Abstract
Major depression is a serious disorder of enormous sociological and clinical relevance. The discovery of antidepressant drugs in the 1950s led to the first biochemical hypothesis of depression, which suggested that an impairment in central monoaminergic function was the major lesion underlying the disorder. Basic research in all fields of neuroscience (including genetics) and the discovery of new antidepressant drugs have revolutionized our understanding of the mechanisms underlying depression and drug action. There is no doubt that the monoaminergic system is one of the cornerstones of these mechanisms, but multiple interactions with other brain systems and the regulation of central nervous system function must also be taken into account In spite of all the progress achieved so far, we must be aware that many open questions remain to be resolved in the future.
Keywords: depression, monoamine, serotonin, norepinephrine, treatment, genetics, neurobiology
Abstract
La depresión mayor es un trasiorno severo, de enorme importancia clínica y sociológica. El descubrimiento de los fármacos antidepresivos en ios años 1950 condujo a la primera hipótesis bioquimica de la depresión, la cual sugería que la principal lesión a la base de este trastorno era un deterioro de la función monoaminérgica central. La investigación básica en todos los campos de las neurociencias (incluyendo la genética) y el descubrimienio de nuevos fármacos antidepresivos han revolucionado nuestra comprensián acerca de los mécanismes fundamentales de la depresión y de la acción de las drogas. No hay dudas que el sistema monoaminérgico es una de las piedras angulares, pero también hay que tomar en cuenta las múltiples interacciones con otros sistemas cérébrales y la regulación de la función del sistema nervioso central. A pesar de todos los progresos alcanzados hasta la fecha, debemos estar conscientes de que existen muchas preguntas que deben ser resueltas en el futuro.
Abstract
La dépression sévère est une maladie grave dont l'impact sociologique et clinique est immense. La découverte des antidépresseurs dans les années 50 a débouché sur la première hypothèse biochimique concernant la dépression, selon laquelle un déficit de la fonction monoaminergique centrale était l'anomalie principale sous-jacente à la maladie. La recherche fondamentale dans tous les domaines de la neuroscience (y compris génétique) et la découverte de nouveaux antidépresseurs ont bouleversé notre compréhension des mécanismes de la dépression et de son traitement Si le système monoaminergique est sans conteste l'une des pierres angulaires de ces mécanismes, les multiples interactions existant avec d'autres systèmes cérébraux et la régulation du système nerveux central doivent également être prises en compte. En dépit des nombreux progrès accomplis jusqu'à maintenant, de nombreuses questions demeurent toujours sans réponse.
Depression is a potentially life -threatening disorder that affects hundreds of millions of people all over the world. It can occur at any age from childhood to late life and is a tremendous cost to society as this disorder causes severe distress and disruption of life and, if left untreated, can be fatal. The psychopathological state involves a triad of symptoms with low or depressed mood, anhedonia, and low energy or fatigue. Other symptoms, such as sleep and psychomotor disturbances, feelings of guilt, low self-esteem, suicidal tendencies, as well as autonomic and gastrointestinal disturbances, are also often present. Depression is not a homogeneous disorder, but a complex phenomenon, which has many subtypes and probably more than one etiology. It includes a predisposition to episodic and often progressive mood disturbances, differences in symptomatology ranging from mild to severe symptoms with or without psychotic features, and interactions with other psychiatric and somatic disorders.
Classification, prevalence, and course of depression
At present, the essence of major depressive disorder is a clinical course that is characterized by one or more major depressive episodes without a history of manic, mixed, or hypomanic episodes, according to the criteria of the Diagnostic and Statistical Manual of Menial Health, Fourth Edition (DSM.-IV).1For an appropriate diagnosis, five of the following nine DSM-IVsymptoms must be present continuously for a minimum 2-week period: (i) depressed mood; (ii) loss of interest or pleasure; (iii) significant weight or appetite alteration; (iv) insomnia or hyposomnia; (v) psychomotor agitation or retardation; (vi) fatigue or loss of energy; (vii) feelings of worthlessness; (viii) diminished ability to think or concentrate or indccisiveness; and (ix) suicidal ideation.
Historically, there has been lengthy discussion on the basis and classification of depression. Two different concepts, Emil Kraepelin's formulation of depression as a disease and Sigmund Freud's view of depression as a manifestation of internalized anger and loss, were the two opposite points of view at the beginning of the 20th century. It was the merit of Sir Martin Roth and the Newcastle Group that contributed to the understanding of depression: they classified the clinical manifestations of depression (from mild to severe psychotic) in a categorical manner, separating them into distinct groups of “endogenous” and “reactive” subtypes of depression.2 This concept was used for decades in biological psychiatric research in order to identify etiologically different subtypes of the disorder. The recent editions of DSM-IV 1 and the International Statistical Classification of Diseases, 10th Revision (ICD-10) 3 follow the results from collaborative projects“-5 in the USA and the UK and distinguish unipolar (depression) from bipolar (manic depressive) disorder.
The lifetime prevalence of depression is as high as 20% in the general population worldwide with a female to male ratio of about 5:2. We have to assume that only about one third of patients are in treatment, maybe not due to ignorance, but due to the fact that symptoms may not be qualitatively different from those of everyday experience. Typically, the course of the disease is recurrent and most patients recover from major depressive episodes.6 However, a substantial proportion of the patients become chronic and after 5 and 10 years of prospective follow-up, 12% and 7%, respectively, are still depressed.7 In patients who do recover, there is a high rate of recurrence and it has been found that approximately 75% of patients experience more than one episode of major depression within 10 years.8,9 Suicide is a considerable risk for mortality in depression, and the rate of suicide is rather high between the age of 15 and 24 years.10 Several lines of evidence indicate an important relationship between depression and cardiovascular disease, together with increased mortality rates. Some studies have demonstrated that depression increases the risk of developing cardiac disease, in particular coronary artery disease, and worsens the prognosis after myocardial infarction.11 Depression also appears to increase the risk for cardiac mortality independently of baseline cardiac status; moreover, the excess mortality risk for major depression was more than twice that for minor depression.12
Another very important aspect of depression is the high rate of comorbidity with other psychiatric disturbances. Anxiety, especially panic disorder, is often associated with affective disorders, while the magnitude of the association with alcohol or drug abuse is less pronounced. Interestingly, the onset of anxiety generally precedes that of depression, whereas alcohol misuse is equally likely to pre- or postdate the onset of depression.13,14
Risk factors for depression
The impact of life events
The influence of chronic stress and adverse life events on the development of depression has been subject of numerous investigations and the work has been influenced by studies of the somatic and endocrine consequences of stress in animals (see reference 15 for a review). Despite much criticism of the methodology (eg, the choice of instruments to obtain life event information, the elimination of events that are consequences of physical illness, or the quantification of stress), most findings show an excess of severely threatening events prior to onset, particularly for events categorized as exit events or undesirable events.15 Life events preceding depression are variable and are probably unrelated to the symptom pattern, which means that there is no clear-cut difference in the presence of events provoking the onset of endogenous or nonendogenous depression.16 There is ongoing discussion on the impact of events on depressive outcome, as positive events were reported to improve outcome, whereas stressful events were shown to lessen improvement and increase the probability of relapse.17 The fact that major depression is more likely in females than in males can, however, not be explained by differing rates or sensitivities to stressful life events.
Although women reported more interpersonal and men more legal- or work-related stressful life events, this cannot be attributed to the greater prevalence of major depression in females.18
Genetic influences
There is abundant evidence from family, twin, and adoption studies that genetic factors play an important role in the etiology of affective disorders. Ill ere is strong epidemiological evidence for a genetic contribution, especially for bipolar disorders, and heritability is estimated to be as high as 80%.19 However, the inheritance does not follow the classical mendelian pattern, which suggests that a single major gene locus may not - or at least only in few families - account for the increased intrafamilial risk for the disorder. More likely is a model of a complex disorder, which postulates that several genes of modest effect interact with each other or with a variety of environmental factors to increase familial susceptibility for the disorder.20 Additional factors further complicate both epidemiological and molecular genetic studies. Among these are various genetic mechanisms, which mainly concern the interaction of different genes that are not sufficient or strong enough alone to lead to a susceptibility to the disease. Further problems arise because of difficulties in ascertaining the clinical phenotype, as phenocopies exist.21 Despite these problems, considerable advances have been made in the last years in linkage studies with bipolar disorder and promising regions have been identified on chromosomes 4, 5, 12, 18, and 21, and the X chromosome.19,21
The influence of genes in major unipolar depression is less clear than for bipolar disorder. Although population-based and hospital register-based twin studies have found a substantial heritability in major depression,20 the variation in liability by nongenetic factors seems to be more pronounced in unipolar major depression than in bipolar disorders. Accordingly, the results of linkage analyses are less convincing for this disease,21 but it is increasingly being proposed that environmental measures and life events tend to be contaminated by genetic components.22
An alternative strategy to linkage analyses is the application of association studies in which candidate genes are investigated in a cohort of patients and compared with healthy controls. This method depends crucially on our understanding of the disease psychopathology and the hypotheses on the underlying biochemical processes and on the selection of ethnically matched controls.23 Association studies with candidate genes seem to be more promising in unipolar depression and analyses of candidate genes of the serotonergic system, such as tyrosine hydroxylase, the serotonin (5-hydroxytryptamine [5-HT]) transporter, and the 5-HT2C receptor, have exhibited interesting results.21,24,25
Molecular genetic studies not only offer the possibility of unraveling the gene or genes responsible for heritability, but also widen our insights into the pathophysiological mechanism. Taking into account the clinical and etiological heterogeneity of depression, the results of these investigations provide the possibility of subtyping and differentiating patients of a diagnostic category according to underlying biological parameters. The recent finding on differing genotype distribution of the 5-HT2A receptor polymorphism in patients with a seasonal pattern of episodes supports this view of genetic and etiological heterogeneity.26 Other approaches may include the different risk for men and women, because it is still not ascertained whether sex modifies the etiological impact of genetic factors. However, heritability seems to be significantly greater in women than in men,27 a fact that should be taken into account in future linkage and association studies.
