Abstract
There is increasing knowledge about considerable comorbidity between psychiatric and somatic diseases, questioning whether variations in genes could be predisposing factors for both conditions. With respect to the multiple interactions between brain and body investigations have centered on variants in several candidate genes for proteins that mediate these interactions and therefore also have implications in psychiatric disorders. The available data, although still preliminary and rare, indicate the importance of polymorphic variants in genes coding for the serotonin (5-hydroxytryptamine, 5-HT) transporter (5-HTT), the 5-HT2A receptor, proinflammatory cytokines, and the angiotensin-converting enzyme (ACE) in migraine, fibromyalgia, cardiovascular disorders, and psychiatric conditions. The role played by these various polymorphisms remains to be determined, as does whether they are indicative of common pathophysiological mechanisms or identify a subgroup of patients with somatic disorders that are more closely related to psychiatric symptoms. Nevertheless, they do at least illustrate the potential influence of genetic differences on illness course and treatment outcome, and might be a rational approach to drug development and treatment paradigms.
Keywords: gene, polymorphism, fibromyalgia, migraine, cardiovascular disorder, psychiatric disorder
Abstract
Existe un conocimiento creciente acerca de la importante comorbilidad entre las enfermedades psiquiátricas y somáticas, lo que permite preguntarse si las variaciones genéticas podrían constituir factores predisponentes para ambas condiciones. En relación con las múltiples interacciones entre el cerebro y el cuerpo, las investigaciones se han centrado en variantes de algunos genes candidato para proteínas que median estas interacciones y que de esta manera tendrían participación en los trastornos psiquiátricos. Los datos disponibles, aunque todavía preliminares y escasos, indican la importancia que tienen las variantes polimórficas en los genes que codifican el transportador de serotonina (5-hidroxitriptamina, 5-HT), el receptor 5-HT2A, las citoquinas proinflamatorias y la enzima convertidora de angiotensina (ECA) en la migraña, la fibromialgia, las enfermedades cardiovasculares y los cuadros psiquiátricos. Queda por determinar el papel que juegan estos diversos polimorfismos; ya que pueden ser indicativos de mecanismos fisio-patológicos comunes o bien pueden servir para identificar un subgrupo de pacientes que presenten trastornos somáticos muy relacionados con síntomas psiquiátricos. No obstante, ellos ilustran al menos la potencial influencia de las diferencias genéticas en el curso de la enfermedad y en la evolución del tratamiento, y podrían proporcionar una aproximación racional para el desarrollo de fármacos y esquemas terapéuticos.
Abstract
Les connaissances sur la comorbidité très importante entre les maladies psychiatriques et somatiques ont progressé, posant la question de l'influence des variations génétiques comme facteurs prédisposants à ces deux types de pathologies. Dans le cadre des interactions multiples entre le corps et le cerveau, l'étude s'est surtout portée sur les variantes de plusieurs gènes candidats codant pour les protéines permettant la transmission des neuromodulations, et qui pourraient de ce fait être impliquées dans les troubles psychiatriques. Les données disponibles, bien qu'encore préliminaires et peu nombreuses, soulignent l'importance des variants génétiques polymorphiques codant pour le transporteur de la sérotonine (5-HTT), le récepteur 5-HT2A, les cytokines pro-inflammatoires et l'enzyme de conversion de l'angiotensine (ECA) dans la migraine, la fibromyalgie, les maladies cardio-vasculaires et les maladies psychiatriques. Il reste encore à déterminer si le rôle joué par ces divers polymorphismes reflète des mécanismes physiopathologiques communs ou s'il identifie un sous-groupe de patients atteints de troubles somatiques apparentés plus étroitement aux symptômes psychiatriques. À tout le moins, ces polymorphismes illustrent l'influence potentielle des variations génétiques sur l'évolution de la maladie ou l'issue du traitement et, à ce titre, pourraient fournir une approche rationnelle pour le développement de médicaments et l'établissement de schémas thérapeutiques.
It is increasingly becoming recognized that somatic and psychiatric disorders frequently cooccur in the same individual and that persons with mental illness or a history of it have more medical conditions during their lifetime than the general population.1 Somatic complaints involving various types of pain, such as headache, stomach pain, vague, poorly localized pain, and back pain, are frequent in various psychiatric conditions, but the relationship between them and the question of whether psychoactive drugs similarly improve both conditions have never been clarified.
