Abstract
We now appreciate that estrogen is a pleiotropic gonadal steroid that exerts profound effects on the plasticity and cell survival of the adult brain. Over the past century, the life span of women has increased, but the age of the menopause remains constant. This means that women may now live over one third of their lives in a hypoestrogenic, postmenopausal state. The impact of prolonged hypoestrogenicity on the brain is now a critical health concern as we realize that these women may suffer an increased risk of cognitive dysfunction and neurodegeneration due to a variety of diseases. Accumulating evidence from both clinical and basic science studies indicates that estrogen exerts critical protective actions against neurodegenerative conditions such as Alzheimer's disease and stroke. Here, we review the discoveries that comprise our current understanding of estrogen action against neurodegeneration. These findings carry far-reaching possibilities for improving the quality of life in our aging population.
Keywords: estrogen; estradiol; estrogen replacement therapy; menopause; stroke; cerebral ischemia; Alzheimer's disease; cognition, brain injury; neuroprotection
Abstract
Actualmente nosotros sabemos que los estrógenos son esteroides gonadales pleiotrópicos que ejercen efectos significativos en la plasticidad y en la sobrevida celular del cerebro adulto. Durante el siglo pasado la duración de la vida de las mujeres ha aumentado, pero la edad de la menopausia se ha mantenido constante. Esto signífica que ahora las mujeres pueden vivir más de un tercio de sus vidas en un estado hipoestrogénico postmenopáusico. El impacto en el cerebro del hipoestrogenismo prolongado constituye ahora una preocupación crítica de salud ya que nos damos cuenta que estas mujeres pueden tener un aumento del ríesgo de disfunción cognitiva y de neurodegeneración debido a una variedad de enfermedades. La evidencia que se ha acumulado tanto de los estudios clínicos como de ciencias básicas índica que los estrógenos ejercen acciones protectoras críticas ante condíciones neurodegenerativas como la enfermedad de Alzheimer y los accidentes vasculares cerebrales. En este artículo nosotros revisamos los descubrímientos que engloban nuestra comprensión actual de la acción de los estrógenos contra la neurodegeneración. Estos hallazgos conducen a posibilidades de gran alcance para mejorar la calidad de vida en nuestra población que envejece.
Abstract
Nous savons maintenant que les estrogènes sont des stéroïdes gonadiques pléiotropes qui exercent d'importants effets sur la plasticité et la survie cellulaire du cerveau de l'adulte. Si l'espérance de vie des femmes a augmenté au siècle dernier, l'âge de la ménopause, quant à lui, reste constant. Cela signifie que les femmes peuvent maintenant passer plus d'un tiers de leur vie dans un climat postménopausique, hypoestrogénique. L'impact sur le cerveau d'une hypoestrogénicité prolongée est devenu un souci de santé préoccupant car nous réalisons que ces femmes peuvent être confrontées à un risque accru de dysfonctionnement cognitif et de neurodégénérescence secondaires à de nombreuses maladies. L'accumulation des constatations issues des études scientifiques cliniques et fondamentales indique que les estrogènes exercent des actions protectrices cruciales contre les pathologies neurodégénératives telles que la maladie d'Alzheimer et l'accident vasculaire cérébral. Nous passons ici en revue les découvertes qui constituent nos connaissances actuelles sur l'action des estrogènes contre la neurodégénérescence. Ces découvertes ouvrent la voie à des possibilités d'une portée considérable pour améliorer la qualité de vie de notre population vieillissante.
Estrogen is a pleiotropic hormone that acts beyond the scope of its reproductive functions and exerts protective actions on multiple tissues including the brain. The protective actions of estrogen carry tremendous implications for the promotion of health and the prevention of disease in postmenopausal women. Since the life span of women has increased from approximately 50 to 80 years, but the age of the menopause remains at about 51 years, women may now live over three decades of their lives in a hypoestrogenic, postmenopausal state. The impact of prolonged hypoestrogenicity is now a critical health concern, since we realize that these women may suffer from an increased vulnerability to a variety of diseases. Conversely, replacement, with estrogen appears to act in the primary prevention of many disease processes, including neurodegeneration. Estrogen, however, is not always beneficial, as high and unopposed levels may increase the risk for certain cancers in some women. Our challenge, therefore, is to design hormone replacement therapies that exert, only beneficial effects in the body. To this end, we must gain a more complete understanding of the spectrum of estrogen's actions and, more specifically, we must dissect the mechanisms that underlie its actions.
The broad spectrum of estrogen's actions includes significant, protection of the brain and primary prevention against neurodegeneration. Clinical observations indicate that estrogen replacement in postmenopausal women can (i) ameliorate cognitive dysfunction, and (ii) decrease the risk and delay the onset of degenerative conditions such as Alzheimer's disease (AD) and stroke.
Basic science studies have revealed a strong cellular and molecular basis for these clinical observations. Recent insights into the molecular events that underlie estrogen-mediated neuroprotection encompass actions that range from its pharmacological, antioxidant mechanisms to its physiological, estrogen receptor (ER)-dependent mechanisms. The results of the studies that reveal estrogen's neuroprotective actions and mechanisms carry exciting and far-reaching possibilities for improving the quality of life of our aging population. As we continue to discover how estrogens act in the brain to promote enhanced neural function and exert protective effects against degeneration, we will be able to design hormones that exert, only beneficial effects in the body.
Estrogen, the menopause, and hormone replacement
Estrogen
Estrogens are synthesized predominantly in the ovary as 18-carbon steroids with an aromatic A-ring. They act on multiple endocrine targets and arc synthesized in many forms. Most clinical and basic science studies have focused attention on the actions of estradiol, the most potent and biologically active form of estrogen that circulates in the body prior to the menopause.
Menopause
Because the menopause impacts the health of so many women, investigators have focused on understanding driving factors that govern this change. For many years, it was accepted that the menopause resulted simply from the depletion of the postmitotic pool of ovarian follicles that is set down during embryonic development.1 Clearly the exhaustion of this reservoir necessarily means that a woman is permanently postmenopausal and can no longer produce offspring with her genetic makeup. As importantly, since the ovarian follicles are not only the source of germ cells, but are also the primary source of estradiol, plasma concentrations of this hormone drop precipitously during the postmenopausal years and remain low for the remainder of a woman's life, unless she chooses to take hormone replacement therapy.
Whether or not the brain plays a role in the transition to the menopause has been a topic of active debate. Results from studies using animal models have suggested that aging of the brain and a declining ability to provide coordinated neurochemical signals that, are required for ovulation contribute to reproductive senescence. However, whether these findings are relevant to the human menopause has been less clear. Recently, an increasing number of researchers have begun to appreciate that the brain may play an important role in the sequence of events leading to menopause in humans. Several findings lead to this conclusion. First, the pattern of luteinizing hormone (LH) secretion and the levels of folliclestimulating hormone (FSH) secretion change before women enter the perimenopausal period. These changes are likely to reflect, changes in the pattern of hypothalamic hormone secretion. Second, the responsiveness of the brain to estradiol treatment, and the ability of this hormone to induce gonadotropin surges are greatly compromised in middle-aged women as they enter the transition to the menopause. Finally, estradiol concentrations remain normal or arc elevated during the perimenopausal period, suggesting that, the ovary remains capable of secreting this critical hormone. Interestingly, these findings are strikingly similar to what has been observed in middle-aged laboratory rodents, as they become less fertile and cease to have reproductive cycles.2 Since many studies to examine the role of the brain cannot be performed in women because they are invasive and involve experimental manipulations that, cannot be performed in humans, laboratory animals provide the only means through which we can gain a better understanding of the role of the brain in the menopause. The striking similarities between many of the events that occur during middle age give us reason to believe that rodents serve as excellent models in which to examine the factors that initiate the process of reproductive aging during middle age. We hope that information gained from these species can be extrapolated to humans and will allow us to uncover and explore concepts that can be generalized to human reproductive aging.
Estrogen replacement therapy in postmenopausal women
The process of reproductive aging in women ultimately results in a hypoestrogenic, postmenopausal state. As our understanding of estrogen action in the body grows, the consequences of prolonged hypoestrogenicity and the profound impact of estrogen replacement therapy (ERT) on postmenopausal women become increasingly clear. We now know that, although estrogen can promote disease in some women,3 it acts in a broad spectrum of tissues to promote health and overall well-being.4-8 Insight into many of the protective actions of estrogen is gained from observations that oophorectomized young women suffer increased pathophysiology, such as bone resorption,9 compared with their counterparts with normal reproductive function. Further, hypoestrogenic, postmenopausal women often suffer increased disease compared with premenopausal women and age-matched men. Studies demonstrate that estrogen acts in the brain to enhance cognitive function and decrease the risk and/or delay the onset of neurodegenerative conditions.6,10-13 Further, estrogen decreases the risk and/or mortality for cardiovascular disease, potentially through its beneficial effects on the lipid profile and on the endothelium,7,14,15 though recent evidence suggests that estrogen-mediated protection of the heart may not persist in women with preexisting cardiovascular disease.16 Finally, estrogen is crucial in the positive remodeling of bone; the loss of estrogen in postmenopausal women is accompanied by a dramatic increase in osteoporosis.5
Estrogen: effects on cognition and Alzheimer's disease
The focus of this review - and the focus of our recent discoveries - is the action of estrogen in the injured and aging brain. Our interest in this area stemmed from clinical observations that estrogen acts beyond its traditional targets in the hypothalamus and pituitary, and influences other facets of brain function involved in memory and neurodegeneration. Since neurodegenerative events occur frequently in elderly women who are chronically hypoestrogenic, the results of these studies carry profound implications in the clinical setting. Thus, it is important, to determine whether and how ERT can ameliorate neural dysfunction.