Biochemical basis of depression
The enormous progress in the field of neuroscience in the 20th century brought us fascinating insights into the nature of mental processes. Starting with neuroanatomy and electrophysiology at the beginning of the 20th century, neuroscience now is an interdisciplinary field occupying many areas of biological investigations, ranging from molecular studies of cell and gene function to brain-imaging techniques, thus broadening our knowledge of the cellular and molecular machinery that regulates behavior.28 For a long time, and especially in the field of psychiatry, little was known about the biological substrates of the disorders and the work of Julius Axelrod, Arvid Carlsson, and several other Nobel Prize winners has significantly contributed to the understanding of brain function, and investigations of psychiatric disorders are now fully based in basic neuroscience.
Synaptic transmission
One of the most important advances in neuroscience was the pioneering work of Otto Loewi and other scientists, ie, that chemical transmission is the major means by which nerves communicate with one another. Today, it is well known that the pre- and postsynaptic events are highly regulated and are the basis for plasticity and learning within the central nervous system (CNS). Chemical transmission requires several steps including synthesis of the neurotransmitters, their storage in secretory vesicles, and their regulated release into the synaptic cleft between pre- and postsynaptic neurones, but also the termination of neurotransmitter action and the induction of the final cellular responses via different steps in the signal transduction cascade.
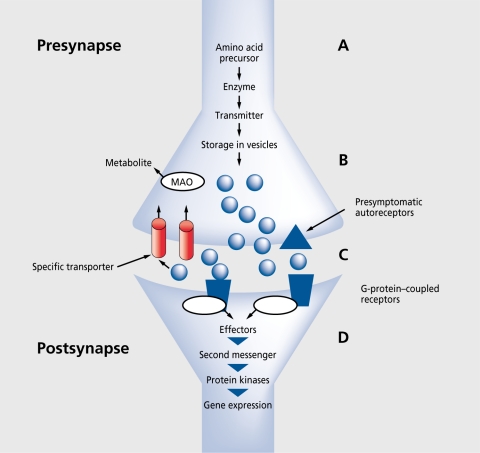
Figure 1is a schematic representation of a synapse for classic neurotransmitters. The initial step of synthesis is the facilitated transport of amino acids from blood to the brain, where precursors are converted via enzymatic reactions into transmitters, which are stored in synaptic vesicles, and finally released into the synaptic cleft by a Ca2+-dependent process. The rate of neurotransmitter release is dependent on the firing rate of the neurones, which means that conditions or drugs that alter the firing rate modify the release of the transmitter. A further important regulatory mechanism of release involves the somatodendritic autoreceptors, since binding of the released transmitter molecules leads to reduced synthesis or further release from the presynapse. The synaptic effects are terminated by binding of the transmitters to specific transporter proteins and reuptake into the presynapse, where they are metabolized by enzymes, for example, monoamine oxidase (MAO), or stored once again in the vesicles.29
Figure 1. Schematic representation of a synapse and the steps of chemical transmission. Precursors are transported from blood into the brain (A), converted into transmitters via enzymatic processes, and stored in synaptic vesicles (B). The transmitters are released into the synaptic cleft (C), where they either react with presynaptic autoreceptors to regulate synthesis and release, or with postsynaptic receptors to induce the events of the downstream signal transduction cascade (D). MAO, monoamine oxidase.
Neurotransmitter molecules do not cross the postsynaptic membrane, but induce a cascade of reactions via their initial binding to surface receptors within the post-synaptic membrane, which are often coupled to guanine nucleotide-binding proteins (G-proteins). These G-proteins represent essential initial regulatory components in transmembrane signaling, because they modulate a number of effector systems within the cells, including adenylylcyclases, phospholipases, and the phosphoinositidemediated system.30 The early cellular events of the signal transduction cascade (ie, increase in concentrations of intracellular calcium ions or second messengers, such as cyclic adenosine monophosphate fcAMP]) initiate a pathway via phosphorylation of protein kinases,31 which in turn regulates many biological responses and controls short- and long-term brain functions by regulation of neuronal ion channels, receptor modulation, neurotransmitter release, and, ultimately, synaptic potentiation and neuronal survival.32,33 Disrupted function in one or more steps of this chemical transmission may be a crucial mechanism underlying depression. On the other hand, it is now well established that these mechanisms are targets of antidepressant action.
Monoamine hypothesis
The first major hypothesis of depression was formulated about 30 years ago and proposed that the main symptoms of depression are due to a functional deficiency of the brain monoaminergic transmitters norepinephrine (NE), 5-HT, and/or dopamine (DA), whereas mania is caused by functional excess of monoamines at critical synapses in the brain.34-36 Evidence for this hypothesis came from clinical observations and animal experiments, which showed that the antihypertensive drug reserpine, which causes a depletion of presynaptic stores of NE, 5-HT, and DA, induced a syndrome resembling depression. In contrast to the effects obtained with reserpine, euphoria and hyperactive behavior were observed in some patients being treated with iproniazid, a compound synthesized for the treatment of tuberculosis, which increased brain concentrations of NE and 5-HT by inhibiting the metabolic enzyme MAO.
Considering the origin of the noradrenergic, serotonergic, and dopaminergic neurones in the brain and their projections into many areas of the brain, it is clear that monoaminergic systems are responsible for many behavioral symptoms, such as mood, vigilance, motivation, fatigue, and psychomotor agitation or retardation. Abnormal function and the behavioral consequences of either depression or the manic state may arise from altered synthesis, storage, or release of the neurotransmitters, as well as from disturbed sensitivity of their receptors or subcellular messenger functions.37
Neurotransmitter concentration
Many attempts have been made to prove the hypothesis of reduced monoamine availability by measurement of neurotransmitters and/or their metabolites in postmortem brain tissues and body fluids, such as cerebrospinal fluid (CSF), blood, and urine.38 Although repeated data showing decreased levels of the NE metabolite a-methoxy-4-hydroxyphenylglycol (MHPG), which indicates NE. turnover in brain, support the hypothesis of a deficient noradrenergic system,38 the results are inconsistent.39 Similarly to the noradrenergic system, the data on determinations of 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) could not prove the hypothesis of exclusively reduced serotonergic transmission. Many studies reported decreased central serotonergic turnover in major depression;40,41 but findings also suggested that reduced 5-HT function may not be present in all depressed patients.42 These discrepancies between studies may reflect both methodological problems, such as difficulties in measuring the amines after various postmortem delays, and the fact that determinations of neurotransmitters or their metabolites in CSF or blood reflect a summation of many events in many brain areas and not in restricted nuclei.43
Similarly to the data on neurotransmitter concentrations, the results on the possibility of impaired activity of the enzymes for synthesis and degradation of monoamines are not convincing. Tyrosine hydroxylase and tryptophan hydroxylase are essential for NE and 5-HT synthesis, respectively, and were found to be up- or downregulated in postmortem brain samples, suggesting a minor importance for transmitter synthesis. Similarly, no conclusive abnormalities were found in the degrading activity of MAO.42
The paradigm of monoamine depletion, which links clinical state to monoamine deficiency, nicely offers the possibility of investigating the effect of decreased monoamine concentration on behavior and gives us much additional information on its impact on the psych opathology of depression. Addition of α-methylparatyrosine, which inhibits the NE-synthesizing enzyme tyrosine hydroxylase, leads to a depletion of NE in the synapse.44 A similar influence on the metabolism of 5-HT is obtained by application of a tryptophan-free amino acid mixture, which induces a rapid cerebral depletion of tryptophan and ultimately a decrease in 5-HT concentrations.45 Interestingly, depletion of monoamines did not induce or worsen the symptoms of depression in healthy controls or unmedicated patients, which means that monoamine deficiency alone is not sufficient for the clinical syndrome. However, in patients currently receiving drug treatment, the antidepressant response was transiently reversed in a manner that was dependent on the class of antidepressant.46 These results support the evidence that antidepressants require an intact monoamine system for their therapeutic action, but the pathophysiology of depression may not be explained by a single monoamine-related mechanism.44,47
Transporters for neurotransmitter reuptake
Transport proteins play a crucial role in monoaminergic transmission: they reduce the availability of neurotransmitters in the synaptic cleft and thus terminate the effect of the neurotransmitters on pre- and postsynaptic receptors. Although much of our knowledge about transporter dysfunction comes from animal and postmortem brain studies, the 5-HT transport system is not restricted to tissues of the CNS, but is also present in human platelets. This gives us the opportunity to investigate its function in vivo and in different states of depression.48 Different substances have been used to mark the protein and other investigations measured the active uptake of 5-HT, and, at least for platelets, there is now consensus about a decreased transporter function in major depression - a finding that was not observed in other psychiatric disorders.42,49 In contrast, the results with postmortem samples are not as convincing as those with platelets,49 possibly due to inconsistencies in the selection of subjects or the much discussed problems of investigating the rapidly degrading proteins after various postmortem delays.
