Abstract
It has become increasingly clear that males and females differ even more dramatically than we previously thought. Not only do they exhibit differing responses to stress and environmental experience, but they can also respond in opposite directions. In rats, it has been shown that exposure to an acute stressful event can enhance subsequent learning in males while dramatically impairing learning in females. These opposite effects of stress on memory formation are accompanied by similarly opposite effects on neuroanatomical measures, such as dendritic spines in the hippocampal formation. Moreover, these opposite effects of stress are mediated by different hormonal systems between the sexes. These unique responses to stressful experience in male versus female rats may be used to model sex differences in mental illness, such as those that exist for depression and posttraumatic stress disorder.
Keywords: stress, memory formation, gender, depression, posttraumatic stress disorder
Abstract
Cada vez se ha demostrado con mayor claridad que los hombres y las mujeres difieren aun más dramáticamente de lo que previamente se pensaba. Ellos no sólo muestran diferencias en las respuestas al estrés y a las experiencias ambientales, sino que también pueden responder en sentidos opuestos. En ratas se ha observado que la exposición a un acontecimiento estresante agudo puede favorecer en los machos en forma consecutiva un aprendizaje; en cambio, en las hembras el aprendizaje se deteriora en forma dramática. Estos efectos opuestos del estrés sobre la formación de la memoria se acompañan del mismo modo de efectos opuestos sobre ciertas mediciones neuroanatómicas como la formación de espinas dendríticas en el hipocampo. Además, estos efectos opuestos del estrés están mediados por sistemas hormonales diferentes en cada sexo. Estas respuestas distintivas para la experiencia estresante en hombres versus mujeres pueden ser utilizadas para modelar diferencias por sexo en las enfermedades mentales, como aquéllas que existen para la depresión y el trastorno por estrés postraumático.
Abstract
Que les hommes et les femmes diffèrent bien plus que nous ne le pensions auparavant est une réalité de plus en plus manifeste. Non seulement leurs réponses au stress et aux changements d'environnement diffèrent mais elles sont parfois opposées, il a été montré chez le rat que l'exposition à un événement aigu stressant pouvait améliorer l'apprentissage ultérieur chez le mâle alors que celui-ci était fortement freiné chez la femelle. Parallèlement, ces effets opposés du stress sur la mémoire sont assortis de résultats opposés en termes de critères neuroanatomiques, tel le nombre des épines dendritiques dans l'hippocampe. Par ailleurs, ces effets opposés du stress sont médiés par des systèmes hormonaux qui diffèrent selon le sexe. Ces réponses caractéristiques aux expériences de stress chez les rats mâles comparés aux femelles peuvent servir à modéliser les différences selon le sexe qui existent dans les maladies mentales, par exemple la dépression et le syndrome de stress posttraumatique.
It is obvious that there are many differences between the sexes, and our external differences only mask those beneath. However, for various reasons, some cultural, it is often assumed that male and female response systems differ only as a matter of degree and not. of direction. Indeed, it is often assumed that differences in our experiences or response to external events stem from differences in habits or belief systems that are malleable and could change by adopting a perspective more like the other sex. In this review, I will present data from a series of studies that indicate that males and females not only differ in the degree of their response, but often in direction too. To illustrate this phenomenon, 1 will focus on behavioral and neuronal responses to stressful experience and learning opportunities. These examples arise from studies conducted in the white albino laboratory rat. This approach eliminates some of the cultural and sociological considerations inherent to many discussions about sex differences in behavior. In addition to behavioral measures, I will present data indicating that anatomical measures of plasticity in the male and female brain can respond in opposite directions to the same environmental event. These behavioral and neuronal differences are dependent, on the presence of sex and stress hormones, but. differing ones for males versus females. Finally, 1 will discuss how these sexually dimorphic and diergic responses to life experience may be used to model sex differences in mental disorders, such as depression and posttraumatic stress disorder.