The mechanisms for these interactions are largely unknown, but a variety of indirect and direct mechanisms, which could also be either concomitants or consequences of one condition, have been proposed. The importance of biological vulnerability factors and environment has been heavily debated, and it was proposed that a substantial proportion of morbidity may be attributed not to a specific risk for one disorder, but to few underlying liability factors that are applicable to both somatic and psychiatric disorders.1 Thus, even if psychiatric and somatic conditions do not affect each other, they might still cosegregate if they share common underlying factors, including genetic factors. Especially in complex disorders with multif actorial pathophysiological mechanisms, the relevance of genes has exceeded the simple identification of a diseaseenabling cause and is now focusing on importance for treatment response, side effects, interactions with the environment, and personality factors. It was further proposed that both the vulnerability for different disorders and the individual's interaction with the environment are influenced by genes (“nature and nurture”).2
Mechanisms of the interaction between brain and body
The dispute over whether the brain or the body predominates can be traced back to ancient times. Although Hippocrates (460-377 bc), the legendary father of medicine, gave an early description of the brain and recognized that each side of the brain controls the opposite side of the body, the ultimate conceptual framework of brain-body interactions was established by the seminal observations of the French philosopher René Descartes (1596-1650). He provided the first articulation of the brain-body interaction by localizing the brain's contact with body in the pineal gland, and thus raised the question of the brain being the body's control center. Today, we are now aware that there are intimate connections and communications between brain and soma, since adaptation to stressful stimuli, maintenance of homeostasis, and ultimately survival require a bidirectional feedback communication among the different components. Thus, the combined actions of the central nervous system (CNS) and closely linked hormonal and immune systems function as a “supercontroller” with the capacity to regulate not only cognition and behavior, but also heart and vasculature, metabolism, and fluid and electrolyte balance.3
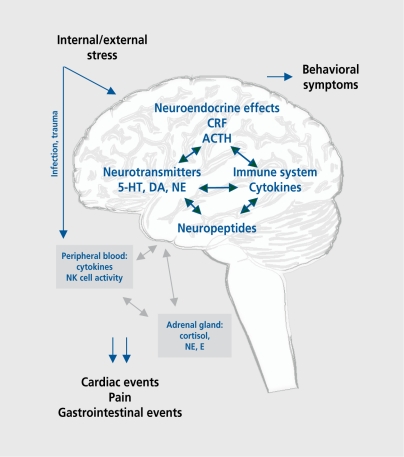
Mental stress, either acute or chronic, produces certain physiological responses via the CNS (Figure 1). The body's adaptive responses to stress stimuli are mediated by an intricate system, which includes the hypothalamus-pituitary-adrenocortical (HPA) axis and the sympathoadrenal system. Dysregulation of the system by repetitive or chronic stress may induce continually increased adrenocortico-tropic hormone (ACTH) and corticosteroid levels, increase the production of monoamines and proinflammatory cytokines within the brain, and thus contribute to a variety of somatic and psychiatric disorders including hypertension, atherosclerosis, functional disorders of the digestive system, several immunological disorders, affective disorders, or anxiety.4 From a clinical point of view, much evidence has accumulated that disturbances in the HPA axis with elevated circulating plasma levels of ACTH and Cortisol play a pivotal role in depression. As it is also known that administered corticosteroids induce hypercholesterolemia, hypertriglyceridemia, and hypertension and that elevated morning Cortisol concentrations are correlated with coronary artherosclerosis,5 a relationship between depression and vascular diseases seems plausible. However, in recent years, a paradoxical phenomenon has emerged from neurobiological studies on the effect of chronic stress, as a number of studies have provided evi dence that the adrenal gland is hypoactive in some stressrelated states, resulting in hypocortisolism. This enhanced negative feedback sensitivity of glucocorticoid receptors or a persistent lack of Cortisol availability can be observed in posttraumatic stress disorder and in other conditions such as chronic fatigue syndrome, fibromyalgia (FM), and rheumatoid arthritis. It was proposed that traumatized or chronically stressed individuals may have an increased vulnerability for stress-related somatic disorders.6
Figure 1. Interactions between brain and body. CRF, corticotropin-releasing factor; ACTH, adrenocorticotropic hormone; E, epinephrine; NE, norepinephrine; 5-HT, 5-hydroxytryptamine (serotonin); DA, dopamine; NK, natural killer.
The monoamine neurotransmitter systems, serotonin (5-hydroxytryptamine, 5-HT), norepinephrine (NE), and dopamine (DA), which are cornerstones of the hypotheses of psychiatric disorders, play important roles in mood, cognition, learning, motor activity, vigilance, reward, sleep, appetite, and cardiovascular function. Although their most important cell bodies are located in relatively small areas of the brain or brain stem, axonal projections are sent throughout the brain along specific pathways to mediate specific functions; when dysfunctional, they generate many symptoms of psychiatric disorders. On the other hand, axonal projections are also sent down the spinal cord, where they act as key homeostatic regulators to vegetative function or sensations coming from the internal milieu of the body. Thus, NE is also a major neurotransmitter in postganglionic sympathetic synapses and alterations in function of their transporters or receptors are compromised in cardiomyopathy, heart failure, hypertension, and ischemia.7
Our understanding of the immunomodulatory activities of numerous neuroendocrine mediators, such as Cortisol, sex hormones, catecholamines, or corticotropin-releasing hormone (CRH) has advanced substantially. Proinflammatory cytokines are also expressed in the brain by microglia, astrocytes, oligodendrocytes, and neurons, and mediate the response to acute and chronic inflammatory CNS diseases. Products of an activated immune systern (eg, cytokines) may affect neuroendocrine and central neurotransmitter processes, and conversely neuroendocrine and central neurotransmitter alterations may have an impact on immune activity.8 It had been shown that the immune system acts like a sensory organ informing the brain of peripheral antigenic challenge, and that immune activation with increased production of proinflammatory cytokines, such as interleukin-1β (IL-1β) and tumor necrosis factor α (TNF-α), increases the activity of the HPA axis and leads to altered monoamine turnover in the brain, thus influencing complex behavioral processes.9 The findings of variations in plasma cytokines in patients with major depression10 or schizophrenia11 are supported by the facts that some antidepressants suppress the inflammatory response10 and clozapine has antiviral properties, at least in vitro.12
With respect to the multiple interactions, it becomes apparent that variations in genes coding for the proteins that are regulating or modulating these interactions might have a tremendous influence on the susceptibility for somatic and psychiatric disorders or for a combined vulnerability. Thus, studies that support linkage or association of variations in the genes implicated in these candidate pathways to psychiatric disorders could also be a hint to somatic distress. Indeed, it is known that certain inherited metabolic disorders, eg, Wilson disease, can impose with psychiatric symptoms as their initialand sometimes as their only manifestation and a variety of psychological and psychiatric manifestations, ranging from lowering of IQ to frank psychosis or mood disturbances can be observed.13 However, these disorders are rare, and the majority of psychiatric and somatic diseases are complex disorders, which means that the underlying pathophysiology is multifactorial with interactions between one or more genes and environmental factors.
Findings in selected disorders
Migraine
Migraine is one of the most common neurovascular disorders affecting between 16% and 23% of the general population. Characteristics of the disease are severe episodes of headache, together with autonomic and neurological symptoms.14 Despite the rare familial hemiplegic migraine, which is probably caused by mutations in the calcium channel α1-subunit,15 two main types can be distinguished: migraine without aura and migraine with aura. The latter has preceding transient focal neurological symptoms, mostly visual.
