Abstract
This review focuses on recent brain imaging and behavioral studies of sensory gating functions, which assess similarities between the effects of classic hallucinogens (eg, psilocybin), dissociative anesthetics (eg, ketamine), and entactogens (eg, 3,4-methylenedioxymethamphetamine [MDMA]) in humans. Serotonergic hallucinogens and psychotomimetic anesthetics produce overlapping psychotic syndromes associated with a marked activation of the prefrontal cortex (hyperfrontality) and other overlapping changes in temporoparietal, striatal, and thalamic regions, suggesting that both classes of drugs act upon a common final pathway. Together with the observation that both hallucinogens and N-methyl-oaspartate (NMDA) antagonists disrupt sensory gating in rats by acting on 5-hydroxytryptamine (serotonin) 5-HT2 receptors located in cortico-striato-thalamic circuitry these findings suggest that disruption of cortico-subcortical processing leading to sensory overload of the cortex is a communality of these psychoses. In contrast to hallucinogens, the entactogen MDMA produces an emotional state of positive mood, concomitant with an activation of prefrontolimbiclparalimbic structures and a deactivation of amygdala and thalamus.
Keywords: hallucinogen, psilocybin, entactogen, MDMA, NMDA antagonist, ketamine brain imaging, behavioral study
Abstract
Esta revisión se centra en recientes estudios con-ductuales y de imágenes cerebrales de las funciones de regulación sensorial, los cuales evalúan semejanzas entre los efectos de los alucinógenos clásicos (por ej, psilocibina), los anestésicos dísocíadores (por ej, ketamina) y los entactógenos (por ej, 3,4-metilendioximetanfe-tamina [MDMA]) en humanos. Los alucinógenos serotoninérgicos y los anestésicos psicotomimé-ticos producen un síndrome psicótico sobrepuesto, el cual se asocia con una marcada activación de la corteza prefrontal (híperfrontalídad) y otros cambios sobrepuestos en las regiones témpora-parietales, estríatales y talámícas, lo que sugiere que ambos grupos de drogas actúan en una vía final común. Junto con la observación que ambos alucinógenos y que los antagonistas del N-metil-D-aspartato (NMDA) desorganizan la regulación sensorial en ratas, al actuar a nivel de los receptores 5-HT2 de 5-hidroxitriptamina (serotonina) localizados en los circuitos córtico-estriado-talámícos, estos hallazgos sugieren que una desorganización del procesamiento córtíco - subcortical que lleve a una sobrecarga de la corteza es común en estas psicosis. En contraste con los alucinógenos, el entactógeno MDMA provoca un estado emocional de ánimo positivo, concomitante con una activación de estructuras prefrontolímbicas I paralímbicas y una desactivación de la amígdala y del tálamo.
Abstract
Cet article passe en revue les études récentes portant sur l'imagerie cérébrale et les aspects comportementaux relatifs aux fonctions de filtrage des voies sensorielles, ayant pour but d'évaluer les similitudes entre les effets des hallucinogènes classiques (par ex, la psilocybine), des anesthésiques dissociatifs (par ex. la kétamine) et les entactogènes (par ex, la 3,4-méthylènedioxyméthamphétamine [MDMA]) chez l'homme. Les hallucinogènes sérotoniner-giques et les anesthésiques psychosomimétiques induisent des syndromes psychotiques largement comparables en rapport avec une activation marquée du cortex préfrontal (hyperfrontalité) et d'autres modifications affectant les régions tempo-ropariétales, striatales et thalamiques, suggérant que les deux classes médicamenteuses agissent sur la même voie finale. Ces résultats, si on les rapproche de la perturbation de la fonction de filtrage des voies sensorielles provoquée chez le rat par les antagonistes du N-méthyl-D-aspartate (NMDA) et les hallucinogènes via leur action sur les récepteurs 5-HT2 [5-hydroxytryptamine (sérotonine)] du circuit cortico-striato-thalamique, suggèrent que la perturbation opérationnelle cortico-sous-corticale responsable de la surcharge sensorielle du cortex est un point commun à ces psychoses. Contrairement aux hallucinogènes, l'entactogène MDMA provoque un état émotionnel d'humeur positive parallèlement à une activation des structures préfronto-limbiques et paralimbiques et à une désactivation des amygdales et du thalamus.
Hallucinogens are a group of chemically heterogeneous compounds, all with the ability to induce altered states of consciousness (ASC) characterized by profound alterations in mood, thought processes, perception, and experience of the self and environment otherwise rarely experienced except in dreams, contemplative and religious exaltation, and acute psychoses. The term hallucinogen seems to be somewhat inappropriate, since not all these drugs reliably produce visual and auditory hallucinations.1,2 Therefore hallucinogens have been also called psychotomimetic (psychosis-mimicking), psycholytic (psyche-loosening), or psychedelic (mind-manifesting), reflecting the widely different attitudes and intentions with which these substances have been approached.
As plant drugs, psychedelic hallucinogens have a long and colorful history. Because of their ability to produce a visionary and ecstatic state, they were often ascribed magical or mystical properties. For centuries, they were used restrictedly as sacraments in religious rites and people in the Western world were hardly aware of their existence. Examples of the use of naturally occurring hallucinogens in various cultures include psilocybin derived from the Aztec sacred magic mushroom teonanacatl, mescaline derived from the peyote cactus taken by Native Americans, or N,N-dimethyltryptamine (DMT), the active ingredient of ayahuasca, a hallucinogenic plant extract employed by Amazonian Indians.3 However, with the discovery of the hallucinogenic properties of the semisynthetic ergoline d-lysergic acid diethylamide (LSD) by the Swiss chemist Albert Hofmann in 1943, hallucinogens and related compounds have become the focus of modern scientific research. The LSD-induced psychosis-like syndrome and the structural similarity between LSD and serotonin (5-hydroxytryptamine [5-HT]) prompted the hypothesis that 5-.H.T is involved in the pathophysiology of schizophrenia. Since then a number of newly discovered hallucinogens or psychotomimetic agents, such as phencyclidine (PCP) and ketaminc, have been used as models to study the neuronal basis of drug-induced ASC and its relation to naturally occurring psychoses.4-6
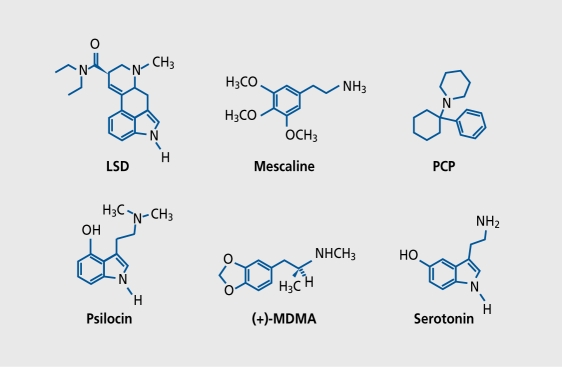
Psychedelic hallucinogens can be classified by either chemical structure or their primary mode of action. The so-called serotonergic hallucinogens include indolamines, such as psilocybin and LSD, and phenylethylamines, such as mescaline and 2,5-dimethoxy-4-iodoamphetamine (DOI) (Figure 1). Serotonergic hallucinogens act primarily upon 5-HT1 ( 5-HT2, 5-HT6, and 5-HT7 receptors and partly upon the dopamine (DA) receptors D1 and D2 and the adrenergicα2 receptors. A second class of drugs with hallucinogenic properties often referred to as psychedelic or dissociative anesthetics includes arykyclohexy lamines, whose most important representatives are PCP and ketamine. These agents primarily act as antagonists of the vV-mcthyl-D-aspartate (NMDA) subtype of the glutamate receptor. Finally, a third class of drugs, the so-called “entactogens,” produce psychedelic-like effects, but virtually no hallucinations. They arc closely related structurally to hallucinogenic phenylethylamines and stimulant amphetamines and include phenyttsopropy lamines, such as 3,4-methylenedioxymethamphetaminc (MDMA), 3,4-methylenedioxyethylamphctamine (MDE), and related compounds.
Figure 1. Chemical structures of some important representatives of hallucinogens. Classic serotonergic hallucinogens include indolamines, such as the semisynthetic lysergic acid diethylamide (LSD) and psilocybin/psilocin (the active principle of the sacred Aztec magic mushrooms), and phenylethylamines, such as mescaline (the active principle of peyote cactus). Indolamines and phenylethylamines share close structural features with the neurotransmitter serotonin (5-hydroxytryptamine [5-HT]). Dissociative or psychedelic anesthetics include phencyclidine (PCP) and related drugs, such as ketamine. Entactogens, such as 3,4-methylenedioxymethamphetamine (MDMA), which produce psychedeliclike symptoms but virtually no hallucinations, are structurally closely related to both serotonergic hallucinogens (mescaline) and classic stimulants (amphetamines).
This review summarizes recent experiments to elucidate the neurobiological basis of the psychological effects of psilocybin, ketamine, and MDMA, each representing one of the three classes of psychedelics. Functional brain imaging with positron emission tomography (PET) was used to identify the brain regions or functional interactions among the neurotransmitter systems involved in the action of these drugs. Furthermore, receptor mechanisms of hallucinogenic and related drugs have been investigated by exploring the effects of specific receptor antagonists on drug-induced psychological alterations and information-processing functions, such as sensorimotor gating as indexed by prepulse inhibition (PPI) of the startle reflex.
The premise of the present review is that many of the shared psychedelic effects of serotonergic hallucinogens and NMDA antagonists can be understood as an effect downstream of a common neurotransmitter system or final pathway. First, both serotonergic hallucinogens and NMDA antagonists produce sufficient overlapping psychologial alterations despite different primary modes of action. Second, there is converging evidence from brain imaging, behavioral, and electrophysiological studies that both serotonergic hallucinogens and NMDA antagonists disrupt information processing within corticostriato-thalamic pathways implicated in the pathogenesis of psychotic disorders. Since entactogens such as MD.M.A are expected to produce only mild psychedelic symptoms, it will be of interest to know to what extent MDMA-induced neurobiological alterations differ from those seen in the states induced by hallucinogens and NMDA antagonists.
