Abstract
Studies on the pathophysiology of schizophrenia have implicated the limbic cortex, using postmortem, structural, and functional data, especially in the hippocampus (HC) and the anterior cingulate cortex (ACC). We have made contributions to the literature consistent with this idea: first, we describe a positive significant correlation between psychotic symptoms in schizophrenia and neuronal activity in the ACC and HC, suggesting the involvement of limbic cortex in the mediation of symptoms in schizophrenia. Second, in the ACC and the anterior HC (but not in the posterior HC), regional cerebral blood flow (rCBF) is abnormal (ie, reduced in the ACC and elevated in the HC) in schizophrenia. Third, the relationship of rCBF to task difficulty in the ACC is altered in schizophrenia, suggesting a failure of participation of the ACC in effortful tasks. Lastly, connectivity between the ACC and HC during the performance of an auditory discrimination task is also lacking, suggesting that cognitive performance in schizophrenia lacks a functional limbic contribution. On the basis of these changes, we studied the effects of antipsychotic drugs in these abnormal areas in persons with schizophrenia. Both first- and second-generation antipsychotics produce functional alterations in these limbic cortical areas, in the direction of normals, putatively acting through the brain's own cortical-subcortical circuits.
Keywords: schizophrenia, limbic cortex, anterior cingulate cortex, hippocampus, antipsychotic medication
Abstract
Los estudios acerca de la fisiopatología de la esquizofrenia utilizando datos postmortem, estructurales y funcionales han comprometido a la corteza límbica, especialmente en el hipocampo (HC) y en la corteza angulada anterior (CCA). Nosotros hemos realizado contribuciones a la literatura consistentes con esta idea: primero, nosotros describimos una correlación positiva significativa entre los síntomas psicótícos de la esquizofrenia y la actividad neuronal en la CCA y el HC, lo que sugiere el compromiso de la corteza límbica en la mediación de síntomas en la esquizofrenia. Segundo, en la esquizofrenia el flujo sanguíneo cerebral regional (FSCr) es anormal en la CCA y el HC anterior (pero no en el HC posterior); está reducido en la CCA y aumentado en el HC. Tercero, la relación entre la dificultad para realizar una tarea y el FSCr en la CCA se altera en la esquizofrenia, lo que sugiere una falla en la participación de la CCA en las tareas de gran esfuerzo. Finalmente, también existe una falta de conexión entre la CCA y el HC durante la ejecución de una tarea de discriminación auditiva, lo que sugiere que el rendimiento cognítívo en la esquizofrenia adolece de una contribución funcional del sistema límbico. En base a estos cambios, nosotros estudiamos los efectos de fármacos antipsicóticos en estas áreas anormales en personas con esquizofrenia. Tanto los antipsicóticos de primera como de segunda generación producen alteraciones funcionales en estas áreas de la corteza límbica, como ocurre en los normales, al actuar putativamente a través de los propios circuitos córtico-subcorticales del cerebro.
Abstract
Les études sur la physiopathologie de la schizophrénie ont impliqué le cortex limbique, sur la base de données post mortem, structurales et fonctionnelles, surtout dans l'hippocampe et le cortex cingulaire antérieur (CCA). Nos contributions à la littérature sont en accord avec cette notion; premièrement nous décrivons une corrélation positive significative entre les symptômes psychotiques dans la schizophrénie et l'activité neuronale dans l'hippocampe et le CCA, suggérant l'implication du cortex limbique dans la médiation des symptômes de la schizophrénie. Deuxièmement, dans le CCA et l'hippocampe antérieur (mais pas dans l'hippocampe postérieur), le débit sanguin cérébral régional (DSCr) est anormal dans la schizophrénie (c'est-à-dire, diminué dans le CCA et élevé dans l'hippocampe). Troisièmement, la relation dans le CCA entre le DSCr et la difficulté de la tâche est altérée au cours de la schizophrénie, ce qui suggère un déficit de participation du CCA dans les tâches difficiles. Enfin, la connexion entre le CCA et l'hippocampe au cours d'une tâche de discrimination auditive est également absente, évoquant un défaut de participation limbique fonctionnelle dans la performance cognitive au cours de la schizophrénie. Sur la base de ces modifications, nous avons étudié les effets des antipsychotiques dans ces zones anormales chez des schizophrènes. Les antipsychotiques de première et de seconde génération entraînent tous deux des modifications fonctionnelles dans ces zones corticales limbiques, s'approchant des résultats des sujets normaux, en agissant, a priori, par l'intermédiaire des propres circuits cérébraux corticaux-sous-corticaux.
