Abstract
In Alzheimer's disease, cognition now responds to several drugs. Anticholinesterases target the acetylcholine deficit. In mild-to-moderate Alzheimer's disease, they all provide significant benefit versus placebo on the Alzheimer's Disease Assessment ScheduleCognitive Section (ADAS-Cog), Side effects, in 5% to 15% of cases, include nausea, vomiting, diarrhea, anorexia, and dizziness. Tacrine, the leading anticholinesterase, caused frequent hepatic enzyme elevation and was withdrawn; once-daily donepezil spares the liver and improves global measures of change in severe dementia; rivasiigmine is indicated in comorbid vascular disease; while galaniamine modulates the cerebral nicotinic acetylcholine receptors that potentiate the response to acetylcholine. Alternative agents include the N-methyl-D-aspartate (NMDA) receptor antagonist, memaniine, licensed in Europe for moderately severe to severe Alzheimer's disease; it acts on a different neurotransmitter system present in 70% of neurons, protecting against pathologic glutamergic activation while preserving or even restoring physiologic glutamergic activation. The clinician's armamentarium in AD has never been greater.
Keywords: Alzheimer's disease, dementia, cholinesterase inhibitor, tacrine, donepezil, rivastigmine, galantamine
Abstract
En la Enfermedad de Alzheimer, ahora los aspectos cognitivos responden a diversas drogas. Los anticolinesterásicos apuntan al déficit de aceiticolina. En la Enfermedad de Alzheimer leve a moderada estos fármacos proveen beneficios significativos respecto al placebo en la sección cognitiva de la Escala de Evaluatión de la Enfermedad de Alzheimer (ADAS-Cog). Los efectos colaterales, en un 5% a 15% de los casos, incluyen náuseas, vómitos, diarrea, anorexia y mareos. La tacrina, el anticolinesterásico guía, provocó un aumento frecuente de las enzimas hepáticas y fue retirada; el donepezil administrado una vez al día no afecta el hígado y mejora las mediciones globales de cambio en la demencia severa; la rivastigmina está indicada en la enfermedad vascular comórbida, mientras que la galantamina modula los receptores nicotínicos de acetilcolina cerebral, los que potencian la respuesta a acetilcolina. Entre los agentes alternativos se incluyen los antagonistas del receptor N-metil-D-aspartato (NMDA), como la memantina, que obiuvo la licencia en Europa para la Enfermedad de Alzheimer moderadamente severa a severa; actúa en un sistema neurotransmisor diferente, présente en el 70% de las neuronas, que al proteger contra la activación glutamatérgica patológica preserva o aun restaura la activación glutamatérgica fisiológica. El arsenal del clínico en la Enfermedad de Alzheimer nunca ha sido más numeroso.
Abstract
La cognition, atteinte au cours de la maladie d'Alzheimer, répond maintenant à plusieurs médicaments. Les anticholinestérasiques ciblent le déficit en acetylcholine. Au cours de la maladie d'Alzheimer légère à modérée, ils procurent tous un bénéfice significatif comparés au placebo sur l'échelle ADASCog (Alzheimer's Disease Assessment Schedule-Cognitive Section, échelle de mesure de la performance cognitive). Les effets secondaires, dans 5 % à 15 % des cas, incluent nausées, vomissements, diarrhée, anorexie et vertiges. La tacrine, le principal anticholinestérasique, provoquait souvent une élévation des enzymes hépatiques et a été retirée du marché ; en dose unique quotidienne, le donépézil respecte le foie et améliore les mesures globales de détérioration dans la démence sévère ; la rivastigmine est indiquée en cas de comorbidité avec la maladie vasculaire ; enfin, la galantamine agit sur les récepteurs cérébraux nicotiniques de l'acétyleholine qui potential isent la réponse à l'acétyleholine. Parmi les autres traitements on peut noter l'antagoniste du récepteur du N-méthyl-D-aspartate (NMDA), la mémantine, autorisée en Europe dans le traitement de la maladie d'Alzheimer modérée à sévère ; elle agit sur un système différent de neuromédiateurs, présent dans 70 % des neurones, protecteur contre l'activation glutamatergique pathologique et respectant ou même rétablissant l'activation glutamatergique physiologique. L'arsenal thérapeutique du praticien n'a jamais été plus vaste dans la maladie d'Alzheimer.
Alzheimer's disease is the commonest cause of dementia and describes a clinical syndrome made up of three domains. First, a neuropsychological domain encompassing those deficits of cognitive function such as amnesia (memory loss), aphasia (language disturbance), apraxia (the inability to carry out motor tasks despite intact motor functions), and agnosia (the inability to recognize people or objects despite intact sensory functions). Second, a group of psychiatric symptoms and behavioral disturbances, which have been termed neuropsychiatrie features,1 noncognitive phenomena, or behavioral and psychological symptoms of dementia (BPSD).2 These consist of psychiatric symptoms (such as delusions, hallucinations, depression, paranoid ideas, and misidentifications) and behavioral disturbances (such as aggression, wandering, and sexual disinhibitions). Third, problems with activities of daily living (ADL), which include instrumental ADI . in the early stages of dementia when the person is unable to carry out complex tasks, such as shopping, driving, and using the telephone, and basic ADL in the later stages of dementia, when a person is unable to go to the toilet or feed, dress, and wash themselves.