The problems of postmortem investigations may be overcome by functional imaging techniques that allow a noninvasive investigation of the 5-HT transporter in the human brain. Using the method of single photon emission computed tomography (SPECT) and the radiolabeled tracer123 I-β-CIT ([123 I]-2β-carbomethoxy-3β-(4iodophcnyl) tropane), the decrease in 5-HT transport that had already been identified in platelets was confirmed for the CNS.50,51 Moreover, there might even be a genetic basis for this dysfunctional 5-HT transport, since a common polymorphism within the promoter region of the 5-HT transporter gene leads to altered transcriptional activity and hence to diminished expression of the gene.52 Interestingly, this polymorphism for “lower function” was found more frequently in depressed patients.53
As regards the NE transporter, few studies have been conducted to measure the NE reuptake sites. Without an ideal peripheral model, most experiments were carried out in postmortem samples and the few results are controversial.54 There was also no relationship to genetic variants of the NF: transporter.55
Neurotransmitter receptors
In addition to monoamine deficiency, an abnormality in transmission can also arise from changes in receptor function, which means either changes in coupling between transmitters and receptors or changes in the downstream signal transduction cascade. For both the noradrenergic and serotonergic system, a multiplicity of receptors have been identified so far, each classified according to its pharmacological or molecular characteristics. NE transmission is regulated via α- or β-adrenoccptors and their various subtypes, with the same pharmacological properties in brain and periphery.29 Receptor classification for the serotonergic system has proceeded rapidly and to date we know of several major categories, ranging from 5-HT1 to 5-HT7 receptors, each with further subtypes.56
Receptors are not static entities: their numbers and affinities are regulated by many factors, for example, the transmitter concentration, which leads to compensatory down- or upregulation in the receptor protein. Despite intensive investigation over the years, our knowledge of alterations in monoamine receptor numbers or affinities in untreated depressed patients is relatively poor and unconvincing. The frequently reported supersensitivity of presynaptic α2 -adrenoceptors, which modulate the release of NE,42 as well as altered numbers and affinities of 5-HT1 and 5-HT2 receptors in brain and/or platelets57 have been the subject of much discussion.
Due to the rapid development of molecular biology, interest has shifted from the mere determination of the receptor numbers or affinities toward the signal transduction cascade. There is mounting evidence for the role of these mechanisms in the modulation of neuronal activity and pathophysiology of mental disorders.58 Using this new approach, several studies in peripheral cell model systems and/or in postmortem brain tissue report alterations in G-proteins,59 at multiple sites of the cAMP pathway,60 and in protein kinases.61 These findings have led to the formulation of a molecular and cellular hypothesis of depression, which proposes that signal transduction pathways are in a pivotal position in the CNS, in that they affect the functional balance between multiple neurotransmitter systems and physiological processes.
Pharmacological treatment of depression
Since Kuhn introduced imipramine in the 1950s, the availability of antidepressant drugs has expanded greatly, not only in terms of number, but also, and especially, in terms of diversity in the associated pharmacological effects. The first-generation antidepressants, the tricyclic antidepressants (TCAs) and MAO inhibitors (MAOIs), increase the concentrations of 5-HT and/or NE and are effective in alleviating the symptoms of depression. Although both types of drugs have been used with great success for many years, there are several undesirable side effects that limit their application. TCAs acts on many other transmitter systems in the CNS and periphery, eg, the histaminergic or acetylcholinergic systems,62 leading to sedation, hypotension, blurred vision, dry mouth, and other unwanted effects. In addition, TCAs may be life -threatening and fatal in overdose, especially due to their effects on the cardiovascular system.63 Also, the irreversible MAOIs have their own problems, such as an interaction with tyramine (the so-called “cheese effect”), which causes potentially lethal hypertension.62 The main problem with the less severe side effects is a reduction in compliance, patients often do not take a sufficient dosage for an adequate period of time and thus remain in an “undertreated” state.
The development of newer antidepressants has aimed to improve the safety and tolerability of the TCAs and reuptake inhibitors, and selectivity for a single monoamine seemed to be the key to this goal. Since the introduction of fluoxetine as the first selective serotoninreuptake inhibitor (SSRI), a great number of similarly acting drugs have followed and SSRIs are now applied in the treatment of several psychiatric disturbances, such as anxiety, panic, or obsessive compulsive disorder, where altered serotonergic transmission is assumed.62 Because preclinical and clinical studies have shown that chronic stimulation of the 5-HT system also affects the NE system and vice versa,64 there has been renewed interest in the role of neurotransmitters other than serotonin. The development of the newest generation of antidepressants, including reboxetine (a selective NE-reuptake inhibitor), venlafaxine (a dual reuptake inhibitor), or the multiple receptor-acting substances mirtazepine, nefazodone, bupropion, and trazodone, may positively influence therapeutic potentials with reduced incidence of side effects due to reduced affinities for other systems.62 Interestingly, one drug, tianeptine, shows a quite atypical mechanism, namely an increase in 5-HT uptake, but most probably this substance predominantly counteracts stress effects in the hippocampus.65
With the use of these new drugs, the incidence of severe side effects was certainly reduced, but there are rather severe, treatment-resistant types of depression, which may not adequately be treated with these drugs. A new drug regimen, augmentation therapy, was introduced some years ago, which is defined as the addition of a second agent to an existing antidepressant to achieve improved clinical response. Popular strategies are augmentation of TCA drugs with Li+, or SSRIs with pindolol. Although the results of these strategies in relieving depressive symptoms are encouraging, more prospective, well-controlled studies will have to clarify the benefits and risks of augmentation strategies.66
The effect of antidepressants on the NE and 5-HT receptor systems has been known for a long time, and the decreased sensitivities of β-adrenoceptors and cortical 5-HT2A receptors have often been suggested to be a prerequisite for antidepressant action.67 The delay in antidepressant response makes it clear that the immediate effects of these drugs are not the main explanation of their antidepressant action, but gradual adaptive changes in neuronal responses might be ultimately responsible for the therapeutic benefits.68 Recent research with SSRIs and dual uptake inhibitors has shifted the research focus beyond the levels of receptors to those of protein kinase-mediated phosphorylation of transcription factors, which ultimately leads to changes in programs of gene expression.69
Considering the currently available drugs for antidepressant treatment, there is now no doubt that the NE and 5-HT system are important in the pathophysiology and treatment of depression, as all the agents interact with one or both of these systems and the net effect is an increase in 5-HT neurotransmission.70 Future antidepressants will have to be developed with pharmacology directed at alternative neurotransmitters or neuromodulators, following novel mechanisms and hypotheses. For example, the involvement of γ-aminobutyric acid (GABA) in depression has long been suspected.71 Another example in the search for better treatment of depression has been the demonstration that a substance-P antagonist had an antidepressant activity equivalent to the SSRI paroxetine.72 Further targets for drugs include corticotropin-releasing factor (CRF; see the article by Holsboer in this issue73 ) or melatonin (see the article by Pevet in this issue74 ); these are currently under investigation and clinical results will be available in the near future. However, the “ideal” antidepressant remains to be discovered: it should not only be effective and safe, but also be well tolerated and contribute to the overall well-being of the patient.
