Abstract
Subtle alterations in brain development caused by genes or early environmental hazards, such as obstetric complications, play a role in projecting some individuals on a trajectory toward schizophrenia. High-risk and cohort studies demonstrate that children destined to develop schizophrenia tend to have delayed milestones and subtle neuromotor and cognitive impairments (particularly in coordination and language). These neurocognitive problems lead to difficulties in interpersonal relations, and their progressive alienation makes these at-risk children more likely to harbor odd or paranoid ideas. This cascade of increasingly deviant development may then be compounded by brain maturational changes during adolescence with a resultant lability of the dopaminergic response to stress. As a result, the individual is more susceptible to the effects of the abuse of dopamine-releasing drugs, and to other risk factors such as migration or stressful life events; social isolation may be a common pathway underlying several of the social risk factors.
Keywords: genes, imaging, neurodevelopment, prenatal risk factor, relatives, risk factor, schizophrenia
Abstract
Alteraciones sutiles del desarrollo cerebral, causadas por genes y daños ambientales tempranos (como por ejemplo complicaciones obstétricas), parecen jugar un papel clave en proyectar ciertos individuos hacia el desarrollo de esquizofrenia. Estudios de cohortes y de sujetos con alto riesgo demuestran que aquellos niños destinados a sufrir esquizofrenia tienen retrasos del desarrollo y sutiles déficits motores y cognitivos (especialmente en coordinación y lenguaje). Estos problemas neurocognitivos causan dificultades en interacciones sociales y el aislamiento progresivo que estos niños experimentan aumenta sus probabilidades de generar ideas paranoides o extrañas. Esta cascada de desarrollo anómalo progresivo probablemente se complementa con alteraciones de la maduración cerebral durante la adolescencia, resultando en una mayor labilidad de los sistemas dopaminérgicos de respuesta al estrés. En consecuencia, estos jóvenes son más vulnerables al abuso de drogas liberadoras de dopamina, así como a otros factores de riesgo como la migración o los acontecimientos vitales estrestantes. El aislamiento social podría ser una vía común para diversos factores de riesgo socioambientales.
Abstract
Des altérations discrètes du développement cérébral d'origine génétique ou liées à des facteurs de risque environnementaux précoces, tels que les complications obstétricales, interviennent en propulsant certains sujets sur la trajectoire menant vers la schizophrénie. Des études de cohorte et portant sur les risques élevés montrent que les enfants prédestinés à la schizophrénie ont tendance à présenter un retard touchant les grandes étapes du développement psychomoteur ainsi que des altérations neuromotrices et cognitives discrètes (touchant en particulier la coordination et le langage). Ces désordres entraînent des difficultés dans leurs relations interpersonnelles et leur aliénation mentale progressive fait que ces enfants à risque sont plus enclins à exprimer des idées bizarres ou paranoïdes. Cet enchaînement de développements déviants croissants pourrait alors être aggravé par des modifications de la maturation cérébrale au cours de l'adolescence entraînant une instabilité de la réponse dopaminergique au stress. De ce fait, le sujet est plus sensible aux effets de l'emploi excessif de médicaments libérant de la dopamine et aux autres facteurs de risque tels que la migration ou les événements de vie stressants ; l'isolement social pourrait être une voie commune sous-jacente à plusieurs facteurs de risque sociaux.
The idea that psychosis can have a developmental origin was common in the latter part of the 19th century,1 but was subsequently displaced by Emil Kracpelin's view of “dementia praecox”2 as a deteriorating illness. In the 1980s, a number of research groups began to speculate that schizophrenia might indeed have a significant developmental component.3-6 In 1987, Murray and Lewis7 summarized the evidence in an editorial in the British Medical Journal entitled “Is schizophrenia a neurodevelopmental disorder?” In the years since then, researchers have increasingly answered the question in the affirmative, but have also become aware that the simple “neurodevelopmental” hypothesis fails to explain all the available data. Therefore, in this paper, we consider how new information has caused the original “doomed from the womb” hypothesis to evolve. We begin by discussing the strongest evidence implicating a role for deviant development, ie, that concerning the characteristics of prcschizophrenic children.
The antecedents of schizophrenia in childhood and adolescence
Neuropsychological development in childhood
Children who go on to develop schizophrenia tend to display early neurological and cognitive problems. The early research focused on children with a family history of psychosis. Indeed, Fish8 pointed out that the increased prevalence of neurological signs in multiple sensorimotors systems in the offspring of schizophrenics was consistent with an “inherited neurointegrative deficit.” High-risk studies concur showing that 25 % to 50 % of children born to mothers with schizophrenia have developmental abnormalities especially poor motor coordination in early childhood, and attention and information processing deficits later.9-11 Walker12 examinded home movies of preschizophrenic children and noted more postural and upper limb movement abnormalities than in their well siblings. These abnormalities were most noticeable in the first 2 years of life, and ameliorated the reafter, raising the possibility of ongoing recovery from an early lesion.13
A series of large birth cohort studies were published through the 1990s. The 1946 British Birth Cohort Study followed up 4 746 children for 43 years. The 30 children who were destined to develop schizophrenia, as a group, had delayed milestones (walking was delayed by 1.2 months), more speech problems, lower educational test scores, and a preference for solitary play.14
As part of the British National Child Development Study, Done et al15 compared the childhood social adjustment of 40 patients with schizophrenia, 35 with affective psychoses, 79 with neurotic disorders, and 1914 controls. Those who later developed schizophrenia had significantly more neurocognitive problems and social maladjustment than controls at the age of 7 years, especially if they were male. Children who went on to develop affective psychoses did not differ from controls. Preneurotic children, especially if female, manifested poorer social adjustment than controls at age 11.
Cannon et al16 compared elementary school records of 400 preschizophrenic children and 400 healthy controls born in Helsinki between 1951 and 1960. Poor performance in sports and handicrafts, which may indicate motor coordination deficits, were risk factors for schizophrenia. This finding is consistent with the high-risk and other birth cohort studies, and also the poor motor coordination seen on childhood videotapes.12,13
A prospective cohort study from Philadelphia compared 72 patients with schizophrenia, 62 of their unaffected relatives, and 7941 controls. Both the patients and their well relatives performed significantly worse than the nonpsychiatric controls (but did not differ from each other) on verbal and nonverbal cognitive tests at the ages of 4 and 7 years. Early social maladjustment, motor coordination deficits, and behavioral and language dysfunction (like echolalia, inappropriate laughter, or unintelligible speech) were significantly associated with both schizophrenia and sibling status. Hence, premorbid social, cognitive, and motor dysfunctions are significant indicators of vulnerability to schizophrenia, such vulnerability being the result of familial (genetic and shared environmental) factors.17-19
In the Dunedin (New Zealand) follow-up study, in which 761 children were regularly studied and followed up till the age of 26 years, the usual neurocognitive risk factors for schizophrenia were found. A child psychiatrist also interviewed the children at age 11 years, with a structured diagnostic interview searching for evidence of psychotic symptoms. Interestingly, those children who reported unusual quasi -psychotic experiences had a 16-fold increased risk for schizophreniform disorder at age 26 years. This study demonstrates a certain continuity of psychotic symptoms from childhood to adulthood and indicates that delusions often have their origins more than a decade before psychosis is formally diagnosed.20 It is also interesting in that this and several of the other cohort studies point to receptive language deficits as being particularly noticeable in preschizophrenic individuals.14,15,18,21 Much research indicates that auditory hallucinations are disorders of inner language.22,23 Perhaps it is not surprising that children who have impaired language perception are more prone to misperceive their own inner speech as voices in adult life.
Isohanni et al24 studied over 12 000 subjects from the 1966 Finnish birth cohort at the time of their 31st birthday. Using a national hospital register, they identified 100 cases of schizophrenia, 55 other psychoses, and 315 nonpsychotic disorders. Data on the ages at which subjects learned to stand, walk, and control bowel and bladder functions were compared across the groups. Delayed milestones increased the risk for later psychosis in a linear fashion (so early milestones implied protection). There was no such association between milestones and nonpsychotic disorders. Apart from the interesting “dose-response effect” described, this study suggests that the childhood precursors may be specific to psychotic disorders as opposed to conveying risk for general psychopathology.
Neuropsychological characteristics in late adolescent/early adult life
David et al25 investigated the association between 10 and the later development of psychosis in a cohort study of nearly 50 000 18-year-old males who were conscripted into theSwedish army in 1969 to 1970. By 1983, 195 subjects in the cohort had been admitted to hospital with schizophrenia and another 192 with nonschizophrenic psychosis. There was a highly significant association between low IQ scores and the subsequent development of schizophrenia. Indeed, the relationship between schizophrenia and IQ was linear, with risk gradually increasing as IQ fell at all levels of intellectual ability. The risk for nonschizophrenic psychoses was also higher in those with lower IQ, but the effect was less marked and nonlinear. The effect size of the low IQ risk factor exceeded that of any other known environmental risk factors. The association between low IQ and schizophrenia could be directly causal with cognitive impairment leading to false beliefs and perceptions. Alternatively, the association could be indirect with factors such as abnormal brain development increasing the risk for schizophrenia, and incidentally causing the lower IQ.
Davidson et al26 examined assessment scores for nearly 10 000 16- and 17-year-old boys entering the Israeli army Deficits in social functioning, organizational ability, and intellectual functioning predicted later hospitalization with schizophrenia. There was a linear relationship between IQ and risk for schizophrenia, and the authors suggest that low IQ is itself a causal factor increasing the risk for schizophrenia; it could act independently or be one of the means by which other genetic or environmental influences exert their effect, or both. The authors suggest that low IQ could compromise information processing, leading eventually to the psychopath ology of schizophrenia, or alternatively that high IQ may be protective.
Risk factors in early life
Obstetric complications
Many small case-control studies reported an excess of obstetric complications (OCs) among patients with schizophrenia. Most of these early data have been summarized in two meta-analyses. Firstly, Geddes and Lawrie27 confirmed an association between OCs and schizophrenia with an odds ratio of approximately 2. Secondly, Geddes et al28 examined 11 studies, which used the Lewis and Murray scale29 to interview mothers retrospectively about their offspring's gestation. Data were available for 700 patients with schizophrenia and 835 controls. Premature rupture of membranes, prematurity, and the use of resuscitation or incubator emerged as significant risk factors for schizophrenia.
