Abstract
Prestin, which is a member of the solute carrier 26 anion transporter family (SLC26A5), is a voltage-dependent membrane-based motor protein that confers electromotility on mammalian cochlear outer hair cells (OHCs).1 OHCs are a mammalian innovation, their presence2 and their endowment with functional prestin is essential for normal hearing of mammals.3 In order to clarify the molecular mechanism underlying the voltage-dependent motility of prestin, precise description of the relation between voltage-induced prestin-associated charge movement and the resulting cell displacement is essential. By simultaneously measuring voltage-dependent charge movement, which is manifested in the nonlinear capacitance (NLC) of the cell membrane, and voltage-induced OHC displacement, we provided compelling experimental evidence that prestin-associated charge movement and the resulting electromotility are fully coupled, and that prestin has at least two voltage-dependent conformational transition steps. These findings provide a basis for understanding the molecular mechanism of prestin. Here we discuss the relevance of our finding in the elucidation of the voltage-dependent motor mechanism of prestin, and speculate about possible voltage sensing mechanisms of the molecule.
Key words: prestin, membrane motor, SLC26, nonlinear capacitance, electromotility, voltage, dipole
Our study provided experimental evidence that prestin's conformational change, representing compacted and expanded states of the molecule, has at least two voltage-dependent steps. How should this finding be appreciated for understanding the underlying molecular mechanism of this novel motor protein, the only member of the SLC family that is motile? Since prestin has evolved from an SLC26 anion transporter ancestor, it is likely that prestin has acquired voltage-dependent motile function by modifying the transporter mechanism. Necessity of intracellular chloride for normal motor function of prestin4 is in agreement with the hypothesis. In fact, in a very recent study, Tang et al. successfully converted pendrin (SLC26A4), which has close sequence similarity to prestin in the SLC26A family, into an electromotile protein by inserting a short amino acid segment that is uniquely found in mammalian prestin.5 In another very recent study, Oliver et al. succeeded in converting a non-motile non-mammalian prestin homolog into an electromotile protein by swapping multiple amino acid segments.6 Quite interestingly, the amino acid segments used by Tang et al. and Oliver et al. did not overlap, implying that there are more ways than one for an SLC26 anion transporter to gain voltage-dependent motility function. It is thus likely that the voltage-dependent motilities of the chimeric proteins used in those studies are induced by different underlying molecular mechanisms. It is conceivable that the two distinct voltage-dependent OHC cell-displacement processes that we observed in our study are intimately related to those potentially distinct voltage-induced motilities seen in the chimeric proteins. It would be interesting to measure the unitary displacements of the chimeric proteins in order to compare to those of wild-type prestin as determined in our study (0.20 nm + 0.34 nm = 0.54 nm step along the axial direction of an OHC).7 It is possible that the unitary displacements of those chimeric motors are much smaller than those found in wild-type prestin, and that prestin has evolved to enhance the voltage-induced displacement by combining multiple (at least two) voltage-dependent displacement processes in a facilitative, possibly nonlinear manner. It would also be interesting to measure the electromechanical coupling efficiencies of those chimeric proteins, and to compare the values to those obtained for wild-type prestin in our study (1.9 nm/aC and 3.5 nm/aC),7 for gaining insights into the efficacies of the chimeric proteins. It may be possible that the electromechanical coupling efficiency of prestin is synergistically enhanced by combining multiple voltage-dependent displacement processes.
