Abstract
Despite the growing means devoted to research and development (R α D) and refinements in the preclinical stages, the efficiency of central nervous system (CMS) drug development is disappointing. Many drugs reach patient studies with an erroneous therapeutic indication andlor in incorrect doses. Apart from the first clinical studies, which are conducted in healthy volunteers and focus only on safety, iolerability, and pharmacokinetics, drug development mostly relies on patient studies. Psychiatric disorders are characterized by heterogeneity and a high rate of comorbidity. It is becoming increasingly difficult to recruit patients for clinical trials and there are many confounding factors in this population, for example, those related to treatments. In order to keep patient exposure and financial expenditure to a minimum, it is important to avoid ill-designed and inconclusive studies. This risk could be minimized by gathering pharmacodynamic data earlier in development and considering that the goal of a phase 1 plan is to reach patient studies with clear ideas about the compound's pharmacodynamic profile, its efficacy in the putative indication (proof of concept), and pharmacokinetic/pharmacodynamic relationships, in addition to safety, tolerability, and pharmacokinetics. Human models in healthy volunteers may be useful tools for this purpose, but their use necessitates a global adaptation of the phase scheme, favoring pharmacodynamic assessments without neglecting safety. We are engaged in an R α D program aimed to adapt existing models and develop new paradigms suitable for early proof of concept substantiation.
Keywords: drug development, proof of concept, model, healthy volunteer, Alzheimer's disease, anxiety, depression, schizophrenia
Abstract
A pesar de los crecientes recursos dedicados a la investigación y desarrollo, y al refinamiento en las etapas preclinicas, la eficiencia del desarrollo de fármacos para el sistema nervioso central (SNC) ha desilusionado, Muchos fármacos llegan a esiudiarse en pacientes con una indicatión terapéutica errónea yfo en dosis incorrectas. Además de los primeros estudios clinicos que se realizan en voluntaries sanos y que se enfocan sólo a la seguridad, tolerancia y farmacocinética, el desarrollo de fármacos depende principalmente de los estudios en pacientes. Los trastornos psiquiátricos se caracterizan por su heterogeneidad y la alta proporción de comorbilidad. Cada vez es más dificil reclutar pacientes para ensayos clinicos y hay muchos factores de confusión en esta población; por ejemplo, los relacionados con los tratamientos. A fin de reducir al minimo la exposición del paciente y el gasto financiero, es importante evitar estudios mal diseñados y no concluyentes. Este riesgo podria ser minimizado al obtener datos precozmente durante el desarrollo y al considerar que el objetivo en la fase 1 del plan es llegar a los estudios en pacientes con las ideas claras acerca del perfil farmacodinámico del compuesto, su eficacia en la probable indicación (proof of concept), y relaciones entre farmacodinámica y farmacocinética, además de la seguridad, tolerancia y farmacocinética, Los modelos humanos en voluntarios sanos pueden constituir herramientas utiles para este propósito, pero su empleo necesita de una adaptación global del esquema de la fase 1, favoreciendo las evaluaciones farmacodinámicas sin descuidar la seguridad. Nosotros estamos comprometidos en un programa de investigación y desarrollo orientado a adaptar modelos existentes y a desarrollar nuevos paradigmas que se adecuen a una precoz verificación de la proof of concept.
Abstract
Malgré l'augmentation des moyens consacrés à la recherche et au développement (R et D) et au perfectionnement des phases précliniques, l'efficacité du développement des médicaments du système nerveux central (SNC) est décevante. De nombreux médicaments atteignent le stade d'étude chez le sujet malade avec une indication thérapeutique fausse et/ou des doses incorrectes. En dehors des premières études cliniques, qui sont conduites chez le volontaire sain et mettent l'accent uniquement sur la sécurité, la tolérance et la pharmacocinétique, le développement des médicaments repose principalement sur les études cliniques chez le sujet malade. Les troubles psychiatriques se caractérisent par une hétérogénéité et un taux élevé de comorbidité. Le recrutement de patients pour des essais cliniques devient de plus en plus difficile et il existe de nombreux facteurs confondants dans cette population, par exemple ceux liés aux traitements. Il est important d'éviter les études mal conçues et peu concluantes pour limiter au maximum l'exposition des patients et les dépenses financières. Ce risque peut être minimisé en recueillant des données pharmacodynamiques plus tôt au cours du développement et en considérant que l'objectif des études de phase 1 est d'aboutir à des études chez le sujet malade avec des idées claires en ce qui concerne le profil pharmacodynamique du composé, son efficacité dans l'indication probable (preuve de concept), et les relations pharmacocinétiquelpharmacodynamie, en plus de la sécurité, de la tolérance et de la pharmacocinétique, À cet effet, les modèles humains chez le volontaire sain peuvent être un outil utile, mais leur utilisation nécessite une adaptation globale du schéma de phase 1, favorisant les évaluations pharmacodynamiques sans négliger la sécurité. Nous sommes engagés dans un programme de R et D visant à adapter les modèles existants et à développer de nouveaux paradigmes capables d'établir à un stade précoce la preuve de concept.
Phase 1 studies constitute a pivotal step in drug development. TTicir goal is to gather enough information to warrant the scientific value of phase 2 studies. The information to be collected includes the pharmacological actions of the drug, its side effects with increasing doses, its pharmacokinetics (PK) and metabolism, its mechanisms of action, and, if possible, early evidence of effectiveness.1 'The classic method of conducting phase 1 studies is much more limited (Table I). First-time-in-man, single -dose, and repeated-dose studies are carried out in healthy volunteers (HV), according to a parallel, double -blind (DB), placebocontrolled design. They are focused on PK, safety, and tolerability, seeking the maximal tolerated dose (MTD), which will be the basis for the choice of doses in subsequent patient studies. Using this scheme, many drugs have been developed in the wrong indication2 or using inappropriate doses,3 which led to failures or irrelevant studies, which then had to be replicated leading to delays, increased costs, and overexposure of patients to drugs.
It seems clear that, gathering data on pharmacodynamics (PD) and PK/PD relationships earlier would minimize these risks, bearing in mind that, in any case, further steps will face other major issues such as patient heterogeneity and placebo response.
Table I. Three ways of conducting phase 1 studies. MTD, maximal tolerated dose; PK, pharmacokinetics; PD, pharmacodynamics; BBB, blood-brain barrier. *Basic PD includes BBB crossing, minimal active dose, dose effect, and non-central nervous system (CNS) PD. **Basic PD without BBB crossing. ***Refined PD (model): proof of concept, minimal active dose, dose effect, and non-CNS PD.
| Classic | curent (usual) | Echanced | |
| Single-dose study | |||
| Population | Young, healthy volunteers | Young, healthy volunteers | Young, healthy volunteers |
| Design | Parallel, double-blind, vs placebo | Parallel, double-blind, vs placebo | Parallel, double-blind, vs placebo |
| Objectives |
|
|
|
| Repeated-dose study | |||
| Population | Healthy volunteers | Healthy volunteers | Healthy volunteers |
| Design | Parallel, double-blind, vs placebo | Parallel, double-blind, vs placebo | Grossover, double-blind, vs placebo |
| Objectives |
|
|
|
| Next step | |||
| Population | Patients | Health volunteers | Patients |
| Design | Parallel, double-blind, vs placebo | Grossover, double-blind, vs placebo | Parallel, double-blind, vs placebo |
| Objectives |
|
|
|
Our usual way of conducting phase 1 studies takes these needs into account (Table II). As early as in the first-inman study, in addition to PK and safety/tolerability evaluation, we collect, basic, central nervous system (CNS) PD data, as well as peripheral PD data (eg, evidence of blood-brain barrier crossing, QTc or cardiac rhythm changes, minimal active dose, and dose effect), and attempt to sketch PK/PD relationships. This information is expanded in repeated-dose studies, which can be followed by PD studies in HV, conducted according to a crossover, DB, placebo-controlled design and using the most, appropriate tools, such as wake or sleep electroencephalography (EEG), cognition or functional imaging according to the molecule and its putative indication (see, for example, references 4 to 10). This allows patient studies to be undertaken with a better knowledge of the drug profile and the most appropriate doses.
