Abstract
The processes of neuronal outgrowth and guidance have typically been studied in classic 2D cell culture systems that do not recapitulate topographical cues present in the in vivo extracellular matrix (ECM). Using microfabrication techniques, we mimicked this ECM topography by presenting laminin on a line pattern with nanometric size features. We found that this not only allows neurite orientation but also robust outgrowth. This depends on dynamic stochastic sensing of the line pattern by growth cone filopodia which allows them to probe their surrounding space by measuring the extent of filopodial adhesion surface with the ECM. Filopodium alignment with an ECM line allows to the formation of a robust F-actin network that leads to its stabilization, allowing steady neurite extension along the line pattern. Because this model system allows exquisitely stereotypic filopodial dynamics, this opens up the possibility to easily study the spatio-temporal dynamics of the signaling networks that regulate this prototypic growth cone navigation system.
Key words: neurite outgrowth, filopodium, actin dynamics, microfabrication, live cell imaging
Proper functioning of the nervous system requires functional connections between neurons. This requires undifferentiated neurons to extend neurites that will subsequently differentiate in axons and dendrites to wire the adult brain. Most of what we know about the biology of neurite outgrowth has been performed in classic 2-dimensional culture dishes, which do not recapitulate the in vivo occurring, 3-dimensional environments in which the extracellular matrix assembles structures with specific geometries and size features on the order of tens of nanometers to micrometers. By example, laminin can build fibrillar tracks on the surface of Schwann cells which are important for neurite outgrowth and neuronal regeneration after a lesion.1 Such ECM tracks display submicrometer size features. It is therefore reasonable to assume that precise topology of the ECM is important for the cell's ability to interact with and perceive its environment.2 However, the importance of the ECM organization and topography at the micro- and nanometer scale is still poorly understood. With recent advances in microfabrication techniques,3,4 this now becomes accessible, and multiple reports document that extension of neuronal processes from neurons are highly dependent on the geometrical topology of the ECM.
We and others show that when ECM cues that induce neurite outgrowth are presented on a number of different scaffolds such as linear ridge groove patterns,5 aligned nanofibers,6 or porous structures,7 all having size features on the order of hundreds of nanometers, neuronal processes can read these patterns and align along them. This is remarkable since, in these experiments, the size features of these structures are smaller than the neuronal process and the growth cone itself, excluding the possibility that orientation of outgrowth is the result of sterically confining the neurite to a specific geometrical domain. This suggests that the neurite is able to sense and read such nanotopographic cues, most likely through its growth cone. Another surprising result is that such nanopatterned substrates not only orient neurite outgrowth but also lead to increase in neuronal process length,5,6 robust axonal specification7 or differentiation of stem cells to the neuronal lineage.8–10 The ability of the cell to polarize (e.g., to directionally extend neurites) seems therefore to be intimately linked with robust neurite extension, and this might in turn allow regulation of cell fate.
In our recently published paper,5 we identify a potential mechanism by which neuronal growth cones are able to interprete such topographic cues to orient neurite outgrowth in a model ridge/groove nanopatterned ECM laminin substrate (refered to as the line substrate throughout this article). We also provide insight into how directionality is linked to robust neuronal process extension. We find that the line substrate leads to a switch in cell morphology, with a drastic decrease in the number and length of filopodia, and the formation of streamlined growth cones that contrast with the flat veil-like growth cones typically observed on classic 2D substrates. Sensing of the line substrate occurs through a dynamic, stochastic search and capture mechanism performed by the growth cone filopodia. The filopodia explore their environment through a highly stereotypic behavior involving protrusion-retraction cycles that are coupled with lateral scanning motions, with a typical filopodium lifecycle on the order of 5 to 10 min. Because of their size features (roughly 2–3 µm length and 200–300 nm width), filopodia will display different extents of interaction with the line substrate (width: 350 nm) depending if they are aligned or not with a pattern ridge. We observe that when a filopodium aligns with the line substrate, this leads to its stabilization for hours in the direction of the pattern. This occurs through the formation of a dense F-actin network in the aligned filopodium that might enable a very efficient cytoskeletal-substrate coupling. In non-aligned filopodia, less efficient cytoskeletal-substrate coupling might lead to filopodia retraction when the retrograde actin flow exceeds actin assembly at the filopodium tip.11 At a global cell level, the consequence of filopodium stabilization is that it allows for steady neurite outgrowth, rather than the neurite protrusion-retraction cycles observed on the classic 2D substrate. This explains how directionality is coupled to robust neuronal outgrowth. Schematics of the dynamic filopodium behavior underlying the sensing process is pictured in Figure 1.