Sex differences in learning and memory
There are numerous reports of sex differences in basic learning processes.1,2 However, they vary greatly depending on the task used and species involved. In general, men tend to outperform women on tasks that require mental and spatial rotation, whereas women tend to outperform men when tested for spatial location in a static environment. Also, men are much more accurate at aiming an object at a target, whereas women often excel at tasks that require fine motor skills. Some of these sex differences in performance, such as those for targeting, also exist in nonhuman primates.3 With respect, to the most common laboratory animal, the rat, sex differences in performance are influenced by natural differences in activity levels. Female rats, who are generally more active than male rats, perform best, on tasks that require activity, such as active avoidance, and do quite poorly on those that require immobility, such as during fear conditioning or passive avoidance (for a review, see reference 4). Because sex differences in activity may confound differences in learning, we have adopted a task that does not depend on voluntary activity: classical conditioning of the eyeblink response. During this task, the animal is exposed to an aversive stimulation of the eyelid, which causes it to blink. During training, the stimulation is preceded by a tone, which predicts the onset, of the stimulation. After repeated exposure to these paired stimuli, the animal “learns” that the tone predicts the eyelid stimulation and blinks in response to it. This task has a number of advantages for studying sex differences in learning. First, the eyeblink is a discrete response that can be accurately measured and quantified. Second, performance of this task is not. dependent on overt activity or exploration. The animal must, emit an unconditioned response to the eyelid stimulation and only upon training elicits a conditioned response to the tone. As an additional advantage, the anatomical substrates that underlie learning the basic response have been identified.5,6 Finally and perhaps most, importantly, the task can be and has been conducted in virtually all animals, from mice to rats to monkeys to humans.7,8 Since results from animal studies often generate novel hypotheses about human behavior, this paradigm affords the possibility of testing them directly in normal and patient, populations. Using this task of classical eyeblink conditioning, we have observed that female rats acquire the learned response faster and emit more learned responses during training than do males.9,10 This sex difference in conditioning is even more prevalent if one takes into account the stages of estrus, the cyclic behavior of hormones associated with ovulation. Female rats have a 4- to 5-day cycle over which estrogen and progesterone levels change fairly dramatically. Proestrus is a stage prior to ovulation when estrogen levels are relatively high. When trained during this stage, females learn faster and condition more than females in other stages.11 These data suggest that estrogen is positively related to performance of this associative learning task.
How do these results compare to others in the literature? Certainly, there are numerous reports that learning (or performance) is related to the presence of sex hormones,12-15 although these effects vary depending on task and species. Women tested during the phase of the menstrual cycle associated with high levels of estrogen score better on tests of verbal fluency and fine motor skills - tests that, women already perform well relative to men.1,16 In rats, females tested during proestrus perform poorly during a spatial memory task that is dependent, on an intact, hippocampal formation, but perform optimally when the task is not dependent on the structure.17 Some report, that females tested during estrus have deficient spatial performance relative to males and females in other stages,18 whereas others report no effect of estrous cycle on learning, though performance variables were affected.19 Some of these effects can be ameliorated by previous familiarization with the task demands,20 suggesting that the stressful nature of some of these tasks contribute to the seemingly variable responses. Given the variation in the task demands, the brain structures involved, as well as the cyclic nature of endogenous hormone levels, it should come as no surprise that the relationship between absolute levels of hormones and learning is inconsistent. Moreover, since hormone levels do vary so frequently over time and experience, their effect on learning could not. be absolute. Rather, hormones modulate learning to varying degrees via. numerous mechanisms and presumably for numerous adaptive reasons.
Sex-specific responses to stress and memory formation
As with learning, there are sex differences in the stress response and these effects are often a matter of degree, not. direction. The most robust, sex difference occurs with endogenous levels of glucocorticoids. In many species, glucocorticoid levels are higher in females than males.21,22 This sex difference is apparent, under unstressed and stressed conditions and in rats, glucocorticoid levels are elevated in females during proestrus relative to other stages of estrus. Stressful experience can also elicit very different, behavioral responses in males versus females.
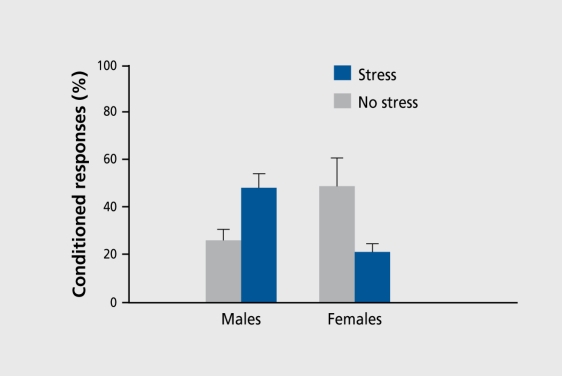
For example, we have shown that female rats exposed to an acute stressful event are severely handicapped in their ability to learn an associative response.9,10 Oddly enough, males respond in the opposite direction to females and thus exhibit enhanced performance after exposure to the same stressful event.23,24 The stressful event consists of cither brief exposure to intermittent tailshocks or brief swim stress (20 min), both of which are common methods for inducing behavioral depression in laboratory animals. As a measure of learning, we again used the classically conditioned eyeblink response. These opposite responses to stress are not limited to simple associative learning as occurs during classical conditioning with overlapping stimuli. As illustrated in Figure 1, they are also evident during trace conditioning, a more difficult task in which the conditioning stimuli are separated in time. This task critically involves the hippocampal formation, and some have even suggested that it involves conscious awareness.25-27
Figure 1. Percentage of conditioned responses measured over training in male rats and female rats tested during proestrus. They were exposed to the acute stressor and 24 hours later were trained on the trace-conditioned eyeblink response.
