Abstract
The aged are an extremely heterogeneous population that is growing worldwide, included are healthy and agile individuals in their early sixties, as well as an increasing number of people over the age of 35. Pharmacotherapy is expected to continue its prominent role in the medical management of a wide range of conditions that affect older people. Adverse consequences of all kinds complicate the use of medications, and such events seem to increase in incidence with polypharmacy. Cognitive impairment can occur during the course of treatment with a wide range of medications and can have a variety of presentations, Both the number of concurrent medications that older individuals routinely use and physiologic changes in these patients render them more susceptible to developing cognitive toxicity. Most of the frequently implicated medications carry documentation of their ability to cause cognitive disturbances in their package labeling, suggesting that the level of vigilance for adverse effects during the course of their use should always be high. Such caution can be used to guide appropriate drug treatment of the aged so that clinicians do not need to opt for undertreatment to avoid toxicity.
Keywords: adverse drug reaction, cognitive toxicity, elderly, risk factor
Abstract
Los ancianos constituyen una población extremadamente heterogénea gue está creciendo en todo el mundo. Incluso hay individuos sanos y ágiles en los inicios de los sesenta como también un número creciente de personas de edad superior a los 85 años. Se espera que la farmacoterapia continúe su papel destacado en el manejo médico de una amplia variedad de condiciones que afectan a la población mayor. Las consecuencias adversas de todo tipo complican el empleo de medicamentos y tales eventos parecen aumentar su incidencia con la polifarmacia. El deterioro cognitivo puede ocurrir durante el curso del tratamiento con una amplia gama de medicamentos y puede tener una variedad de presentaciones. Tanto el número de fármacos combinados que los individuos mayores rutinariamente utilizan, como los cambios fisiológicos en estos pacientes contribuyen a hacerlos más susceptibles de presentar una toxicidad cognitiva. La mayoría de los medicamentos frecuentemente implicados en esta toxicidad llevan documentación acerca de su capacidad para provocar alteraciones cognitivas rotuladas en el envase, lo que sugiere que el nivel de vigilancia de estos efectos durante el curso de su empleo debe ser siempre alto. Esta preocupación puede tenerse en cuenta para orientar apropiadamente acerca del tratamiento con fármacos en el anciano, de tal forma que los clínicos no necesiten optar por un subtratamiento para evitar la toxicidad.
Abstract
Les personnes âgées constituent une population très hétérogène qui augmente dans le monde entier. Elle comprend aussi bien des individus alertes et en bonne santé de la soixantaine, qu'un nombre croissant de personnes dépassant les 35 ans, La pharmacothérapie est appelée à poursuive son rôle de premier plan dans la prise en charge médicale de nombre de pathologies touchant les personnes âgées. Des effets indésirables de toutes sortes compliquent l'utilisation des médicaments, et la polymédication semble augmenter leur incidence. Un grand nombre de médicaments peuvent entraîner l'apparition d'une détérioration cognitive, cette dernière pouvant d'ailleurs se présenter sous diverses formes. Tant le nombre de médicaments différents pris quotidiennement par les sujets âgés que les modifications physiologiques inhérentes à l'âge sont responsables, chez ces patients, d'une susceptibilité particulière au développement d'une toxicité cognitive. Les notices de la plupart des médicaments courants impliqués signalent la possibilité de l'apparition de troubles cognitifs, ce qui implique que le niveau de vigilance relatif aux événements indésirables pendant le traitement doit toujours rester élevé. Cette vigilance permettra aux médecins de prescrire les traitements appropriés chez les sujets âgés plutôt que de les sous-traiter par peur de l'apparition d'une toxicité.
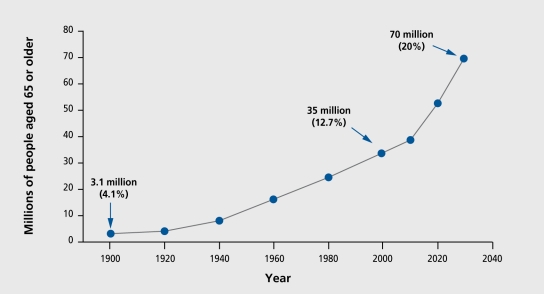
A prominent feature of the current, and projected populations in developed and developing countries is the increase in the relative and absolute numbers of aging individuals (Figure 1). 1 Defined by various organizations as those over age 60, or alternatively 65, this heterogeneous population is estimated by the World Health Organization to increase to ewer one billion worldwide by the year 2020.2 Europe is expected to increase its percentage of aged residents from its current 20% to 25%. rIli c population of Japan is expected to be over 30% aged. Projections for North America, East Asia, Latin America, and South Asia are 23%, 17%, 12%, and 10%, respectively.2 The most rapid increases are expected in developing countries. Whereas France increased its aged population from 7% to 17% ewer the course of 115 years (1865-1980), estimates are that China will double its number in the same demographic group from 10% to 20% in the 20 years between 2000 and 2027.2 Causes of death in developing countries are expected to be largely age-related by 2020, coming from noncommunicable diseases such as cancer, diabetes, and cardiovascular disease,2 conditions which have been heavily dependent on drug therapy for management, in developed countries.
Figure 1. Actual and projected demographic data from the US population' shown as a representative example of cross-national demographic shifts. Ciosed circles connected by solid lines are the actual numbers of individuals aged 65 years or older, with anticipated numbers projected to the year 2030. In parentheses are the numbers expressed as a percentage of the total population. By 2030, it is expected that 70 million Americans will be aged 65 years or older, comprising 20% of the total population. Reproduced from reference 1: Profile of older Americans: 2000. Washington, DC: Administration on Aging. June 2001. www.aoa.dhhs.gov/aoa/stats/profile/. Copyright © 2001, Administration on Aging.
In addition, the number of individuals over age 85 will rise dramatically The US government expects these “oldest of the old” to grow by 56% to 5.7 million between 1995 and 2010, as compared with the 13% increase in those aged 65 to 84.3 Projections are that the cumulative growth rate for this particular “oldest” subset from 1995 to 2050 will be greater than 400%, constituting nearly 5% of the total US population.3 In both the old and oldest groups, the majority are female. In 1995, there were more than two times as many women as men in the US age 85 and ewer group.3 Women are expected to continue to outnumber men in all age categories. Data from the United Nations4 show that, the life expectancy for women is greater in virtually all developed and developing countries, suggesting that most elderly populations will have some degree of female majority.