Although the pathophysiology of schizophrenia remains unknown, clues about its mechanisms are emerging.1 It is one of the most studied human illnesses in the field of neuroscience. Moreover, the most sophisticated modern techniques have been brought to bear on answering its question: cellular and molecular techniques,2,3 genetics,4 and in vivo imaging.5 We know that it is a complex genetic illness with little gross pathology or replicated markers of dysfunction.
Investigators in our laboratory, among others, have been studying the localization of functional pathology in this illness. In the future, this information will allow a more detailed histological, cellular, and molecular examination of changes in those target regions. Moreover, it will provide an experimental framework for future studies of drug action and family studies.
Limbic cortex: the ACC and the HC
Our first suggestion that the limbic cortex could be a player in the functional pathology of schizophrenia came from the correlation that we identified between neuronal activity in the anterior cingulate cortex (ACC) and hippocampus (HC) (measured by [18F]deoxyglucosc positron emission tomography) and the magnitude of psychosis score (measured on the Brief Psychiatric Rating Scale [BPRS]) (r=0.590; P=0.03).This correlation between psychosis and neuronal activity was only obtained when the study volunteers were drug-free, but was entirely obscured
by antipsychotic medication. These findings suggest that the symptoms of psychosis, in this case the positive symptoms, are mediated in some way by these brain areas. Fortunately, this correlation between regional cerebral blood flow (rCBF) and schizophrenia symptoms falls in a brain region often noted to be abnormal in schizophrenia,5,8 increasing its face validity.
Moreover, in schizophrenia, the ACC and the HC show altered levels of neuronal activity when at rest and when performing a task relative to normals, so long as they are in a medication-free condition.9,10 During an auditory recognition task, where performance was carefully matched and the task trained between the schizophrenia and the normal volunteers, the only area that showed a significant difference from normal in task-activated neuronal activity was the ACC. In this case, rCBF was lower in the schizophrenia group.11
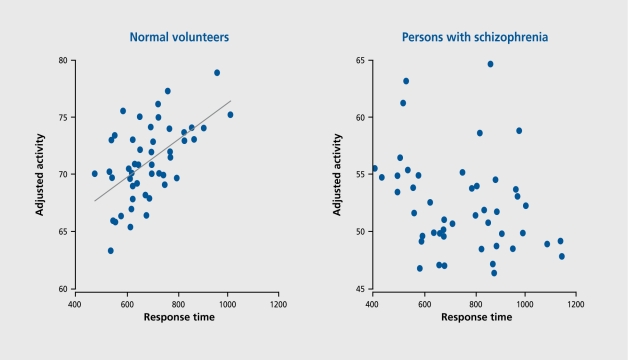
Not only was the magnitude of activation reduced, but also, in contrast to the normal volunteers, the activations were irregularly related to performance. In the normal group, there was a significant and positive correlation between task difficulty and rCBF in the ACC, a region critical to task performance. However, in the schizophrenia group, there was no such relationship; in fact, there was even a hint of an inverse correlation between rCBF and the level of task difficulty (Figure 1).
Figure 1. Correlations between performance and the anterior cingulate cortex (ACC) in normal volunteers and persons with schizophrenia.
The ACC lies on the medial surface of the frontal lobes, and the HC is on the medial surface of the temporal lobe. The HC is a small structure in terms of volume, but it plays a critical role in human learning and memory.12 In schizophrenia, the function of this structure is abnormal as measured by an increase in neuronal activity relative to the normal volunteer in the anterior region only, with the middle and posterior sections of the structure showing normal rCBF.10 Again, this difference in schizophrenia only appears in the medication-free condition, since treatment with an antipsychotic (either first- or secondgeneration) reduced this abnormal rCBF in the anterior HC.13 Moreover, when probed with noncompetitive N methyl-D-aspartate (NMD A) blockade, specifically ketamine, rCBF in the HC was reduced, whereas no change occurred with ketamine in normal volunteers (H. H. Holcomb, manuscript in preparation). This observation suggests that the hippocampal cortex in schizophrenia may lack a normal NMDA-antagonism buffer, making this region more susceptible to glutamate blockade at the NMDA receptor in the illness.