In the last years, the necessity for a proof of concept (POC) approach has emerged. Simply stated, POC means that at some stage of the development, before launching large patient studies, it should be demonstrated that the drug does what it is supposed to do. Classically, POC studies are patient studies. Psychiatric disorders are highly heterogeneous syndromes, with a high rate of comorbidity (eg, dementia or schizophrenia coexist with affective or anxiety disorders, affective disorders with anxiety disorders, different anxiety disorders together, etc). Therefore, essentially two strategies are possible to improve patient recruitment. The first is to recruit, small, homogeneous groups of highly characterized patients (no comorbidity, homogeneous symptomatic profile, imaging or biological characteristics, such as genotype, if applicable, etc), which is an ambitious and lengthy operation. The second is to increase the sample size, with the hope that, number will compensate for heterogeneity. This also makes the trial longer and more expensive, and there is no guarantee that. the compensation will be obtained. In addition, concern about, withdrawing an ongoing treatment on the part of ethics committees, physicians, nurses, families, and patients further hampers recruitment.
Studies in HVs have advantages that, compensate for these difficulties. HVs arc easier to recruit. They make more homogeneous - or less heterogeneous - populations than patients and, since more subjects are available, homogeneity can be improved through specific selection criteria. By definition, these subjects are healthy, have a lower risk of complications, and tolerate procedures better. In addition, they have no expectations about, the treatment, which can minimize placebo/nocebo effects. By definition, they arc also volunteers and get paid, and are thus more compliant.
Conducting POC studies in HVs implies the recourse to models or symptom provocation. These challenges can be understood as the provocation not of a complete clinical picture, but of some core signs and symptoms. The goal can be to produce and study functional markers because they are often more sensitive - and hopefully more reliable - than clinical signs and symptoms. Therefore, it is possible to use less stressful provocative procedures, which further increases subjects' comfort and compliance. Symptom provocation in HVs must comply with some basic rules (Table II), as stated by D'Souza et al.11 In the context of drug development, a model must, also induce target signs and symptoms in a reasonable proportion of HVs. 'These signs and symptoms must be accompanied with reliable functional changes, which can be used as biomarkcrs and display low intcrindividual and mostly intraindividual variability, to warrant good test-retest reliability and permit, the assessment, of drugs effects in a crossover, placebo-controlled design.
Table II . Criteria to justify symptom provocation in humans.11 .
|
|
|
|
Although the principle of a POC approach in HVs is appealing, we must be cautious in putting it into practice. Even when the provocation procedure is simple (eg, a single agent with well-known neurochemical properties, in the case of a pharmacological model), the totality of the neurochemical consequences of its administration are seldom known. The same holds for the new compound studied and for its interaction with the challenge. As a consequence, a positive result (ie, reversal or prevention of the challenge's effects by the new drug) is undoubtedly a clue to efficacy, but. a negative result can hardly be taken as the basis for a “no-go” decision. This often makes pharmaceutical companies reluctant to add a POC study in HVs to their development plan, arguing that, in case of negative results, it could merely delay it. and increase costs. In fact, introducing POC studies in HVs implies further enhancing the global phase 1 scheme (Table I). In this “enhanced development plan,” the single-dose study has the same design and goals as the regular one. The repeated-dose study merges the former repeated-dose and PD HV studies, ie, it. is conducted according to a crossover (per dose), placebo-controlled design. Provided that a single administration study has shown good tolerability in an HV group close to the target population (eg, elderly HVs for cognitive enhancers), this study can be conducted in such a group. A model, if available, can also be used in this study, by adding an administration the day after the classic PK and PD assessments. This avoids wasting HVs and resources, and maximizes the chances of positive results; however, it requires paying close attention to tolerability and safety, which must, be verified before a challenge is added to a repeated-administration study.
Models at FORENAP
Few models are available for routine use in drug development. We have launched a program to adapt existing models for this purpose (Table III), following the principles described above. Below we discuss the rationale for each of these models, as well as preliminary results when available, at. least those which are not covered by confidentiality agreements.
Table III . Models available or in development at FORENAP. AD, Alzheimer's disease; CCK-4, cholecystokinin tetrapeptide; EEG, electroencephalography; ERP, event-related potential; fMRI, functional magnetic resonance imaging; MEG, magnetoencephalography.
| Indication | Method | Marker | Status |
| AD/cognition | |||
| Scopolamine | EEG/ERPs | Routine | |
| Lorazepam | EEG/ERPs | Routine | |
| Low-dose ketamine | EEG/ERPs | Validation underway | |
| Nonpharmacological method | fMRI | Validation underway | |
| Anxiety | |||
| Panic | CCK-4 | fMRI | Validation underway |
| Anticipatory | Behavioral | fMRI | validation underway |
| Depression | |||
| Tryptophan depletion | Sleep EEG | validation completed | |
| Schizophrenia | |||
| Apomorphine | EEG | Routine | |
| Ketamine | EEG/MEG | validation underway |
Alzheimer's disease and age-related cognitive impairment
The scopolamine model
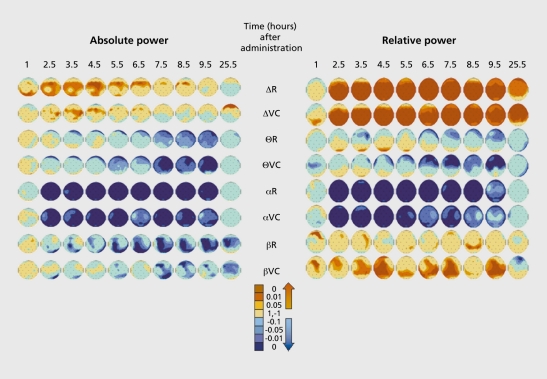
The scopolamine model is based on the cholinergic hypothesis of aging and Alzheimer's disease (AD). Its theoretical drawback is that scopolamine is a nonselective muscarinic blocker, whereas selective muscarinic Mr blockade could be considered to better modelize the status of the cholinergic system in AD.12 Nevertheless, it is a well-established model, producing cognitive defects close to those observed in mild AD and FRG changes consisting of an increase in 5 and - to a lesser extent - 6 bands, and a decrease in a and p power.13 The scopolamine model has been widely used in clinical experimental pharmacology,14-23 as well as in the assessment, of cognitive enhancers.24-40 In our hands, a 0.5-mg subcutaneous injection of scopolamine in young HVs induced impairment, in immediate and delayed word recall, multiple choice reaction time and accuracy, and the digit symbol substitution test. In quantified EEG, it. reduced total power and induced an increase in S and a decrease in 6, a, and p absolute power; in relative power analysis, the 8 and p band activity was increased and that of the 6 and a bands decreased (Figure 1.) An interesting feature of this model is that is can be reversed or prevented not. only by cholinomimetic drugs, but. also, as we have found, by compounds without direct, cholinergic effects.30,31,38,41
Figure 1. Effect of scopolamine (0.5 mg subcutaneously)on electroencephalogram (EEG) in 12 healthy young men. Placebo and scopolamine were administered according to a crossover, double-blind design. EEG was recorded from 28 electrodes during the first 3 min in vigilance-controlled conditions, and then at rest. Analog filtering 1 to 70 Hz, 12 dB/octave; digitalization with a 256-Hz sampling frequency; fast Fourier transform on each 2-s artifact-free epochs. δ, 0.5-3.5 Hz; Θ, 4-7.5 Hz; α, 8-12.5 Hz; β, 13-32 Hz. Maps displayed are P values after scopolamine-placebo comparison. Scopolamine reduced total power (not shown) and induced an increase in 6 and a decrease in Θ, α, and β absolute power; in relative power analysis, the δ and β bands' activity was increased and that of the Θ and a bands decreased. R, at rest; VC, in vigilance-controlled conditions.