Serotonergic hallucinogens and NMDA antagonists
Psychological effects
Among the key psychological functions that are altered by hallucinogens or NMDA antagonists are: (i) psychoticlike symptoms; (ii) changes in mood; and (iii) changes in perception of time, self, and environment, including both threatening or pleasant experiences of derealization and depersonalization phenomena. Ill esc psychological functions share many aspects of prominent psychiatric symptoms of disorders such as schizophrenia, or delusional disorder, and can be assessed via standard psychiatric or psychological rating scales.1
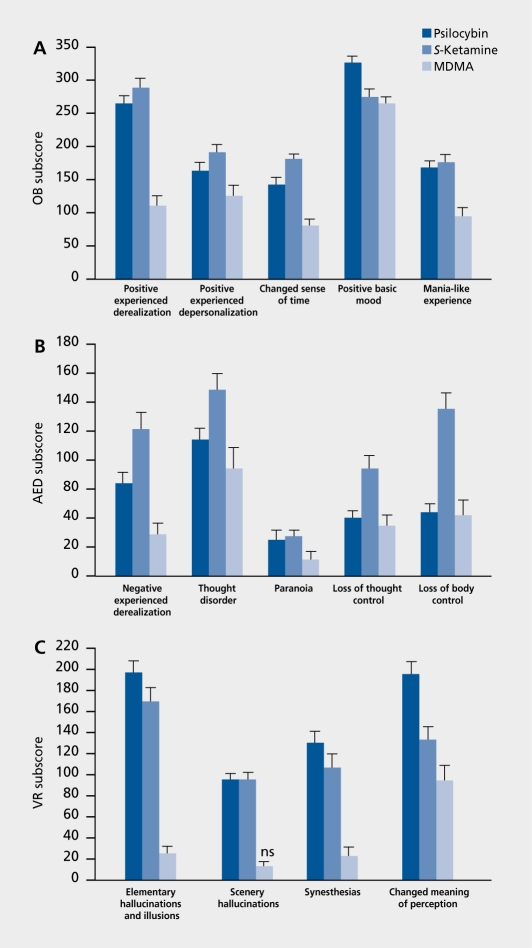
According to the work of Dittrich,7 the common nucleus of drug-induced ASC can be described by three dimensions (factors) of the APZ questionnaire, which is an ASC rating scale.2,8 These dimensions are: (i) oceanic boundlessness (OB), referring to dissolution of ego boundaries associated with positive emotions ranging from heightened mood to sublime happiness and serenity or grandiosity; (ii) anxious ego-dissolution (AED), including thought disorder and loss of autonomy and self-control variously associated with arousal, anxiety, and paranoid ideations; and (iii) visionary reslrucluralizaiion (VR) referring to auditory and visional illusions, hallucinations, and altered meaning of perception.2,8 As seen in (Figure 2)., both psilocybin and ketamine produce either loss of ego boundaries associated with positive emotions or negative ego-disintegration associated with thought disorder and loss of autonomy and self-control.9-12
Figure 2. Subscale scores of the altered states of consciousness (APZ) questionnaire forS-ketamine (n=68; 0.012 mg/kg/min IV), psilocybin (n=99; 0.26 mg/kg PO), and 3,4-methylenedioxymethamphetamine (MDMA) (n=74; 1.5-1.7 mg/kg PO) in healthy volunteers. With the exception of complex hallucinations after MDMA, S-ketamine-, psilocybin-, and MDMA-induced scores are all significant compared with placebo. Values are means±SE, all P<0.05 or less. A. The oceanic boundlessness (OB) scale measures derealization and depersonalization associated with a positive basic mood ranging from heightened feelings to exaltation and alterations in the sense of time. B. The anxious ego-dissolution (AED) scale measures ego-disintegration and loss of autonomy and self-control associated with arousal and anxiety. C. The visionary restructuralization (VR) scale measures alterations in perception and meaning.
The ego-disintegration and the loss of self-control over thought process and intentionality, and the uncertainty or lack in differentiating between ego and noncgo spheres observed in psilocybin- and ketamine-induced psychoses are highly reminiscent of acute schizophrenic decompensation.13-17 Also, the finding of heightened awareness associated with euphoria in psilocybin- and kctaminetreated subjects is consistent with the view that the earliest affective changes in schizophrenic patients are often pleasurable or exhilarating.18-21 Furthermore, prospective22 and comparative studies indicate that perceptual disturbances including the heightened sensitivity, auditory and visual illusions, and hallucinations reported by ke famine - and psilocybin- treated subjects are prominent features of prodromal, early, and acute schizophrenic patients.21,23-25 Similar findings were reported in comparable studies in healthy volunteers receiving psilocybin or the phenylethyamine hallucinogen mescaline.26,27
Thus, the present evidence suggests that hallucinogeninduced ASC share many common phenomenological features with the early acute stages of the schizophrenic disorders and may provide useful models to elucidate the neuronal basis of productive symptoms of schizophrenic pathophysiology. However, despite the number of similarities between the psilocybin and ketamine models of psychoses, substantial differences have also become apparent in the limited human studies.
Specifically, it appears that both 5-ketamine and racemic ketamine produced more pronounced anxiety, thought disturbances, and ego-disintegration than psilocybin. Moreover, in contrast to psilocybin, both 5-ketamine and racemic ketamine produced transient apathy, emotional withdrawal, and feelings of indifference, which resembled the negative symptoms of schizophrenia in many ways. This finding is consistent with the view that ketamine and PCP induce thought disturbances and cognitive impairments in healthy subjects, which mimic those seen in schizophrenia, including deficits in working memory, attention, abstract reasoning, decision making, and planning.28-31 Thus, it has frequently been argued that the state produced by NM'DA antagonists may more closely mimic naturally occurring schizophrenias (Table I)..10-12,28-41
Table I. Comparison of effects of psilocybin (0.2-0.24 mg/kg PO), S-ketamine (0.01-0.02 mg/kg/min), and 3,4-methylenedioxymethamphetamine (MDMA) (1.5-1.7 mg/kg PO), and symptoms in schizophrenias (summarized from references 10-12, 28-31, and 33-41). 5-HT, 5 hydroxytryptamine; GABA, γ-aminobutyric acid; NMDA, N-methy!-D-aspartate; mGluR, metabotropic glutamate receptor; D1, D2, dopamine receptors; H1, histamine receptor; α2, α2 adrenergic receptor. * MDMA has highest affinity for the 5-HT transporter (Ki= 0.61 µM)and lesser for α2 (Ki=3.6 µM) and 5-HT2 receptors (Ki5.1µM) in rat brain. * * Chronic administration of NMDA antagonists in rats decreases frontal cortical activity.
| Psilocybin | Ketamine | MDMA | Schizophrenias | |
| Receptor level | ||||
| Primary locus of action | 5-HT2A, 5-HT1A | NMDA | 5-HT transporter,* | Unknown |
| 5-HT2A, 5-HT1A | ||||
| α2, H1 | ||||
| Downstream effects on | GABA, D1 | 5-HT2A | D1, D2 | |
| D2, mGluR | GABA, D1, D2 | |||
| mGluR | ||||
| Psychopathology | ||||
| Positive symptoms | ||||
| • Hallucination/illusions | ++ | + | - | ++ |
| • Delusions | + | + | - | ++ |
| • Thought disorder | + | ++ | + | ++ |
| Negative symptoms | ||||
| • Blunted affect | 0- + | + - ++ | - | ++ |
| • Withdrawal | + | + - ++ | - | ++ |
| Depersonalization | + - ++ | ++ | + | ++ |
| Derealization | + | ++ | + | ++ |
| Neuropsychology | ||||
| • Attention disturbance | + - ++ | + | + | ++ |
| • Distractibility | + | ++ | - | ++ |
| • Working memory | + | ++ | ? | ++ |
| • Associative deficits | + | + - ++ | ? | ++ |
| • Planning/mental flexibility | ++ | ? | ? | ++ |
| Cortical activity | ||||
| • Frontal (PET) | ++ (acute) | ++ (acute) | (+) | ++ (acute) |
| - - (chronic)** | - - (chronic) |
Cortico-striato-thalamic loops: a common pathway?
Theories regarding the neuronal basis of the symptomatology of schizophrenic psychoses have often suggested that deficits in early information processing may underlie the diversity of psychotic symptoms and cognitive disturbances observed in the group of schizophrenias.42,28-44 Such theories posit that a fundamental feature of information processing dysfunction in psychosis is the inability of these patients to screen out, inhibit, filter, or gate extraneous stimuli and to attend selectively to salient features of the environment. Gating deficits may cause these subjects to become overloaded with excessive exteroceptive and interoceptive stimuli, which, in turn, could lead to a breakdown of cognitive integrity and difficulty in distinguishing self from nonself.44,45
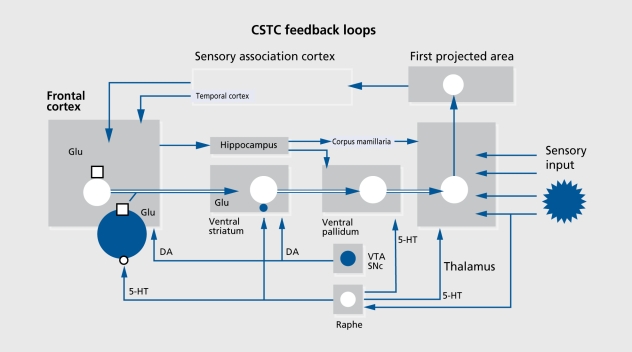
In recent years, this theoretical construct has been successfully operationalized by measuring the behavioral plasticity of acoustic startle responses, such as PPI and habituation.46 Symptomatic schizophrenia patients exhibit deficits in both PPI and habituation. Extensive lesion and drug studies in rodents have demonstrated that sensorimotor gating functions, such as PPI, are subject to considerable forebrain modulation from cortical, limbic, striatal, pallidal, and thalamic structures, including cortico-striato-pallido-thalamic (CSPT) circuitry.46,47 Moreover, animal studies indicate that hallucinogens, amphetamines including MDM.A, and NMDA antagonists disrupt sensorimotor gating in rats by interacting with different components of the CSPT loop. These findings are consistent with the “thalamic filter hypothesis of psychosis,” advanced by Carlsson and Carlsson.48 This theory proposes that corticostriatal pathways exert a modulatory influence on the thalamic gating of sensory information to the cerebral cortex (Figure 3). 49 Theoretically, an impairment of thalamic filtering should result in sensory overload of the cortex, leading to a breakdown of integrative cortical functions, and subsequently to positive symptoms such as delusions, hallucinations, thought disturbances, persecution, and loss of a coherent ego experience. In addition, various negative symptoms, such as emotional and social withdrawal, could result from - and be understood as - efforts to protect from input overload.
