Abstract
On the basis of extensive basic and clinical studies, corticotropin-releasing hormone (CRH) and its related family members are considered to play a pivotal role in stress-related disorders, such as anxiety and depression. CRH is regarded as the principal mediator in the brain of the stress response, as it mediates neuroendocrine, autonomic, and behavioral responses to stressful challenges. Recently, this neuropeptide family has expanded due to the discovery of two new members, urocortin II (also termed stresscopin-related peptide) and urocortin III (also termed stresscopin), which are selective agonists for the CRH receptor type 2. They show a discrete neuroanatomical localization and are involved in stress-coping responses, such as anxiolysis. Here, on the basis of recent developments, we suggest that CRH, the urocortins, and their receptors form a complex system in the brain, which is recruited during both the acute and the recovery phases of the stress response.
Keywords: corticotropin-releasing hormone, urocortin, anxiety, depression, hypothalamic-pituitary-adrenocortical axis, corticosteroid receptor, antidepressant, autonomic nervous system
Abstract
En base a numerosos estudios clínicos y básicos se consídera que la hormona liberadora de corticotropina (CRH) y las sustancias de la misma familia juegan un papel central en los trastornos relacionados con el estrés, como la ansiedad y la depresión. La CRH es considerada el mediador principal en el cerebro de la respuesta de estrés, ya que media las respuestas neuroendocrinas, autonómicas y conductuales a los estímulos estresantes. Recientemente esta familia de neuropéptidos se ha incrementado debido al descubrimiento de dos nuevos miembros, la urocortina II (también denominada “stresscopin-related peptide”) y la urocortina III (también llamada “stresscopin”), las cuales son agonistas selectivos de los receptores de CRH tipo 2. Ellas muestran una localización anatómica reducida y participan en las respuestas de adaptación al estrés como la ansiolisis. Según esto, en base a descubrimientos recientes, nosotros sugerimos que la CRH, las urocortinas y sus receptores forman un sistema complejo en el cerebro, el cual interviene tanto en la fase aguda como en la fase de recuperación de la respuesta de estrés.
Abstract
De grandes études cliniques ont montré que la corticolibérine (CRH) et les substances de la même famille jouent un rôle central dans les troubles liés au stress tels que l'anxiété et la dépression. La CRH est considérée comme le principal médiateur cérébral de réponse au stress, en particulier pour ce qui concerne les réponses neuroendocriniennes, autonomes et comportementales aux situations de stress. Récemment, cette famille de neuropeptides s'est agrandie grâce à la découverte de deux molécules agonistes sélectifs du récepteur de la CRH de type 2, l'urocortine II et l'urocortine III (désignées par nous respectivement sous les termes de «stresscopin-related peptide» et «stresscopin»). Ces molécules manifestent une discrète localisation neuroanatomique et sont impliquées dans les réponses d'adaptation au stress telles que l'anxiolyse. Sur la base de découvertes récentes, nous avançons l'hypothèse que la CRH, les urocortines et leurs récepteurs forment, dans le cerveau, un système complexe qui entre en jeu tant dans les phases aiguës que lors des phases de récupération de la réponse au stress.
Stress-related psychiatric disorders such as anxiety and major depression impair the lives of approximately 10% to 15% of the population. For many years, the success of the pharmacological treatment of these disorders has been restrained by various factors, including long latency of clinical effect, treatment resistance, adverse side effects, and, in the case of the anxiolytic benzodiazepines, tolerance and addictive potential. Although stress has continuously been a subject of research since the 1940s, the pharmacological principle of action (ie, the interaction with the classic neurotransmitters such as serotonin, noradrenaline, and γ-aminobutyric acid [GABA]) of the antidepressant and anxiolytic drugs prescribed today still stems from work carried out as early as the 1950s.
A new epoch began in 1981 with the discovery of corticotropin-releasing hormone (CRH) as the principal mediator of the effects of stress on the hypothalamicpituitary-adrenocortical (HPA) axis and behavior.1 Not surprisingly, clinical studies soon demonstrated that this neuropeptide is implicated in depression and anxiety disorders.2-5 Moreover, basic research studies have presented evidence that elevated central CRH levels are involved in the etiology of stress-related physiological and behavioral disorders.6 In pharmacology, however, the discovery of two types of CRH-binding receptors (CRHR1 and CRHR2) and a non-receptor CRH-binding protein (CRHBP) was an immense breakthrough. With the recent discovery of more - endogenous - ligands beside CRH, the concept is dawning that CRH, its congeners, and their receptors form an intricate network in the brain, which provides a variety of potential targets for drug intervention.
Here, we review recent developments regarding the functions of CRHR1, CRHR2, and their ligands in the brain. On the basis of the new reported findings, we put forward a new concept of the role of CRHR1, CRHR2, and their ligands in both the acute and recovery phases of the stress response. This concept is also presented as a hypothetical model for the pathophysiology of anxiety and major depressive disorders.
The expanding family of CRH and its receptors
The number of known members of the CRH family of neuropeptides has considerably grown during recent years. Until recently, it also comprised peptides structurally related to CRH, including urocortin (Ucn),7 fish urotensin I,8 and amphibian sauvagine.9 The biological actions of CRH and Ucn are mediated via binding to two types of G-protein-coupled receptors, CRHR1 and CRHR2, which have different expression patterns and physiological functions.10-12 Regarding CRHR2, two different splice variants were identified, CRHR2α and CRHR2β, which have an uneven distribution between brain (CRHR2α) and periphery (CRHR2β) in rodents.13
CRHR1 binds both CRH and Ucn with high affinity, whereas CRHR2 shows a clear preference for Ucn (Table I) 10,11,14,15 The next question was whether a neuropeptide that selectively binds to CRHR2 exists in mammalian brain.
Table I. Binding properties and functional activities of members of the corticotropin-releasing hormone (CRH) neuropeptide family. Data are extracted from references 10, 11, 14, and 15. Values were determined using transiently transfected COS-M6 cells (h/rCRH only), transiently or stably transfected mouse Ltk - cells (h/rCRH only), or stably transfected Chinese hamster ovary cells (cAMP measurements) or their membranes (binding experiments). *Binding experiments were conducted with α- and β-splice variant of the human CRHR2. For more details, see the mentioned publications. (-), data not available. Ucn, urocortin; CRHR, corticotropin-releasing hormone receptor; h, human; m, murine; r, rat; Ki dissociation constant; EC50, median effective concentration.
| Peptide | Binding hCRHR1 (Ki, nM) | Binding rCRHR2α (Ki, nM) | Binding mCRHR2β (Ki, nM) | cAMP hCRHR1 (EC50, nM) | cAMP rCRHR2α (EC50, nM) | cAMP mCRHR2β (EC50, nM) |
| CRH (rat/human) | 3.3 | 42 | 47 | 4 | 20 | - |
| CRH (sheep) | 1.1 | 230* | 320* | - | - | - |
| Ucn (rat) | 0.32 | 2.2 | 0.62 | 0.15 | 0.063 | 0.087 |
| Ucn (human) | 0.4 | 0.3* | 0.5* | - | - | - |
| Ucn II (human) | >100 | 1.7 | 0.50 | >100 | 0.26 | 0.42 |
| Ucn II (mouse) | >100 | 2.1 | 0.66 | >100 | 0.14 | 0.05 |
| Ucn III (human) | >100 | 21.7 | 13.5 | >100 | 0.16 | 0.12 |
| Ucn III (mouse) | >100 | 5.0 | 1.8 | >100 | 0.073 | 0.081 |
| Urotensin I (fish) | 0.4 | 1.8* | 5.7* | - | - | - |
| Sauvagine (frog) | 0.7 | 0.5* | 2.1* | - | - | - |
This question was answered by the very recent discovery of two selective ligands for CRHR2, Ucn II (also termed stresscopin-related peptide16,17) and Ucn III (also called stresscopin14,17) using mouse and human cDNA libraries (Table I). In contrast to CRH and Ucn, neither Ucn II nor Ucn III bind to CRHBP.14 The murine Ucn II (mUcn II) gene encodes a 112-amino acid (aa) precursor containing a putative 38-aa mature peptide at the C terminus.16 Amino acid sequences in the coding region of mUcn II show 76%, 47%, 34%, and 42% homology with human urocortin-related peptide (URP [GenBank accession No. BE622276], which Lewis et al14 proposed should be called hUcn II; henceforth this nomenclature is adopted in this paper), pufferfish (Takifugu rubripes) URP,18 human/rat CRH (h/rCRH), and rat Ucn (rUcn), respectively. The putative 38-aa sequence of hUcn III and mUcn III share 40% and 37% sequence homology with mUcn II and hUcn II, respectively.14 Their rather distant relationship with CRH and Ucn is illustrated by their modest homology with h/rCRH (32% and 26%) and hUcn and mUcn (21% and 18%). hUcn III and mUcn III share an identity of 42% and 37% with hUcn II and m Ucn, whereas, out of three pufferfish peptide sequences, two (GenBank accession Nos.AJ251323 and AL175143) were highly related to hUcn III and mUcn III, and the third one resembled mostly fish (eg, flounder) urotensin I.14