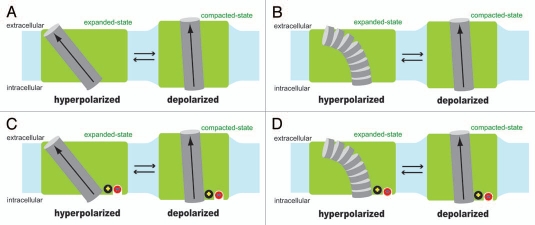
Very little is known about the electromechanical coupling mechanism of prestin. Even the identity of the voltage-sensing moiety remains to be determined. Extrinsic anions such as chloride were first proposed as the voltage sensor of prestin,4 however, there is now growing evidence that the voltage sensing charges are intrinsic to the prestin molecule but require allosteric regulation by chloride.8–10 However, systematic mutations of charged amino acids to neutral ones have not yielded the identity of the key charged amino acid residues.4,11,12 In other words, the voltage-dependent charge movement of prestin cannot be eliminated by any mutation of charged amino acids, singly or in groups, tested so far except in non-germane cases of impaired membrane targeting caused by some mutations. One possible voltage sensor is the α-helix dipole that places partial positive charge (+0.5) near the N-terminus and partial negative charge (−0.5) near the C-terminus.13 A model that assumes the α-helix dipole as the voltage sensor was previously proposed to explain the underlying molecular mechanism of OHC electromotility.14 Although the model was introduced in a context that is no longer tenable, the helix dipole-based voltage-sensing model itself can be applicable to prestin. Voltage-sensing α-helix dipoles have not been found in any voltage-dependent ion channel or transporter studied so far. However, such mechanism is theoretically possible and, as discussed below, it can explain prestin's operation. It is also possible that charged amino acids and the α-helix dipole work in concert to serve as the aggregate voltage sensor of prestin. Partial attenuation of prestin's voltage sensitivity was found in some prestin mutants whose charged amino acids were mutated to neutral ones. The residual may be due to the remaining α-helix dipole. Figure 1A shows a model where an α-helix dipole serves as the voltage sensor. Interaction energy of the α-helix dipole (µ) and the electric field (E) is calculated as µEcosθ, where θ is the angle between the µ and E vectors. The magnitude of E can be determined by the membrane potential (Vm) divided by the thickness of the membrane. If the thickness of the membrane (Lm) and the length of the voltage sensing α-helix (Lh) are assumed to be similar as in Figure 1A, the calculation of the µ-E interaction energy becomes insensitive to Lm and Lh [µEcosθ = (0.5 × 1.6 × 10−19 × Lh) × (Vm/Lm) × cosθ = 0.5 × 1.6 × 10−19 × Vm × cosθ]. The µ-E interaction energy is maximized when θ ≅ 0° (Fig. 1A, right). If the position and the angle of the voltage sensing α-helix in the membrane shown in the right side of Figure 1A did not change by Vm, the voltage-dependent difference in the µ-E interaction energy between the expanded-state (Fig. 1A, left) vs. the compacted-state (Fig. 1A, right) would be calculated as 19 kBT/V, where kB and T are the Boltzmann constant and absolute temperature, respectively. Since our model in Figure 1A assumes greater θ (0° << θ < 90°) for the expanded-state (Fig. 1A, left), the voltage-dependent energy difference of the µ-E interaction between the two distinct prestin conformations would become smaller than 19 kBT/V. However, the experimentally determined voltage sensitivity of prestin is typically 30–35 kBT/V for the simple 2-state Boltzmann model, and 25.2 kBT/V and 23.9 kBT/V for the 3-state Boltzmann model.7 All these values are greater than 19 kBT/V, supporting the possibility of the α-helix dipole serving as the voltage sensor in the prestin molecule. Another possibility is shown in Figure 1B, where the formation of an α-helix is disrupted (Fig. 1B, left) or induced (Fig. 1B, right) by Vm change. It is known that the stability of an α-helix is significantly affected by the charge-dipole interaction.15 Therefore, if there were a transmembrane segment in prestin, whose α-helix forming propensity is moderate and thus greatly influenced by the magnitude and the polarity of Vm, it is possible that the helix-non-helix transition induced by Vm is used as the voltage sensing mechanism.
Figure 1.