Figure 3. The limbic cortico-striato-thalamic-cortical (CSTC) feedback loops are involved in memory, learning, and self-nonself discrimination by linkage of cortically categorized exteroceptive perception with internal stimuli of the value system. The filter function of the thalamus, which is under the control of the CSTC feedback/reentry loops, is postulated to protect the cortex from exteroceptive sensory information overload, as well as from interna! overarousal. The model predicts that sensory overload of the cortex and psychosis may result from thalamic gating deficits, which may be caused by ketamine by blockade of N-methyl-D-aspartate (NMDA)-mediated glutamatergic (Glu) corticostriatal and/or by increasing mesolimbic dopaminergic (DA) neurotransmission. Excessive stimulation of serotonin 5-HT2 receptors (for example, by psilocybin) may lead to a similar neurotransmitter imbalance in the CSTC loops, which again results in an opening of the thalamic filter, sensory overload of the cortex, and psychosis. VTA, ventral tegmental area; SNc, substantia nigra pars compacta; GABA, γ-aminobutyric acid; , NMDA receptor. Modified from reference 49: Vollenvveider FX, Greyer MA. A systems model of altered consciousness: integrating natural and drug-induced psychoses. Brain Res Bull. 2001:56:495507. Copyright © 2001, Elsevier Science.
On the basis of these findings and the thalamic filter model, ACSs induced by hallucinogens and NMDA antagonists in humans can be conceptualized as complex disturbances that arise from more elementary deficits of sensory information processing in corticostriato-thalamo-cortical (CSTC) feedback loops.6,50 The model proposes that NMDA antagonists may disrupt thalamic filter functions and produce sensory overload of cortical areas, particularly of the prefrontal cortex, by blocking NMDA receptors located on corticostriatal pathways, while serotonergic hallucinogens may alter thalamocortical transmission by stimulation of 5-HT2 receptors located in several components of the CSTC loop, including the prefrontal cortex, striatum, nucleus accumbens, and thalamus (for details, see reference 49).
Brain imaging studies
Until recently, many neural circuit models were based on animal studies, and implications for the effects of hallucinogenic drugs or disease models in humans were based on inferences from these studies. However, functional neuroimaging studies enable one to examine these neural circuit models directly and test specific hypotheses about the role of specific neural systems in the expression of ASC.
PET with the radiotracer 18F-fluorodcoxyglucose (18FDG) was used to assess drug-induced changes in the regional cerebral metabolic rate of glucose (CMRglu), as an index of cerebral activity. We found that a hallucinogenic dose of racemic ketamine increased neuronal activity in the prefrontal cortex (hyperfrontality) and associated limbic regions, as well as in striatal and thalamic structures in healthy volunteers, giving the first evidence that functional alterations in CSTC loops may underlie the symptomatology of drug-induced ASC.50 This hyperfrontality finding was corroborated and extended in subsequent studies in healthy volunteers in which the effects of hallucinogens and NMDA antagonists including psilocybin, racemic ketamine, and S-ketamine were compared.
In particular, we found that, despite different primary mechanisms of action, the two classes of drugs produced strikingly similar brain activation patterns as indexed by normalized CMRglu. Both psilocybin and ketamine markedly increased brain activity bilaterally in the frontomedial and frontolateral cortex, including the anterior cingulate. Lesser increases were found in the temporomedial, superior, and inferior parietal cortices, striatum, and thalamus. Decreases were found in the left caudate nucleus, bilaterally in the ventral striatum, occipital lobe, and visual pathway.9-11 A correlational analysis revealed that the metabolic hyperfrontality in ketamine and psilocybin subjects was associated with a depersonalization/derealization syndrome, thought disturbances, and mania-like symptoms.9-11 The hyperfrontality finding in ASC was further supported by evidence from brain imaging studies with ketamine and psilocybin in healthy volunteers27,51 and was also found in subjects treated with the classic pheny le thyl amine hallucinogen mescaline.52
Correlations between cerebral activity and psychological alterations
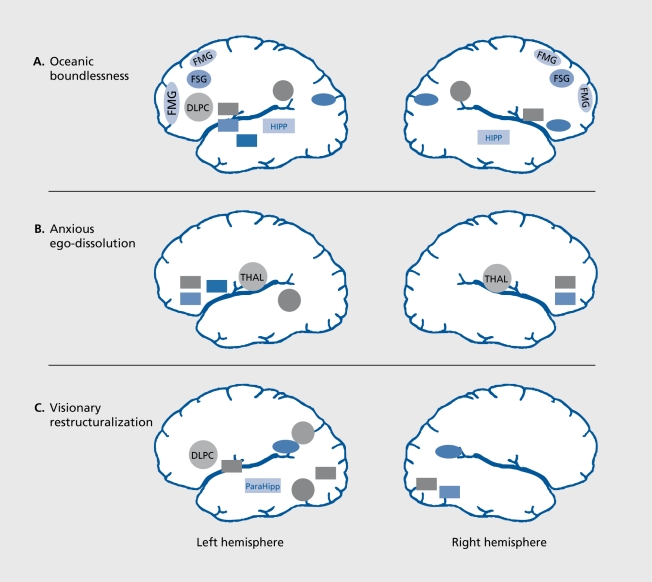
The correlation of changes in cerebral activation with changes in self-assessment enables one to further corroborate the role of specific neural substrates in these psychological functions. Correlational analysis between normalized metabolic activity and psychological scores of the APZ questionnaire revealed that the severity of OB correlated positively with CMRglu bilaterally in frontomedial superior, frontolateral, and left inferolatcrai prefrontal cortex, anterior cingulate, as well as bilaterally in inferior parietal and occipitomedial cortex.6 There were negative correlations between OB and CMRglu bilaterally in the hippocampus and caudate nucleus, and left amygdala and ventral striatum (Figure 4A Figure 4).
Figure 4. Correlations between the three dimensions of the APZ questionnaire for altered states of consciousness (oceanic boundlessness [OB], anxious ego-dissolution [AED], and visionary restructuralization [VR]) and regional brain activity (cerebral metabolic rate of glucose [CMRglu]) in healthy volunteers under psilocybin (0.26 mg/kg PO)orS-ketamine(0.012 mg/kg/min IV) challenge (n=52, P<0.0001). A. The OB dimension. The activation of a pref rontal-parietal network in parallel with the deactivation of a striato-limbic-amygdala-centered network correlated with the OB dimesion measuring derealization and depersonalization associated with positive emotions ranging from enhanced mood to feelings of happiness and serenity, or grandiosity. B. The AED dimension. Thalamic hyperactivity in conjunction with decreased activity in orbitofrontal and ventral anterior cingulate cortex and left putamen correlated with the AED dimension measuring thought disorder and ego-disintegration, and loss of self-control variously associated with anxiety, panic, and paranoid ideations. C. The VR dimension. Activation of the dorsolateral prefrontal cortex (DLPC) and of components of the dorsal (inferioparieta! cortex [IPC], angular gyrus [GA], supramarginal gyrus [GS]) and ventral stream (inferiotemporal cortex [ITC]) of higher order visual processing (ITC) in parallel with deactivation of strital and limbic regions correlated with VR comprising visual hallucinations, synesthesias and changed meaning of percepts. Positive correlations are indicated by circles and negative correlations by rectangles. FMG, f rontomedial gyrus; FSG, frontosuperior gyrus; IPL, inferiorparietal lobe; OCM, occipitomedial cortex; CAU, caudate nucleus; NAC, nucleus accumbens; AMY, amygdala; HIPP, hippocampus; OF, orbitofrontal cortex; AC, anterior cingulate; PUT, putamen; TMG, temporomedial gyrus; THAL, thalamus; GL, lingual gyrus; GF, fusiform gyrus; GPE, globus pallidus; ParaHipp, parahippocampus.
The OB dimension, which relates to the altered perception of time and space as well as the pleasurable experience of dissolution of ego-boundaries and which can culminate in transcendental or “mystical” states, substantially relates to functional alterations in an extended frontolimbic-parieto-striatal network including the amygdala. Indeed, according to current views, in conjunction with parietal and limbic areas, the frontal cortex is critical for the construction and maintenance of a coherent self. In its executive faculty, the frontal cortex, including the anterior cingulate, has an active role in structuring time, directing attention to relevant exteroceptive or interoceptive stimuli, and initiating and expressing appropriate behaviors.53-55 The parietal cortex is important for determining the relationship of the self to extrapersonal space, based on visuospatial input from the dorsal stream of visual information processing.56 Together with motor and somatosensory cortical areas, the frontolimbic-parietal network is sometimes called “central neural authority”57 to express the idea that it constitutes a functional system crucially involved in ego-structuring processes and the formation and representation of a coherent self that is defined in time and space. On the basis of these theoretical concepts, it appears plausible that overstimulation of the central neural authority may lead to profound alterations of sclf-cxpericncc and space/time perception, as reflected by the increased OB scores in hallucinogen-induced ASC. Finally, the concomitant decrease in amygdala activity may account for the more pleasurable experiences associated with the OB dimension.