Thus, in mammalian brain, the CRH/Ucn receptor network comprises two receptor types and four ligands, of which three (CRH, Ucn II, and Ucn III) are pharmacologically monogamous and one (Ucn) is, at least regarding CRHR1 and CRHR2, promiscuous. The complexity is further increased by the presence of a binding protein, which presumably constrains the biological activity of CRH and Ucn.19
CRHR1 and CRHR2 in the brain: receptor vs ligand distribution
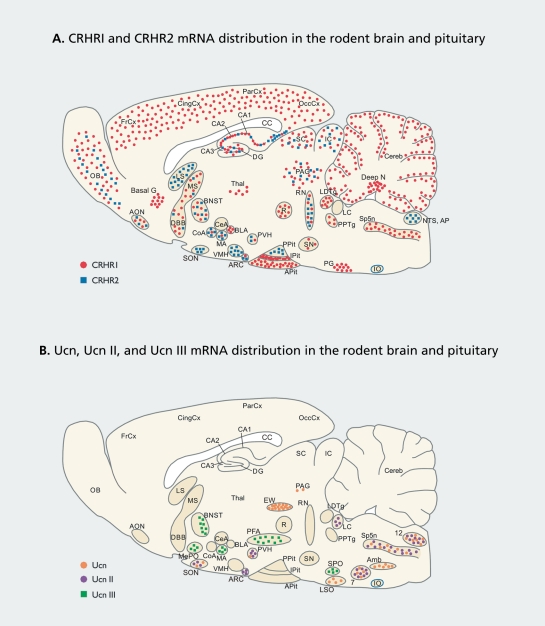
CRHR1 and CRHR2 mRNA show a distinct, but overlapping, distribution in the brain as revealed by in situ hybridization histochemistry studies (Figure 1A).12,20-23
Figure 1. Distribution of CRHR1 and CRHR2 mRNA (A), and Ucn, Ucn II, and Ucn III mRNA (B) in a sagittal section of the rodent brain. The presented mRNA distribution is based on in situ hybridization studies.12,14,16,20-23 The drawn sagittal sections are only 2D schematic representations and, therefore, cannot be neuroanatomically exact. Abbreviations: 7, facia! nucleus; 12, hypoglossal nucleus; Amb, ambiguus nucleus; AON, anterior olfactory nucleus; AP, area postrema; APit, anterior pituitary; ARC, arcuate nucleus; Basal G, basal ganglia; BLA, basolateral amygdala; BNST, bed nucleus stria terminalis; CA1-3, fields CA1-3 of Ammon horn; CC, corpus callosum; CeA, central amygdaloid nucleus; Cereb, cerebellum; CingCx, cingulate cortex; CoA, cortical nucleus amygdala; Deep N, deep nuclei; DG, dentate gyrus; DBB, diagonal band of Broca; EW, Edinger-Westphal nucleus; FrCx, frontal cortex; IC, inferior colliculi; 10, inferior olive; IPit, intermediate lobe of the pituitary; LC, locus ceruleus; LDTg, laterodorsal tegmental nucleus; LS, lateral septum; LSO, lateral superior olive; MA, medial nucleus amygdala; MePO, median preoptic area; MS, medial septum; NTS, nucleus tractus solitarius; OB, olfactory bulb; OccCx, occipital cortex; PAG, periaqueductal gray area; ParCx, parietal cortex; PFA, perifornical area; PG, pontine gray, PPit, posterior pituitary; PPTg, pedunculopontine tegmental nucleus; PVH, paraventricular hypothalamus; R, red nucleus; RN, raphe nuclei; SC, superior colliculi; SN, substantia nigra; SON, supraoptic nucleus; SP5n: spina! trigeminal nucleus; SPO: superior paraolivary nucleus; Thai, thalamus; VMH, ventromedial hypothalamic nucleus.
CRHR1 displays a widespread distribution in the central nervous system (CNS) regions involved in sensory information processing and motor control, such as the cortical mantle, olfactory bulb and related regions, hippocampus, amygdala (mainly basolateral, medial, and cortical nuclei), basal ganglia, certain thalamic nuclei, medial and lateral hypothalamic nuclei, periaqueductal gray area, many brainstem nuclei (sensory, motor, and reticular), and cerebellum (Figure 1A). In contrast, CRHR2 is virtually restricted to subcortical structures such as the lateral septum (LS),bed nucleus of the stria terminalis (BNST), the ventromedial hypothalamic nucleus (VMH), and certain amygdaloid nuclei (medial and cortical nuclei). Moderate levels of both receptors are expressed in the dorsal and median raphe nuclei, whereas only low levels are found in the hypothalamic paraventricular nucleus (PVH).12,20-22 In the anterior pituitary, CRHR1 mediates the effects of CRH on adrenocorticotropic hormone (ACTH) release and, thus, on glucocorticoid hormone secretion (Figure 1A). 10,22
Due to its pharmacologically characterized effects in the brain in terms of autonomic, neuroendocrine, behavioral, and emotional changes,24 CRH is regarded as the principal mediator of the stress response. However, as pointed out by Bittencourt and Sawchenko,21 a puzzling issue remains that neuronal activation (in terms of Fos expression) is found in nuclei known to be pertinent for eliciting the stress response (eg, the central nucleus of the amygdala, paraventricular nucleus, nucleus tractus solitarius (NTS), ventrolateral medulla, locus ceruleus), but not containing appreciable amounts of either CRHR mRNA expression12,23 or CRH binding.25 A possible explanation may be the occurrence of transsynaptic effects via structures that do contain CRHR1 or CRHR2, but this notion can only be partly satisfactory given the multitude and potency of CRH-induced responses.
A mismatch has been found with regard to the localization of Ucn-immunoreactive (ir) fibers and CRHR2 distribution. Brain nuclei expressing highest levels of Ucn mRNA, ie, the Edinger-Westphal nucleus (EW),the lateral olivary nucleus, and the supraoptic nucleus (Figure 1B), mainly project caudally; this is in the face of high concentrations of CRHR2 in forebrain areas, such as the BNST, LS, and VMH.20 However, a Ucn-ir projection stemming from the EW was found terminating in the intermediate lateral septal nucleus (iLS),21 but the projection ended in a region medially localized from the ventrolateral part to which CRHR2 is confined.20 With the recent discovery of the CRHR2-selective ligands Ucn II and Ucn III, the issue regarding the localization of the endogenous ligands of forebrain CRHR2 can be addressed.
The distribution of Ucn II mRNA is distinctly subcortical, including regions known to be involved in physiological and behavioral responses to stress, such as the PVH (HPA axis and autonomic control26), the locus ceruleus (arousal and anxiety27), and the arcuate nucleus (food intake and energy balance28), and is partly overlapping that of CRH (PVH29) and Ucn (brainstem and spinal motor nuclei) (Figure 1B).20 Intracerebroventricular (ICV) injection of Ucn II induces Fos expression in the BNST, PVH, central nucleus of the amygdala, parabrachial nucleus, and NTS, but not in other CRHR2-rich locations, such as the LS, raphe nuclei, and VMH.16 In view of the high affinity of Ucn II for CRHR2, the latter observation was unexpected and a solid explanation is still lacking. The disagreement may indicate the requirement of additional factors necessary for activation of the neuron, at least in terms of Fos. Alternatively, these CRHR2-expressing neurons may display activation of signal transduction pathways not ultimately leading to synthesis of Fos. For instance, we have recently found that phosphorylation of cAMP response element-binding protein (CREB), a transcription factor activated through CRHR1 and CRHR2, is not necessarily correlated with Fos expression (BilangBleuel et al, unpublished data). Nevertheless, given the evident similarity between the localizations of Ucn II and CRH, it seems pertinent that Ucn II participates in responses to stress.16 However, their differential binding preference for CRHR1 and CRHR2 suggests that CRH and Ucn II have different functions in the stress response.
The localization of Ucn III (Figure 1B) 14,17 in the brain is different from that of CRH,29 Ucn,20 and Ucn II.16 This latest discovered member of the CRH neuropeptide family is found in the median preoptic area, the rostral perifornical area (a region lateral from the PVN in the hypothalamus), the posterior part of the BNST, and the medial nucleus of the amygdala (Figure 1B).14 Until now, unfortunately, no Fos studies have been published with Ucn III. It is relevant to note though that parts of the perifornical region project into the (CRHR2-rich) LS, an area in which both Ucn-ir and piscine urotensin I-ir can be found.20 However, within the LS, Ucn-ir and urotensin I are differentially localized with Ucn-ir prevailing in the medial aspect of the iLS and urotensin I-ir concentrating in the ventrolateral aspect of this nucleus, ic, the site where CRH-R2 mRNA is also found (see also above). It may be speculated that, given the structural relationship between urocortins and urotensin, the immunoreactivity in the ventrolateral aspect of the iLS as revealed with the piscine urotensin I antiserum may actually be Ucn III. Recently, Ucn III-ir fibers were indeed found in this region of the LS (and in the VMH), which corresponds well with the sites of CRHR2 mRNA expression (P. E. Sawchenko, personal communication).
