Abstract
Diagnosis of Alzheimer's disease is often missed or delayed in clinical practice; thus, methods to improve early detection would provide opportunities for early intervention, symptomatic treatment, and improved patient function. Emerging data suggest that the disease process begins years before clinical diagnostic confirmation. This paper reviews current research focusing on methods for more specific and sensitive early detection using measures of genetic risk for Alzheimer's disease and functional brain imaging. This approach aims to identify patients in a presymptomatic stage for early treatment to delay progressive cognitive decline and disease onset.
Keywords: Alzheimer's disease, brain imaging, genetic risk, positron emission tomography, magnetic resonance imaging
Abstract
El diagnóstico de la enfermedad de Alzheimer habitualmente es erróneo o se realiza tardíamente en la práctica clínica; por lo tanto, los métodos que ayuden a la detección precoz facilitarán las opciones de una intervención oportuna, de un tratamiento sintomático y de una mejoría en el funcionamiento del paciente. La información reciente sugiere que el proceso patológico comienza años antes de la confirmación del diagnóstico clínico. Este artículo revisa la investigación actual, focalizándose en métodos para una detección precoz más especifica y sensible, a través de mediciones del riesgo genético para la enfermedad de Alzheimer y neuroimágenes cerebrales funcionales. Esta aproximación ayuda a identificar pacientes en una etapa presintomática, para un tratamiento precoz que ayude a retardar el deterioro cognitivo progresivo y la aparición de la enfermedad.
Abstract
En pratique clinique, dans la maladie d'Aizheimer, le diagnostic n'est pas toujours posé ou est souvent retardé; le développement de méthodes diagnostiques précoces pourrait permettre une prise en charge dès le début de la maladie, la prescription d'un traitement symptomatique et l'amélioration de l'état fonctionnel du patient. Les nouvelles données dont nous disposons suggèrent que le processus pathologique débute plusieurs années avant que le diagnostic clinique ne soit confirmé. Cet article concerne les recherches en cours sur des techniques de diagnostic précoce plus spécifiques et plus sensibles associant l'évaluation du risque génétique de développer la maladie et l'imagerie cérébrale. Cette approche a pour objectif d'identifier les patients atteints à un stade présymptomatique afin de les traiter précocement pour retarder l'apparition du déficit cognitifet le début de la maladie.
Advances in medical technology have led to an aging population. A major impact of this “age revolution” is a dramatic increase in persons afflicted with Alzheimer's disease (AD), the most common form of late-life mental decline. In the United States, the disease strikes approximately 4 million persons.1 Patients and their caregivers suffer emotionally and bear a large proportion of the economic burden, estimated to approach $90 billion each year.2
Despite the prevalence of AD, the diagnosis is often overlooked or mistaken.3 Barrett and associates4 reported that as few as 40% of primary care physicians even knew that AD was the most common cause of dementia in late life. Several types of diagnostic error may occur, including incorrectly applying a dementia diagnosis, positively diagnosing the disease when, in fact, it is not present, or not recognizing dementia when it is present. Such errors may result from a lack of attention to cognitive functioning in routine medical examinations and to misperceptions about the normal aging process.5 Early AD detection advances would not only offer presymptomatic disease recognition but likely improve diagnostic accuracy once cognitive impairment progresses to a dementia diagnosis.