There were many methodological criticisms of this early work. However, in the last few years, a number of large register-based longitudinal studies (summarized in Table I 30-43) have been published. Despite occasional inconsistencies, the new evidence overwhelmingly supports the notion that exposure to OCs is a risk factor for schizophrenia. Although the overall effect of OCs is modest, some studies suggest that the association may be stronger among male patients36,44 and among cases with an early onset,37,38,45,46 but not everyone believes this.43
Table I. Register-based studies of obstetric complications (OCs) and schizophrenia. NCPP, National Collaborative Perinatal Project; ECA, Epidemiologic Catchment Area; RR, relative risk; CI, confidence interval.30 .
| Study | Sample and method | Main findings |
| Jones et al,31 1998 | 1966-born cohort with 11 017 Finnish subjects. Linked psychiatric and obstetric case registers. FoIlow-up at age 28 years | 76 subjects had developed schizophrenia at age 28. Schizophrenic patients were significantly more likely than controls to have had perinatal brain damage or to be premature |
| Hultman et al,32 1999 | Swedish population-based cohort study. Comparison of obstetric records for 167 patient/control pairs linked to psychiatric register | Schizophrenic patients were more likely than controls to have OCs, especially if male. Schizophrenia was associated with multiparity and maternal bleeding in pregnancy |
| Dalman et al,33 1999 | Same cohort as Hultman et al.32 Follow-up of 507 516 children born in the late 1970s | 238 subjects developed schizophrenia during follow-up. Schizophrenia was associated with OCs. Preeclampsia was the strongest individual risk factor |
| Kendell et al,34 1996 | 115 case-control pairs from the Scottish population born in 1971 to 1974. Linked obstetric and psychiatric records | Schizophrenia was associated with a significant excess of OCs, especially preeclampsia and infants requiring hospital neonatal care |
| Kendell et al,35 2000 | Reanalysis of same Scottish sample34 | Selection bias was detected in the control group of the 1996 study. Reanalysis showed no significant association between OCs and schizophrenia |
| Byrne et al,36 2000 | Compared obstetric records of 431 patient/control pairs. Linked with psychiatric case register | Global rate of OCs did not differ between patients and controls. Male patients with a young onset had significantly more OCs than controls |
| Cannon et al,37 2000 | Prospective cohort study (NCPP). Comparison of 72 patients, 63 unaffected siblings, and 7 941 controls | The odds of schizophrenia increase linearly with increasing number of hypoxia-associated OCs |
| Rosso et al,38 2000 | 1955 Finnish birth cohort. 80 patients, 61 unaffected siblings, and 56 matched controls | Hypoxia-associated OCs significantly increase the odds of early-onset schizophrenia (but not of later-onset cases) |
| Zornberg et al,39 2000 | Prospective cohort study (NCPP). 603 individuals born between 1959 and 1966 were followed for 23 years after early childhood assessments | Hypoxic/ischemia-related fetal/neonatal OCs were associated with striking increase in risk for schizophrenia and other nonaffective psychoses (5.7 % vs 0.4 % for nonexposed) |
| Brown et al,40 2001 | Comparison of 70 young adults from the rubella-exposed 1964 birth cohort, against 1 510 unexposed controls from the ECA and the Albany/Saratoga studies | The subjects prenatally exposed to rubella had a substantially higher risk for nonaffective psychosis compared with unexposed controls (RR: 5.2) |
| Wahlbeck et al,41 2001 | Cohort study of 7 086 Finnish subjects. Linked obstetric and school health records with psychiatric register | Intrauterine and childhood malnutrition was associated with increased lifetime risk for schizophrenia |
| Dalman et al,42 2001 | Case-control studies recruiting from the Stockholm psychiatric register: 524 patients with schizophrenia and 1 043 controls matched by age, gender, hospital, and parish of birth. OC data were obtained from birth records | There was a strong association between signs of asphyxia at birth and schizophrenia with an odds ratio of 4.4 (95 % Cl: 1.9-10.3), after adjusting for other OCs, maternal history of psychosis, and social class |
| Thomas et al,43 2001 | The increased risk of schizophrenia associated with OCs (asphyxia in particular) was not significantly modified by gender, maternal history of psychosis, or age of onset. Limited statistical power |
The mechanism underlying the link between OCs and schizophrenia remains elusive, but recent long-term cohort studies with detailed obstetric information point to fetal/neonatal hypoxia.33,38,39 According to Cannon et al,37 the odds of schizophrenia increase linearly with an increasing number of hypoxic/ischemic complications. A plausible model is that those with a genetic liability to schizophrenia may be especially sensitive to the excitotoxic effects of hypoxia on the fetal/neonatal brain.37,47
Markers of prenatal deviant development
It is well established that the morphogeneses of the brain, the craniofacial region, and the epidermal ridges are intimately related. Minor physical anomalies (small alterations of ectodermal development, such as defects on the head, facial features, hands, and feet) are known to occur during the first and second trimesters of life.48 An increase in minor physical anomalies is a consistent finding among patients with schizophrenia49-51 and this has been interpreted as a marker of altered development.
Epidermal ridges appear on the hand between weeks 12 and 15 of life and after this period they remain unchanged.52 Dermatoglyphic abnormalities are found in excess in schizophrenia.53-55 Preliminary support for the idea they may be indirect indicators of aberrant brain development came from Van Os et al,56 who reported an association between dermatoglyphic ridge counts and cerebral structural abnormalities measured by magnetic resonance imaging (MRI) in patients with schizophrenia. Similarly, compared with controls, patients with schizophrenia present a dermatoglyphic deviance (excessive fluctuating asymmetry of ridge counts), which correlates with their excessive mixed handedness. These can be interpreted as two related markers pointing toward greater developmental instability in schizophrenia.57
Are the brain deviances in schizophrenia developmental in origin?
The most consistent deviances described in schizophrenia are an increase in ventricular size and subtle global and regional cortical volume reductions.58-62 Wright et al63 carried out a meta-analysis of 58 MRI studies, which included 1 588 patients with schizophrenia. The mean lateral ventricular volume was greater (126 %) than that of controls and the mean cerebral volume was smaller (98 %). Relative to the cerebral volume reductions, the regional volumes of the subjects with schizophrenia were 98 % for the frontal lobes, 94 % for the amygdala/hippocampus, and 96.5 % for the thalamus. Recently, voxel-based methods of analyzing structural MRI images have enabled the whole brain to be examined, and implicated particularly the medial temporal region, insula, and anterior cingulate.64,65
Certainly, brain changes are present near to the onset of the frank psychosis. This is demonstrated by the numerous reports of ventricular enlargement and other deviations in first-onset cases of schizophrenia66-68 and in earlyonset cases too.69,70 Furthermore, there is an excess of congenital lesions in patients with schizophrenia, such as aqueduct stenosis, arachnoid and septal cysts, and agenesis of the corpus callosum and cavum septum pellucidum.71,72 However, such abnormalities are distinctly unusual and the findings are generally much more subtle. Indeed, it is an open question whether these differences in brain structure found between people with schizophrenia and normal subjects are “brain abnormalities,” which are an intrinsic part of the disease process itself. Alternatively, they might be regarded as deviations within the normal range, which increase the risk of developing schizophrenia.
One piece of evidence does, however, point to fetal life. The normal brain is typically asymmetrical, and the development of brain asymmetry is usually complete by the middle of the third trimester of gestation. A number of studies revealed a reduced asymmetry in several brain areas in schizophrenia.73-75 A recent meta-analysis, reviewing handedness, language, and anatomical studies, confirms the decreased anatomical and functional cerebral lateralization in schizophrenia.76 This reduced asymmetry found in several brain structures in schizophrenia is likely therefore to originate during fetal life. Neuropathological studies have largely confirmed the neuroimaging data concerning gross anatomy,77 but there has been much less consistency concerning histopathologica! findings. Three influential papers reported cytoarchitcctonic findings suggestive of altered neuronal migration during fetal life.78-82 Unfortunately, none of these initial reports has been fully replicated.83-87 The other histopathological evidence frequently cited in support of a fetal origin of schizophrenia is the absence of gliosis in postmortem schizophrenic brain.88-90 Certainly, the absence of fibrillary gliosis strongly argues against an adult-onset degenerative process; however, it does not prove a developmental one.91 Thus, we can conclude that there are currently no replicated histopathological findings that unequivocally implicate aberrant neurodevelopmental in fetal life.
The causes of structural brain deviances in schizophrenia: nature or nurture?
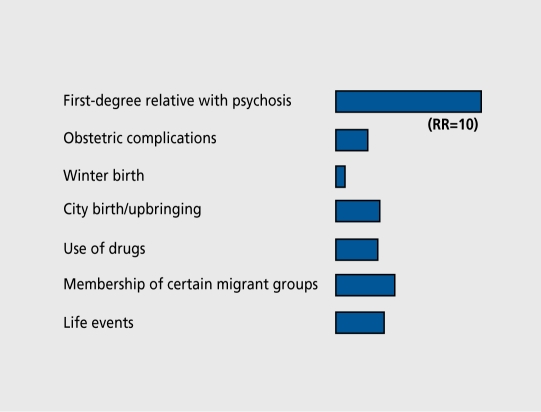
The environmental factors that we discussed earlier have only a modest risk-increasing effect, and generally operate in the context of genetic risk (Figure 1).92 The genetic predisposition to schizophrenia has been well established and, recently, two large twin studies have confirmed that a high proportion of the variance in liability to schizophrenia is due to additive genetic effects.93-95
Figure 1. Individual risk factors and their effect sizes. RR, relative risk. Modified from reference 92: Van Os J, Jones P, Sham P, Bebbington P, Murray RM. Psychosis as a continuum of variation in dimensions of psychopathology. In: Haffner H, Grattaz W, eds. Search for the Causes of Schizophrenia. Vol 4. Berlin, Germany: Springer: 1999:59-80. Copyright © 1999, Springer.
A number of investigators have asked whether the firstdegree relatives of people with schizophrenia show any of the same brain structural deviations as their schizophrenic kin. The Maudsley Family Study96 examined patients and well relatives in families with several schizophrenic members, ie, families assumed to transmit a high genetic load. Sharma et al96 carried out. MRI scans in patients, well relatives, and controls. They further divided the relatives into standard relatives and presumed “obligate carriers” (relatives who, although well themselves, have psychotic offspring and psychotic siblings or parents and therefore appear to be transmitting genetic risk). The so-called obligate carriers showed a similar increase in lateral ventricular volume to the patients themselves; other relatives were midway between patients and controls, as one might expect from a group in which some, but not all, carry susceptibility genes.97,98
Stefanis et al99 used the Maudsley Family Study samples to show that this is not the whole story. They compared hippocampal volumes in (i) people with familial schizophrenia, but no pregnancy or birth complications (OCs); (ii) people with schizophrenia with no family history but severe OCs; and (iii) controls. Reduction in the left hippocampal volume was associated with the diagnosis of schizophrenia, but this was accounted for by the patients with a history of severe OCs. Patients from the familial group did not differ from controls. This study confirms the work of McNeil et al100 showing that, decreased hippocampal volume in schizophrenia is in part, a consequence of early environmental damage and points toward one causal mechanism, ie, severe OCs lead to hypoxia, which causes left, hippocampal (and other cerebral) damage. This work contrasts with other studies that found that the unaffected relatives of people with schizophrenia have decreased hippocampal volume.101-103 It may be that the discrepancy lies in whether or not it was just the hippocampus or the hippocampal-amygdal complex that was measured.