The severity of anxious ego-dissolution (AED) was positively correlated with CMRglu in the thalamus and left temporomedial gyrus, and negatively correlated with CMRglu bilaterally in orbitofrontal cortex and adjacent anterior cingulate. Thus, it appears that AED and the associated thought disorder depend mainly on thalamic overactivity and orbitofrontal underactivity (Figure 4B Figure 4). This finding may indicate enhanced thalamic transmission and support the view that deficient thalamic gating leads to sensory overload of the cortex and psychosis. In fact, thalamic (and anterior cingulate-parietal) overactivity was associated with disorganization in schizophrenic patients.58 Malfunction of the orbitofrontal cortex may account for the continuing intrusion of irrelevant stimuli into the stream of mental activity and lead to the perseverations, thought blocking, and difficulty concentrating that are typically associated with AED.59
The severity of VR (including hallucinations) was positively correlated with CMRglu in the left dorsolateral prefrontal and inferior temporal cortex, bilaterally in temporo-parietal association cortex. Negative correlations were found in left globus pallidus and parahippocampus, and bilaterally in visual pathways (gyrus fusiformis and lingualis). Thus, it appears that visual hallucinations are associated with abnormal prefrontal activation in conjunction with activation of sensory modality-specific cerebral structures involved in normal perception, which is similar to the situation reported in patients with auditory hallucinations (Figure 4C Figure 4).60
Hyperfrontality as an index of acute psychoses
The hyperfrontality finding and its association with positive psychotic symptoms seen in drug-induced ASC is of particular interest because it appears to parallel similar findings in some studies in acutely ill schizophrenic and nonschizophrenic psychotic patients.36,38,61,62 Interestingly, one of these studies reported that hyperperfusion in the frontal, anterior cingulate, parietal, and temporal cortices, which correlates with positive symptoms including formal thought disorder and grandiosity in drugnaive schizophrenic patients, was normalized after neuroleptic treatment, and that persisting negative symptoms correlated with frontal, cingulate, basal, and thalamic hypoperfusion.38 An activation of prefrontal and cingulate cortex with transient exacerbation of positive psychotic symptoms was also reported in chronic schizophrenics during ketamine challenge.63 These findings suggest that metabolic hyperfrontality (rather than hypofrontality, as seen in chronic schizophrenia) is a pathophysiological manifestation of certain acute psychotic symptoms in drug-induced and naturally occurring psychoses. This view is further supported by the finding that pretreatment with the atypical antipsychotic clozapine reduced 5-ketamine-induced hyperfrontality and thalamic activation associated with psychotic symptoms in normal volunteers.64 In the light of such evidence, it would be expected that drugs that reduce or prevent excessive prefrontal activation might be useful for treating positive and cognitive symptoms of schizophrenia.
Convergence on neurotransmitter systems
The hyperfrontality common to the psilocybin and ketamine models of psychoses also supports the idea, that psychedelic hallucinogens and psychotomimetic NMDA antagonists may mediate some of their effects through a common final pathway or neurotransmitter system, downstream of their primary locus of action. In particular, the similarity of the effects of psilocybin and ketamine on ego functions, cognition, and perception underscore recent animal and human findings suggesting a convergence in their behavioral effects, despite the differences in their primary mechanisms of action.
Of particular relevance to sensory overload theories of drug-induced ASC are behavioral measures of sensorimotor gating functions, such as PPI of the startle response.65 Hie cross-species study of homologue gating functions such as PPI in animal and human models of psychosis offers a unique possibility for the exploration of neurobiological substrates relevant to schizophrenia. Symptomatic schizophrenics and never-medicated firstepisode schizophrenia patients exhibit deficits in PPI, which have been suggested to be central to the psychotic symptomatology of the illness.42,66 Indeed, the most striking correlate of deficient PPI in schizophrenia is a measure of thought disorder derived from the Rorschach test.67 Similarly, in rats, both serotonergic hallucinogens and NMDA antagonists produce deficits in PPI.68 Extensive pharmacological studies in animals demonstrate that PPI is modulated by multiple interacting neurotransmitters, including the dopaminergic, serotonergic, cholinergic, GABAergic, and glutamatergic systems within CSPT pathways.46
Role of dopamine
In keeping with the DA hyperactivity hypothesis of schizophrenia, we hypothesized that increased striatal DA activity could also contribute to the 5-ketamineand psilocybin-induced symptomatology in humans, although 5-ketamine and psilocybin have no affinity for D2 receptors.69,70 This hypothesis has been tested using PET and [nC]raclopride. Reduction in [“C]raclopride binding potential (BP) has been well established as an indirect measure of the change in synaptic DA concentration in animal and human studies.71,72 Indeed, both S ketamine and psilocybin significantly reduced [nC]raclopride BP in ventral striatum consistent with an increase in striatal DA concentration.73,74 Moreover, these changes in fnC]raclopridc BP significantly correlated with depersonalization, supporting the view that excessive DA transmission at D2 receptors contributes to the generation of positive psychotic symptoms in ketamine- and psilocybin-treated subjects. However, the DA-mediated change in [11C]raclopride BP at D2 receptors explained only about 36% of the variance of positive symptoms, indicating that other neurotransmitter systems contribute to the pathogenesis of ketamine- and psilocybininduced symptomatology. In support of this view, we found that the D2 antagonist haloperidol has virtually no effect on psilocybin-induced cognitive impairments and reduced psychotic symptoms by only about 30% in psilocybin-treated subjects.12 Similarly, recent results in healthy subjects demonstrate that ketamine psychosis is not ameliorated by haloperidol pretreatment:41 Comparably, haloperidol had also virtually no effect on the PPI-disruptive effect of the hallucinogenic 5-HT2 agonist DOT and the NMDA antagonist PCP in animal models of psychosis.65,75 Given these findings, it appears that increased DA activity may play a minor role in both psilocybin- and ketamine-induced ASC.
Role of serotonin
During the last decade, accumulating evidence from binding, electrophysiological, and behavioral studies in animals suggested that indoleamine and phenylethylamine hallucinogens may produce their psychological effects via the 5-HT2A receptors in the brain (for details, see references 76 and 77). However, although the preponderance of evidence suggested that hallucinogens are agonists at 5-HT2A receptors, this issue was clouded by studies that demonstrated LSD to be a partial agonist78 or even an antagonist79 at 5-HT2A receptors. Moreover, since LSD, 5-methoxy-DMT, DMT, and psilocin have been shown to display high affinity for, and to act as agonists at, 5-HT1A receptors, the role of 5-HT1A and 5-HT2A receptors in the generation of hallucinosis in man remains elusive.
The important question as to whether serotonergic hallucinogens are agonists or antagonists at 5-HT2A and 5-HT2C receptors has recently been answered. Consistent with animal studies, we have demonstrated that the psychological effects of psilocybin in humans can be completely blocked by the preferential 5-HT2A antagonist ketanserin.12 In addition, preliminary data demonstrate that the metabolic hyperfrontality and PPI disruptive effects of psilocybin in humans can be reversed by ketanserin.71,81 Since ketanserin has no affinity for 5-HT1A receptors, this finding suggests that serotonergic hallucinogens produce their central effects through a common action upon 5-HT2 receptors. The fact that ketanserin has about 100-fold greater antagonistic potency at 5-HT2A than at 5-TTT2C receptors indicates that the psychological effects of psilocybin are mediated by 5-HT2A rather than 5-HT2C receptor activation. This interpretation is corroborated by the finding that the highly selective 5-HT2A receptor antagonist M100,907, but not 5-HT2C antagonists, blocks the disruption of PPI in rats produced by serotonergic hallucinogens.82,83 Moreover, the effects of serotonergic hallucinogens (ESD and DOI) on sensorimotor gating in rats are mediated, at least in part, through 5-HT2A receptors located within the ventral pallidum,83,84 a component of the CSPT loop.85 These findings suggest that both indolamine and phenylethylamine hallucinogens may alter thalamic filter functions through 5-HT2A receptors associated with paleostriatal input to the thalamus. They also support the view that antagonist actions at the 5HT2A receptors may have an important contribution to the unique clinical efficacy of atypical antipsychotics such as clozapine in the treatment of the schizophrenias.86
Although psychotomimetic NMDA antagonists (eg, ketamine) act primarily through a noncompetitive NMDA blockade of the NMDA subtype of the glutamate receptor, there is converging evidence implicating 5-HT mechanisms, particularly those involving 5-HT2A receptors, in the action of NM'DA antagonists. For example, it has been shown that the psychological effects of ketamine arc ameliorated by the mixed 5-HT2/D2 and atypical antipsychotic clozapine, but are virtually insensitive to typical antipsychotics that have preferential actions at D2 receptors, such as haloperidol.87 Moreover, preliminary data from our laboratory show that clozapine reduces 5-ketamine-induced metabolic hyperfrontality and associated psychotic symptoms in healthy human volunteers.64,80 These findings parallel observations in animal studies demonstrating that the PPI -disruptive effects of NMDA antagonists in rats are blocked by the atypical antipsychotics (eg, clozapine or olanzapine),88,89 but are generally insensitive to typical antipsychotics (eg, haloperidol).90. Moreover, the fact that the highly selective 5-HT2A receptor antagonist Ml100,907 is also effective in blocking the PPI-disruptive effects of NMDA antagonists in rats91 strongly suggests that the psychotomimetic effects of NMDA antagonists in humans involve 5-HT2 receptor activation. Finally, studies in rats have indicated that the NMDA antagonists produce these gating deficits by actions within particular parts of the CSPT circuitry, including the frontal cortex and hippocampus.92 Interestingly, NMDA antagonists, like serotonergic hallucinogens,85 appear to be ineffective when administered directly into the DA-rich nucleus accumbens.92
Role of glutamate
Recent electrophysiological studies have produced new evidence that both psychedelic hallucinogens and NMDA antagonists activate the serotonergic system and enhance glutamatergic transmission via non-NMDA receptors in the frontal cortex.93,94 Whether this common mechanism contributes to the higher-level cognitive, perceptual, and affective effects of serotonergic hallucinogen and NMDA antagonists warrants further investigation.40
Taken together, serotonergic hallucinogens and psychotomimetic NM..DA antagonists produce schizophrenia-like deficits in behavioral measures of sensory gating such as PPI, and do so by actions localized to different parts of the CSPT circuitry. Despite their different primary mechanisms and sites of action, however, a common denominator of the effects of these drug classes is that they alter the dynamics of the integrated CSPT circuitry such that normal information processing is distorted by deficits in fundamental forms of sensorimotor gating.