Epidemiological studies demonstrate a higher psychiatric comorbidity for anxiety, personality trait disorders, and suicide attempts in persons suffering from migraine than in persons without.16 Further, the lifetime prevalence of major depression was approximately three times increased in patients with migraine with a significant bidirectional relationship between the two disorders.17
Migraine is believed to be the manifestation of a hereditary abnormal sensitivity of neurovascular reaction to sudden changes in the internal or external environment, or to cyclic changes in the CNS. Although the real underlying mechanisms remain obscure, there is much support for a role of 5-HT, which is not only involved in regulation of behavioral functions, but also in gastrointestinal mobility and vascular resistance. Thus, acute migraine attacks can effectively be treated with triptans, highly selective 5-H1B/1D receptor agonists, which act mainly via vasoconstriction of dilated cerebral blood vessels, inhibition of the release of neuropeptides such as substance P, and inhibition of nociceptive neurotransmission. Typical antidepressants, eg, amitriptyline or fluoxetine, are also effective in the preventive treatment of migraine, probably via their influence on the neurotransmitter systems. These findings are a hint for possible common pathophysiological mechanisms for migraine and depression, and lead to the question of whether polymorphisms relevant for affective disorders might also be involved in migraine.
Genes of the serotonergic system
Among the most frequently investigated candidate genes of the serotonergic pathway is the serotonin transporter (5-HTT), which cleaves the synaptic cleft from neurotransmitters and thus limits the duration of 5-HT function. Two polymorphic sites within the 5-HTT gene are partieularly interesting: one located in the promoter region with a deletion/insertion variation of 44 bp, creating short (S) and long (L) alleles, the 5-HTT-linked promoter region (HTTLPR); and a variable number of tandem repeats (VNTR) polymorphism, located in the second intron of the gene.18 The presence of the S allele of the HTTLPR is associated with decreased 5-HT reuptake, which, in turn, results in a longer duration of the serotonergic activity.19 Although the results with psychiatric patients are not conelusive, the polymorphism may be of some importance in anxiety-related personality traits,20 depression,21 and sulcidality 22 However, for migraine sufferers, no association has been found to date for this genetic variant of the 5-HTT.23,24
In contrast, the VNTR polymorphism, whose functional significance remains to be elucidated, seems to play a role in migraine, as the frequency of the ST12.10 allele was increased.23,24 Moreover, differences were observed between migraine with and without aura, thus being in concordance with the assumption that these forms of the disorder might be etiologically distinct.24 These data support the view that susceptibility to migraine has a genetic component, which may, in some cases, be associated with a locus at or near 5-HTT.
Further studies investigated genetic variations in the 5-HT receptors. Whereas no association was observed for the 5-HT2c subtype,25 a polymorphism in the 5-HT2A receptor gene (a T to C transition at position 102), which has been repeatedly associated with psychiatric disorders and treatment response,26,27 also seems to be relevant in migraine. Although the distribution of the 5-HT2A T102C genotypes could not be directly related to an increased risk of migraine, the C/C genotypes were more frequently observed in migraine with aura,28,29 thus confirming the previously observed etiologic dichotomy of the two subtypes.
Genes of the immune system
The association between pain and inflammation has led physicians to suspect a connection between immunological mechanisms and headache syndromes for many years. Several immunological abnormalities, such as changes in serum levels of complement and immunoglobulins or increased TNF-α, have been described in body fluids of patients with migraine and may be related to susceptibility to increased infection.30 The cause for this increased susceptibility is unclear, but was discussed as a result of chronic stress, a well-known suppressor of the immune system. Stress relief enhances immune activity and triggers a burst of circulating vasoactive neuropeptides (such as substance P or neurokinin A), which function as mediators of inflammation and potential precipitators of a migraine attack in vulnerable subjects.30
It is well known that the production of cytokines is also regulated by genes,31 which might in turn have implications on the age of onset of several disorders as rheumatoid arthritis32 or Alzheimer's disease.33 A recent observation indicated that migraine patients with aura who are carrying the T/T genotype of the interleukin-la C889T polymorphism have about 10 years earlier age of onset of their migraine attacks. This supports the hypothesis of a genetically driven sterile inflammation as one etiological factor.34 Moreover, an association was found for the cytokines produced by TNF genes with the TNFβ2 allele, but only in migraine sufferers without aura.35 These findings support the assumption that the abnormalities in immunological parameters are not only a consequence of the headache attacks, as has been repeatedly hypothesized, but can also modify the clinical course and the phenotypic expression of the disease.
Fibromyalgia
FM is a syndrome characterized by chronic widespread, persistent pain associated with increased tenderness to palpation due to lowering of the mechanical pain threshold and additional symptoms such as stiffness, fatigue, and psychological distress.36 Several additional clinical features of FM, including depression, anxiety, and sleep disturbances, as well as the fact that it runs in families and shows an increased familial loading with depressive disorders, have even lead to the suggestion that FM might be a “depressive spectrum disorder.”37 This assumption was further supported by positive therapeutic response to antidepressant drugs, as well as by the fact that pain perception threshold was found to be decreased during depression,38 which was considered as being attributable to dysfunction in several neurotransmitter systems.39
The etiology of FM is unknown, but a possible contribution of 5-HT has been suggested on the basis of multiple biological findings, as for example, low levels of serum 5mfjT40 and low 5-hydroxyindole acetic acid (5-HIAA) levels in cerebrospinal fluid (CSF) of idiopathic pain patients.41 Further, since tryptophan supplementation was shown to improve not only depression and anxiety, but also somatic pains in a variety of patient cohorts, the concept of decreased flux through the 5-HT pathway in FM patients has been proposed.42 A recent finding in FM patients demonstrated strong negative correlations between the serum concentrations of substance P and 5-HIAA and between substance P and serum tryptophan.43 These findings support the hypothesis of a systemic involvement of 5-HT and substance P in FM.