Other early environmental effects
A slight, increase in risk for schizophrenia exists among individuals born in late winter/early spring.104,105 These results point towards an etiological agent acting during gestation, birth, or early childhood rather than around the time of onset. While some studies suggested this seasonal effect could be secondary to exposure to influenza in the uterus during winter ,45,106,107 other research failed to find such a link.108 Intrauterine rubella infection has also been put forward as a potential risk factor for schizophrenia:40,109 Buka et al110 studied blood samples of mothers of 27 cases with psychosis and 54 matched controls as part of the Providence Collaborative Perinatal Project. Maternal blood samples collected during pregnancies in the early sixties were retrieved and analyzed for evidence of perinatal pathogens capable of affecting brain development. The offspring of mothers who had elevated levels of IgG and IgM immunoglobulins and antibodies to herpes simplex type 2 during pregnancy were at increased risk of developing schizophrenia and other psychotic illnesses in adulthood.
Other potential early hazards described are maternal malnutrition,111 maternal diabetes mellitus,112 and maternal stress.“113,114 Finally, Rantakallio et al115 and Westergaard et al108 demonstrated that the window of opportunity for risk-increasing insults is wider than was previously thought, as those exposed to childhood viral central nervous system (CNS) infections were five times more likely to develop schizophrenia than those not exposed. There is also some evidence that brain injury in childhood may increase the risk of developing schizophrenia.116
What the simple neurodevelopmental model fails to explain
Why does damage occurring in early life cause symptoms only decades later?
Brain maturation is a prolonged process that continues until well after adolescence, so one possible explanation for the late onset of symptoms is that lesions could lie silent until maturation affects the neuronal circuits that were deviant, but normally not fonctional, in chilhood.117-121 Animal models have given some support to this view; Lipska et al120 reported that hippocampal lesions made to newborn rats remained relatively silent until adult life.
However, an alternative model suggests that at Ieast some of the crucial developmental events occur in adolescence. Recent neuropathological studies report a decrease in the volume of the cortex, an increase in neuronal density, and also loss of synaptic markers, findings compatible with synaptic loss.122-124 Keshavan and colleagues125 have used magnetic resonance spectroscopy (MRS) to show that schizophrenics show a phosphomonoesterase pattern suggestive of failure of new synapse production and excessive synaptic reduction. They suggest that the ventricular enlargement and cortical volume decrement may arise in part from an excess of the normal cortical pruning that occurs in normal adolescence.126 This has been christened the “late” (as opposed to “early”) neurodevelopmental model.127
This model could perhaps explain the seemingly contradictory findings concerning whether brain deviance in schizophrenia occurs prior to illness onset, or is progressive.128,129 The initial evidence suggested the former, but in recent years there have been suggestions that some cerebral ventricular enlargement and volumetric reductions in the temporal lobes in schizophrenia, may have a progressive course,130,131 which is possibly limited in time.132 Lieberman et al133 examined 107 first-onset cases, of which 51 were followed over 1 to 6 years. They confirmed the presence of ventricular enlargement and hippocampal volume reductions at the time of the first episode. In addition, they reported a progressive increase in ventricular volume in those patients with poor outcome only, but found no further reductions in cortical or hippocampal volumes on follow-up. Another longitudinal study focusing on childhood-onset schizophrenia, reported that brain abnormalities were progressing during adolescence, but became stable in early adulthood.134 Further work from the same group using high-resolution MRI scans showed that, compared with 12 matched controls, 12 adolescent early-onset patients had accelerated gray matter loss over a 5-year follow-up period. The authors interpret the dynamics of their anatomical findings in the light of family and twin imaging studies. They conclude that early neurodevelopmental deviances and later gray-matter loss are probably related and influenced by common genes, while they also support the notion that nongenetic triggers contribute to the onset and initial progression of the illness.135 Possibly, first-onset patients presenting in childhood or adolescence may show the brain structural consequences of synaptic pruning, but those samples that, comprise adult patients may show little change. This is an interesting view, but the supporting evidence so far is sparse. Allin and Murray136 point out that many questions remain on the progression of brain morphology deviances in schizophrenia. Finding answers will require larger samples, controlling for the interactions of clinical outcome and medication, and longer follow-up periods.
Later-onset schizophrenia
As noted earlier, a key difficulty for the “early” neurodevelopmental model is why the onset, of frank psychosis does not occur until several decades after the supposed developmental lesion(s).The “late” developmental model attempts to explain the onset in the second decade of life by invoking brain maturational events in adolescence, but, of course, cases with such an onset remain a minority. People develop schizophrenia throughout adult life into old age, and late-onset patients show relatively normal premorbid adjustment.137 Furthermore, according to Howard et al,138 the relatives of late-onset psychosis cases seem to carry less genetic loading for schizophrenia and are at higher risk for affective disorders. A potential explanation is that patients with late-onset schizophrenia may in fact have a different illness, possibly with etiological factors in common with affective psychosis; a second possibility is that the symptoms may arise from brain degeneration.139
What factors influence the age of onset, of psychosis? Onset is generally earlier in males than in females.137,140 Furthermore, those patients with a family history of schizophrenia tend to have an earlier age of onset than cases with less genetic risk,141,142 regardless of their gender.143 As noted earlier, OCs may also be associated with early onset,36,-45,144 as are other indicators of aberrant neurodevelopment, such as premorbid cognitive and behavioral deficits, minor physical anomalies, smaller brains, and larger cerebral ventricles.145,146
One may conclude that the role of neurodevelopmental impairment is most, marked in early-onset schizophrenia, but it becomes progressively less obvious in patients with increasing age of onset. In other words, only a proportion of the variance in liability to schizophrenia can be attributed to impaired brain development.
Is schizophrenia more than a brain disorder? The role of social risk factors
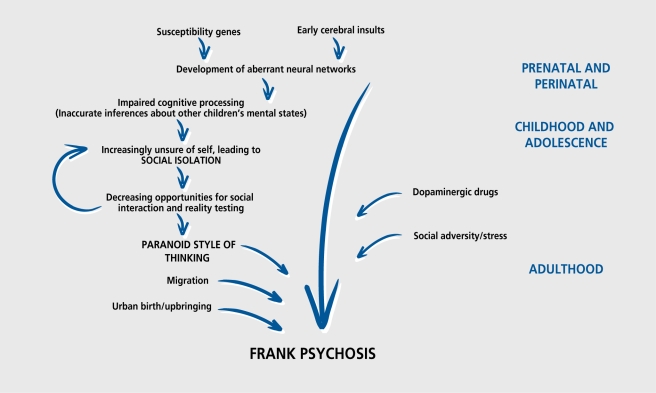
The view that, schizophrenia, is simply a brain disorder remained unchallenged from the late 1970s to the late 1990s. Thus, the simple neurodevelopmental model implied that, schizophrenic symptoms are simply a consequence of the development of aberrant neural networks.147 However, there is increasingly robust evidence that social risk factors play a crucial role in the development, of schizophrenia (Figure 2).30 However, for most social risk factors, while there is a clear association with schizophrenia, the direction of causality has not been demonstrated.
Figure 2. The cascade of increasingly abnormal function that culminates in the onset of full-blown psychosis, including the main risk factors for psychosis over life.30 .
Could an adverse upbringing convey higher risk for schizophrenia?
In the British 1946 Birth Cohort, those 4-year-old children rated as having a poor mother-child relationship had a 6-fold increase in risk for schizophrenia, later in life. Of course, this does not tell us whether poor mothering was a causal risk factor, or whether the preschizophrenic child was so deviant as to be unable to form a close bond with the mother.14 However, children of schizophrenic mothers who were adopted away and then reared in adverse circumstances have a higher risk than those brought, up in loving homes by stable adoptive parents.148 Furthermore, Mirsky et al149 noted that children with known genetic risk for schizophrenia were more likely to develop the disorder if they lived on a kibbutz, rather than in a family home. Overall, kibbutz children did not have a higher risk, suggesting that high-risk children carry a genetic vulnerability to the social environment.
The effect of being born or brought up in a city
Several studies in the 1990s indicated that being born or brought up in a city increases the risk for schizophrenia.150 In one of the most impressive, Mortensen et al151 examined a Danish national sample and showed that the relative risk for schizophrenia, associated with urban birth was 2.4 and that, there was a dose-response relationship (the larger the town of birth, the greater the risk) suggesting a causal effect. Mortensen and colleagues pointed out that because so many people are born and live in cities, a relatively small increase in risk would cause a large increase in the numbers of people with the disease. Indeed, they calculated that the population-attributable risk (PAF) for urban birth was 34.6 %, compared with 9 % or 7 % for having a mother or father with schizophrenia, respectively. The estimate of PAF appears robust, as a similar PAF has previously been reported in data from another country,152 where, in addition, it was shown that the effect of urban birth/upbringing is not confounded by urban residence in adult life.153 Finally, in a further analysis of the above Danish study, Pederscn and Mortensen154 have shown that the association between urbanization and schizophrenia is based on continuous or repeated exposures during upbringing (not just, urban birth), and that there is indeed a dose-response relationship between urban upbringing and risk for schizophrenia.
The risk associated with isolation
The Swedish conscript study discussed above also looked at the interaction of premorbid personality and social isolation. Young men who felt, they were more sensitive than their peers, had fewer than two close friends, and did not have a girlfriend had an increased risk of later developing the disorder.155 Once again this raises the question of whether these characteristics are an expression of a schizoid or schizotypal personality or whether they are in themselves independent risk factors. Until proven otherwise, it is wise to consider that both may be true, ie, individuals with a schizoid or schizotypal personality may be less able to make social relationships, and then the social isolation itself may cause them to become increasingly deviant. Van Os et al156 found that people who were single had a slightly higher risk of developing psychosis if they lived in a neighborhood with fewer single people, compared with a neighborhood with many other single people. The authors suggested that single status might, give rise to perceived (or actual) social isolation if most other people are living with a partner. The question of whether social isolation may increase the risk for schizophrenia (or rather whether a close relationship may be protective) is also raised by Jablensky et al,157 who showed that marriage had a protective effect for males, and that this was not simply a consequence of better-adjusted males being able to marry.
