types.
Key words: extracellular matrix, cytokinesis, hemicentin, cleavage furrow, Caenorhabditis elegans, mouse embryo
The extracellular matrix (ECM) is an organized meshwork of glycoproteins and proteoglycans that provides a structural context and regulatory signals to growing cells to help pattern the four-dimensional development of complex tissues. Interactions between the ECM and cytoskeleton, mediated by transmembrane receptors, regulate cell and tissue shape and are critical for nearly all aspects of development.1 Although extensive modification in cell shape occurs during cytokinesis, no role for cell-ECM interactions have been incorporated into detailed models of the cytokinesis process.2,3
In a recent paper, we show that hemicentins, large highly conserved extracellular matrix proteins, assemble in the cleavage furrow of dividing mouse blastomeres and C. elegans germ cells. In both cell types, loss of hemicentin results in membrane instability, cleavage furrow retraction and multinucleate cells.4
Genetic analyses in C. elegans and zebrafish suggest that hemicentin functions as an extracellular adhesive, forming anchorages that hold cells together and maintain tissue integrity.5,6 For example, hemicentin assembles into lineshaped structures between somatic cells in C. elegans, forming cell-ECM-cell “sandwiches” that anchor uterus, mechanosensory neurons and intestine to the epidermis. Hemicentin also co-assembles with fibulin-1D to form flexible elastic fiber-like structures that are 7–9 µm long and extend from anterior pharynx to surrounding bodywall muscle.5,7 In the absence of hemicentin several cell anchorages fail with catastrophic consequences. For example, defects in hemidesmosome mediated anchorages that anchor the uterus to epidermal tissue result in uterine detachment and prolapse through the vulva.5
Is there a simple model that can accomodate the adhesive and elastic functions of hemicentin in somatic tissues and the function of hemicentin in cytokinesis? In both cases, hemicentin may promote assembly of cytoskeletal structures in the cell cortex through interactions with an integrin, dystroglycan and/or syndecan, transmembrane glycoproteins that have been implicated in cytokinesis (Fig. 1).8–11 It is possible that hemicentins assemble at the leading edge of the cleavage furrow, forming an elastic band around the periphery that stabilizes and prevents retraction of the cleavage furrow. In this scenario, hemicentin polymers would most likely assemble parallel to the membrane at the leading edge of the cleavage furrow, although other arrangements are also possible (Fig. 1).
Figure 1.
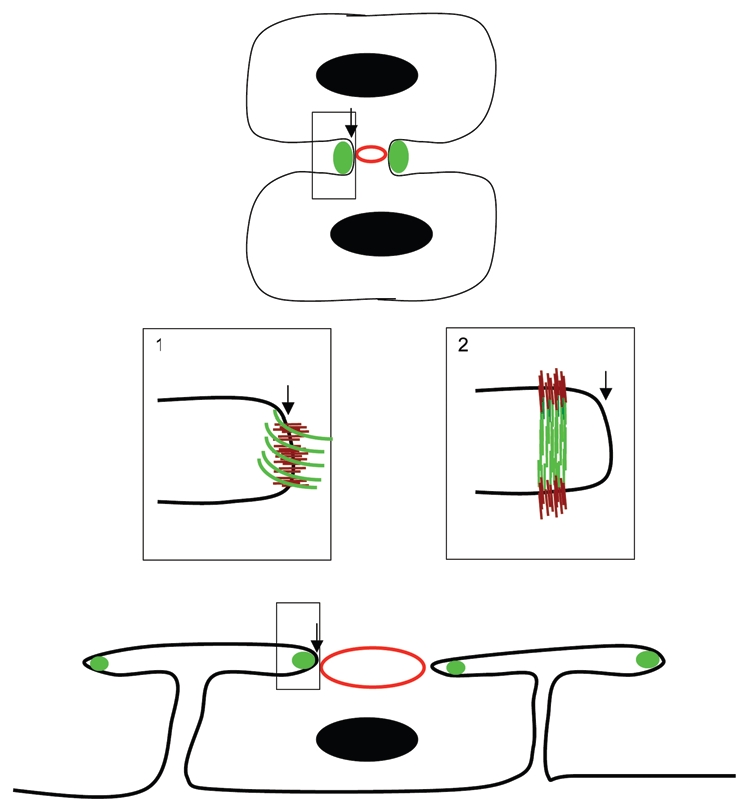
Hemicentins assemble in the cleavage furrow. Schematic diagram of hemicentin (green) in the incomplete cleavage furrow of a conventional cell (e.g., mouse blastomere, top) and a syncytial germ cell of the C. elegans gonad (bottom). An expanded view (box 1) shows a simple model in which hemicentin (green) assembles as an elastic ring around the periphery of the cleavage furrow leading edge. An unconventional model (box 2) shows hemicentin in a distal position from the leading edge of the cleavage furrow. Hemicentin polymers may be assembled perpendicular to the membrane, linking the two cellular products of cytokinesis, a distribution that is more consistent with the role of hemicentins as elastic connectors between somatic cells in C. elegans. Also shown are nuclei (black circles) contractile rings (red circles) and putative transmembrane receptors (brown lines).