Medications are an integral part of the clinical management of the health problems of older individuals. In developed countries, drugs from virtually every therapeutic class, including antibiotics, cardiovascular, psychotropics, and antiinflammatory drugs, are used extensively and often in combination in this group of patients, even though historically almost, none of the drug development data have been collected in this demographic group. In a five-country survey including Australia, New Zealand, Canada, United Kingdom, and the US, approximately 75% of those ewer the age of 65 in all five countries took at least, one prescription drug on a regular and ongoing basis for a chronic medical condition.5 Many investigators have documented the prevalence of polypharmacy in populations of similar demographics.6-8 One group estimates that, although the aged were 13% of the US population in 1998, they received 34% of dispensed prescription drugs, and the average number of prescriptions filled per year for an older person was expected to reach 28.5 in the year 2000.8 In addition, older individuals also use nonprescription medications, including herbal and nutritional supplements.9-12
Although drug therapy has contributed significantly to the management, of numerous medical conditions in older patients, a substantial number of these individuals will experience some sort of adverse drug reaction (ADR).13-15 ADRs have been recognized as a serious health problem, and one US government, report estimated that, 10% to 15% of geriatric hospital admissions were caused by ADRs.16 Other data also support this.17,18 Documentation and classification of these events has been hampered by a lack of common terminology and agreed-upon definitions. In a recent address before a US Senate Committee on adverse drug events, the Director of the Center for Drug Evaluation and Research of the Food and Drug Administration stated that the term “adverse drug reaction” connotes a potential relationship between a medication and an undesircd outcome.19 In addition, she noted that the overwhelming majority of ADRs reported are side effects that have already been identified and described in the product, label and can be expected to occur under certain clinical conditions. Some reports suggest, that, although particular drugs are repeatedly implicated in ADRs among older patients, they continue to be used in ways which are problematic.20 Overall, the most important, indicator of risk for an ADR has consistently been shown to be the number of medications a given individual takes.13 The relative odds of an ADR specifically related to cognitive impairment in older individuals have also been found to increase as the number of prescription drugs increased.21 Whether age itself is an independent predictor of risk of ADRs in general has been difficult to assess. Prospective studies conducted by the Gruppo Italiano di Farmacovigilanza nell'Anziano (GI.FA) suggest that, age may be an independent, risk factor only in the most, advanced age-groups.17
Cognitive impairment is a broadly definable ADR, which is extremely important in older people and one to which they seem to have heightened susceptibility. Symptomatology includes disorders that can be termed “psychiatric” and/or “neurologic,” and often occurs on a continuum. Some drugs that, are linked with discretely classifiable outcomes, such as depression and suicide or seizures, are often also noted to cause a variety of more subtle central nervous system (CNS) disturbances as well, such as confusion or decreased sensorium. Such symptoms arc more difficult to assess and could clearly have an impact on cognitive abilities. However, these drugs may more routinely be considered in the context of their most dramatic adverse sequelae, and may be overlooked when considering drugs that can impair “cognition.”
Many manifestations of cognitive toxicity can be considered, from overt delirium and dementia to potential consequences, such as falls and automobile accidents. Even the more subtle manifestations, which could involve mood or memory, can have dramatic consequences if the ability of the individual to perform the activities necessary for independent living is compromised. The definition of toxicity may be somewhat, arbitrary and difficult, to differentiate categorically from expected clinical effect. Drugs used for sedation, for example, may impair cognition in the course of exerting their therapeutic effect without, an undesired outcome if the setting is proper and the effect terminates in a predictable and expected manner. That same impairment in other contexts, however, may lead to serious adverse consequences and be regarded as toxicity.
As noted abewe, the fact, that aged individuals are commonly on multiple medications increases the risk of all ADRs,13,14 including those resulting in impaired cognition. Many of the commonly used medications, such as digoxin, psychotropics, and those with anticholinergic (muscarinic-blocking) properties, have been well documented as causes of cognitive disturbances, even when used alone.13,14,22-24 A number of intrinsic physiologic alterations also put older individuals at increased risk for cognitive toxicity, including changes in neuroplasticity with resulting changes in drug sensitivity,25-27 and changes in drug distribution and elimination with subsequent pharmacokinetic toxicity.28-40 These factors form the basis for the aged's increased risk for the development of cognitive problems from medications.
Manifestations of cognitive toxicity
As with many ADRs, a clear association between drug and cognitive disturbance can be difficult, to definitively establish, particularly if the disturbance is subtle and if the impairment is in fact, multifactorial in origin.41,42 Acute confusional states (delirium) have been most clearly documented by clinical report, but dementia has also been shown to be a presentation of drug toxicity. Delirium is characterized by disturbed consciousness with reduced ability to focus, sustain, or shift, attention.43,44 Onset is usually rapid with fluctuations in levels of impairment, over the course of a day. Such patients also frequently exhibit confusion, agitation, delusions, and/or hallucinations. Many medications have been reported to cause delirium, such as those with anticholinergic activity, as well as opioids, sedatives, anxiolytics, and others. It is also important, to recall that withdrawal from some sedative-hypnotics and anxiolytics has also been reported to precipitate delirium.
Dementia associated with medication use involves multiple cognitive deficits, including memory impairment, with accompanying deficits in speech, recognition, motor and sensory ability, or other executive functions (such as planning, organizing, or abstracting).43,44 Onset is generally insidious, and progression is slow. Drug treatment, may not be ongoing at the time the condition is identified, but, in general, has previously been prolonged and intensive. Sedative-hypnotics, anxiolytics, anticonvulsants, and intrathecal methotrexate have all been reported to cause dementia.
Investigators examining the effect of particular drugs in controlled settings are able to assess less global or drastic, but, still definitive effects by using formal testing to measure effects on memory, attention/concentration, reaction time, and executive function in relation to drug exposure.45 For example, impairment, of mental skills in older subjects has been found to be greater and more persistent than in younger subjects following administration of some benzodiazepines, such as triazolam and alprazolam.46,47 Some data suggest that the aged are more sensitive to the effects of any given plasma level of these drugs. Similarly, cognitive decrements have been measured following oxybutynin and diphenhydramine.48 These deficits can be assessed using various methods, such as arithmetic calculation, digit recognition, simple reaction time, and the digit symbol substitution test. Although clearly showing cognitive impairment, these individuals do not, have the more dramatic level of symptomatology seen in drug-induced delirium.
Whether or not drug-induced impairment may have a role in falls or other accidents is less certain.49-52 Although falls in older persons are most often multifactorial in origin, many psychotropic medications, including some without any known propensity for causing orthostatic hypotension, have been consistently linked to an increased risk of falling and, by some, to overall mortality.53 Sedating drugs can clearly impair awareness of hazards and diminish reaction time, and overall sedation and reaction time have been recognized as two potentially important factors in falls. Some investigators have found that a history of a recent, fall was independently associated with involvement in an automobile crash, suggesting that both incidents could share risk factors.54 A number of retrospective and epidemiologic studies have found that, older drivers who used opioid analgesics and cyclic antidepressants had an increased risk for injurious motor vehicle collisions without any evidence of dose-related effects.55 Conflicting results have been found for the benzodiazepines, with some investigators finding that the risk of crash involvement, is increased, while others finding that, it is not.55-57
While it, remains uncertain as to what degree drug-induced cognitive toxicity is involved in such discretely definable events as accidents, it is clear that the spectrum of cognitive impairment ranges from the more obvious presentations of delirium to the less discernible deficits that can occur in reaction time, computational skill, symbol recognition, and memory. The latter may only be considered or identified outside formal clinical investigations when dramatic sequelae, such as a fail, occur. In addition, affective or behavioral toxicity may occur with manifestations such as depression or agitation. It is possible that these less severe cognitive manifestations also have a potentially substantial, though undoubtedly variable, effect, on activities of daily living and quality of life. Such consequences, however, are difficult to measure and even more difficult to relate to experimental tests of performance.