Endocrine processes in depression
A variety of hormonal abnormalities, such as altered levels of Cortisol, growth hormone (GH), or thyroid hormones, indicate the existence of endocrine disturbances, especially dysfunctions in the hypothalamuspituitary-adrenal (HPA) axis and/or the regulation of thyroid function. The consistent finding that a significant subpopulation of depressed patients hypersecrete Cortisol during the depressed state but not after recovery75 led to intensive investigation and analysis of the HPA system. The observations include hypersecretion of hypothalamic corticotropin-releasing hormone (CRH) and inadequate glucocorticoid feedback, increased Cortisol levels, and impaired suppression of the HPA axis in response to exogenous glucocorticoid administration.76-78 A more refined analysis recently led to formulation of the concept that impaired corticosteroid receptor signaling is a key mechanism in the pathogenesis of depression.79
Investigations of hormonal responses to noradrenergic stimulation provided useful information about the possible role of NE and pituitary and adrenal hormone secretion in depression. These strategies involve measurement of the response of hormones such as GH and Cortisol to agents that directly or indirectly modulate noradrenergic activity. We have long known that GH release is stimulated by catecholaminergic mechanisms, among others. For almost 30 years now, different GH stimulation tests have been used to prove whether GH response in depressed patients differs from controls and subjects with other psychiatric diseases. Most revealed significant differences between patients with major depression and healthy subjects or patients with minor depression, using various specific substances to challenge GH response. Patients with recurrent major depression exhibited a blunted GH response, which could be interpreted as cither decreased DA receptor sensitivity (challenge with apomorphine) or decreased α2-adrenoceptor sensitivity (challenge with clonidine).80 It was further suggested that this blunted GH response to clonidine was a trait marker that persists in depressed patients following their recovery.81 -82 However, as challenge with different α2-adrenoceptor-selective agents resulted in a normal GH response, an intrinsic abnormality in the GH system was also suggested as opposed to decreased a2-adrenoceptor sensitivity.83
Alterations in thyroid function have been repeatedly linked to depression and the administration of triiodothyronine (T3 ) seems to be an effective adjunctive treatment for many patients.84,85 The relationship between thyroid hormones and neurotransmitters have mainly focused on the noradrenergic and serotonergic systems and it was shown that thyroid hormone application increases cortical serotonin release86 and may act as a cotransmitter to NE in the adrenergic nervous system.87 However, the exact mechanism of this interaction is not clear. Especially intriguing was the observation that 5-HT function was especially reduced in patients without hypothalamus-pituitary-thyroid axis abnormalities, which suggests that mechanisms that are not serotonergic might be involved in the reduced secretion of thyroid-stimulating hormone (TSH).84
A further hint on the influence of hormones comes from the fact that the immediate postpartum period is a time of highly increased risk for the onset or relapse of depression.88 Several results underline the influence of estrogen and progesterone,89 thyroid hormones,90 or alterations in the HPA axis,91 but the direct mechanisms have not been clarified. In addition, recurrent depressive symptoms can be limited to the premenstrual period and more enduring depression is typically exacerbated premenstrually. These findings on possible disturbances in sex hormones could give an explanation for the increased incidence in women.
Neuroimmune mediators
The clinical course of depression is that of a variable disease with long periods of recovery between periods of depression in many patients, but it can also involve closely spaced episodes that finally lead to a severe and unremitting course. It has been repeatedly suggested that this variable course of the disorder might be explained by inflammatory processes.10 Traditionally, both stress and depression have been associated with impaired immune function and increased susceptibility to infectious and neoplastic disease.92 Despite the initial findings of immunosuppression in depression, some studies have indicated that immune activation could also be present and might even play a role in the onset of depressive symptoms.93 This hypothesis was underlined by findings of increased plasma cytokine and acute phase protein concentrations in the blood of depressed patients.94 In addition to the immunological alterations reported in patients with major depression, a number of studies have examined the hypothesis that exposure to stressful life events, such as academic examinations, divorce, or bereavement, causes impairment in various aspects of cellular immune function, such as lymphocyte and natural killer (NK) cell activity.95
Concerning the underlying mechanism of this interaction, we now recognize that the immune system is a key mediator of brain-body interactions. Cytokines influence various CNS functions that are dysregulated in major depression, such as sleep, food intake, cognition, temperature, and neuroendocrine regulation.96,97 Experimental administration of interleukin-1 (IL-1) into the CNS produces stress-like effects on behavior, monoamine transmitters, HPA axis activity, and immune function; IL-1 is also a regulator of the 5-HT transporter gene.95 Another hint to the link between immune system and mood came from observations that a large number of previously psychiatrically healthy individuals treated with exogenous cytokines such as interleukin-2 (IL-2) and interferon-α. (IFN-α) develop depression-like symptoms, such as depressed mood, increased somatic complaints, and stress reaction, cognitive impairment, and difficulties with motivation and flexible thinking.95 The fact that these are transient alterations, which disappear after termination of therapy, implies that cytokines may play a causal role in producing these symptoms. Future research will have to examine the causal link between depression and the action of cytokines, as well as the effect of antidepressants on cytokine hypersecretion.
Neurotrophins and depression
One hypothesis for the pathophysiology and treatment of depression involves adaptation or plasticity of neuronal systems. Depression could thus result from an inability to make the appropriate adaptive responses to stress or other aversive stimuli, and antidepressants may act by correcting this dysfunction or by directly inducing the appropriate adaptive responses.98
The neurotrophic factors are among the growth factors that have been studied for their role in the adult nervous system. Of these endogenous proteins, brainderived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) have been shown to promote the function and growth of 5-HT-containing neurones in the adult brain.99 Chronic, but not acute, infusions of these substances have impressive effects on serotonergic neurone growth and regeneration, and further induced sprouting of 5-HT nerve terminals.99 In animal experiments, BDNF ameliorated learned helplessness, an effect that is normally observed with antidepressant treatment.100 Further studies underlined these interrelations and it was shown that treatment with antidepressants, including specific inhibitors of 5-HT or NE uptake as well as MAOIs, elevates BDNF mRNA levels in the rat hippocampus via the 5-HT2A and the β-adrenoceptor subtypes, and prevents the stress-induced decreases in BDNF mRNA.101 Interestingly, this effect became evident after 3 weeks of treatment, but not after a single dose, thus being reminiscent of the delay in treatment response. The results of these animal experiments were confirmed by a recent postmortem finding of increased BDNF expression in patients being treated with antidepressants.102 Understanding the mechanism of how these drugs elevate BDNF mRNA could be particularly important, since BDNF concentration cannot be increased by exogenous neurotrophins, which are relatively large lipophobic proteins that do not cross the blood-brain barrier. However, small molecules that pass through the blood-brain barrier and subsequently boost endogenous neurotrophin levels could represent a new generation of antidepressants.103
Interaction between monoamines and other neurotransmitters and neuropeptides
Three decades after its formulation, the monoamine hypothesis of depression underwent various adaptations. Although it has contributed to our understanding of the regulation of neuronal function in general, there is no doubt that a dysfunction in one of the monoaminergic systems alone does not provide an adequate explanation for the pathophysiology of depression or the mechanism of drug action. One of the intriguing problems of therapy is the fact that it takes several days to weeks before the antidepressant effect becomes apparent, although the neurotransmitter concentrations are increased within hours of a single dose of reuptake inhibitor. The results of depletion studies further support the hypothesis that a simple change in the level of one of the monoamines or their receptor affinity is sufficient to induce or alleviate depression.104
It is now well established that there are considerable interactions of monoaminergic neurones with each other and with other systems in the brain, and there are many behavioral overlaps that reflect interactions among these neurotransmitters. Although NE.controls vigilance, like 5-HT, it also influences anxiety and irritability. In addition, impulsive behavior appears to be controlled by 5-HT, and yet it shares with DA an influence on appetite, sex, and aggression. Moreover, DA and NE. appear to affect euphoria and pleasure, thus influencing motivation and energy. These overlaps make it difficult to assign full responsibility to any particular neurotransmitter.105 As it has been demonstrated that even selective reuptake inhibitors affect both systems,106,107 leading to alterations of neuronal firing and postsynaptic receptor responses, a clear assignment to several symptoms or response to treatment seems impossible. Monoaminergic systems are also modulated by other factors, eg, CRH, vasopressin, neuropeptide Y, cytokines, excitatory amino acids, or neurotrophic factors.83 Therefore, a plausible model for the pathophysiology of depression and the action of antidepressant treatment needs to take into account the complexity of the regulation of CNS function. Moreover, chronic stress, which is doubtless an important precipitating factor in depression, has many effects, not only on behavior, but also on the endocrine, immune, and neurotransmitter systems,108 and the data implicate a close link between stress and changes in the HPA axis and the central NE system. Accordingly, depression may result from dysfunctions in the areas of the brain that are modulated by these systems, such as the frontal cortex, hippocampus, amygdala, and basal ganglia. It is also well known that these areas are highly sensitive to the effects of stress, possibly accounting for the adverse impact of life events on depression.105 Thus, many different factors could lead to a selective or generalized dysfunction in these brain areas, accounting for the probable heterogeneity of depression.
