Abstract
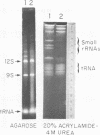
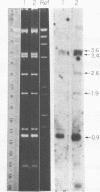
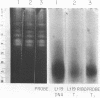
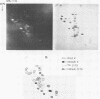
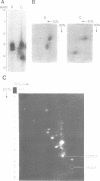
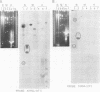
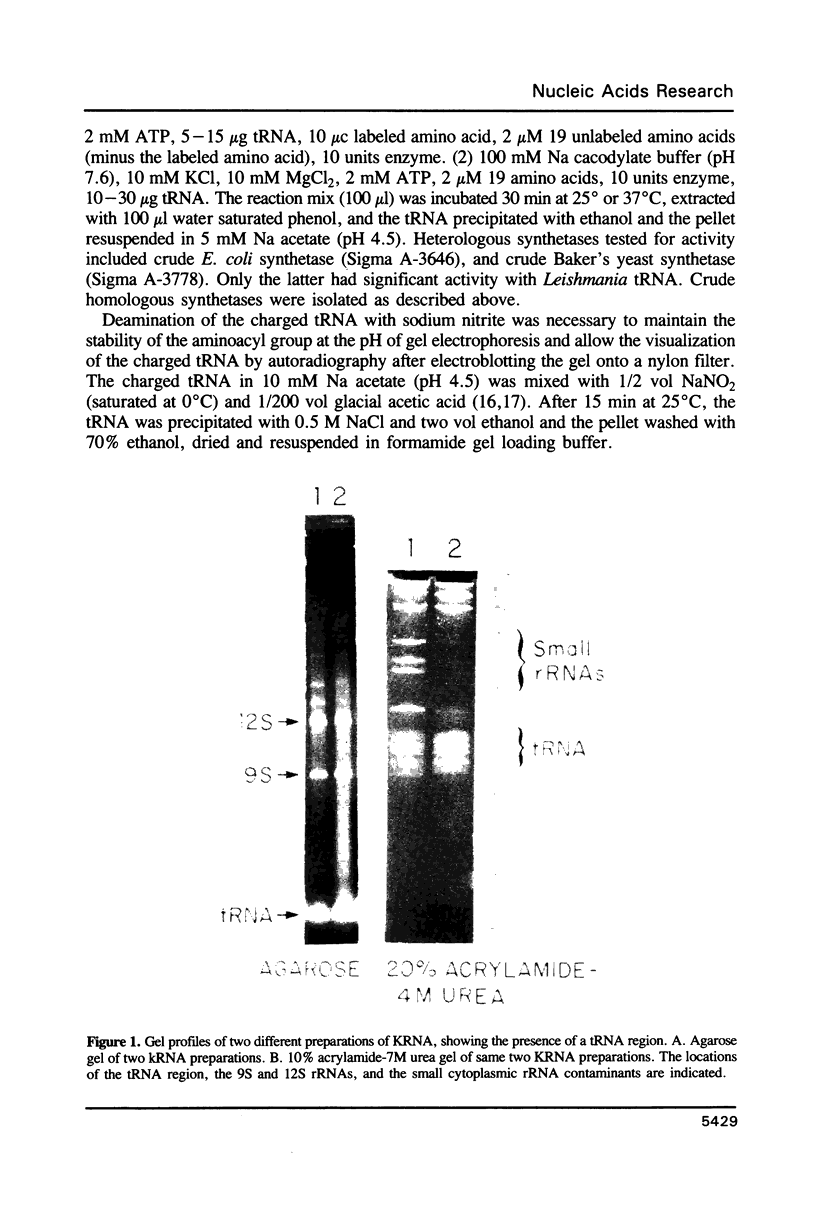
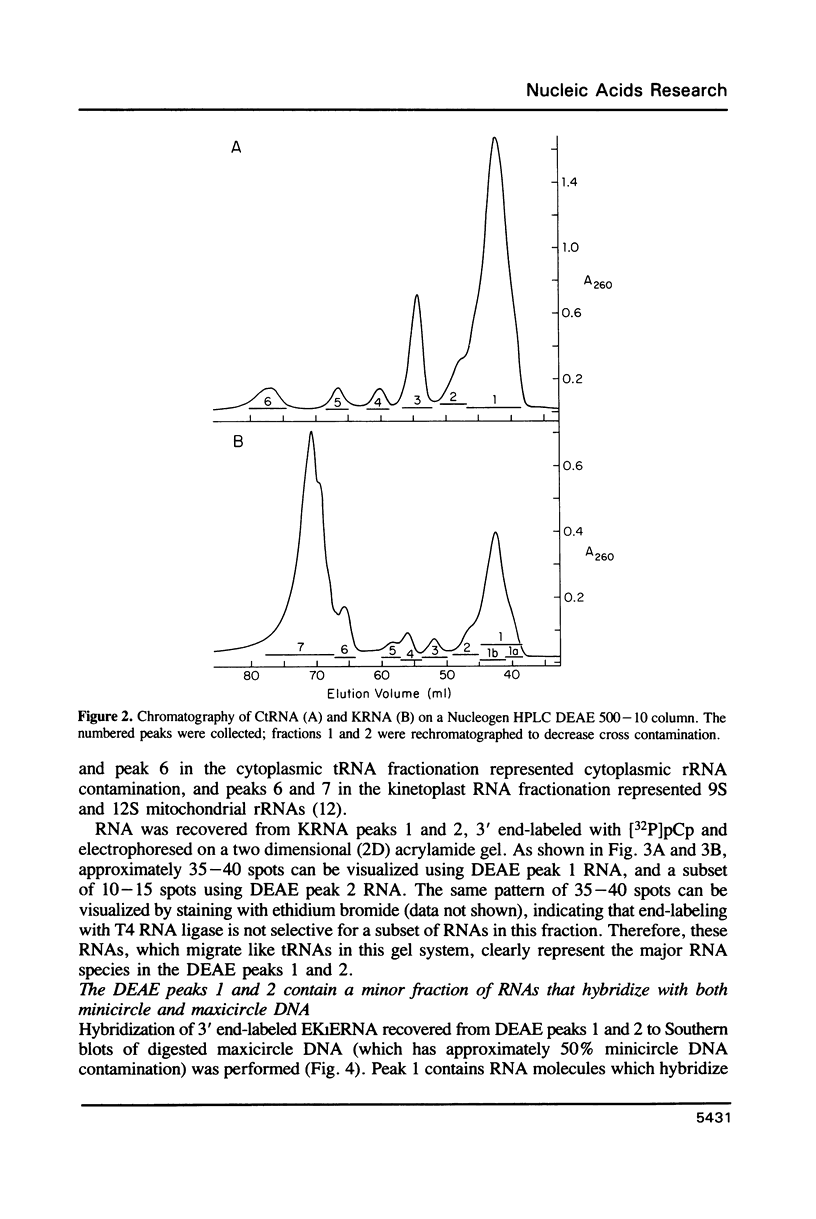
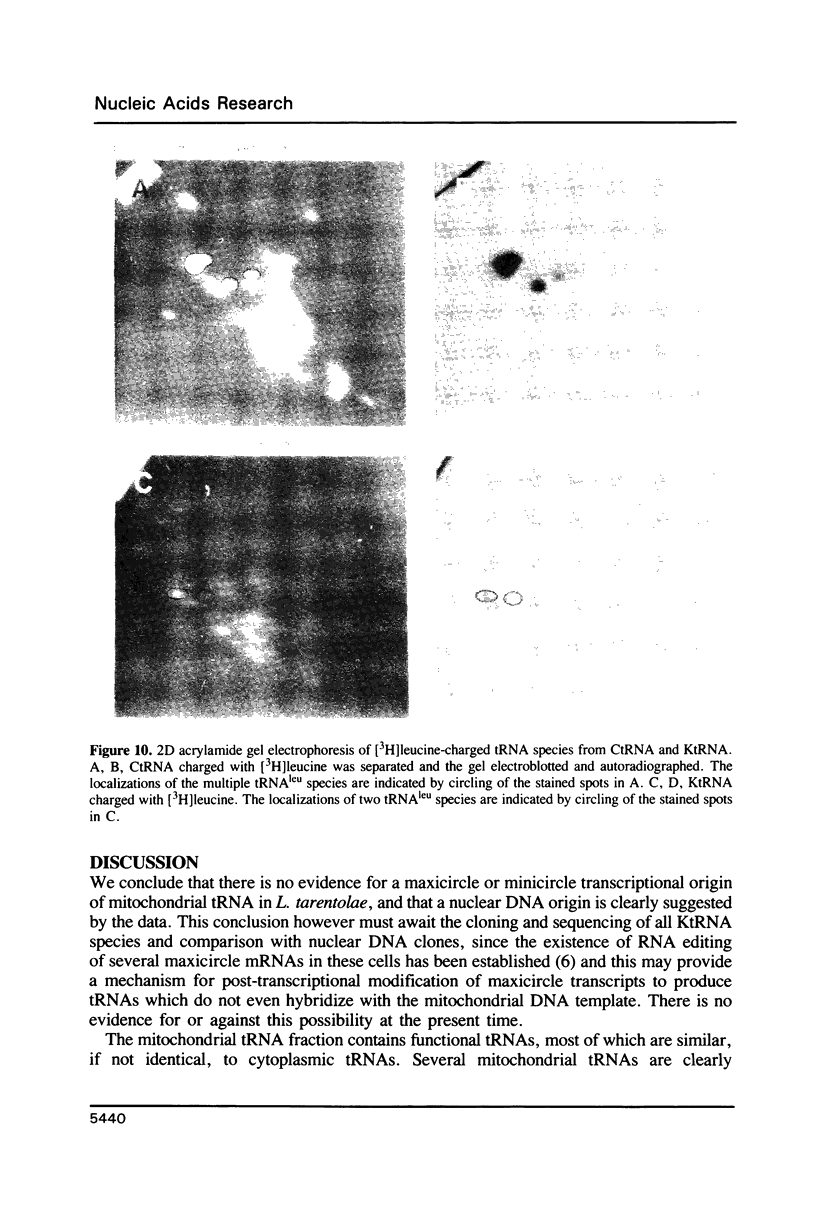
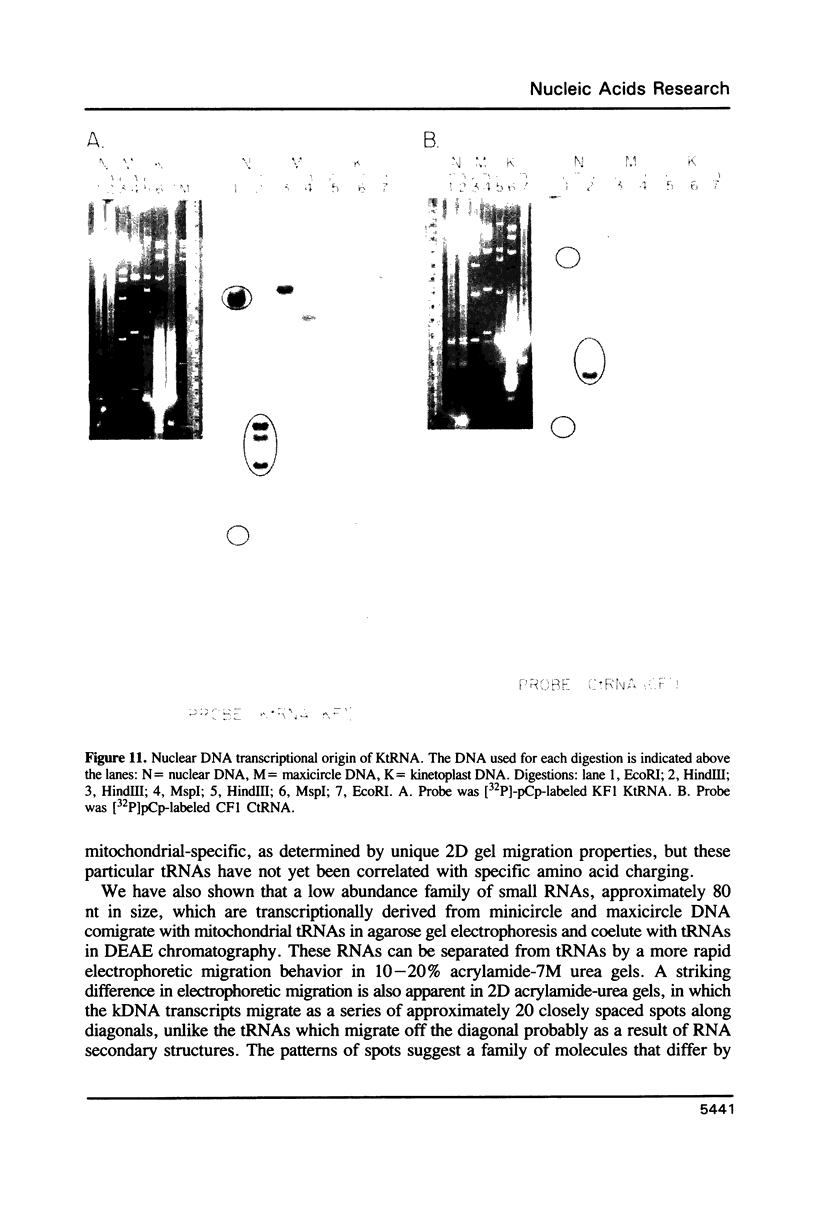
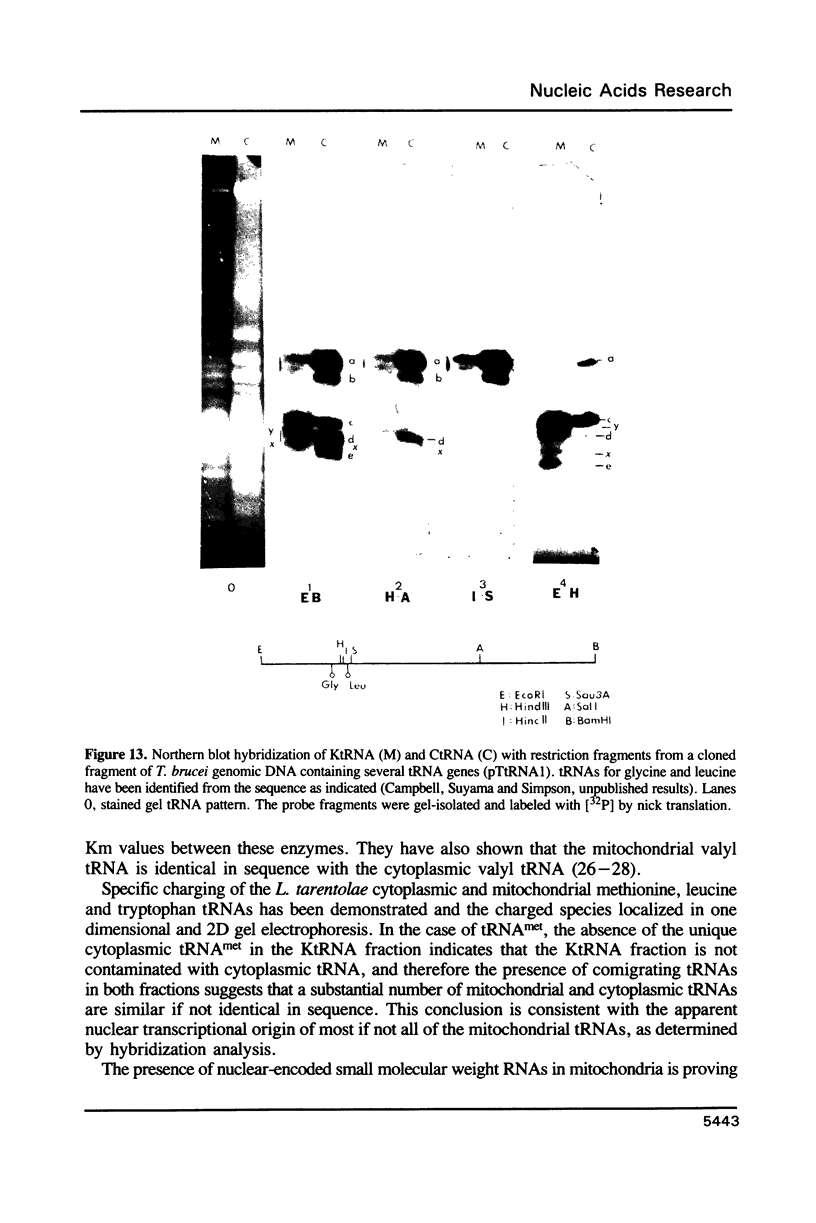
The mitochondrion of Leishmania tarentolae contains approximately 35-40 tRNAs many of which comigrate with cytoplasmic tRNAs. Both mitochondrial (KtRNA) and cytoplasmic (CtRNA) tRNAs are functional, as they could be acylated either by mitochondrial or cytoplasmic synthetase extracts. There are two methionyl tRNA species in the cytoplasmic and mitochondrial fractions, one of which is unique to each fraction, indicating that the KtRNA fraction is free of CtRNA contamination. Leucyl and glycyl tRNAs were identified by hybridization with a genomic clone from Trypanosoma brucei. KtRNA hybridizes with nuclear chromosomes, but not with minicircle or maxicircle