Although no cure exists for AD, early disease detection offers a number of potential benefits. Accurate differential diagnosis will identify patients with depression, thyroid disease, or other treatable conditions. Once diagnosed, AD patients can then receive treatment for cognitive losses as well as associated behavioral problems. Drug treatments for cognitive impairment and nonpharmacological and pharmacological treatments for the behavioral problems associated with dementia can also enhance quality of life.1
Several lines of research suggest that AD actually begins years before its clinical manifestations are obvious. Positron emission tomography (PET) studies of glucose metabolism combined with genetic risk assessment show regional glucose abnormalities in middle-aged persons with a genetic risk for AD.6 Studies of structural images suggest that regional atrophy of hippocampus and other medial temporal regions may be an early predictor of future cognitive decline.7 Brain autopsy studies of normal aging and older persons with mild cognitive impairment also indicate very early, preclinical accumulation of neuritic plaques (NPs) and neurofibrillary tangles (NFTs), the neuropathological hallmarks of AD, years before a clinical diagnosis can be confirmed.8,9 Finally, findings from a study of 93 nuns also support the notion of subtle preclinical functional abnormalities. In that study,10 a systemic assessment of these nuns' early autobiographies (mean age 22 years) and their later (age 7595) cognitive performances found that low idea density and lack of grammatical complexity in early life predicted low cognitive test scores in late life.
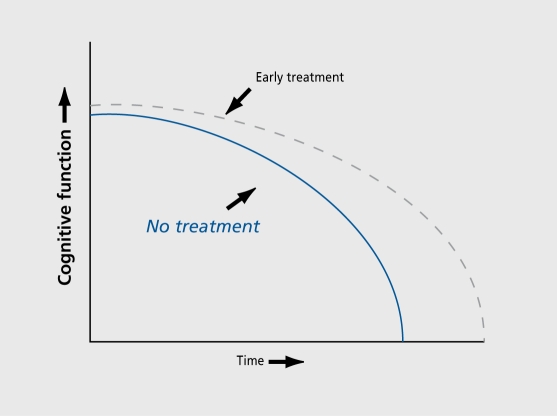
Identifying AD in a preclinical state before the condition can be confirmed using consensus diagnostic criteria also has several potential benefits. When early detection assessments are negative, people with mild memory complaints can be reassured that their forgetfulness reflects a normal age-related change that probably will not progress. In addition, many people would like to know about a poor prognosis while still in a mildly impaired state in order to plan their futures while mental faculties remain. The most compelling argument for preclinical detection strategies is to identify candidates for antidementia treatments before extensive neuronal death develops, since new antidementia treatments are more likely to delay disease progression than to reverse neuronal death. Patients with mild memory losses, who are at risk for AD progression are ideal candidates for antidementia interventions. Although current cholinergic treatments result in symptomatic rather than disease-altering or structural effects, it would certainly be of interest to initiate treatments very early, as disease-modifying interventions emerge. Moreover, both the expense and potential risks of treatment make it reasonable to reserve treatment only for those people who are at the greatest risk for developing the disease (Figure 1).
Figure 1. Hypothetical decline curve for two patients with Alzheimer's disease, one who received treatment (dotted line) another who did not (solid line). A major goal of early detection is to intervene early in the course of the disease, even in presymptomatic stages.
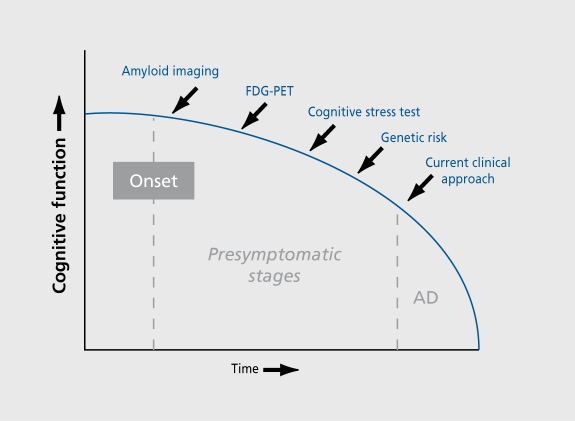
Our group has focused on early detection of AD by combining measures of genetic risk and brain imaging. This paper summarizes information on genetic discoveries and recent work using three different brain-imaging approaches: measures of glucose metabolism using fluorodeoxyglucose positron emission tomography (FDG-PET) during mental rest, functional magnetic resonance imaging (fMRI) during memory performance tasks, and in vivo imaging of NPs and NFTs using PET and small molecule probes (Figure 2).