An alternative and less conventional model is that hemicentins may assemble perpendicular to membrane slightly distal to the leading edge of the cleavage furrow where it might form stabilizing links that connect the two cellular products of cytokinesis as they are forming (Fig. 1). In this orientation, hemicentins could hold the membranes between the two cells at a fixed distance, thereby stabilizing the shape of the nascent cleavage furrow.12 Although there is little or no direct evidence for it, this type of arrangement may be worth contemplating since it is consistent with the distribution and function of hemicentin as an extracellular linker between different somatic cell types.5
Since the ECM is an interdependent network of glycoproteins and proteoglycans it is quite likely that hemicentin interacts with other ECM components to perform its function in membrane stability. It is possible that proteoglycans and other secreted and transmembrane glycoproteins collaborate with hemicentin and may substitute for hemicentin to promote cleavage furrow stability in cells that do not express hemicentin and organisms without a hemicentin ortholog.13–16 However, it should be pointed out that the role of hemicentins appears to be distinct from that of some extracellular proteoglycans and polysaccharides that appear to stabilize the cleavage furrow by providing osmotic support, a role that is consistent with the function of proteoglycans in forming hydrated gels that promote tissue expansion and provide resistance to compressive forces in diverse tissues (e.g., cartilage). Future studies are likely to shed light on how hemicentins and other ECM components stabilize and promote the dramatic changes in cell shape that occur as one cell is cleaved into two.
Acknowledgments
This work was supported by funding from the National Science Foundation (MCB0744838) and the National Institutes of Health (GM65184).
References
- 1.Ingber DE. Mechanical control of tissue morphogenesis during embryological development. Int J Dev Biol. 2006;50:255–266. doi: 10.1387/ijdb.052044di. [DOI] [PubMed] [Google Scholar]
- 2.Pollard TD. Mechanics of cytokinesis in eukaryotes. Curr Opin Cell Biol. 2010;22:50–56. doi: 10.1016/j.ceb.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glotzer M. The molecular requirements for cytokinesis. Science. 2005;307:1735–1739. doi: 10.1126/science.1096896. [DOI] [PubMed] [Google Scholar]
- 4.Xu X, Vogel BE. A secreted protein promotes cleavage furrow maturation during cytokinesis. Curr Biol. 2011;21:114–119. doi: 10.1016/j.cub.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogel BE, Hedgecock EM. Hemicentin, a conserved extracellular member of the immunoglobulin superfamily, organizes epithelial and other cell attachments into oriented lineshaped junctions. Development. 2001;128:883–894. doi: 10.1242/dev.128.6.883. [DOI] [PubMed] [Google Scholar]
- 6.Carney TJ, Feitosa NM, Sonntag C, Slanchev K, Kluger J, Kiyozumi D, et al. Genetic analysis of fin development in zebrafish identifies furin and hemicentin1 as potential novel Fraser syndrome disease genes. PLoS Genet. 2010;6:e1000907. doi: 10.1371/journal.pgen.1000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muriel JM, Dong C, Hutter H, Vogel BE. Fibulin-1C and Fibulin-1D splice variants have distinct functions and assemble in a hemicentin-dependent manner. Development. 2005;132:4223–4234. doi: 10.1242/dev.02007. [DOI] [PubMed] [Google Scholar]
- 8.Aszodi A, Hunziker EB, Brakebusch C, Fässler R. Beta1 integrins regulate chondrocyte rotation, G1 progression and cytokinesis. Genes Dev. 2003;17:2465–2479. doi: 10.1101/gad.277003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reverte CG, Benware A, Jones CW, LaFlamme SE. Perturbing integrin function inhibits microtubule growth from centrosomes, spindle assembly and cytokinesis. J Cell Biol. 2006;174:491–497. doi: 10.1083/jcb.200603069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higginson JR, Thompson O, Winder SJ. Targeting of dystroglycan to the cleavage furrow and mid-body in cytokinesis. Int J Biochem Cell Biol. 2008;40:892–900. doi: 10.1016/j.biocel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Keller-Pinter A, Bottka S, Timar J, Kulka J, Katona R, Dux L, et al. Syndecan-4 promotes cytokinesis in a phosphorylation-dependent manner. Cell Mol Life Sci. 2010;67:1881–1894. doi: 10.1007/s00018-010-0298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson DN, Spudich JA. Mechanics and regulation of cytokinesis. Curr Opin Cell Biol. 2004;16:182–188. doi: 10.1016/j.ceb.2004.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olson SK, Bishop JR, Yates JR, Oegema K, Esko JD. Identification of novel chondroitin proteoglycans in Caenorhabditis elegans: embryonic cell division depends on CPG-1 and CPG-2. J Cell Biol. 2006;73:985–994. doi: 10.1083/jcb.200603003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan SN, Olson S, Canman JC. Cytokinesis: thinking outside the cell. Curr Biol. 2011;21:119–121. doi: 10.1016/j.cub.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt M. Survival and cytokinesis of Saccharomyces cerevisiae in the absence of chitin. Microbiology. 2004;150:3253–3260. doi: 10.1099/mic.0.27197-0. [DOI] [PubMed] [Google Scholar]
- 16.White J, Bednarek S. Cytokinesis: GAGs form the walls that separate our parts. Curr Biol. 2003;13:717–718. doi: 10.1016/j.cub.2003.08.048. [DOI] [PubMed] [Google Scholar]