DNA. KtRNA isolated by DEAE chromatography or agarose gel electrophoresis contains additional small RNAs which hybridize with both minicircle and maxicircle DNA. These transcripts do not migrate like tRNAs in acrylamide gels and their functions is unknown. We suggest that most if not all mitochondrial tRNAs in L. tarentolae are nuclear-encoded and imported into the mitochondrion.
Full text
PDF



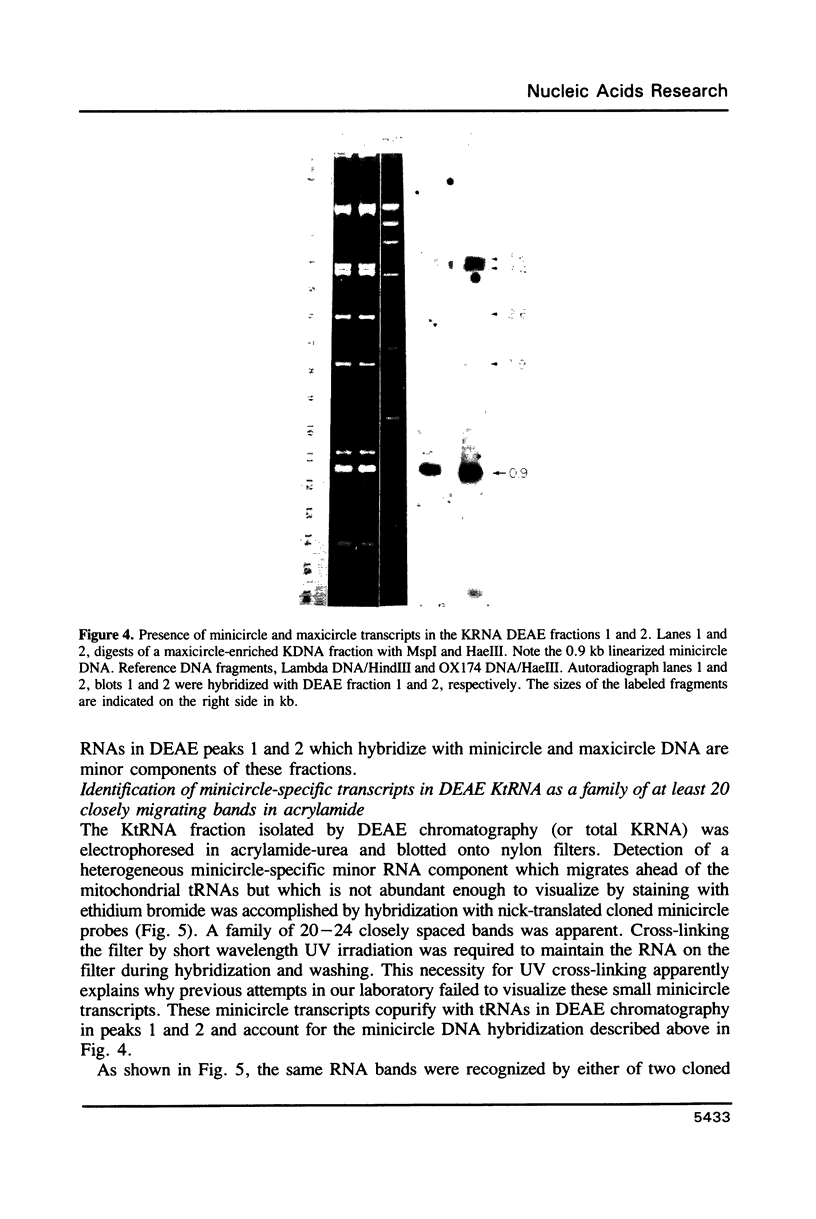

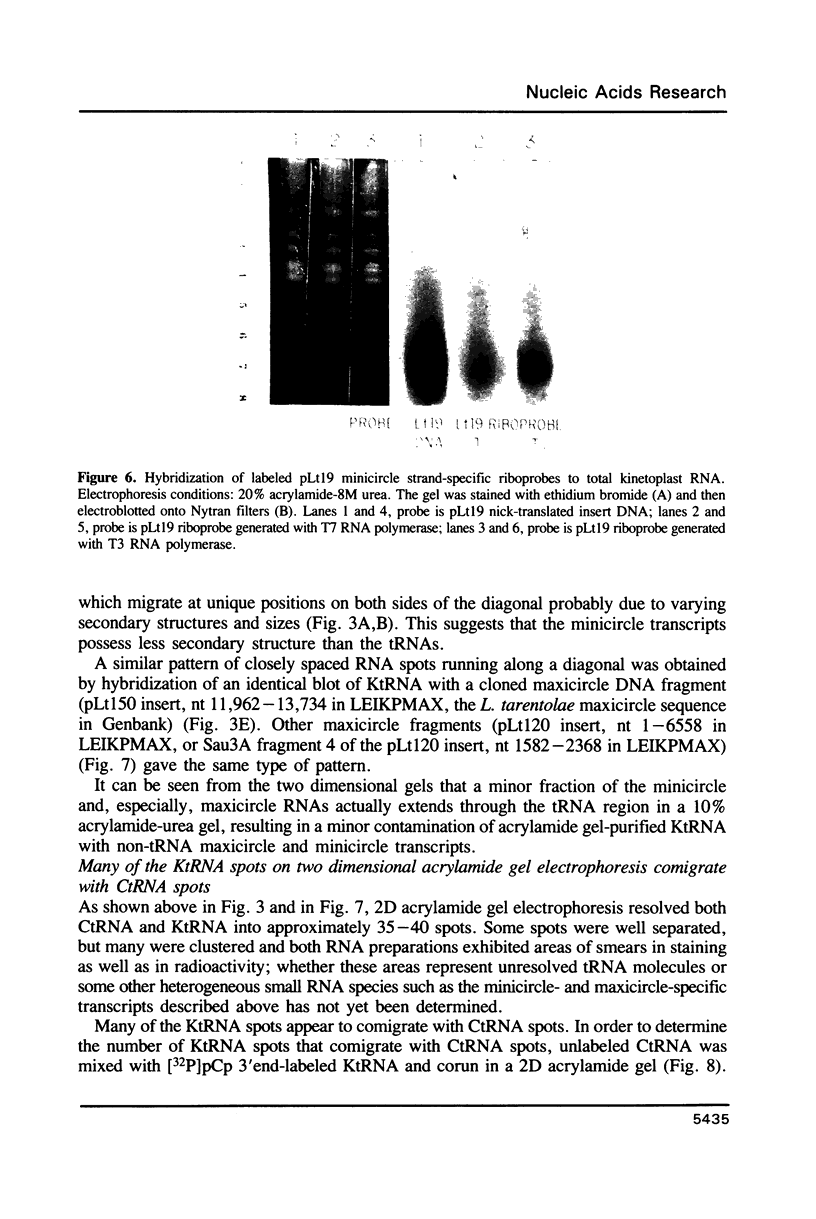

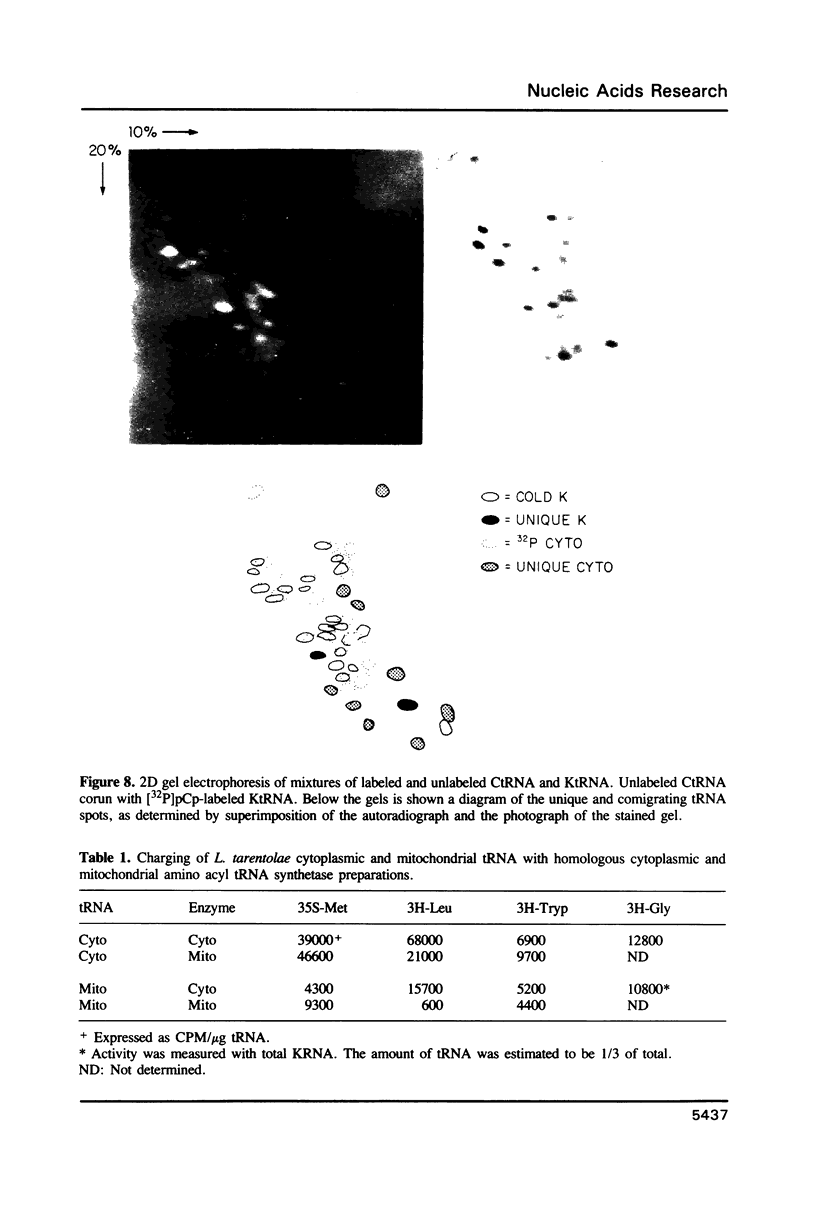
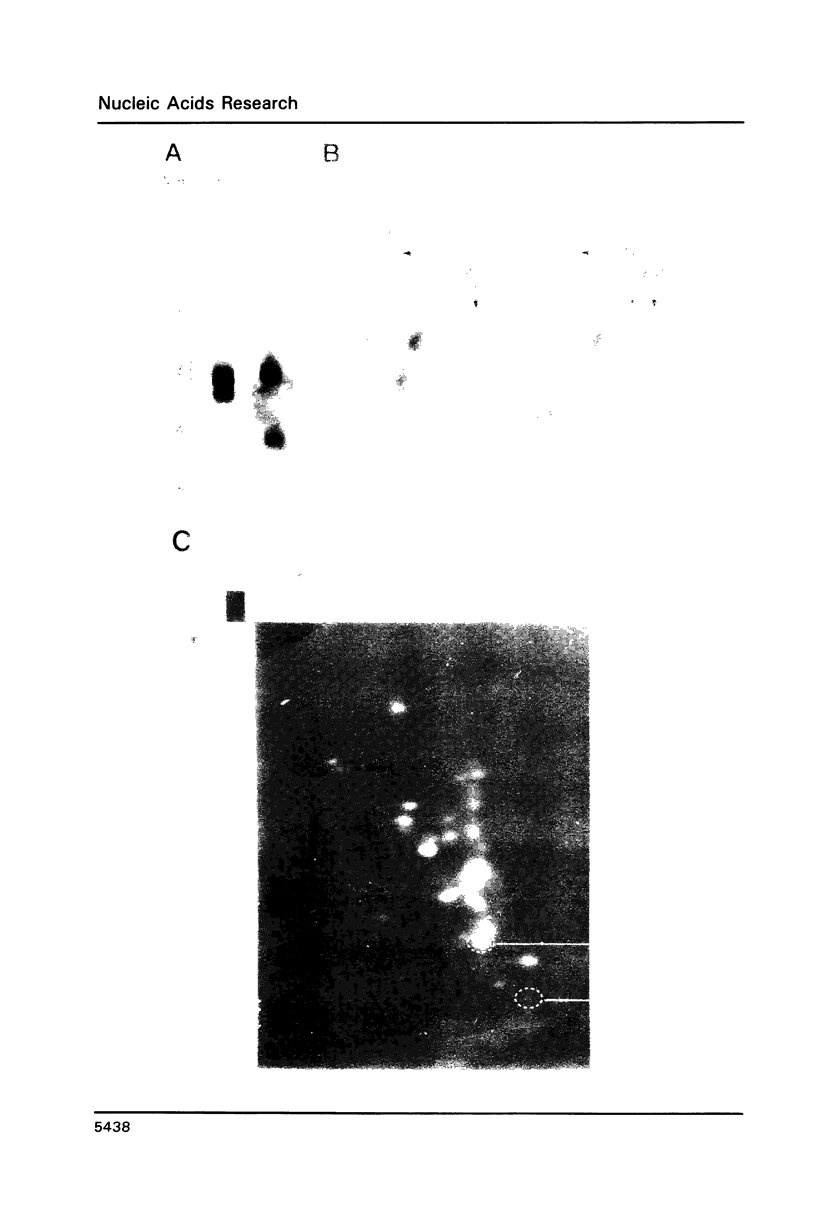




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aujame L., Wallace R. B., Freeman K. B. Chemical and physical properties of mammalian mitochondrial aminoacyl-transfer RNAs. I. Molecular weights of mitochondrial leucyl- and methionyl-transfer RNAs. Biochim Biophys Acta. 1978 Apr 27;518(2):308–320. doi: 10.1016/0005-2787(78)90187-9. [DOI] [PubMed] [Google Scholar]
- Benne R., Sloof P. Evolution of the mitochondrial protein synthetic machinery. Biosystems. 1987;21(1):51–68. doi: 10.1016/0303-2647(87)90006-2. [DOI] [PubMed] [Google Scholar]