The lorazepam model
Benzodiazepines (BZDs) are known to induce sedation, psychomotor impairment, and anterograde amnesia, leaving retention and retrieval spared.42 Although cognitive impairment is a class effect, differences between different BZDs have been reported, independently of their elimination half-lives.43-45 A dissociation between the cognitive and sedative effects of drugs has also been described.46 Lorazepam has dose-related memory- and attention-impairing effects.43,44,47 It has been suggested48 that the profile of lorazepam-induced cognitive impairment is close to that observed in Korsakoff's syndrome, whereas scopolamine rather mimics AD. Some studies47,49 were unable to distinguish the effects of lorazepam from those of scopolamine. Both drugs were shown to have similar effects on verbal priming50 and in a face-name associative encoding task,51 and as well as on associated functional magnetic resonance imaging (fMRI) activation patterns. On the other hand, differential effects were found on logical reasoning, immediate and delayed recall,52 and priming for human faces.53
BZDs have well-known effects on EEG. Changes in p amplitudes seem to reflect their interaction and intrinsic efficacy at the GABAA-BZD (GABA, y-aminobutyric acid) receptor complex; their effects on p and a activity their anxiolytic, anticonvulsant, and sedative properties; and 8-induced changes their hypnotic action.54
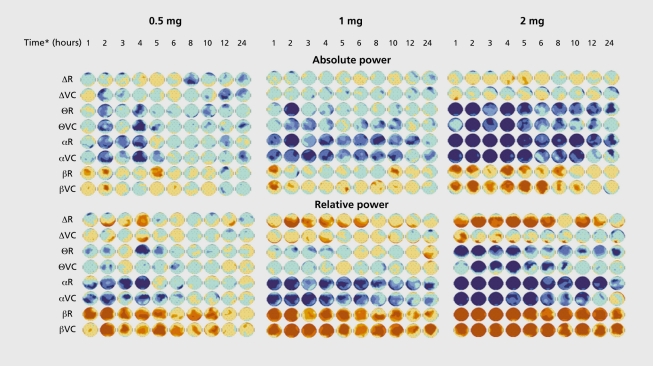
In our hands, an oral dose of 2 mg lorazepam impaired immediate and delayed word recall, multiple choice reaction time and accuracy, and digit, symbol substitution test, but. had no effect, on flicker fusion frequency. In quantified EEG, lorazepam's effects were dose -dependent in length and intensity, increasing 8 and P power, and decreasing the power of the 6 and a frequency bands (Figure 2.)
Figure 2. Effects of three oral doses of lorazepam on electroencephalogram (EEG) in 20 young healthy male volunteers. Lorazepam 0.5 mg, 1 mg, and 2 mg, and placebo were administered according to a crossover, double-blind design. Maps displayed are P values after lorazepam-placebo comparison. In absolute power analysis, lorazepam dose-dependently decreased Θ and α power, and increased β activity. Relative power disclosed an additional effect on δ power which was, also dose-dependently, increased. See Figure 1 for methods and maps color code. *Time post -administration. R, at rest; VC, in vigilance-controlled conditions.
The low-dose ketamine model
Ketamine infusion produces positive, negative, and cognitive symptoms reminiscent, of those observed in schizophrenia.55-65 A hypoglutamatcrgic state has also been proposed as the substratum of late-stage AD.66 Studies focused on ketamine-induced cognitive impairment, should separate the latter from the psychotomimetic effects of ketamine, which is possible using lower doses.64
Nonpharmacological approaches
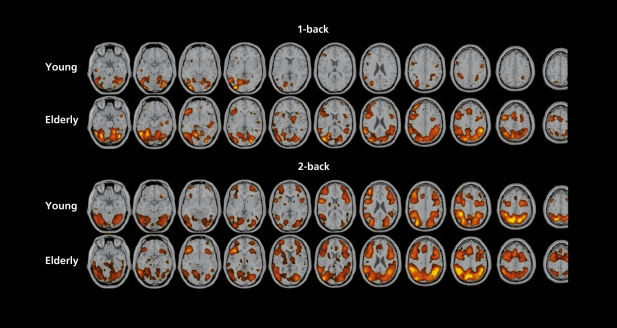
Functional (positron emission tomography [PET] and fMRI) studies on the neural correlates of cognitive aging basically describe two cases.67 In one, performance and brain activation during the task are lower than in young controls; this is also the case for episodic memory and conflict, resolution tasks. The second consists of preserved performances associated with enlarged activation, engaging more brain regions, such as during working memory tasks. Our fMRI activation maps, obtained during a spatial “n-back” working memory challenge are in agreement, with these data (Figure 3.) Our hypothesis is that activation patterns in elderly volunteers should be closer to those of young volunteers after administration of a cognitive enhancer. Indeed, PPT scan and fMRI studies in young volunteers have shown that physostigmine infusion improved working memory performances and reduced task -related activation.68-70
Figure 3. Statistical parametric maps (SPMs) of the group analysis (4 young and 4 elderly healthy male volunteers, 3 functional magnetic resonance imaging [fMRI] exams per subject) during “n-back” spatial working memory task versus control. In the control condition (“O-back”), subjects had to respond when a square appeared in a given position on the computer screen; in the “1-back” condition, when it appeared in the same place as 1 screen before; in the “2-back” condition when it appeared in the same place as 2 screens before. In both the 1 -back and the 2-back tasks, accuracy score was similar in elderly and young subjects. Reaction time was similar in the two groups during the 1 -back task, but significantly longer in the elderly group during the 2-back task. Significant activity (t-score>4) is displayed upon anatomical axial T1 images after being transformed to standardized Talairach space.
Anxiety
Panic attack model: CCK-4
The idea of using cholecystokinin tetrapeptide (CCK-4) as a panic probe came from experiments showing that BZDs antagonized CCK-8S in the rat,71 as well as from the serendipitous finding that a 70-ug CCK-4 injection produced panic-like feeling in healthy humans.72 In subsequent studies,73-91 CCK-4 induced panic attacks in 0% to 70% of HVs and these attacks were quantitatively and qualitatively similar to those reported by patients. Attack incidence and severity of symptoms were dose-dependent, although discordant results have been described with the same dose and a considerable overlap exists in the rate of response to different doses. The dose of 50 ug seems to give the most homogeneous response rate, ranging from 47% to 65%. Test-retest reliability has been poorly assessed. Two studies - although not specifically designed for this purpose - reported a decrease in the number and intensity of panic symptoms,79,88 as well as in the incidence of panic attacks.79
In HVs, lorazepam prevented CCK-4-induced panic,73 as did the CCK-4 receptor antagonist. CI988,80 propranolol,87 ondansetron after acute but not repeated administration,88 atrial natriuretic peptide,89 and vigabatrin.90 PET scan studies found an increased cerebral blood flow concomitant of anxiety response in the claustrum-insularamygdala region bilaterally and in cerebellar vermis and anterior cingulate gyrus.92,93 Our fMRI results confirm these data.