Figure 2. Technological developments (blue) offer the potential of identifying Alzheimer's disease prior to the time point when clinicians currently arrive at a clinical diagnosis. Combining various approaches is likely to improve early diagnostic accuracy.
Genetic issues
Genetic studies have found an association between the apolipoprotein E-4 (APOE-4) allele on chromosome 19 and the common form of AD that begins after age 60.11 APOE has three allelic variants (2, 3, and 4) and five common genotypes (2/3, 3/3, 2/4, 3/4, and 4/4). The APOE-4 allele has a dose-related effect on increasing risk and lowering onset age for late-onset familial and sporadic AD,11, 12 while APOE-2 confers protection.13 The APOE-4 allele is associated with decline in delayed recall in older adults11, and is a strong predictor of future cognitive decline in people with mild cognitive impairment (MCI).15 However, APOE-4 may have only a modest effect in predicting cognitive decline in many older persons; thus, APOE genotype alone is not considered a useful predictor in nondemented persons.16 The APOE gene explains only 50% of the genetic variability in AD,17 and investigators have searched for other linkage regions18 and candidate genes.19, 20
Combining PET scanning and genetic risk measures
The discovery of the APOE-4 genetic risk for AD was extremely helpful in our early AD detection efforts with PET imaging. In 1995, our group first reported results combining PET imaging and APOE genetic risk in people with a family history of AD/1 We studied 12 nondemented relatives with APOE-4 and 19 relatives without APOE-4, and compared them with 7 probable AD patients. “At-risk” subjects had mild memory complaints, normal cognitive performance, and at least two relatives with AD. Subjects with APOE-4 did not differ from those without APOE-4 in mean age at exam (56.4 vs 55.5 years) or in neuropsychological performance. Parietal metabolism was significantly lower and left-right parietal asymmetry higher in at-risk subjects with APOE-4 than in those without APOE-4. Patients with dementia had significantly lower parietal metabolism than did at-risk subjects with APOE-4. We concluded that the APOE-4 allele was associated with reduced cerebral parietal metabolism and increased asymmetry in relatives at risk for AD.
The following year, Reiman and associates22 replicated these results and extended them to other brain regions, including temporal, prefrontal, and posterior cingulate in a study of 11 APOE-4 homozygotes (4/4 genotype) and 22 APOE-3 homozygotes (3/3 genotype) of similar ages to those in our own initial study (mid-fifties). They also applied an automated image analysis method, wherein metabolic reductions were standardized using threedimensional (3D) stereotactic surface projections from FDG-PRT scans of AD patients compared with controls. The results from these two studies21, 22 provided independent confirmation of an association between genetic risk and regional cerebral glucose hypometabolism.
More recently, our group23 performed FDG-PET scans during mental rest on 65 middle-aged and older persons (mean, SD: 67.3, 9.4 years; range 50-84 years) with mild memory complaints or probable AD.24 Subjects (61 Caucasians, 3 Asians, and 1 Hispanic) were right-handed, in the 50 to 84 year age range (mean, SD: 67.3, 9.4 years), and recruited through newspaper ads and various referrals. Of the 573 persons who volunteered because of minor memory concerns (eg, difficulty remembering names), 508 were excluded for a variety of reasons (eg, medication use, concurrent medical illness). The 27 subjects with age-associated memory impairment (AAMI) and APOE-4 were matched according to age and educational level to 27 AAMI subjects without APOE-4. The 11 AD patients were included as a comparison group without regard to APOE status, since cerebral metabolic patterns do not vary according to APOE genotype in AD patients.25 Subject groups were similar in mean age at examination, sex ratio, frequency of family history of AD, dementia onset age within families, and educational achievement level. Both verbal (Buschke-Fuld)26 and visual (Benton)27 memory performance scores were significantly lower in the demented group but not significantly different between the two nondemented groups (verbal: t=0.51, df=52, P=0.18; visual: t=1.05, df=52, P=0.61).