Estrogen and cognition
Clinical studies demonstrate that estrogen influences memory and cognition,6,17-22 and can protect against neurodegenerative diseases such as AD.10,11,23-30 These findings, however, are not without controversy. As the results of more clinical studies become available, we are beginning to appreciate that, the protective actions of estrogen do not apply in all situations.31-33
Many studies have examined whether ERT improves cognitive function. The majority of data show that estrogen can enhance cognitive function in both young22 and older women.19,21,34 It appears that by maintaining normal cognitive function, estrogen may further act to decrease the risk and delay the onset, of AD.10,26,28-35 Importantly, estrogen docs not exert actions on all aspects of memory. It is critical to consider that memory is a broad term describing several distinct neural functions, many of which originate in different and overlapping regions of the brain. Thus, it is not surprising that estrogen may influence specific subtypes of memory. For example, some,17,19,34 but not all,36 studies show that ERT appears to specifically enhance immediate and delayed recall of verbal information. Other reports indicate that the beneficial actions of ERT on cognition include improvement of visuospatial memory.37,38
Estrogen and Alzheimer's disease
Several studies have examined whether ERT decreases the risk, delays the onset, or stops the progression of neurodegeneration caused by AD. Estrogen has been shown to decrease the risk for AD10 or induce a modest improvement, in cognitive function in individuals with AD.11,35,39,40 However, other studies have reported no difference in cognitive function between estrogen- and placebo-treated individuals.41-43 A recent cohort, study43 failed to detect slowing of AD progression or improvement of cognitive and functional outcomes in women with mild-to-modcratc AD treated with Premarin®. The results of this study and those of other studies31-33 strongly suggest, that estrogen may fail to reverse or even halt a disease process that has already been initiated. Therefore, while it is clear that estrogen can influence certain aspects of cognitive function and decrease the risk for AD, it remains controversial whether it can act against an existing neurodegenerative condition. Taken together, the findings of these studies indicate that estrogen may protect the brain through primary prevention.
Estrogen and stroke
Our studies on estrogen action against neurodegeneration have focused attention on whether estradiol plays a role in stroke injury. We, and others, have aggressively investigated this question since stroke: (i) is a major form of neurodegeneration that grossly impacts our aging population, and (ii) has been successfully reproduced in several animal models. To date, most clinical studies have assessed whether ERT alters the risk and mortality of stroke,44 but have not addressed whether estradiol decreases the degree of brain injury resulting from stroke.
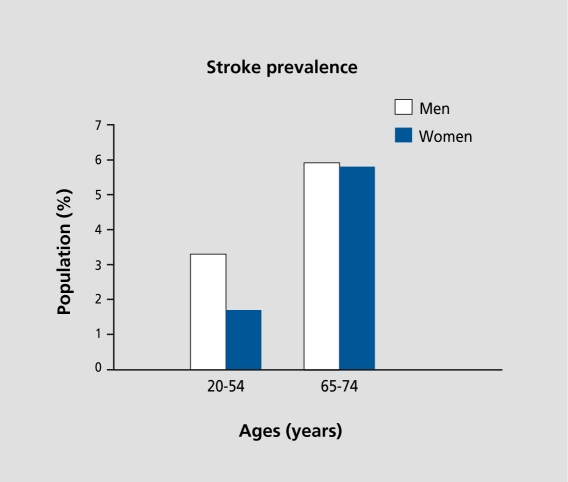
Stroke is a neurodegenerative condition that greatly impacts the health and livelihood of our aging population. It is the third leading cause of death for middleaged and older women, and a major health problem that affects 500 000 Americans each year.45 Interestingly, premenopausal women are at a lower risk for stroke than men of the same age.46 However, after the menopause the incidence of cerebrovascular disease rises rapidly.47 These clinical observations parallel statistics on the prevalence of stroke with regard to age and sex: at a younger age, women appear to be protected against stroke, compared with men, but lose this advantage in their postmenopausal years (Figure 1).48 Together, these data suggest that endogenous estrogen plays a protective role against stroke. Since stroke imposes major morbidity and mortality in postmenopausal women, it is critical to determine whether ERT may decrease the risk and/or severity of cerebrovascular disease.
Figure 1. Endogenous estrogen may decrease the risk for stroke. Prior to the menopause, women appear to be protected against the occurrence of stroke, compared with age-matched men (left). However, this protection is lost at some time after the menopause (right), suggesting that endogenous estrogen may play a protective role against stroke. Data modified from the American Heart Association (2001).48 .
Classification of stroke
Stroke results in infarction of the brain. Major causes and risk factors for stroke include coronary artery disease, cardiac failure, diabetes, hypertension, atherosclerosis, and thrombotic conditions. The overlapping and often mixed etiologies of stroke can result in two major types of pathology: ischemic stroke or hemorrhagic stroke. Briefly, clot(s) in the cerebro vasculature produce ischemic infarct, and the bursting of cerebral vessel (s) causes subarachnoid hemorrhage.
Estrogen and stroke risk
Several lines of evidence suggest that ERT may reduce the likelihood for stroke by modifying risk factors that underlie both stroke and coronary heart, disease (CHD).44 For example, estrogen may protect by exerting beneficial effects on diabetes and on the scrum lipid profile.49,50 Interestingly, CHD doubles the risk for stroke and ERT greatly reduces the risk for CHD (by 30% to 40%). It thus follows that estrogen may decrease the risk for stroke in parallel with its protective actions on CHD.
In contrast to protection, estrogen may, under some circumstances, impose an increased risk for stroke by influencing coagulation and fibrinolysis. Concerns of the thrombotic potential of estrogen arose from early observations that oral contraceptives appeared to increase the risk of venous thrombosis, pulmonary embolism, and stroke.51 Similarly, ERT in postmenopausal women appears to be associated with a higher risk of venous thrombosis during the first year of use.52 However, whether ERT imposes a risk for ischemic stroke in postmenopausal women is unclear. We now understand that the dose of estrogen administered and the route of estrogen delivery are key components in determining clotting potential. At higher doses, oral estrogen, which enters the body via the enterohepatic system, can stimulate the production of thrombogenic factors53,54 predominantly through its actions on the liver. Alternatively, lower doses of estrogen, delivered orally or transdermally, may not significantly affect hemostasis.53,55-57 Importantly, transdermal delivery of estrogen bypasses enterohepatic circulation and may thus prevent estrogen-mediated stimulation of thrombogenic factors in the liver. Collectively, these findings highlight the importance of low, physiological doses in estrogen replacement of postmenopausal women.
ERT and stroke: overview of clinical studies
Studies have only begun to explore the actions of hormone replacement on stroke in the clinical setting. Data from several human studies clearly indicate that estrogen exerts protection against stroke58-61; however, many studies report cither protective trends or no significant, effect, of estrogen58,59,62-72 and few report deleterious effects of estrogen.62,66,73,74 Preliminary results from the latest clinical study, the Women's Estrogen for Stroke Trial (WEST), indicate that estrogen docs not protect against the rate of either nonfatal stroke or death in postmenopausal women with a history of stroke.75 In parallel with studies that fail to detect estrogen-mediated protection of the heart in women with cardiovascular disease,16 or of the brain in women with AD,43 the results of WEST suggest that estrogen does not effectively protect against, or reverse a disease process that has already been initiated.
To date, clinical studies have mainly probed whether ERT significantly affects the incidence and mortality of stroke. The outcomes of many of these studies are varied and often appear to be contradictory. Thus, we cannot yet draw clear conclusions from the existing data due to several confounding issues. The lack of uniformity among the data in clinical reports may result, from several inconsistencies.44 First, stroke is a mixed group of diseases with varying etiologies. If ERT decreases or increases the risk of specific stroke subtypes, effects of estrogen may be distorted and/or masked when strokes arc grouped together and classified differently among the studies. Second, it is important to distinguish between ever and current users of ERT, as these categories represent two distinct, populations of women; ever users of ERT may demonstrate long-lasting actions of estrogen, while current users of ERT may exhibit primarily short-term actions of estrogen. Clearly, mixing the two populations can obscure important effects of estrogen. Finally, it is critical to identify and probe the effects of different modes and regimens of ERT since estrogen action can vary dramatically if it is administered (i) in physiological versus pharmacological doses; (ii) alone or combined with progesterone (cyclic or continuous); and (iii) orally or transdermally. Since the distinctions among ERT regimens are not clear in most, clinical studies, it is often difficult to interpret results or, further, to compare results with the outcomes of other studies.