Brain imaging
Despite the increasing number of biochemical and molecular biological studies in depression research, the advances in neuroimaging techniques now offer the possibility of studying anatomical alterations in living patients. Application of magnetic resonance imaging (MRI) techniques and positron emission tomography (PET) has disclosed a battery of abnormalities in the brains of patients with major depression. Several studies have suggested that a large proportion of patients with major depression do indeed have signs of brain atrophy. Increased ventricle-brain volumes have been discussed, as have localized atrophy in the frontal lobes, especially in patients with late -life depression.109,110 Functional studies have revealed reduced blood flow in specific brain regions, particularly the frontal lobe and the basal ganglia.111 One of the brain structures that has been extensively studied with regard to the action of stress, depression, and antidepressant actions is the hippocampus - a brain area that is involved in learning and memory.112 Recent imaging studies have shown that the hippocampus undergoes selective volume reduction in stressrelated neuropsychiatrie disorders, such as recurrent depression; it has been suggested that this is related to hypercortisolemia.113
Pharmacogenetics
Large interindividual differences in outcome and side effects are quite common during treatment with antidepressants and, also at recommended doses, the clinical response to drugs might range continuously from “good effect” to “no response” or even “deterioration” with a high incidence of adverse effects. Although several factors, such as age, gender, body fat, alcohol intake, and nicotine consumption, account for the patient-topatient differences, there is increasing evidence that genetic factors also underlie the differences in psych opharmacological drug response.114,115 This hypothesis is further supported by observations of comparable responses to antidepressant therapy among relatives.116 Thus, the concept of pharmacogenetics as originally defined by Vogel 1959,117 which means heritable differences in metabolism and activity of exogenous agents, might help unravel the variability in drug response and metabolism.
Relevant genetic polymorphisms are found in drugmetabolizing enzymes, neurotransmitter receptors, and transport proteins. These variants result in no effect or in a change in the rate of metabolism, as well as in altered protein binding and/or function.118 Accordingly, most studies focus on the cytochrome P-450 isoenzymes (CYP), neurotransmitter receptors, and selective transporters, following the hypotheses of pathophysiological and drug action mechanisms. However, newer concepts such as the drug's site of action, the signal transduction cascade, or neuropeptides are also gaining importance in this field of research.
Metabolizing enzymes have long been recognized as a major source of pharmacokinetic variability, since they influence the interindividual variation in elimination rates, steady-state concentrations, and biotransformation. More than 30 isoforms of the cytochrome P-450 isoenzymes are known today, but few have clinical significance in psychiatry: CYP3A, CYP2D6, CYP2C19, and CYP2C9.118 Different drugs are metabolized by different enzymes and variants in these genes can lead to three possible phenotypes: poor metabolizers (PM), normal metabolizers (NM), and extensive metabolizers (EM). About 7% of Caucasians, 1% of Asians, and 7% to 8% of Africans are classified as PM, who might exhibit increased concentrations of metabolized drugs at conventional doses.119 Genotyping of metabolizing enzymes might have clinical implications, as combinations of drugs that are metabolized by one enzyme may lead to dangerous pharmacokinetic interactions, particularly in PMs.120 Thus, the knowledge of an individual's metabolic rate will help adjust therapeutic doses or combinations accordingly.
The genetic basis of pharmacodynamic variability is becoming a focus of future research. Interesting directions include variants in genes that regulate monoamine uptake, the function of receptors, or the events of the signal transduction cascade.30
Although many investigations have shown that genetic variations in target proteins influence their interaction with psychotropic drugs, these results are still inconclusive and far from the original concept of tailoring the drug regimen to an individual's predisposition and predicting a patient's response to therapeutic agents. However, considering that many other factors besides allelic variants may contribute to the final phenotype of “responder” or “nonresponder,” we cannot assume a one-to-one relationship between genotype and phenotype. Moreover, we must take into account that, like the pathophysiological mechanisms of complex psychiatric disorders, different genes are interacting and modulating each other in drug response, in addition to environmental factors. Nevertheless, the field of pharmacogenetics is expanding rapidly, and the elucidation of the disease processes through genomics, the identification of novel drug targets, and the subtyping of patient populations are all ambitious methods that may help us individualize pharmacological therapy.
Conclusion
Understanding the biology of major depression is a challenging scientific problem with enormous sociological and clinical relevance. The discovery of antidepressant drugs and the investigation of their mechanism of action has revolutionized our understanding of neuronal functioning and the possible mechanisms underlying depression. There is no doubt that the monoaminergic system is one of the cornerstones of research, but any hypothesis for the pathophysiology of depression must take into account the many interactions with other brain systems and the complexity of the regulation of the CNS function. In spite of all the progress that has been achieved in the last decades, we must be aware that there are still today considerable problems in understanding and treating severe depression, and knowing the cause of treatment-resistant depression.
Selected abbreviations and acronyms
- BDNF
brain-derived neurotrophic factor
- CRH
corticotropin-releasing hormone
- DA
dopamine
- GABA
γ-aminobutyric acid
- GH
growth hormone
- HPA
hypothalamus-pituitary-adrenal
- S-HT
5-hydroxy try ptamine (serotonin)
- MAOI
monoamine oxidase inhibitor
- NE
norepinephrine
- NK
natural killer (cell)
- SSRI
selective serotonin-reuptake inhibitor
- TCA
tricyclic antidepressant
REFERENCES
- 1.American Psychiatrie Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 2.Garside RF., Kay DW., Wilson IC., Deaton ID., Roth M. Depressive syndromes and the classification of patients. Psychol Med. 1971;1:333–338. doi: 10.1017/s0033291700042306. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. The ICD-10 Classification of Mental and Behavioral Disorders. Clinical Descriptions and Diagnostic Guidelines. Geneva, Switzerland: World Health Organization; 1992.