If these effects of uncontrollable stressful experience on learning in rats are relevant, to the human condition, they should possess some characteristics of mental illness, particularly those associated with stressful experience. One that comes to mind is posttraumatic stress disorder (PTSD). After experiencing a traumatic stressful event, some humans develop a series of behaviors that are maladaptive and cause distress and dysfunction,28 such as avoidance, reduced responsiveness, increased arousal, anxiety, and guilt. Of those that develop PTSD, more than twice as many are women.29 Often-times, they reexperience frightening aspects of the traumatic event, particularly if presented with cues that are associated with the event. To determine whether the effects of stress on learning in rats were sensitive to these factors, we exposed rats to cues associated with the stressful event days after it had ceased and at a time when the effects of stress would have dissipated. Indeed, days after the stressor, males reintroduced to the stress context, were further enhanced in their performance, whereas females were further impaired.10,30 Minimally, these results suggest that, the effects of acute stress on learning are not. entirely dependent on sensory stimulation, but rather can be stimulated by associations that were established during stressful environment. More generally, they suggest that the effects of acute stress on later learning in rats may model some disrupting effects of trauma on cognitive processes in humans such as occurs during PTSD.
Stress hormones and stress effects on memory formation
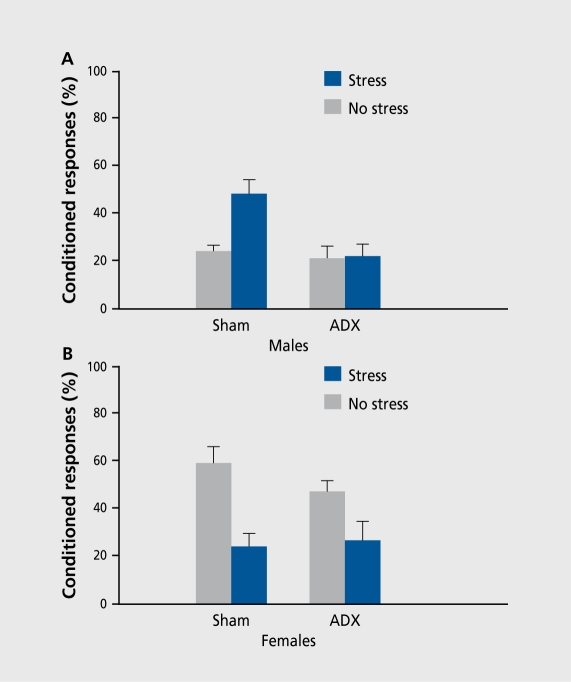
There are numerous examples of sex differences in behavior, but few demonstrating an opposite response to the same stimulus between sexes. What could be responsible for inducing these opposite responses? When exposed to a stressor, the organism responds by activating a complex series of physiological and behavioral responses that arc mediated by the sympathetic branch of the autonomic nervous system (ANS) and the hypothalamic-pituitary-adrenal (HPA) axis. The release of glucocorticoids (corticosterone [CORT] in rats) by the adrenal glands is an important part, of the organism's ability to deal with stress.31 Among other effects, increased levels of corticosterone potentiate the release of adrenaline, increase cardiovascular tone, and mobilize the energy needed for fight and flight responses. In a series of experiments, we directly evaluated the potential role of glucocorticoids in the sex and stress effects on conditioning. After removing endogenous glucocorticoids via adrenalectomy, male and female rats were stressed and trained on the classically conditioned eyeblink response. Somewhat surprisingly, adrenalectomy prevented the enhancing effect of stress on learning in males, but did not alter the female response to stress (Figure 2).10,32 Thus, exposure to the acute stressful event, not only has opposite effects on this measure of performance in males and females, but these effects are mediated by different hormonal systems.
Figure 2. Contribution of adrenal hormones to the opposite effects of stress on learning in males versus females. A. Males adrenalectomized (ADX) prior to stressor exposure were not affected by stress, while those exposed to a sham surgery showed an enhanced response rate. B. In contrast, females adrenalectomized (ADX) prior to the stressor exposure showed impaired response after stress, as did the females exposed to a sham surgery.
How do these results compare to others in the literature? This is a difficult question since there are many different types and effects of stress; they are enhancing or disruptive depending on the task, training conditions, and sex of the animal.10,33-36 Despite the differences in response, many are assumed to occur via glucocorticoid activity and most often by activity within the hippocampal formation. The hippocampus has an abundance of glucocorticoid receptors, particularly the type I or mineralocorticoid receptor,37 and the structure is implicated in feedback of the HPA axis.38 Thus, our results regarding the male response to stress are generally consistent, with much of the literature. That the female response is not dependent on the presence of glucocorticoids may be an aberration or simply reflect the fact that so few studies have been conducted in the female.