Anticipatory anxiety: behavioral model
Recent research suggests that the neurophysiological mechanisms underlying anxiety disorders are closely related - if not identical - to those underlying the emotion of fear.94 This provides the rationale for using behavioral models based on fear induction or anticipation of an avcrsive stimulus. In behavioral models, an anxious state is induced by presentation of stimuli having an aversive emotional content, via any sensory modality. A major drawback of using intrinsically fearful stimuli is that, the fear or aversion elicited can vary according to volunteers' traits and experiences. Aversive conditioning (in which an emotionally neutral or conditioned stimulus [CS] is paired with an aversive - or unconditioned - one [UCS], usually in different sensory modalities) allows for a more homogeneous response within the subject population by adjusting the aversive nature of the UCS on an individual basis. Although amygdala activation is considered to be central to anticipatory anxiety,94-96 other regions are also activated during classical conditioning, eg, right, orbitofrontal, dorsolateral prefrontal, inferior and superior frontal, inferior and middle temporal cortices, and left superior frontal cortices,97,98 anterior cingulatc, and insula,99 according to the paradigm used. The study of many regions together can lessen the consequences of “missing” the amygdaloid complex activation, which is transient even when the UCS continues to be presented in association with CS.95,98,99 Our results confirm the merits of this approach.
Depression: the tryptophan depletion challenge
The rationale and results of a recent study with this model are given elsewhere in this volume.100
Schizophrenia
The apomorphine model
For decades, dopamine transmission abnormalities have been thought to be involved in the pathophysiology of schizophrenia,101 justifying the stimulation of dopaminergic pathways as a model of schizophrenia in HVs. Apomorphine, a nonselective dopaminergic agonist, has a rapid phase of absorption and distribution in the periphery (20 min) as well as the brain compartment. (30 min) in humans102 and is an ideal pharmacological tool because it has minor psychotropic effects in both HVs and psychiatric patients. We have characterized apomorphineinduced topographic changes in neurophysiological markers using a 28-lead multielectrode montage in HVs. To ensure that, observed modifications are of central and not of peripheral origin, subjects were pretrcated with domperidone, a dopamine antagonist that does not cross the blood-brain barrier. We assessed drug-induced modifications in EEG/event-related potential measurements at different time points after subcutaneous injection of apomorphine.103 As expected, the effects of apomorphine on EEG were partially opposite to those reported for classic104 and atypical105,106 neuroleptics, with an increase in fast P power and decrease in 6 relative power. This model was validated using haloperidol, which antagonized the acute effects of apomorphine.107
The ketamine model
N-Methyl-D-aspartate (NMDA) receptor blockade by ketamine infusion in HVs is acknowledged to be a good model of schizophrenia, reproducing positive, negative, and cognitive symptoms.55-65 Despite evidence that ketamine modulates dopamine striatal concentration,108-111 its clinical effects were not reversed by haloperidol in patients112 or in HVs,61 or olanzapine,113 but were blunted by clozapine in patients with schizophrenia.114 This inconsistent effect of antipsychotics could be dose-related. The above studies used ketamine doses of 0.1 to 0.9 mg/kg in bolus or 1-h infusion, whereas we use 0.16 to 0.54 mg/kg in a 2-h infusion.
Conclusion
There is an agreement on the need to increase the efficiency of drug development. Whatever the improvements in the chemical and preclinical steps, clinical development strategy remains critical. Human models in HVs are obviously not a panacea. They are not applicable to any situation and the validity of the different provocation procedures is uneven. Their optimal use is within what we call an “enhanced development plan,” which requires improvements in safety data processing. Nevertheless, when properly used, human models can secure phase 1 study results, be of help in a “go” (more than in a “no-go”) decision, and therefore improve the safety and efficiency of patient studies, leading to a reduction in both time and resources.
Selected abbreviations and acronyms
- AD
Alzheimer's disease
- BZD
benzodiazepine
- DB
double-blind (study)
- fMRI
functional magnetic resonance imaging
- HV
healthy volunteer
- MTD
maximal tolerated dose
- PD
pharmacodynamics
- PK
pharmacokinetics
- POC
proof of concept
Contributor Information
Christian Gilles, FORENAP, Institute for Research in Neuroscience and Neuropsychiatry, Rouffach, France.
Thérèse Schunck, FORENAP, Institute for Research in Neuroscience and Neuropsychiatry, Rouffach, France.
Gilles Erb, FORENAP, Institute for Research in Neuroscience and Neuropsychiatry, Rouffach, France.
Izzie Jacques Namer, FORENAP, Institute for Research in Neuroscience and Neuropsychiatry, Rouffach, France.
Yann Hodé, FORENAP, Institute for Research in Neuroscience and Neuropsychiatry, Rouffach, France.
Jean-François Nedelec, FORENAP, Institute for Research in Neuroscience and Neuropsychiatry, Rouffach, France.
Peter Boeijinga, FORENAP, Institute for Research in Neuroscience and Neuropsychiatry, Rouffach, France.
Remy Luthringer, FORENAP, Institute for Research in Neuroscience and Neuropsychiatry, Rouffach, France.
Jean-Paul Mâcher, FORENAP, Institute for Research in Neuroscience and Neuropsychiatry, Rouffach, France.