Cerebral ischemia; an animal model of stroke
Realistically, even the best, well-designed clinical studies may not benefit from the experimental advantages of many basic science studies including clear and unconfounded controls, well-controlled environments, and lack of selection or recall bias. Thus, investigators have developed animal models of stroke to investigate the pathophysiology and potential treatments for stroke.
Cerebral artery occlusion
Experimental methods developed to emulate stroke in animal models produce brain ischemia by blocking blood flow to the cerebral vasculature. The varieties of techniques used to induce ischemia differ with regard to the means of producing ischemia, the site of occlusion, and the duration of occlusion. We utilized an animal model of stroke, permanent middle cerebral artery occlusion (MCAO), to examine the effects of estrogen in neurodegeneration. Since ischemic infarcts represent the majority (>70%) of cerebrovascular disease in the aging population, we adopted an animal model that reproduces ischemic infarcts. MCAO has been thoroughly developed and characterized to study the pathophysiology and therapeutic possibilities of ischemic injury. Occlusion, or the blocking of blood flow, of the middle cerebral artery predominantly affects two major areas: the cortex and underlying striatum. Permanent blockage of this artery at its base causes severe metabolic impairment in the striatum because this region receives no alternative blood perfusion; this characterizes the “ischemic core.” The cortex, on the other hand, undergoes moderate metabolic impairment and is potentially salvageable by effective therapeutic agents because it receives some perfusion from the anterior cerebral artery and the vertebral artery76; this characterizes “ischemic penumbra.”
Ischemia results in cell death
The ischemic brain is exposed to excessive amounts of glutamate, which leads to massive influxes of calcium into cells. Although the exact, mechanisms of ischemic injury are not clear, glutamate neurotoxicity is a key player in the pathogenesis of an ischemic lesion.77-79 Inappropriate rises in intracellular calcium due to glutamate and ion dyshomeostasis can cause moderate or irreversible injury, depending on the severity of the insult.79 An evolving view of trauma in focal cerebral ischemia is that the severely impaired “ischemic core” dies by necrotic cell death, a mode of death characterized by inflammation and cellular bursting. The moderately injured “ischemic penumbra” dies, in part, by apoptotic cell death, an orchestrated event of cellular signaling that results in distinct morphological changes resembling autodigestion.80 While the exact modes of ischemic cell death are controversial, several apoptotic factors have been identified as pathogenic or survival components in ischemic injury.81-87 As discussed below, many studies, including our own, have investigated whether estradiol can attenuate cell death resulting from ischemic injury and whether the mechanisms of protection against cell death involve suppression of apoptotic signaling.
Estrogen and neuroprotection: insights from basic science studies
Estrogen protects against in vivo brain injury
In 1991, a single in vivo report suggested that estradiol may play a role in protection of the brain. This study, carried out by Hall and colleagues, demonstrated that female gerbils sustained less neuronal pathology following global ischemia than males.88 Since then, the field of estrogen and neuroprotection has rapidly expanded and numerous laboratories have demonstrated that estrogen exerts profound neuroprotective actions in a variety of paradigms of brain injury.89 The results of these studies have clearly shown that that estradiol decreases the severity of injury in several in vivo models including cerebral ischemia,90-95 cerebral contusion,96-98 hypoxia,99 and drug-induced toxicity.100
Studies performed using animal models of stroke provide strong evidence that estradiol is a neuroprotective factor that, profoundly attenuates the degree of ischemic brain injury. These studies clearly establish that females uniformly endure less stroke injury than males. Female gerbils demonstrate less neuronal pathology than males after ischemia induced by unilateral carotid artery occlusion.88 Likewise, gonadally intact female rats sustain over 50% less infarction than gonadally intact males and ovariectomized female rats following ischemia induced by transient occlusion of the middle cerebral artery.94,101 Further, gonadectomized females90-93,102 and males97 that are treated with estradiol suffer less MCAO-induced injury than estradiol-depleted controls.
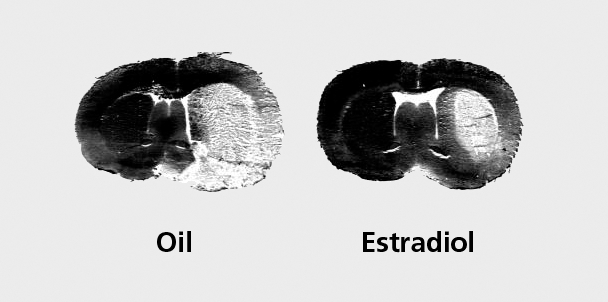
Our work has significantly contributed to the understanding of the neuroprotective actions of physiological levels of estradiol. We have found that low, physiological doses of estradiol replacement are sufficient to exert dramatic protection in the brains of young female rats (Figure 2).90 Further, we found that, middle-aged female rats remain responsive to the neuroprotective effects of low estradiol levels.103 Collectively, the results of these studies suggest that postmenopausal women that are estrogen-replaced may suffer a decreased degree of brain injury following a stroke, compared with their hypoestrogenic counterparts. However, we must be careful in extrapolating from rodents to humans until the appropriate clinical studies are performed.
Figure 2. Physiological levels of estradiol decrease ischemic brain damage following stroke injury. Representative coronal sections obtained from oil-treated (left) and estradiol-treated (right) rat brains collected 24 h after the onset of ischemia and stained with hemotoxylin and eosin. Ischemic injury, produced by permanent middle cerebral artery occlusion, appears unstained and is distributed across the cerebral cortex and striatum. Pretreatment with low levels of estradiol dramatically decreases the extent of stroke injury, compared with oil-treated controls.
Estrogen protects against in vitro neural injury
In addition to in vivo studies, several in vitro studies have greatly contributed to our understanding of estrogen action against degeneration. Many paradigms have been utilized to investigate whether estrogen can protect neural cells, in vitro. Studies have been performed in primary neuronal cultures, mixed astrocyte/neuron cultures, cell lines, and organotypic cultures. Using these paradigms, investigators have aimed to reproduce the deleterious environments found in various neurodegenerative conditions such as AD and stroke and have then tested whether estrogen protects against cell death.
In vitro studies clearly establish that estrogen exerts profound protective effects against a variety of neurotoxic insults. Studies have induced injury through conditions that mimic AD toxicity,104-107 hypoxia, and oxidative stress,107-113 excitotoxicity,107,111,114-116 and physical injury.117 Thus, studies have examined whether estradiol can salvage cells from death induced by inhibition of mitochondrial function, suppression of glucose metabolism, alteration of nitric oxide production, or administration of substances such as β-amyloid peptide, excitatory amino acids, free radicals, and glycoprotein 120. Though the differing modes of injury are distinct, they may share similar mechanisms of toxicity and face final common pathways in the induction of cell death. It remains to be determined whether estradiol protects against cell death through parallel or divergent pathways in the different modes of injury.
Estrogen does not always protect
It is important to appreciate that estrogen does not always exert beneficial effects. The actions of estrogen appear to be dictated by the type of estrogen administered, dose of estrogen given, and the animal model utilized. The type of estrogen administered impacts the efficacy of its neuroprotective actions. Most, studies have focused attention on the effects of 17β-cstradiol since it is the most, biologically active and potent endogenous estrogen. However, we have gained major insight into estrogen action through studies that have probed the effects of 17α-estradiol, an “inactive” stereoisomer that, does not effectively bind and activate ERs. The studies show that at physiological levels, 17β-estradiol protects and 17α-estradiol fails to protect against, brain injury,110 indicating that ERs arc critical to the mechanisms of hormone-mediated protection.12,118 However, the picture becomes more complex when we consider the dose of estrogen administered. Although physiological levels of 17α-estradiol fail to protect, pharmacological levels of both 17β- and 17α-estradiol act to protect12,119 through mechanisms that are likely to bypass ERs. The results of these findings highlight the importance of dose and type/potency of estrogen administered in achieving neuroprotection. Since a variety of estrogenic compounds are components of ERT preparations and several “designer” estrogens are administered or being developed, it will be critical to assess the efficacies of the wide variety of estrogens in promoting beneficial actions in the brain.
Under certain circumstances, 17β-estradiol can either fail to protect or even harm the brain. While estradiol can decrease brain injury in the vast majority of studies, estradiol fails to attenuate cell death in some animal models.96,120,121 It is possible that when the degree of injuryis too severe, as may be the case in the hippocampus following prolonged global ischemia,120 the actions of estradiol are not sufficient to prevent cell death. Under other circumstances, estradiol can be deleterious to neural function. In animal models of epilepsy, estradiol lowers the threshold for seizures and facilitates the induction and duration of excitatory neural firing.115,122 These data suggest that ERT may not always exert, only beneficial actions in the brains of postmenopausal women, particularly in those with a medical history of epilepsy. As we continue to learn about, the complexity of estrogen action with regards to dose, type of estrogen, and neurological condition, we will be better able to modify and transform estrogen replacement into therapy that exerts only beneficial actions in the brains of postmenopausal women.
Molecular mechanisms of estrogen-mediated neuroprotection
Estrogen may exert neuroprotective effects through several mechanisms: estrogen can act through ER-depcndent and ER-independent, genomic as well as nongenomic means to attenuate neural injury. Collectively, studies demonstrate that the pathway of estrogen action is influenced by the dose of estrogen administered. In general, pharmacological levels of estradiol protect the brain through mechanisms that do not require PRs, while physiological levels of estradiol protect the brain through mechanisms that depend upon ERs, as discussed below.