- Boer P. H., Gray M. W. Scrambled ribosomal RNA gene pieces in Chlamydomonas reinhardtii mitochondrial DNA. Cell. 1988 Nov 4;55(3):399–411. doi: 10.1016/0092-8674(88)90026-8. [DOI] [PubMed] [Google Scholar]
- Braly P., Simpson L., Kretzer F. Isolation of kinetoplast-mitochondrial complexes from Leishmania tarentolae. J Protozool. 1974 Nov;21(5):782–790. doi: 10.1111/j.1550-7408.1974.tb03752.x. [DOI] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. An electrophoretic karyotype for yeast. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3756–3760. doi: 10.1073/pnas.82.11.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. Mouse RNAase MRP RNA is encoded by a nuclear gene and contains a decamer sequence complementary to a conserved region of mitochondrial RNA substrate. Cell. 1989 Jan 13;56(1):131–139. doi: 10.1016/0092-8674(89)90991-4. [DOI] [PubMed] [Google Scholar]
- Chiu N., Chiu A., Suyama Y. Native and imported transfer RNA in mitochondria. J Mol Biol. 1975 Nov 25;99(1):37–50. doi: 10.1016/s0022-2836(75)80157-4. [DOI] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Doersen C. J., Guerrier-Takada C., Altman S., Attardi G. Characterization of an RNase P activity from HeLa cell mitochondria. Comparison with the cytosol RNase P activity. J Biol Chem. 1985 May 25;260(10):5942–5949. [PubMed] [Google Scholar]
- England T. E., Bruce A. G., Uhlenbeck O. C. Specific labeling of 3' termini of RNA with T4 RNA ligase. Methods Enzymol. 1980;65(1):65–74. doi: 10.1016/s0076-6879(80)65011-3. [DOI] [PubMed] [Google Scholar]
- Entelis N. S., Maslov D. A., Bol'shakova E. V., Zaitseva G. N. Pervichnaia struktura neobychnogo gena valinovoi tRNK mitokhondrii Crithidia oncopelti. Dokl Akad Nauk SSSR. 1987;297(6):1498–1501. [PubMed] [Google Scholar]
- Gomez-Eichelmann M. C., Holz G., Jr, Beach D., Simpson A. M., Simpson L. Comparison of several lizard Leishmania species and strains in terms of kinetoplast minicircle and maxicircle DNA sequences, nuclear chromosomes, and membrane lipids. Mol Biochem Parasitol. 1988 Jan 15;27(2-3):143–158. doi: 10.1016/0166-6851(88)90034-5. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Snijders A., Janssen J. W., Borst P. Transcription of kinetoplast DNA in Trypanosoma brucei bloodstream and culture forms. Plasmid. 1981 May;5(3):329–350. doi: 10.1016/0147-619x(81)90009-3. [DOI] [PubMed] [Google Scholar]
