Abstract
The acquisition of intestinal microbiota in the immediate postnatal period has a defining impact on the development and function of many immune and metabolic systems integral to health and well-being. Recent research has shown that the presence of gut microbiota regulates the set point for hypothalamic-pituitary-adrenal (HPA) axis activity.1 Accordingly, we sought to investigate if there were other changes of brain function such as behavioral alterations in germ free (GF) mice, and if so, to compare these to behavior of mice with normal gut microbiota. Our recent paper showed reduced anxietylike behavior in the elevated plus maze (EPM) in adult GF mice when compared to conventionally reared specific pathogen-free (SPF) mice.2 Here, we present data collected when we next colonized the adult GF mice with SPF feces thereby introducing normal gut microbiota, and then reassessed anxiety-like behavior. Interestingly, the anxiolytic behavioral phenotype observed in GF mice persisted after colonization with SPF intestinal microbiota. These data show that gut-brain interactions are important to CNS development of stress systems and that a critical window may exist after which reconstitution of microbiota and the immune system does not normalize the behavioral phenotype.
Key words: germ free, microbiota, anxiety-like behavior, gut-brain, elevated plus maze
It is well established that the gut and brain communicate in a bidirectional manner through the autonomic nervous system, immune system and the HPA axis.3–5 Gut-brain communication is important to human gastrointestinal and psychiatric illness, which is highlighted by the increased comorbidity found between mood and anxiety disorders and both inflammatory bowel disease and the functional bowel disorders.6–8 Indeed the focus of Rome III, a diagnostic instrument designed to aid clinicians in the diagnosis of functional bowel disorders such as irritable bowel syndrome (IBS), is on gut, brain and spinal cord interactions and their involvement in the generation of symptoms of pain and intestinal dysfunction.9 Luminal contents have also become a focus of study in the etiology of functional bowel disorders, with a number of studies pointing to variations in the composition of gut microbiota in patients suffering from IBS compared to controls.10 A recent report found that compared to specific pathogen-free (SPF) mice, adult germ free mice showed an exaggerated stress response, as evidenced by increased plasma corticosterone (CORT) and adrenocorticotrophic hormone (ACTH) levels in response to restraint stress.1 Clearly the study of the impact of gut microbiota on the development of HPA dysfunction and potentially anxiety-like behavior has important clinical applications in the study of both gastrointestinal and psychiatric health and disease, as they are essentially involved in the communication between the gut and the brain.
We propose that the intestinal microbiota housed within the gastrointestinal tract may act as a mediator in the communication existing between the gut and the brain. In our recent study,2 we examined behavior in germ free (GF) mice and provided evidence that gut microbiota are important to the development of stress circuitry and related behaviors. We demonstrated that GF mice show reduced anxiety-like behavior in the elevated plus maze (EPM) in comparison to specific pathogen-free (SPF) mice, a phenotype that was accompanied by changes in plasticity-related genes in the hippocampus and amygdala.2 Our results provide support for the idea that normal healthy gut bacteria may influence the development of the CNS and thereby its function, as reflected by both the behavioral and molecular changes seen in GF mice. Our findings are consistent with another recent report linking gut microbiota with behavior and neurochemistry.11 In both the original work by Sudo et al. and the recent work by Heijtz et al. the authors addressed the issue of critical windows of development. Sudo et al. demonstrated reconstitution of germ free mice with SPF flora at 6 weeks (young adult) but not at 14 weeks (adult) reversed the HPA axis response to restraint stress.1 Heijtz et al. showed that reconstitution of germ free mice early in life reversed the locomotor and anxiety-like behaviors in the EPM, however, increased exploratory behavior in the light/dark box persisted in these mice.11 In a subset of GF mice used in our original study, we colonized the GF mice with SPF faeces thereby introducing normal gut microbiota and reassessed anxiety-like behavior. Conventionalization of GF mice (CONV-GF) occurred by daily mixing the GF mouse's home bedding with SPF mouse bedding and stool. Studies have shown that this intervention will reconstitute the full complement of microbiota to a GF animal.12,13 As shown in Figure 1, CONV-GF mice showed a persistent anxiolytic behavioral phenotype, as shown by increased time spent in the open arms of the EPM and increased open arm entries (t-test, p < 0.05). Given that we observed no change in the altered behavioral phenotype post-conventionalizion in the adult GF mouse, it is likely that neural pathways are altered early in development and that there is a critical period postnatally in which HPA axis dysfunction and behavioral traits become relatively hard-wired into adulthood.
Figure 1.
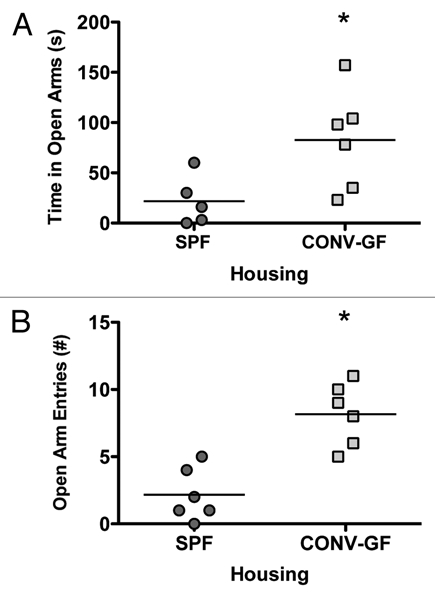
EPM testing of SPF and conventionalized GF mice (CONVGF) showed increased open arm (A) and increased open arm entries (B) in CONV-GF mice compared to SPF mice. *p < 0.05.