Functional connectivity in the limbic cortex
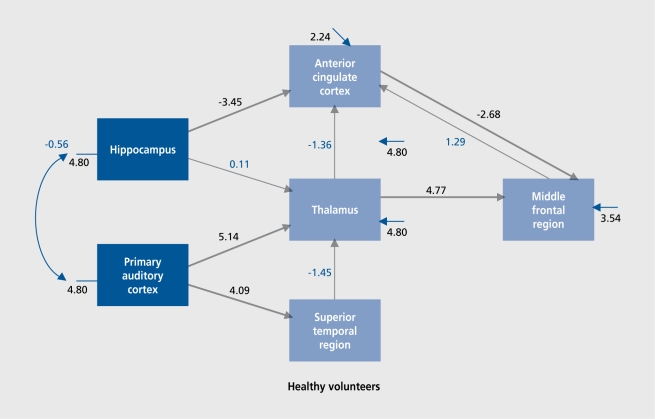
The data so far suggest functional abnormalities in both limbic cortical structures, the ACC and the HC. On the basis of these data, we hypothesize that the functional connectivity between structures would be altered. Therefore, we used a statistical technique called structural equation modeling (SEM) to test the connectivity within limbic cortex during the performance of an effortful task, an auditory discrimination task. We used scans acquired from 12 normal volunteers and 18 volunteers with schizophrenia during task performance and rest. First, by combining all scans (ie,both groups) into a single analysis, we defined task-activated regions. Then, using an exploratory factor analysis, we examined which regions showed a correlation with each other. These data, plus the information already known about connectivity with auditory cortex, were used to construct an a priori hypothesized circuit (albeit simplified), which could mediate the cerebral events associated with task performance. We tested this hypothesized circuit (Figure 2) for “activity” in mediating task performance in the healthy volunteer group and in the schizophrenia group (D. R. Medoff, manuscript in preparation).
Figure 2. Functional connectivity: hypothesized circuit.
In the normal volunteers, connectivity was evident between the primary auditory cortex, forward through the thalamus and to the middle frontal region, where most likely, the short-term memory aspects of the task were mediated. Also, the limbic cortex was significantly activated, connected within itself, and connected to the thalamocortical structures, to add a “top down” limbic component to the performance of the discrimination task. However, within the schizophrenia group, while there was evidence of an effective pathway carrying the auditory information forward through the thalamus to the middle frontal cortex, the limbic component, was missing. In schizophrenia, there was no significant connectivity within the limbic cortex (between the ACC and the HC) or between the limbic structures and the thalamocortical regions mediating the memory aspect of the task. Behaviorally, the group of volunteers with schizophrenia were actually performing the task equivalent.lv to the normal volunteers, but. apparently without the added benefit of their limbic cortex.
These data suggest, a failure of limbic activity in schizophrenia to coordinate with neocortical activation for the purpose of effortful task performance in schizophrenia. These results suggest, that persons with schizophrenia accomplish effortful mental behaviors without the benefit of normal limbic cortex activity, even when performing the behaviors equivalent to normals. This localization information will allow us in future studies to develop a focus on molecular and cellular abnormalities within these regions. It is this ability to focus human postmortem studies on brain regions likely to contain pathology that is one of the values of this kind of localization information. Moreover, we are proceeding to examine elements of the inhibitory (GABAergic [GABA, γ-aminobutyric acid]) and excitatory (glutamatergic, especially NMDA -mediated) systems in the limbic cortex in schizophrenia.3
Effects of antipsychotic medication
This idea that, functional pathology in schizophrenia can be captured in altered limbic cortex function raises the question of antipsychotic medication effects and the regions in which those actions are manifest. With haloperidol (the protypical first-generation antipsychotic), our research showed that increases in neuronal activity in the basal ganglia (caudate and putamen) and in the thalamus, particularly the anterior portion, were associated with haloperidol administration affecting the ACC and frontal cortex first in what we interpreted as a tertiary action.14 Regional decreases in neuronal activity were associated with haloperidol in the ACC and in the frontal cortex, particularly the middle and the inferior portions. When we evaluated clozapine (the prototypical second-generation drug), we saw that the common areas of activation with haloperidol were the caudate, the ventral striatum, and the anterior thalamus, and the common areas of inhibition were the HC and the ACC.9 These observations suggest, that antidopaminergic antipsychotic medications act. within the limbic and the limbic-related cortex to produce their antipsychotic action, ie, in the very regions that are dysfunctional in the disease itself. We have far to go in explaining what the medications are actually doing in these limbic regions (presumably, secondary to the dopamine receptor blockade in the ventral striatum). In addition to action in these common “antidopaminergic” regions, we saw greater activation in frontal ACC and medial frontal cortex with clozapine, perhaps accounting for its superior antipsychotic actions.