Risk factors for developing drug-induced cognitive impairment
As noted above, the risk of drug-related cognitive toxicity increases with the number of medications prescribed, and many older persons concurrently take numerous drugs as part of their medical regimens. However, there are also factors that are intrinsic to aging individuals that increase the likelihood of undesirable cognitive side effects. There is evidence that both neurotransmission and signal transduction undergo changes during aging, leading to changes in regulation, sensitivity, and efficiency of the entire neurotransmission process.25-27 Data suggest that there is probable reduced transmission in many systems, including the cholinergic, GABAergic (GABA, γ-aminobutyric acid), serotonergic, dopaminergic, and noradrenergic systems.58 Some data indicate that this may be due to loss of neurons or synapses, while other data indicate that, there is neuronal dysfunction.25,26 Loss of proteins that regulate synaptic plasticity has been documented both in the normal aging brain and in Alzheimer's disease.27 Such alterations may render the older individual more vulnerable to drugs that further perturb these systems. An anticholinergic drug administered to an aged person, for example, blocks postsynaptic acetylcholine receptors in a CNS that, already has compromised cholinergic system activity. The result can be confusion, disorientation, and memory loss, which would not, occur in a younger person with more baseline acetylcholine neurotransmission.
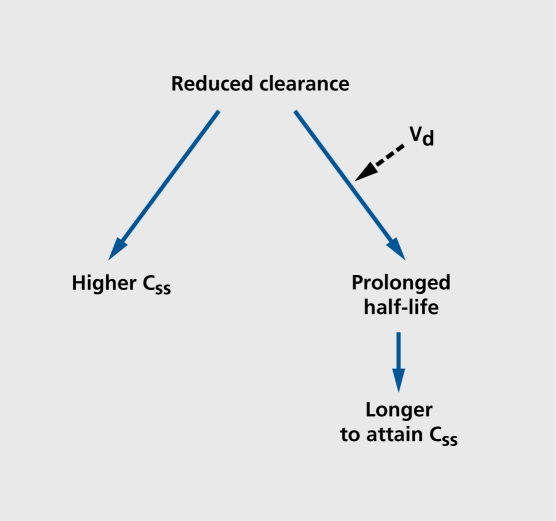
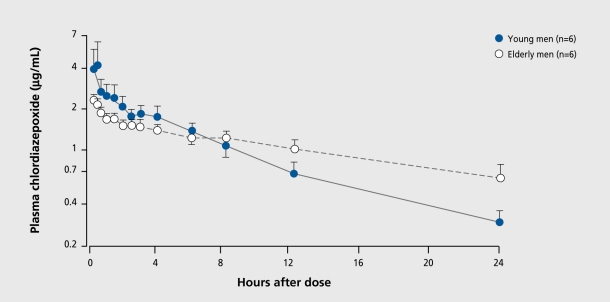
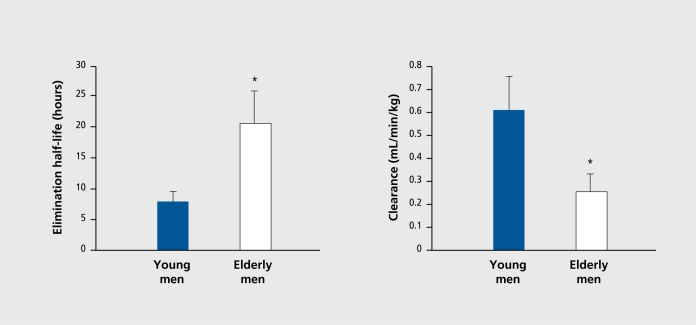
Other fundamental changes that occur outside the CNS also increase the vulnerability of aging people to cognitive toxicity. Older individuals, especially the oldest, of the old, have changes in the way they distribute and clear drugs, which can lead to altered pharmacokinetics and, ultimately, pharmacodynamics.28 The most important, involves the capacity to remove drugs from the body. Clearance (intrinsic to organ function) and dosing (controlled by the clinician) will determine the amount, of drug accumulation in the body as well as contribute to the determination of elimination half-life. For drugs that cross the blood-brain barrier, higher plasma levels will lead to higher CNS drug concentrations with the accompanying risk of toxicity. Identical dosing regimens given to older and younger patients will result, in different concentrations at, steady state if clearance rates differ (Figure 2). As age increases, renal blood flow and glomerular filtration rate decrease, and drugs eliminated by the kidneys generally exhibit, reduced clearance. Similary, a number of drugs cleared in the liver by oxidative metabolism also show reduced clearance because of reductions in enzymatic activity (Figure 3 and Figure 4). 59 One particularly important route of hepatic clearance involves metabolism by cytochrome P450-3A4 (CYP3A4).This enzyme is found in the liver and small intestine and is solely or significantly involved in the clearance of the majority of drugs in clinical use today. Examples of psychoactive drugs that utilize this pathway to some important, degree include alprazolam, diazepam, triazolam, Zolpidem, citalopram, amitripty line, nefazodone, trazodone, and haloperidol. Most have been found to have impaired clearance in aging populations.28 Though drug transport proteins, such as P-glycoprotcin, the multidrug resistant (mdrl) gene product, are increasingly identified as being importantly involved in the distribution and clearance of many drugs, such as digoxin, virtually nothing is known about the effect of normal aging on their expression or function.60,61 Further examination of their behavior in the intestine, liver, kidney, and blood-brain barrier may be important in explaining both kinetic and dynamic sensitivity in older people.
Figure 2. Consequences of reduced clearance in the elderly. For any given drug, impairment of the capacity for drug elimination (reduced clearance) will cause an elevation in steady-state concentrations (Css) with a resulting increase in the likelihood of toxicity. Reduced clearance also may cause a prolongation of elimination half-life, and a consequent delay in the rate of attainment of the steady-state condition. An increase in volume of distribution (Vd) may also contribute to a prolongation of elimination half-life.
Figure 3. Mean (±SE) plasma chlordiazepoxide concentrations in a series of young and elderly male volunteers who received a single 50-mg intravenous dose of chlordiazepoxide hydrochloride.59 Note the slower elimination of chlordiazepoxide in the elderly group.