The migration effect
As far back as the 1930s, Odegaard158 noted that Norwegian migrants to the USA were at increased risk for schizophrenia, while as recently as 1999, Mortensen et al151 reported that children born in Greenland to Danish mothers had a relative risk of 3.7 for schizophrenia. However, the most, striking findings have come from the UK, where numerous studies have reported an increased incidence of schizophrenia among African-Caribbean people.159 Misdiagnosis,160 drug abuse,161 and increased neurodevelopmental insult162-164 have been largely ruled out as possible explanations. A high genetic predisposition seems unlikely, since the increased risk is not shared by those living in the Caribbean.165 Indeed, Hutchinson et al166 found that morbid risks for schizophrenia were similar for parents and siblings of white and first-generation African-Caribbean patients. However, morbid risk for siblings of second-generation African-Caribbean psychotic probands was approximately 7 times higher than that for their white counterparts. This study, which almost exactly replicates the work of Sugarman and Craufurd,167 suggests the operation of an environmental agent, that is operating on this population in the UK, but not in the Caribbean; social isolation and alienation are plausible candidates. Finally, Boydell et al168 noted that the incidence of schizophrenia, in migrants is greatest when they live in areas with few other migrants; again one possible explanation is relative isolation and lack of social support.
Stressful life events
Three prospective studies have found an association between life events and onset, of psychosis.169-171 Stressful life events in the 3 weeks preceding onset or relapse seemed important, although the effect size was greater in affective psychosis than in schizophrenia. However, it is difficult, to exclude the possibility that some of these events may have been caused by the patient, and thus reflect his/her inherited personality characteristics.
Further environmental risks: the impact of drugs
It is well known that abuse of dopamine-releasing drugs such as amphetamines and cocaine can precipitate psychosis; cannabis appears to have similar risk-increasing effects, though over a longer period of time. Again, there is the question of whether individuals with certain genotypes may selectively expose themselves to drugs to whose psychotogenic effects they are particularly vulnerable. For example, in the Swedish conscript, study discussed above, Andreasson et al172 found that heavy cannabis consumption at the age of 18 was associated with a 6-fold increased risk of developing schizophrenia over the next 13 years. The dose-response relationship suggested causality. But might, the 18-year-olds have been taking cannabis because they were already disturbed? Over half of those who admitted to heavy cannabis use at age 18 already had a psychiatric diagnosis. However, even when these individuals were excluded, cannabis consumption remained a risk factor for later psychosis. This finding has recently been replicated in a cohort, of children followed up in Dunedin (New Zealand). Cannabis consumption at age 15 years was associated with a significantly increased risk for later schizophreniform disorder. Again there was an interaction with psychiatric symptoms, so that those who had shown mild but quasi-psychotic ideas at age 11 years were especially vulnerable to the risk-increasing effects of cannabis. As in the Swedish study, the effect of cannabis consumption remained significant when this was taken into account.173
McGuire161 et al showed that the relatives of patients with cannabis-associatcd psychosis had an increased morbid risk themselves. Chen174 noted similar findings for methamphetamine use in a large Taiwanese sample. Those methamphetamine abusers who developed a psychosis were distinguished from those who did not by a greater frequency of schizoid and schizotypal traits in childhood and by having more relatives affected with schizophrenia. Thus, it may be that some individuals abuse drugs because they already have psychiatric problems and, among them, it is those who have a genetic predisposition to psychosis who are particularly likely to develop a schizophreniform psychosis.
Conclusion
Genetic epidemiological and molecular studies both imply that liability to schizophrenia is inherited not through a single major gene, but through a number of genes of small effect. Some of those genes are likely to be involved in the control of neurodevelopment175 and some are probably shared with other psychotic conditions such as bipolar disorder.94 Studies of the relatives of patients with schizophrenia, indicate that families transmit minor developmental deviations, which are relatively innocent in themselves; for example, slight, alterations in brain structure,96 in neurophysiology,176 or in neurocognition.97 However, when a child is unlucky enough to inherit several of these traits, and is also exposed to environmental insults to the developing brain, such as OCs, then their cumulative effect puts that individual on a trajectory of increasing deviance. Well-designed, whole-population-based studies have confirmed that children destined to develop schizophrenia show an excess of minor emotional, neuromotor, and cognitive difficulties, and that these lead them to become increasingly isolated from their peers and to harbor odd ideas. There arc some interesting but as yet unconfirmed claims that this early developmental deviance may then be compounded by maturational brain changes during adolescence, which result in a lability of the dopaminergic response to stress. The developmentally compromised individual is then especially vulnerable to (and indeed may selectively expose him/herself to) certain stresses during adolescent or adult, life, such as abuse of drugs and social adversity, especially isolation.
Contributor Information
Elvira Bramon, Division of Psychological Medicine, Institute of Psychiatry, London, UK.
Robin M. Murray, Division of Psychological Medicine, Institute of Psychiatry, London, UK.
REFERENCES
- 1.Clouston T. The Neuroses of Development. Edinburgh, UK: Oliver & Boyd. 1891 [Google Scholar]
- 2.Kraepelin E. Dementia praecox. The Clinical Roots of the Schizophrenia Concept. 5th ed. Leipzig, Germany: Cambridge University Press. 1896:426–441. [Google Scholar]
- 3.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982-1983;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 4.Schulsinger F., Parnas J., Petersen ET., et al. Cerebral ventricular size in the offspring of schizophrenic mothers - a preliminary study. Arch Gen Psychiatry. 1984;41:602–606. doi: 10.1001/archpsyc.1984.01790170076008. [DOI] [PubMed] [Google Scholar]
- 5.Murray RM., Lewis SW., Reveley AM. Towards an etiological classification of schizophrenia. Lancet. 1985;1:1023–1026. doi: 10.1016/s0140-6736(85)91623-x. [DOI] [PubMed] [Google Scholar]
- 6.Weinberger D. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 7.Murray RM., Lewis SW. Is schizophrenia a neurodevelopmental disorder? BMJ (Clin Res Ed). 1987;295:681–682. doi: 10.1136/bmj.295.6600.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fish B. Neurobiological antecedents of schizophrenia in childhood. Arch Gen Psychiatry. 1977;34:1297–1313. doi: 10.1001/archpsyc.1977.01770230039002. [DOI] [PubMed] [Google Scholar]
- 9.Amminger GP., Pape S., Rock D., et al. Relationship between childhood behavioral disturbance and later schizophrenia in the New York High-Risk Project. Am J Psychiatry. 1999;156:525–530. doi: 10.1176/ajp.156.4.525. [DOI] [PubMed] [Google Scholar]
- 10.Erlenmeyer-Kimling L., Rock D., Roberts SA., et al. Attention, memory, and motor skills as childhood predictors of schizophrenia-related psychoses. The New York High-Risk Project. Am J Psychiatry. 2000;157:1416–1422. doi: 10.1176/appi.ajp.157.9.1416. [DOI] [PubMed] [Google Scholar]
- 11.Ott SL., Allen J., Erlenmeyer-Kimling L. The New York High-Risk Project. Observations on the rating of early manifestations of schizophrenia. Am J Med Genet. 2001;105:25–27. [PubMed] [Google Scholar]
- 12.Walker EF. Neurodevelopmental aspects of schizophrenia. Schizophr Res. 1993;9:151–152. [Google Scholar]
- 13.Walker EF., Lewine RRJ., Neumann C. Childhood behavioral characteristics and adult brain morphology in schizophrenia. Schizophr Res. 1996;22:93–101. doi: 10.1016/s0920-9964(96)00070-9. [DOI] [PubMed] [Google Scholar]
- 14.Jones P., Rodgers B., Murray R., Marmot M. Child developmental risk-factors for adult schizophrenia in the British 1946 Birth Cohort. Lancet. 1994;344:1398–1402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- 15.Done DJ., Crow TJ., Johnstone EC., Sacker A. Childhood antecedents of schizophrenia and affective-illness - social adjustment at ages 7 and 11. BMJ. 1994;309:699–703. doi: 10.1136/bmj.309.6956.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannon M., Jones P., Huttunen MO., et al. School performance in Finnish children and later development of schizophrenia - a population-based longitudinal study. Arch Gen Psychiatry. 1999;56:457–463. doi: 10.1001/archpsyc.56.5.457. [DOI] [PubMed] [Google Scholar]