Estrogen receptor-mediated mechanisms
ERs are critical to our understanding of the mechanisms of estrogen action. Two ER subtypes, or ERs, exist: the classic ERoc and the recently discovered ERβ. Although, portions of ERα and ERβ are quite similar in structure, their distributions throughout the body and the brain are unique. Their unique regional distributions suggest that the receptors play very different roles in the body.
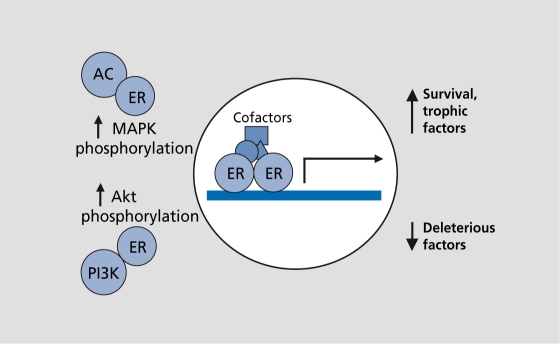
Both ERα and ERβ are transcription factors. They bind estradiol through their ligand-binding domains and, upon activation, homodimerize or heterodimerize through zinc finger structures located in the DNA and ligand-binding domains. The ER dimer then translocates to the nucleus and binds to the estrogen-responsive elements located in the promoters of estrogen-responsive genes to regulate transcription (Figure 3). ER-regulated transcription is enhanced by cofactors (coactivators and corepressors) that, bind the ER-DNA complex to either amplify or diminish transcriptional activation or repression (Figure 3).
Figure 3. Estrogen receptors (ERs) act through traditional and novel mechanisms. This diagram illustrates ERs in their classical roles as transcription factors and in their newfound roles as components of signal transduction pathways. As transcription factors, ERs bind estradiol, dimerize, and then translocate to the nucleus. In the nucleus, ERs bind to estrogen-responsive elements in the promoters of target genes to induce or to suppress transcription. Cofactors can modulate the ER-DNA interactions to either amplify or diminish transcription. In addition to gene transactivation, ERs may also activate signal transduction pathways. For example, estradiol stimulates adenyly! cyclase (AC), one of several ways that it increases mitogen-activated protein kinase (MAPK) phosphorylation. Further, estradiol stimulates phosphoinositol-3-kinase (PI3K), which increases Akt (protein kinase B) phosphorylation. These effects on signal transduction may turn key proteins on or off, or ultimately induce genomic actions.
Our long-standing and traditional view of ER action123 is rapidly transforming as we discover novel and unique roles for ERs, beyond direct, transcriptional modulation. We now know that ERs interact with signal transduction pathways,124,125 such as adenylyl cyclase, phosphoinositol 3-kinase, and/or mitogen-activated kinase (MAPK), or involve cross-talk with growth factor receptors, such as trkA and insulin-like growth factor-I (IGF-I) receptor.114,124,126-128 These novel ER-mediated mechanisms may lead to downstream altered gene expression and/or altered phosphorylation of proteins to promote estradiol action (Figure 3).
These traditional and novel ER-mediated interactions may induce a variety of cellular responses that, promote trophic and protective effects in the brain. Physiological levels of estradiol can enhance synaptic plasticity,129-133 regulate the expression of neurotrophins and cognate receptors,134-137 and elevate the expression of cell survival factors.106,138,139 Any or all ER-mediated actions of estradiol that enhance the integrity and plasticity of the brain may ultimately promote neuroprotection.
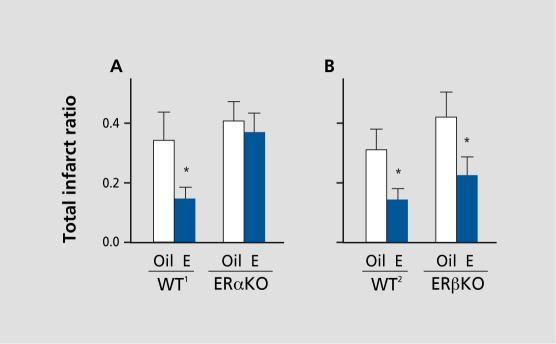
We investigated the functional roles for ERs in estradiolmediated protection against stroke injury and discovered a novel and unique role for ERα in the brain. Our data revealed that physiological levels of estradiol require ERs to exert, protection against cerebral ischemia.110,140 Specifically, we utilized transgenic mice that were knocked out for cither ERα or ERβ and found that the classic ER, ERα, is the critical mechanistic link in the ability of low levels of estradiol to exert neuroprotection (Figure 4). We have begun to identify the repertoire of downstream genomic targets of estradiol action through ERs and, to date, have reported that estradiol modulates the expression of a several players in ischemic brain injury including survival factors,139 immediate early genes,141 neuropeptides,142 and trophic factors.143
Figure 4. Estrogen receptor-α(ERα) is critical in estradiol-mediated protection of the brain following stroke injury. Estradiol (E) reduces ischemic infarct in both wildtype mice, WT1 (A) and WT2 (B), compared with corresponding oil-treated controls (n=6-10/group) (*P<0.02). Estradiol fails to protect in ERα knock-out (ERαKO) mice, compared with oil-treated controls (n=1 3/group) (A), but continues to protect in (B) ERβKO mice, compared with oil-treated controls (n=8-9/group) (*P<0.02). Values represent means±SE. Reproduced from reference 140: Dubai DB, Zhu B, Yu B, et al. Estrogen receptor-ot, not -3, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci U S A. 2001;98:1952-1957. Copyright © 2001, National Academy of Sciences, USA.
Estrogen receptor-independent mechanisms and actions
Though our studies focus attention on the mechanisms of ER-dependent, physiological actions of estrogen, it is very important to understand the mechanisms underlying pharmacological actions of estrogen since these effects are rapid, immediate, and may be critical to development, of acute treatment of brain injury.
While physiological levels of estradiol generally require receptor-mediated genomic or nongcnomic function for neuroprotection, pharmacological levels of estradiol appear to protect through non-ER-mediated effects. Pharmacological levels of estradiol can rapidly and revcrsibly decrease N-methyl-D-aspartate (NMDA)induced currents,116 suggesting that it may reduce excitatory cell death caused by neurodegenerative injury. Furthermore, estrogens can influence members of the nitric oxide synthase family to induce vasodilatory actions on cerebral blood vessels144 and thus improve blood flow to compromised brain regions. Estrogens can also act as potent, antioxidants and inhibit, lipid peroxidation88,105,107,112,145-148 through actions that have been shown to occur via the C3 hydroxyl group located on the phenolic A-ring of the steroids.112,145 These studies88,105,112,145,146 confirm that this antioxidant, mechanism requires supraphysiological levels of estrogen, and these findings may be key in the development of therapeutic approaches aimed at achieving neuroprotection against injury induced by oxidative stress.
Conclusion
In summary, a large breadth of clinical and basic science studies have led to a new appreciation that estradiol acts far beyond the reproductive axis and exerts profound protective actions in the adult and aging brain. Though we have only just begun to identify potential cellular and molecular mechanisms of this protection, our growing knowledge of estrogen action in the injured brain will ultimately lead to a more complete understanding of the precise mechanisms underlying estradiol-mediated protection. This knowledge is crucial to developing both preventative and acute therapies for neurodegenerative conditions and carries great promise for improving the quality of lives in our aging population.
This work was supported by a Merck Geriatric Scholarship (DBD) and the National Institutes of Health: AG00242, AG02224, AG17164, and RR1 5592 (PMW).
Contributor Information
Dena B. Dubal, Department of Physiology, University of Kentucky College of Medicine, Lexington, KY, USA.
Phyllis M. Wise, Division of Biological Sciences, University of California Davis, Davis, Calif, USA.