Figure 4. Mean (±SE) values of chlordiazepoxide elimination half-life (left) and clearance (right) in young and elderly male volunteers as determined in the study described in Figure 3 59. The asterisk (*) indicates a statistically significant difference between young and elderly groups.
In addition to changes in specific organs, such as the kidney and the liver, more general changes in body habitus also take place. There is an overall increase in adipose tissue, which leads to an increased volume of distribution for lipophilic drugs. Gender is an important, factor, since women have a greater proportion of adipose tissue than men, regardless of age. Such changes do not affect absolute drug accumulation, but, they do affect elimination half-life, which means that the time until a steadystate situation is reached will be increased. Consequently, the time from the initiation of drug therapy or dosage change until the plasma levels have arrived at the new higher (or lower) steady -state will be prolonged. Time to desired clinical effect can also be expected to be prolonged. Furthermore, when a given medication effect (such as a sign of toxicity) occurs later than expected, it may lead to the erroneous conclusion that, it, is not medication-related, since the patient was already considered (erroneously) to be “stabilized” on a particular medication. Given that the majority of the aged are female, substantial differences in volumes of distribution can be expected. For drugs whose initial pharmacokinetic profiles have been determined in younger, predominantly male populations,62 the differences between actual and expected half-lives could be striking. For lipophilic drugs that require renal excretion or hepatic oxidation, the combination of reduced clearance and increased volume of distribution will lead to profound increases in half-life. The familiar adage, “start low, go slow,” suggesting lower starting doses with slower and smaller incremental changes, becomes almost a clinical imperative.
Frequently implicated medications
A number of medications seem to have a predictable potential for causing cognitive toxicity in aging individuals. Often this information is clearly presented in the drug's product labeling.63 This should not be misconstrued to mean that these medications are never appropriate for use in aging people. Close management, with consideration of the specific patient, and clinical circumstances and particular risk-benefit balance may result in efficacy with minimal or acceptable side effects. Generally, drugs that are predominantly used in older populations will reveal any toxicities in that same population. It may not be clear whether older individuals are at greater risk. Medications that arc used in all age-groups seem to be more likely to have been studied with regard to whether the elderly are more likely to develop these toxicities.
Further, there are medications not individually discussed that arc sporadically linked with toxic effects. As examples, nonsteroidal antiinflammatory drugs and histamine receptor antagonists are both widely used in their prescription and over-the-counter forms. Both are occasionally mentioned as causes of confusion. For some medications, conflicting data exist regarding whether the medication itself can be independently implicated in causing cognitive impairment, (eg, histamine receptor antagonists)64 or whether the elderly are more sensitive to a particular undesirable effect, (eg, alprazolam).65
Some medications may indirectly participate in causing cognitive difficulties by impairing normal excretion of a drug with CNS effects.66 Such drug interactions arc most common with the very potent inhibitors of drug metabolism (eg, ketoconazole inhibition of CYP3 A4).67 The same may prove to be true of inhibition of drug transport. For herbal and other dietary supplements, there are few data available to make any kind of assessment. In spite of assigned “likelihood” for causing undesirable CNS effects, any change in cognitive function that occurs during the course of any drug or “health aid” therapy should immediately prompt the consideration that medication or supplements may be involved. This is particularly true for the frail elderly and those hospitalized in critical care settings.
Medications with anticholinergic characteristics
These medications can cause a wide range of symptomatology ranging from deficits in attention and memory to florid delirium. Anticholinergic activity can be found in drugs across many therapeutic classes. Scopolamine is used to model the memory deficits found in Alzheimer's disease.68 Atropine and scopolamine can cause delirium even in low doses and when used as mydriatics.22 Oxybutynin, cyclobenzaprine, diphenhydramine, trihexyphenidyl, benztropine, doxepin, amitriptyline, clomipramine, trimipraminc, imipramine, protriptylinc, clozapine, chlorpromazine, chlorprothixene, and thiothixene are just some of the drugs that possess significant anticholinergic activity.63 Psychotropic characteristics of some of the above, such as the tricyclic antidepressants and neuroleptics, may be additive with the anticholinergic properties in causing undesirable symptomatology. It should be noted that proper drug treatment, of geriatric depression has been shown to improve cognitive abilities even when accompanied by slight increases in serum anticholinergicity.69
Sedative-hypnotics
A variety of effects are detectable and vary with the use pattern and particular drug. Some “toxicity” can be viewed as an extension of therapeutic effect. The benzodiazepines have received extensive study.28,37,38 Following acute and chronic benzodiazepine administration, aged individuals may achieve higher plasma levels, with consequently more pronounced sedation and performance impairment. In addition, the aged may exhibit increased sensitivity to some benzodiazepines. Data have linked long-acting benzodiazepines with an increased risk of falls, while other investigations suggest that dosing has a greater effect than duration of drug action. Withdrawal has been accompanied by delirium. In settings of single-dose administration, such as for insomnia or discrete anxiety episodes, appropriate drug choice and dosing can virtually ensure that drug effect, and its associated impairment will terminate at a reasonably predictable time. Simply substituting highly anticholinergic drugs or older, less studied medications such as meprobamate68,70 in place of benzodiazepines will not in itself reduce the risk of cognitive toxicity.
Other medications
The following drugs or drug classes have been implicated in the concurrence of cognitive toxicity.
Selegiline. The most frequent problems include delirium, hallucinations, agitation, and overall sedation.71
L-dopa. Used as a sole agent or in combination with carbidopa, a variety of cognitive problems have been reported to be associated with its use.72,73
Amantadine. Used as an antiviral as well as in Parkinson's disease, therapy has been linked to suicide attempts in patients with and without, previous psychiatric problems. These patients exhibit, a variety of abnormal mental states, including confusion, depression, paranoia, personality changes, and aggressive behavior.74,75 In aging populations, where its use would most likely occur, clearance is reduced and plasma levels are higher at standard doses.
Phenytoin. The CNS is the most common site of toxicity, which appears to be dose-related, but can occur even within the usual effective serum concentration range of 40 to 79 µmol/L. Confusion as well as speech and coordination difficulties are common.76
Digoxin. Some data indicate that this drug ranks first, in the number of prescriptions made out. to the elderly in the US.77 A spectrum of CNS-related effects can occur, including depression and anxiety as well as confusion and delirium with hallucinations. Such symptoms may appear in the absence of cardiac toxicity and at therapeutic plasma levels (0.6-2.6 nmol/L).78 Clearance of digoxin correlates with renal function as determined by creatinine clearance, which generally declines with age.