REFERENCES
- 1.Food and Drug Administration. www.fda.gov/cder/handbook/phase1.htm. FDA: Rockville, Md. Accessed 2003 Jan 30; [Google Scholar]
- 2.Rapeport G. Proof of concept in big pharma and the biotechnology industry. 6th EUFEPS Conference, Basel, Switzerland. 1999 November 30 to December 2 [Google Scholar]
- 3.Rolan P. The contribution of clinical pharmacology surrogates and models to drug development - a critical appraisal. Br J Pharmacol. 1997;44:219–225. doi: 10.1046/j.1365-2125.1997.t01-1-00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.d'Ardhuy XL., Boeijinga P., Renault B., et al. Effects of serotonin-selective and classical antidepressants on the auditory P300 cognitive potential. Neuropsychobiology. 1999;40:207–213. doi: 10.1159/000026621. [DOI] [PubMed] [Google Scholar]
- 5.Luthringer R., Dago KT., Patat A., et al. Pharmacoelectroencephalographic profile of bef loxatone, a new reversible MÂO-A inhibitor, in healthy subjects. Neuropsychobiology. 1996;34:98–105. doi: 10.1159/000119299. [DOI] [PubMed] [Google Scholar]
- 6.Boeijinga P., Muzet M., Gamand S., d'Aniello F., Luthringer R., Mâcher JP. Double-blind, randomized, placebo-controlled study of the effects on wake EEG of 2 doses of Praxilene* using a cross-over design in elderly healthy male volunteers. Eur Neuropsychopharmacol. 2001;10 (suppl 3):S363. [Google Scholar]
- 7.Staner L., Luthringer R., Mâcher JP. The effects of antidepressant drugs on sleep EEG in major depression: mechanisms and therapeutic implications. CNS Drugs. 1999;11:49–60. [Google Scholar]
- 8.Luthringer R., Toussaint M., Schaltenbrand N., et al. A double-blind, placebo-controlled evaluation of the effects of orally administered venlafaxine on sleep in inpatients with major depression. Psychopharmacol Bull. 1996;32:637–646. [PubMed] [Google Scholar]
- 9.Bolo N., Nedelec JF., Muzet M., et al. Central effects of acamprosate. Part 2. Acamprosate modifies the brain in-vivo proton magnetic resonance spectrum in healthy young male volunteers. Psychiatry Res. 1998;82:115–127. doi: 10.1016/s0925-4927(98)00017-1. [DOI] [PubMed] [Google Scholar]
- 10.Bolo N., Hode Y., Nedelec JF., Laine E., Wagner G., Mâcher JP. Brain pharmacokinetics and tissue distribution in vivo of f luvoxamine and fluoxetine by fluorine magnetic resonance spectroscopy. Neuropsychopharmacology. 2000;23:428–438. doi: 10.1016/S0893-133X(00)00116-0. [DOI] [PubMed] [Google Scholar]
- 11.D'Souza DC., Berman RM., (Crystal JH., Charney DS. Symptom provocation studies in psychiatric disorders: scientific value, risks, and future. Biol Psychiatry. 1999;46:1060–1080. doi: 10.1016/s0006-3223(99)00209-7. [DOI] [PubMed] [Google Scholar]
- 12.Gilles C., Ertlé S. Pharmacological models in Alzheimer's disease research. Dialogues Clin Neurosci. 2000;2:247–255. doi: 10.31887/DCNS.2000.2.3/cgilles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebert U., Kirch W. Scopolamine model of dementia: electroencephalogram findings and cognitive performance. EurJ Clin Invest. 1998;28:944–949. doi: 10.1046/j.1365-2362.1998.00393.x. [DOI] [PubMed] [Google Scholar]
- 14.Richardson JS., Miller PS., Lemay JS., et al. Mental dysfunction and the blockade of muscarinic receptors in the brain of the normal elderly. Prog Neuropsychopharmacol Biol Psychiatry. 1985;9:651–654. doi: 10.1016/0278-5846(85)90034-x. [DOI] [PubMed] [Google Scholar]
- 15.Sunderland T., Tariot P., Murphy DL., Weingartner H., Mueller EA., Cohen RM. Scopolamine challenges in Alzheimer's disease. Psychopharmacology. 1985;87:247–249. doi: 10.1007/BF00431817. [DOI] [PubMed] [Google Scholar]
- 16.Sunderland T., Tariot PN., Cohen RM., Weingartner H., Mueller EA III., Murphy DL. Anticholinergic sensitivity in patients with dementia of the Alzheimer type and age-matched controls. A dose-response study. Arch Gen Psychiatry. 1987;44:418–426. doi: 10.1001/archpsyc.1987.01800170032006. [DOI] [PubMed] [Google Scholar]
- 17.Zemishlany Z., Thorne AE. Anticholinergic challenge and cognitive functions: a comparison between young and elderly normal subjects, far. J Psychiatry Re/at So. 1991;28:32–41. [PubMed] [Google Scholar]
- 18.Flicker C., Ferris SH., Serby M. Hypersensitivity to scopolamine in the elderly. Psychopharmacology. 1992;107:437–441. doi: 10.1007/BF02245172. [DOI] [PubMed] [Google Scholar]
- 19.Gitelman DR., Prohovnik I. Muscarinic and nicotinic contributions to cognitive function and cortical blood flow. Neurobiol Aging. 1992;13:313–318. doi: 10.1016/0197-4580(92)90044-x. [DOI] [PubMed] [Google Scholar]
- 20.Ray PG., Meador KJ., Loring DW., Zamrini EW., Yang XH., Buccafusco JJ. Central cholinergic hypersensitivity in aging. J Geriatr Psychiatry Neurol. 1992;5:72–77. doi: 10.1177/002383099200500203. [DOI] [PubMed] [Google Scholar]
- 21.Molchan SE., Martinez RA., Hill JL., et al. Increased cognitive sensitivity to scopolamine with age and a perspective on the scopolamine model. Brain Res Brain Res Rev. 1992;17:215–226. doi: 10.1016/0165-0173(92)90017-g. [DOI] [PubMed] [Google Scholar]
- 22.Rabey JM., Neufeld MY., Treves TA., Sifris P., Korczyn AD. Cognitive effects of scopolamine in dementia. J Neural Transrn Gen Sect. 1996;103:873–881 . doi: 10.1007/BF01273365. [DOI] [PubMed] [Google Scholar]
- 23.Tariot PN., Patel SV., Henderson RE. Age-related decline in central cholinergic function demonstrated with scopolamine. Psychopharmacology. 1996;125:50–56. doi: 10.1007/BF02247392. [DOI] [PubMed] [Google Scholar]
- 24.Ghoneim MM., Mewaldt SP. Studies on human memory: the interactions of diazepam, scopolamine and physostigmine. Psychopharmacology. 1977;52:1–6. doi: 10.1007/BF00426592. [DOI] [PubMed] [Google Scholar]
- 25.Anisman H. Time-dependent changes in activity, reactivity, and responsivity during shock: effects of cholinergic and catecholaminergic manipulations. Neurology. 1977;27:783–790. doi: 10.1016/s0091-6773(77)92215-5. [DOI] [PubMed] [Google Scholar]
- 26.Liljequist R., Mattila MJ. Effect of physostigmine and scopolamine on the memory functions of chess players. Med Biol. 1979;57:42–405. [PubMed] [Google Scholar]
- 27.Mewaldt SP., Ghoneim MM. The effects and interactions of scopolamine and methamphetamine on human memory. Pharmacol Biochem Behav. 1979;10:205–210. doi: 10.1016/0091-3057(79)90088-1. [DOI] [PubMed] [Google Scholar]
- 28.Preston GC., Ward C., Lines CR., Poppleton P., Haigh JR., Traub M. Scopolamine and benzodiazepine models of dementia: cross reversals by RO 15-1788 and physostigmine. Psychopharmacology. 1989;98:487–494. doi: 10.1007/BF00441947. [DOI] [PubMed] [Google Scholar]
- 29.Wesnes K., Simpson PM., Kidd AG. The use of a scopolamine model to study the nootropic effects of tenilsetam (CAS 997) in man. Med Sci Res. 1987;15:1063–1064. [Google Scholar]