Genes of the serotonergic system
As regards the 5-HTT gene HTTLPR polymorphism, we have observed a higher frequency of the SS genotype in FM patients compared with healthy controls and, interestingly, patients with the SS genoytpe exhibited higher levels of depression and psychological distress.44 This preliminary finding was recently confirmed in patients of two different ethnicities (Jewish and Arabian populations), and extended by the observation that the FM patients were characterized by extremes of temperament extensions, especially harm avoidance.45 These results are in concordance with our previous findings, as the personality trait of harm avoidance and neuroticism are correlates of clinical depression and anxiety-related disorders. Taken together, these findings suggest that the correlation between FM and the short allele (S) of the HTTLPR polymorphism could either (i) be indicative of common pathophysiological mechanisms with depression; or (ii) be mediated by depressionand anxietyrelated traits, and thus indicative of a subgroup of patients more likely to suffer depression.
The 5-HT2A receptor also seems to be of great interest, since a recent finding with animal experiments suggested that the 5-HT2a receptor is involved in the thermal hyperalgesic mechanism of 5-HT in periphery46 The facts that the cerebral expressions of the 5-HT2A, 5-HT2C, and 5-HT6 receptors in rats are colocalized extensively with synthesis and expression of the neuropeptides enkephalin, substance P, and dynorphin47 and that substance P immunoreactivity was found to be higher in lumbar CSF of FM patients48 further highlight the importance of the 5-HT2 receptor family in the underlying pathophysiological process of this disorder. In a large cohort of FM patients, we showed a significantly different genotype distribution in FM patients with a decrease in T/T and an increase in T/C and C/C genotypes of the 5-HT2A C102T polymorphism. Although there were no correlations between genotype and age of onset, duration of the disorder, or psychopathological symptoms rated on the Beck Depression Inventory, we found a reduced pain threshold in patients with the T/T genotype.49 Recently, the significant correlation between the 5-HT2A TT genotype and increased pain perception was replicated in a different sample, but no overall differences between patients and controls were observed.50 Although these findings indicate that the 102T allele of the 5-HT2A receptor gene polymorphism is involved in the complex circuits of nociception, this genetic variant is apparently not directly involved in the etiology of FM, but is supposed to be in linkage disequilibrium with the true variant nearby, which remains to be unraveled.
Depression and cardiovascular disorders
There is burgeoning literature on the relationship between mood disorders and cardiovascular disease (CVD). Several studies have demonstrated that depression increases the risk of developing cardiac disease, in particular coronary artery disease, and to worsen prog- nosis after myocardial infarction.51 The impact of depression was mostly related to the premorbid cardiac disease
status with a twoto fourfold increased risk of mortality during the first 6 months following myocardial infarction, but a recent analysis has shown that depression increases the risk for cardiac mortality independently of baseline cardiac status.52
The mechanisms of increased cardiac risk attributable to depressive illness are at present uncertain, but activation of the sympathetic nervous system with increased levels of monoamines,53 exaggerated platelet activity, and/or enhanced inflammatory-mediated atherogenesis are likely to be of primary importance.51,53
The 5-HTT gene
Activation of platelets is pivotal to the development of hemostasis and thrombosis, and plays a role in the development of atherosclerosis via multiple interactions with endothelial vessel walls and plasma coagulation factors.54 However, patients with depression also exhibit altered platelet function and increased aggregation, and thus predispose depressed patients to clotting diathesis.51 Thus, it was proposed that increased platelet activation in depression might be the mechanism by which depression becomes a significant risk factor for CVD. The mechanisms by which platelet activation is increased in depression remain unknown, but one possibility involves the 5-HT system, because 5-HT activates platelet aggregation thus leading to thrombus formation.55 In this context, it would be remarkable if the 5-HTTLPR polymorphism influenced the degree of platelet activation, as homozygosity for the long allele (L/L) could be associated with platelet activation, increased platelet factor 4, and thromboglobulin levels in elderly, depressed patients.56 On the basis of this finding, it was proposed that platelets in persons with the L/L genotype are more efficient in uptake and storage of 5-HT in their dense granules, followed by increased 5-HT release upon activation, which may consequently lead to a greater thrombus formation and finally to myocardial infarction.56
The possible importance of the 5-HTTLPR polymorphism was further underlined by a study investigating the impact of indices of CNS 5-HT function on cardiovascular reactivity to mental stress. The study was carried out in healthy volunteers and showed that persons with one or two L alleles had higher CSF levels of the 5-HT metabolite 5-HIAA than those with the S/S genotype and exhibited greater blood pressure and heart rate responses to a mental stress protocol. Thus, the 5-HTTLPR polymorphism affects not only central 5-HT function, but also seems to be involved in the regulation of biobehavioral characteristics.57
The G-protein-β3 subunit (Gβ3) gene
Neurotransmitter molecules do not cross the postsynaptic membranes, but induce a cascade of reactions via their initial binding to surface receptors within the postsynaptic membrane, which are often coupled to guanine-nucleotide-binding proteins (G proteins). These G proteins represent initial regulatory components in transmembrane signaling and are thus key elements in signal transduction, regulating many biological responses.58 In one subunit of these G proteins, the Gβ3 subunit, a polymorphism was identified (a C to T exchange at position 825 in exon 10), which leads to the occurrence of a splice variant (Gβ3-s) with deletion of 41 amino acids. It is now fairly well established that the T allele of this polymorphism, which results in increased ion flux across the membrane and increased signal transduction, is associated with hypertension and obesity.59,60 However, this genetic variant is not just important for somatic disorders, because we also found an increased frequency of the T allele in patients with affective psychosis.61 In an extended sample, using the DNA of 201 patients with major depression without increased proportions of hypertensives, we were able to replicate our previous results of increased frequency of allele T and increase in TT homozygotes (χ2=14.8; df=2; P=0.0006). Thus, our results are consistent with the hypothesis that disturbances in the signal transduction cascade on the level of G proteins are involved as contributing factor in the pathophysiology of major depression, despite its importance in essential hypertension.62
The ACE gene
Angiotensin-converting enzyme (ACE) is a zinc metallopeptidase involved in blood pressure regulation via the angiotensin-renin cascade, generating angiotensin II (ATII) from angiotensin I, and via degradation of the powerful vasodilator bradykinin. However, the effects of this enzyme are not restricted to the vasculature, as several studies have demonstrated that ACE might also be involved in HPA axis regulation and catecholamine production via the generation of ATII63 and is thus required for sympathoadrenal activation during stress.