REFERENCES
- 1.vom Saal FS., Finch CE., Nelson JF. Natural history and mechanisms of reproductive aging in humans, laboratory rodents, and other selected vertebrates. In: Knobil E, Neill JD, eds. The Physiology of Reproduction. New York, NY: Raven Press; 1994:1213–1314. [Google Scholar]
- 2.Wise PM. New understanding of the complexity of the menopause and challenges for the future. In: Bellino FL, ed. Proceedings of the International Symposium on the Biology of the Menopause. Norwell, Mass: Springer; 2000:1–8. [Google Scholar]
- 3.Speroff L. Postmenopausal hormone therapy and breast cancer. Obstet Gynecol. 1996;87:44S–53S. doi: 10.1016/0029-7844(95)00428-9. [DOI] [PubMed] [Google Scholar]
- 4.Hammond CB. Menopause and hormone replacement therapy: an overview. Obstet Gynecol. 1996;87:2S–15S. doi: 10.1016/0029-7844(95)00429-7. [DOI] [PubMed] [Google Scholar]
- 5.Lindsay R. The menopause and osteoporosis. Obstet Gynecol. 1996;87:16S–19S. doi: 10.1016/0029-7844(95)00430-0. [DOI] [PubMed] [Google Scholar]
- 6.Sherwin BB. Hormones, mood, and cognitive functioning in postmenopausal women. Obstet Gynecol. 1996;87:20S–26S. doi: 10.1016/0029-7844(95)00431-9. [DOI] [PubMed] [Google Scholar]
- 7.Wild RA. Estrogen: effects on the cardiovascular tree. Obstet Gynecol. 1996;87:27S–35S. doi: 10.1016/0029-7844(95)00434-3. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan JM., Fowlkes LP. The clinical aspects of estrogen and the cardiovascular system. Obstet Gynecol. 1996;87:365–435. doi: 10.1016/0029-7844(95)00432-7. [DOI] [PubMed] [Google Scholar]
- 9.Prior JC., Vigna YM., Wark JD., et al. Premenopausal ovariectomy-related bone loss: a randomized, double-blind, one-year trial of conjugated estrogen or medroxyprogesterone acetate. J Bone Miner Res. 1997;12:1851–1863. doi: 10.1359/jbmr.1997.12.11.1851. [DOI] [PubMed] [Google Scholar]
- 10.Kawas C., Resnick S., Morrison A., et al. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer's disease: the Baltimore Longitudinal Study of Aging. Neurology. 1997;48:1517–1521. doi: 10.1212/wnl.48.6.1517. [DOI] [PubMed] [Google Scholar]
- 11.Henderson VW., Watt L., Buckwalter JG. Cognitive skills associated with estrogen replacement in women with Alzheimer's disease. Psychoneuroendocrinology. 1996;21:421–430. doi: 10.1016/0306-4530(95)00060-7. [DOI] [PubMed] [Google Scholar]
- 12.Wise PM., Dubai DB., Wilson ME., Rau SW., Liu Y. Estrogens: trophic and protective factors in the adult brain. Front Neuroendocrine!. 2001;22:33–66. doi: 10.1006/frne.2000.0207. [DOI] [PubMed] [Google Scholar]
- 13.Wise PM., Dubai DB., Wilson ME., Rau SW., Bôttner M. Minireview: Neuroprotective effects of estrogen - new insights into mechanisms of action. Endocrinology. 2001;142:969–973. doi: 10.1210/endo.142.3.8033. [DOI] [PubMed] [Google Scholar]
- 14.Shlipak MG., Angeja BG., Go AS., Frederick PD., Canto JG., Grady D. Hormone therapy and in-hospital survival after myocardial infarction in postmenopausal women. Circulation. 2001;104:2300–2304. doi: 10.1161/hc4401.98414. [DOI] [PubMed] [Google Scholar]
- 15.Hodis HN., Mack WJ., Lobo RA., et al. Estrogen in the prevention of atherosclerosis: a randomized, double-blind, placebo-controlled trial. Circulation. 2001;104:2300–2304. doi: 10.7326/0003-4819-135-11-200112040-00005. [DOI] [PubMed] [Google Scholar]
- 16.Hulley S., Grady D., Bush T., et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 17.Sherwin BB. Estrogen effects on cognition in menopausal women. Neurology. 1997;48:521–526. doi: 10.1212/wnl.48.5_suppl_7.21s. [DOI] [PubMed] [Google Scholar]
- 18.Sherwin BB., Carlson LE. Estrogen and memory in women. J Soc Obstet Gynecol Can. 1997;19:7–13. [Google Scholar]
- 19.Kampen DL., Sherwin BB. Estrogen use and verbal memory in healthy postmenopausal women. Obstet Gynecol. 1998;83:979–983. doi: 10.1097/00006250-199406000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Sherwin BB. Estrogenic effects on memory in women. Ann N Y Acad Sci. 1994;743:213–231. doi: 10.1111/j.1749-6632.1994.tb55794.x. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt R., Fazekas F., Reinhart B., et al. Estrogen replacement therapy in older women: a neuropsychological and brain MRI study. J Am Geriatr Soc. 1996;44:1307–1313. doi: 10.1111/j.1532-5415.1996.tb01400.x. [DOI] [PubMed] [Google Scholar]
- 22.Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- 23.Birge SJ. The role of estrogen in treatment of Alzheimer's disease. Neurology. 1997;48:S36–S41. doi: 10.1212/wnl.48.5_suppl_7.36s. [DOI] [PubMed] [Google Scholar]
- 24.Henderson VW., Paganini-Hill A. Estrogen and Alzheimer's disease. J Soc Obstet Gynecol Can. 1997;19:21–28. [Google Scholar]
- 25.Fillit H. Estrogens in the pathogenesis and treatment of Alzheimer's disease in postmenopausal women. Ann N Y Acad Sci. 1994;743:233–239. doi: 10.1111/j.1749-6632.1994.tb55795.x. [DOI] [PubMed] [Google Scholar]
- 26.Paganini-Hill A., Henderson VW. Estrogen replacement therapy and risk of Alzheimer disease. Arch Intern Med. 1996;156:2213–2217. [PubMed] [Google Scholar]
- 27.Paganini-Hill A., Henderson VW. Estrogen deficiency and risk of Alzheimer's disease in women. Am J Epidemiol. 1994;140:256–261. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- 28.Tang MX., Jacobs D., Stern Y., et al. Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet. 1996;348:429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- 29.Asthana S., Craft S., Baker LD., et al. Cognitive and neuroendocrine response to transdermal esrogen in postmenopausal women with Alzheimer's disease: results of a placebo-controlled, double-blind, pilot study. Psychoneuroendocrinology. 1999;24:657–677. doi: 10.1016/s0306-4530(99)00020-7. [DOI] [PubMed] [Google Scholar]
- 30.Waring SC., Rocca WA., Petersen RC., O'Brien PC., Tangalos EG., Kokmen E. Postmenopausal estrogen replacement therapy and risk of AD. A population-based study. Neurology. 1999;52:965–970. doi: 10.1212/wnl.52.5.965. [DOI] [PubMed] [Google Scholar]
- 31.Henderson VW., Paganini-Hill A., Miller BL., et al. Estrogen for Alzheimer's disease in women. Randomized, double-blind, placebo-controlled trial. Neurology. 2000;54:295–301. doi: 10.1212/wnl.54.2.295. [DOI] [PubMed] [Google Scholar]
- 32.Marder K., Sano M. Estrogen to treat Alzheimer's disease: too little, too late? So what's a woman to do? Neurology. 2000;54:2035–2037. doi: 10.1212/wnl.54.11.2035. [DOI] [PubMed] [Google Scholar]
- 33.Wang PN., Liao SQ., Liu RS., et al. Effects of estrogen on cognition, mood, and cerebral blood flow in AD. A controlled study. Neurology. 2000;54:2061–2066. doi: 10.1212/wnl.54.11.2061. [DOI] [PubMed] [Google Scholar]
- 34.Robinson D., Friedman L., Marcus R., Tinklenberg J., Yesavage J. Estrogen replacement therapy and memory in older women. J Am Geriatr Soc. 1994;42:919–922. doi: 10.1111/j.1532-5415.1994.tb06580.x. [DOI] [PubMed] [Google Scholar]
- 35.Henderson VW., Paganini-Hill A., Emanuel CK., Dunn ME., Buckwalter JG. Estrogen replacement therapy in older women. Arch Neurol. 1994;51:896–900. doi: 10.1001/archneur.1994.00540210068014. [DOI] [PubMed] [Google Scholar]
- 36.Shaywitz SE., Shaywitz BA., Pugh KR., et al. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA. 1999;281:1197–1202. doi: 10.1001/jama.281.13.1197. [DOI] [PubMed] [Google Scholar]
- 37.Resnick SM., Maki PM., Golski S., Kraut MA., Zonderman AB. Effects of estrogen replacement therapy on PET cerebral blood flow and neuropsychological performance. Horm Behav. 1998;34:171–182. doi: 10.1006/hbeh.1998.1476. [DOI] [PubMed] [Google Scholar]
- 38.Resnick SM., Metter EJ., Zonderman AB. Estrogen replacement therapy and longitudinal decline in visual memory. A possible protective effect? Neurology. 1997;49:1491–1497. doi: 10.1212/wnl.49.6.1491. [DOI] [PubMed] [Google Scholar]
- 39.Honjo H., Tanaka K., Kashiwagi T., et al. Senile dementia-Alzheimer's type and estrogen. Horm Metab Res. 1995;27:204–207. doi: 10.1055/s-2007-979941. [DOI] [PubMed] [Google Scholar]