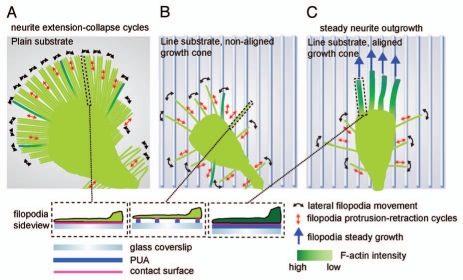
Figure 1.
Model of neurite guidance in response to nanotopographical cues. (A) Plain substrate. Unrestricted access to ECM leads to a large amount of long filopodia, none of which can be stabilized by a robust F-actin cytoskeleton. This is accompanied with a high frequency of neurite collapse events. (B) Line substrate, unaligned growth cone. Filopodia scan the line substrate through lateral scanning and protrusion/retraction events. Only few filopodia align on the lines and thus almost all filopodia sense only discrete adhesion points to the ECM. (C) Line substrate, aligned growth cone. Through stochastic sensing, multiple filopodia have aligned on the line substrate and have assembled an F-actin rich cytoskeleton that stabilizes them. On the distal part of the growth cone, non-aligned, unstable filopodia continue to operate, suggesting a crosstalk between both filopodia populations. This stabilizes the growth cone leading to steady neurite outgrowth. Figure reproduced with permission from Jang et al.5
An important clue that the stochastic search and capture mechanism we describe is relevant, is that in vivo imaging of neuronal guidance,12 reveals similar streamlined growth cones with lateral filopodial scanning as we observe on the line substrate (Fig. 2). This clearly contrasts with the flat, veil-like growth cones that are typically observed in 2D substrates. Our data clearly suggests that the key feature of stochastic search and capture performed by filopodia is that it serves as an “alignment sensor” for the line substrate. This is possible because the filopodium has similar size features than the line substrate and might therefore sense different extent of adhesion depending on if it is aligned or not with the asymmetric line nanostructure. Given the large literature about filopodium cytoskeletal dynamics and growth cone guidance, an important question that comes to mind is why this striking behavior was not observed before. The simple answer is that, on classic 2D substrates, the isotropic nature of the ECM surrounding filopodia does not allow them to sense local differences in adhesive contact. Because each filopodia engage an identical amount of ECM, this does not allow to trigger the signal amplification mechanisms that allow filopodium stabilization and steady neurite outgrowth. This also applies to stripe assays that are commonly used as an experimental paradigm for directional neurite outgrowth in the neurobiology community. Such stripe assays typically consist of alternating zones of non-permissive (e.g., poly-D-ornithine) and permissive substrates (e.g., laminin) with size features of tens of microns,13 that are locally experienced as an isotropic environment by growth cone filopodia. In this case, orientation of neurite outgrowth on the permissive substrate might be the result of mechanically sensing the rigidity of the environment by evaluating the differential traction forces exerted by integrins on the permissive or non-permissive substrate, rather than local changes in adhesive contact as on a nanometer-sized line substrate. Not surprisingly, this has been shown to require myosin activity.13 In contrast, we find that myosin and Rho kinase activities are dispensible for neurite orientation on the nanometer-sized line substrate, suggesting that integrin molecules might not work as mechanosensors, but maybe simply evaluate differences in the extent of adhesive contact. These lines of evidence point to the existence of highly diverse modes of ECM sensing that depend on the geometry and size features of the ECM that the growth cone encounters.
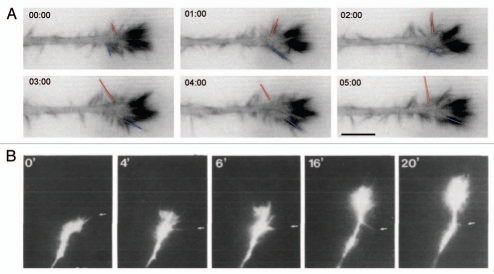
Figure 2.
Comparison of growth cone dynamics on the line substrate and in a developing retina. (A) Growth cone dynamics of N1E-115 neuron-like cells on the line substrate. N1E-115 were transfected with GFP-lifeact, an F-actin marker. Red and blue lines indicates the lateral movement of non-aligned filopodia. Note the high F-actin content in aligned filopodia. Timescale is in minutes:seconds. Part reproduced with permission from Jang et al.5 (B) Growth cone dynamics of Xenopus retinal axons imaged live in the developping retina. Note streamlined appearance of growth cone and left-right lateral movement of lateral filopodia (indicated by arrows). Part reproduced with permission from.12 Scale bars = 10 mm.