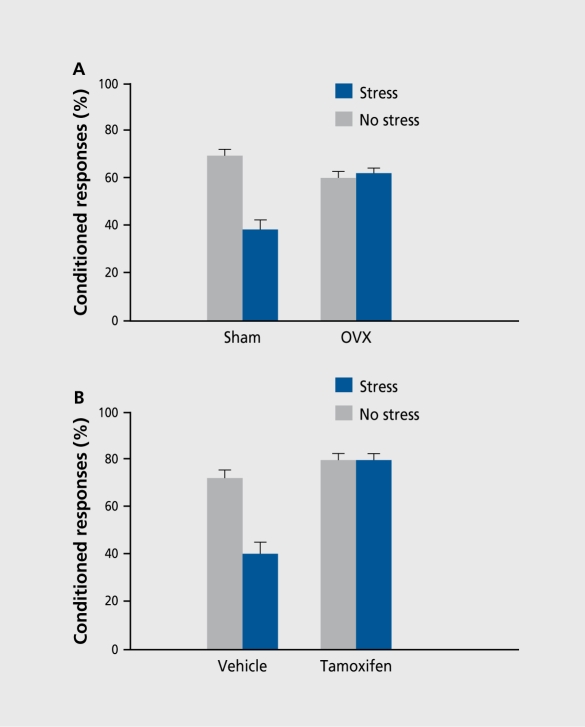
Since glucocorticoids are not critically involved in the stress effect in females, we considered other potential modulators, the first being ovarian hormones. As shown in Figure 3, their removal via ovariectomy prevented the stress effect on conditioning, suggesting that their presence is necessary for observing an impairment, after stress. Of the two primary ovarian hormones, we evaluated a specific role for estrogen. Figure 3 shows that treatment with the estrogen antagonist tamoxifen prevented the stress effect on conditioning.9 Together these data suggest that estrogen is critically involved in the stress effect on conditioning in females. We have also determined that the detrimental effect, of stress on learning is dependent on the stage of estrus in which the learning occurs. Of the stages, females that were trained during proestrus (stressed 24 hours earlier in di estrus) were most impaired by stressor exposure.11 Since this stage is associated with elevated levels of estrogen, the hormone is again implicated in these stress effects on conditioning.
Figure 3. Contribution of ovarian hormones to the stress effect on learning in females. A. Females that were ovariectomized (OVX) prior to stressor exposure and training were not impaired by stress and exhibited a similar response to those exposed to a sham surgery. B. Treatment with the estrogen antagonist tamoxifen prevented the stress effect on conditioning in females.
Recall that females under normal unstressed conditions learn faster in proestrus than in other stages. How might estrogen contribute to both enhanced learning under unstressed conditions and impaired learning after stress? It may be useful to consider the effect of stress on learning from a slightly different perspective in which stress does not impair conditioning directly, but rather prevents the enhancement, that normally occurs when estrogen levels are elevated.
Neuroanatomical correlates of stress and sex differences in learning
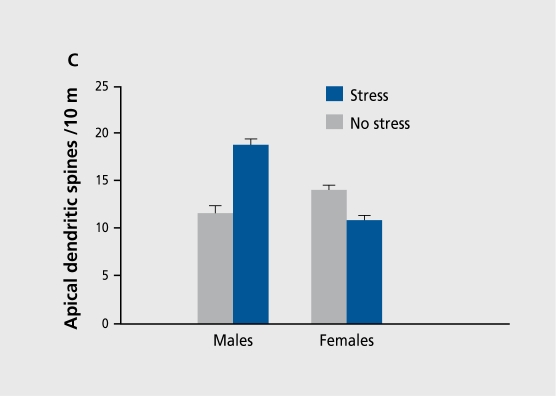
These opposite effects of stress in males and females pose some interesting questions, one being whether there is a neuronal or anatomical substrate that can account for these opposite responses to stress. .First, we considered a potential role for dendritic spines, tiny protrusions on many dendrites in the brain, which are a source of excitatory input.39 Because they enable connections and associations to be made between adjacent neurons, it has been hypothesized that they are involved in the formation of associative memories. Despite the pervasiveness of the hypothesis in the literature, there are minimal data in support of this. In fact, the most. potent modulator of dendritic spines so far established is estrogen. Acute exposure to estradiol enhances spine density in the hippocampus of ovariectomized females; moreover, females in proestrus have a greater spine density than females in other stages.40,41 The effect of estrus on spine density is rapid and dramatic, varying as much as 30% over the 5-day cycle. Recently, we compared the changes in spine density across the estrous cycle in females with that, of males. As shown previously,42 females in proestrus had a greater density of dendritic spines on apical dendrites in area. CA1 of the hippocampus. As shown in Figure 4, we also observed that females in proestrus have a greater density of spines in the hippocampus than do males.43 As discussed, it has long been assumed that dendritic spines participate in learning processes. So docs this change in spine density across the estrous cycle and between the sexes relate to learning ability? At. least as measured with classical eyeblink conditioning, there is a positive relationship between spine density and performance in females: females in proestrus outperform females in other stages and thus the variation in spine density correlates with their ability to acquire the learned response.11
Figure 4. A. Photograph of a hippocampal pyramidal cell impregnated with Golgi. Original magnification: 400x. B. A higher magnification (1 000x) of the dendrite illustrates the spines. C. Effects of exposure to an acute stressful event on density of dendritic spines in area CA1 of the hippocampus showing increased density in males, but a decreased density in females during proestrus.