CRHR1 and CRHR2 in anxiety, sleep/electroencephalographic regulation and HPA axis control: significance for clinical anxiety and depression
In recent years, many studies have been performed to delineate the specific role of CRHR1 and CRHR2 in stress-related physiological and behavioral processes to gain insight into anxiety and major depressive disorders. Various strategies have been employed including pharmacological approaches, mutant mice with functional deletions in one of the receptors, and antisense oligodeoxynucleotide (ODN) technology. These investigations have provided insight into the complexity of the contributions of CRHR1 and CRHR2 in the regulation of emotional behavior, HPA axis activity, and autonomic function. For some processes, the roles of CRHR1 and CRHR2 seem clear, whereas for others they still need to be clarified.
Anxiety
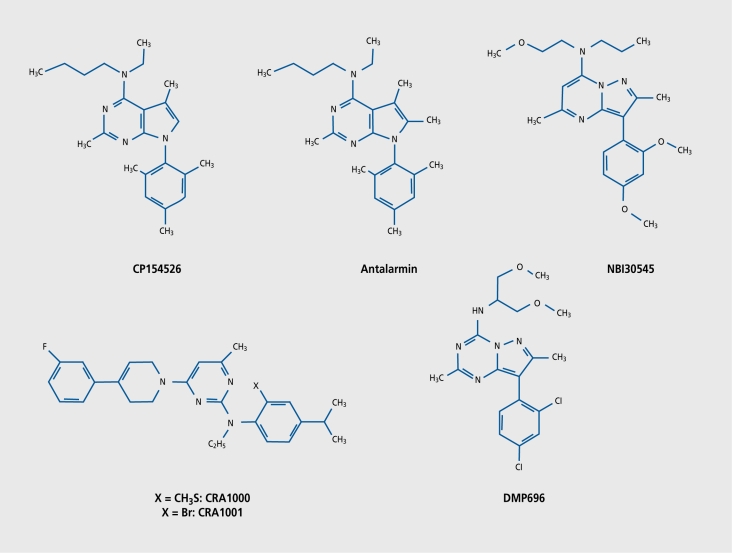
CRH is highly implicated in the regulation of anxietyrelated behavior and is thought to play a pivotal role in anxiety and depressive disorders.24,30,31 Several lines of evidence point to the participation of CRHR1 in the effects of CRH. First, CRHR1 binds CRH with high affinity, in contrast to CRHR2. Second, CRHRl-deficient mice show decreased anxiety-related behavior.32,33 Third, transgenic mice overexpressing CRH show increased anxiety-related behavior34 (van Gaalen et al, unpublished data). Fourth, central administration of CRHR1 antisense ODNs inhibit CRH- and social defeat-elicited anxiety-related behaviors and evoke anxiolytic-like effects in certain anxiety tests, whereas CRHR2 antisense ODNs did not produce any significant effects in these tests.35-38 Fifth, the selective (nonpeptidergic) CRHR1 antagonists (Figure 2), NBI-27914, CRA1000, CRA1001 (all anilinopyrimidines), and CP154526 (a pyrrolopyrimidine) inhibit the anxiogenic action of CRH39,40 (for review, see reference 41). However, it should be noted that, in many cases, the antagonists had no anxiolytic effect under nonstressed conditions in behavioral paradigms such as the light/dark box or elevated plus-maze, but did so after the animals had been preexposed to stress.39,40,42 Thus, it seems that the antagonists need the release of endogenous CRH or CRH-like peptide for their action to come about, also underlining that these compounds do not have any intrinsic (cf, reverse agonist) activity. Anxiolytic effects have also been observed with the more novel antagonists, R121919 (formerly called NBI-30775, a phenylpyrimidine),42,43 antalarmin (a pyrrolopyrimidine derivative),44,45 DMP904 (a pyrazolopyrimidine), and DMP696 (a pyrazolotriazine).46-48 Due to recent achievements in the development of nonpeptidergic CRHR1 ligands for single photon emission computed tomography (SPECT) and positron emission tomography (PET), in vivo monitoring of CRHR1 in the living brain may soon become possible.49,50
Figure 2. Chemical structures of several nonpeptidergic corticotropin-releasing hormone receptor type 1 (CRHR1) antagonists.
In summary, there is robust evidence that CRHR1 is highly involved in anxiety-related behavior. Nevertheless, a role of CRHR2 in this cannot be excluded. The three lines of CRHR2-deficient mice,51-53 unfortunately, do not provide an unambiguous answer to the question about the role of this receptor in anxiety. In two lines of CRHR2-deficient mice, increased anxiety-related behavior was observed,52,53 whereas in the third no changes were found.51 This disparity may be caused by differences in genetic background, environmental factors, and the behavioral test conditions used.54
Recent pharmacological experiments, however, point to a much more complex involvement of CRHR2 in anxiety. The picture is emerging that CRHR2 activation can result in anxiolysis or anxiogenesis depending on the timing of the animal test and, possibly, the localization of the receptor. Radulovic et al55 found that injection of a high (500 ng/mouse) CRHR2-binding dose of h/rCRH into the iLS of mice increases anxiety-like behavior in the plus-maze 30 min postinjection, which was prevented by pretreatment with the CRHR2 antagonist antisauvagine-30. Increased anxiety in the plus-maze was also observed 30 min after a 60-min immobilization trial, which was prevented by intraseptal, but not intradorsohippocampal, administration of antisauvagine-30 before the stress procedure.55 Thus, acutely CRHR2-mediated signaling in the iLS results in anxiogenesis. Physiologically, the CRHR2 in this nucleus is activated by Ucn stemming from the EW,20 and, likely more so, by Ucn III from the perifornical region (P. E. Sawchenko, personal communication).14 In contrast, ICV administration of the selective CRHR2 agonists mUcn II56 or mUcn III (E. Zorrilla, personal communication) results in decreased anxiety-related behavior in the plus-maze not acutely, but after 4 hours. Thus, CRHR2 in the brain is capable of decreasing anxiety in a delayed fashion.
Thus, the anxiogenic and anxiolytic properties of CRHR2 are certainly not paradoxical, because they operate in different time domains poststress. Together, it may be hypothesized that during the acute phase of the stress response, the increase in emotionality is evoked by CRH-mediated CRHR1 activation and lienor Ucn IIl-mediated CRHR2 activation, presumably in the amygdala, BNST, and/or iLS. However, as part of the recovery phase, CRHR2, following activation by Ucn, Ucn II, and/or Ucn III, participates in reducing emotionality some hours after the stressful experience. Thus, CRHR2 mediates a dual mode of action on anxietyrelated behavior. A challenge for the future will be to resolve the exact neural circuitry involved, the underlying molecular and cellular mechanisms, and the manner in which this dual action program is tuned by afferent neural (eg, from the frontal cortex, hippocampus, hypothalamus, and autonomic centers) and humoral (eg, glucocorticoid hormones) input.
Sleep/electroencephalographic regulation
Sleep disturbances are often seen after exposure to stress57,58 and are also commonly observed in major depressive disorders.59 The disturbances observed in depressed patients include a disinhibition of rapid eye movement (REM) sleep (encompassing reduced REM latency, reduced REM density, and prolonged first REM sleep period), decreases in slow-wave-sleep (SWS), increases in wakefulness, and disturbed sleep continuity.59 Evidence is accumulating that the sleep disturbances seen in depressed patients are at least in part due to a hypersecretion of CRH or CRH-like peptides in the CNS. Administration of CRH to rats or humans increases wakefulness and decreases SWS,60-62 whereas, conversely, ICV application of a-helical CRH (9-41) (a peptidergic, predominantly CRHR1, antagonist) to rats decreases wakefulness and increases SWS.57 Moreover, pretreatment with α-helical CRH (9-41) abolished the stress-induced increases in REM sleep in rats.58
Recently performed basic and clinical studies using R121919 have provided further insight into the role of CRHR1 in sleep/electronencephalographic (EEG) regulation and sleep disturbances in depressed patients. In rats selectively bred for increased innate anxiety, R121919 abolished the increases in plasma ACTH and corticosterone levels and the decreases in sleep induced by the administration of the vehicle (a citrate buffer, pH 4.8, an acid vehicle causing mild pain) (M. Lancel et al, unpublished data).63 This observation points to a role of CRHR1 in stress-induced changes in sleep. In depressed patients treated for a time period of 30 days with R121919, increases in SWS and decreases in the number of awakenings and REM density were found.64 By the end of the treatment, an inverse correlation was found between the duration of SWS and the severity level of the depression (expressed as the Hamilton score).64 However, plasma levels of Cortisol (which is known to affect sleep architecture) were hardly changed during the course of treatment (H. E. Künzel et al, unpublished data). These findings strongly suggest an involvement of CRHR1 in the sleep disturbances seen in major depressive disorders. Moreover, the sleep and endocrine data together suggest that the hypersecretion of Cortisol does not have a major impact on sleep in depressed patients. Currently, no information is available on the role of CRHR2 in sleep regulation. Recently, the effects of ICV administered urocortin on the EEG and on event-related potentials (ERPs) of awake rats was evaluated.65 It was observed that the neuropeptide enhances arousal, as determined by EEG, and modulates the speed of stimulus evaluation as measured by ERPs.65 Clearly, insight into the function of CRHR2 in sleep/EEG regulation awaits further investigations.