- Kidane G. Z., Hughes D., Simpson L. Sequence heterogeneity and anomalous electrophoretic mobility of kinetoplast minicircle DNA from Leishmania tarentolae. Gene. 1984 Mar;27(3):265–277. doi: 10.1016/0378-1119(84)90071-4. [DOI] [PubMed] [Google Scholar]
- Martin R. P., Schneller J. M., Stahl A. J., Dirheimer G. Import of nuclear deoxyribonucleic acid coded lysine-accepting transfer ribonucleic acid (anticodon C-U-U) into yeast mitochondria. Biochemistry. 1979 Oct 16;18(21):4600–4605. doi: 10.1021/bi00588a021. [DOI] [PubMed] [Google Scholar]
- Maréchal-Drouard L., Weil J. H., Guillemaut P. Import of several tRNAs from the cytoplasm into the mitochondria in bean Phaseolus vulgaris. Nucleic Acids Res. 1988 Jun 10;16(11):4777–4788. doi: 10.1093/nar/16.11.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H., Simpson L., Rosenblatt H., Simpson A. M. Restriction map, partial cloning and localization of 9S and 12S kinetoplast RNA genes on the maxicircle component of the kinetoplast DNA of Leishmania tarentolae. Gene. 1979 May;6(1):51–73. doi: 10.1016/0378-1119(79)90085-4. [DOI] [PubMed] [Google Scholar]