Comparisons among the three subject groups (region of interest [ROI] analysis) indicated the lowest metabolic rates in the AD group, intermediate rates in the nondemented group with APOE-4, and highest rates in the nondemented group without APOE-4. These differences were bilateral and significant (ANOVAs; df=2.59) in inferior parietal (left hemisphere: F=9.2, P=0.0003; right hemisphere: F=15.6, P<0.0001), posterior cingulate (left: F=14.6, F<0.0001; right: P=17.7, P<0.0001), dorsolateral prefrontal (left: F=13.7, P<0.0001; right: F=5.6, P=0.006), and inferior temporal regions (left: F=43, P=0.018; right: F=3 A, P=0.040). Significant group differences present only in the left hemisphere were found in the medial temporal (F = 4.9,P=0.011) and superior temporal (F=6.0, P=0.004) regions. Further comparisons between the two nondemented groups indicated significantly lower metabolism in subjects with APOE-4 in the inferior parietal region for both the right (t = 2.6, df = 52, P=0.011) and left (t=2.2, df=52, P=0.035) hemispheres compared with those without APOE-4. These differences remained significant even if we eliminated from the comparison the two subjects homozygous for APOE-4. Statistical parametric mapping (SPM) analysis comparing nondemented groups showed similar results with APOE-4 subjects having significantly lower metabolism than those without APOE-4, particularly in the left inferior parietal, lateral temporal, and posterior cingulate regions.28 The peak voxels were in Brodmann's area 21 at (-68, -38, -16) with a secondary focus at (-70, 48,0).
Genetic risk and fMRI results
Activation imaging during memory task performance may reveal subtle alterations in brain function, perhaps prior to the emergence of mild memory impairments. This approach has been described as a “cognitive stress test” for the brain. The approach is based on the hypothesis that cognitive activity may uncover subtle brain functional abnormalities not present during resting cognitive states, similar to how the electrocardiogram stress test reveals subtle cardiac abnormalities when the heart is stressed from treadmill exercise. Functional MRI (fMRI) provides measures of relative cerebral blood flow (rCBF) during memory or other cognitive task performance, and has the advantages of high resolution in space and time and lack of radiation exposure.29 Thirty middle-aged and older subjects with mild memory complaints but normal memory performance received APOE genotyping. The 14 subjects with the APOE-4 genetic risk for AD did not differ significantly from the 16 subjects without APOE-4 group in age, prior educational achievement, or rates of AD family history. During fMRI scanning on a 3-tesla unit, subjects performed an unrelated paired associate learning task. Brain activation patterns were determined during both learning and retrieval task periods and analyzed using both between-group and within-subject approaches. Compared with subjects without APOE-4, those at genetic risk showed significantly greater magnitude and spatial extent of rCBF during memory retrieval in regions affected by AD: left medial temporal and bilateral parietal and prefrontal. Longitudinal data indicated that baseline brain activation correlated with verbal memory decline assessed 2 years later. Relative cerebral blood flow responses to a memory challenge may reflect compensatory cognitive efforts for emerging functional deficits in people at genetic risk for AD. These results suggest that combining brain activation and genetic risk measures may provide information that eventually predicts future cognitive decline.