If spine density is positively related to learning ability (of this task), then manipulations other than estrogen that modulate this type of learning may be expected to have effects on spine density. Initially, we considered the effects of stress. As discussed, exposure to an acute stressful event, enhances later performance in males, but impairs performance in females. In a series of experiments, we tested whether exposure to one of these stressors would affect spine density in the hippocampus and whether the effect would be sex-dependent. As illustrated in Figure 4, males exposed to the acute stressful event, of intermittent tailshocks possess a greater den-sity of spines than their unstressed male controls. Conversely, proestrous females who normally possess a high density of spines exhibit a decrease after exposure to the stressful event.43 Thus, spine density is positively related to performance under these specific conditions. To review, females in proestrus have a greater density than females in other stages and males, and they condition more. In response to stress, males have a greater density of spines than unstressed males and they condition more. In response to stress, females have a reduced density of spines and they condition poorly. These data do not indicate that spines are necessary for learning or that their presence mandates that learning will occur. Rather, they suggest, that the presence of spines may enhance the potential for learning - should the opportunity arise.
Sex differences in depression
What do these dramatically different behavioral and neuronal responses in male and female rats tell us about human behavior and adaptation to stressful experience? Minimally, they indicate that we must be very careful in generalizing results obtained from males to females. A relevant, example of this problem concerns the phenomenon of “learned helplessness.” In the 1960s, a number of influential behavioral scientists came upon an interesting observation. They had been using inescapable and escapable shocks in dogs to study the processes of Pavlovian (or classical) conditioning. During their experiments, they noticed that the dogs that were previously exposed to inescapable shock were less likely to learn a later task in which escape was then possible.44,45 These animals, as well as the many other species tested in this paradigm, displayed a number of features characteristic of depression. They did not eat. as much, had sleeping problems, and were generally inactive. In essence, it appeared as if they had “given up” and no longer had the motivation to learn. A number of psychologists picked up on these similarities and thereafter promoted this “learned helplessness” phenomenon in animals as a model of depression in humans.46 This model had such wide appeal that it is included in nearly every general and abnormal psychology textbook and was eventually developed into a more sophisticated model of depression known as learned hopelessness.46
The incidence and prevalence of depression is higher in women than in men. It. would thus be interesting to test for learned helplessness behaviors in females. Unfortunately, only a few studies have done so. In most, of these studies, rats were tested in a shuttle-box avoidance paradigm, in which the animal must, “learn” to escape from a footshock on one side of the cage. In order to terminate the shock, the animal must escape through an opening to the other side of the cage and back to the initial side. After exposure to inescapable shocks, male rats were impaired in their performance, whereas the females were not affected.47,48 Although these results suggest that females are not learning impaired, it. is difficult to prove this conclusively. This is in part because females are generally more active than males, thus the sex difference may simply reflect differences in behavior not relevant to learning, per se. Nonetheless, this paradigm is a commonly accepted animal model for depression in humans. That it may not adequately model female behavior suggests that alternative models may be warranted.
Although women are more likely than men to experience major depression in their lifetime, the course of that depression may not differ.49 There is no sex difference in duration of the first, episode, time to recovery, time to first, recurrence, and severity of symptoms. These data contrast, with those observed for manic-depressive illness, with no apparent, difference in prevalence, but. rather one of course. It is reported that, women cycle from mania to depression more rapidly than do men and they may have more depressive episodes and dysphoria.50 The increased prevalence of unipolar and course of bipolar depression as well as general changes in personality are often associated with or exacerbated by changes in ovarian hormones levels such as occur prior to ovulation, after pregnancy, and during menopause.50-52 It is in this context that we again present our findings regarding the effects of stress on learning in the females, this time highlighting its relationship to changing levels of estrogen. In a typical experiment, female rats are exposed to an inescapable stressor such as intermittent tailshocks or swimming, and we then measure learning 24 hours later. As discussed, exposure to these stressors dramatically impairs subsequent learning in the female rat.9-11 This effect, most. pronounced when females are stressed during diestrus and trained in proestrus, a time period over which estradiol levels are changing. Thus, the effect. of stress is dependent, on the stage of estrus and potentially on changing levels of ovarian hormones.11 Initially, we hypothesized that exposure to the stressful event, altered the cycle, perhaps by decreasing the release of estrogen. However, experiments to test, this hypothesis indicated that acute stress did not disrupt the cycle. We did observe an increase in estrogen levels after its cessation.53 However, injection of stress levels of estradiol did not. impair learning as did the stressor. Thus, the effect of stress on memory formation in the female depends less on absolute levels of estrogen and more on their fluctuation during and shortly after the traumatic event. Consistent, with some of the emotional disturbances that can occur during menstruation, postpartum, and menopause, these data suggest, that females are particularly susceptible to the deleterious consequences of stress when ovarian hormones are fluctuating.