HPA axis control
Evidence has been accumulating that disturbances in the regulatory control of the HPA axis play a pivotal role in the etiology of major depression.66,67 Moreover, studies on depressed patients have indicated that there appears to be a close correlation between a stable remission of the clinical symptomatology and a normalization of HPA regulation.68 The cause for the aberrant - in most cases, hyperactive - HPA axis is presumably a hyperactive central CRH system (for review, see references 24, 30, and 69 to 71) and defunct brain and pituitary corticosteroid receptor systems.66,72,73 Although it was more than a decade ago that a reduced CRH receptor density was found in the frontal cortex of depressed patients who had committed suicide,3 efforts have only recently started to delineate CRHR1 and CRHR2 expression in postmortem brains of depressed patients. In a recent study, investigators observed in pituitaries of suicide victims a shift in the ratio of CRHR1 (less)/CRHR2 (more) mRNA levels, but it was unclear whether the victims had a history of major depressive illness.74 Studies on the role of CRHR1 and CRHR2 in HPA regulation have mainly been performed in rodents.
Recent studies on CRHR1- and CRHR2-deficicnt mice indicate that these receptors play different roles in the HPA axis. CRHR1-deficient mice are unable to mount a stress-induced HPA response in terms of circulating ACTH and corticosterone, whereas baseline ACTH levels are normal and baseline corticosterone levels virtually undetectable.32,33,75,76 Thus, CRHR1 is crucial for stress-induced HPA responsiveness, but not for the baseline hypothalamic-pituitary drive. For the latter, vasopressin seems to be necessary and its expression was even found to be increased in the PVH of CRHR1 mutants.76 The observation that baseline corticosterone levels were undetectable throughout the circadian cycle revealed a role of CRHR1 in the development of the adrenal medulla and adrenocortical sensitivity to ACTH.32 In the PVH, only low levels of CRHR1 mRNA can be found, but levels are induced in response to stress22,77-80 or ICV CRH administration.81 This induction of CRHR1 mRNA may be implemented in the positive feedback action of CRH on paraventricular neurons, but the evidence needs to be further solidified.
Exposure of the CRHR2-deficient and wildtype mice to restraint stress revealed changes in HPA axis regulation at different levels in two out of three mutant lines.51-53 Presumably, due to single-time -point analysis, Kishimoto et al53 did not observe any changes in stressinduced HPA activity. The other two CRHR2 mutant mouse lines showed increased responses in plasma ACTH and corticosterone levels to restraint stress.51,52 The plasma ACTH levels in the mutant mice decreased within just 10 min of onset of stress, which is in sharp contrast to the wildtype animals, whereas corticosterone levels continued to rise reaching higher levels than the wildtypes.51,52 At 90 min poststress, corticosterone levels were still higher in the mutant mice. It is clear from these data that there is an array of changes in the HPA axis of CRHR2 mutant mice that may explain the different hormonal responses: (i) hypersensitivity of the corticotrophs to hypothalamic secretogogues; (ii) higher glucocorticoid levels cause ACTH levels to fall earlier due to higher negative feedback inhibition; and (iii) the adrenal cortex of the mutant mice is possibly hypersensitive to ACTH.51,52 In summary, these changes in HPA responses to stress suggest that CRHR1 and CRHR2 arc acting in an antagonistic manner with CRHR1 acting proactively and CRHR2 acting attenuatively. The sites of these antagonistic actions are currently unknown, but may include the pituitary gland, the PVH, brain areas providing afferent input to the PVH such as the amygdala, BNST, and the lateral septum, and the sympathetic motor nuclei driving the sympathoadrenomedullary pathway. Studies on the HPA axis of recently created CRHR1- and CRHR2-double mutant mice confirm the data obtained with the single gene mutants, with the CRHR1 mutation nevertheless having a dominating influence, presumably due to its “key” position on the anterior pituitary corticotrophs.82
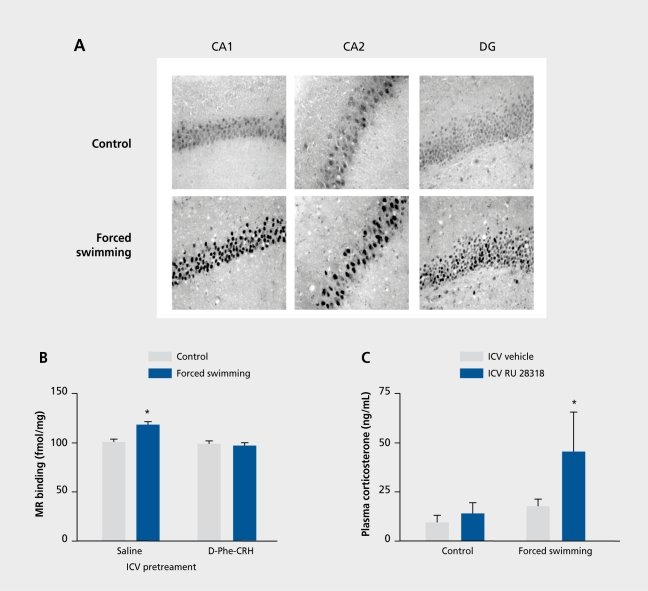
Beside the CRH receptors, corticosteroid receptors are also key elements in the regulation of the HPA axis.72,73 They can be distinguished in two types of glucocorticoid-binding receptors: the mineralocorticoid receptor (MR or type I) and the glucocorticoid receptor (GR or type II).83 MRs are mainly localized in the hippocampus, whereas GRs have a widespread distribution in the CNS. They have different functions in HPA regulation with MRs mediating the tonic inhibitory influence of the hippocampus (via the BNST) on parvicellular PVH neurons and GRs mediating the negative feedback action of glucocorticoids on HPA activity.72,73,83 Recently, we discovered a new mechanism of cross-talk between the CRH neuropeptide systems and the hippocampal MR. It was found that, within 8 h poststress, acute stressors via a CRHR-mediated action cause an elevation in MR levels in the hippocampus, which was associated with an augmented MR-mediated inhibition of HPA activity (Figure 3.).84 Thus, CRHRs are involved in strengthening an important control instrument (ie,MR) of the HPA axis. Although the effect of stress was mimicked by an ICV injection of CRH, pointing to an involvement of CRHR1 (Figure 3),84 exactly which CRH receptor- CRHR1 or CRHR2- is the mediator of this phenomenon needs to be clarified, as much as the localization of these receptors. Furthermore, we have postulated that, given the eminent role of the CRH-MR pathway in maintaining control of HPA axis activity poststress, in patients suffering from a stressrelated disorder, such as major depression, HPA hyperactivity may have developed due to desensitization of MR-inducibility by CRH or CRH-like neuropeptides.73,84
Figure 3. Effect of forced swimming stress on rat hippocampal mineralocorticoid (MR) receptor levels and its consequences for MR-mediated hypothalamic-pituitary-adrenocortical (HPA) axis regulation. A. Within 24 h, forced swimming induces an increase in MR immunoreactivity in nuclei of pyramidal and granular neurons in the CA1, CA2, and CA3 fields of the Ammon horn (not shown) hippocampal pyramidal layers and dentate gyrus (DG). This effect was stressor-specific, as it was also seen after novelty stress, but not after exposure to a cold environment (not shown). B. Intracerebroventricular (ICV) pretreatment with the nonselective corticotropin-releasing hormone receptor (CRHR) antagonist D-Phe12, Nle21,38, α-Me-Leu37)-CRH12-41. (D-Phe-CRH12-41) prevented the forced swimming-induced increase in hippocampal MRs. Moreover, ICV corticotropin-releasing hormone (CRH) treatment mimics the effect of stress on hippocampal MR levels (not shown). C. As shown in an RU 28318 challenge test, the forced swimming-induced elevation in hippocampal MR levels is associated with a potentiated MR-mediated inhibition of HPA activity. RU 28318 is a selective MR antagonist and, after ICV administration and by blocking the MR, induces a release of the HPA axis from the tonic inhibitory control elicited by hippocampal MRs. The experiment was based on the idea that, when MRs are upregulated poststress, the release of HPA activity by RU 28362 would be exaggerated compared with the control, unstressed, situation. Thus, rats were stressed by forced swimming and 24 h later injected ICV with RU 28362. Trunk blood was collected 30 min postinjection. Indeed, the results show that the release of HPA activity in terms of plasma corticosterone levels was larger when rats had been stressed 24 h earlier, indicating that the stress-induced increase in hippocampal MR levels is associated with an enhanced MR-mediated tonic inhibitory control of HPA axis activity. For further details, see reference 84. Reproduced from reference 84: Gesing A, Bilang-Bleuel A, Droste SK, Linthorst ACE, Holsboer F, Reul JMHM. Psychological stress increases hippocampal mineralocorticoid receptor levels: involvement of corticotropin-releasing hormone. J Neurosci. 2001;21:4822-4829. Copyright © 2001, Society for Neuroscience.
To summarize, CRHR1 plays a critical role in the acute phase of the stress-induced HPA response, whereas CRHR2 is involved in the recovery phase. The stressevoked increase in hippocampal MR expression appears to be part of the recovery phase, but whether this clement is mediated by CRHR1 or CRHR2 needs clarification.