Serotonergic amphetamines; MDMA
Psychological effects
In contrast to serotonergic hallucinogens and NMDA antagonists, a typical recreational and nontoxic dose of MDMA (1.5-7 mg/kg PO) produces an affective state of enhanced mood, profound well-being, happiness, increased extroversion and sociability, slight derealization and depersonalization, little anxiety, and moderate thought disturbances, but no hallucinations in normal volunteers.95 Depersonalization phenomena are mild and, in contrast to hallucinogens (eg, psilocybin), not experienced as problematic or psychotic fusion, but experienced as a pleasurable state of loosened ego boundaries as measured by the APZ questionnaire (Figure 2). Similar findings were reported with MDMA and its congener MDE in healthy volunteers.96-100
Brain imaging studies
To identify the functional neuroanatomy involved in the action of MDMA in humans, the effect of MDMA (1.7 mg/kg) versus placebo on regional cerebral blood flow (C.BF) was investigated in MDMA-naive human subjects using PET and [H2 15O]-PET.101 M.DMA moderately increased brain activity as indexed by CBF bilaterally in the ventromedial prefrontal cortex, the ventral anterior cingulate, the inferior temporal lobe, and the medial occipital cortex and in the cerebellum. Decreases in CBF were found bilaterally in the motor and somatosensory cortex, the superior temporal lobe, the dorsal cingulate cortex, the insula, and the thalamus. Unilateral decreases were found in the left amygdala, and the right parahippocampus. This activation pattern and associated affective state, which was characterized by heightened mood, increased extroversion, slight derealization, and intensification of vision, substantially differ from those seen in ketamine- and psilocybin-induced psychosis-like syndromes.
The activation of prefrontal and related limbic/paralimbic structures in conjunction with deactivation of the amygdala may underlie the emotional effects of MDMA. This view is consistent with findings implicating the amygdala,102,103 orbitofrontal cortex,103 ventral anterior cingulate cortex,103,104 prefrontal cortex, temporal lobe, and thalamus104 in the regulation of mood and emotion. In this network, the amygdala appears to play a pivotal role in the mediation of both positive and negative emotions.102,103,105 Acute administration of M..DMA also facilitated social communication, as measured by a significant increase in the “extroversion” subscale of the Adjective Mood Rating Scale. This increase correlated with CBF in the temporal cortex, amygdala, and orbitofrontal cortex. These brain regions are richly interconnected and together form the basolateral circuit which, according to current theories, is involved in the mediation of social communication.106,107 Lesions or disturbances of this circuit can lead to decreased social interaction, inadequate social behavior, or even the inability to decode social cues.108-108 The marked modulation of activity in the basolateral circuit produced by M.DMA and its association with increased extroversion provide further support for a critical role of the basolateral circuit in the processing of socially relevant information.
The present findings suggest that an amygdala-centered network including ventral-frontal and temporal cortices underlies the cooccurrence of pleasurable emotion and enhanced social communication, providing a rationale for the interrclatcdness of emotional and social processes. Thus, further research into the neurochemical mechanisms of MDMA could advance our understanding of the neuroanatomical regulation of mood and social interaction.
Neurotransmitter systems involved in the effects of MDMA
On the basis of mechanistic studies in animals, it has been widely assumed that the psychological effects of MDMA in humans might be mediated through its potent ability to release serotonin, and to a lesser extent DA.111 In addition, MDMA has moderate affinity for the serotonergic 5-HT2 and adrenergic α2 receptors.76 To elucidate the contribution of neurotransmitter and receptor systems in the action of MDMA, the blocking effects of specific receptor antagonists on MDMA-induced psychological and behavioral alterations were investigated. In these studies, we found that pretreatment with the selective serotonin-reuptake inhibitor (SSRI) citalopram markedly reduced all of the psychological effects of MDMA in healthy volunteers, indicating that the effects of MDMA in humans are largely due to 5-HT transporter-mediated enhanced 5-HT release.112 The 5-HT2 antagonist ketanserin only moderately attenuated the MDM'A experience, but significantly abolished the perceptual effects.113 This suggests that stimulation of 5-HT2 receptors mediates the mild hallucinogen-like action of MDMA in humans, such as intensification of colors. Finally, the D2 antagonist haloperidol only partly reduced the euphoric effects of MDMA suggesting that DA contributes little to the psychological effects of MDMA at the dose tested.114,115
Surprisingly, MDMA dose-dependently reduced sensorimotor gating, as indexed by the PPI of startle in rats, but increased PPI in healthy human subjects under comparable conditions.116 This disparity between the effects of MDMA in rats and humans may reflect a speciesspecific difference in the mechanism of action of MDMA or in the behavioral expression of a similar pharmacological effect, or both. In accordance with animal studies, we recently demonstrated that this PPIenhancing effect of MDMA in normals is markedly reduced by the SSRI citalopram, but is not affected by the D2 antagonist haloperidol or the 5-HT2A/C antagonist ketanserin.117 Thus, it appears that the effect of MDMA on PPI in humans is - like in animals - due to MDMA-induced release of serotonin. However, it is also obvious that some of the functional consequences of the released serotonin differ between rats and humans, since MDMA has opposite effects on PPI. In fact, there is more recent evidence that species-specific differences may contribute to the opposite effects of MDMA on PPI in rats and humans. Specifically, it was found that 5-HT1A agonists disrupt PPI in rats, but increase PPI in mice.118,119 Thus, the role of 5-HT1A receptors in mediating effects of MDMA on PPI in humans remains to be elucidated. Furthermore, whether the indirect agonistic effects of MDMA on 5-HT1A receptors ameliorate psychotic symptom formation needs to be clarified. The present data also demonstrate the compelling need for comparison studies in animals and humans to increase our understanding of the role of the serotonergic systems involved in the regulation of information processing in health and disease.
Conclusions
The present review discussed evidence that similar neural systems are altered by serotonergic hallucinogens and psychotomimetic NMDA antagonists, despite the differences in the primary sites of action of these drug classes. Furthermore, these same systems appear to exhibit abnormalities in incipient stages of naturally occurring psychoses. Thus, the elucidation of common mechanisms downstream from 5-HT2A or NMDA receptors can provide new targets for investigating the pathophysiology of naturally occurring psychoses such as schizophrenia. Present evidence suggests that the effects of a typical recreational dose of MDMA on regional brain activity and sensory gating functions can be delineated from those seen with psychedelic hallucinogens. The data also indicate that excessive serotonergic activation is not sufficient to produce psychosis.
Select abbreviations and acronyms
- AED
anxious ego-dissolution
- ASC
altered states of consciousness
- CMRglu
cerebral metabolic rate of glucose
- CSPT
cortico-striato-pallido-thalamic
- CSTC
cortico -striata -thalamo -cortical
- DA
dopamine
- DMT
N.N-dimethyltryptamine
- DOI
2,5-dimethoxy-4-iodoamphetamine
- 18FDG
18F -fluor odeoxy glucose
- 5-HT
5 -hydroxy try ptamine
- LSD
d-lysergic acid diethylamide
- MDE
3,4-methylenedioxyethamphetamine
- MDMA
3,4-methylenedioxymethamphetamine
- NMDA
N-methyl-D-aspartate
- OB
oceanic boundlessness
- PCP
phencyclidine
- PPI
prepulse inhibition
- VR
visionary restructuralization
The author would like to thank M. F. I. Vollenweider-Scherpenhuyzen, MD, for critical comments on the manuscript; the subjects who participated in these studies; his collaborators from the University of California San Diego (Mark A. Geyer, PhD, Martin Paulus, MD), the University of Groningen (K. L. Leenders, MD), and the PET Center University of Zurich (Alfred Buck, MD); and David E. Nichols, PhD for reading the manuscript and providing chemical structure drawings. Some of the work summarized here was supported by the Swiss National Science Foundation (SNF 32-040900, 32-53001.97, and 31-52989.97), the Swiss Federal Office of Health (BAG: 316.98.0686 and 316.98.0724), and the Heffter Research Institute, USA (Grant HRI-02.99).
The author would like to dedicate this article to Dr Albert Hofmann on the occasion of his 95th birthday.