Hypothetical voltage sensing mechanisms of prestin. Two different structural states of prestin (expanded vs. compacted) are shown in green with a hypothetical voltage sensing α-helix (gray cylinder), whose N-terminus faces the extracellular side. The α-helix dipole, the direction of which is from the C-terminus (negatively charged) to the N-terminus (positively charged) by definition, is shown with arrow. Shown in light blue is the cell membrane. The length of the hypothetical voltage sensing α-helix is assumed to be similar to the thickness of the cell membrane in the models. If the typical cell membrane thickness of ∼3 nm were used, and if the partial charge present at the N-terminus and the C-terminus were assumed to be +0.5 and −0.5, respectively, the magnitude of the α-helix dipole moment would be calculated as 2.4 × 10−28 Cm (computation: 0.5 × 1.6 × 10−19 × 3 × 10−9). In model (A and C), the structure of the hypothetical voltage sensing α-helix is assumed to be maintained during physiological membrane potential changes, whereas, in (B and D), the propensity of the α-helix formation is assumed to be moderate or low, and thus is assumed to be significantly reduced to induce destruction of the helix structure under hyperpolarized membrane potential. In (C and D), a hypothetical intrinsic positive charge (black circle) and a hypothetical extrinsic anion binding site with bound chloride (red circle) are included for explaining the observed Vpk shifts induced by chloride substitution with various anions (see text for detail).
How should chloride, which is essential for normal function of prestin, be included in the models considered above? At this point, it is hard to speculate how chloride confers normal motor function on prestin due to the lack of knowledge regarding prestin-motor function in the absence of extrinsic anion binding. Variable shifts in the voltage operating point (Vpk; the membrane potential at the peak of the nonlinear capacitance function and maximum slope of the motility function) both in the hyperpolarizing direction and depolarizing direction with different degrees and changes in the magnitude of prestin-associated charge movement are observed even if endogenous anions are substituted with large non-halide anions such as 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pentane sulfonate, gluconate, malate and even salicylate, suggesting that those substituted anions are capable of binding to prestin, and differently modulating its motor function.8,9,16 By focusing on the fact that most substituted anions tested so far significantly affect Vpk, chloride is included in our models in an effort to provide a tentative picture of how extrinsic anions could affect Vpk (Fig. 1C and D). The models shown in Figure 1C and D are based on Figure 1A and B, respectively, but assume a positively charged component that is situated in the close vicinity or on the C-terminus of the hypothetical voltage sensing helix. The identity of the positive charge can be a positively charged amino acid or a partial positive charge derived from the N-terminus of another α-helix that is in the reversed configuration. The extrinsic anion-binding site is assumed to be situated close to the positive charge. In the absence of extrinsic anion binding, the positive charge further stabilizes the voltage sensing α-helix by charge-dipole interaction but attenuates the magnitude of the α-helix dipole in Figure 1C, while it increases the α-helix forming propensity in Figure 1D. In both models, binding of an extrinsic anion to prestin is expected to screen the positive charge, and thus is expected to affect Vpk of prestin by either increasing the magnitude of the α-helix dipole in Figure 1C or reducing the α-helix forming propensity in Figure 1D. The different degrees and directions of Vpk shifts observed in the chloride substitution experiments with various anions4,8,9,16 can be explained by different degrees of the positive charge screening in our models.
The validity of the voltage sensor models described above can be tested by introducing a charged amino acid at either (or both) end of a potentially α-helix forming transmembrane segment to enhance/attenuate the α-helix dipole, or to increase/decrease α-helix forming propensity. Designing such mutants requires precise structural information of prestin. Unfortunately, the structure of prestin is not yet solved, although some prestin topology models have been proposed in references 17 and 18. Recently, the detailed transmembrane topology of archetypal BicA transporter from Synechococcus, which is a member of the sulfate permease (SulP) family that includes the mammalian SLC26 family, was determined.19 Meantime, until the structure of prestin becomes available, these topology models would be useful to some extent for designing prestin mutants to test the above considered voltage sensor models of prestin.
Acknowledgments
This work was supported by the Hugh Knowles Center and National Institutes of Health Grant DC00089-40 (to P.D.).