PET imaging of plaques and tangles in AD
New research is under way to develop additional early detection strategies with greater sensitivity and specificity, including studies aimed at imaging the neuropathological hallmarks of AD. Intraneuronal NFTs and extracellular P-amyloid-rieh senile plaques (SPs) have been implicated as central components of the pathogenic cascade in AD, which highlights the importance of noninvasive in vivo assessment of SP and NFT deposition. A hydrophobic radiofluorinated derivative of 1,1-dicyano2-[6-(dimethylamino)naphthalen-2-yl]propene (FDDNP) was used in conjunction with PET to determine the localization and load of NFTs and SPs in the living brain of AD patients (n=7) and controls (n=3).30 Fluorescence microscopy also was used to determine SP and NFT binding in AD brain specimens. Greater accumulation and slower FDDNP clearance was observed in SP/NFTrieh brain areas, particularly the hippocampus-amygdalaentorhinal complex, but also temporal and parietal cortex in advanced disease stages. In vitro fluorescence microscropy showed excellent visualization of NFTs, SPs, and diffuse amyloid in AD, matching results with thioflavin T obtained in the same specimens. The availability of this noninvasive technique may allow longitudinal evaluation of SP and NFT deposition, permitting more accurate diagnosis and evaluation of therapies.
Conclusions
Evidence is emerging that suggests the eventual utility of combining genetic risk and brain-imaging measures to identify people with very mild memory loss that may be progressive. With such surrogate markers, clinical trials will be possible to determine efficacy of antidementia treatments in presymptomatic stages. The goal is to delay progressive memory loss upon dementia onset so that older people can live longer lives with improved functioning and mental capacities.
Selected abbreviations and acronyms
- AD
Alzheimer's disease
- FDG-PET
fluorodeoxyglucose positron emission tomography
- ∫MRI
functional magnetic resonance imaging
- MCI
mild cognitive impairment
- PET
positron emission tomography
- SP
senile plaque
- NFT
neurofibrillary tangle
- NP
neuritic plaque
Supported by the Montgomery Street Foundation, San Francisco, Calif; the Fran and Ray Stark Foundation Fund for Alzheimer's Disease Research, Los Angeles, Calif; the Department of Energy; NIH grants MH52453, AG10123, AG13308, and the Alzheimer's Association grant IIRG94101. The views expressed are those of the authors and do not necessarily represent those of the Department of Veterans Affairs.
REFERENCES
- 1.Small GW., Rabins PV., Barry PP., et al. Diagnosis and treatment of Alzheimer disease and related disorders. Consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer's Association, and the American Geriatrics Society. JAMA. 1997;278:1363–1371. [PubMed] [Google Scholar]
- 2.Ernst RL., Hay JW. The US economic and social costs of Alzheimer's disease revisited. Am J Pub Health. 1994;84:1261–1264. doi: 10.2105/ajph.84.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross GW., Abbott RD., Petrovitch H., et al. Frequency and characteristics of silent dementia among elderly Japanese - American men: the Honolulu-Asia Aging Study. JAMA. 1997;277:800–805. [PubMed] [Google Scholar]
- 4.Barrett JJ., Haley WE., Harrell LE., Powers RE. Knowledge about Alzheimer disease among primary care physicians, psychologists, nurses, and social workers. Alzheimer Dis Assoc Disord. 1997;11:99–106. doi: 10.1097/00002093-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Mant A., Eyland EA., Pond DC., Saunders NA., Chancellor AH. Recognition of dementia in general practice: comparison of general practitioners' opinions with assessments using the Mini-Mental State Examination and the Blessed dementia rating scale. Fam Pract. 1988;5:184–188. doi: 10.1093/fampra/5.3.184. [DOI] [PubMed] [Google Scholar]
- 6.Small GW. Neuroimaging and genetic assessment for early diagnosis of Alzheimer's disease. J Clin Psychiatry. 1996;57suppl 14):9–13. [PubMed] [Google Scholar]