- 30.Wesnes KA., Simpson PM., Christmas L., Anand R., McClelland GR. The effects of moclobemide on cognition. J Neural Transrn Suppl. 1989;28:91–102. [PubMed] [Google Scholar]
- 31.Wesnes K., Anand R., Lorscheid T. Potential of moclobemide to improve cerebral insufficiency identified using a scopolamine model of aging and dementia. Acta Psychiatr Scand Suppl. 1990;360:71–72. doi: 10.1111/j.1600-0447.1990.tb05338.x. [DOI] [PubMed] [Google Scholar]
- 32.Wesnes K., Anand R., Simpson P., Christmas L. The use of a scopolamine model to study the potential nootropic effects of aniracetam and piracetam in healthy volunteers. Int J Geriatr Psychiatry. 1990;6:95–102. doi: 10.1177/026988119000400406. [DOI] [PubMed] [Google Scholar]
- 33.Wesnes KA., Simpson PM., White L., et al. Cholinesterase inhibition in the scopolamine model of dementia. Ann N Y Acad Sci. 1991;640:268–271 . doi: 10.1111/j.1749-6632.1991.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 34.Mohs RC., Davis KL. Interaction of choline and scopolamine in human memory. Life Sci. 1985;37:193–197. doi: 10.1016/0024-3205(85)90423-0. [DOI] [PubMed] [Google Scholar]
- 35.Patat A., Klein MJ., Surjus A., Hucher M., Granier J. RU 41656 does not reverse the scopolamine-induced cognitive deficit in healthy volunteers. Eur J Clin Pharmacol. 1991;41:225–231. doi: 10.1007/BF00315434. [DOI] [PubMed] [Google Scholar]
- 36.Canal N., Franceschi M., Alberoni M., Castiglioni C., De Moliner P., Longoni A. Effect of L-alpha-glyceryl-phosphorylcholine on amnesia caused by scopolamine. Int J Clin Pharmacol Ther Toxicol. 1991;29:103–107. [PubMed] [Google Scholar]
- 37.Preda L., Alberoni M., Bressi S., et al. Effects of acute doses of oxiracetam in the scopolamine model of human amnesia. Psychopharmacology. 1993;110:421–426. doi: 10.1007/BF02244648. [DOI] [PubMed] [Google Scholar]
- 38.Jones RW., Wesnes KA., Kirby J. Effects of NMDA modulation in scopolamine dementia. Ann N Y Acad Sci. 1991;640:241–244. doi: 10.1111/j.1749-6632.1991.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 39.Brass EP., Polinsky R., Sramek JJ., et al. Effects of the cholinomimetic SDZ ENS-163 on scopolamine-induced cognitive impairment in humans. J Clin Psychopharmacol. 1995;15:58–62. doi: 10.1097/00004714-199502000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Duka T., Ott H., Rohloff A., Voet B. The effects of a benzodiazepine receptor antagonist beta-carboline ZK-93426 on scopolamine-induced impairment on attention, memory and psychomotor skills. Psychopharmacology. 1996;123:361–373. doi: 10.1007/BF02246647. [DOI] [PubMed] [Google Scholar]
- 41.Molchan SE., Mellow AM., Lawlor BA., et al. TRH attenuates scopolamineinduced memory impairment in humans. Psychopharmacology. 1990;100:84–89. doi: 10.1007/BF02245795. [DOI] [PubMed] [Google Scholar]
- 42.Lister RG. The amnestic action of benzodiazepines in man. Neurosci BiobehavRev. 1985;9:87–94. doi: 10.1016/0149-7634(85)90034-x. [DOI] [PubMed] [Google Scholar]
- 43.Curran HV., Schiwy W., Lader M. Differential amnesic properties of benzodiazepines: a dose-response comparison of two drugs with similar elimination half-lives. Psychopharmacology. 1987;92:358–364. doi: 10.1007/BF00210844. [DOI] [PubMed] [Google Scholar]
- 44.Curran HV. Benzodiazepines, memory and mood: a review. Psychopharmacology. 1991;105:1–8. doi: 10.1007/BF02316856. [DOI] [PubMed] [Google Scholar]
- 45.Roehrs T., Zorick F., Sicklesteel L., Wittig R., Hartse K., Roth T. Effects of hypnotics on memory./. Clin Psychopharmacol. 1983;3:310–313. [PubMed] [Google Scholar]
- 46.Curran HV., Pooviboonsuk P., Dalton JA., Lader MH. Differentiating the effects of centrally acting drugs on arousal and memory: an event-related potential study of scopolamine, lorazepam and diphenhydramine. Psychopharmacology. 1998;135:27–36. doi: 10.1007/s002130050482. [DOI] [PubMed] [Google Scholar]
- 47.Preston GC., Broks P., Traub M., Ward C., Poppleton P., Stahl SM. Effects of lorazepam on memory, attention and sedation in man. Psychopharmacology ; 1988;95:208–215. doi: 10.1007/BF00174511. [DOI] [PubMed] [Google Scholar]
- 48.Weingartner H. Models of memory dysfunctions. Ann N Y Acad Sci. 1985;444:359–369. doi: 10.1111/j.1749-6632.1985.tb37601.x. [DOI] [PubMed] [Google Scholar]
- 49.Curran HV., Schifano F., Lader M. Models of memory dysfunction? A comparison of the effects of scopolamine and lorazepam on memory, psychomotor performance and mood. Psychopharmacology. 1991;103:83–90. doi: 10.1007/BF02244079. [DOI] [PubMed] [Google Scholar]
- 50.Thiel CM., Henson RN., Morris JS., Friston KJ., Dolan RJ. Pharmacological modulation of behavioral and neuronal correlates of repetition priming. J Neurosci. 2001;21:6846–6852. doi: 10.1523/JNEUROSCI.21-17-06846.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sperling R., Grève D., Dale A., et al. Functional MRI detection of pharmacologically induced memory impairment. Proc Natl Acad Sci USA. 2002;99:455–460. doi: 10.1073/pnas.012467899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duka T., Redeman B., Voet B. Scopolamine and lorazepam exert different patterns of effects in a test battery assessing stages of information processing. Psychopharmacology. 1995;119:315–324. doi: 10.1007/BF02246298. [DOI] [PubMed] [Google Scholar]
- 53.Thiel CM., Henson RN., Dolan RJ. Scopolamine but not lorazepam modulates face repetition priming: a psychopharmacological fMRI study. Neuropsychopharmacology. 2002;27:282–292. doi: 10.1016/S0893-133X(02)00316-0. [DOI] [PubMed] [Google Scholar]
- 54.Mandema JW., Danhof M. Electroencephalogram effect measures and relationships between pharmacokinetics and pharmacodynamics of centrally active drugs. Clin Pharmacokinet. 1992;23:191–215. doi: 10.2165/00003088-199223030-00003. [DOI] [PubMed] [Google Scholar]
- 55.Harris JA., Biersner RJ., Edwards D., Bailey LW. Attention, learning, and personality during ketamine emergence: a pilot study. Anesth Analg. 1975;54:169–172. [PubMed] [Google Scholar]
- 58.Pandit SK., Kothary SP., Kumar S. Low dose intravenous infusion technique with ketamine. Anesthesia. 1980;35:669–675. doi: 10.1111/j.1365-2044.1980.tb03882.x. [DOI] [PubMed] [Google Scholar]
- 57.Ghoneim MM., Hinrichs JV., Mewaldt SP., Petersen RC. Ketamine: behavioral effects of subanesthetic doses. J Clin Psychopharmacol. 1985;5:70–77. [PubMed] [Google Scholar]
- 56.Oye I., Paulsen O., Maurset A. Effects of ketamine on sensory perception: evidence for a role of W-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 1992;260:1209–1213. [PubMed] [Google Scholar]