References
- 1.Zheng J, Shen W, He D, Long K, Madison L, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405:149–155. doi: 10.1038/35012009. [DOI] [PubMed] [Google Scholar]
- 2.Ryan A, Dallos P. Effect of absence of cochlear outer hair cells on behavioral auditory threshold. Nature. 1975;253:44–46. doi: 10.1038/253044a0. [DOI] [PubMed] [Google Scholar]
- 3.Dallos P, Wu X, Cheatham M, Gao J, Zheng J, Anderson C, et al. Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron. 2008;58:333–339. doi: 10.1016/j.neuron.2008.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliver D, He D, Klocker N, Ludwig J, Schulte U, Waldegger S, et al. Intracellular anions as the voltage sensor of prestin, the outer hair cell motor protein. Science. 2001;292:2340–2343. doi: 10.1126/science.1060939. [DOI] [PubMed] [Google Scholar]
- 5.Tang J, Tan X, Pecka J, Beisel K, He D. Creation of a novel pendrin protein with both transporter and motor functions; 34th meeting of the Association for Research in Otolaryngology; Baltimore, MD. 2011. p. 67. [Google Scholar]
- 6.Oliver D, Schaechinger T, Gorbunov D, Halaszovich C, Kugler S, Fakler B. A synthetic prestin reveals protein domains and molecular operation of outer hair cell piezoelectricity; 34th meeting of the Association for Research in Otolaryngology; February 19–23, 2011; Baltimore MD. p. 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Homma K, Dallos P. Evidence that prestin has at least two voltage-dependent steps. J Biol Chem. 2011;286:2297–2307. doi: 10.1074/jbc.M110.185694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rybalchenko V, Santos-Sacchi J. Cl-flux through a non-selective, stretch-sensitive conductance influences the outer hair cell motor of the guinea-pig. J Physiol. 2003;547:873–891. doi: 10.1113/jphysiol.2002.036434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rybalchenko V, Santos-Sacchi J. Anion control of voltage sensing by the motor protein prestin in outer hair cells. Biophys J. 2008;95:4439–4447. doi: 10.1529/biophysj.108.134197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song L, Santos-Sacchi J. Conformational state-dependent anion binding in prestin: evidence for allosteric modulation. Biophys J. 2010;98:371–376. doi: 10.1016/j.bpj.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai J, Navaratnam D, Samaranayake H, Santos-Sacchi J. En bloc C-terminal charge cluster reversals in prestin (SLC26A5): effects on voltage-dependent electromechanical activity. Neurosci Lett. 2006;404:270–275. doi: 10.1016/j.neulet.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 12.Bai JP, Surguchev A, Montoya S, Aronson PS, Santos-Sacchi J, Navaratnam D. Prestin's anion transport and voltage-sensing capabilities are independent. Biophys J. 2009;96:3179–3186. doi: 10.1016/j.bpj.2008.12.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Holde K, Johnson C, Ho P. Principles of physical biochemistry. New Jersey: Prentice-Hall, Inc.; 1998. pp. 112–114. [Google Scholar]
- 14.Ashmore JF, Géléoc GS, Harbott L. Molecular mechanisms of sound amplification in the mammalian cochlea. Proc Natl Acad Sci USA. 2000;97:11759–11764. doi: 10.1073/pnas.97.22.11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shoemaker KR, Kim PS, York EJ, Stewart JM, Baldwin RL. Tests of the helix dipole model for stabilization of alpha-helices. Nature. 1987;326:563–567. doi: 10.1038/326563a0. [DOI] [PubMed] [Google Scholar]
- 16.Song L, Seeger A, Santos-Sacchi J. On membrane motor activity and chloride flux in the outer hair cell: lessons learned from the environmental toxin tributyltin. Biophys J. 2005;88:2350–2362. doi: 10.1529/biophysj.104.053579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navaratnam D, Bai J, Samaranayake H, Santos-Sacchi J. N-terminal-mediated homomultimerization of prestin, the outer hair cell motor protein. Biophys J. 2005;89:3345–3352. doi: 10.1529/biophysj.105.068759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deák L, Zheng J, Orem A, Du G, Aguiñaga S, Matsuda K, et al. Effects of cyclic nucleotides on the function of prestin. J Physiol. 2005;563:483–496. doi: 10.1113/jphysiol.2004.078857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shelden MC, Howitt SM, Price GD. Membrane topology of the cyanobacterial bicarbonate transporter, BicA, a member of the SulP (SLC26A) family. Mol Membr Biol. 2010;27:12–23. doi: 10.3109/09687680903400120. [DOI] [PubMed] [Google Scholar]