- 4.Katz MM., Secunda SK., Hirschfeld RM., Koslow SH. NIMH clinical research branch collaborative program on the psychobiology of depression. Arch Gen Psychiatry. 1979;36:765–771. doi: 10.1001/archpsyc.1979.01780070043004. [DOI] [PubMed] [Google Scholar]
- 5.Gurland B. Aims, organization, and initial studies of the Cross-National Project. IntJ Aging Hum Dev. 1976;7:283–293. doi: 10.2190/n00c-hvd6-w8j8-7kxy. [DOI] [PubMed] [Google Scholar]
- 6.Weissman MM., Bland RC., Canino GJ., et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276:293–299. [PubMed] [Google Scholar]
- 7.Keller MB., Hirschfeld RM., Hanks D. Double depression: a distinctive subtype of unipolar depression. J Affect Disord. 1997;45:65–73. doi: 10.1016/s0165-0327(97)00060-8. [DOI] [PubMed] [Google Scholar]
- 8.Angst J. How recurrent and predictible is depressive illness? In: Montgomery SA, Rouillon F, eds. Long-Term Treatment in Depression. New York, NY: John Wiley. 1992:29–39. [Google Scholar]
- 9.Lavori PW., Keller MB., Scheftner W., Fawcett J., Mueller Tl. Recurrence after recovery in unipolar MDD. An observational follow-up study of clinical predictors and somatic treatment as a mediating factor. int J Methods Psychiatry Res. 1994;4:211–229. [Google Scholar]
- 10.Wong ML., Licinio J. Research and treatment approaches to depression. Nat RevNeurosci. 2001;2:343–351. doi: 10.1038/35072566. [DOI] [PubMed] [Google Scholar]
- 11.Musselman DL., Evans DL., Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- 12.Penninx BW., Beekman AT., Honig A., et al. Depression and cardiac mortality: results from a community-based longitudinal study. Arch Gen Psychiatry. 2001;58:221–227. doi: 10.1001/archpsyc.58.3.221. [DOI] [PubMed] [Google Scholar]
- 13.Angst J. Depression and anxiety: implications for nosology, course, and s treatment. J Clin Psychiatry. 1997;58(suppl 8):3–5. [PubMed] [Google Scholar]
- 14.Merikangas KR., Angst J., Eaton W., et al. Comorbidity and boundaries of affective disorders with anxiety disorders and substance misuse: results of an international task force. Br J Psychiatry Suppl. 1996:58–67. [PubMed] [Google Scholar]
- 15.Paykel ES. The evolution of life events research in psychiatry. J Affect Disord. 2001;62:141–149. doi: 10.1016/s0165-0327(00)00174-9. [DOI] [PubMed] [Google Scholar]
- 16.Brown GW., Harris TO., Hepworth C. Life events and endogenous depression. A puzzle reexamined. Arch Gen Psychiatry. 1994;51:525–534. doi: 10.1001/archpsyc.1994.03950070017006. [DOI] [PubMed] [Google Scholar]
- 17.Paykel ES., Cooper Z., Ramana R., Hayhurst H. Life events, social support and marital relationships in the outcome of severe depression. Psychol Med. 1996;26:121–133. doi: 10.1017/s0033291700033766. [DOI] [PubMed] [Google Scholar]
- 18.Kendler KS., Thornton LM., Prescott CA. Gender differences in the rates of exposure to stressful life events and sensitivity to their depressogenic effects. Am J Psychiatry. 2001;158:587–593. doi: 10.1176/appi.ajp.158.4.587. [DOI] [PubMed] [Google Scholar]
- 19.Berrettini W. Molecular linkage studies in bipolar disorder. Dialogues ClinNeurosci. 1999;1:12–21. doi: 10.31887/DCNS.1999.1.1/wberrettini. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craddock N., Khodel V., Van Eerdewegh P., Reich T. Mathematical limits of multilocus models: the genetic transmission of bipolar disorder. Am J Hum Genet. 1995;57:690–702. [PMC free article] [PubMed] [Google Scholar]
- 21.Souery D., Rivelli SK., Mendlewicz J. Molecular genetic and family studies in affective disorders: state of the. art. J Affect Disord. 2001;62:45–55. doi: 10.1016/s0165-0327(00)00350-5. [DOI] [PubMed] [Google Scholar]
- 22.Malhi GS., Moore J., McGuffin P. The genetics of major depressive disor! der. Curr Psychiatry Rep. 2000;2:165–169. doi: 10.1007/s11920-000-0062-y. [DOI] [PubMed] [Google Scholar]
- 23.Jones I., Craddock N. Candidate gene studies of bipolar disorder. Ann Med. 2001;33:248–256. doi: 10.3109/07853890108998753. [DOI] [PubMed] [Google Scholar]
- 24.Ho LW., Furlong RA., Rubinsztein JS., Walsh C., Paykel ES., Rubinsztein DC. Genetic associations with clinical characteristics in bipolar affective disorder and recurrent unipolar depressive disorder. Am J Med Genet. 2000;96:36–42. [PubMed] [Google Scholar]
- 25.Lerer B., Macciardi F., Segman RH., et al. Variability of 5-HT2c receptor cys23ser polymorphism among European populations and vulnerability to affective disorder. Mol Psychiatry. 2001;6:579–585. doi: 10.1038/sj.mp.4000883. [DOI] [PubMed] [Google Scholar]
- 26.Arias B., Gutierrez B., Pintor L., Gasto C., Fananas L. Variability in the 5-HT(2A) receptor gene is associated with seasonal pattern in major depression. Mol Psychiatry. 2001;6:239–242. doi: 10.1038/sj.mp.4000818. [DOI] [PubMed] [Google Scholar]
- 27.Kendler KS., Gardner CO., Neale MC., Prescott CA. Genetic risk factors for major depression in men and women: similar or different heritabilities and same or partly distinct genes? Psychol Med. 2001;31:605–616. doi: 10.1017/s0033291701003907. [DOI] [PubMed] [Google Scholar]
- 28.Kandel ER., Squire LR. Neuroscience: breaking down scientific barriers to the study of brain and mind. Science. 2000;290:1113–1120. doi: 10.1126/science.290.5494.1113. [DOI] [PubMed] [Google Scholar]
- 29.Kuhar MJ., Couceyro PR., Lambert PD. Catecholamines. In: Siegel GJ, Agranoff BW, Albers L, Fisher SK, Uhler MD, eds. Basic Neurochemistry Philadelphia, Pa: Lippincott Williams & Wilkins. 2001:243–262. [Google Scholar]
- 30.Duman RS., Heninger GR., Nestler EJ. A molecular and cellular theory of depression [see comments]. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 31.Perez J., Tardito D., Mori S., Racagni G., Smeraldi E., Zanardi R. Abnormalities of cAMP signaling in affective disorders: implication for pathophysiology and treatment. Bipolar Disord. 2000;2:27–36. doi: 10.1034/j.1399-5618.2000.020104.x. [DOI] [PubMed] [Google Scholar]
- 32.Popoli M., Brunello N., Perez J., Racagni G. Second messenger-regulated protein kinases in the brain: their functional role and the action of antidepressant drugs. J Neurochem. 2000;74:21–33. doi: 10.1046/j.1471-4159.2000.0740021.x. [DOI] [PubMed] [Google Scholar]
- 33.Battaini F. Protein kinase C isoforms as therapeutic targets in the nervous system disease states. Pharmacol Res. 2001;44:353–361. doi: 10.1006/phrs.2001.0893. [DOI] [PubMed] [Google Scholar]
- 34.Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122:509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- 35.Matussek N. Biochemistry of depression [in German]. J Neural Transm. 1972;33:223–234. doi: 10.1007/BF01245319. [DOI] [PubMed] [Google Scholar]
- 36.Coppen A. The biochemistry of affective disorders. Br J Psychiatry. 1967;113:1237–1264. doi: 10.1192/bjp.113.504.1237. [DOI] [PubMed] [Google Scholar]
- 37.Stahl SM. Basic psychopharmacology of antidepressants, part 1: Antidepressants have seven distinct mechanisms of action. J Clin Psychiatry. 1998;59(suppl4):5–14. [PubMed] [Google Scholar]
- 38.Potter WZ., Scheinin M., Golden RN., et al. Selective antidepressants and cerebrospinal fluid. Lack of specificity on norepinephrine and serotonin metabolites. Arch Gen Psychiatry. 1985;42:1171–1177. doi: 10.1001/archpsyc.1985.01790350045009. [DOI] [PubMed] [Google Scholar]
- 39.Schatzberg AF., Schildkraut JJ. Recent studies on norepinephrine system in mood disorders. In: Bloom FE, Kupfer DJ, eds. Psychopharmacology The Fourth Generation in Progress. New York, NY: Raven Press. 1995:911–920. [Google Scholar]
- 40.Maes M., Meltzer HY. The serotonin hypothesis of major depression. In: Maes M, Meltzer HY, eds. Psychopharmacology; The Fourth Generation of Progress. New York, NY: Raven Press. 1994:933–944. [Google Scholar]
- 41.Cheetham SC., Katona CL., Horton RW. Postmortem studies of neurotransmitter biochemistry in depression and suicide. In: Horton RW, Katona CL, eds. Biological Aspects of Affective Disorders.London, UK: Academic Press. 1991:192–221. [Google Scholar]
- 42.Leonard BE. Evidence for a biochemical lesion in depression. J Clin Psychiatry. 2000;61(suppl 6):12–17. [PubMed] [Google Scholar]