β-Blockers. Symptoms ranging from depression to memory disturbances and pseudodementia have been attributed to individual drugs, including propranolol and local use of timolol in glaucoma.79,80
Lidocaine. Symptoms ranging from confusion to delirium are common manifestations of toxicity.81
Antibiotics. Penicillins, cephalosporins, quinoloncs, and imipenem/cilastatin have all been shown to cause cognitive disturbances, particularly at high doses in renal insufficiency, severely ill patients, and/or patients with increased blood-brain barrier permeability. Quinoloncs such as ciprofloxacin can cause events such as anxiety and agitation, while imipenem can precipitate confusion (as well as convulsions).82-89
Corticosteroids. Particularly at higher doses, drugs such as prednisone can precipitate psychosis. Memory and attention deficits have also occurred during chronic therapy.87,88
Immunochemotherapy. Both interferon-alpha and interleukin-2 have been linked with serious depression.89,90
Opiate analgesic. Symptoms ranging from overt sedation to depression and delirium have occurred with many of the narcotics and vary with the clinical setting (postoperative vs chronic pain management). Some investigators feel that meperidine may be more likely to cause symptoms because of the anticholinergic nature of its metabolite, normeperidine.91,92 However, all opiate agonists have anticholinergic effects, which in turn may precipitate delirium. Long-term codeine use has been associated with depressive symptoms.93
Comment
As clinicians in adult medicine settings worldwide see an increasingly aging patient population, it will be necessary to remain abreast of which medications or health aids, both prescription and nonprescription, can cause disorders of cognition, as well as to recognize the variety of presentations. It should not be necessary to undertrcat the elderly and deprive them of the benefits of pharmacotherapy in order to avoid toxicity.94 A high level of care and vigilance should keep the therapy that is intended to extend life and enhance its quality from diminishing vital cognitive capacity.
Supported by Grants MH-58435, MH-01237, DA-05258, DA-13209, DA-06889, DK-58496, RR-00054, and MH-34223 from the United States Department of Health and Human Services, the Canadian Institutes for Health Research, the Centre for Addiction and Mental Health Research, and the Centre for Research in Women's Health, Canada. We are grateful for the collaboration and support of Richard I. Shader and Jerold S. Harmatz.
Contributor Information
Lisa L. von Moltke, Department of Pharmacology and Experimental Therapeutics, Tufts University School of Medicine, and the Division of Clinical Pharmacology, New England Medical Center, Boston, Mass, USA.
David J. Greenblatt, Department of Pharmacology and Experimental Therapeutics, Tufts University School of Medicine, and the Division of Clinical Pharmacology, New England Medical Center, Boston, Mass, USA.
Myroslava K. Romach, Sunnybrook and Women's College Health Sciences Centre and the University of Toronto, Ontario, Canada.
Edward M. Sellers, Sunnybrook and Women's College Health Sciences Centre and the University of Toronto, Ontario, Canada.
REFERENCES
- 1.Profile of older Americans: 2000. Washington, DC: Administration on Aging. June 2001. wvwv.aoa.dhhs.gov/aoa/stats/profile/. Accessed August 6, 2001. [Google Scholar]
- 2.Population ageing - a public health challenge. Fact Sheet No 135. Geneva, Switzerland: World Health Organization. September 1998. www.who.int /inf-fs/en/fact135.html. Accessed August 6, 2001. [Google Scholar]
- 3.Aging into the 21st century: demographic changes. Washington, DC: Administration on Aging. May 1996. www.aoa.dhhs.gov/aoa/stats/aging21/demography.html. Accessed August 6, 2001. [Google Scholar]
- 4.InfoNation. United Nations, www.un.org/. Accessed 30 October 2001. [Google Scholar]
- 5.Schoen C., Strumpf E., Davis K., Osborn R., Donelan K., Blendon RJ. The Elderly's Experiences with Health Care in Five Nations. New York, NY: The Commonwealth Fund. May 2000. www.cmwf.org/programs/international/schoen_5nat_387.asp. Accessed August 6, 2001. [Google Scholar]
- 6.Walop W., Amos S., Dalziel W., et al. Prescription and nonprescription drug use among at-risk community-dwelling seniors in Ottawa-Carleton. Can J Clin Pharmacol. 1999;6:93–100. [PubMed] [Google Scholar]
- 7.Thomas HF., Sweetnam PM., Janchawee B., Luscombe DK. Polypharmacy among older men in South Wales. Eur J Clin Pharmacol. 1999;55:411–415. doi: 10.1007/s002280050649. [DOI] [PubMed] [Google Scholar]
- 8.Families USA. cost overdose: growth in drug spending for the elderly, 19922010. Washington, DC: Families USA Foundation. July 2000. Publication 00107. www.familiesusa.org/media/pdf/drugod.pdf. Accessed August 6, 2001. [Google Scholar]
- 9.Sproule BA., Busto UE., Buckle C., Herrmann N., Bowles S. The use of non-prescription sleep products in the elderly. Int J Geriatr Psychiatry. 1999;14:851–857. [PubMed] [Google Scholar]
- 10.McRae S. Elevated serum digoxin levels in a patient taking digoxin and Siberian ginseng. Can Med Assoc J. 1996;155:293–295. [PMC free article] [PubMed] [Google Scholar]
- 11.Matthews HB., Lucier GW., Fisher KD. Medicinal herbs in the United States: research needs. Environ Health Perspect. 1999;107:773–778. doi: 10.1289/ehp.99107773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lantz MS., Buchalter E., Giambanco V. St. John's wort and antidepressant drug interactions in the elderly. J Geriatr Psychiatry Neurol. 1999;12:7–10. doi: 10.1177/089198879901200103. [DOI] [PubMed] [Google Scholar]
- 13.Atkin PA., Veitch PC., Veitch EM., Ogle SJ. The epidemiology of serious adverse drug reactions among the elderly. Drugs Aging. 1999;14:141–152. doi: 10.2165/00002512-199914020-00005. [DOI] [PubMed] [Google Scholar]