- 40.Fillit H., Weinreb H., Cholst I., et al. Observations in a preliminary open trial of estradiol therapy for senile dementia-Alzheimer's type. Psychoneuroendocrinology. 1986;11:337–345. doi: 10.1016/0306-4530(86)90019-3. [DOI] [PubMed] [Google Scholar]
- 41.Barrett-Connor E., Kritz-Silverstein D. Estrogen replacement therapy and cognitive function in older women. JAMA. 1993;269:2637–2641. [PubMed] [Google Scholar]
- 42.Brenner DE., Kukull WA., Stergachis A., et al. Postmenopausal estrogen replacement therapy and the risk of Alzheimer's disease: a populationbased case-control study. Am J Epidemiol. 1994;140:262–267. doi: 10.1093/oxfordjournals.aje.a117245. [DOI] [PubMed] [Google Scholar]
- 43.Mulnard RA., Cotman CW., Kawas C., et al. Estrogen replacement therapy for treatment of mild to moderate Alzheimer's disease: a randomized controlled study. Alzheimer's Disease Cooperative Study. JAMA. 2000;283:1007–1015. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- 44.Paganini-Hill A. Hormone replacement therapy and stroke: risk, protection or no effect? Maturitas. 2001;38:243–261. doi: 10.1016/s0378-5122(01)00167-0. [DOI] [PubMed] [Google Scholar]
- 45.Paganini-Hill A. Estrogen replacement therapy and stroke. Prog Cardiovasc Dis. 1995;38:223–242. doi: 10.1016/s0033-0620(95)80014-x. [DOI] [PubMed] [Google Scholar]
- 46.Wenger NK., Speroff L., Packard B. Cardiovascular health and disease in women. N Engl J Med. 1993;329:247–256. doi: 10.1056/NEJM199307223290406. [DOI] [PubMed] [Google Scholar]
- 47.Kannel WB., Thorn TJ. The incidence, prevalence and mortality of cardiovascular disease. In: Schlant RC, Alexander RW, ed. The Heait. 8th ed. New York, NY: McGraw-Hill; 1994:185–197. [Google Scholar]
- 48.American Heart Association. 2001 Heart and Stroke Statistical Update. Dallas, Texas: American Heart Association; 2001 [Google Scholar]
- 49.Cefalu WT. The use of hormone replacement therapy in postmenopausal women with type 2 diabetes mellitus. J Womens Health Gend Based Med. 2001;10:241–255. doi: 10.1089/152460901300139998. [DOI] [PubMed] [Google Scholar]
- 50.Palin SL., Kumar S., Sturdee DW., Barnett AH. HRT in women with diabetes - review of the effects on glucose and lipid metabolism. Diabetes Res ClinPract. 2001;54:67–77. doi: 10.1016/s0168-8227(01)00277-7. [DOI] [PubMed] [Google Scholar]
- 51.Glllum LA., Mamidipudi SK., Johnston SC. Ischemic stroke risk with oral contraceptives. JAMA. 2000;284:72–78. doi: 10.1001/jama.284.1.72. [DOI] [PubMed] [Google Scholar]
- 52.Gutthann SP., Garcia Rodriguez LA., Castellsague J., Oliart AD. Hormone replacement therapy and risk of venous thromboembolism: populationbased case-control study. BMJ. 1997;314:796–800. doi: 10.1136/bmj.314.7083.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scarabin PY., Alhenc-Gelas M., Plu-Bureau G., Taisne P., Agher R., Aiach M. Effects of oral and transdermal estrogen/progesterone regimens on blood coagulation and fibrinolysis in postmenopausal women. A randomized clinial trial. Arterioscler Thromb Vase Biol. 1997;17:3071–3078. doi: 10.1161/01.atv.17.11.3071. [DOI] [PubMed] [Google Scholar]
- 54.Luyer MDP., Khosla S., Owen WG., Miller VM. Prospective randomized study of effects of unopposed estrogen replacement therapy on markers of coagulation and inflammation in postmenopausal women. J Clin Endocrinol Metab. 2001;86:3629–3634. doi: 10.1210/jcem.86.8.7768. [DOI] [PubMed] [Google Scholar]
- 55.Schwartz SM., Petitti DB., Siscovick DS., et al. Stroke and use of low-dose oral contraceptives in young women. A pooled analysis of two US studies. Stroke. 1998;29:22–84. doi: 10.1161/01.str.29.11.2277. [DOI] [PubMed] [Google Scholar]
- 56.Giltay EJ., Gooren LJG., Emeis JJ., Kooistra T., Stehouwer CD. Oral, but not transdermal, administration of estrogens lowers tissue-type plasminogen activator levels in humans without affecting endothelial synthesis. Arterioscler Thromb Vase Biol. 2001;20:1396–1403. doi: 10.1161/01.atv.20.5.1396. [DOI] [PubMed] [Google Scholar]
- 57.Petitti DB., Sidney S., Bernstein A., Wolf S., Quesenberry C., Ziel HK. Stroke in users of low-dose oral contraceptives. N Engl J Med. 1996;335:8–15. doi: 10.1056/NEJM199607043350102. [DOI] [PubMed] [Google Scholar]
- 58.Petitti DB., Sidney S., Quensenberry CP., Jr Bernstein A. Ischemic stroke and use of estrogen and estrogen/progestogen as hormone replacement therapy. Stroke. 1998;29:23–28. doi: 10.1161/01.str.29.1.23. [DOI] [PubMed] [Google Scholar]
- 59.Falkeborn M., Persson I., Terent A., Adami HO., Lithell H., Bergstrom R. Hormone replacement therapy and the risk of stroke. Arch Intern Med. 1993;153:1201–1209. [PubMed] [Google Scholar]
- 60.Schairer C., Adami HO., Hoover R., Persson I., Manson JE. Cause-specific mortality in women receiving hormone replacement therapy. Epidemiology. 1997;8:59–65. doi: 10.1097/00001648-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 61.Paganini-Hill A., Ross RK., Henderson BE. Postmenopausal oestrogen treatment and stroke: a prospective study. BMJ. 1988;297:519–522. doi: 10.1136/bmj.297.6647.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pederson AT., Lidegaard 0., Kreiner S., Ottesen B. Hormone replacement therapy and risk of non-fatal stroke. Lancet. 1997;350:12–83. doi: 10.1016/S0140-6736(97)06005-4. [DOI] [PubMed] [Google Scholar]
- 63.Paganini-Hill A., Perez Barreto M. Stroke risk in older men and women: aspirin, estrogen, exercise, vitamins, and other factors. J Gend Specif Med. 2001;4:18–28. [PubMed] [Google Scholar]
- 64.Stampfer MJ., Colditz GA., Willett WC., et al. Postmenopasual estrogen replacement therapy and cardiovascular disease. Ten-year follow-up from the Nurses' Health Study. N Engl J Med. 1991;325:756–762. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- 65.Grodstein F., Stampfer MJ., Colditz GA., et al. Postmenopausal hormone therapy and mortality. N. Engl J Med. 1997;336:1769–1775. doi: 10.1056/NEJM199706193362501. [DOI] [PubMed] [Google Scholar]
- 66.Grodstein F., Manson JE., Colditz GA., Willett WC., Speizer FE. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933–941. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- 67.O'Keefe JH., Kim SC., Hall RR., Cochran VC., Lawhorn SL., McCallister BD. Estrogen replacement therapy after coronary angioplasty in women. J Am Coll Cardiol. 1997;29:1–5. doi: 10.1016/s0735-1097(96)00443-3. [DOI] [PubMed] [Google Scholar]
- 68.Cauley JA., Seeley DG., Browner WS., et al. Estrogen replacement therapy and mortality among older women. The study of osteoporotic fractures. Arch Intern Med. 1997;157:2181–2187. [PubMed] [Google Scholar]
- 69.Fung MM., Barrett-Conner E., Bettencourt RR. Hormone replacement therapy and stroke risk in older women. J Womens Health. 1999;8:359–364. doi: 10.1089/jwh.1999.8.359. [DOI] [PubMed] [Google Scholar]
- 70.Rodriguez C., Galle EE., Patel AV., Tatharn LM., Jacobs EJ., Thun MJ. Effect of body mass on the association between estrogen replacement therapy and mortality among elderly US women. Am J Epidemiol. 2001;153:145–152. doi: 10.1093/aje/153.2.145. [DOI] [PubMed] [Google Scholar]
- 71.Simon JA., Hsia J., Cauley JA., et al. Postmenopausal hormone therapy and risk of stroke. Circulation. 2001;103:638–642. doi: 10.1161/01.cir.103.5.638. [DOI] [PubMed] [Google Scholar]
- 72.Viscoli CM., Brass LM., Kernan WN., Sarrel PM., Horowitz Rl. Estrogen after ischemic stroke: effect of estrogen replacement on risk of recurrent stroke and death in the Women's estrogen for Stroke Trial (WEST). Stroke. 2001;32:329. [Google Scholar]
- 73.Wilson PW., Garrison RJ., Castelli WP. Postmenopausal estrogen use, cigarette smoking, and cardiovascular morbidity in women over 50. The Framingham Study. N Engl J Med. 1985;313:1038–1043. doi: 10.1056/NEJM198510243131702. [DOI] [PubMed] [Google Scholar]
- 74.Hart RG., Pearce LA., McBride R., Rothbart RM., Asinger RW. Factors associated with ischemic stroke during aspirin therapy in atrial fibrillation. Analysis of 2012 participants in the SPAF Mil clinical trials. Stroke. 1999;30:1223–1229. doi: 10.1161/01.str.30.6.1223. [DOI] [PubMed] [Google Scholar]