Evidence of behavioral alterations in conjunction with adaptive immune deficits have been demonstrated by Cushman et al. who showed that deletion of the recombinase activating gene (RAG-1) in mice which results in absent antibody synthesizing capability, caused increased exploration in the open field and decreased open arm avoidance in the EPM. However, it should be noted that RAG-1 is also expressed in the CNS15 where its function has yet to be elucidated. It is possible that the known immaturity of the adaptive immune system of GF mice somehow contributes to the behavioral phenotype we observed. The sensory arm of the autonomic nervous system may be an alternate route whereby gut microbiota may affect brain function. Several reports make this link. Oral ingestion of a bifidobacterium by conventional Sprague Dawley (SD) rats resulted in evidence of changes in serotonin metabolism in the brain stem.16 Consumption of Lactobacillus reuteri (LR) by conventional SD rats inhibited the perception of pain consequent to visceral distension suggesting that commensal bacteria can affect nerve function and pathways in conventional animals.17 Recent work in our laboratories has shown that oral treatment of SD rats with LR consistently activated calcium dependent potassium channels in a specific subset of enteric neurons in the colonic myenteric plexus,18 thus affording a direct link between luminal commensals and the enteric nervous system. In related studies using minimal doses of two different potentially pathogenic bacteria, citrobacter rodentium and campylobacter jejuni, Lyte and colleagues19,20 observed vagally mediated activation in the brain stem of mice in the absence of any evidence of inflammation in the gut, again emphasizing the effects of intestinal microbes upon the gut-brain communication pathway.
We consider that the behavioral and molecular alterations that we have observed in GF mice may have occurred due to the absence of microbial direct or indirect neural communication. Further work exploring these mechanistic possibilities may lead to novel insights into the complex roles of commensal bacteria in the development and function of the CNS. Unraveling these pathways may eventually lead to new therapeutic approaches in dealing with both gastrointestinal and psychiatric illness.
References
- 1.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ free mice. Neurogastroenterol Motil. 2011;23:255–264. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 3.Brthoud HR. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil. 2008;20:64–72. doi: 10.1111/j.1365-2982.2008.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drossman DA. What does the future hold for irritable bowel syndrome and the functional gastrointestinal disorders? J Clin Gastroenterol. 2005;39:251–256. doi: 10.1097/01.mcg.0000156107.13247.69. [DOI] [PubMed] [Google Scholar]
- 5.Shanahan F. Brain-gut axis and mucosal immunity: a perspective on mucosal psychoneuroimmunology. Semin Gastrointest Dis. 1999;10:8–13. [PubMed] [Google Scholar]
- 6.Creed F, Guthrie E. Psychological factors in the irritable bowel syndrome. Gut. 1987;28:1307–1318. doi: 10.1136/gut.28.10.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker JR, Ediger JP, Graff LA, Greenfeld JM, Clara I, Lix L, et al. The Manitoba IBD cohort study: a population-based study of the prevalence of lifetime and 12-month anxiety and mood disorders. Am J Gastroenterol. 2008;103:1989–1997. doi: 10.1111/j.1572-0241.2008.01980.x. [DOI] [PubMed] [Google Scholar]
- 8.Wood JD. Neuropathophysiology of functional gastrointestinal disorders. World J Gastroenterol. 2007;13:1313–1332. doi: 10.3748/wjg.v13.i9.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grundy D, Al-Chaer ED, Aziz Q, Collins SM, Ke M, Tache Y, et al. Fundamentals of neurogastroenterology: basic science. Gastroenterology. 2006;130:1391–1411. doi: 10.1053/j.gastro.2005.11.060. [DOI] [PubMed] [Google Scholar]
- 10.Matto J, Maunuksela L, Kajander K, Palva A, Korpela R, Kassinen A, et al. Composition and temporal stability of gastrointestinal microbiota in irritable bowel syndrome—a longitudinal study in IBS and control subjects. FEMS Immunol Med Microbiol. 2005;43:213–222. doi: 10.1016/j.femsim.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Heijtz RD, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 13.Cebra JJ. Influences of microbiota on intestinal immune system development. Am J Clin Nutr. 1999;69:1046–1051. doi: 10.1093/ajcn/69.5.1046s. [DOI] [PubMed] [Google Scholar]
- 14.Cushman J, Lo J, Huang Z, Wasserfall C, Petitto JM. Neurobehavioral changes resulting from recombinase activation gene 1 deletion. Clin Diagn Lab Immunol. 2003;10:13–18. doi: 10.1128/CDLI.10.1.13-18.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chun JJ, Schatz DG, Oettinger MA, Jaenisch R, Baltimore D. The recombination activating gene-1 (RAG-1) transcript is present in the murine central nervous system. Cell. 1991;64:189–200. doi: 10.1016/0092-8674(91)90220-s. [DOI] [PubMed] [Google Scholar]
- 16.Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43:164–174. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Kunze W, Mao Y, Forsythe P, Bienenstock J. Effect of Lactobacillus SP. (Lb) probiotic ingestion on membran properties of intrinsic sensory neurons in the rat colon. Bethesda MD: American College of Gastroenterology; 2006. [Google Scholar]
- 18.Kunze WA, Mao YK, Wang B, Huzinga JD, Ma XJ, Forsythe P, et al. Oral ingestion of Lactobacillus reuteri enhances excitability of colonic AH sensory neurons through inhibition of the slow afterhyperpolarization by inhibiting calcium dependent potassium channel (IKCa) opening. J Cell Mol Med. 2011;13:2261–2270. doi: 10.1111/j.1582-4934.2009.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goehler LE, Gaykema RP, Opitz N, Reddaway R, Badr N, Lyte M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immun. 2005;19:334–344. doi: 10.1016/j.bbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Lyte M, Li W, Opitz N, Gaykema RP, Goehler LE. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol Behav. 2006;89:350–357. doi: 10.1016/j.physbeh.2006.06.019. [DOI] [PubMed] [Google Scholar]