- 59.Krystal JH., Karper LP., Seibyl JP., et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 60.Krystal JH., Karper LP., Bennet A., et al. Interactive effects of subanesthetic ketamine and subhypnotic lorazepam in humans. Psychopharmacology. 1998;135:213–229. doi: 10.1007/s002130050503. [DOI] [PubMed] [Google Scholar]
- 61.Krystal JH., D'Souza DC., Karper LP., et al. Interactive effects of subanesthetic ketamine and haloperidol in healthy humans. Psychopharmacology. 1999;145:193–204. doi: 10.1007/s002130051049. [DOI] [PubMed] [Google Scholar]
- 62.Malhotra AK., Finals DA., Weingartner H., et al. IMMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology. 1996;14:301–307. doi: 10.1016/0893-133X(95)00137-3. [DOI] [PubMed] [Google Scholar]
- 63.Adler CM., Goldberg TE., Malhotra AK., Pickar D., Breier A. Effects of ketamine on thought disorder, working memory and semantic memory in healthy volunteers. Biol Psychiatry. 1998;43:811–816. doi: 10.1016/s0006-3223(97)00556-8. [DOI] [PubMed] [Google Scholar]
- 64.Newcomer JW., Farber NB., Jevtovic-Todorovic V., et al. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999;20:106–118. doi: 10.1016/S0893-133X(98)00067-0. [DOI] [PubMed] [Google Scholar]
- 65.Lahti AC., Weiler MA., Michaelidis T., et al. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25:455–467. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- 66.Farber NB., Newcomer JW., Olney JW. The glutamate synapse in neuropsychiatrie disorders. Focus on schizophrenia and Alzheimer's disease. Prog Brain Res. 1998;116:421–437. doi: 10.1016/s0079-6123(08)60453-7. [DOI] [PubMed] [Google Scholar]
- 67.Reuter-Lorenz PA. New visions of the aging mind and brain. Trends Cogn Sci. 2002;6:394–400. doi: 10.1016/s1364-6613(02)01957-5. [DOI] [PubMed] [Google Scholar]
- 68.Furey ML., Pietrini P., Haxby JV., et al. Cholinergic stimulation alters performance and task-specific regional cerebral blood flow during working memory. Proc Natl Acad Sci U S A. 1997;94:6512–6516. doi: 10.1073/pnas.94.12.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Furey ML., Pietrini P., Alexander GE., Schapiro MB., Horwitz B. Cholinergic enhancement improves performance on working memory by modulating the functional activity in dinstinct brain regions: a positron emission tomography regional cerebral blood flow study in healthy humans. Brain Res Bull. 2000;51:213–218. doi: 10.1016/s0361-9230(99)00219-1. [DOI] [PubMed] [Google Scholar]
- 70.Furey ML., Pietrini P., Haxby JV. Cholinergic enhancement and increased selectivity of perceptual processing during working memory. Science. 2000;290:2315–2319. doi: 10.1126/science.290.5500.2315. [DOI] [PubMed] [Google Scholar]
- 71.Bradwejn J., de >Montigny C. Benzodiazepines antagonize cholecystokinin-induced activation of rat hippocampal neurons. Nature. 1984;312:363–364. doi: 10.1038/312363a0. [DOI] [PubMed] [Google Scholar]
- 72.Rehfeld JF. Cholecystokinin and anxiety, an overview. In: Dourish CT, Cooper SJ, Iversen SD, Iversen LL eds. Multiple Cholecystokinin Receptors in CNS. New York, NY: Oxford University Press. 1992:117–120. [Google Scholar]
- 73.de Montigny C. Cholecystokinin tetrapeptide induces panic-like attacks in healthy volunteers. Preliminary findings. Arch Gen Psychiatry. 1989;46:511–517. doi: 10.1001/archpsyc.1989.01810060031006. [DOI] [PubMed] [Google Scholar]
- 74.Koszycki D., Bradwejn J., Bourin M. Comparison of the effects of cholecystokinin-tetrapeptide and carbon dioxide in healthy volunteers. Eur Neuropsychopharmacol. 1991;1:137–414. doi: 10.1016/0924-977x(91)90715-7. [DOI] [PubMed] [Google Scholar]
- 75.Koszycki D., Cox BJ., Bradwejn J. Anxiety sensitivity and response to cholecystokinin tetrapeptide in healthy volunteers. Am J Psychiatry. 1993;150:1881–1883. doi: 10.1176/ajp.150.12.1881. [DOI] [PubMed] [Google Scholar]
- 76.Koszycki D., Zacharko RM., Le Medello JM., Bradwejn J. Behavioral, cardiovascular, and neuroendocrine profiles following CCK-4 challenge in healthy volunteers: a comparison of panickers and nonpanickers. Depress Anxiety. 1998;8:1–7. [PubMed] [Google Scholar]
- 77.Bradwejn J., Koszycki D., Shriqui C. Enhanced sensitivity to cholecystokinin tetrapeptide in panic disorder. Clinical and behavioral findings. Am J Psychiatry. 1991;151:603–610. doi: 10.1001/archpsyc.1991.01810310021005. [DOI] [PubMed] [Google Scholar]
- 78.Bradwejn J., Koszycki D., Bourin M. Dose-ranging study of the effects of cholecystokinin in healthy volunteers. J Psychiatr Neurosci. 1991;16:91–95. [PMC free article] [PubMed] [Google Scholar]
- 79.Bradwejn J., Koszycki D., Couëtoux du Tertre A., paradis M., Bourin M. Effects of flumazenil on cholecystokinin-tetrapeptide-induced panic symptoms in healthy volunteers. Psychopharmacology. 1994;114:257–261. doi: 10.1007/BF02244846. [DOI] [PubMed] [Google Scholar]
- 80.Bradwejn J., Koszycki D., Paradis M., Reece P., Hinton J., Sedman A. Effect of CI-988 on cholecystokinin tetrapeptide-induced panic symptoms in healthy volunteers. Biol Psychiatry. 1995;38:742–746. doi: 10.1016/0006-3223(95)00081-X. [DOI] [PubMed] [Google Scholar]
- 81.Bradwejn J., LeGrand JM., Koszycki D., Bates JHT., Bourin M. Effects of cholecystokinin tetrapeptide on respiratory functions in healthy volunteers. Am J Psychiatry. 1998;155:280–282. doi: 10.1176/ajp.155.2.280. [DOI] [PubMed] [Google Scholar]
- 82.Schruers K., Caycedo N., Overbeek T., Bûchold H., Bourin M., Griez E. Effects of low dose cholecystokinin on respiratory function in healthy volunteers. Eur Neuropsychopharmacol. 2000;10:419–421. doi: 10.1016/s0924-977x(00)00101-2. [DOI] [PubMed] [Google Scholar]
- 83.Shlik J., Vasar V., Âluoja A., et al. The effect of cholecystokinin tetrapeptide on respiratory resistance in healthy volunteers. Biol Psychiatry. 1997;42:206–212. doi: 10.1016/s0006-3223(96)00334-4. [DOI] [PubMed] [Google Scholar]
- 84.Shlik J., Zhou Y., Koszycki D., Vaccarino F., Bradwejn J. Effects of CCK-4 infusion on the acoustic eye-blink startle and psychophysiological measures in healthy volunteers. J Psychopharmacol. 1999;13:385–390. doi: 10.1177/026988119901300409. [DOI] [PubMed] [Google Scholar]
- 85.Jerabek I., Boulenger JP., Bradwejn J., Lavallée YJ., Jolicoeur FB. CCK4 induced panic in healthy subjects I. Psychological and cardiovascular effects. Eur Neuropsychopharmacol. 1999;9: 149–155 . doi: 10.1016/s0924-977x(98)00020-0. [DOI] [PubMed] [Google Scholar]
- 86.Flint AJ., Koszycki D., Vaccarino FJ., Cadieux A., Boulenger JP., Bradwejn J. Effect of aging on cholecystokinin-induced panic. Am J Psychiatry. 1998;155:283–285. doi: 10.1176/ajp.155.2.283. [DOI] [PubMed] [Google Scholar]