- 17.Cannon TD., Bearden CE., Hollister JM., Rosso IM., Sanchez LE., Hadley T. Childhood cognitive functioning in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophr Bull. 2000;26:379–393. doi: 10.1093/oxfordjournals.schbul.a033460. [DOI] [PubMed] [Google Scholar]
- 18.Bearden CE., Rosso IM., Hollister JM., Sanchez LE., Hadley T., Cannon TD. A prospective cohor study of childhood behavioral deviance and language abnormalities as predictors of adult schizophrenia. Schizophr Bull. 2000;26:395–410. doi: 10.1093/oxfordjournals.schbul.a033461. [DOI] [PubMed] [Google Scholar]
- 19.Rosso IM., Bearden CE., Hollister JM., et al. Childhood neuromotor dysfunction in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophr Bull. 2000;26:367–378. doi: 10.1093/oxfordjournals.schbul.a033459. [DOI] [PubMed] [Google Scholar]
- 20.Poulton R., Caspi A., Moffitt TE., Cannon M., Murray R., Harrington H. Children's self-reported psychotic symptoms and adult schizophreniform disorder-a 15-year longitudinal study. Arch Gen Psychiatry. 2000;57:1053–1058. doi: 10.1001/archpsyc.57.11.1053. [DOI] [PubMed] [Google Scholar]
- 21.Hollis C. Child and adolescent (juvenile-onset) schizophrenia - a casecontrol study of premorbid developmental impairments. Br J Psychiatry. 1995;166:489–495. doi: 10.1192/bjp.166.4.489. [DOI] [PubMed] [Google Scholar]
- 22.Shergill SS., Bullmore E., Simmons A., Murray R., McGuire P. Functional anatomy of auditory verbal imagery in schizophrenic patients with auditory hallucinations. Am J Psychiatry. 2000;157:1691–1693. doi: 10.1176/appi.ajp.157.10.1691. [DOI] [PubMed] [Google Scholar]
- 23.Shergill SS., Brammer MJ., Williams SCR., Murray RM., McGuire PK. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry. 2000;57:1033–1038. doi: 10.1001/archpsyc.57.11.1033. [DOI] [PubMed] [Google Scholar]
- 24.Isohanni M., Jones PB., Moilanen K., et al. Early developmental milestones in adult schizophrenia and other psychoses. A 31-year follow-up of the Northern Finland 1966 Birth Cohort. Schizophr Res. 2001;52:1–19. doi: 10.1016/s0920-9964(00)00179-1. [DOI] [PubMed] [Google Scholar]
- 25.David AS., Malmberg A., Brandt L., Allebeck P., Lewis G. IQ and risk for schizophrenia: a population-based cohort study. Psychol Med. 1997;27:1311–1323. doi: 10.1017/s0033291797005680. [DOI] [PubMed] [Google Scholar]
- 26.Davidson M., Reichenberg A., Rabinowitz J., Weiser M., Kaplan Z., Mark M. Behavioral and intellectual markers for schizophrenia in apparently healthy male adolescents. Ami J Psychiatry. 1999;156:1328–1335. doi: 10.1176/ajp.156.9.1328. [DOI] [PubMed] [Google Scholar]
- 27.Geddes JR., Lawrie SM. Obstetric complications and schizophrenia: a meta-analysis. Br J Psychiatry. 1995;167:786–793. doi: 10.1192/bjp.167.6.786. [DOI] [PubMed] [Google Scholar]
- 28.Geddes JR., Verdoux H., Takei N., et al. Schizophrenia and complications of pregnancy and labor: an individual patient data meta-analysis. Schizophr Bull. 1999;25:413–423. doi: 10.1093/oxfordjournals.schbul.a033389. [DOI] [PubMed] [Google Scholar]
- 29.Lewis SW., Owen MJ., Murray RM. Obstetric complications and schizophrenia: methodology and mechanisms. In: Schulz SC, Tamminga CA, eds. Schizophrenia: A Scientific Focus. New York, NY: Oxford University Press. 1989 [Google Scholar]
- 30.Bramon E., Kelly J., van Os J., Murray R. The cascede of increasingly deviant development that culminates in the onset of schizophrenia. Neurosci News. 2001;4:5–19. [Google Scholar]
- 31.Jones PB., Rantakallio P., Hartikainen AL., Isohanni M., Sipila P. Schizophrenia as a long-term outcome of pregnancy, delivery, and perinatal complications. A 28-year follow-up of the 1966 North Finland general population birth cohort. Am J Psychiatry. 1998;155:355–364. doi: 10.1176/ajp.155.3.355. [DOI] [PubMed] [Google Scholar]
- 32.Hultman CM., Sparen P., Takei N., Murray RM., Cnattingius S. Prenatal and perinatal risk factors for schizophrenia, affective psychosis, and reactive psychosis of early onset: case-control study. BMJ. 1999;318:421–426. doi: 10.1136/bmj.318.7181.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalman C., Allebeck P., Cullberg J., Grunewald C., Koster M. Obstetric complications and the risk of schizophrenia - a longitudinal study of a national birth cohort. Arch Gen Psychiatry. 1999;56:234–240. doi: 10.1001/archpsyc.56.3.234. [DOI] [PubMed] [Google Scholar]
- 34.Kendell RE., Juszczak E., Cole SK. Obstetric complications and schizophrenia: a case control study based on standardised obstetric records. Br J Psychiatry. 1996;168:556–561. doi: 10.1192/bjp.168.5.556. [DOI] [PubMed] [Google Scholar]
- 35.Kendell RE., Mclnneny K., Juszczak E., Bain M. Obstetric complications and schizophrenia - two case-control studies based on structured obstetric records. Br J Psychiatry. 2000;176:516–522. doi: 10.1192/bjp.176.6.516. [DOI] [PubMed] [Google Scholar]
- 36.Byrne M., Browne R., Mulryan N., et al. Labour and delivery complications and schizophrenia - case-control study using contemporaneous labour ward records. Br J Psychiatry. 2000;176:531–536. doi: 10.1192/bjp.176.6.531. [DOI] [PubMed] [Google Scholar]
- 37.Cannon TD., Rosso IM., Hollister JM., Bearden CE., Sanchez LE., Hadley T. A prospective cohort study of genetic and perinatal influences in the etiology of schizophrenia. Schizophr Bull. 2000;26:351–366. doi: 10.1093/oxfordjournals.schbul.a033458. [DOI] [PubMed] [Google Scholar]
- 38.Rosso IM., Cannon TD., Huttunen T., Huttunen MO., Lonnqvist J., Gasperoni TL. Obstetric risk factors for early-onset schizophrenia in a Finnish birth cohort. Am J Psychiatry. 2000;157:801–807. doi: 10.1176/appi.ajp.157.5.801. [DOI] [PubMed] [Google Scholar]
- 39.Zornberg GL., Buka SL., Tsuang MT. Hypoxic-ischemia-related fetal/neonatal complications and risk of schizophrenia and other nonaffective psychoses: a 19-year longitudinal study. Am J Psychiatry. 2000;157:196–202. doi: 10.1176/appi.ajp.157.2.196. [DOI] [PubMed] [Google Scholar]
- 40.Brown AS., Cohen P., Harkavy-Friedman J., et al. Prenatal rubella, premorbid abnormalities, and adult schizophrenia. Biol Psychiatry. 2001;49:473–486. doi: 10.1016/s0006-3223(01)01068-x. [DOI] [PubMed] [Google Scholar]
- 41.Wahlbeck K., Forsen T., Osmond C., Barker DJP., Eriksson JG. Association of schizophrenia with low maternal body mass index, small size at birth, and thinness during childhood. Arch Gen Psychiatry. 2001;58:48–52. doi: 10.1001/archpsyc.58.1.48. [DOI] [PubMed] [Google Scholar]
- 42.Dalman C., Thomas HV., David AS., Gentz J., Lewis G., Allebeck P. Signs of asphyxia at birth and risk of schizophrenia - population-based case-control study. Br J Psychiatry. 2001;179:403–408. doi: 10.1192/bjp.179.5.403. [DOI] [PubMed] [Google Scholar]
- 43.Thomas HV., Dalman C., David AS., Gentz J., Lewis G., Allebeck P. Obstetric complications and risk of schizophrenia - effect of gender, age at diagnosis and maternal history of psychosis. Br J Psychiatry. 2001;179:409–414. doi: 10.1192/bjp.179.5.409. [DOI] [PubMed] [Google Scholar]
- 44.Castle DJ., Murray RM. The neurodevelopmental basis of sex differences in schizophrenia. Psychol Med. 1991;21:565–575. doi: 10.1017/s0033291700022194. [DOI] [PubMed] [Google Scholar]
- 45.Verdoux H., Geddes JR., Takei N., et al. Obstetric complications and age at onset in schizophrenia: an international collaborative meta-analysis of individual patient data. Am J Psychiatry. 1997;154:1220–1227. doi: 10.1176/ajp.154.9.1220. [DOI] [PubMed] [Google Scholar]
- 46.Kotlicka-Antczak M., Gmitrowicz A., Sobow TM., Rabe-Jablonska J. Obstetric complications and Apgar score in early-onset schizophrenic patients with prominent positive and prominent negative symptoms. J Psychiatr Res. 2001;35:249–257. doi: 10.1016/s0022-3956(01)00022-x. [DOI] [PubMed] [Google Scholar]
- 47.Fearon P., Cotter D., Murray R. Is the association between obstetric complications and schizophrenia mediated by glutamatergic excitotoxic damage in the fetal/neonatal brain? In: Reveley AM, Deakin J, eds. The Psychopharmacology of Schizophrenia. 1st ed. London, UK: Arnold. 2000:21–40. [Google Scholar]
- 48.Waddington JL., Lane A., Larkin C., O'Callaghan E. The neurodevelopmental basis of schizophrenia: clinical clues from cerebro-craniofacial dysmorphogenesis, and the roots of a lifetime trajectory of disease. Biol Psychiatry. 1999;46:31–39. doi: 10.1016/s0006-3223(99)00055-4. [DOI] [PubMed] [Google Scholar]
- 49.O'Callaghan E., Larkin C., Kinsella A., Waddington JL. Familial, obstetric, and other clinical correlates of minor physical anomalies in schizophrenia. Am J Psychiatry. 1991;148:479–483. doi: 10.1176/ajp.148.4.479. [DOI] [PubMed] [Google Scholar]
- 50.Griffiths TD., Sigmundsson T., Takei N., et al. Minor physical anomalies in familial and sporadic schizophrenia: the Maudsley family study. J Neurol Neurosurg Psychiatry. 1998;64:56–60. doi: 10.1136/jnnp.64.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McNeil TF., Cantor-Graae E., Weinberger DR. Relationship of obstetric complications and differences in size of brain structures in monozygotic twin pairs discordant for schizophrenia. Am J Psychiatry. 2000;157:203–212. doi: 10.1176/appi.ajp.157.2.203. [DOI] [PubMed] [Google Scholar]
- 52.Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 53.Fananas L., Moral P., Bertranpetit J. Quantitative dermatoglyphics in schizophrenia - study of family history subgroups. Hum Biol. 1990;62:421–427. [PubMed] [Google Scholar]