Significance for anxiety disorders and depression
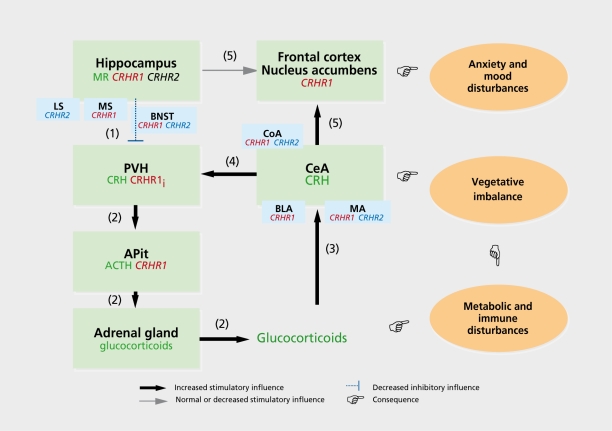
A CRH hyperfunction in the brain appears to be a characteristic often seen in major depression and anxiety disorders. This notion originates from cerebrospinal fluid (CSF) CRH measurements, CRH binding, and CRH challenge tests.4,85 Comparison of a variety of studies on CSF CRH measurements revealed that this was not an equivocal finding in all studies, but seemed to depend on certain factors associated with depressive illness. It is especially those patients showing melancholia, psychosis, hypercortisolemia, and dexamethasone nonsuppression who present elevated CSF CRH levels (for reviews, see references 69 and 70). It is presently still unclear where in the brain the elevated levels of CRH in the CSF stem from. It is, however, unlikely that they are derived exclusively from the PVH. The hypersecreted CRH may originate from the central amygdaloid nucleus, in which the neuropeptide's synthesis is known to be under positive control by glucocorticoid hormones.86 Thus, those depressed patients who hypersecrete glucocorticoids - at least in part due to defunct hippocampal inhibition of HPA activity (see above and Figure 4) - also have increased CRH synthesis in the central amygdaloid nucleus. Indeed, hypercortisolemia in depressed patients is associated with elevated CSF CRH levels (see above). The increased expression of CRH in the central amygdaloid nucleus may be responsible for the increase in emotionality and anxiety, and the neurovegetative instability often associated with major depression.87,88 Moreover, the central amygdaloid nucleus exerts a stimulatory influence on the HPA axis, via its direct and indirect (via the BNST) connections to the PVN.73 It may be speculated that in depressed patients a positive feed-forward loop may have been established between the amygdala and the HPA axis. Given that the neural and humoral components of this loop have uncountable interactions with other - central and peripheral - systems, the consequences will be manifold, including effects on mood, cognition, libido, the cardiovascular system, immune system, and metabolism (Figure 4).
Figure 4. Shift in limbic afferent control of the hypothalamic-pituitary-adrenocortical (HPA) axis and its consequences for affective states and physiological functioning. This figure presents a working hypothesis on limbic-HPA axis interactions in anxiety and depressive disorders. On the basis of established afferent and efferent regulatory interactions between the HPA axis and its limbic “partners” (the hippocampus and the amygdala), we propose a framework giving a neuropharmacological basis to the psychiatric, neurovegetative, and physiological disturbances seen in anxiety and depressive disorders. Patients suffering from major depression or pathological anxiety often show a dysregulated - mostly hyperactive - HPA axis which is associated with increased cerebrospinal fluid (CSF) levels of corticotropin-releasing hormone (CRH).30,69,70 Apart from intrinsic HPA axis disturbances at the hypothalamic, pituitary, or adrenal level, the reason for the HPA hyperactivity may well derive from defunct processes in the hippocampus and central amygdaloid nucleus (CeA) known to provide direct and indirect efferent projections to the hypothalamic paraventricular nucleus (PVH).73,89 As postulated (see text), chronic stressful life events result in a loss of capacity of CRH or CRH-related peptides to upregulate hippocampal mineralocorticoid receptor (MR) levels (1) leading to a loosening of the tonic inhibitory influence on parvicellular neurons in the PVH.73 In depressed patients and in a variety of animal models, a reduced expression of MR has indeed been found.72,73,90 (2) Consequently, levels of CRH and coexpressed vasopressin will increase in these neurons (for a review, see references 65, 72, and 74) providing an enhanced drive on HPA activity, anterior pituitary (APit)CRH-binding receptor (CRHRI) desensitization, and adrenal hyperplasia.66,73 (3) Subsequently, the elevated circulating glucocorticoid levels will raise CRH expression in the CeA,87 (4) resulting in an enhanced stimulatory influence on the PVH. In this manner, a positive feed-forward loop develops accelerating the establishment of a state of sustained HPA hyperactivity. Importantly, regarding the activity of the efferent amygdaloid and hippocampal projections to the nucleus accumbens, (5) a shift may occur toward an augmented input from the CeA relative to that from the hippocampus. Such a shift will result in an increased emotionality likely to develop in vulnerable subjects to pathological anxiety and mood disorders. Finally, the enhanced - caudally directed - CeA activity leading to an increased sympathetic outflow in combination with the excess glucocorticoid levels can result in a variety of neurovegetative (eg, cardiovascular or gastrointestinal problems), metabolic, and immune disturbances, often seen in anxiety, mood, and psychosomatic disorders. Also, CeA- and hippocampus-associated structures are drawn such as the cortical nucleus amygdala (CoA), basolateral amygdala (BLA), and medial nucleus amygdala (MA) with regard to the CeA, and the lateral septum (LS)and media! septum (MS) with regard to the hippocampus because they participate strongly in CeA and hippocampus functioning. In addition, their own function is modulated by incoming signals via CRHR1 and/or CRHR2. The bed nucleus stria terminalis (BNST), containing both CRHR1 and CRHR2, is drawn because it is an important relay station for hippocampal output to the PVH. Thus, this model can be used as a framework to investigate in an integrative manner the anxiogenic, anxiolytic, HPA-driving, HPA-restraining, and other actions of CRH, urocortin (Ucn), Ucn II, and Ucn III. Such studies should also implement interactions of these neuropeptides with the classic neurotransmitters, which, for sake of clarity, were not depicted in the scheme, as were other, possibly participating, structures such as, for instance, the thalamic paraventricular nucleus which contains low-to-moderate CRHR1 levels.22,91 The elucidation of the interactions between the various components of this network including the CRHRs and their ligands will bring forward new pharmacotherapeutic strategies for the treatment of anxiety and depressive disorders. Note: The green boxes comprise primary elements (ie, brain regions and parts of the HPA axis) of the system of limbic-HPA axis interactions. The orange ovals represent the different categories of pathological disturbances resulting (as indicated by the pointing hands) from aberrant functioning of certain elements of the network. The localization in certain elements (eg, brain regions, APit, adrenal gland) of CRHR1 is indicated in red, CRHR2 in blue, whereas other important components such as CRH, adrenocorticotropic hormone (ACTH), glucocorticoids, and MR, are indicated in dark green. With (1) to (5) the sequence of the progressive disturbances are denoted (see main text of legend). CRHR1i: CRHR1 mRNA inducible by stress or CRH (see text).
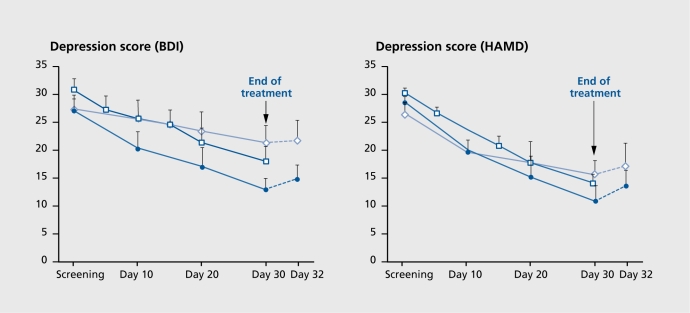
Above, we postulated that CRHR1 and CRHR2 play different roles in stress-evoked anxiety, in which both receptors operate, possibly in different regions of the brain (eg, central amygdaloid nucleus, BNST, intermediate LS), in the acute (anxiogenic) phase of the stress response, and in which CRHR2 promotes anxiolysis during the stress recovery phase. We have also described a parallel mechanism for the role of these receptors in the stress-induced HPA response. As mentioned, there are strong indications for a CRH-evoked CRHR1-mediated hypersignaling in the brain of patients suffering from anxiety and depressive disorders. This condition is thought to be responsible for the increases in emotionality and HPA activity, and neurovegetative and sleep disturbances seen in these patients. Indeed, a preliminary exploratory clinical study in our clinical department at the Max Planck Institute of Psychiatry in which depressed patients were treated with the nonpeptidergic CRHR1 antagonist R121919 showed that blocking CRHRl signaling in these patients had beneficial effects (Figure 5). Beside the effects on sleep architecture (see above),64 the treatment resulted in a substantial reduction in the depression (Figure 5) and anxiety scores.92 The current status of research promises that CRHRl antagonism represents a novel pharmacotherapeutic strategy to treat depression, pathological anxiety such as phobias, panic, and posttraumatic, stress disorder. This new development in the pharmacological treatment of major depressive and anxiety disorders is a significant step toward the formation of basic science-driven therapies. It is to be expected that this development is also an important step on the way to the ultimate goal of a pharmacotherapy with rapid onset of clinical effect, negligible side effects, and absent treatment resistance.