- Rohrer S. P., Michelotti E. F., Torri A. F., Hajduk S. L. Transcription of kinetoplast DNA minicircles. Cell. 1987 Jun 5;49(5):625–632. doi: 10.1016/0092-8674(87)90538-1. [DOI] [PubMed] [Google Scholar]
- Simpson L., Braly P. Synchronization of Leishmania tarentolae by hydroxyurea. J Protozool. 1970 Nov;17(4):511–517. doi: 10.1111/j.1550-7408.1970.tb04719.x. [DOI] [PubMed] [Google Scholar]
- Simpson L. Kinetoplast DNA in trypanosomid flagellates. Int Rev Cytol. 1986;99:119–179. doi: 10.1016/s0074-7696(08)61426-6. [DOI] [PubMed] [Google Scholar]
- Simpson L., Neckelmann N., de la Cruz V. F., Simpson A. M., Feagin J. E., Jasmer D. P., Stuart K. Comparison of the maxicircle (mitochondrial) genomes of Leishmania tarentolae and Trypanosoma brucei at the level of nucleotide sequence. J Biol Chem. 1987 May 5;262(13):6182–6196. [PubMed] [Google Scholar]
- Simpson L., Shaw J. RNA editing and the mitochondrial cryptogenes of kinetoplastid protozoa. Cell. 1989 May 5;57(3):355–366. doi: 10.1016/0092-8674(89)90911-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L., Simpson A. G. Kinetoplast RNA of Leishmania tarentolae. Cell. 1978 May;14(1):169–178. doi: 10.1016/0092-8674(78)90311-2. [DOI] [PubMed] [Google Scholar]
- Simpson L. The mitochondrial genome of kinetoplastid protozoa: genomic organization, transcription, replication, and evolution. Annu Rev Microbiol. 1987;41:363–382. doi: 10.1146/annurev.mi.41.100187.002051. [DOI] [PubMed] [Google Scholar]
- Suyama Y., Hamada J. The mitochondrial and cytoplasmic valyl tRNA synthetases in Tetrahymena are indistinguishable. Arch Biochem Biophys. 1978 Dec;191(2):437–443. doi: 10.1016/0003-9861(78)90382-x. [DOI] [PubMed] [Google Scholar]
- Suyama Y. The origins of mitochondrial ribonucleic acids in Tetrahymena pyriformis. Biochemistry. 1967 Sep;6(9):2829–2839. doi: 10.1021/bi00861a025. [DOI] [PubMed] [Google Scholar]
- Suyama Y. Two dimensional polyacrylamide gel electrophoresis analysis of Tetrahymena mitochondrial tRNA. Curr Genet. 1986;10(5):411–420. doi: 10.1007/BF00418415. [DOI] [PubMed] [Google Scholar]