Further evidence suggests an involvement of the brain renin-angiotensin system in regulation of mood, because of the colocalization of angiotensin with dopamine-synthesizing neurons,64 the fact that ACE is involved in the metabolism of the neuropeptide substance P, which, in turn, is supposed to play a role in depression,65 and the clinical observation that the application of ACE inhibitors in hypertensives can induce euphoric or depressive states.66
The ACE I/D polymorphism is characterized by the insertion (I) or deletion (D) of a 287-bp sequence within intron 16 of the gene; and the D allele is associated with increased levels of circulating ACE.67 Numerous studies have implicated the ACE DD genotype with cardiovascular disorders, including myocardial infarction,68 hypertension, and left ventricular hypertrophy,69 and also affective disorders,70 but these associations were not consistent in all studies. A review of the literature revealed a moderate degree of increased risk for myocardial infarction and coronary heart disease associated with the ACE DD genotype in most populations.71 Recently, the impact of the ACE DD genotype on myocardial infarction was reevaluated using the paradigm of gene-gene interaction between the ACE gene variants together with the C825T polymorphism in the Gβ3 subunit in patients with coronary artery disease with and without myocardial infarction. In the combined analysis of the ACE and Gβ3 polymorphisms, the highest relative risk (odds ratio [OR] 7.5) was found in Gβ3-TT/ACE-DD carriers, suggesting that the interaction between the Gβ3 825T and the ACE D alleles increases the risk more than sevenfold and are thus a possible contributing factor for myocardial infarction.72
On the basis of our own previous results regarding an association of the Gβ3 825T allele with affective disorders61 and a possible influence of the ID polymorphism of the ACE gene polymorphism on therapeutic outcome in affective disorders,73 we studied the interaction of both gene variants in 201 patients with unipolar major depression and 161 ethnically matched controls. Interestingly, in depressed patients, we observed a combined action of ACE and Gβ3 genotypes: ACE-ID and DD/Gβ3-TT carriers were more than four times more frequent in the depressed group than in the controls (crude OR=5.83; 95% confidence interval 1.99-17.08; P=0.0002).62 As our study was carried out in depressed patients without serious cardiovascular impairment, we are currently unable to predict whether this combined action of the ACE ID/DD and Gβ3 TT genotype increases the risk for both disorders. Nevertheless, our study is the first report of the same allelic combination of two genes that increase the risk for myocardial infarction72 and the vulnerability for depressive disorder, and this could be one missing link for the interaction.
Summary and conclusion
With respect to the multiple interactions between brain and body, it is plausible that polymorphisms in genes coding for proteins that regulate or modulate these interactions have a tremendous influence on susceptibility, pathophysiology, or comorbidity of somatic and psychiatric disorders. Thus, variants in several candidate genes that have repeatedly been associated with psychiatric disorders may also be successfully investigated in somatic disorders or vice versa. The available data, although being still preliminary and rare, implicate the importance of the serotonergic and catecholaminergic systems, the various components of the HPA axis as well as the immune system and ACE as possible common genetic risk factors for psychiatric and somatic disorders. Interestingly, polymorphisms within the 5-HTT and 5-HT2A receptor gene were found to be related to migraine, FM, cardiovascular events, and several psychiatric conditions, thus underlining the multiple effects of this neurotransmitter in brain and periphery. Neither the immunological system nor the ACE gene have yet been extensively investigated, but a similar importance for these proteins could be anticipated.
The role played by these various polymorphisms remains to be determined. Some may not be specific for disorders, but could increase susceptibility to the disorder and induce endophenotypic vulnerability markers. Although it is questionable whether these findings have immediate clinical implications, they do at least illustrate the potential influence of genetic differences on illness course and treatment outcome, and help elucidate the biological underpinnings of the diseases, which allows a more rationale approach to drug development and treatment paradigms.
Selected abbreviations and acronyms
- ACE
angiotensin-converting enzyme
- CRH
corticotropin-releasing hormone
- DA
dopamine
- FM
fibromyalgia
- 5-HIAA
5 -hydroxy indole acetic acid
- HPA
hypothalamus-pituitary adrenocortical (axis)
- 5-HT
5 -hydroxy try ptamine (serotonin)
- 5-HTT
serotonin transporter
- IL-1β
interleukin-1β
- NE
norepinephrine
- TNF-α
tumor necrosis factor α
REFERENCES
- 1.Neeleman J., Ormel J., Bijl RV. The distribution of psychiatrie and somaticill health: associations with personality and socioeconomic status. Psychosom Med. 2001;63:239–247. doi: 10.1097/00006842-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Kendler KS., McGuire M., Gruenberg AM., O'Hare A., Spellman M., Walsh D. The Roscommon Family Study. IV. Affective illness, anxiety disorders, and alcoholism in relatives. Arch Gen Psychiatry. 1993;50:952–960. doi: 10.1001/archpsyc.1993.01820240036005. [DOI] [PubMed] [Google Scholar]