It will be important to clarify the modality by which filopodia relay signals to the cytoskeleton to orient neurite outgrowth in this specific mode of growth cone guidance. Importantly, in a 2D environment, in which every filopodium engages identical amount of ECM, its stabilization cannot occur. This suggests that on the line substrate, the growth cone senses the local asymmetry of the ECM by globally integrating signals emanating from aligned and non-aligned filopodia which display different extents of adhesion with the substrate. These signals must then be integrated to locally switch on the robust F-actin network that allows filopodium stabilization and, ultimately, robust directional neurite outgrowth. To understand this system, we must therefore identify the players that allow directional sensing by integrins and the logic of this signal integration machinery. At the same time, we must also gain insight in the signaling events that allow the formation of the robust actin network that stabilizes aligned filopodia. An important feature of our experimental model system is that its filopodia morphodynamics are exquisitely robust and stereotypic compared to the 2D environment. Furthermore, because the streamlined growth cone displays a reduced number of filopodia, this allows to easily observe each phases of their cycles of growth/retraction, lateral scanning motion and stabilization (Fig. 2A and B). This is not the case on the 2D substrate in which the high filopodia density leads them to laterally bump into each other, restricts their movement, and thus precludes the observation of these events (Fig. 2C). The robustness of this system should therefore allow to unambigously quantify each dynamic stage of the filopodium life cycle such as growth and retraction, left-right lateral movement and stabilization through assembly of a robust F-actin network. This should allow to decipher the signaling networks that regulate each filopodium behavior using genetic perturbations and visualization of spatio-temporal signaling events using fluorescent biosensors. Since the neurite proteome of our N1E-115 neuronal-like cell system has been elucidated,14 we have a catalogue of components as a template that can be tested by RNA interference screens. A complementary technique will be to visualize the fine dynamics of signaling proteins such as Rho GTPases, which are essential regulators of the cytoskeleton, and have previously shown to occur on spatial and temporal scales of micrometer and tens of seconds.15 We therefore envision that dynamic activation patterns of Rho GTPase signaling will correlate with different morphogenetic phases of filopodium movement. Finally, computer vision techniques are also available to merge dynamics datasets from different experiments in one universal model.16 This could then be ultimately used for modeling the whole process.
Beyond its capacity to allow efficient directional neurite outgrowth, the line substrate mentioned in this study has also been shown to induce robust differentiation of human embryonic stem cells to the neuronal lineage without the use of differentiation-inducing agents such as cocktails of growth factors.8 Furthermore, differentiation is not efficient on classic 2D substrates that use the same ECM protein, suggesting that ECM nanotopology plays an important role. How can this occur? The important parameters here might be cell intrinsic, mechanical cues, which have been recently proposed to be equally important in stem cell fate regulation than the classically studied extrinsic cues such as specific soluble growth factors and ECM components.17,18 Such cell intrinsic signals are produced by mechanical cycles of extension and retraction that allow cells to sense their environment, initiate intracellular signaling events that can ultimately be integrated to produce long term responses such as transcriptional regulation and cell fate specification.18 Obviously, the different morphodynamic cell behaviors observed on the 2D (neurite growth/retraction cycles) and the nanopattern (steady neurite outgrowth) immediatly suggest that there will be production of distinct cell-intrinsic, mechano-chemical signals in both modes of neurite outgrowth. How could such morphodynamic behaviors be integrated in a global cellular response? One possibility is that during neurite retraction, in which the growth cone de-adheres, the integrin signaling that normally occurs in the adherent growth cone during neurite protrusion is simply turned off because of a lack of integrin engagement with the ECM. There are numerous examples of retrograde signaling mechanisms between the growth cone and the nucleus,19 and one might easily imagine that such processes are depending on integrin signaling in the growth cone (e.g., that the growth cone is in its adhesive state). Continuous integrin engagement during processive neurite outgrowth on the nanopattern might therefore ensure constant retrograde signaling from the growth cone to the nucleus, and allow robust cell specification or other cellular responses such as cell survival and quiescence. At a different spatial and temporal scale, the unstable versus stable filopodia behaviors on the 2D versus the nanopattern could also lead to production of similar cell-intrinsic mechano-chemical signals.
To conclude, we believe that the nanopattern technology presented here, combined with a variety of contemporary cell biology techniques such as live cell imaging and large scale perturbation studies has the potential to provide an integrated view of the spatio-temporal signaling networks that regulate a prototypical growth cone guidance process. Additionally, this model system also opens up the possibility to study how these spatio-temporal signaling networks are globally integrated by the cell to regulate cell fate.
Acknowledgments
This work was supported by grants from the Swiss National Science Foundation and Human Frontiers Science Program to Olivier Pertz.