REFERENCES
- 1.Leuner H. Halluzinogene: Psychische Grenzzustànde in Forschung und Thérapie. 1st ed. Bern, Switzerland: Hans Huber; 1981 [Google Scholar]
- 2.Dittrich A. The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry. 1998;31(suppl 2):80–84. doi: 10.1055/s-2007-979351. [DOI] [PubMed] [Google Scholar]
- 3.Schultes RE., Hofmann À. The Botany and Chemistry of Hallucinogens. 2nd ed. Springfield, III: Charles C Thomas; 1980 [Google Scholar]
- 4.Gouzoulis E., Hermle L., Thelen B., Sass H. History, rationale and potential of human experimental hallucinogenic drug research in psychiatry. Pharmacopsychiatry. 1998;31:63–68. doi: 10.1055/s-2007-979348. [DOI] [PubMed] [Google Scholar]
- 5.Hermle L., Oepen G., Spitzer M. Zur Bedeutung der Modellpsychosen. Fortschr Neurol Psychiatr. 1988;56:48–58. doi: 10.1055/s-2007-1001217. [DOI] [PubMed] [Google Scholar]
- 6.Vollenweider FX. Advances and pathophysiological models of hallucinogen drug actions in humans: a preamble to schizophrenia research. Pharmacopsychiatry. 1998;31(suppl 2):92–103. doi: 10.1055/s-2007-979353. [DOI] [PubMed] [Google Scholar]
- 7.Dittrich À. Àtiologie-unabhàngige Strukturen verànderter Wachbewusstseinszustànde. 2nd ed. Stuttgart, Germany: VWB-Verlag fur Wissenschaft und Bildung; 1996 [Google Scholar]
- 8.Dittrich À., von Arx S., Staub S. International study on altered states of consciousness (ISASC). Summary of the results. German J Psychol. 1985;9:319–339. [Google Scholar]
- 9.Vollenweider FX., Leenders KL., Scharfetter C., et al. Metabolic hyperfrontality and psychopathology in the ketamine model of psychosis using positron emission tomography (PET) and [F-18]-fluorodeoxyglocose (FDG) Eur Neuropsychopharmacol. 1997;7:9–24. doi: 10.1016/s0924-977x(96)00039-9. [DOI] [PubMed] [Google Scholar]
- 10.Vollenweider FX., Antonini A., Leenders KL., Oye I., Hell D., Angst J. Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (fi)-ketamine in healthy volunteers measured by FDG-PET. Eur Neuropsychopharmacol. 1997;7:25–38. doi: 10.1016/s0924-977x(96)00042-9. [DOI] [PubMed] [Google Scholar]
- 11.Vollenweider FX., Leenders KL., Scharfetter C., Maguire P., Stadelmann O., Angst J. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology. 1997;16:357–372. doi: 10.1016/S0893-133X(96)00246-1. [DOI] [PubMed] [Google Scholar]
- 12.Vollenweider FX., Vollenweider-Scherpenhuyzen MFI., Bàbler A., Vogel H., Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9:3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- 13.Bowers MB., Freedman DX. “Psychedelic” experiences in acute psychoses. Arch Gen Psychiatry. 1966;15:240–248. doi: 10.1001/archpsyc.1966.01730150016003. [DOI] [PubMed] [Google Scholar]
- 14.Heimann H. Models of experience and behavior in psychotic disorders. Pharmacopsychiatry. 1986;19:128–133. doi: 10.1055/s-2007-1017170. [DOI] [PubMed] [Google Scholar]
- 15.Keeler MH. Similarity of schizophrenia and the psilocybin syndrome as determined by objective methods. IntJ Neuropsychiatry. 1965;1:630–634. [PubMed] [Google Scholar]
- 16.Martindale C., Fischer R. The effects of psilocybin on primary process content in language. Confin Psychiatr. 1977;20:195–202. [PubMed] [Google Scholar]
- 17.Rûrnmele W., Gnirss F. Untersuchungen mit Psilocybin, einer psychotropen Substanz aus Psilocybe Mexicana. Schweiz Arch Neurol Psychiatr. 1961;87:365–385. [PubMed] [Google Scholar]
- 18.Bowers MB. Pathogenesis of acute schizophrenic psychosis. Arch Gen Psychiatry. 1968;15:240–248. doi: 10.1001/archpsyc.1968.01740090092009. [DOI] [PubMed] [Google Scholar]
- 19.Chapman J. The early symptoms of schizophrenia. Br J Psychiatry. 1966;112:225–251. doi: 10.1192/bjp.112.484.225. [DOI] [PubMed] [Google Scholar]
- 20.Hermle L., Spitzer M., Borchardt D., Gouzoulis E. Beziehungen der Modell- bzw. Drogenpsychosen zu schizophrenen Erkrankungen. Fortschr Neurol Psychiatr. 1992;60:383–392. doi: 10.1055/s-2007-1000662. [DOI] [PubMed] [Google Scholar]
- 21.Gouzoulis E., Hermle L., Sass H. Psychedelische Erlebnisse zu Beginn produktiver Episoden endogener Psychosen. Nervenarzt. 1994;65:198–201. [PubMed] [Google Scholar]
- 22.Klosterkotter J., Ebel H., Schultze-Lutter F., Steinmeyer EM. Diagnostic validity of basic symptoms. Eur Arch Psychiatr Clin Neurosci. 1996;246:147–154. doi: 10.1007/BF02189116. [DOI] [PubMed] [Google Scholar]
- 23.Freedman B., Chapman LJ. Early subjective experiences in schizophrenic episodes. J Abnorm Psychol. 1973;82:46–54. doi: 10.1037/h0034952. [DOI] [PubMed] [Google Scholar]
- 24.McCabe MS., Fowler RC., Cadoret RJ., Winokur G. Symptom differences in schizophrenia with good and poor prognosis. Am J Psychiatry. 1972;128:1239–1243. doi: 10.1176/ajp.128.10.1239. [DOI] [PubMed] [Google Scholar]
- 25.Gouzoulis-Mayfrank E., Habermeyer E., Hermle L., Steinmeyer AM., Kunert HJ., Sass H. Hallucinogenic drug-induced states resemble acute endogenous psychoses: results of an empirical study. Eur Psychiatry. 1998;13:399–406. doi: 10.1016/S0924-9338(99)80686-5. [DOI] [PubMed] [Google Scholar]
- 26.Hermle L., Gouzoulis E., Spitzer M. Blood flow and cerebral laterality in the mescaline model of psychosis. Pharmacopsychiatry. 1998;31:85–91. doi: 10.1055/s-2007-979352. [DOI] [PubMed] [Google Scholar]
- 27.Gouzoulis-Mayfrank E., Schreckenberger M., Sabri O., et al. Neurometabolic effects of psilocybin, 3,4-methylenedioxyethylamphetamine (MDE) and d-rnethamphetamine in healthy volunteers. Neuropsychophannacology. 1999;20:565–581. doi: 10.1016/S0893-133X(98)00089-X. [DOI] [PubMed] [Google Scholar]
- 28.Crystal JH., Karper LP., Seibyl JP., et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 29.Krystal JH., Bennett A., Abi-Saab D., et al. Dissociation of ketamine effects on rule acquisition and rule implementation: possible relevance to NMDA receptor contributions to executive cognitive functions. Biol Psychol. 2000;47:137–143. doi: 10.1016/s0006-3223(99)00097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malhotra AK., Finals DA., Weingartner H., et al. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology. 1996; 14:301–307. doi: 10.1016/0893-133X(95)00137-3. [DOI] [PubMed] [Google Scholar]
- 31.Krystal JH., Karper LP., D'Souza DC., et al. Interactive effects of subanesthetic ketamine and subhypnotic lorazepam in humans: psychotomimetic. perceptual, cognitive and neuroendocrine responses. Psychopharmacology. 1998;135:213–229. doi: 10.1007/s002130050503. [DOI] [PubMed] [Google Scholar]
- 32.Collier BB. Ketamine and the conscious. mind. Anaesthesia. 1972;27:120–137. doi: 10.1111/j.1365-2044.1972.tb08186.x. [DOI] [PubMed] [Google Scholar]
- 33.Malhotra AK., Finals DA., Adler CM., et al. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychophannacology. 1997;17:141–150. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- 34.Lahti AC., Weiler MA., Michaelidis T., Parwanl A., Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25:455–467. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- 35.Sprague JE., Everman SL., Nichols DE. An intergrated hypothesis for the serotonergic axonal loss induced by 3,4-methylenedioxymethamphetamine. Neurotoxicology. 1998;19:427–441. [PubMed] [Google Scholar]
- 36.bmeier KP., Lawrie SM., Blackwood DH., Johnstone EC., Goodwin GM. Hypofrontality revisited: a high resolution single photon emission computed tomography study in schizophrenia. J Neurol Neurosurg Psychiatry. 1995;58:452–456. doi: 10.1136/jnnp.58.4.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parellada E., Catafau AM., Bernardo M., Lomena F., Gonzâlez-Monclûs E., Setoain J. Prefrontal dysfunction in young acute neuroleptic-naive schizophrenic patients: a resting and activation SPECT study. Psychiatry Res Neuroimaging. 1994;55:131–139. doi: 10.1016/0925-4927(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 38.Sabri O., Erkwoh R., Schreckenberger M., Owega A., Sass H., Buell U. Correlation of positive symptoms exclusively to hyperperfusion or hypoperfusion of cerebral cortex in never-treated schizophrenics. Lancet. 1997;349:1735–1739. doi: 10.1016/S0140-6736(96)08380-8. [DOI] [PubMed] [Google Scholar]
- 39.Liddle PF., Friston KJ., Frith CD., Frackowiak RSJ. Cerebral blood flow and mental processes in schizophrenia. J R Soc Med. 1992;85:224–227. doi: 10.1177/014107689208500415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anand A., Charney DS., Oren DA., et al. Attenuation of the neuropsychiatrie effects of ketamine with lamotrigine: support for hyperglutamatergic effects of A/-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry. 2000;57:270–276. doi: 10.1001/archpsyc.57.3.270. [DOI] [PubMed] [Google Scholar]
- 41.Krystal JH., D'Souza DC., Karper LP., et al. Interactive effects of subanesthetic ketamine and haloperidol in healthy humans. Psychopharmacology. 1999;145:193–204. doi: 10.1007/s002130051049. [DOI] [PubMed] [Google Scholar]