Table I. Sex differences in mental illness.
| Disorder | Male:female ratio | Reference |
| • Disorders of infancy, childhood, or adolescence | ||
| Attention-deficit hyperactivity disorder | 3.00:1.00 | 54 |
| Autistic disorder | 3.63:1.00 | 55 |
| Developmental disorder not otherwise specified | 3.62:1.00 | 55 |
| • Psychotic disorders | ||
| Schizophrenia | 2.64:1.00 | 56 |
| Schizophrenia-refated disorders | 2.23:1.00 | 56 |
| • Cognitive disorders | ||
| Alzheimer's disease | 1.00:1.56 | 57 |
| Dementia | 1.00 1.18 | 57 |
| • Mood disorders | ||
| Unipolar depression | 1.00:2.20 | 58 |
| Bipolar depression | 1.00:1.00 | 59 |
| • Anxiety disorders | ||
| Generalized anxiety disorder | 1.00:1.34 | 60 |
| Agoraphobia and panic disorders | 1.00.1.00 | 60 |
| Posttraumatic stress disorder | 1.00:3.00 | 61 |
Conclusion: sex differences in mental illness
That females are different from males may come as no surprise. Nor that, their brains are different. What might. be unexpected is that they would respond in opposite directions to the same environmental event, and that their brains would follow in course. In the face of such divergence, perhaps we should reconsider sex differences in mental illness (Table I). Females are not only more likely to experience depression, but. also phobias, generalized anxiety disorder, and posttraumatic stress disorder. They are more often diagnosed with eating disorders, as well as borderline and histrionic personality disorders. Males, on the other hand, are more likely to experience autism and antisocial and narcissistic personality disorders, as well as attention deficit, disorder and mental retardation. It may be instructive that the mental disorders more common in women are related to affect whereas those more common in men are related to cognition. Exactly how information about sex differences in emotional and cognitive responses in rats can be used to understand or promote mental health in humans is unclear, but a greater appreciation of our differences can only enhance our ability to treat our common afflictions.
Supported by grants from the National Institutes of Mental Health (MH59970) and (MH 59740), National Alliance for Research on Schizophrenia and Depression (NARSAD), and the van Arneringen Foundation.
REFERENCES
- 1.Hampson E., Kimura D. Reciprocal effects of hormonal fluctuations on human motor and perceptual-spatial skills. Behav Neurosci. 1988;102:456–459. doi: 10.1037//0735-7044.102.3.456. [DOI] [PubMed] [Google Scholar]
- 2.Kimura D. Sex and Cognition. Cambridge, Mass: MIT Press; 1999 [Google Scholar]
- 3.Goodall J. The Chimpanzees of Gombe. Cambridge, Mass: Harvard University Press; 1986 [Google Scholar]
- 4.Shors TJ. Stress and sex effects on associative learning: for better or for worse. The Neuroscientist. 1998;4:353–364. [Google Scholar]
- 5.Thompson RF., Krupa DJ. Organization of memory traces in the mammalian brain. Ann Rev Neurosci. 1994;17:519–549. doi: 10.1146/annurev.ne.17.030194.002511. [DOI] [PubMed] [Google Scholar]
- 6.Steinmetz JE., Rosen JB., Chapman PF., Lavond DG., Thompson RF. Classical conditioning of the eyeblink response with a mossy fiber stimulation CS. I. Pontine nuclei and middle cerebellar peduncle stimulation. Behav Neurosci. 1986;100:871–880. doi: 10.1037//0735-7044.100.6.878. [DOI] [PubMed] [Google Scholar]
- 7.Thompson RF., Kim JJ. Memory systems in the brain and localization of memory. Proc Natl Acad Sci USA. 1996;93:13438–13444. doi: 10.1073/pnas.93.24.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodruff-Pak DS. Eyeblink classical conditioning in HM: delay and trace paradigms. Behav Neurosci. 1993;107:911–925. doi: 10.1037//0735-7044.107.6.911. [DOI] [PubMed] [Google Scholar]
- 9.Wood GE., Shors TJ. Stress facilitates classical conditioning in males but impairs conditioning in females through activational influences of ovarian hormones. Proc Natl Acad Sci USA. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood GE., Beylin AV., Shors TJ. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav Neurosci. 2001;115:175–187. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- 11.Shors TJ., Lewczyk C., Paczynski M., Mathew PR., Pickett J. Stages of estrous mediate the stress-induced impairment of associative learning in the male rat. Neuroreport. 1998;9:419–423. doi: 10.1097/00001756-199802160-00012. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham SG., Rissman EF., Foster TC. Sex differences in the activational effects of ERalpha on spatial learning. Horm Behav. 1998;34:163–170. doi: 10.1006/hbeh.1998.1475. [DOI] [PubMed] [Google Scholar]