- 7.Jack CR., Peterson RC., Xu YC., et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braak H., Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 9.Price JL., Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 10.Snowdon DA., Kemper SJ., Mortimer JA., Greiner LH., Wekstein DR., Markesbery WR. Linguistic ability in early life and cognitive function and Alzheimer's disease in late life. Findings from the Nun Study. JAMA. 1996;275:528–532. [PubMed] [Google Scholar]
- 11.Saunders AM., Strittmatter WJ., Schmechel D., et al. Association of apolipoprotein E allele E4 with late onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 12.Corder EH., Saunders AM., Strittmatter WJ., et al. Gene dose of apolipoprotein E type allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 13.Corder EH., Saunders AM., Risch MJ., et al. Apolipoprotein E type 2 allele and the risk of late onset Alzheimer's disease. Wat Genet. 1994;7:180–183. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- 14.O'Hara R., Yesavage JA., Kraemer HC., Mauricio M., Friedman LF., Murphy GMJ. The APOE epsilon4 allele is associated with decline on delayed recall performance in community-dwelling older adults. J Am Geriatr Soc. 1998;46:1493–1498. doi: 10.1111/j.1532-5415.1998.tb01532.x. [DOI] [PubMed] [Google Scholar]
- 15.Petersen RC., Smith GE., Waring SC., Ivnik RJ., Tangalos EG., Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 16.Relkin NR., Tanzi R., Breitner J., et al. Apolipoprotein E genotyping in Alzheimer's disease: position statement of the National Institute on Aging/Alzheimer's Association Working Group. Lancet. 1996;347:1091–1095. [PubMed] [Google Scholar]
- 17.Roses AD., Devlin B., Conneally PM., et al. Measuring the genetic contribution of APOE in late-onset Alzheimer disease (AD). Am J Hum Genet. 1995;57(suppl):A202. [Google Scholar]
- 18.Pericak-Vance MA., Bass MP., Yamaoka LH., et al. Complete genomic screen in late-onset familial Alzheimer disease. Evidence for a new locus on chromosome 12 [see comments], JAMA. 1997;278:1237–1241. [PubMed] [Google Scholar]
- 19.Scott WK., Yamaoka LH., Locke PA., et al. No association or linkage between an intronic polymorphism of presenilin-1 and sporadic or lateonset familial Alzheimer disease. Genet Epidemiol. 1997;14:307–315. doi: 10.1002/(SICI)1098-2272(1997)14:3<307::AID-GEPI8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Small GW., Noble EP., Matsuyama SS., et al. D2 dopamine receptor A1 allele in Alzheimer disease and aging. Arch Neurol. 1997;54:281–285. doi: 10.1001/archneur.1997.00550150041014. [DOI] [PubMed] [Google Scholar]
- 21.Small GW., Mazziotta JC., Collins MT., et al. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA. 1995;273:942–947. [PubMed] [Google Scholar]
- 22.Reiman EM., Caselli RJ., Yun LS., et al. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E [see comments]. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 23.Small GW., Ercoli LM., Huang SC., et al. PET and genetic risk for Alzheimer disease. J Nucl Med. 1999;40(suppl):70p. [Google Scholar]
- 24.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 25.Corder EH., Jelic V., Basun H., et al. No difference in cerebral glucose metabolism in patients with Alzheimer disease and differing apolipoprotein E genotypes. Arch Neurol. 1997;54:273–277. doi: 10.1001/archneur.1997.00550150035013. [DOI] [PubMed] [Google Scholar]
- 26.Buschke H., Fuld PA. Evaluating storage, retention and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 27.Benton AL. The Revised Visual Retention Test. New York: Psychological Corporation; 1974 [Google Scholar]
- 28.Silverman DHS., Ercoli LM., Komo S., Huang SC., Phelps ME., Small GW. Influence of single apolipoprotein E type 4 allele on regional brain metabolism of healthy subjects. 1 Nucl Med. 1999;40(suppl):70P. [Google Scholar]
- 29.Small GW., Bookheimer SY., Strojwas MH., et al. Functional MRI of memory tasks in older persons with APOE-4. Biol Psychiatry. 1999;45:49S. [Google Scholar]
- 30.Barrio JR., Huang SC., Cole GM., Satyamurthy N., Petric A., Small GW. PET imaging of tangles and plaques in Alzheimer disease. J Nucl Med. 1999;40(suppl):70P–71P. [Google Scholar]