- 14.Beyth RJ., Shorr Rl. Epidemiology of adverse drug reactions in the elderly by drug class. Drugs Aging. 1999;14:231–239. doi: 10.2165/00002512-199914030-00005. [DOI] [PubMed] [Google Scholar]
- 15.Uppal R., Jhaj R., Malhotra S. Adverse drug reactions among inpatients in a north Indian referral hospital. Natl Med J India. 2000;13:16–18. [PubMed] [Google Scholar]
- 16.Interventions to reduce drug-related reactions among communityresident elderly Medicare and Medicaid patients. Washington, DC: United States Department of Health and Human Services. May 1993. www.hhs.gov/aspe/pic/7/pic4477.txt. Accessed August 6, 2001. [Google Scholar]
- 17.Zuccala G., Onder G., Carbonin P., Bernabei R. Adverse drug reactions in the elderly: need for dedicated databases. Arch Intern Med. 2000;160:1700–1701. doi: 10.1001/archinte.160.11.1700. [DOI] [PubMed] [Google Scholar]
- 18.Mannesse CK., Derkx FH., de Ridder MA., Man in't Veld AJ., van der Cammen TJ. Contribution of adverse drug reactions to hospital admission of older patients. Age Ageing. 2000;29:35–39. doi: 10.1093/ageing/29.1.35. [DOI] [PubMed] [Google Scholar]
- 19.Woodcock J. Testimony on medical errors: understanding adverse drug events. Washington, DC: Department of Health and Hman Services. February 2000. www.hhs.gov/asl/testify/t000201a.html. Accessed August 6, 2001. [Google Scholar]
- 20.van Eijk ME., Bahri P., Dekker G., et al. Use of prevalence and incidence measures to describe age-related prescribing of antidepressants with and without anticholinergic effects. J Clin Epidemiol. 2000;53:645–651. doi: 10.1016/s0895-4356(99)00194-8. [DOI] [PubMed] [Google Scholar]
- 21.Larson EB., Kukull WA., Buchner D., Reifler BV. Adverse drug reactions associated with global cognitive impairment in elderly persons. Ann Intern Med. 1987;107:169–173. doi: 10.7326/0003-4819-107-2-169. [DOI] [PubMed] [Google Scholar]
- 22.Moore AR., O'Keeffe ST. Drug-induced cognitive impairment in the elderly. Drugs Aging. 1999;15:15–28. doi: 10.2165/00002512-199915010-00002. [DOI] [PubMed] [Google Scholar]
- 23.Sleeper R., Bond CA., Rojas-Fernandez C. Psychotropic drugs and falls: new evidence pertaining to serotonin reuptake inhibitors. Pharmacotherapy. 2000;20:308–317. doi: 10.1592/phco.20.4.308.34885. [DOI] [PubMed] [Google Scholar]
- 24.Hanratty CG., McGlinchey P., Johnston GD., Passmore AP. Differential pharmacokinetics of digoxin in elderly patients. Drugs Aging. 2000;17:353–362. doi: 10.2165/00002512-200017050-00003. [DOI] [PubMed] [Google Scholar]
- 25.Pedigo NW., Jr. Neurotransmitter receptor plasticity in aging. Life Sci. 1994;55:1985–1991. doi: 10.1016/0024-3205(94)00378-5. [DOI] [PubMed] [Google Scholar]
- 26.Hatanpaa K., Isaacs KR., Shirao T., Brady DR., Rapoport SI. Loss of proteins regulating synaptic plasticity in normal aging of the human brain and in Alzheimer disease. J Neuropathol Exp Neurol. 1999;58:637–643. doi: 10.1097/00005072-199906000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Fowler CJ., Cowburn RF., Joseph JA. Alzheimer's, ageing and amyloid: an absurd allegory?. Gerontology. 1997;43:132–142. doi: 10.1159/000213841. [DOI] [PubMed] [Google Scholar]
- 28.von Moltke LL., Abernethy DR., Greenblatt DJ. Kinetics and dynamics of psychotropic drugs in the elderly. In: Salzman C, ed. Clinical Geriatric Psychopharmacology Baltimore, Md: Williams & Wilkins. 1998:70–93. [Google Scholar]
- 29.von Moltke LL., Greenblatt DJ., Harmatz JS., Shader Rl. Psychotropic drug metabolism in old age: principles and problems of assessment. In: Bloom FE, Kupfer DJ, eds. Psychopharmacology: The Fourth Generation of Progress. New York, NY: Raven Press. 1995:1461–1469. [Google Scholar]
- 30.von Moltke LL., Greenblatt DJ., Shader Rl. Clinical pharmacokinetics of antidepressants in the elderly: therapeutic implications. Clin Pharmacokinet. 1993;24:141–160. doi: 10.2165/00003088-199324020-00004. [DOI] [PubMed] [Google Scholar]
- 31.von Moltke LL., Greenblatt DJ. Pharmacokinetics of psychotropic drugs in the elderly. Ann Rev Gerontol Geriatr. 1999;19:53–71. [Google Scholar]
- 32.Hämmerlein A., Derendorf H., Lowenthal DT. Pharmacokinetic and pharmacodynamic changes in the elderly: clinical implications. Clin Pharmacokinet. 1998;35:49–64. doi: 10.2165/00003088-199835010-00004. [DOI] [PubMed] [Google Scholar]
- 33.Vestal RE. Aging and pharmacology. Cancer. 1997;80:1302–1310. doi: 10.1002/(sici)1097-0142(19971001)80:7<1302::aid-cncr16>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 34.Durnas C., Loi CM., Cusack BJ. Hepatic drug metabolism and aging. Clin Pharmacokinet. 1990;19:359–389. doi: 10.2165/00003088-199019050-00002. [DOI] [PubMed] [Google Scholar]
- 35.Montamat SC., Cusack BJ., Vestal RE. Management of drug therapy in the elderly. N Engl J Med. 1989;321:303–309. doi: 10.1056/NEJM198908033210507. [DOI] [PubMed] [Google Scholar]
- 36.Pollock BG. Psychotropic drugs and the aging patient. Geriatrics. 1998;53(suppl 1):S20–S24. [PubMed] [Google Scholar]
- 37.Greenblatt DJ., Harmatz JS., Shader Rl. Clinical pharmacokinetics of anxiolytics and hypnotics in the elderly: therapeutic considerations (Part I). Clin Pharmacokinet. 1991;21:165–177. doi: 10.2165/00003088-199121030-00002. [DOI] [PubMed] [Google Scholar]
- 38.Greenblatt DJ., Harmatz JS., Shader Rl. Clinical pharmacokinetics of anxiolytics and hypnotics in the elderly: therapeutic considerations (Part II). Clin Pharmacokinet. 1991;21:262–273. doi: 10.2165/00003088-199121040-00003. [DOI] [PubMed] [Google Scholar]
- 39.Greenblatt DJ., Sellers EM., Shader Rl. Drug disposition in old age. N Engl J Med. 1982;306:1081–1088. doi: 10.1056/NEJM198205063061804. [DOI] [PubMed] [Google Scholar]
- 40.Sellers EM., Flecker RC., Romach MK. Drug metabolism in the elderly: confounding of age, smoking, and ethanol effects. Drug Metab Rev. 1983;14:225–250. doi: 10.3109/03602538308991390. [DOI] [PubMed] [Google Scholar]