- Svensson I., Isaksson L., Henningsson A. Aminoacylation and polypeptide synthesis with tRNA lacking ribothymidine. Biochim Biophys Acta. 1971 May 13;238(2):331–337. doi: 10.1016/0005-2787(71)90100-6. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Schatz G. DNA-protein conjugates can enter mitochondria via the protein import pathway. Nature. 1989 Mar 9;338(6211):170–172. doi: 10.1038/338170a0. [DOI] [PubMed] [Google Scholar]
- White T. C., Rudenko G., Borst P. Three small RNAs within the 10 kb trypanosome rRNA transcription unit are analogous to domain VII of other eukaryotic 28S rRNAs. Nucleic Acids Res. 1986 Dec 9;14(23):9471–9489. doi: 10.1093/nar/14.23.9471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitseva G. N., Mett I. L., Maslov D. A., Lunina L. D., Koleshnkov A. A. O nalichii genov ribosomnykh i transportnykh RNK v kinetoplastnoi DNK dvukh vidov kritidii. Biokhimiia. 1979 Nov;44(11):2073–2082. [PubMed] [Google Scholar]
- de la Cruz V. F., Neckelmann N., Simpson L. Sequences of six genes and several open reading frames in the kinetoplast maxicircle DNA of Leishmania tarentolae. J Biol Chem. 1984 Dec 25;259(24):15136–15147. [PubMed] [Google Scholar]