- 75.Viscoli CM., Brass LM., Kernan WN., Sarrel PM., Suissa S., Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243–1249. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 76.FitzGerald MJT. Blood supply of the brain. In: Neuroanatomy - Basic and Clinical. London, UK: WB Saunders. 1997:42–52. [Google Scholar]
- 77.Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 78.Choi DW., Hartley DM. Calcium and glutamate-induced cortical neuronal death. Res Publ Assoc Res Nerv Ment Dis. 1993;71:23–34. [PubMed] [Google Scholar]
- 79.Choi DW. Calcium: still center-stage in hypoxic-ischemic neuronal death. Trends Neurosci. 1995;18:58–60. [PubMed] [Google Scholar]
- 80.Patel T., Gores GJ., Kaufmann SH. The role of proteases during apoptosis. FASEB. 1996;10:587–597. doi: 10.1096/fasebj.10.5.8621058. [DOI] [PubMed] [Google Scholar]
- 81.Linnik MD., Zobrist RH., Hatfield MD. Evidence supporting a role for programmed cell death in focal cerebral ischemia in rats. Stroke. 1993;24:2002–2009. doi: 10.1161/01.str.24.12.2002. [DOI] [PubMed] [Google Scholar]
- 82.MacManus JP., Hill IE., Huang ZG., Rasquinha I., Xue D., Buchan AM. DNA damage consistent with apoptosis in transient focal ischemic neocortex. Neuroreport. 1994;5:493–496. doi: 10.1097/00001756-199401120-00031. [DOI] [PubMed] [Google Scholar]
- 83.Hisahara S., Shoji S., Okano H., Miura M. ICE/CED-3 family executes oligodendrocyte apoptosis by tumor necrosis factor. J Neurochem. 1997;69:10–20. doi: 10.1046/j.1471-4159.1997.69010010.x. [DOI] [PubMed] [Google Scholar]
- 84.Hara H., Fink K., Endres M., et al. Attenuation of transient focal cerebral ischemic injury in transgenic mice expressing a mutant ICE inhibitory protein. J Cereb Blood Flow Metab. 1997;17:370–375. doi: 10.1097/00004647-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 85.Krajewski S., Mai JK., Krajewska M., Sikorska M., Mossakowski MJ., Reed JC. Upregulation of bax protein levels in neurons following cerebral ischemia. J Neurosci. 1995;15:6364–6375. doi: 10.1523/JNEUROSCI.15-10-06364.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Asahi M., Hoshimaru M., Uemura Y., et al. Expression of interleukin-1b converting enzyme gene family and bcl-2 gene family in the rat brain following permanent occlusion of the middle cerebral artery. J Cereb Blood Flow Metab. 1997;17:11–18. doi: 10.1097/00004647-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 87.Martinou JC., Dubois-Dauphin M., Staple JK., et al. Overexpression of bcl-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron. 1994;13:1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 88.Hall ED., Pazara KE., Linseman KL. Sex differences in postischemic neuronal necrosis in gerbils. J Cereb Blood Flow Metab. 1991;11:292–298. doi: 10.1038/jcbfm.1991.61. [DOI] [PubMed] [Google Scholar]
- 89.Roof RL., Hall ED. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J Neurotrauma. 2000;17:367–388. doi: 10.1089/neu.2000.17.367. [DOI] [PubMed] [Google Scholar]
- 90.Dubai DB., Kashon ML., Pettigrew LC., et al. Estradiol protects against ischemic injury. J Cereb Blood Flow Metab. 1998;18:1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 91.Rusa R., Alkayed NJ., Grain BJ., et al. 173-Estradiol reduces stroke injury in estrogen-deficient female animals. Stroke. 1999;30:1665–1670. doi: 10.1161/01.str.30.8.1665. [DOI] [PubMed] [Google Scholar]
- 92.Toung TJK., Traystman RJ., Hum PD. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke. 1998;29:1666–1670. doi: 10.1161/01.str.29.8.1666. [DOI] [PubMed] [Google Scholar]
- 93.Simpkins JW., Rajakumar G., Zhang YQ., et al. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg. 1997;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- 94.Zhang YQ., Shi J., Rajakumar G., Day, Simpkins JW. Effects of gender and estradiol treatment on focal brain ischemia. Brain Res. 1998;784:321–324. doi: 10.1016/s0006-8993(97)00502-7. [DOI] [PubMed] [Google Scholar]
- 95.Wang Q., Santizo R., Baughman VL., Pelligrino DA., ladecola C. Estrogen provides neuroprotection in transient forebrain ischemia through perfusion-independent mechanisms in rats. Stroke. 1999;30:630–637. doi: 10.1161/01.str.30.3.630. [DOI] [PubMed] [Google Scholar]
- 96.Emerson CS., Headrick JP., Vink R. Estrogen improves biochemical and neurologic outcome following traumatic brain injury in male rats, but not females. Brain Res. 1993;608:95–100. doi: 10.1016/0006-8993(93)90778-l. [DOI] [PubMed] [Google Scholar]
- 97.Garcia-Estrada J., DelRio JA., Luquin S., Soriano E., Garcia-Segura LM. Gonadal hormones down-regulate reactive gliosis and astrocyte proliferation after a penetrating brain injury. Brain Res. 1993;628:271–278. doi: 10.1016/0006-8993(93)90964-o. [DOI] [PubMed] [Google Scholar]
- 98.Roof RL., Duvdevani R., Stein DG. Gender influences outcome of brain injury: progesterone plays a protective role. Brain Res. 1991;607:333–336. doi: 10.1016/0006-8993(93)91526-x. [DOI] [PubMed] [Google Scholar]
- 99.Saiyed M., Riker WK. Cholinergic and anticholinergic drug effects on survival during hypoxia: significant gender differences. J Pharmacol Exp Ther. 1993;264:1146–1153. [PubMed] [Google Scholar]
- 100.Cadet K., Ladenheim B., Baum I., Carlson E., Epstein C. CuZn-superoxide dismutase (CuZnSOD) transgenic mice show resistance to the lethal effects of methylenedioxyamphetamine (MDÂ) and of methylenedioxymethamphetamine (MDMA). Brain Res. 1994;655:259–262. doi: 10.1016/0006-8993(94)91624-1. [DOI] [PubMed] [Google Scholar]
- 101.Alkayed NJ., Harukuni I., Kimes AS., London ED., Traystman RJ., Hum PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–166. doi: 10.1161/01.str.29.1.159. [DOI] [PubMed] [Google Scholar]
- 102.Yang SH., Shi J., Day, Simpkins JW. Estradiol exerts neuroprotective effects when administered after ischemic insult. Stroke. 2000;31:745–750. doi: 10.1161/01.str.31.3.745. [DOI] [PubMed] [Google Scholar]
- 103.Dubai DB., Wise PM. Neuroprotective effects of estradiol in middleaged female rats. Endocrinology. 2001;142:43–48. doi: 10.1210/endo.142.1.7911. [DOI] [PubMed] [Google Scholar]
- 104.Green SG., Gridley KE., Simpkins JW. Estradiol protects against p-amyloid (25-35)-induced toxicity in SK-N-SH human neuroblastoma cells. Neurosci Lett. 1996;218:165–168. doi: 10.1016/s0304-3940(96)13148-7. [DOI] [PubMed] [Google Scholar]
- 105.Gridley KE., Green PS., Simpkins JW. Low concentrations of estradiol reduce β-amyloid (2 5-3 5)- induced toxicity, lipid peroxidation and glucose utilization in human SK-N-SH neuroblastoma cells. Brain Res. 1997;778:158–165. doi: 10.1016/s0006-8993(97)01056-1. [DOI] [PubMed] [Google Scholar]
- 106.Pike CJ. Estrogen modulates neuronal Bcl-xL expression and p-amyloid-induced apoptosis: relevance to Alzheimer's disease. J Neurochem. 1999;72:1552–1563. doi: 10.1046/j.1471-4159.1999.721552.x. [DOI] [PubMed] [Google Scholar]
- 107.Goodman Y., Bruce AJ., Cheng B., Mattson MP. Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid ppeptide toxicity in hippocampal neurons. J Neurochem. 1996;66:1836–1844. doi: 10.1046/j.1471-4159.1996.66051836.x. [DOI] [PubMed] [Google Scholar]
- 108.Vedder H., Anthes N., Stumm G., Wurz C., Behl C., Krieg JC. Estrogen hormones reduce lipid peroxidation in cells and tissues of the central nervous system. J Neurochem. 1999;72:2531–2538. doi: 10.1046/j.1471-4159.1999.0722531.x. [DOI] [PubMed] [Google Scholar]
- 109.Zaulyanov LL., Green PS., Simpkins JW. Glutamate receptor requirement for neuronal cell death from anoxia-reoxygenation: an in vitro model for assessment of the neuroprotective effects of estrogens. Cell Mol Neurobiol. 1999;19:705–718. doi: 10.1023/a:1006948921855. [DOI] [PubMed] [Google Scholar]
- 110.Wilson ME., Dubai DB., Wise PM. Estradiol protects against injuryinduced cell death in cortical expiant cultures: a role for estrogen receptors. Brain Res. 2000;873:235–242. doi: 10.1016/s0006-8993(00)02479-3. [DOI] [PubMed] [Google Scholar]
- 111.Regan RF., Guo Y. Estrogens attenuate neuronal injury due to hemoglobin, chemical hypoxia, and excitatory amino acids in murine cortical cultures. Brain Res. 1997;764:133–140. doi: 10.1016/s0006-8993(97)00437-x. [DOI] [PubMed] [Google Scholar]