- 42.Braff DL., Geyer MA. Sensorimotor gating and schizophrenia: human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- 43.Geyer MA., Braff DL. Startle habituation and sensorimotor gating in schizophrenia and related animal models. Schizophr Bull. 1987;13:643–668. doi: 10.1093/schbul/13.4.643. [DOI] [PubMed] [Google Scholar]
- 44.McGhie A., Chapman J. Disorders of attention and perception in early schizophrenia. Br J Med Psychol. 1961;34:103–116. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- 45.Karper LP., Freeman GK., Grillon C., Morgan CA., Charney DS., Krystal JH. Preliminary evidence of an association between sensorimotor gating and distractibility in psychosis. J Neuropsychiatry Clin Neurosci. 1996;8:60–66. doi: 10.1176/jnp.8.1.60. [DOI] [PubMed] [Google Scholar]
- 46.Swerdlow NR., Geyer MA. Using an animal model of deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia. Schizophr Bull. 1998;24:285–301. doi: 10.1093/oxfordjournals.schbul.a033326. [DOI] [PubMed] [Google Scholar]
- 47.Swerdlow NR., Koob GF. Dopamine, schizophrenia, mania, and depression: toward a unified hypothesis of cortico-striato-pallido-thalamic function. Behav Brain Sci. 1987;10:197–245. [Google Scholar]
- 48.Carlsson M., Carlsson A. Schizophrenia: a subcortical neurotransmitter imbalance syndrome? Schizophr Bull. 1990;16:425–432. doi: 10.1093/schbul/16.3.425. [DOI] [PubMed] [Google Scholar]
- 49.Vollenweider FX., Geyer MA. A systems model of altered consciousness: integrating natural and drug-induced psychoses. Brain Res Bull. 2001;56:495–507. doi: 10.1016/s0361-9230(01)00646-3. [DOI] [PubMed] [Google Scholar]
- 50.Vollenweider FX. The use of psychotomimetics in schizophrenia research with special emphasis on the PCP/ketamine model psychosis [in German], Sucht. 1992;38:389–409. [Google Scholar]
- 51.Breier A., Malhotra AK., Finals DA., Weisenfeld NI., Pickar D. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Pychiatry. 1997;154:805–811. doi: 10.1176/ajp.154.6.805. [DOI] [PubMed] [Google Scholar]
- 52.Hermle L., Fûnfgeld M., Oepen G., et al. Mescaline-induced psychopathological, neuropsychological, and neurometabolic effects in normal subjects: experimental psychosis as a tool for psychiatric research. Biol Psychiatry. 1993;32:976–991. doi: 10.1016/0006-3223(92)90059-9. [DOI] [PubMed] [Google Scholar]
- 53.Fuster JM. The Prefrontal Cortex. 2nd ed. New York, NY: Raven Press; 1989 [Google Scholar]
- 54.Mllner B., Pertrides M., Smith ML. Frontal lobes and the temporal organisation of memory. Hum Neurobiol. 1985;4:137–142. [PubMed] [Google Scholar]
- 55.Posner Ml., Petersen SE. The attention system of the human brain. Ann. Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 56.Mllner AD., Goodale MA. Visual pathways to perception and action. Prog Brain Res. 1993;95:317–337. doi: 10.1016/s0079-6123(08)60379-9. [DOI] [PubMed] [Google Scholar]
- 57.Hernegger R. Wahrnehmung und Bewusstsein. Ein Diskussionsbeitrag zur Neuropsychologie. 1st ed. Berlin, Germany: Spectrum Akademischer Verlag; 1995 [Google Scholar]
- 58.Liddle PF., Friston K., Frith C., Hirsch S., Frackowiak R. Patterns of regional cerebral blood flow in schizophrenia. Br J Psychiatry. 1992;160:179–186. doi: 10.1192/bjp.160.2.179. [DOI] [PubMed] [Google Scholar]
- 59.Schnider A., Treyer V., Buck A. Selection of currently relevant memories by the human posterior medial orbitofrontal cortex. J Neurosci. 2000;20:5880–5884. doi: 10.1523/JNEUROSCI.20-15-05880.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weiss AP., Heckers S. Neuroimaging of hallucinations: a review of the literature. Psychiatry Res. 1999;92:61–74. doi: 10.1016/s0925-4927(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 61.Cleghorn JM., Garnett ES., Nahmias C., et al. Increased frontal and reduced parietal glucose metabolism in acute untreated schizophrenia. Psychiatry Res. 1989;28:119–133. doi: 10.1016/0165-1781(89)90040-1. [DOI] [PubMed] [Google Scholar]
- 62.Catafau AM., Parellada E., Lomena FJ., et al. Prefrontal and temporal blood flow in schizophrenia: resting and activation technetium-99mHMPAO SPECT patterns in young neuroleptic-naive patients with acute disease. . J Nucl Med. 1994;35:935–941. [PubMed] [Google Scholar]
- 63.Lahti AC., Holcomb HH., Medoff DR., Tamminga CA. Ketamine activates psychosis and alters limbic blood flow in schizophrenia. Neuroreport. 1995;6:869–872. doi: 10.1097/00001756-199504190-00011. [DOI] [PubMed] [Google Scholar]
- 64.Vollenweider FX., Bâchle D., Leenders KL., Missimer J., Hell D. 5-HT receptor activation: a common denominator of the NMDA antagonist and 5HT2A agonist models of psychosis? Biol Psychiatry. 2000;47(suppl):129S–130S. [Google Scholar]
- 65.Geyer MA. Behavioral studies of hallucinogenic drugs in animals: implications for schizophrenia research. Pharmacopsychiatry. 1998;31:73–79. doi: 10.1055/s-2007-979350. [DOI] [PubMed] [Google Scholar]
- 66.Ludewig K., Ludewig S., Vollenweider FX. Decreased habituation of the acoustic startle reflex in unmedicated first episode schizophrenia. Pharmacopsychiatry. 2001;5:187. [Google Scholar]
- 67.Perry W., Geyer MA., Braff DL. Sensorimotor gating and thought disturbance measured in close temporal proximity in schizophrenic patients. Arch Gen Psychiatry. 1999;56:277–281. doi: 10.1001/archpsyc.56.3.277. [DOI] [PubMed] [Google Scholar]
- 68.Geyer MA., Swerdlow DL. Multiple transmitters modulate prepulse inhibition of startle: relevance to schizophrenia. In: Palomo T, Beninger RJ, Archer T, eds. interactive Monoaminergic Disorders. Madrid, Spain: Fundacion Cerebro y Mente; 1999:343–354. [Google Scholar]
- 69.Oye I., Hustveit O., Maurset A., Ratti Moberg E., Paulsen O., Skoglund LA. The chiral forms of ketamine as probes for NMDA-receptor function in humans. In: Kameyama T, Nabeshima T, Domino EF, eds. NMDA Receptor Related Agents; Biochemistry, Pharmacology And Behavior. Ann Arbor, Mich: NPP Books; 1991:381–389. [Google Scholar]
- 70.McKenna DJ., Repke DB., Peroutka SJ. Differential interactions of indolealkylamines with 5-hydroxytryptamine receptor subtypes. Neuropharmacology. 1990;29:193–198. doi: 10.1016/0028-3908(90)90001-8. [DOI] [PubMed] [Google Scholar]
- 71.Volkow ND., Fowler JS., Wang GJ., et al. Reproducibility of repeated measures of carbon-11C-raclopride binding in the human brain. . J Nucl Med. 1993;34:609–613. [PubMed] [Google Scholar]
- 72.Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 73.Vollenweider FX., Vontobel P., Hell D., Leenders KL. 5-HT modulation of dopamine release in basal ganglia in psilocybin-induced psychosis in man: a PET study with [“Cjraclopride. Neuropsychopharmacology. 1999;20:424–433. doi: 10.1016/S0893-133X(98)00108-0. [DOI] [PubMed] [Google Scholar]
- 74.Vollenweider FX., Vontobel P., Oye I., Hell D., Leenders KL. Effects of S-ketamine on striatal dopamine release: a [“Cjraclopride PET study of a model psychosis in humans. J Psychiatr Res. 2000;34:35–43. doi: 10.1016/s0022-3956(99)00031-x. [DOI] [PubMed] [Google Scholar]
- 75.Padich RA., McCloskey TC., Kehne JH. 5-HT modulation of auditory and visual sensorimotor gating. II. Effects of the 5-HT2A antagonist MDL 100,907 on disruption of sound and light prepulse inhibition produced by 5-HT agonists in Wistar rats. Psychopharmacology. 1996;124:107–116. doi: 10.1007/BF02245610. [DOI] [PubMed] [Google Scholar]
- 76.Nichols DE. Role of serotonergic neurons and 5-HT receptors in the action of hallucinogens. In: Baumgarten HG, Gôthert M, eds. Serotonergic Neurons and 5-HT Receptors in the CNS. Berlin, Germany: Springer Verlag; 1997:563–585. [Google Scholar]
- 77.Aghajanian GK., Marek GJ. Serotonin and hallucinogens. Neuropsychopharmacology. 1999;21(2 suppl):16S–23S. doi: 10.1016/S0893-133X(98)00135-3. [DOI] [PubMed] [Google Scholar]
- 78.Sanders-Bush E., Burries KD., Knoth K. Lysergic acid diethylamide and 2,5dimethoxy-4-methylamphetamine are partial agonists at serotonin receptors linked to phosphoinositide hydrolysis. J Pharmacol Exp Ther. 1988;246:924–928. [PubMed] [Google Scholar]
- 79.Pierce PA., Peroutka SJ. Antagonist properties of d-LSD at 5-hydroxytryptamine-2 receptors. Neuropsychopharmacology. 1990;3:503–508. [PubMed] [Google Scholar]
- 80.Vollenweider FX., Umbricht D., Geyer MA., Hell D. Effects of NMDAantagonists and 5-HT2A-agonists on prepulse inhibition in human volunteers. Schizophr Res. 2000;41:147. [Google Scholar]
- 81.Vollenweider FX., Leenders KL., Scharfetter C., Hell D. A systems model of altered consciousness: integrating natural and drug-induced psychoses. Eur J Psychiatry. 2000;15(suppl 2):318S. doi: 10.1016/s0361-9230(01)00646-3. [DOI] [PubMed] [Google Scholar]
- 82.Geyer MA., Krebs-Thomson K., Varty GB. The effects of M 100907 in pharmacological and developmental animal models of prepulse inhibition deficits in schizophrenia. Neuropsychopharmacology. 1999;21(6 suppl 2):134–142. [Google Scholar]
- 83.Sipes TE., Geyer MA. DOI disruption of prepulse inhibition of startle in the rat by 5-HT2Aand not by 5-HT2c receptors. Behav Pharmacol. 1995;6:839–842. [PubMed] [Google Scholar]