- 54.Bracha HS., Torrey EF., Bigelow LB., Lohr JB., Linington BB. Subtle signs of prenatal maldevelopment of the hand ectoderm in schizophrenia - a preliminary monozygotic twin study. Biol Psychiatry. 1991;30:719–725. doi: 10.1016/0006-3223(91)90017-g. [DOI] [PubMed] [Google Scholar]
- 55.Fananas L., van Os J., Hoyos C., McGrath J., Mellor CS., Murray R. Dermatoglyphic a-b ridge count as a possible marker for developmental disturbance in schizophrenia: replication in two samples. Schizophr Res. 1996;20:307–314. doi: 10.1016/0920-9964(95)00013-5. [DOI] [PubMed] [Google Scholar]
- 56.Van Os J., Woodruff PWR., Fananas L., et al. Association between cerebral structural abnormalities and dermatoglyphic ridge counts in schizophrenia. Compr Psychiatry. 2000;41:380–384. doi: 10.1053/comp.2000.8999. [DOI] [PubMed] [Google Scholar]
- 57.Reilly JL., Murphy PT., Byrne M., et al. Dermatoglyphic fluctuating asymmetry and atypical handedness in schizophrenia. Schizophr Res. 2001;50:159–168. doi: 10.1016/s0920-9964(00)00044-x. [DOI] [PubMed] [Google Scholar]
- 58.Johnstone E., Crow T., Frith C., Husband J., Kreel L. Cerebral ventricular size and cognitive impeirment in chronic schizophrenia. Lancet. 1976;2:924–926. doi: 10.1016/s0140-6736(76)90890-4. [DOI] [PubMed] [Google Scholar]
- 59.Lewis S. Computerised topography in schizophrenia 15 years on. Br J Psychiatry. 1990;157(suppl9):16–24. [PubMed] [Google Scholar]
- 60.Woodruff P., Murray R. The aetiology of brain abnormalities in schizophrenia. In: Ancil RJ, Holliday S, Higenbottam J, eds. Schizophrenia: Exploring the Spectrum of Psychosis. Chichester, UK: Wiley. 1994:95–144. [Google Scholar]
- 61.Chua SE., McKenna PJ. Schizophrenia - a brain disease - a critical review of structural and functional cerebral abnormality in the disorder. Br J Psychiatry. 1995;166:563–582. doi: 10.1192/bjp.166.5.563. [DOI] [PubMed] [Google Scholar]
- 62.Van Horn J., McManus I. Ventricular enlargement in schizophrenia. A meta-analysis of studies of the ventricle:brain ratio (VBR). Br J Psychiatry. 1992;160:687–697. doi: 10.1192/bjp.160.5.687. [DOI] [PubMed] [Google Scholar]
- 63.Wright IC., Rabe-Hesketh S., Woodruff PWR., David AS., Murray RM., Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 64.Wright IC., McGuire PK., Poline JB., et al. A voxel-based method for the statistical analysis of gray and white matter density applied to schizophrenia. Neuroimage. 1995;2:244–252. doi: 10.1006/nimg.1995.1032. [DOI] [PubMed] [Google Scholar]
- 65.Sigmundsson T., Suckling J., Maier M., et al. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am J Psychiatry. 2001;158:234–243. doi: 10.1176/appi.ajp.158.2.234. [DOI] [PubMed] [Google Scholar]
- 66.Turner S., Toone B., Brett-Jones J. Computerised tomographic scan changes in early schizophrenia. Psychol Med. 1986;16:219–225. doi: 10.1017/s003329170000266x. [DOI] [PubMed] [Google Scholar]
- 67.Delisi LE., Hoff AL., Schwartz JE., et al. Brain morphology in 1st-episode schizophrenic-like psychotic patients - a quantitative magnetic resonance imaging study. Biol Psychiatry. 1991;29:159–175. doi: 10.1016/0006-3223(91)90044-m. [DOI] [PubMed] [Google Scholar]
- 68.Ettinger U., Chitnis XA., Kumari V., et al. Magnetic resonance imaging of the thalamus in first-episode psychosis. Am J Psychiatry. 2001;158:116–118. doi: 10.1176/appi.ajp.158.1.116. [DOI] [PubMed] [Google Scholar]
- 69.Friedman L., Findling RL., Kenny JT., et al. An MRI study of adolescent patients with either schizophrenia or bipolar disorder as compared to healthy control subjects. Biol Psychiatry. 1999;46:78–88. doi: 10.1016/s0006-3223(98)00351-5. [DOI] [PubMed] [Google Scholar]
- 70.Aoyama F., lida J., Inoue M., et al. Brain imaging in childhood- and adolescence-onset schizophrenia associated with obsessive-compulsive symptoms. Acta Psychiatr Scand. 2000;102:32–37. doi: 10.1034/j.1600-0447.2000.102001032.x. [DOI] [PubMed] [Google Scholar]
- 71.O'Callaghan E., Buckley P., Redmond O., et al. Abnormalities of cerebral structure in schizophrenia on magnetic-resonance-imaging - interpretation in relation to the neurodevelopmental hypothesis. J R Soc Med. 1992;85:227–231. doi: 10.1177/014107689208500416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rajarethinam R., Miedler J., Dequardo J., et al. Prevalence of cavum septum pellucidum in schizophrenia studied with MRI. Schizophr Res. 2001;48:201–205. doi: 10.1016/s0920-9964(00)00110-9. [DOI] [PubMed] [Google Scholar]
- 73.Crow TJ., Ball J., Bloom SR., et al. Schizophrenia as an anomaly of development of cerebral asymmetry - a postmortem study and a proposal concerning the genetic basis of the disease. Arch Gen Psychiatry. 1989;46:1145–1150. doi: 10.1001/archpsyc.1989.01810120087013. [DOI] [PubMed] [Google Scholar]
- 74.Bilder RM., Wu HW., Bogerts B., et al. Absence of regional hemispheric volume asymmetries in first-episode schizophrenia. Am J Psychiatry. 1994;151:1437–1447. doi: 10.1176/ajp.151.10.1437. [DOI] [PubMed] [Google Scholar]
- 75.Bullmore E., Brammer M., Harvey I., Murray R., Ron M. Cerebral hemispheric asymmetry revisited - effects of handedness, gender and schizophrenia measured by radius of gyration in magnetic resonance images. Psychol Med. 1995;25:349–363. doi: 10.1017/s0033291700036254. [DOI] [PubMed] [Google Scholar]
- 76.Sommer I., Aleman A., Ramsey N., Bouma A., Kahn R. Handedness, language lateralisation and anatomical asymmetry in schizophrenia - metaanalysis. Br J Psychiatry. 2001;178:344–351. doi: 10.1192/bjp.178.4.344. [DOI] [PubMed] [Google Scholar]
- 77.Bogerts B. The neuropathology of schizophrenic diseases: historical aspects and present knowledge. Eur Arch Psychiatry Clin Neurosci. 1999;249:2–13. doi: 10.1007/pl00014181. [DOI] [PubMed] [Google Scholar]
- 78.Kovelman J., Scheibel A. A neurobiological correlate of schizophrenia. Biol Psychiatry. 1984;19:1601–1621. [PubMed] [Google Scholar]
- 79.Jakob H., Beckmann H. Prenatal developmental disturbances in the limbic allocortex in schizophrenia. J Neural Transm. 1986;65:303–326. doi: 10.1007/BF01249090. [DOI] [PubMed] [Google Scholar]
- 80.Akbarian S., Bunney WE., Potkin SG., et al. Altered distribution of nicotinamide-adenine dinucleotide phosphate diaphorase cells in frontal lobe of schizophrenics implies disturbances of cortical development. Arch Gen Psychiatry. 1993;50:169–177. doi: 10.1001/archpsyc.1993.01820150007001. [DOI] [PubMed] [Google Scholar]
- 81.Akbarian S., Vinuela A., Kim JJ., Potkin SG., Bunney WE., Jones EG. Distorted distribution of nicotinamide-adenine dinucleotide phosphate diaphorase neurons in temporal lobe of schizophrenics implies anomalous cortical development. Arch Gen Psychiatry. 1993;50:178–187. doi: 10.1001/archpsyc.1993.01820150016002. [DOI] [PubMed] [Google Scholar]
- 82.Akbarian S., Jones EG. Developmental abnormalities of the prefrontal cortex in schizophrenia. Biol Psychiatry. 1996;39:200. [Google Scholar]
- 83.Christison GW., Casanova MF., Weinberger DR., Rawlings R., Kleinman JE. A quantitative investigation of hippocampal pyramidal cell size, shape, and variability of orientation in schizophrenia. Arch Gen Psychiatry. 1989;46:1027–1032. doi: 10.1001/archpsyc.1989.01810110069010. [DOI] [PubMed] [Google Scholar]
- 84.Benes F., Sorenson I., Bird E. Reduced neuronal size in posterior hippocampus of schizophrenic patients. Schizophr Bull. 1991;17:597–608. doi: 10.1093/schbul/17.4.597. [DOI] [PubMed] [Google Scholar]
- 85.Arnold SE., Franz BR., Gur RC., et al. Smaller neuron size in schizophrenia in hippocampal subfields that mediate cortical-hippocampal interactions. Am J Psychiatry. 1995;152:738–748. doi: 10.1176/ajp.152.5.738. [DOI] [PubMed] [Google Scholar]
- 86.Cotter D., Kerwin R., Doshi B., Martin CS., Everall IP. Alterations in hippocampal non-phosphorylated MAP2 protein expression in schizophrenia. Brain Res. 1997;765:238–246. doi: 10.1016/s0006-8993(97)00575-1. [DOI] [PubMed] [Google Scholar]
- 87.Akil M., Lewis DA. Cytoarchitecture of the entorhinal cortex in schizophrenia. Am J Psychiatry. 1997;154:1010–1012. doi: 10.1176/ajp.154.7.1010. [DOI] [PubMed] [Google Scholar]
- 88.Falkai P., Bogerts B. Cell loss in the hippocampus of schizophrenics. Eur Arch Psychiatry Clin Neurosci. 1986;236:154–161. doi: 10.1007/BF00380943. [DOI] [PubMed] [Google Scholar]
- 89.Roberts GW., Colter N., Lofthouse R., Johnstone EC., Crow TJ. Is there gliosis in schizophrenia? Investigation of the temporal lobe. Biol Psychiatry. 1987;22:1459–1468. doi: 10.1016/0006-3223(87)90104-1. [DOI] [PubMed] [Google Scholar]
- 90.Roberts G., Harrison P. Gliosis and its implications for the disease process. In: Harrison P, Roberts G, eds. The Neuropathology of Schizophrenia. 1st ed. New York, NY: Oxford University Press. 2000:137–145. [Google Scholar]
- 91.Harrison PJ. The neuropathology of schizophrenia - a critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 92.Van Os J., Jones P., Sham P., Bebbington P., Murray RM. Psychosis as a continuum of variation in dimensions of psychopathology. In: Haffner H, Grattaz W, eds. Search for the Causes of Schizophrenia. 1999;Vol 4. Berlin, Germany: Springer:59–80. [Google Scholar]