- 112.Behl C., Skutella T., Lezoualch F., et al. Neuroprotection against oxidative stress by estrogens: structure-activity relationship. Mol Pharmacol. 1997;51:535–541. [PubMed] [Google Scholar]
- 113.Behl C., Widmann M., Trapp T., Holsboer F. 17p-Estradiol protects neurons from oxidative stress-induced cell death in vitro. Biochem Biophys Res Commun. 1998;216:473–482. doi: 10.1006/bbrc.1995.2647. [DOI] [PubMed] [Google Scholar]
- 114.Singer CA., Figueroa-Masot CD., Batchelor RH., Dorsa DM. The mitogenactivated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J Neurosci. 1999;19:2455–2463. doi: 10.1523/JNEUROSCI.19-07-02455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Singer CA., Rogers KL., Strickland TM., Dorsa DM. Estrogen protects primary cortical neurons from glutamate toxicity. Neurosci Lett. 1996;212:13–16. doi: 10.1016/0304-3940(96)12760-9. [DOI] [PubMed] [Google Scholar]
- 116.Weaver CE., Jr, Park-Chung M., Gibbs TT., Farb DH. 17βEstradiol protects against NMDA-induced excitotoxicity by direct inhibition of NMDA receptors. Brain Res. 1997;761:338–341. doi: 10.1016/s0006-8993(97)00449-6. [DOI] [PubMed] [Google Scholar]
- 117.Stoppini L., Buchs PA., Muller D. Lesion-induced neurite sprouting and synapse formation in hippocampal organotypic cultures. Neuroscience. 1993;57:985–994. doi: 10.1016/0306-4522(93)90043-f. [DOI] [PubMed] [Google Scholar]
- 118.Zhang Y., Tounekti 0., Akerman B., Goodyer CG., LeBlanc A. 17β-Estradiol induces an inhibitor of active caspases. J Neurosci. 2001;21:1–6. doi: 10.1523/JNEUROSCI.21-20-j0007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Green PS., Bishop J., Simpkins JW. 17α-Estradiol exerts neuroprotective effects on SK-N-SH cells. J Neurosci. 1997;17:511–515. doi: 10.1523/JNEUROSCI.17-02-00511.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Harukuni I., Hurn PD., Grain BJ. Deleterious effect of beta-estradiol in a rat model of transient forebrain ischemia. Brain Res. 2001;900:137–142. doi: 10.1016/s0006-8993(01)02278-8. [DOI] [PubMed] [Google Scholar]
- 121.Kondo Y., Suzuki K., Sakuma Y. Estrogen alleviates cognitive dysfunction following transient brain ischemia in ovariectomized gerbils. Neurosci Lett. 1997;238:45–48. doi: 10.1016/s0304-3940(97)00847-1. [DOI] [PubMed] [Google Scholar]
- 122.Lason W. Effects of estrogens on seizures and neurotoxicity. Pol J Pharmacol. 2000;52:59–62. [PubMed] [Google Scholar]
- 123.Tsai MJ., O'Malley B. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Ann Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 124.Toran-Allerand CD., Singh M., Setalo G. Novel mechanisms of estrogen action in the brain: new players in an old story. Front Neuroendocrinol. 1999;20:97–121. doi: 10.1006/frne.1999.0177. [DOI] [PubMed] [Google Scholar]
- 125.Green PS., Simpkins JW. Neuroprotective effects of estrogens: potential mechanisms of action. IntJ Dev Neurosci. 2000;18:347–358. doi: 10.1016/s0736-5748(00)00017-4. [DOI] [PubMed] [Google Scholar]
- 126.Singh M., Setalo G., Jr, Guan X., Warren M., Toran-Allerand CD. Estrogeninduced activation of mitogen-activated protein kinase in cerebral cortical expiants: convergence of estrogen and neurotrophin signaling pathways. J Neurosci. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Murphy DD., Segal M. Morphological plasticity of dendritic spines in central neurons is mediated by activation of cAMP response element binding protein. Proc Natl Acad Sci USA. 1997;94:1482–1487. doi: 10.1073/pnas.94.4.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou Y., Watters JJ., Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 1996;137:2163–2166. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]
- 129.McEwen BS., Alves SE., Bulloch K., Weiland NG. Ovarian steroids and the brain: implications for cognition and aging. Neurology. 1997;48:58–515. doi: 10.1212/wnl.48.5_suppl_7.8s. [DOI] [PubMed] [Google Scholar]
- 130.Woolley CS., McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Murphy DD., Cole NB., Segal M. Brain-derived neurotrophic factor mediates estradiol-induced dendritic spine formation in hippocampal neurons. Proc Natl Acad Sci U SA. 1998;95:11412–11417. doi: 10.1073/pnas.95.19.11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McEwen BS., Tanapat P., Weiland NG. Inhibition of dendritic spine induction on hippocampal CA1 pyramidal neurons by a nonsteroidal estrogen antagonist in female rats. Endocrinology. 1999;140:1044–1047. doi: 10.1210/endo.140.3.6570. [DOI] [PubMed] [Google Scholar]
- 133.Gould E., Woolley CS., Frankfurt M., McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sohrabji F., Miranda RC., Toran-Allerand CD. Estrogen differentially regulates estrogen and nerve growth factor receptor mRNAs in adult sensory neurons. J Neurosci. 1994;14:459–471. doi: 10.1523/JNEUROSCI.14-02-00459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Singh M., Meyer EM., Simpkins JW. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology. 1995;136:2320–2324. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- 136.McMillan PJ., Singer CA., Dorsa DM. The effects of ovariectomy and estrogen replacement on trkA and choline acetyltransferase mRNA expression in the basal forebrain on the adult female Sprague-Dawley rat. J Neurosci. 1996;16:1860–1865. doi: 10.1523/JNEUROSCI.16-05-01860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gibbs RB., Wu D., Hersh LB., Pfaff DW. Effects of estrogen replacement on the relative levels of choline acetyltransferase, trkA, and nerve growth factor messenger RNAs in the basal forebrain and hippocampal formation of adult rats. Exp Neurol. 1994;129:70–80. doi: 10.1006/exnr.1994.1148. [DOI] [PubMed] [Google Scholar]
- 138.Singer CA., Rogers KL., Dorsa DM. Modulation of Bcl-2 expression: a potential component of estrogen protection in NT2 neurons. Neuroreport. 1998;9:2565–2568. doi: 10.1097/00001756-199808030-00025. [DOI] [PubMed] [Google Scholar]
- 139.Dubai DB., Shughrue PJ., Wilson ME., Merchenthaler I., Wise PM. Estradiol modulates Bcl-2 in cerebral ischemia: a potential role for estrogen receptors. J, Neurosci. 1999;19:6385–6393. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dubai DB., Zhu B., Yu B., et al. Estrogen receptor-a, not -p, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci USA. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rau SW., Dubai DB., Wise PM. Immediate early gene regulation by estradiol in ischemic injury. Soc Neurosci. 2000;26:778. [Google Scholar]
- 142.Dubai DB., Wilson ME., Shughrue PJ., Merchenthaler I., Wise PM. Induction of galanin gene expression in estradiol-mediated neuroprotection against cerebral ischemia. Soc Neurosci. 1999;25:1449. [Google Scholar]
- 143.Bôttner M., Dubai DB., Rau SW., Wise PM. Activin gene expression increases after stroke injury: modulation by estradiol. Soc Neurosci Abstr. 2001;27:1165. [Google Scholar]
- 144.Pelligrino DA., Galea E. Estrogen and cerebrovascular physiology and pathophysiology. Jpn J Pharmacol. 2002;86:137–158. doi: 10.1254/jjp.86.137. [DOI] [PubMed] [Google Scholar]
- 145.Green PS., Gordon K., Simpkins JW. Phenolic A ring requirement for the neuroprotective effects of steroids. J Steroid Biochem Mol Biol. 1997;63:229–235. doi: 10.1016/s0960-0760(97)00124-6. [DOI] [PubMed] [Google Scholar]
- 146.Subbiah MT., Kessel B., Agrawal M., Rajan R., Abplanalp W., Rymaszewski A. Antioxidant potential of specific estrogens on lipid peroxidation. J Clin Endocrinol Metab. 1993;77:1095– 1097. doi: 10.1210/jcem.77.4.8408459. [DOI] [PubMed] [Google Scholar]
- 147.Green PS., Gridley KE., Simpkins JW. Nuclear estrogen receptor-independent neuroprotection by estratrienes: a novel interaction with glutathione. Neuroscience. 1998;84:7–10. doi: 10.1016/s0306-4522(97)00595-2. [DOI] [PubMed] [Google Scholar]
- 148.Culmsee C., Vedder H., Ravati A., et al. Neuroprotection by estrogens in a mouse model of focal cerebral ischemia and in cultured neurons: evidence for a receptor-independent antioxidative mechanism. J Cereb Blood Flow Metab. 1999;19:1263–1269. doi: 10.1097/00004647-199911000-00011. [DOI] [PubMed] [Google Scholar]