The “systems” approach in schizophrenia
These results emphasize the “systems” aspect of pathophysiology and therapeutics in schizophrenia. Schizophrenia is not merely an illness of a single brain region, and probably not. of any one neurotransmitter system. Rather, in persons with the illness, it. appears that an entire neural system (in our hands, the limbic system) behaves abnormally during mental tasks in association with disease symptoms, and abnormally influences the related neocor tical and subcortical brain. It is the ACC and the HC that seem to misfunction most regularly in psychotic states of the illness, at rest, and with task stimulation. Without having yet. identified specifically where or what, is the lesion, it can be said that changes in neuronal activity in the limbic pathways are associated with the symptoms of psychosis and cognitive change.
We have carried out the number of studies with the different techniques described above to discover and evaluate the systems component of cerebral activation, and compared normal and schizophrenia groups on rCBF parameters. These approaches have led us to conclude that limbic system function, especially around tasks of learning and memory, is abnormal in schizophrenia. It is our contention that, treatments for the illness also should be targeted toward a “system,” to correct the neuronal activity of an entire “psychosis” system in schizophrenia. The decades of research into the neural basis of Parkinson's disease has clarified much of the neural substrate used by dopamine to exert, its effects on frontal cortex through the basal ganglia thalamocortical (BGTC) circuits.15 Studies in humans of the actions of haloperidol on regional neuronal activity are consistent with the idea, that when antipsychotics act in striatum to block the dopamine D2 receptors, a signal is transmitted from the striatum, through the thalamus, to the frontal cortex, including particularly the anterior cingulate and the dorsolateral frontal.14 It is in these areas of frontal cortex where the full and final action of these antipsychotic drugs is probably exerted on cortically mediated human behaviors, but mediated through the BGTC circuit. Antipsychotics with additional monoaminergic and other actions probably exert these actions directly in the frontal cortex, in addition to the dopamine-mediated circuit actions. The latter extrastriatal effects may represent an important difference between first- and second-generation antipsychotic drugs.16
Dopamine and glutamate each appear to possibly have preferential systems of influence: the frontal cortexBGTC circuit for dopamine and the limbic circuit for glutamate. Especially the NMDA-sensitive glutamate receptor, when blocked with a noncompetitive antagonist, alters function primarily in limbic and frontal cortex (as measured by immediate early gene alterations in laboratory rodents in response to phencyclidine17 or by rCBF alterations in humans in response to ketamine).18 Thus, even if the initiation of this NMDA antagonist change is in the limbic cortex, the extensive influence of the limbic system on related neocortical and subcortical structures is so potent, that it alters function in frontal cortex and even in the limbic striatum when hippocampal firing changes. Thus, a convergent projection area of both of these systems - the frontal neocortex and limbic cortex - is common to both dopaminergic and NMDA-sensitive glutamatergic transmission. Thus, while dopamine and glutamate system pharmacologies are similar, each has its preferential primary action systems and each delivers its “information” to diverse brain regions in a highly interactive/overlapping fashion, through the welldescribed and existing neuronal circuits.
Conclusion
Schizophrenia is a disease of disordered mental productivity and organization, not of a single neurotoxic or neurodegenerative pathogen, and, can be formulated entirely as a neural systems disorder of the central nervous system (CNS).This suggests that, the function of the system overall, not of any single component, may be abnormal in the illness and could result, in the symptoms of the illness. Thus, we have formulated our current antipsychotic treatment actions as a systems approach to treating, not necessarily the primary pathology of schizophrenia, but the disordered system “output.” Because dopamine and glutamate strongly modulate neural systems that have overlapping tertiary targets (ie, the frontal cortex and limbic striatum), the same kind of pharmacological action (ie, “antipsychotic”) could be delivered to regions regulating the behaviors of the CNS, be they motor, cognitive, or affective, through, for example, the frontal cortex. Thus, it is not only a drug action at a regional target within the brain, but also, its overall action on a related neural system in the brain that determines its overall actions on neurally mediated behaviors and illnesses, like schizophrenia. Known antipsychotic drugs that block D2 receptors likely have their therapeutic action on functions of the frontal cortex, mediated through the BGTC neuronal circuit. Psychotomimetic agents like ketamine also appear to have their actions (antitherapeutic, in this case) within the limbic cortex, but. these actions also extend into the frontal cortical regions. It. would follow then that neither of these drug effects seems to be exerted primarily in the area of delivery, but as an indirect projection effect to frontal cortex from different, but overlapping, neuronal networks. The development, and testing of drugs that modulate the neuronal circuits of interest, for psychosis may provide novel approaches for modulating neuronal activity in the frontal cortex and provide novel kinds of antipsychotic effects.