- 93.Cannon TD., Kaprio J., Lonnqvist J., Huttunen M., Koskenvuo M. The genetic epidemiology of schizophrenia in a Finnish twin cohort - a population-based modeling study. Arch Gen Psychiatry. 1998;55:67–74. doi: 10.1001/archpsyc.55.1.67. [DOI] [PubMed] [Google Scholar]
- 94.Cardno AG., Marshall EJ., Coid B., et al. Heritability estimates for psychotic disorders - The Maudsley Twin Psychosis Series. Arch Gen Psychiatry. 1999;56:162–168. doi: 10.1001/archpsyc.56.2.162. [DOI] [PubMed] [Google Scholar]
- 95.Cardno A., Sham P., Murray P., McGuffin P. A twin study of clinical variables in psychotic disorders. Am J Med Genet. 2000;96:P82. [Google Scholar]
- 96.Sharma T., Lancaster E., Lee D., et al. Brain changes in schizophrenia - volumetric MRI study of families multiply affected with schizophrenia - The Maudsley Family Study 5. Br J Psychiatry. 1998;173:132–138. doi: 10.1192/bjp.173.2.132. [DOI] [PubMed] [Google Scholar]
- 97.Faraone SV., Green AI., Seidman LJ., Tsuang MT. “Schizotaxia”: clinical implications and new directions for research. Schizophr Bull. 2001;27:1–18. doi: 10.1093/oxfordjournals.schbul.a006849. [DOI] [PubMed] [Google Scholar]
- 98.McDonald C., Grech A., Toulopoulou T., et al. Brain volumes in familial and nonfamilial schizophrenic probands and their unaffected relatives. Neuropsychiatr Genet. In press doi: 10.1002/ajmg.10604. [DOI] [PubMed] [Google Scholar]
- 99.Stefanis N., Frangou S., Yakeley J., et al. Hippocampal volume reduction in schizophrenia: Effects of genetic risk and pregnancy and birth complications. Biol Psychiatry. 1999;46:697–702. doi: 10.1016/s0006-3223(99)00089-x. [DOI] [PubMed] [Google Scholar]
- 100.McNeil TF., Cantor-Graae E., Weinberger DR. Relationship of obstetric complications and differences in size of brain structures in monozygotic twin pairs discordant for schizophrenia. Am J Psychiatry. 2000;157:203–212. doi: 10.1176/appi.ajp.157.2.203. [DOI] [PubMed] [Google Scholar]
- 101.Seidman LJ., Faraone SV., Goldstein JM., et al. Thalamic and amygdala-hippocampal volume reductions in first-degree relatives of patients with schizophrenia: an MRI-based morphometric analysis. Biol Psychiatry. 1999;46:941–954. doi: 10.1016/s0006-3223(99)00075-x. [DOI] [PubMed] [Google Scholar]
- 102.Lawrie SM., Whalley HC., Abukmeil SS., et al. Brain structure, genetic liability, and psychotic symptoms in subjects at high risk of developing schizophrenia. Biol Psychiatry. 2001;49:811–823. doi: 10.1016/s0006-3223(00)01117-3. [DOI] [PubMed] [Google Scholar]
- 103.O'Driscoll GA., Florencio PS., Gagnon D., et al. Amygdala-hippocampal volume and verbal memory in first-degree relatives of schizophrenic patients. Psychiatry Res Neuroimaging. 2001;107:75–85. doi: 10.1016/s0925-4927(01)00095-6. [DOI] [PubMed] [Google Scholar]
- 104.Bradbury T., Miller G. Season of birth in schizophrenia: a review of the evidence, methodology and etiology. Psychol Bull. 1985;98:569–594. [PubMed] [Google Scholar]
- 105.Davies GJ., Welham J., Torrey EF., McGrath J. Season of birth effect and latitude: a systematic review and meta-analysis of northern hemisphere schizophrenia studies. Schizophr Res. 2000;41:A54. doi: 10.1093/oxfordjournals.schbul.a007030. [DOI] [PubMed] [Google Scholar]
- 106.Sham PC., Maclean CJ., Kendler KS. Risk of schizophrenia and age difference with older siblings - evidence for a maternal viral infection hypothesis. Br J Psychiatry. 1993;163:627–633. doi: 10.1192/bjp.163.5.627. [DOI] [PubMed] [Google Scholar]
- 107.Takei N., Mortensen PB., Klaening U., et al. Relationship between in utero exposure to influenza epidemics and risk of schizophrenia in Denmark. Biol Psychiatry. 1996;40:817–824. doi: 10.1016/0006-3223(95)00592-7. [DOI] [PubMed] [Google Scholar]
- 108.Westergaard T., Mortensen PB., Pedersen CB., Wohlfahrt J., Melbye M. Exposure to prenatal and childhood infections and the risk of schizophrenia - suggestions from a study of sibship characteristics and influenza prevalence. Arch Gen Psychiatry. 1999;56:993–998. doi: 10.1001/archpsyc.56.11.993. [DOI] [PubMed] [Google Scholar]
- 109.Brown AS., Susser ES., Cohen P., Greenwald S. Schizophrenia following prenatal rubella exposure: gestational timing and diagnostic specificity. Schizophr Res. 1998;29:17–18. [Google Scholar]
- 110.Buka SL., Tsuang MT., Torrey EF., Klebanoff MA., Bernstein D., Yolken RH. Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry. 2001;58:1032–1037. doi: 10.1001/archpsyc.58.11.1032. [DOI] [PubMed] [Google Scholar]
- 111.Susser E., Hoek HW., Brown A. Neurodevelopmental disorders after prenatal famine - The story of the Dutch Famine Study. Am J Epidemiol. 1998;147:213–216. doi: 10.1093/oxfordjournals.aje.a009439. [DOI] [PubMed] [Google Scholar]
- 112.Wright P., Sham PC., Gilvarry CM., et al. Autoimmune diseases in the pedigrees of schizophrenic and control subjects. Schizophr Res. 1996;20:261–267. doi: 10.1016/0920-9964(96)82950-1. [DOI] [PubMed] [Google Scholar]
- 113.Myhrman A., Rantakallio P., Isohanni M., Jones P., Partanen U. Unwantedness of a pregnancy and schizophrenia in the child. Br J Psychiatry. 1996;169:637–640. doi: 10.1192/bjp.169.5.637. [DOI] [PubMed] [Google Scholar]
- 114.Van Os J., Selten JP. Prenatal exposure to maternal stress and later schizophrenia. The May 1940 invasion of The Netherlands. Br J Psychiatry. 1998;172:324–326. doi: 10.1192/bjp.172.4.324. [DOI] [PubMed] [Google Scholar]
- 115.Rantakallio P., Jones P., Moring J., VonWendt L. Association between central nervous system infections during childhood and adult onset schizophrenia and other psychoses: a 28-year follow-up. int J Epidemiol. 1997;26:837–843. doi: 10.1093/ije/26.4.837. [DOI] [PubMed] [Google Scholar]
- 116.Sachdev P., Smith JS., Cathcart S. Schizophrenia-like psychosis following traumatic brain injury: a chart-based descriptive and case-control study. Psychol Med. 2001;31:231–239. doi: 10.1017/s0033291701003336. [DOI] [PubMed] [Google Scholar]
- 117.Benes FM. Myelination of cortical-hippocampal relays during late adolescence. Schizophr Bull. 1989;15:585–593. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- 118.Feinberg I. Schizophrenia - caused by a fault in programmed synaptic elimination during adolescence. J Psychiatr Res. 1983;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 119.Olney JW., Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 120.Lipska BK., Jaskiw GE., Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage - a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- 121.Lipska BK., Weinberger DR. Genetic variation in vulnerability to the behavioral effects of neonatal hippocampal damage in rats. Proc Natl Acad Sci USA. 1995;92:8906–8910. doi: 10.1073/pnas.92.19.8906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Selemon LD., Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit-based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 123.Glantz LA., Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 124.Akil M., Weinberger DR. Gliosis and its implications for the disease process. In: Harrison P, Roberts G, eds. The Neuropathology of Schizophrenia. 1st ed. New York, NY: Oxford University Press. 2000:137–145. [Google Scholar]
- 125.Keshavan MS., Anderson S., Pettegrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J Psychiatr Res. 1994;28:239–265. doi: 10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 126.McGlashan TH., Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- 127.Keshavan MS. Development, disease and degeneration in schizophrenia: a unitary pathophysiological model. J Psychiatr Res. 1999;33:513–521. doi: 10.1016/s0022-3956(99)00033-3. [DOI] [PubMed] [Google Scholar]
- 128.Vita A., Giobbio GM., Dieci M., et al. Stability of cerebral ventricular size from the appearance of the first psychotic symptoms to the later diagnosis of schizophrenia. Biol Psychiatry. 1994;35:960–962. doi: 10.1016/0006-3223(94)91243-2. [DOI] [PubMed] [Google Scholar]
- 129.Gur RE., Cowell P., Turetsky Bl., et al. A follow-up magnetic resonance imaging study of schizophrenia - relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry. 1998;55:145–152. doi: 10.1001/archpsyc.55.2.145. [DOI] [PubMed] [Google Scholar]
- 130.Woods BT. Is schizophrenia a progressive neurodevelopmental disorder? Toward a unitary pathogenetic mechanism. Am J Psychiatry. 1998;155:1661–1670. doi: 10.1176/ajp.155.12.1661. [DOI] [PubMed] [Google Scholar]
- 131.DeLisi L. Defining the course of brain structural change and plasticity in schizophrenia. Psychiatry Res. 1999;92:1–9. doi: 10.1016/s0925-4927(99)00033-5. [DOI] [PubMed] [Google Scholar]
- 132.Lieberman JA. Is schizophrenia a neurodegenerative disorder? A clinical and neurobiological perspective. Biol Psychiatry. 1999;46:729–739. doi: 10.1016/s0006-3223(99)00147-x. [DOI] [PubMed] [Google Scholar]
- 133.Lieberman J., Chakos M., Wu HW., et al. Longitudinal study of brain morphology in first-episode schizophrenia. Biol Psychiatry. 2001;49:487–499. doi: 10.1016/s0006-3223(01)01067-8. [DOI] [PubMed] [Google Scholar]
- 134.Giedd JN., Jeffries NO., Blumenthal J., et al. Childhood-onset schizophrenia: progressive brain changes during adolescence. Biol Psychiatry. 1999;46:892–898. doi: 10.1016/s0006-3223(99)00072-4. [DOI] [PubMed] [Google Scholar]