- 41.Foy A., O'Connell D., Henry D., Kelly J., Cocking S., Halliday J. Benzodiazepine use as a cause of cognitive impairment in elderly hospital inpatients. J Gerontol A Biol Sci Med Sci. 1995;50:M99–M106. doi: 10.1093/gerona/50a.2.m99. [DOI] [PubMed] [Google Scholar]
- 42.Pullen R. Psychopharmaka und Fahrtauglichkeit bel alteren Patienten. Versicherungsmedizin. 1999;51:71–74. [PubMed] [Google Scholar]
- 43.American Psychiatric Asssociation. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association Press. 1994 [Google Scholar]
- 44.Jacobson S., Shader Rl. Delirium and dementia. In: Shader Rl, ed. Manual of Psychiatric Therapeutics. 3rd ed. Boston, Mass: Little Brown and Co. 2001 [Google Scholar]
- 45.Morgan K. Hypnotic drugs, psychomotor performance and aging. J Sleep Res. 1994;3:1–15. doi: 10.1111/j.1365-2869.1994.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 46.Greenblatt DJ., Harmatz JS., Shapiro L., Engelhardt N., Gouthro TA., Shader Rl. Sensitivity to triazolam in the elderly. N Engl J Med. 1991;324:1691–1698. doi: 10.1056/NEJM199106133242403. [DOI] [PubMed] [Google Scholar]
- 47.Bertz RJ., Kroboth PD., Kroboth FJ., et al. Alprazolam in young and elderly men: sensitivity and tolerance to psychomotor, sedative and memory effects. J Pharmacol Exp Ther. 1997;281:1317–1329. [PubMed] [Google Scholar]
- 48.Katz IR., Sands LP., Bilker W., DiFilippo S., Boyce A., D'Angelo K. Identification of medications that cause cognitive impairment in older people: the case of oxybutynin chloride. J Am Geriatr Soc. 1998;46:8–13. doi: 10.1111/j.1532-5415.1998.tb01006.x. [DOI] [PubMed] [Google Scholar]
- 49.Thapa PB., Gideon P., Fought RL., Ray WA. Psychotropic drugs and risk of recurrent falls in ambulatory nursing home residents. Am J Epidemiol. 1995;142:202–211. doi: 10.1093/oxfordjournals.aje.a117619. [DOI] [PubMed] [Google Scholar]
- 50.Mendelson WB. The use of sedative/hypnotic medication and its correlation with falling down in the hospital. Sleep. 1996;19:698–701. [PubMed] [Google Scholar]
- 51.Campbell AJ. Drug treatment as a cause of falls in old age: a review of the offending agents. Drugs Aging. 1991;1:289–302. doi: 10.2165/00002512-199101040-00005. [DOI] [PubMed] [Google Scholar]
- 52.Gumming RG. Epidemiology of medication-related falls and fractures in the elderly. Drugs Aging. 1998;12:43–53. doi: 10.2165/00002512-199812010-00005. [DOI] [PubMed] [Google Scholar]
- 53.Kripke DF., Klauber MR., Wingard DL., Fell RL., Assmus JD., Garfinkel L. Mortality hazard associated with prescription hypnotics. Biol Psychiatry. 1998;43:687–693. doi: 10.1016/s0006-3223(97)00292-8. [DOI] [PubMed] [Google Scholar]
- 54.Sims RV., Owsley C., Allman RM., Ball K., Smoot TM. A preliminary assessment of the medical and functional factors associated with vehicle crashes by older adults. J Am Geriatr Soc. 1998;46:556–561. doi: 10.1111/j.1532-5415.1998.tb01070.x. [DOI] [PubMed] [Google Scholar]
- 55.Leveille SG., Buchner DM., Koepsell TD., McCloskey LW., Wolf ME., Wagner EH. Psychoactive medications and injurious motor vehicle collisions involving older drivers. Epidemiology. 1994;5:591–598. doi: 10.1097/00001648-199411000-00006. [DOI] [PubMed] [Google Scholar]
- 56.McGwin G., Sims RV., Pulley L., Roseman JM. Relations among chronic medical conditions, medications, and automobile crashes in the elderly: a population-based case-control study. Am J Epidemiol. 2000;152:424–431. doi: 10.1093/aje/152.5.424. [DOI] [PubMed] [Google Scholar]
- 57.“Benzodiazepine/Driving” Collaborative Group. Are benzodiazepines a risk factor for road accidents?. Drug Alcohol Depend. 1993;33:19–22. doi: 10.1016/0376-8716(93)90029-p. [DOI] [PubMed] [Google Scholar]
- 58.Sunderland T. Neurotransmission in the aging central nervous system. In: Salzman C, ed. Clinical Geriatric Psychopharmacology. Baltimore, Md: Williams & Wilkins. 1998:51–69. [Google Scholar]
- 59.Greenblatt DJ., Divoll MK., Abernethy DR., Ochs HR., Harmatz JS., Shader Rl. Age and gender effects on chlordiazepoxide kinetics: relation to antipyrine disposition. Pharmacology. 1989;38:327–334. doi: 10.1159/000138553. [DOI] [PubMed] [Google Scholar]
- 60.von Moltke LL., Greenblatt DJ. Drug transporters in psychopharmacology-are they important?. J Clin Psychopharmacol. 2000;20:291–294. doi: 10.1097/00004714-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 61.Schinkel AH., Wagenaar E., van Deemter L., Mol CA., Borst P. Absence of the mdrla P-glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest. 1995;96:1698–1705. doi: 10.1172/JCI118214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parikh C. Antidepressants in the elderly: challenges for study design and their interpretation. Br J Clin Pharmacol. 2000;49:539–547. doi: 10.1046/j.1365-2125.2000.00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Physicians Desk. Reference. 55th ed. Ordell, NJ: Medical Economics Company. 2001 [Google Scholar]
- 64.Patten SB., Love EJ. Drug-induced depression. Psychother Psychosom. 1997;66:63–73. doi: 10.1159/000289110. [DOI] [PubMed] [Google Scholar]
- 65.Kaplan GB., Greenblatt DJ., Ehrenberg BL., Goddard JE., Harmatz JS., Shader Rl. Single-dose pharmacokinetics and pharmacodynamics of alprazolam in elderly and young subjects. J Clin Pharmacol. 1998;38:14–21. doi: 10.1002/j.1552-4604.1998.tb04370.x. [DOI] [PubMed] [Google Scholar]
- 66.Greenblatt DJ., von Moltke LL. Sedative-hypnotic, anxiolytic agents. In: Levy RH, Thummel KE, Trager WF, Hansten PD, Eichelbaum M, eds. Metabolic Drug Interactions. Philadelphia, Pa: Lippincott, Williams and Wilkins. 2000:259–270. [Google Scholar]
- 67.Greenblatt DJ., Wright CE., von Moltke LL., et al. Ketoconazole inhibition of triazolam and alprazolam clearance: differential kinetic and dynamic consequences. Clin Pharmacol Ther. 1998;64:237–247. doi: 10.1016/S0009-9236(98)90172-2. [DOI] [PubMed] [Google Scholar]