- 43.Agranoff BW., Cotman CW., Uhler MD. Learning and memory. In: Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD, eds. Basic Neurochemistry. Philadelphia, Pa: Lippincott Williams & Wilkins. 1998:1027–1052. [Google Scholar]
- 44.Miller HL., Delgado PL., Salomon RM., et al. Clinical and biochemical effects of catecholamine depletion on antidepressant-induced remission of depression. Arch Gen Psychiatry. 1996;53:117–128. doi: 10.1001/archpsyc.1996.01830020031005. [DOI] [PubMed] [Google Scholar]
- 45.Neumeister A., Praschak RN., Willeit M., Stastny J., Kasper S. Monoamine depletion in non-pharmacological treatments for depression. AdvExp Med Biol. 2000;467:29–33. doi: 10.1007/978-1-4615-4709-9_4. [DOI] [PubMed] [Google Scholar]
- 46.Delgado PL. Depression: the case for a monoamine deficiency. J Clin Psychiatry. 2000;61 (suppl 6):7–11. [PubMed] [Google Scholar]
- 47.Van der Does AJ. The effects of tryptophan depletion on mood and psychiatric symptoms. J Affect Disord. 2001;64:107–119. doi: 10.1016/s0165-0327(00)00209-3. [DOI] [PubMed] [Google Scholar]
- 48.Lesch KP., Wolozin BL., Murphy DL., Reiderer P. Primary structure of the human platelet serotonin uptake site: identity with the brain serotonin transporter. J Neurochem. 1993;60:2319–2322. doi: 10.1111/j.1471-4159.1993.tb03522.x. [DOI] [PubMed] [Google Scholar]
- 49.Owens MJ., Nemeroff CB. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem. 1994;40:288–295. [PubMed] [Google Scholar]
- 50.Malison RT., Price LH., Berman R., et al. Reduced brain serotonin transporter availability in major depression as measured by [*23l]-2]5-carbomethoxy-3p-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry. 1998;44:1090–1098. doi: 10.1016/s0006-3223(98)00272-8. [DOI] [PubMed] [Google Scholar]
- 51.Staley JK., Malison RT., Innis RB. Imaging of the serotonergic system: interactions of neuroanatomical and functional abnormalities of depression. Biol Psychiatry. 1998;44:534–549. doi: 10.1016/s0006-3223(98)00185-1. [DOI] [PubMed] [Google Scholar]
- 52.Heils A., Wichems C., Mossner R., et al. Functional characterization of the murine serotonin transporter gene promoter in serotonergic raphe neurons. J Neurochem. 1998;70:932–939. doi: 10.1046/j.1471-4159.1998.70030932.x. [DOI] [PubMed] [Google Scholar]
- 53.Collier DA., Stober G., Li T., et al. A novel functional polymorphism within the promoter of the serotonin transporter gene: possible role in susceptibility to affective disorders [see comments]. Mol Psychiatry. 1996;1:453–460. [PubMed] [Google Scholar]
- 54.Klimek V., Stockmeier C., Overholser J., et al. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J Neurosci. 1997;17:8451–8458. doi: 10.1523/JNEUROSCI.17-21-08451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hadley D., Hoff M., Holik J., et al. Manic-depression and the norepinephrine transporter gene. Hum Hered. 1995;45:165–168. doi: 10.1159/000154279. [DOI] [PubMed] [Google Scholar]
- 56.Frazer A., Hensler JG. Serotonin. In: Siegel GJ, Agranoff BW, Albers L, Fisher DA, Uhler MD, eds. Basic Neurochemistry. Philadelphia, Pa: Lippincott Williams & Wilkins. 1998:263–292. [Google Scholar]
- 57.Hrdina PD., Bakish D., Ravindran A., Chudzik J., Cavazzoni P., Lapierre YD. Platelet serotonergic indices in major depression: up-regulation of 5-HT2A receptors unchanged by antidepressant treatment. Psychiatry Res. 1997;66:73–85. doi: 10.1016/s0165-1781(96)03046-6. [DOI] [PubMed] [Google Scholar]
- 58.Manji HK., McNamara R., Chen G., Lenox RH. Signalling pathways in the brain: cellular transduction of mood stabilisation in the treatment of manicdepressive illness. Aust N Z J Psychiatry. 1999;33(suppl):S65–S83. doi: 10.1111/j.1440-1614.1999.00670.x. [DOI] [PubMed] [Google Scholar]
- 59.Yatham LN., Srisurapanont M., Zis AP., Kusumakar V. Comparative studies of the biological distinction between unipolar and bipolar depressions. Life Sci. 1997;61:1445–1455. doi: 10.1016/s0024-3205(97)00432-3. [DOI] [PubMed] [Google Scholar]
- 60.Duman RS., Malberg J., Thome J. Neural plasticity to stress and antidepressant treatment. Biol Psychiatry. 1999;46:1181–1191. doi: 10.1016/s0006-3223(99)00177-8. [DOI] [PubMed] [Google Scholar]
- 61.Coull MA., Lowther S., Katona CL., Horton RW. Altered brain protein kinase C in depression: a post-mortem study. Eur Neuropsychopharmacol. 2000;10:283–288. doi: 10.1016/s0924-977x(00)00084-5. [DOI] [PubMed] [Google Scholar]
- 62.Feighner JP. Mechanism of action of antidepressant medications [see discussion]. J Clin Psychiatry. 1999;60(suppl 4):4–11. [PubMed] [Google Scholar]
- 63.Glassman AH., Shapiro PA. Depression and the course of coronary artery disease. Am J Psychiatry. 1998;155:4–11. doi: 10.1176/ajp.155.1.4. [DOI] [PubMed] [Google Scholar]
- 64.Murphy DL., Andrews AM., Wichems CH., Li Q., Tohda M., Greenberg B. Brain serotonin neurotransmission: an overview and update with an emphasis on serotonin subsystem heterogeneity, multiple receptors, interactions with other neurotransmitter systems, and consequent implications for understanding the actions of serotonergic drugs. J Clin Psychiatry. 1998;59(suppl 15):4–12. [PubMed] [Google Scholar]
- 65.McEwen BS., Conrad CD., Kuroda Y., Frankfurt M., Magarinos AM., McKittrick C. Prevention of stress-induced morphological and cognitive consequences. Fur Neuropsychopharmacol. 1997;7(suppl 3):S323–S328. doi: 10.1016/s0924-977x(97)00064-3. [DOI] [PubMed] [Google Scholar]
- 66.Schweitzer I., Tuckwell V., Johnson G. A review of the use of augmentation therapy for the treatment of resistant depression: implications for the clinician. Aust N Z J Psychiatry. 1997;31:340–352. doi: 10.3109/00048679709073843. [DOI] [PubMed] [Google Scholar]
- 67.Peroutka SJ., Snyder SH. Long-term antidepressant treatment decreases spiroperidol-labeled serotonin receptor binding. Science. 1980;210:88–90. doi: 10.1126/science.6251550. [DOI] [PubMed] [Google Scholar]
- 68.Blier P., Abbott FV. Putative mechanisms of action of antidepressant drugs in affective and anxiety disorders and pain. J Psychiatry Neurosci. 2001;26:37–43. [PMC free article] [PubMed] [Google Scholar]
- 69.Manier DH., Shelton RC., Sulser F. Cross-talk between PKA and PKC in human fibroblasts: what are the pharmacotherapeutic implications? J Affect Disord. 2001;65:275–279. doi: 10.1016/s0165-0327(00)00278-0. [DOI] [PubMed] [Google Scholar]
- 70.Frazer A. Antidepressants. J Clin Psychiatry. 1997;58(suppl 6):9–25. [PubMed] [Google Scholar]
- 71.Sanacora G., Mason GF., Rothman DL., et al. Reduced cortical gammaaminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 72.Kramer MS., Cutler N., Feighner J., et al. Distinct mechanism for antidepressant activity by blockade of central substance-P receptors [see comments]. Science. 1998;281:1640–1645. doi: 10.1126/science.281.5383.1640. [DOI] [PubMed] [Google Scholar]
- 73.Reul JMHM., Holsboer F. On the role of corticotropin-releasing hormone receptors in anxiety and depression. Dialogues Clin Neurosci. 2002;4:31–40. doi: 10.31887/DCNS.2002.4.1/jreul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pevet A. Melatonin. Dialogues Clin Neurosci. 2002;4:57–72. doi: 10.31887/DCNS.2002.4.1/ppevet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rubin RT., Poland RE., Lesser IM., Martin DJ. Neuroendocrine aspects of primary endogenous depression. V. Serum prolactin measures in patients and matched control subjects. Biol Psychiatry. 1989;25:4–21. doi: 10.1016/0006-3223(89)90142-x. [DOI] [PubMed] [Google Scholar]
- 76.Holsboer F., Barden N. Antidepressants and hypothalamic-pituitaryadrenocortical regulation. Endocr Rev. 1996;17:187–205. doi: 10.1210/edrv-17-2-187. [DOI] [PubMed] [Google Scholar]
- 77.Holsboer F., Gerken A., von Bardeleben U., et al. Human corticotropinreleasing hormone in depression - correlation with thyrotropin secretion following thyrotropin-releasing hormone. Biol Psychiatry. 1986;21:601–611. doi: 10.1016/0006-3223(86)90121-6. [DOI] [PubMed] [Google Scholar]
- 78.Holsboer F., Liebl R., Hofschuster E. Repeated dexamethasone suppression test during depressive illness. Normalisation of test result compared with clinical improvement. J Affect Disord. 1982;4:93–101. doi: 10.1016/0165-0327(82)90039-8. [DOI] [PubMed] [Google Scholar]