Figure 5. Changes in the self-rating Beck Depression Inventory (BDI; left) and the 21-item Hamilton Depression Rating Scale (HAMD; right) during and after treatment with the nonpeptidergic CRHRI antagonist R121919 (formerly called NBI-30775) and the selective serotonin reuptake inhibitor (SSRI) paroxetine. During the screening period, all psychoactive medication was stopped for a minimum of 5 days. Patients were enrolled in two dose escalation panels. In panel 1, the dose range increased from 5 to 40 mg (open diamonds), whereas, in panel 2, the dose range increased from 40 to 80 mg (closed circles). The increases took place within 30 days. The panel 2 treatment regimen resulted in a response rate (ie, 50% reduction of the initial severity of depression) equivalent to that found in paroxetine-treated patients (open squares; historical data for patients matched according to age, gender, and severity of depression; patients had been treated with 20 to 40 mg paroxetine under clinical drug study conditions). In both panels, HAMD and BDI rating scales worsened after discontinuation of R121919. Data are means ± standard error of the mean. Reproduced from reference 92: Zobel AW, Nickel T, Kunzel HE, et al. Effects of the high-affinity corticotropin-releasing hormone receptor I antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res. 2000;34:171-181. Copyright © 2000, Elsevier Science.
Nevertheless, extrapolating the aforementioned hypothesis, it cannot be excluded that, apart from CRHRl hyperfunction, a condition of CRHR2 hypofunction may exist in depressed patients. Due to impaired CRHR2-mediated anxiolysis, the subject might remain in an extended state of anxiety and arousal. Possibly, other stress recovery processes could also be impaired by the defunct CRHR2, including HPA regulation and autonomic processes.17,51-53,93,94
Figure 4 present a working hypothesis based on an integration of the previously described issues. Our hypothetical model basically proposes a mechanism in which the development of anxiety and mood disorders is caused by a shift in the balance between the effects of the hippocampus and the central amygdaloid nucleus initially on the HPA axis, but eventually also on the nucleus accumbens and frontal cortex, brain regions involved in the regulation of affective states. The altered state of amygdaloid output is also expected to affect autonomic outflow which, in combination with the enhanced glucocorticoid secretion, could be responsible for the physiological, metabolic, and immune disturbances often seen in depressed and anxious patients. The CRH neuropeptide family and their receptors are major participants in this network and, with the recent growth of this family (ie, Ucn II and Ucn III), a major step has been made toward the elucidation of the roles of CRHRl and CRHR2 in anxiety and depression.
Concluding remarks
Overall, the pattern that is emerging is one of a network subserving the acute and the recovery phase of the stress-coping response. Recent advances with regard to the growth of the CRH neuropeptide family, the dual function of CRHR1 and CRHR2 in anxiety and HPA regulation, and the CRH-MR regulatory shunt in HPA axis control have provided the cornerstones for a significant leap in our understanding of the wiring and timings of the stress-coping response. Of utmost importance here is the acquired knowledge about the stress defense mechanisms underpinning anxiolysis, HPA control, and autonomic stability. These advances open the way for the development of novel classes of antidepressant drugs not just targeting the acute response systems, but also acting as supports to the stress defense mechanisms. To address this goal, substantial investments are required to further elucidate the regulatory pathways and players governing the network both in health and disease. In view of the recent development in the fields of functional and pharmacogenomics (for instance, see reference 95), and proteomics, together with the experience of several decades of research in stress physiology, pharmacology, neuroanatomy, and molecular biology, this challenge can be surmounted.
Selected abbreviations and acronyms
- ACTH
adrenocorticotropic hormone
- BNST
bed nucleus of the stria terminalis
- CREB
cAMP response element-binding protein
- CRH
corticotropin-releasing hormone
- CRHR
CRH-binding receptor
- ERPs
event-related potentials
- EW
Edinger-Westphal nucleus
- GR
glucocorticoid receptor
- HPA
hypothalamic-pituitary-adrenocortical (axis)
- ICV
intracerebroventricular
- iLS
intermediate lateral septal nucleus
- ir
immunoreactive
- LS
lateral septum
- MR
mineralocorticoid receptor
- NTS
nucleus tractus solitarius
- ODN
oligodeoxynucleotide
- PVH
hypothalamic paraventricular nucleus
- Ucn
urocortin
- VMH
ventromedial hypothalamic nucleus
Contributor Information
Johannes M. H. M. Reul, Max Planck Institute of Psychiatry, Munich, Germany.
Florian Holsboer, Max Planck Institute of Psychiatry, Munich, Germany.
REFERENCES
- 1.Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and betaendorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 2.Nemeroff CB., Widerlöv E., Bissette G., et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 3.Nemeroff CB., Owens MJ., Bissette G., Andorn AC., Stanley M. Reduced corticotropin-releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry. 1988;45:577–579. doi: 10.1001/archpsyc.1988.01800300075009. [DOI] [PubMed] [Google Scholar]
- 4.Holsboer F., Von Bardeleben U., Gerken A., Stalla GK., Müller OA. Blunted corticotropin and normal Cortisol response to human corticotropin-releasing factor in depression. N Engl J Med. 1984;311:1127. doi: 10.1056/NEJM198410253111718. [DOI] [PubMed] [Google Scholar]
- 5.Holsboer F., Von Bardeleben U., Wiedemann K., Müller OA., Stalla GK. Serial assessment of corticotropin-releasing hormone response after dexamethasone in depression implications for pathophysiology of DST nonsuppression. Biol Psychiatry. 1987;22:228–234. doi: 10.1016/0006-3223(87)90237-x. [DOI] [PubMed] [Google Scholar]
- 6.Linthorst ACE., Flachskamm C., Hopkins SJ., et al. Long-term intracerebroventricular infusion of corticotropin-releasing hormone alters neuroendocrine, neurochemical, autonomic, behavioral, and cytokine responses to a systemic inflammatory challenge. J Neurosci. 1997;17:4448–4460. doi: 10.1523/JNEUROSCI.17-11-04448.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaughan J., Donaldson C., Bittencourt J., et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- 8.Lederis K., Vale WW., Rivier JE., et al. Urotensin I - a novel CRF-like peptide in Catostomus commersoni urophysis. Proc West Pharmacol Soc. 1982;25:223–227. [PubMed] [Google Scholar]
- 9.Montecucchi PC., Anastasi A., de Castiglione R., Erspamer V. Isolation and amino acid composition of sauvagine. An active polypeptide from methanol extracts of the skin of the South American frog Phyllomedusa sauvagei. Int J Peptide Protein Res. 1980;16:191–199. [PubMed] [Google Scholar]
- 10.Chen R., Lewis KA., Perrin MH., Vale WW. Expression of cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci USA. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovenberg TW., Liaw CW., Grigoriadis DE., et al. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci USA. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chalmers DT., Lovenberg TW., De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF(2)) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF(1) receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovenberg TW., Chalmers DT., Liu CG., Desouza EB. CRF(2 alpha) and CRF(2 beta) receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology. 1995;136:4139–4142. doi: 10.1210/endo.136.9.7544278. [DOI] [PubMed] [Google Scholar]
- 14.Lewis K., Li C., Perrin MH., et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dautzenberg FM., Kilpatrick GJ., Hauger RL., Moreau JL. Molecular biology of the CRH receptors - in the mood. Peptides. 2001;22:753–760. doi: 10.1016/s0196-9781(01)00388-6. [DOI] [PubMed] [Google Scholar]
- 16.Reyes TM., Lewis K., Perrin MH., et al. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu SY., Hsueh AJW. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med. 2001;7:605–611. doi: 10.1038/87936. [DOI] [PubMed] [Google Scholar]
- 18.Brunner B., Grutzner F., Yaspo ML., Ropers HH., Haaf T., Kalscheuer VM. Molecular cloning and characterization of the Fugu rubripes MEST/COPG2 imprinting cluster and chromosomal localization in Fugu and Tetraodon nigroviridis. Chromosome Res. 2000;8:465–476. doi: 10.1023/a:1009263504671. [DOI] [PubMed] [Google Scholar]