- 68.Martinez R., Molchan SE., Lawlor BA., et al. Minimal effects of dextroamphetamine on scopolamine-induced cognitive impairments in humans. Biol Psychiatry. 1997;41:50–57. doi: 10.1016/0006-3223(95)00674-5. [DOI] [PubMed] [Google Scholar]
- 69.Nebes RD., Pollock BG., Mulsant BH., Butters MA., Zmuda MD., Reynolds CF. Cognitive effects of paroxetine in older depressed patients. J Clin Psychiatry. 1999;60(suppl 20):26–29. [PubMed] [Google Scholar]
- 70.Littrell RA., Hayes LR., Stillner V. Carisoprodol (Soma): a new and cautious perspective on an old agent. South Med J. 1993;86:753–756. doi: 10.1097/00007611-199307000-00006. [DOI] [PubMed] [Google Scholar]
- 71.Montastruc JL., Chaumerliac C., Desboeuf K., et al. Adverse drug reactions to selegiline: a review of the French pharmacovigilance database. Clin Neuropharmacol. 2000;23:271–275. doi: 10.1097/00002826-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 72.Young BK., Camicioli R., Ganzini L. Neuropsychiatrie adverse effects of antiparkinsonian drugs. Characteristics, evaluation and treatment. Drugs Aging. 1997;10:367–383. doi: 10.2165/00002512-199710050-00005. [DOI] [PubMed] [Google Scholar]
- 73.Factor SA., Molho ES., Podskalny GD., Brown D. Parkinson's disease: druginduced psychiatric states. Adv Neurol. 1995;65:115–138. [PubMed] [Google Scholar]
- 74.Guay DR. Amantadine, rimantadine prophylaxis of influenza A in nursing homes. Atolerability perspective. Drugs Aging. 1994;5:8–19. doi: 10.2165/00002512-199405010-00002. [DOI] [PubMed] [Google Scholar]
- 75.Postma JU., Van Tilburg W. Visual hallucinations and delirium during treatment with amantadine (Symmetrel). J Am Geriatr Soc. 1975;23:212–215. doi: 10.1111/j.1532-5415.1975.tb00187.x. [DOI] [PubMed] [Google Scholar]
- 76.Wong ICK., Tavernor SJ., Tavernor RME. Psychiatric adverse effects of anticonvulsant drugs: incidence and therapeutic implications. CNS Drugs. 1997;8:492–509. [Google Scholar]
- 77.Families USA. Still rising: drug price increases for seniors 1999-2000. Washington DC: Families USA Foundation. Publication 00-103. April 2000. www.familiesusa.org/media/pdf/pdrug.pdf. Accessed August 6, 2001. [Google Scholar]
- 78.Miura T., Kojima R., Sugiura Y., Mizutani M., Takatsu F., Suzuki Y. Effect of aging on the incidence of digoxin toxicity. Ann Pharmacother. 2000;34:427–432. doi: 10.1345/aph.19103. [DOI] [PubMed] [Google Scholar]
- 79.Munroe WP., Rindone JP., Kershner RM. Systemic side effects associated with the ophthalmic administration of timolol. Drug Intell Clin Pharm. 1985;19:85–89. doi: 10.1177/106002808501900201. [DOI] [PubMed] [Google Scholar]
- 80.Rogers TK., Bowman CE. Cognitive impairment associated with betablockade in the elderly. Postgrad Med J. 1990;66:1050–1052. doi: 10.1136/pgmj.66.782.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Turner WM. Lidocaine and psychotic reactions. Ann Intern Med. 1982;97:149–150. doi: 10.7326/0003-4819-97-1-149_2. [DOI] [PubMed] [Google Scholar]
- 82.Sternbach H., State R. Antibiotics: neuropsychiatrie effects and psychotropic interactions. Harv Rev Psychiatry. 1997;5:214–226. doi: 10.3109/10673229709000304. [DOI] [PubMed] [Google Scholar]
- 83.Andrejak M., Schmit JL., Tondriaux A., Hary L., Debailleux S., Moore N. Neurologic side effects of fluoroquinolones. Apropos of 9 cases concerning pefloxacin [In French]. Thérapie. 1992;47:415–418. [PubMed] [Google Scholar]
- 84.Ball P., Tillotson G. Tolerability of fluoroquinolone antibiotics. Past, present and future. Drug Safety. 1995;13:343–358. doi: 10.2165/00002018-199513060-00004. [DOI] [PubMed] [Google Scholar]
- 85.Schliamser SE., Cars O., Norrby SR. Neurotoxicity of beta-lactam antibiotics: predisposing factors and pathogenesis. J Antimicrob Chemother. 1991;27:405–425. doi: 10.1093/jac/27.4.405. [DOI] [PubMed] [Google Scholar]
- 86.Norrby SR. Neurotoxicity of carbapenem antibacterials. Drug Saf. 1996;15:87–90. doi: 10.2165/00002018-199615020-00001. [DOI] [PubMed] [Google Scholar]
- 87.Varney NR., Alexander B., Maclndoe JH. Reversible steroid dementia in patients without steroid psychosis. Am J Psychiatry. 1984;141:369–372. doi: 10.1176/ajp.141.3.369. [DOI] [PubMed] [Google Scholar]
- 88.Lewis DA., Smith RE. Steroid-induced psychiatric syndromes. A report of 14 cases and a review of the literature. J Affect Disord. 1983;5:319–332. doi: 10.1016/0165-0327(83)90022-8. [DOI] [PubMed] [Google Scholar]
- 89.Maes M., Capuron L., Ravaud A., et al. Lowered serum dipeptidyl peptidase IV activity is associated with depressive symptoms and cytokine production in cancer patients receiving interleukin-2-based immunotherapy. Neuropsychopharmacology. 2001;24:130–140. doi: 10.1016/S0893-133X(00)00168-8. [DOI] [PubMed] [Google Scholar]
- 90.Malaguarnera M., Di Fazio I., Restuccia S., Pistone G., Ferlito L., Rampello L. Interferon alpha-induced depression in chronic hepatitis C patients: comparison between different types of interferon alpha. Neuropsychobiology. 1998;37:93–97. doi: 10.1159/000026485. [DOI] [PubMed] [Google Scholar]
- 91.Forman WB. Opiold analgesic drugs in the elderly. Clin Geriatr Med. 1996;12:489–500. [PubMed] [Google Scholar]
- 92.Eisendrath SJ., Goldman B., Douglas J., Dimatteo L., Van Dyke C. Meperidine-induced delirium. Am J Psychiatry. 1987;144:1062–1065. doi: 10.1176/ajp.144.8.1062. [DOI] [PubMed] [Google Scholar]
- 93.Romach MK., Sproule BA., Sellers EM., Sorrier G., Busto UE. Long-term codeine use is associated with depressive symptoms. J Clin Psychopharmacol. 1999;19:373–376. doi: 10.1097/00004714-199908000-00015. [DOI] [PubMed] [Google Scholar]
- 94.Lebowitz BD., Pearson JL., Schneider LS., et al. Diagnosis and treatment of depression in late life. JAMA. 1997;278:1186–1190. [PubMed] [Google Scholar]