- 3.Fava M. Somatic symptoms, depression, and antidepressant treatment. J Clin Psychiatry. 2002;63:305–307. doi: 10.4088/jcp.v63n0406. [DOI] [PubMed] [Google Scholar]
- 4.Vanitallie TB. Stress: a risk factor for serious illness. Metabolism. 2002;51:40–45. doi: 10.1053/meta.2002.33191. [DOI] [PubMed] [Google Scholar]
- 5.Troxler RG., Sprague EA., Albanese RA., Fuchs R., Thompson AJ. The association of elevated plasma Cortisol and early atherosclerosis as demonstrated by coronary angiography. Atherosclerosis. 1977;26:151–162. doi: 10.1016/0021-9150(77)90098-3. [DOI] [PubMed] [Google Scholar]
- 6.Heim C., Ehlert U., Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 7.Hahn MK., Blakely RD. Monoamine transporter gene structure and polymorphisms in relation to psychiatric and other complex disorders. PharmacogenomicsJ. 2002;2:217–235. doi: 10.1038/sj.tpj.6500106. [DOI] [PubMed] [Google Scholar]
- 8.Clarke BL., Moore DR., Blalock JE. Adrenocorticotropic hormone stimulates a transient calcium uptake in rat lymphocytes. Endocrinology. 1994;135:1780–1786. doi: 10.1210/endo.135.5.7956901. [DOI] [PubMed] [Google Scholar]
- 9.Brebner K., Hayley S., Zacharko R., Merali Z., Anisman H. Synergistic effects of interleukin-1p, interleukin-6, and tumor necrosis factor-oc: central monoamine, corticosterone, and behavioral variations. Neuropsycho-pharmacology. 2000;22:566–580. doi: 10.1016/S0893-133X(99)00166-9. [DOI] [PubMed] [Google Scholar]
- 10.Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- 11.Muller N., Riedel M., Gruber R., Ackenheil M., Schwarz MJ. The immune system and schizophrenia. An integrative view. Ann N Y Acad Sci. 2000;917:456–467. doi: 10.1111/j.1749-6632.2000.tb05410.x. [DOI] [PubMed] [Google Scholar]
- 12.Yolken RH., Karlsson H., Yee F., Johnston-Wilson NL., Torrey EF. Endogenous retroviruses and schizophrenia. Brain Res Brain Res Rev. 2000;31:193–199. doi: 10.1016/s0165-0173(99)00037-5. [DOI] [PubMed] [Google Scholar]
- 13.Colomb M. Psychiatric symptoms in metabolic and other genetic disorders: is our organic workup complete? Harvard Rev Psychiatry. 2002;10:242–248. [PubMed] [Google Scholar]
- 14.Ferrari MD. Migraine. Lancet. 1998;351:9108–9151. doi: 10.1016/S0140-6736(97)11370-8. [DOI] [PubMed] [Google Scholar]
- 15.Sjostrand C., Giedratis V., Ekbom K., Waldenlind E., Hillert J. CACNA1A gene polymorphisms in cluster headache. Cephalalgia. 2001;21:10–18. doi: 10.1046/j.1468-2982.2001.00281.x. [DOI] [PubMed] [Google Scholar]
- 16.Guillem E., Pelissolo A., Lepine JP. Troubles mentaux et migraine: données epidemiologiques [in French]. Encéphale. 1999;25:436–442. [PubMed] [Google Scholar]
- 17.Breslau N., Schultz LR., Stewart WF., Upton RB., Lucia VC., Welch KM. Headache and major depression: is the association specific to migraine? Neurology. 2000;54:308–313. doi: 10.1212/wnl.54.2.308. [DOI] [PubMed] [Google Scholar]
- 18.Lesch KP., Wiesmann M., Hoh A., et al. 5-HT1A receptor-effector system responsivity in panic disorder. Psychopharmacol Berl. 1992;106:111–117. doi: 10.1007/BF02253597. [DOI] [PubMed] [Google Scholar]
- 19.Heils A., Teufel A., Petri S., et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 20.Lesch KP., Bengel D., Heils A., et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region [see comments]. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 21.Collier DA., Stober G., Li T., et al. A novel functional polymorphism within the promoter of the serotonin transporter gene: possible role in susceptibility to affective disorders [see comments]. Mot Psychiatry. 1996;1:453–460. [PubMed] [Google Scholar]
- 22.Bondy B., Erfurth A., de Jonge S., Kruger M., Meyer H. Possible association of the short allele of the serotonin transporter promoter gene polymorphism (5-HTTLPR) with violent suicide. Mol Psychiatry. 2000;5:193–195. doi: 10.1038/sj.mp.4000678. [DOI] [PubMed] [Google Scholar]
- 23.Yilmaz M., Erdal ME., Herken H., Cataloluk O., Barlas O., Bayazït YA. Significance of serotonin transporter gene polymorphism in migraine. J Neurol Sci. 2001;186:27–30. doi: 10.1016/s0022-510x(01)00491-9. [DOI] [PubMed] [Google Scholar]
- 24.Ogilvie AD., Russell MB., Dhall P., et al. Altered allelic distributions of the serotonin transporter gene in migraine without aura and migraine with aura. Cephalalgia. 1998;18:23–26. doi: 10.1046/j.1468-2982.1998.1801023.x. [DOI] [PubMed] [Google Scholar]
- 25.Burnet PW., Harrison PJ., Goodwin GM., et al. Allelic variation in the serotonin 5-HT2C receptor gene and migraine. Neuroreport. 1997;8:2651–2653. doi: 10.1097/00001756-199708180-00003. [DOI] [PubMed] [Google Scholar]
- 26.Coccaro EF., Kavoussi RJ., Sheline Yl., Berman ME., Csernansky JG. Impulsive aggression in personality disorder correlates with platelet 5-HT2A receptor binding. Neuropsychopharmacology. 1997;16:211–216. doi: 10.1016/S0893-133X(96)00194-7. [DOI] [PubMed] [Google Scholar]
- 27.Arranz M., Collier D., Sodhi M., et al. Association between clozapine response and allelic variation in 5-HT2A receptor gene [see comments]. Lancet. 1995;346:281–282. doi: 10.1016/s0140-6736(95)92168-0. [DOI] [PubMed] [Google Scholar]