Abbreviations
- ECM
extracellular matrix
References
- 1.Tsiper MV, Yurchenco PD. Laminin assembles into separate basement membrane and fibrillar matrices in Schwann cells. J Cell Sci. 2002;115:1005–1015. doi: 10.1242/jcs.115.5.1005. [DOI] [PubMed] [Google Scholar]
- 2.Ingber DE. Mechanical control of tissue morphogenesis during embryological development. Int J Dev Biol. 2006;50:255–266. doi: 10.1387/ijdb.052044di. [DOI] [PubMed] [Google Scholar]
- 3.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci USA. 2006;103:2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sniadecki NJ, Desai RA, Ruiz SA, Chen CS. Nanotechnology for cell-substrate interactions. Ann Biomed Eng. 2006;34:59–74. doi: 10.1007/s10439-005-9006-3. [DOI] [PubMed] [Google Scholar]
- 5.Jang KJ, Kim MS, Feltrin D, Jeon NL, Suh KY, Pertz O. Two distinct filopodia populations at the growth cone allow to sense nanotopographical extracellular matrix cues to guide neurite outgrowth. PLoS One. 2010;5:15966. doi: 10.1371/journal.pone.0015966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel S, Kurpinski K, Quigley R, Gao H, Hsiao BS, Poo MM, et al. Bioactive nanofibers: synergistic effects of nanotopography and chemical signaling on cell guidance. Nano Lett. 2007;7:2122–2128. doi: 10.1021/nl071182z. [DOI] [PubMed] [Google Scholar]
- 7.Cho WK, Kang K, Kang G, Jang MJ, Nam Y, Choi IS. Pitch-dependent acceleration of neurite outgrowth on nanostructured anodized aluminum oxide substrates. Angew Chem Int Ed Engl. 2010 doi: 10.1002/anie.201003307. [DOI] [PubMed] [Google Scholar]
- 8.Lee MR, Kwon KW, Jung H, Kim HN, Suh KY, Kim K, et al. Direct differentiation of human embryonic stem cells into selective neurons on nanoscale ridge/groove pattern arrays. Biomaterials. 2010;31:4360–4366. doi: 10.1016/j.biomaterials.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Xie J, Willerth SM, Li X, Macewan MR, Rader A, Sakiyama-Elbert SE, et al. The differentiation of embryonic stem cells seeded on electrospun nanofibers into neural lineages. Biomaterials. 2009;30:354–362. doi: 10.1016/j.biomaterials.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yim EK, Pang SW, Leong KW. Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp Cell Res. 2007;313:1820–1829. doi: 10.1016/j.yexcr.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mallavarapu A, Mitchison T. Regulated actin cytoskeleton assembly at filopodium tips controls their extension and retraction. J Cell Biol. 1999;146:1097–1106. doi: 10.1083/jcb.146.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris WA, Holt CE, Bonhoeffer F. Retinal axons with and without their somata, growing to and arborizing in the tectum of Xenopus embryos: a time-lapse video study of single fibres in vivo. Development. 1987;101:123–133. doi: 10.1242/dev.101.1.123. [DOI] [PubMed] [Google Scholar]
- 13.Turney SG, Bridgman PC. Laminin stimulates and guides axonal outgrowth via growth cone myosin II activity. Nat Neurosci. 2005;8:717–719. doi: 10.1038/nn1466. [DOI] [PubMed] [Google Scholar]
- 14.Pertz JC, Wang Y, Yang F, Wang W, Gay LJ, Gristenko MA, et al. Spatial mapping of the neurite and soma proteomes reveals a functional Cdc42/Rac regulatory network. Proc Natl Acad Sci USA. 2008;105:1931–1936. doi: 10.1073/pnas.0706545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pertz O. Spatio-temporal Rho GTPase signaling—where are we now? J Cell Sci. 2010;123:1841–1850. doi: 10.1242/jcs.064345. [DOI] [PubMed] [Google Scholar]
- 16.Sabouri-Ghomi M, Wu Y, Hahn K, Danuser G. Visualizing and quantifying adhesive signals. Curr Opin Cell Biol. 2008;20:541–550. doi: 10.1016/j.ceb.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mammoto A, Ingber DE. Cytoskeletal control of growth and cell fate switching. Curr Opin Cell Biol. 2009;21:864–870. doi: 10.1016/j.ceb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Vogel V, Sheetz MP. Cell fate regulation by coupling mechanical cycles to biochemical signaling pathways. Curr Opin Cell Biol. 2009;21:38–46. doi: 10.1016/j.ceb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markus A, Patel TD, Snider WD. Neurotrophic factors and axonal growth. Curr Opin Neurobiol. 2002;12:523–531. doi: 10.1016/s0959-4388(02)00372-0. [DOI] [PubMed] [Google Scholar]