- 13.Galea LAM., Kavaliers M., Ossenkopp KP., Innés D., Hargreaves EL. Sexually dimorphic spatial learning varies seasonally in two populations of deer mice. Brain Res. 1994;634:18–26. doi: 10.1016/0006-8993(94)91419-2. [DOI] [PubMed] [Google Scholar]
- 14.Williams CL., Meek WH. The organizational effects of gonadal steroids on sexually dimorphic spatial ability. Psychoneuroendocrinology. 1991;16:155–176. doi: 10.1016/0306-4530(91)90076-6. [DOI] [PubMed] [Google Scholar]
- 15.Sherry DF., Hampson E. Evolution and the hormonal control of sexually dimorphic spatial abilities in humans. Trends Cog Neurosci. 1997;1:50–56. doi: 10.1016/S1364-6613(97)01015-2. [DOI] [PubMed] [Google Scholar]
- 16.Hampson E. Estrogen-related variations in human spatial and articulatory-motor skills. Psychoneuroendocrinology. 1990;15:97–111 . doi: 10.1016/0306-4530(90)90018-5. [DOI] [PubMed] [Google Scholar]
- 17.Warren SG., Juraska JM. Spatial and nonspatial learning across the rat estrous cycle. Behav Neurosci. 1997;111:259–266. doi: 10.1037//0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- 18.Frye CA. Estrus-associated decrements in water maze task are limited to acquisition. Physiol Behav. 1995;57:5–14. doi: 10.1016/0031-9384(94)00197-d. [DOI] [PubMed] [Google Scholar]
- 19.Berry B., McMahan R., Gallagher M. Spatial learning and memory at defined points of the estrous cycle: effects on performance of a hippocampal-dependent task. Behav Neurosci. 1997;111:267–274. doi: 10.1037//0735-7044.111.2.267. [DOI] [PubMed] [Google Scholar]
- 20.Perrot-Sinal TS., Kostenuik MA., Ossenkopp KP., Kavaliers M. Sex differences in performance in the Morris water maze and the effects of initial nonstationary hidden platform training. Behav Neurosci. 1996;110:1309–1320. doi: 10.1037//0735-7044.110.6.1309. [DOI] [PubMed] [Google Scholar]
- 21.Viau V., Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- 22.Critchlow V., Liebelt RA., Bar-Sela M., Mountcastle W., Lipscomb HS. Sex difference in resting pituitary-adrenal function in the rat. Am J Physiol. 1963;205:807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- 23.Shors TJ., Weiss C., Thompson RF. Stress-induced facilitation of classical conditioning. Science. 1992;257:537–539. doi: 10.1126/science.1636089. [DOI] [PubMed] [Google Scholar]
- 24.Shors TJ. Acute stress rapidly and persistently enhances memory formation in the male rat. Neurobiol Learn Mem. 2001;75:10–29. doi: 10.1006/nlme.1999.3956. [DOI] [PubMed] [Google Scholar]
- 25.Beylin AV., Talk AC., Gandhi CC., Wood GE., Matzel LD., Shors TJ. The role of the hippocampus in trace conditioning: temporal incongruity or task difficulty? Neurobiol Learn Mem. 2001;76:447–461. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- 26.Solomon PR., van der Schaaf ER., Thompson RF., Weisz D. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behav Neurosci. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- 27.Clark RE., Squire LR. Classical conditioning and brain systems: the role of awareness. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- 28.Breslau N. The epidemiology of posttraumatic stress disorder: what is the extent of the problem? J Clin Psychiatry. 2001;62:16–22. [PubMed] [Google Scholar]
- 29.Foa EB., Street GP. Women and traumatic events. J Clin Psychiatry. 2001;62:29–34. [PubMed] [Google Scholar]
- 30.Shors TJ., Servatius RJ. The contribution of stressor intensity, duration, and context to the stress-induced facilitation of associative learning. Neurobiol Learn Mem. 1997;67:92–96. doi: 10.1006/nlme.1997.3763. [DOI] [PubMed] [Google Scholar]
- 31.Munich A., Guyre PM., Holbrook NJ. Physiological function of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;1:339–376. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- 32.Shors TJ., Beylin AV., Wood GE., Gould E. The modulation of Pavlovian memory. Behav Brain Res. 2000;110:39–52. doi: 10.1016/s0166-4328(99)00183-7. [DOI] [PubMed] [Google Scholar]
- 33.de Quervain DJF., Roozendaal B., McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- 34.Johnston AN., Rose SP. Isolation-stress-induced facilitation of passive avoidance in the day-old chick. Behav Neurosci. 1998;112:929–936. doi: 10.1037//0735-7044.112.4.929. [DOI] [PubMed] [Google Scholar]
- 35.Diamond DM., Rose GM. Stress impairs LTP and hippocampal-dependent memory. Ann N Y Acad Sci. 1994;30:411–414. doi: 10.1111/j.1749-6632.1994.tb39271.x. [DOI] [PubMed] [Google Scholar]