- 135.Thompson PM., Vidal C., Giedd JN., et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very earlyonset schizophrenia. Proc Natl Acad Sci USA. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Allin M., Murray R. Schizophrenia: a neurodevelopmental or neurodegenerative disorder? Curr Opin Psychiatry. 2002;15:9–15. [Google Scholar]
- 137.Castle D., Sham B., Murray R. Differences in distribution of ages of onset in males and females with schizophrenia. Schizophr Res. 1998;33:179–183. doi: 10.1016/s0920-9964(98)00070-x. [DOI] [PubMed] [Google Scholar]
- 138.Howard RJ., Graham C., Sham P., et al. A controlled family study of late-onset non-affective psychosis (late paraphrenia). Br J Psychiatry. 1997;170:511–514. doi: 10.1192/bjp.170.6.511. [DOI] [PubMed] [Google Scholar]
- 139.Murray RM., Ocallaghan E., Castle DJ., Lewis SW. A neurodevelopmental approach to the classification of schizophrenia. Schizophr Bull. 1992;18:319–332. doi: 10.1093/schbul/18.2.319. [DOI] [PubMed] [Google Scholar]
- 140.Castle DJ., Murray RM. The epidemiology of late-onset schizophrenia. Schizophr Bull. 1993;19:691–700. doi: 10.1093/schbul/19.4.691. [DOI] [PubMed] [Google Scholar]
- 141.Sham PC., Jones P., Russell A., et al. Age at onset, sex, and familial psychiatric morbidity in schizophrenia - Camberwell Collaborative Psychosis Study. Br J Psychiatry. 1994;165:466–473. doi: 10.1192/bjp.165.4.466. [DOI] [PubMed] [Google Scholar]
- 142.Konnecke R., Hafner H., Maurer K., Loffler W., an der Heiden W. Main risk factors for schizophrenia: increased familial loading and pre- and peri-natal complications antagonize the protective effect of oestrogen in women. Schizophr Res. 2000;44:81–93. doi: 10.1016/s0920-9964(99)00139-5. [DOI] [PubMed] [Google Scholar]
- 143.Walsh C., Asherson P., Sham P., et al. Age of onset of schizophrenia in multiply affected families is early and shows no sex difference. In: Holliday S, Ancill R, MacEwan G, eds. Schizophrenia: Breaking Down the Barriers. New York, NY: Wiley. 1996:81–97. [Google Scholar]
- 144.O'Callaghan E., Gibson T., Colohan HA., et al. Risk of schizophrenia in adults born after obstetric complications and their association with early onset of illness-a controlled study. BMJ. 1992;305:1256–1259. doi: 10.1136/bmj.305.6864.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Johnstone EC., Owens DGC., Bydder GM., Colter N., Crow TJ., Frith CD. The spectrum of structural brain changes in schizophrenia - age of onset as a predictor of cognitive and clinical impairments and their cerebral correlates. Psychol Med. 1989;19:91–103. doi: 10.1017/s0033291700011053. [DOI] [PubMed] [Google Scholar]
- 146.Green MF., Satz P., Soper HV., Kharabi F. Relationship between physical anomalies and age at onset of schizophrenia. Am J Psychiatry. 1987;144:666–667. doi: 10.1176/ajp.144.5.666. [DOI] [PubMed] [Google Scholar]
- 147.Bullmore ET., Frangou S., Murray RM. The dysplastic net hypothesis: an integration of developmental and dysconnectivity theories of schizophrenia. Schizophr Res. 1997;28:143–156. doi: 10.1016/s0920-9964(97)00114-x. [DOI] [PubMed] [Google Scholar]
- 148.Tienari P. Interaction between genetic vulnerability and family environment - the Finnish Adoptive Family Study of Schizophrenia. Acta Psychiatr Scand. 1991;84:460–465. doi: 10.1111/j.1600-0447.1991.tb03178.x. [DOI] [PubMed] [Google Scholar]
- 149.Mirsky A., Silberman E., Latz A., Nagler S. Adult outcomes of high-risk children: differential effects of town and kibbutz rearing. Schizophr Bull. 1985;11:150–154. doi: 10.1093/schbul/11.1.150. [DOI] [PubMed] [Google Scholar]
- 150.Lewis G., David A., Andreasson S., Allebeck P. Schizophrenia and city life. Lancet. 1992;340:137–140. doi: 10.1016/0140-6736(92)93213-7. [DOI] [PubMed] [Google Scholar]
- 151.Mortensen PB., Pedersen CB., Westergaard T., et al. Effects of family history and place and season of birth on the risk of schizophrenia. N Engl J Med. 1999;340:603–608. doi: 10.1056/NEJM199902253400803. [DOI] [PubMed] [Google Scholar]
- 152.Marcelis M., Navarro-Mateu F., Murray R., Selten JP., van Os J. Urbanization and psychosis: a study of 1942-1978 birth cohorts in the Netherlands. Psychol Med. 1998;28:871–879. doi: 10.1017/s0033291798006898. [DOI] [PubMed] [Google Scholar]
- 153.Marcelis M., Takei N., van Os J. Urbanization and risk for schizophrenia: does the effect operate before or around the time of illness onset. Psychol Med. 1999;29:1197–1203. doi: 10.1017/s0033291799008983. [DOI] [PubMed] [Google Scholar]
- 154.Pedersen CB., Mortensen PB. Evidence of a dose-response relationship between urbanicity during upbringing and schizophrenia risk. Arch Gen Psychiatry. 2001;58:1039–1046. doi: 10.1001/archpsyc.58.11.1039. [DOI] [PubMed] [Google Scholar]
- 155.Malmberg A., Lewis G., David A., Allebeck P. Premorbid adjustment and personality in people with schizophrenia. Br J Psychiatry. 1998;172:308–313. doi: 10.1192/bjp.172.4.308. [DOI] [PubMed] [Google Scholar]
- 156.Van Os J., Driessen G., Gunther N., Delespaul P. Neighbourhood variation in incidence of schizophrenia - evidence for person-environment interaction. Br J Psychiatry. 2000;176:243–248. doi: 10.1192/bjp.176.3.243. [DOI] [PubMed] [Google Scholar]
- 157.Jablensky A., Cole SW. Is the earlier age at onset of schizophrenia in males a confounded finding? Results from a cross-cultural investigation. Br J Psychiatry. 1997;170:234–240. doi: 10.1192/bjp.170.3.234. [DOI] [PubMed] [Google Scholar]
- 158.Odegaard O. Emigration and insanity: a study of mental disease among Norwegian born population in Minnesota. Acta Psychiatr Neurol Scand. 1932;(suppl4):1–206. [Google Scholar]
- 159.Harrison G., Owens D., Holton D., Nielson D., Boot D. Schizophrenia in the UK Afro-Caribbean Population. Schizophr Res. 1988;1:119–119. [Google Scholar]
- 160.Hickling FW., McKenzie K., Mullen R., Murray R. A Jamaican psychiatrist evaluates diagnoses at a London psychiatric hospital. Br J Psychiatry. 1999;175:283–285. doi: 10.1192/bjp.175.3.283. [DOI] [PubMed] [Google Scholar]
- 161.McGuire PK., Jones P., Harvey I., Williams M., McGuffin P., Murray RM. Morbid risk of schizophrenia for relatives of patients with cannabis-associated psychosis. Schizophr Res. 1995;15:277–281. doi: 10.1016/0920-9964(94)00053-b. [DOI] [PubMed] [Google Scholar]
- 162.Glover GR. Why is there a high rate of schizophrenia in British Caribbeans. Br J Hosp Med. 1989;42:48–51. [PubMed] [Google Scholar]
- 163.Harrison G. Searching for the causes of schizophrenia - the role of migrant studies. Schizophr Bull. 1990;16:663–671. doi: 10.1093/schbul/16.4.663. [DOI] [PubMed] [Google Scholar]
- 164.Hutchinson G., Takei N., Bhugra D., et al. Increased rate of psychosis among African-Caribbeans in Britain is not due to an excess of pregnancy and birth complications. Br J Psychiatry. 1997;171:145–147. doi: 10.1192/bjp.171.2.145. [DOI] [PubMed] [Google Scholar]
- 165.Wessely S., Castle D., Der G., Murray R. Schizophrenia and Afro-Caribbeans - a case-control study. Br J Psychiatry. 1991;159:795–801. doi: 10.1192/bjp.159.6.795. [DOI] [PubMed] [Google Scholar]
- 166.Hutchinson G., Takei N., Fahy TA., et al. Morbid risk of schizophrenia in first-degree relatives of white and African-Caribbean patients with psychosis. Br J Psychiatry. 1996;169:776–780. doi: 10.1192/bjp.169.6.776. [DOI] [PubMed] [Google Scholar]
- 167.Sugarman PA., Craufurd D. Schizophrenia in the Afro-Caribbean community. Br J Psychiatry. 1994;164:474–480. doi: 10.1192/bjp.164.4.474. [DOI] [PubMed] [Google Scholar]
- 168.Boydell J., van Os J., McKenzie K., et al. Incidence of schizophrenia in ethnic minorities in London: ecological study into interactions with environment. BMJ. 2001;323:1336–1338. doi: 10.1136/bmj.323.7325.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ventura J., Neuchterlein K., Lukoff D., Hardesty J. A prospective study of stressful life events and schizophrenic relapse. J Abnorm Psychol. 1989;98:407–411. doi: 10.1037//0021-843x.98.4.407. [DOI] [PubMed] [Google Scholar]
- 170.Malla AK., Norman RMG. Relationship of major life events and daily stressors to symptomatology in schizophrenia. J Nerv Ment Dis. 1992;180:664–667. [PubMed] [Google Scholar]
- 171.Bebbington P., Wilkins S., Jones P., et al. Life events and psychosis - initial results from the Camberwell Collaborative Psychosis Study. Br J Psychiatry. 1993;162:72–79. doi: 10.1192/bjp.162.1.72. [DOI] [PubMed] [Google Scholar]
- 172.Andreasson S., Engstrom A., Allebeck P., Rydberg U. Cannabis and schizophrenia - a longitudinal- study of Swedish conscripts. Lancet. 1987;2:1483–1486. doi: 10.1016/s0140-6736(87)92620-1. [DOI] [PubMed] [Google Scholar]
- 173.Arseneault L., Cannon M., Poulton R., Murray RM., Caspi A., Moffitt TE. Cannabis use in adolescence and risk for adult psychosis. Lancet. In press. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen C. Predisposing Factors to Methamphetamine-induced Psychosis. PhD thesis. University of London. 2001 [Google Scholar]
- 175.Jones P., Murray RM. The genetics of schizophrenia is the genetics of neurodevelopment. Br J Psychiatry. 1991;158:615–623. doi: 10.1192/bjp.158.5.615. [DOI] [PubMed] [Google Scholar]
- 176.Virdi G., Bramon E., Croft R., et al. Mismatch negativity in schizophrenia: a family study. Schizophr Res. 2001;59(suppl):211. doi: 10.1016/s0920-9964(03)00132-4. [DOI] [PubMed] [Google Scholar]