- 84.Ouagazzal AM., Grottick AJ., Moreau JL., Higgins GA. Effects of LSD on prepulse inhibition and spontaneous behavior in the rat: a pharmacological analysis and comparison between two rat strains. Neuropsychopharmacology. 2001;25:565–575. doi: 10.1016/S0893-133X(01)00282-2. [DOI] [PubMed] [Google Scholar]
- 85.Sipes TE., Geyer MA. DOI disrupts prepulse inhibition of startle in rats via 5-HT2A receptors in the ventral pallidum. Brain Res. 1997;761:97–104. doi: 10.1016/s0006-8993(97)00316-8. [DOI] [PubMed] [Google Scholar]
- 86.Meltzer HY., McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull. 1999;25:233–255. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- 87.Malhotra AK., Adler CM., Kennison SD., Elman I., Pickar D., Breier A. Clozapine blunts W-methyl-D-aspartate antagonist-induced psychosis: a study with ketamine. Biol Psychiatry. 1997;42:664–668. doi: 10.1016/s0006-3223(96)00546-x. [DOI] [PubMed] [Google Scholar]
- 88.Bakshi VP., Geyer MA. Antagonism of phencyclidine-induced deficits in prepulse inhibition by the putative atypical antipsychotic olanzapine. Psychopharmacology. 1995;122:198–201. doi: 10.1007/BF02246096. [DOI] [PubMed] [Google Scholar]
- 89.Swerdlow NR., Bakshi V., Waikar M., Taaid N., Geyer MA. Seroquel, clozapine and chlorpromazine restore sensorimotor gating in ketamine-treated rats. Psychopharmacology. 1998;140:75–80. doi: 10.1007/s002130050741. [DOI] [PubMed] [Google Scholar]
- 90.Keith V., Mansbach RS., Geyer MA. Failure of haloperidol to block the effects of phencyclidine and dizocilpine on prepulse inhibition of startle. Biol Psychiatry. 1991;30:557–566. doi: 10.1016/0006-3223(91)90025-h. [DOI] [PubMed] [Google Scholar]
- 91.Varty GB., Bakshi VP., Geyer MA. M100907, a serotonin 5-HT2A receptor antagonist and putative antipsychotic, blocks dizocilpine-induced prepulse inhibition deficits in Sprague-Dawley and Wistar rats. Neuropsychophannacology. 1999;20:311–321. doi: 10.1016/S0893-133X(98)00072-4. [DOI] [PubMed] [Google Scholar]
- 92.Bakshi VP., Geyer MA. Multiple limbic regions mediate the disruption of prepulse inhibition produced in rats by the noncompetitive NMDA antagonist dizocilpine. J Neurosci. 1998;18:8394–8401. doi: 10.1523/JNEUROSCI.18-20-08394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aghajanian GK., Marek GJ. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Brain Res Rev. 2000;31:302–312. doi: 10.1016/s0165-0173(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 94.Moghaddam B., Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 95.Vollenweider FX., Gamma A., Liechti ME., Huber T. Psychological and cardiovascular effects and short-term sequelae of MDMA (“Ecstasy”) on MDMA-naive healthy volunteers. Neuropsychopharmacology. 1998;19:241–251. doi: 10.1016/S0893-133X(98)00013-X. [DOI] [PubMed] [Google Scholar]
- 96.Gouzoulis-Mayfrank E., Thelen B., Habermeyer E., et al. Psychopathological, neuroendocrine and autonomic effects of 3,4-methylenedioxyethylamphetamine (MDE), psilocybin and d-methamphetamine in healthy volunteers. Psychopharmacology. 1999;142:41–50. doi: 10.1007/s002130050860. [DOI] [PubMed] [Google Scholar]
- 97.Gouzoulis-Mayfrank E., Thelen B., Maier S., Habermeyer E., Spitzer M., Sass H. Modulation of semantic priming effects by psilocybin, MDE (ecstasy), and d-methamphetamine. Neurol Psychiatr Brain Res. 1998;6:19–28. [Google Scholar]
- 98.Greer G., Tolbert R. Subjective reports on the effects of MDMA in a clinical setting. J Psychoactive Drugs. 1986;18:319–327. doi: 10.1080/02791072.1986.10472364. [DOI] [PubMed] [Google Scholar]
- 99.Mas M., Magi F., De la Torre R., et al. Cardiovascular and neuroendocrine effects and pharmacokinetics of 3, 4-methylenedioxymethamphetamine in humans. J Pharmacol Exp Ther. 1999;290:136–145. [PubMed] [Google Scholar]
- 100.Hermle L., Spitzer M., Borchardt D., Kovar KA., Gouzoulis E. Psychological effects of MDE in normal subjects. Are entactogens a new class of psychoactive agents? Neuropsychopharmacology. 1993;8:171–176. doi: 10.1038/npp.1993.19. [DOI] [PubMed] [Google Scholar]
- 101.Gamma A., Buck A., Berthold T., Hell D., Vollenweider FX. 3,4-Methylenedioxymethamphetamine (MDMA) modulates cortical and limbic brain activity as measured by (H2:“0)-PET in healthy humans. Neuropsychopharmacology. 2000;23:388–395. doi: 10.1016/S0893-133X(00)00130-5. [DOI] [PubMed] [Google Scholar]
- 102.Schneider F., Gur RE., Mozley LH., et al. Mood effects on limbic blood flow correlate with emotional self-rating: a PET study with oxygen-1 5 labeled water. Psychiatry Res Neuroimaging. 1995;61:265–283. doi: 10.1016/0925-4927(95)02678-q. [DOI] [PubMed] [Google Scholar]
- 103.Ketter TA., Andreason PJ., George MS., et al. Anterior paralimbic mediation of procaine-induced emotional and psychosensory experiences. Arch Gen Psychiatry. 1996;53:59–69. doi: 10.1001/archpsyc.1996.01830010061009. [DOI] [PubMed] [Google Scholar]
- 104.George MS., Ketter TA., Parekh PI., Horwitz B., Herscovitch P., Post RM. Brain activity during transient sadness and happiness in healthy women. Am J Pychiatry. 1995;152:341–351. doi: 10.1176/ajp.152.3.341. [DOI] [PubMed] [Google Scholar]
- 105.Le Doux JE. Emotion: clues from the brain. Ann Rev Psychol. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- 106.Brothers L. Brain mechanisms of social cognition. . J Psychopharmacol. 1996;10:2–8. doi: 10.1177/026988119601000102. [DOI] [PubMed] [Google Scholar]
- 107.Deakin JFW. 5-HT, antidepressant drugs and the psychosocial origins of depression. J Psychopharmacol. 1996;10:31–38. doi: 10.1177/026988119601000106. [DOI] [PubMed] [Google Scholar]
- 108.Franzen EA., Myers RE. Neural control of social behavior: prefrontal and anterior temporal cortex. Neuropsychologia. 1973;11:141–157. doi: 10.1016/0028-3932(73)90002-x. [DOI] [PubMed] [Google Scholar]
- 109.Raleigh MJ., Steklis HD. Effects of orbital frontal and temporal neocortical lesions on affiliative behavior of vervet monkeys . (Cercopithecus aethiops sabaeus). Exp Neurol. 1981;73:378–389. doi: 10.1016/0014-4886(81)90273-9. [DOI] [PubMed] [Google Scholar]
- 110.Adolphs R., Tranel D., Damasio AR. The human amygdala in social judgement. Nature. 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- 111.Geyer MA., Callaway CW. Behavioral pharmacology of ring-substituted amphetamine analogs. In: Cho AK, Segal DS, eds. Amphetamine and Its Analogs: Psychopharmacology, Toxicology, and Abuse. San Diego, Calif: Academic Press; 1994:177–208. [Google Scholar]
- 112.Liechti ME., Baumann C., Gamma A., Vollenweider FX. Acute psychological effects of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) are attenuated by the serotonin uptake inhibitor citalopram. Neuropsychopharmacology. 2000;22:513–521. doi: 10.1016/S0893-133X(99)00148-7. [DOI] [PubMed] [Google Scholar]
- 113.Liechti ME., Saur MR., Gamma A., Hell D., Vollenweider FX. Psychological and physiological effects of MDMA (“Ecstasy”) after pretreatment with the 5-HT2 antagonist ketanserin in healthy humans. Neuropsychopharmacology. 2000;23:396–405. doi: 10.1016/S0893-133X(00)00126-3. [DOI] [PubMed] [Google Scholar]
- 114.Liechti ME., Vollenweider FX. Acute psychological and physiological effects of MDMA (“Ecstasy”) after haloperidol pretreatment in healthy humans. Eur Neuropsychopharmacol. 2000;10:289–295. doi: 10.1016/s0924-977x(00)00086-9. [DOI] [PubMed] [Google Scholar]
- 115.Frei E., Gamma A., Pascual-Marqui RD., Lehmann D., Hell D., Vollenweider FX. Localization of MDMA-induced brain activity in healthy volunteers using low resolution brain electromagnetic tomography (LORETA) Hum Brain Mapping. 2001;14:152–165. doi: 10.1002/hbm.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vollenweider FX., Remensberger S., Hell D., Geyer MA. Opposite effects of 3,4-methylenedioxymethamphetamine (MDMA) on sensorimotor gating in rats versus healthy humans. Psychopharmacology. 1999;143:365–372. doi: 10.1007/s002130050960. [DOI] [PubMed] [Google Scholar]
- 117.Liechti ME., Geyer MA., Hell D., Vollenweider FX. Effects of 3,4-methylenedioxy-W-methylamphetamine (MDMA) on prepulse inhibition and habituation of startle in humans after pretreatment with citalopram, haloperidol, or ketanserin. Neuropsychopharmacology. 2001;24:240–252. doi: 10.1016/S0893-133X(00)00199-8. [DOI] [PubMed] [Google Scholar]
- 118.Sipes TA., Geyer MA. 8-OH-DPAT disruption of prepulse inhibition in rats: reversal with (+)WAY 100,135 and localization of site of action. Psychopharmacology. 1995;117:41–48. doi: 10.1007/BF02245096. [DOI] [PubMed] [Google Scholar]
- 119.Dulawa SC., Hen R., Scearce-Levie K., Geyer MA. Serotonin 1B receptor modulation of startle reactivity, habituation, and prepulse inhibition in wild-type and serotonin 1B receptor knockout mice. Psychopharmacology. 1997;132:125–134. doi: 10.1007/s002130050328. [DOI] [PubMed] [Google Scholar]