- 87.Le Mellédo JM., Bradwejn J., Koszycki D., Bichet DG., Bellavance F. The role of the α-noradrenergic system in chlolecystokinin-tetrapeptideinduced panic symptoms. Biol Psychiatry. 1998;44:364–366. doi: 10.1016/s0006-3223(97)00536-2. [DOI] [PubMed] [Google Scholar]
- 88.Depot D., Caillé G., Mukherjee J., Katzman MA., Cadieux A., Bradwejn J. Acute and chronic role of 5-HT3 neuronal system on behavioral and neuroendocrine changes induced by intravenous cholecystokinin tetrapeptide administration in humans. Neuropsychopharmacology. 1999;20:177–187. doi: 10.1016/S0893-133X(98)00074-8. [DOI] [PubMed] [Google Scholar]
- 89.Wiedemann K., Jahn H., Yassouridis A., Kellner M. Anxiolytic-like effects of atrial natriuretic peptide on cholecystokinin tetrapeptide-induced panic attacks. Preliminary findings. Arch Gen Psychiatry. 2001;58:371–377. doi: 10.1001/archpsyc.58.4.371. [DOI] [PubMed] [Google Scholar]
- 90.Kellner M., Yassouridis A., Hua Y., Wendrich M., Jahn H., Wiedemann K. Intravenous C-type natriuretic peptide augments behavioral and endocrine effects of cholecystokinin tetrapeptide in healthy men. J Psychiatr Res. 2002;36:1–6. doi: 10.1016/s0022-3956(01)00042-5. [DOI] [PubMed] [Google Scholar]
- 91.Zwanzger P., Baghai TC., Schuele C., Strohle A., Padberg F., Kathmann N. Vigabatrin decreases cholecystokinin-tetrapeptide (CCK-4) induced panic in healthy volunteers. Neuropsychopharmacology. 2001;25:699–703. doi: 10.1016/S0893-133X(01)00266-4. [DOI] [PubMed] [Google Scholar]
- 92.Benkelfast C., Bradwejn J., Meyer E., et al. Functional neuroanatomy of CCK4-induced anxiety in normal healthy volunteers. Am J Psychiatry. 1995;152:1180–1184. doi: 10.1176/ajp.152.8.1180. [DOI] [PubMed] [Google Scholar]
- 93.Javanmard M., Shlik J., Kennedy SH., Vaccarino FJ., Houle S., Bradwejn J. Neuroanatomy correlates of CCK-4-induced panic attacks in healthy humans: a comparison of two time points. Biol Psychiatry. 1999;45:872–882. doi: 10.1016/s0006-3223(98)00348-5. [DOI] [PubMed] [Google Scholar]
- 94.LeDoux J. Fear and the brain: where have we been, and where are we going?. Biol Psychiatry. 1998;44:1229–1238. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- 95.Whalen PJ. Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Curr Dir Psychol Sci. 1998;7:177–188. [Google Scholar]
- 96.Fanselow MS., Le Doux J. Why we think plasticity underlying pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- 97.Hugdahl K., Berardi A., Thompson WL., et al. Brain mechanisms in human classical conditioning: a PET blood flow study. Neuroreport. 1995;6:1723–1728. doi: 10.1097/00001756-199509000-00005. [DOI] [PubMed] [Google Scholar]
- 98.LaBar KS., Gatenby JC., Gore JC., LeDoux JE., Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 99.Bûchel C., Morris J., Dolan RJ., Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- 100.Staner L. Sleep-wake mechanisms and drug discovery: sleep EEG as a tool for the development of CNS-acting drugs. Dialogues Clin Neurosci. 2002;4:342–350. doi: 10.31887/DCNS.2002.4.4/lstaner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kahn RS., Davis KL. New development in dopamine and schizophrenia. In: Bloom FE, Kupfer DJ, eds. Psychopharmacology: The Fourth Generation of Progress. New York, NY: Raven Press. 1995:1193–1203. [Google Scholar]
- 102.Przedborski S., Levivier M., Raftopoulos C., Naini AB., Hildebrand J. Peripheral and central pharmacokinetics of apomorphine and its effects on dopamine metabolism in humans. Mov Disord. 1995;10:28–36. doi: 10.1002/mds.870100107. [DOI] [PubMed] [Google Scholar]
- 103.Luthringer R., Rinaudo G., Toussaint M., et al. Electroencephalographs characterization of brain dopaminergic stimulation by apomorphine in healthy volunteers. Neuropsychobiology. 1999;39:49–56. doi: 10.1159/000026560. [DOI] [PubMed] [Google Scholar]
- 104.McClelland GR., Cooper SM., Pilgrim AJ. A comparison of the central nervous system effects of haloperidol, chlorpromazine and sulpiride in normal volunteers. Br J Clin Pharmacol. 1990;30:795–803. doi: 10.1111/j.1365-2125.1990.tb05444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hughes AM., Lynch P., Rhodes J., Ervine CM. Yates RA Electroencephalographic and psychomotor effects of chlorpromazine and risperidone relative to placebo in normal healthy volunteers. Br J Clin Pharmacol. 1999;48:323–330. doi: 10.1046/j.1365-2125.1999.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Galderisi S., Mucci A., Bucci P., Mignone ML., Maj M. Multilead quantitative EEG profile of clozapine in resting and vigilance-controlled conditions. Psychiatry Res. 1996;67:113–122. doi: 10.1016/0925-4927(96)02883-1. [DOI] [PubMed] [Google Scholar]
- 107.Luthringer R. . Evaluation de la sphère dopaminergique cérébrale chez l'homme; Mise au point validation, caractérisation et intérêt du test à l'apomorphine sosu surveillance électroencéphalographique [Doctoral thesis], Strasbourg, France: Louis Pasteur University. 1998 [Google Scholar]
- 108.Smith GS., Schloesser R., Brodie JD., et al. Glutamate modulation of dopamine measured in vivo with positron emission tomography (PET) and 11C-raclopride in normal human subjects. Neuropsychopharmacology. 1998;18:18–25. doi: 10.1016/S0893-133X(97)00092-4. [DOI] [PubMed] [Google Scholar]
- 109.Vollenweider FX., Vontobel P., Oye I., et al. Effects of (S)-ketamine on striatal dopamine: a [“Cjraclopride PET study of a model psychosis in humans. J Psychiatr Res. 2000;34:35–43. doi: 10.1016/s0022-3956(99)00031-x. [DOI] [PubMed] [Google Scholar]
- 110.Breier A., Adler CM., Weisenfeld N., et al. Effects of NMDA antagonism on striatal dopamine release in healthy subjects: application of a novel PET approach. Synapse. 1998;29:142–147. doi: 10.1002/(SICI)1098-2396(199806)29:2<142::AID-SYN5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 111.Kegeles LS., Abi-Dargham A., Zea-Ponce Y., et al. Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: implications for schizophrenia. Biol Psychiatry. 2000;48:627–640. doi: 10.1016/s0006-3223(00)00976-8. [DOI] [PubMed] [Google Scholar]
- 112.Lahti AC., Koffel B., Laporte D., et al. Subanesthetic doses of ketamine simulate psychosis in schizophrenia. Neuropsychopharmacology. 1994;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- 113.Tamminga CA., Medoff DR. Studies in schizophrenia: pathophysiology and treatment. Dialogues Clin Neurosci. 2002;4:000–000. doi: 10.31887/DCNS.2002.4.4/ctamminga. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Malhotra AK., Adler CM., Kennison SD., et al. Clozapine blunts W-methylD-aspartate antagonist-induced psychosis: a study with ketamine. Biol Psychiatry. 1997;42:664–668. doi: 10.1016/s0006-3223(96)00546-x. [DOI] [PubMed] [Google Scholar]