- 28.Erdal ME., Herken H., Yilmaz M., Bayazït YA. Association of the T102C polymorphism of 5-HT2A receptor gene with aura in migraine. J Neurol Sci. 2001;188:99–101. doi: 10.1016/s0022-510x(01)00556-1. [DOI] [PubMed] [Google Scholar]
- 29.Nyholt DR., Curtain RP., Gaffney PT., Brimage P., Goadsby PJ., Griffiths LR. Migraine association and linkage analyses of the human 5-hydroxytryptamine (5-HT2a) receptor gene. Cephalalgia. 1996;16:463–467. doi: 10.1046/j.1468-2982.1996.1607463.x. [DOI] [PubMed] [Google Scholar]
- 30.Kemper RH., Meijler WJ., Korf J., Ter Horst GJ. Migraine and function of the immune system: a meta-analysis of clinical literature published between 1966 and 1999. Cephalalgia. 2001;21:549–557. doi: 10.1046/j.1468-2982.2001.00196.x. [DOI] [PubMed] [Google Scholar]
- 31.Shirodaria S., Smith J., McKay IJ., Kennett CN., Hughes FJ. Polymorphisms in the IL-1A gene are correlated with levels of interleukin-1 alpha protein in gingival crevicular fluid of teeth with severe periodontal disease. J Dent Res. 2000;79:11–19. doi: 10.1177/00220345000790110801. [DOI] [PubMed] [Google Scholar]
- 32.McDowell TL., Symons JA., Ploski R., Forre O., Duff GW. A genetic association between juvenile rheumatoid arthritis and a novel interleukin-1a polymorphism. Arthritis Rheum. 1995;38:221–228. doi: 10.1002/art.1780380210. [DOI] [PubMed] [Google Scholar]
- 33.Grimaldi LM., Casadeï VM., Ferri C., et al. Association of early-onset Alzheimer's disease with an interleukin-1 a gene polymorphism. Ann Neurol. 2000;47:361–365. [PubMed] [Google Scholar]
- 34.Rainero I., Pinessi L., Salani G., et al. A polymorphism in the interleukin1a gene influences the clinical features of migraine. Headache. 2002;42:337–340. doi: 10.1046/j.1526-4610.2002.02103.x. [DOI] [PubMed] [Google Scholar]
- 35.Trabace S., Brioli G., Lulli P., et al. Tumor necrosis factor gene polymorphism in migraine. Headache. 2002;42:341–345. doi: 10.1046/j.1526-4610.2002.02104.x. [DOI] [PubMed] [Google Scholar]
- 36.Wolfe F., Smythe HA., Yunus MB., et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee [see comments]. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 37.Hudson JI., Pope HG. The concept of affective spectrum disorder: relationship to fibromyalgia and other syndromes of chronic fatigue and chronic muscle pain. Balllleres Clin Rheumatol. 1994;8:839–856. doi: 10.1016/s0950-3579(05)80051-2. [DOI] [PubMed] [Google Scholar]
- 38.Ward NG., Bloom VL., Dworkin S., Fawcett J., Narasïmhacharï N., Friedel RO. Psychobiological markers in coexisting pain and depression: toward a unified theory. J Clin Psychiatry. 1982;43:32–41. [PubMed] [Google Scholar]
- 39.van Praag HM., Asnïs GM., Kahn RS., et al. Monoamines and abnormal behaviour. A multi-aminergic perspective. Br J Psychiatry. 1990;157:723–734. doi: 10.1192/bjp.157.5.723. [DOI] [PubMed] [Google Scholar]
- 40.Wolfe F., Russell IJ., Vipraio G., Ross K., Anderson J. Serotonin levels, pain threshold, and fibromyalgia symptoms in the general population. J Rheumatol. 1997;24:555–559. [PubMed] [Google Scholar]
- 41.Almay BG., Von KL., Oreland L. Platelet MAO in patients with idiopathic pain. disorders. J Neural Transm. 1987;69:243–253. doi: 10.1007/BF01244345. [DOI] [PubMed] [Google Scholar]
- 42.Juhl JH. Fibromyalgia and the serotonin pathway. Altern Med Rev. 1998;3:367–375. [PubMed] [Google Scholar]
- 43.Schwarz MJ., Spaeth M., Mùller-Bardorff H., Pongratz DE., Bondy B., Ackenheil M. Relationship of substance P, 5-hydroxyindolic acetic acid and tryptophan in serum of fibromyalgia patients. Neurosci Lett. 1999;259:196–198. doi: 10.1016/s0304-3940(98)00937-9. [DOI] [PubMed] [Google Scholar]
- 45.Cohen H., Buskila D., Neumann L., Ebstein RP. Confirmation of an association between fibromyalgia and serotonin transporter promoter region (5-HTTLPR) polymorphism, and relationship to anxiety-related personality traits. Arthritis Rheum. 2002;46:845–847. doi: 10.1002/art.10103. [DOI] [PubMed] [Google Scholar]
- 44.Offenbaecher M., Bondy B., De JS., et al. Possible association of fibromyalgia with a polymorphism in the serotonin transporter gene regulatory region. Arthritis Rheum. 1999;42:2482–2488. doi: 10.1002/1529-0131(199911)42:11<2482::AID-ANR27>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 46.Tokunaga A., Saïka M., Senba E. 5-HT2A receptor subtype is involved in the thermal hyperalgesic mechanism of serotonin in the periphery. Pain. 1998;76:349–355. doi: 10.1016/S0304-3959(98)00066-9. [DOI] [PubMed] [Google Scholar]
- 47.Ward RP., Dorsa DM. Colocalization of serotonin receptor subtypes 5-HT2A, 5-HT2C and 5-HT6 with neuropeptides in rat striatum. J Comp Neurol. 1996;370:405–414. doi: 10.1002/(SICI)1096-9861(19960701)370:3<405::AID-CNE10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 48.Almay BG., Johansson F., Von Knorring L., Le Grèves P., Terenius L. Substance P in CSF of patients with chronic pain syndromes. Pain. 1988;33:3–9. doi: 10.1016/0304-3959(88)90197-2. [DOI] [PubMed] [Google Scholar]
- 49.Bondy B., Spaeth M., Offenbaecher M., et al. The T102C polymorphism of the 5-HT2A receptor gene in fibromyalgia. Neurobiol Dis. 1999;6:433–439. doi: 10.1006/nbdi.1999.0262. [DOI] [PubMed] [Google Scholar]