- 19.Seasholtz AF., Burrows HL., Karolyi IJ., Camper SA. Mouse models of altered CRH-binding protein expression. Peptides. 2001;22:743–751. doi: 10.1016/s0196-9781(01)00387-4. [DOI] [PubMed] [Google Scholar]
- 20.Bittencourt JC., Vaughan J., Arias C., Rissman RA., Vale WW., Sawchenko PE. Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol. 1999;415:285–312. [PubMed] [Google Scholar]
- 21.Bittencourt JC., Sawchenko PE. Do centrally administered neuropeptides access cognate receptors? An analysis in the central corticotropin-releasing factor system. J Neurosci. 2000;20:1142–1156. doi: 10.1523/JNEUROSCI.20-03-01142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Pett K., Viau V., Bittencourt JC., et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 23.Potter E., Sutton S., Donaldson C., et al. Distribution of corticotropinreleasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci USA. 994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owens MJ., Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev. 1991;43:425–473. [PubMed] [Google Scholar]
- 25.De Souza EB., Insel TR., Perrin MH., Rivier J., Vale W., Kuhar MJ. Corticotropin-releasing factor receptors are widely distributed within the rat central nervous system: an autoradiographic study. J Neurosci. 1985;5:3189–3203. doi: 10.1523/JNEUROSCI.05-12-03189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swanson LW., Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Ann Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 27.Bremner JD., Krystal JH., Southwick SM., Charney DS. Noradrenergic mechanisms in stress and anxiety. I. Preclinical studies. Synapse. 1996;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 28.Elmquist JK., Maratos-Flier E., Saper CB., Flier JS. Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci. 1998;1:445–450. doi: 10.1038/2164. [DOI] [PubMed] [Google Scholar]
- 29.Swanson LW., Sawchenko PE., Rivier J., Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 30.Holsboer F. The rationale for corticotropin-releasing hormone receptor (CRH-R) antagonists to treat depression and anxiety. J Psychiatr Res. 1999;33:181–214. doi: 10.1016/s0022-3956(98)90056-5. [DOI] [PubMed] [Google Scholar]
- 31.Steckler T., Holsboer F. Corticotropin-releasing hormone receptor subtypes and emotion. Biol Psychiatry. 1999;46:1480–1508. doi: 10.1016/s0006-3223(99)00170-5. [DOI] [PubMed] [Google Scholar]
- 32.Timpl P., Spanagel R., Sillaber I., et al. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- 33.Smith GW., Aubry JM., Dellu F., et al. Corticotropin-releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- 34.Stenzel-Poore MP., Heinrichs SC., Rivest S., Koob GF., Vale WW. Overproduction of corticotropin-releasing factor in transgenic mice: a genetic model of anxiogenic behavior. J Neurosci. 1994;14:2579–2584. doi: 10.1523/JNEUROSCI.14-05-02579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinrichs SC., Lapansky J., Lovenberg TW., De Souza EB., Chalmers DT. Corticotropin-releasing factor CRF1, but not CRF2, receptors mediate anxiogenic-like behavior. Regul Pept. 1997;71:15–21. doi: 10.1016/s0167-0115(97)01005-7. [DOI] [PubMed] [Google Scholar]
- 36.Liebsch G., Landgraf R., Gerstberger R., et al. Chronic infusion of a CRH(1) receptor antisense oligodeoxynucleotide into the central nucleus of the amygdala reduced anxiety-related behavior in socially defeated rats. Regul Pept. 1995;59:229–239. doi: 10.1016/0167-0115(95)00099-w. [DOI] [PubMed] [Google Scholar]
- 37.Liebsch G., Landgraf R., Engelmann M., Lörscher P., Holsboer F. Differential behavioural effects of chronic infusion of CRH 1 and CRH 2 receptor antisense oligonucleotides into the rat brain. J Psychiatr Res. 1999;33:153–163. doi: 10.1016/s0022-3956(98)80047-2. [DOI] [PubMed] [Google Scholar]
- 38.Skutella T., Probst JC., Renner U., Holsboer F., Behl C. Corticotropin-releasing hormone receptor (type 1) antisense targeting reduces anxiety. Neuroscience. 1998;85:795–805. doi: 10.1016/s0306-4522(97)00682-9. [DOI] [PubMed] [Google Scholar]
- 39.Smagin GN., Dunn AJ. The role of CRF receptor subtypes in stressinduced behavioural responses. Eur J Pharmacol. 2000;405:199–206. doi: 10.1016/s0014-2999(00)00553-7. [DOI] [PubMed] [Google Scholar]
- 40.Okuyama S., Chaki S., Kawashima N., et al. Receptor binding, behavioral, and electrophysiological profiles of nonpeptide corticotropin-releasing factor subtype 1 receptor antagonists CRA 1000 and CRA 1001. J Pharmacol Exp Then. 1999;289:926–935. [PubMed] [Google Scholar]
- 41.Holsboer F. CRHR1 Antagonists as novel treatment strategies. CNS Spectrums. 2001;6:590–594. doi: 10.1017/s1092852900002133. [DOI] [PubMed] [Google Scholar]
- 42.Keck ME., Welt T., Wigger A., et al. The anxiolytic effect of the CRH1 receptor antagonist R121919 depends on innate emotionality in rats. Eur J Neurosci. 2001;13:373–380. doi: 10.1046/j.0953-816x.2000.01383.x. [DOI] [PubMed] [Google Scholar]
- 43.Heinrichs SC., De Souza EB., Schulteis G., Lapsansky JL., Grigoriadis DE. Brain penetrance, receptor occupancy and anti-stress in vivo efficacy of a small molecule corticotropin-releasing factor 1 receptor selective antagonist. Soc Neurosci Abst. 2000;26:2149. doi: 10.1016/S0893-133X(02)00299-3. [DOI] [PubMed] [Google Scholar]
- 44.Deak T., Nguyen KT., Ehrlich AL., et al. The impact of the nonpeptide corticotropin-releasing hormone antagonist antalarmin on behavioral and endocrine responses to stress. Endocrinology. 1999;140:79–86. doi: 10.1210/endo.140.1.6415. [DOI] [PubMed] [Google Scholar]
- 45.Fiorino DF., Kagaya T., Shibata H., Nishizawa Y. The CRF receptor 1 antagonist, analarmin, decreases anxiety as assessed by the shock-probe burying test in rats. Soc Neurosci Abst. 2000;26:2265. [Google Scholar]
- 46.Gilligan PJ., Baldauf CA., Cocuzza AJ., et al. Pyrazolo-[1,5-a]-pyrimidine CRF antagonists: synthesis and structure-activity relationships. 216th American Chemical Society National Meeting, Boston, Mass, August 23-27, 1998 [Google Scholar]
- 47.Gilligan PJ., Robertson DW., Zaczek R. Corticotropin releasing factor (CRF) receptor modulators: progress and opportunities for new therapeutic agents. J Med Chem. 2000;43:1641–1660. doi: 10.1021/jm990590f. [DOI] [PubMed] [Google Scholar]
- 48.He L., Gilligan PJ., Zaczek R., et al. 4-(1,3-Dimethoxyprop-2-ylamino)-2,7dimethyl-8-(2,4-dichlorophenyl)pyrazolo[1,5-a]-1,3,5-triazine: a potent, orally bioavailable CRF(1) receptor antagonist. J Med Chem. 2000;43:449–456. doi: 10.1021/jm9904351. [DOI] [PubMed] [Google Scholar]
- 49.Tian X., Hsin LW., Webster E., et al. The development of a potential single photon emission computed tomography (SPECT) imaging agent for the corticotropin-releasing hormone receptor type 1. Bioorg Med Chem Lett. 2001;11:331–333. doi: 10.1016/s0960-894x(00)00661-2. [DOI] [PubMed] [Google Scholar]
- 50.Hsin LW., Webster EL., Chrousos GP., et al. Synthesis and biological activity of fluoro-substituted pyrrolo[2,3-d]pyrimidines: the development of potential positron emission tomography imaging agents for the corticotropin-releasing hormone type 1 receptor. Bioorg Med Chem Lett. 2000;10:707–710. doi: 10.1016/s0960-894x(00)00071-8. [DOI] [PubMed] [Google Scholar]
- 51.Coste SC., Kesterson RA., Heldwein KA., et al. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropinreleasing hormone receptor-2. Nat Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- 52.Bale TL., Contarino A., Smith GW., et al. Mice deficient for corticotropinreleasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- 53.Kishimoto T., Radulovic J., Radulovic M., et al. Deletion of CRHR2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:415–419. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- 54.Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- 55.Radulovic J., Rûhmann A., Liepold T., Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J Neurosci. 1999;19:5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valdez GR., Inoue K., Koob GF., Rivier J., Vale WW., Zorrilla EP. Human urocortin II: effects of a novel, selective CRF-R2 receptor agonist on anxiety and motor activation in rats. Soc Neurosci Abst. 2001:27.Abstract number 320.13. [Google Scholar]
- 57.Opp MR. Corticotropin-releasing hormone involvement in stressorinduced alterations in sleep and in the regulation of waking. Adv Neuroimmunol. 1995;5:127–143. doi: 10.1016/0960-5428(95)00004-l. [DOI] [PubMed] [Google Scholar]
- 58.Gonzalez MM., Valatx JL. Effect of intracerebroventricular administration of alpha-helical CRH (9-41) on the sleep/waking cycle in rats under normal conditions or after subjection to an acute stressful stimulus. J Sleep Res. 1997;6:164–170. doi: 10.1046/j.1365-2869.1997.00042.x. [DOI] [PubMed] [Google Scholar]