- 79.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 80.Matussek N. Catecholamines and mood: neuroendocrine aspects. Curr Top Neuroendocrinol. 1988;8:141–182. [Google Scholar]
- 81.Hoehe M., Valido G., Matussek N. Growth hormone response to clonidine in endogenous depressive patients: evidence for a trait marker in depression. In: Shagass C, Josiassen RC, Bridger WH, eds. Biological Psychiatry Amsterdam, The Netherlands: Elsevier. 1986:862–864. [Google Scholar]
- 82.Charney DS., Heninger GR., Sternberg DE. The effect of mianserin on alpha-2 adrenergic receptor function in depressed patients. Br J Psychiatry. 1984;144:407–416. doi: 10.1192/bjp.144.4.407. [DOI] [PubMed] [Google Scholar]
- 83.Anand A., Charney DS. Norepinephrine dysfunction in depression. J Clin Psychiatry. 2000;61(suppl 10):16–24. [PubMed] [Google Scholar]
- 84.Duval F., Mokrani MC., Bailey P., et al. Thyroid axis activity and serotonin function in major depressive episode. Psychoneuroendocrinology. 1999;24:695–712. doi: 10.1016/s0306-4530(99)00022-0. [DOI] [PubMed] [Google Scholar]
- 85.Altshuler LL., Bauer M., Frye MA., et al. Does thyroid supplementation accelerate tricyclic antidepressant response? A review and meta-analysis of the literature. Am J Psychiatry. 2001;158:1617–1622. doi: 10.1176/appi.ajp.158.10.1617. [DOI] [PubMed] [Google Scholar]
- 86.Gur RE., Lerer B., Newman ME. Chronic clomipramine and triiodothyronine increase serotonin levels in rat frontal cortex in vivo: relationship to serotonin autoreceptor activity. J Pharmacol Exp Ther. 1999;288:81–87. [PubMed] [Google Scholar]
- 87.Gordon JT., Kaminski DM., Rozanov CB., Dratman MB. Evidence that 3,3',5-triiodothyronine is concentrated in and delivered from the locus coeruleus to its noradrenergic targets via anterograde axonal transport. Neuroscience. 1999;93:943–954. doi: 10.1016/s0306-4522(99)00146-3. [DOI] [PubMed] [Google Scholar]
- 88.Stotland NL., Stotland NE. Depression in women. Obstet Gynecol Surv. 1999;54:519–525. doi: 10.1097/00006254-199908000-00022. [DOI] [PubMed] [Google Scholar]
- 89.Bloch M., Schmidt PJ., Danaceau M., Murphy J., Nieman L., Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157:924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- 90.Lucas A., Pizarro E., Granada ML., Salinas I., Foz M., Sanmarti A. Postpartum thyroiditis: epidemiology and clinical evolution in a nonselected population. Thyroid. 2000;10:71–77. doi: 10.1089/thy.2000.10.71. [DOI] [PubMed] [Google Scholar]
- 91.Susman EJ., Schmeelk KH., Worrall BK., Granger DA., Ponirakis A., Chrousos GP. Corticotropin-releasing hormone and Cortisol: longitudinal associations with depression and antisocial behavior in pregnant adolescents. J Am Acad Child Adolesc Psychiatry. 1999;38:460–467. doi: 10.1097/00004583-199904000-00020. [DOI] [PubMed] [Google Scholar]
- 92.Licinio J., Wong ML. Pathways and mechanisms for cytokine signaling of the central nervous system. J Clin invest. 1997;100:2941–2947. doi: 10.1172/JCI119846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- 94.Maes M., Bosnians E., De Jongh R., Kenis G., Vandoolaeghe E., Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment-resistant depression. Cytokine. 1997;9:853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 95.Connor TJ., Leonard BE. Depression, stress and immunological activation: the role of cytokines in depressive disorders. Life Sci. 1998;62:583–606. doi: 10.1016/s0024-3205(97)00990-9. [DOI] [PubMed] [Google Scholar]
- 96.Sternberg EM. Emotions and disease: from balance of humors to balance of molecules. Nat Med. 1997;3:264–267. doi: 10.1038/nm0397-264. [DOI] [PubMed] [Google Scholar]
- 97.Rothwell NJ., Hopkins SJ. Cytokines and the nervous system II: Actions and mechanisms of action. Trends Neurosci. 1995;18:130–136. doi: 10.1016/0166-2236(95)93890-a. [DOI] [PubMed] [Google Scholar]
- 98.Duman RS., Malberg J., Thome J. Neural plasticity to stress and antidepressant treatment. Biol Psychiatry. 1999;46:1181–1191. doi: 10.1016/s0006-3223(99)00177-8. [DOI] [PubMed] [Google Scholar]
- 99.Altar CA. Neurotrophins and depression. Trends Pharmacol Sci. 1999;20:59–61. doi: 10.1016/s0165-6147(99)01309-7. [DOI] [PubMed] [Google Scholar]
- 100.Siuciak JA., Lewis DR., Wiegand SJ., Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF). Pharmacol Biochem Behav. 1997;56:131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- 101.Duman RS., Heninger GR., Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 102.Chen B., Dowlatshahi D., MacQueen GM., Wang JF., Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 1999;20:59–61. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- 103.Altar CA. Neurotrophins and depression. Trends Pharmacol Sci. 1999;20:59–61. doi: 10.1016/s0165-6147(99)01309-7. [DOI] [PubMed] [Google Scholar]
- 104.Delgado PL., Moreno FA. Role of norepinephrine in depression. J Clin Psychiatry. 2000;61(suppl 1):5–12. [PubMed] [Google Scholar]
- 105.Blier P. Crosstalk between the norepinephrine and serotonin systems and its role in antidepressant response. J Psychiatry Neurosci. 2001;26:3–10. [PMC free article] [PubMed] [Google Scholar]
- 106.Szabo ST., de Montigny C., Blier P. Modulation of noradrenergic neuronal firing by selective serotonin reuptake blockers. Br J Pharmacol. 1999;126:568–571. doi: 10.1038/sj.bjp.0702343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haddjerl N., Szabo ST., de Montigny C., Blier P. Increased tonic activation of rat forebrain 5-HT(1A) receptors by lithium addition to antidepressant treatments. Neuropsychopharmacology. 2000;22:346–356. doi: 10.1016/S0893-133X(99)00138-4. [DOI] [PubMed] [Google Scholar]
- 108.Pike JL., Smith TL., Hauger RL., et al. Chronic life stress alters sympathetic, neuroendocrine, and immune responsivity to an acute psychological stressor in humans. Psychosom Med. 1997;59:447–457. doi: 10.1097/00006842-199707000-00015. [DOI] [PubMed] [Google Scholar]
- 109.Narayan M., Bremner JD., Kumar A. Neuroanatomy substrates of latelife mental disorders. J Geriatr Psychiatry Neurol. 1999;12:95–106. doi: 10.1177/089198879901200303. [DOI] [PubMed] [Google Scholar]
- 110.Videbech P. MRI findings in patients with affective disorder: a metaanalysis. Acta Psychiatr Scand. 1997;96:157–168. doi: 10.1111/j.1600-0447.1997.tb10146.x. [DOI] [PubMed] [Google Scholar]
- 111.Bench CJ., Friston KJ., Brown RG., Scott LC., Frackowiak RS., Dolan RJ. The anatomy of melancholia - focal abnormalities of cerebral blood flow in major depression. Psychol Med. 1992;22:607–615. doi: 10.1017/s003329170003806x. [DOI] [PubMed] [Google Scholar]
- 112.McEwen BS., Magarinos AM. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol. 2001;16:S7–S19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- 113.Bremner JD., Narayan M., Anderson ER., Staib LH., Miller HL., Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 114.Catalano M. The challenges of psychopharrnacogenetics. Am J Hum Genet. 1999;65:606–610. doi: 10.1086/302559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smith MW., Mendoza RP. Ethnicity and pharmacogenetics. Mt Sinai J Med. 1996;63:285–290. [PubMed] [Google Scholar]
- 116.Franchini L., Serretti A., Gasperini M., Smeraldi E. Familial concordance of fluvoxamine response as a tool for differentiating mood disorder pedigrees. J Psychiatr Res. 1998;32:255–259. doi: 10.1016/S0022-3956(98)00004-1. [DOI] [PubMed] [Google Scholar]
- 117.Vogel F. Moderne Problème der Humangenetik. Ergeb inn Med Kinderheilk. 1959;12:52–125. [Google Scholar]
- 118.Wieczorek SJ., Tsongalis GJ. Pharmacogenomics: will it change the field of medicine? Clin Chim Acta. 2001;308:1–8. doi: 10.1016/s0009-8981(01)00419-3. [DOI] [PubMed] [Google Scholar]
- 119.Cichon S., Nothen MM., Rietschel M., Propping P. Pharmacogenetics of schizophrenia. Am J Med Genet. 2000;97:98–106. doi: 10.1002/(sici)1096-8628(200021)97:1<98::aid-ajmg12>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 120.Alfaro CL., Lam YW., Simpson J., Ereshefsky L. CYP2D6 status of extensive metabolizers after multiple-dose fluoxetine, fluvoxamine, paroxetine, or sertraline. J Clin Psychopharmaco! 1999;19:155–163. doi: 10.1097/00004714-199904000-00011. [DOI] [PubMed] [Google Scholar]