- 50.Gursoy S., Erdal E., Herken H., Madenci E., Alasehirli B. Association of T102C polymorphism of the 5-HT2A receptor gene with psychiatric status in fibromyalgia syndrome. Rheumatol Int. 2001;21:58–61. doi: 10.1007/s002960100130. [DOI] [PubMed] [Google Scholar]
- 51.Musselman DL., Evans DL., Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- 52.Frasure-Smith N., Lesperance F., Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA. 1993;270:1819–1825. [PubMed] [Google Scholar]
- 53.Carney RM., Freedland KE., Veith RC., et al. Major depression, heart rate, and plasma norepinephrine in patients with coronary heart disease. Biol Psychiatry. 1999;45:458–463. doi: 10.1016/s0006-3223(98)00049-3. [DOI] [PubMed] [Google Scholar]
- 54.Lefkovits J., Plow EF., Topol EJ. Platelet glycoprotein llb/llla receptors in cardiovascular medicine. N Engl J Med. 1995;332:1553–1559. doi: 10.1056/NEJM199506083322306. [DOI] [PubMed] [Google Scholar]
- 55.del Zoppo GJ. The role of platelets in ischemic stroke. Neurology. 1998;51(suppl3):S9–S14. doi: 10.1212/wnl.51.3_suppl_3.s9. [DOI] [PubMed] [Google Scholar]
- 56.Whyte EM., Pollock BG., Wagner WR., et al. Influence of serotonin-transporter-linked promoter region polymorphism on platelet activation in geriatric depression. Am J Psychiatry. 2001;158:2074–2076. doi: 10.1176/appi.ajp.158.12.2074. [DOI] [PubMed] [Google Scholar]
- 57.Williams RB., Marchuk DA., Gadde KM., et al. Central nervous system serotonin function and cardiovascular responses to stress. Psychosom Med. 2001;63:300–305. doi: 10.1097/00006842-200103000-00016. [DOI] [PubMed] [Google Scholar]
- 58.Duman RS., Heninger GR., Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 59.Siffert W., Rosskopf D., Siffert G., et al. Association of a human G-protein beta3 subunit variant with hypertension [see comments]. Nat Genet. 1998;18:45–48. doi: 10.1038/ng0198-45. [DOI] [PubMed] [Google Scholar]
- 60.Siffert W., Naber C., Walla M., Ritz E. G protein beta3 subunit 825T allele and its potential association with obesity in hypertensive individuals. J Hypertens. 1999;17:1095–1098. doi: 10.1097/00004872-199917080-00008. [DOI] [PubMed] [Google Scholar]
- 61.Zill P., Baghai TC., Zwanzger P., et al. Evidence for an association between a G protein (33-gene variant with depression and response to antidepressant treatment. Neuroreport. 2000;11:1893–1897. doi: 10.1097/00001756-200006260-00018. [DOI] [PubMed] [Google Scholar]
- 62.Bondy B., Baghai TC., Zill P., et al. Combined action of the ACE D- and Gproteïn B3 T-allele in major depression: a possible link to cardiovascular disease? Mol Psychiatry. 2002;7:1120–1126. doi: 10.1038/sj.mp.4001149. [DOI] [PubMed] [Google Scholar]
- 63.Aguilera G., Kiss A., Luo X. Increased expression of type 1 angiotensin II receptors in the hypothalamic paraventricular nucleus following stress and glucocorticoid administration. J Neuroendocrinol. 1995;7:775–783. doi: 10.1111/j.1365-2826.1995.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 64.Jenkins TA., Mendelsohn-Frederick AO., Chai SY. Angiotensin-converting enzyme modulates dopamine turnover in the striatum. J Neurochem. 1997;68:1304–1311. doi: 10.1046/j.1471-4159.1997.68031304.x. [DOI] [PubMed] [Google Scholar]
- 65.Kramer MS., Cutler N., Feighner J., et al. Distinct mechanism for antidepressant activity by blockade of central substance P receptors [see comments]. Science. 1998;281:1640–1645. doi: 10.1126/science.281.5383.1640. [DOI] [PubMed] [Google Scholar]
- 66.Gunduz H., Georges JL., Fleishman S. Quinapril and depression. Am J Psychiatry. 1999;156:1114–1115. doi: 10.1176/ajp.156.7.1114a. [DOI] [PubMed] [Google Scholar]
- 67.Rigat B., Hubert C., Alhenc-Gelas F., Cambien F., Corvol P., Soubrier F. An insertion/deletion polymorphism in the angiotensin l-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cambien F., Poirier O., Lecerf L., et . Deletion polymorphism in the gene for angiotensin-converting enzyme is a potent risk factor for myocardial infarction. Nature. 1992;359:641–644. doi: 10.1038/359641a0. [DOI] [PubMed] [Google Scholar]
- 69.Corvol P., Soubrier F., Jeunemaitre X. Molecular genetics of the reninangiotensin-aldosterone system in human hypertension. Pathol Biol (Paris). 1997;45:229–239. [PubMed] [Google Scholar]
- 70.Arinami T., Li L., Mitsushio H., Itokawa M., Hamaguchi H., Toru M. An insertion/deletion polymorphism in the angiotensin-converting enzyme gene is associated with both brain substance P contents and affective disorders. Biol Psychiatry. 1996;40:1122–1127. doi: 10.1016/s0006-3223(95)00597-8. [DOI] [PubMed] [Google Scholar]
- 71.O'Malley JP., Maslen CL., lllingworth DR. Angiotensin-converting enzyme DD genotype and cardiovascular disease in heterozygous familial hypercholesterolemia. Circulation. 1998;97:1780–1783. doi: 10.1161/01.cir.97.18.1780. [DOI] [PubMed] [Google Scholar]
- 72.Naber CK., Husing J., Wolfhard U., Erbel R., Siffert W. Interaction of the ACE D allele and the GNB3 825T allele in myocardial infarction. Hypertension. 2000;36:986–989. doi: 10.1161/01.hyp.36.6.986. [DOI] [PubMed] [Google Scholar]
- 73.Baghai T., Schule C., Zwanzger P., et al. Possible influence of the insertion/deletion polymorphism in the angiotensin l-converting enzyme gene on therapeutic outcome in affective disorders. Mol Psychiatry. 2001;6:258–259. doi: 10.1038/sj.mp.4000857. [DOI] [PubMed] [Google Scholar]