- 59.Reynolds CF III., Kupfer DJ. Sleep research in affective illness: state of the art circa 1987. Sleep. 1987;10:199–215. doi: 10.1093/sleep/10.3.199. [DOI] [PubMed] [Google Scholar]
- 60.Ehlers CL., Reed TK., Henriksen SJ. Effects of corticotropin-releasing factor and growth hormone-releasing factor on sleep and activity in rats. Neuroendocrinology. 1986;42:467–474. doi: 10.1159/000124489. [DOI] [PubMed] [Google Scholar]
- 61.Holsboer F., Von Bardeleben U., Steiger A. Effects of intravenous corticotropin-releasing hormone upon sleep-related growth hormone surge and sleep EEG in man. Neuroendocrinology. 1988;48:32–38. doi: 10.1159/000124986. [DOI] [PubMed] [Google Scholar]
- 62.Born J., Späth-Schwalbe E., Schwakenhofer H., Kern W., Fehm HL. Influences of corticotropin-releasing hormone, adrenocorticotropin, and Cortisol on sleep in normal man. J Clin Endocrinol Metab. 1989;68:904–911. doi: 10.1210/jcem-68-5-904. [DOI] [PubMed] [Google Scholar]
- 63.Yu AW., Leung CB., Li PK., Lui SF., Lai KN. Pain perception following subcutaneous injections of citrate-buffered and phosphate-buffered epoetin alpha. Int J Artificial Organs. 1998;21:341–343. [PubMed] [Google Scholar]
- 64.Held K., Künzel HE., Ising M., Murck H., Holsboer F., Steiger A. Sleep EEG changes among depressed patients treated with the CRHR1-antagonist NBI30775. J Psychiatr Res. in press. 2001 doi: 10.1016/s0022-3956(03)00076-1. [DOI] [PubMed] [Google Scholar]
- 65.Slawecki CJ., Somes C., Rivier JE., Ehlers CL. Neurophysiological effects of intracerebroventricular administration of urocortin. Peptides. 1999;20:211–218. doi: 10.1016/s0196-9781(98)00160-0. [DOI] [PubMed] [Google Scholar]
- 66.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 67.Steckler T., Holsboer F., Reul JMHM. Glucocorticoids and depression. Baillieres Clin Endocrinol Metab. 1999;13:107–124. doi: 10.1053/beem.1999.0046. [DOI] [PubMed] [Google Scholar]
- 68.Zobel AW., Nickel T., Sonntag A., Uhr M., Holsboer F., Ising M. Cortisol response in the combined dexamethasone/CRH test as predictor of relapse in patients with remitted depression: a prospective study. J Psychiatr Res. 2001;35:83–94. doi: 10.1016/s0022-3956(01)00013-9. [DOI] [PubMed] [Google Scholar]
- 69.Keck ME., Holsboer F. Hyperactivity of CRH neuronal circuits as a target for therapeutic interventions in affective disorders. Peptides. 2001;22:835–844. doi: 10.1016/s0196-9781(01)00398-9. [DOI] [PubMed] [Google Scholar]
- 70.Kasckow JW., Baker D., Geracioti TD Jr. Corticotropin-releasing hormone in depression and post-traumatic stress disorder. Peptides. 2001;22:845–851. doi: 10.1016/s0196-9781(01)00399-0. [DOI] [PubMed] [Google Scholar]
- 71.Swaab DF. Hypothalamic peptides in human brain diseases. Trends Endocrinol Metab. 1999;10:236–244. doi: 10.1016/s1043-2760(99)00158-7. [DOI] [PubMed] [Google Scholar]
- 72.De Kloet ER., Vreugdenhil E., Oitzl MS., Joëls M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 73.Reul JMHM., Gesing A., Droste S., et al. The brain mineralocorticoid receptor: greedy for ligand, mysterious in function. Eur J Pharmacol. 2000;405:235–249. doi: 10.1016/s0014-2999(00)00677-4. [DOI] [PubMed] [Google Scholar]
- 74.Hiroi N., Wong ML., Licinio J., et al. Expression of corticotropin releasing hormone receptors type I and type II mRNA in suicide victims and controls. Mol Psychiatry. 2001;6:540–546. doi: 10.1038/sj.mp.4000908. [DOI] [PubMed] [Google Scholar]
- 75.Penalva RG., Flachskamm C., Zimmermann S., et al. Corticotropin-releasing hormone receptor type 1 deficiency enhances hippocampal serotonergic neurotransmission: an in vivo microdialysis study in mutant mice. Neuroscience. 2001;109:253–266. doi: 10.1016/s0306-4522(01)00475-4. [DOI] [PubMed] [Google Scholar]
- 76.Müller MB., Landgraf R., Preil J., et al. Selective activation of the hypothalamic vasopressinergic system in mice deficient for the corticotropinreleasing hormone receptor 1 is dependent on glucocorticoids. Endocrinology. 2000;141:4262–4269. doi: 10.1210/endo.141.11.7767. [DOI] [PubMed] [Google Scholar]
- 77.Luo X., Kiss A., Makara G., Lolait SJ., Aguilera G. Stress-specific regulation of corticotropin releasing hormone receptor expression in the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Neuroendocrinol. 1994;6:689–696. doi: 10.1111/j.1365-2826.1994.tb00636.x. [DOI] [PubMed] [Google Scholar]
- 78.Rivest S., Laflamme N., Nappi RE. Immune challenge and immobilization stress induce transcription of the gene encoding the CRF receptor in selective nuclei of the rat hypothalamus. J Neurosci. 1995;15:2680–2695. doi: 10.1523/JNEUROSCI.15-04-02680.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Makino S., Schulkin J., Smith MA., Pacak K., Palkovits M., Gold PW. Regulation of corticotropin-releasing hormone receptor messenger ribonucleic acid in the rat brain and pituitary by glucocorticoids and stress. Endocrinology. 1995;136:4517–4525. doi: 10.1210/endo.136.10.7664672. [DOI] [PubMed] [Google Scholar]
- 80.Imaki T., Katsumata H., Miyata M., Naruse M., Imaki J., Minami S. Expression of corticotropin-releasing hormone type 1 receptor in paraventricular nucleus after acute stress. Neuroendocrinology. 2001;73:293–301. doi: 10.1159/000054646. [DOI] [PubMed] [Google Scholar]
- 81.Mansi JA., Rivest S., Drolet G. Regulation of corticotropin-releasing factor type 1 (CRF1) receptor messenger ribonucleic acid in the paraventricular nucleus of rat hypothalamus by exogenous CRF. Endocrinology. 1996;137:4619–4629. doi: 10.1210/endo.137.11.8895325. [DOI] [PubMed] [Google Scholar]
- 82.Preil J., Müller MB., Gesing A., et al. Regulation of the hypothalamic-pituitary-adrenocortical system in mice deficient for CRH receptors 1 and 2. Endocrinology. 2001;142:1–10. doi: 10.1210/endo.142.11.8507. [DOI] [PubMed] [Google Scholar]
- 83.Reul JMHM., De Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2512. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 84.Gesing A., Bilang-Bleuel A., Droste SK., Linthorst ACE., Holsboer F., Reul JMHM. Psychological stress increases hippocampal mineralocorticoid receptor levels: involvement of corticotropin-releasing hormone. J Neurosci. 2001;21:4822–4829. doi: 10.1523/JNEUROSCI.21-13-04822.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amsterdam JD., Maislin G., Winokur A., Kling M., Gold P. Pituitary and adrenocortical responses to the ovine corticotropin releasing hormone in depressed patients and healthy volunteers. Arch Gen Psychiatry. 1987;44:775–781. doi: 10.1001/archpsyc.1987.01800210019003. [DOI] [PubMed] [Google Scholar]
- 86.Schulkin J., Gold PW., McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23:219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- 87.Davis M. The role of the amygdala in conditioned fear. In: Aggleton JP, ed. The Amygdala. New York, NY: Wiley-Liss; 1992:255–306. [Google Scholar]
- 88.Gray TS. The organization and possible function of amygdaloid corticotropin-releasing factor pathways. In: De Souza EB, Nemeroff CB, eds. Corticotropin-releasing Factor: Basic and Clinical studies of a Neuropeptide. Boca Raton, Fia: CRC Press; 1990:53–68. [Google Scholar]
- 89.Bhatnagar S., Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- 90.Lopez JF., Chalmers DT., Little KY., Watson SJ. Regulation of serotonin(1 A), glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus - implications for the neurobiology of depression. Biol Psychiatry. 1998;43:547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- 91.Bhatnagar S., Viau V., Chu A., Soriano L., Meijer OC., Dallman MF. Acholecystokinin-mediated pathway to the paraventricular thalamus is recruited in chronically stressed rats and regulates hypothalamic-pituitary-adrenal function. J Neurosci. 2000;20:5564–5573. doi: 10.1523/JNEUROSCI.20-14-05564.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zobel AW., Nickel T., Kûnzel HE., et al. Effects of the high-affinity corticotropin-releasing hormone receptor I antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res. 2000;34:171–181 . doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]
- 93.Martínez V., Taché Y. Role of CRF receptor 1 in central CRF-induced stimulation of colonic propulsion in rats. Brain Res. 2001;893:29–35. doi: 10.1016/s0006-8993(00)03277-7. [DOI] [PubMed] [Google Scholar]
- 94.Briscoe RJ., Cabrera CL., Baird TJ., Rice KC., Woods JH. Antalarmin blockade of corticotropin releasing hormone-induced hypertension in rats. Brain Res. 2000;881:204–207. doi: 10.1016/s0006-8993(00)02742-6. [DOI] [PubMed] [Google Scholar]
- 95.Gratacòs M., Nadal M., Martin-Santos R., et al. A polymorphic genomic duplication on human chromosome 15 is a susceptibility factor for panic and phobic disorders. Cell. 2001;106:367–379. doi: 10.1016/s0092-8674(01)00447-0. [DOI] [PubMed] [Google Scholar]