- 36.de Kloet ER., Oitzl MS., Joels M. Stress and cognition: are corticosteroids good or bad guys? 7rencfe. Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- 37.Reul JHM., de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differentail occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 38.Sapolsky RM., Krey L., McEwen B. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress-response. Proc Natl Acad Sci USA. 1984;81:6174–6178. doi: 10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shephard GMG., Harris KM. Three-dimensional structure and composition of CA3/CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J Neurosci. 1998;18:8300–8310. doi: 10.1523/JNEUROSCI.18-20-08300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gould E., Woolley CS., Frankfurt M., McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woolley CS., Gould E., Frankfurt M., McEwen BS. Naturally occurring fluctuations in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woolley CS., McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shors TJ., Chua C., Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Overmier JB., Seligman MEP. Effects of inescapable shock on subsequent escape and avoidance learning. J Comp Physiol Psychol. 1967;63:23–33. doi: 10.1037/h0024166. [DOI] [PubMed] [Google Scholar]
- 45.Seligman MEP., Maier SF. Failure to escape traumatic shock. J Comp Physiol Psychol. 1967;74:1–9. doi: 10.1037/h0024514. [DOI] [PubMed] [Google Scholar]
- 46.Seligman MEP. Helplessness. San Francisco, Calif: Freeman; 1975 [Google Scholar]
- 47.Steenbergen HL., Heinsbroek RPW., van Hest A., van de Poll NE. Sexdependent effects of inescapable shock administration on shuttle-box escape performance and elevated plus-maze behavior. Physiol Behav. 1990;48:571–576. doi: 10.1016/0031-9384(90)90302-k. [DOI] [PubMed] [Google Scholar]
- 48.Kirk RC., Blampied NM. Activity during inescapable shock and subsequent escape avoidance learning: female and male rats compared. NZJ Psychol. 1985;14:9–14. [Google Scholar]
- 49.Simpson HB., Nee JC., Endicott J. First-episode major depression: few sex differences in course. Arch Gen Psychiatry. 1997;54:633–639. doi: 10.1001/archpsyc.1997.01830190059006. [DOI] [PubMed] [Google Scholar]
- 50.Leibenluft MD. Issues in the treatment of women with bipolar illness. J Clin Psychiatry. 1997;58:5–11 . [PubMed] [Google Scholar]
- 51.Bloch M., Schmidt PJ., Danaceau M., Murphy J., Nieman L., Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157:924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- 52.Berlin RE., Raju JD., Schmidt PJ., Adams LF., Rubinow DR. Effects of the menstrual cycle on measures of personality in women with premenstrual syndrome: a preliminary study. J Clin Psychiatry. 2001;62:337–342. doi: 10.4088/jcp.v62n0505. [DOI] [PubMed] [Google Scholar]
- 53.Shors TJ., Pickett J., Wood GE., Paczynski M. Acute stress enhances estrogen levels in the female rat. Stress. 1999;3:163–171. doi: 10.3109/10253899909001120. [DOI] [PubMed] [Google Scholar]
- 54.Biederman J., Mick E., Faraone SV., et al. Influence of gender on attention deficit hyperactivity disorder in children referred to a psychiatric clinic. Am J Psychiatry. 2002;159:36–42. doi: 10.1176/appi.ajp.159.1.36. [DOI] [PubMed] [Google Scholar]
- 55.Volkmar FR., Szatmari P., Sparrow SS. Sex differences in pervasive developmental disorders. J Autism Dev Disord. 1993;23:579–591. doi: 10.1007/BF01046103. [DOI] [PubMed] [Google Scholar]
- 56.Iacono WG., Beiser M. Are males more likely than females to develop schizophrenia? Am J Psychiatry. 1992;149:1070–1074. doi: 10.1176/ajp.149.8.1070. [DOI] [PubMed] [Google Scholar]
- 57.Gao S., Hendrie HC., Hall KS., Hui S. The relationships between age, sex and the incidence of dementia and Alzheimer's disease. Arch Gen Psychiatry. 1998;55:809–815. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- 58.Burt VK., Steio K. Epidemiology of depression throughout the female life cycle. J Clin Pract. 2002;63:9–15. [PubMed] [Google Scholar]
- 59.Gater R., Tansella M., Korten A., Tiemens BG., Mavreas VG., Olatawura MO. Sex differences in the prevalence of detection of depressive and anxiety disorders in general health care settings. Arch Gen Psychiatry. 1998;55:405–413. doi: 10.1001/archpsyc.55.5.405. [DOI] [PubMed] [Google Scholar]
- 80.Weissman MM., Leaf PJ., Tischler GL., Blazer DG., Karno M., Bruce ML., Florio LP. Affective disorders in five United States communities. Psychol Med. 1988;18:141–153. doi: 10.1017/s0033291700001975. [DOI] [PubMed] [Google Scholar]
- 61.Stein MB., Walker JR., Forde DR. Gender differences in susceptibility to posttraumatic stress disorder. Behav Res Ther. 2000;38:619–628. doi: 10.1016/s0005-7967(99)00098-4. [DOI] [PubMed] [Google Scholar]