REFERENCES
- 1.Tamminga CA., Thaker GK., Medoff DR. Neuropsychiatrie aspects of schizophrenia. In: Yudofsky SC, Hales RE, eds. The American Psychiatric Publishing Textbook of Neuropsychiatry and Clinical Neurosciences. Washington, DC: American Psychiatric Press; 2002:989–1020. [Google Scholar]
- 2.Benes FM., Khan Y., Vincent SL., Wickramasinghe R. Differences in the subregional and cellular distribution of GABAa receptor binding in the hippocampal formation of schizophrenic brain. Synapse. 1996;22:338–349. doi: 10.1002/(SICI)1098-2396(199604)22:4<338::AID-SYN5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 3.Gao XM., Sakai K., Roberts RC., Conley RR., Dean B., Tamminga CA. Ionotropic glutamate receptors and expression of A/-methyl-D-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. Am J Psychiatry. 2000;157:1141–1149. doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- 4.Egan MF., Goldberg TE., Kolachana BS., et al. Effect of COMT Val108/158Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heckers S., Rauch SL., Goff D., Savage CR., Schacter DLFAJ., Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nature. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- 6.Harrison PJ., Eastwood SL. Preferential involvement of excitatory neurons in medial temporal lobe in schizophrenia. Lancet. 1998;352:1669–1673. doi: 10.1016/S0140-6736(98)03341-8. [DOI] [PubMed] [Google Scholar]
- 7.Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122(Pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 8.Benes FM. Neurobiological investigations in cingulate cortex of schizophrenic brain [Review]. Schizophr Bull. 1993;19:537–549. doi: 10.1093/schbul/19.3.537. [DOI] [PubMed] [Google Scholar]
- 9.Holcomb HH., Lahti AC., Weiler M., Medoff DR., Tamminga CA. Neuroleptic treatment of schizophrenic patients: how do haloperidol and clozapine normalize brain blood flow patterns associated with a difficult tone recognition task? In: Gattz WF, Hafner H, eds. Search for the Causes of Schizophrenia. Vol IV. Balance of the Century Darmstadt, Germany: Steinkopff Verlag; 1999:355–365. [Google Scholar]
- 10.Medoff DR., Holcomb HH., Lahti AC., Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001:543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- 11.Holcomb HH., Lahti AC., Medoff DR., Weiler M., Dannals RF., Tamminga CA. Brain activation patterns in schizophrenic and comparison volunteers during a matched-performance auditory recognition task. Am J Psychiatry. 2000;157:1634–1645. doi: 10.1176/appi.ajp.157.10.1634. [DOI] [PubMed] [Google Scholar]
- 12.Martin SP., Grimwood PD., Morris RGM. Synaptic plasticity and memory: an evaluation of the hypothesis. In: Cowan WM, Shooter EM, Stevens CF, Thompson RF, eds. Annual Review of Neuroscience. Palo Alto, Calif: Annual Reviews; 2000:649–712. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 13.Lahti AC., Holcomb HH., Weiler MA., Medoff DR., Tamminga CA. Functional effects of antipsychotic drugs: comparing clozapine with haloperidol. Biol Psychiatry. In press. doi: 10.1016/s0006-3223(02)01602-5. [DOI] [PubMed] [Google Scholar]
- 14.Holcomb HH., Cascella NG., Thaker GK., Medoff DR., Dannals RF., Tamminga CA. Functional sites of neuroleptic drug action in the human brain: PET/FDG studies with and without haloperidol. Am J Psychiatry. 1996;153:41–49. doi: 10.1176/ajp.153.1.41. [DOI] [PubMed] [Google Scholar]
- 15.Alexander GE., DeLong MR., Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex [Review], Annu RevNeurosci. 1986;9:357–381 . doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 16.Tamminga CA. Principles of the pharmacotherapy of schizophrenia. In: Bunney BS, ed. Neurobiology of Psychiatric Disorders. New York, NY: Oxford University Press; 1998:272–285. [Google Scholar]
- 17.Gao XM., Hashimoto T., Tamminga CA. Phencyclidine (PCP) and dizocilpine (MK801) exert time-dependent effects on the expression of immediate early genes in rat brain. Synapse. 1998;29:14–28. doi: 10.1002/(SICI)1098-2396(199805)29:1<14::AID-SYN2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 18.Lahti AC., Koffel B., LaPorte D., Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]