Causes of dementia
The relative frequency of causes of dementia vary depending on the population under study. Alzheimer's disease is probably the commonest form (about 50%), followed by vascular dementia (about 25%) and dementia with Lewy bodies (about 20%), with the other 5% being made up of reversible dementias and rarer forms of dementia, such as frontal lobe dementia or Creutzfeldt-Jakob disease. There is increasing evidence that there is a significant overlap between the two commonest causes - Alzheimer's disease and vascular disease. Clinically, it is common for individuals to have features of both disorders. Epidemiological studies suggest that the risk factors for vascular disease are also associated with the development of Alzheimer's disease.3 Histological studies have shown that in many patients there is a coexistence of vascular and Alzheimer's changes and that, even in the presence of Alzheimer's disease histologically, vascular changes significantly influence the clinical picture in terms of the presence of dementia.4
Assessment of dementia
There are now a number of established standardized tools for the assessment of features of dementia and measurement of change.
Cognitive function
Cognitive function is at the core of the assessment of Alzheimer's disease. The most widely used assessment is the Alzheimer's Disease Assessment Schedule - Cognitive Section (ADAS-Cog5), which assesses a number of domains in addition to memory and is sensitive to change. Scores range from zero (no impairment) to 70 (severe impairment). Generally speaking, patients with mild-to-moderate Alzheimer's disease show an increase in ADAS-Cog scores of between 6 to 12 points a year (the ADAS-Cog is scored in the same way as the original Blessed Scale,6 which measures the number of errors rather than the number of correct answers, hence a higher score indicates better cognitive function, in distinction to most other tests). In the later stages of dementia, the Severe Impairment Battery7 is able to measure cognitive function with a score from zero to 100.8 The Mini-Mental State Examination (MMSE)9 is also used as both a measure of change and a descriptor of the severity of the illness (scores of less than 10 out of 30 equate with severe dementia, 10-18 with moderate dementia, and 18-23 mild dementia; scores of 24 and above indicate normality).
Neuropsychiatrie features
Neuropsychiatrie features have been included in studies more recently as recognition of their importance grows. One of the most popular assessments is the Neuropsychiatrie Inventory (NPI),1 which is a 12-item scale that measures a range of noncognitive features. Ratings of frequency and severity are included giving a total score of 144.
Activities of daily living
Several scales have been developed to measure what many regard as the most important feature of Alzheimer's disease and where improvement will have a major positive impact on the life of the patient and their carer. Scales that measure ADL include the Progressive Deterioration Scale (PDS, a 29-item assessment with a score of 1 -100),10 the Interview for Deterioration in Daily living activities in Dementia (IDDD),11 and the Alzheimer's Disease Cooperative Study Activities of Daily living Scale (ADCS/ADL).12 Measures of ADL need to be sufficiently sensitive to assess activities over a range of severities, as well as being a sensitive measure of change.
Global function
There are two types of global function scales. First, there are those that capture the severity and stage of the disease (ie, mild, moderate, and severe) and, second, those that assess changes over the course of the illness. The Clinical Dementia Rating (CDR)13,14 measures the stage of dementia over six domains (the sum of boxes and memory; orientation; judgment and problem solving; community affairs; home and hobbies; personal care) and gives a rating of questionable dementia (0.5), mild dementia (1), moderate dementia (2), and severe dementia (3). The Global Deterioration Scale (GDS)15 gives a similar rating of severity, but with an emphasis on the more severe forms of disease. The concept of a global assessment of change was developed to overcome the criticism that clinical trials that only measured cognitive function were failing to capture (in a global sense) the changes that were the most important to patients and their families. There are a number of measures that have been developed, all of which are based on the premise that if a clinician is able to detect a change, then that change in itself is significant. The basic format of the assessments is the same - a 7-point scale with an anchor point in the middle for no change and three measures of improvement and three measures of deterioration (Clinical Global Impression of Change16). Some standardization has been introduced, which has tended to improve the reliability of the measures (Clinicians' Interview-based Impression of Change [CIBIC]17), but part of the validity is that the score reflects the view of the individual rater, rather than being a scale where answers are simply recorded onto a form. A development is the introduction of information from the caregiver, which allows the independent clinician marking the scale to reflect changes that impinge on the patient and their carer in a global sense (CIBIC+, which includes information from the carer).
Pharmacological approaches to the management of Alzheimer's disease
Cholinesterase inhibitors
These drugs were introduced on the basis of ample neurochemical evidence that there is a significant acetylcholine deficit in Alzheimer's disease. One of the drugs' main actions is to inhibit the enzyme acetylcholinesterase, which breaks down acetylcholine, thus effectively raising the level of the neurotransmitter. Four drugs of this type have been established in Alzheimer's disease: tacrine, donepezil, rivastlgminc, and galantamine. They vary in their pharmacological action. Tacrine is an acridine-based compound (its liver toxicity probably results from this), donepezil is piperidine based and a selective acetylcholinesterase inhibitor, whereas tacrine and galantamine have significant activity on butyrylcholinesterase. Rivastigmine is a carbonate -based compound and is relatively free of drug interactions, and galantamine is an alkaloid. Donepezil has the longest plasma half-life at about 70 hours compared with 6 hours for galantamine, 3 hours for tacrine, and 1.5 hours for rivastigmine (this has the practical advantage that it is excreted quickly from the body and so relief from side effects is much more speedy than with the longer-acting compounds). The half-life also has implications for the daily dosing regimen: the advantage of donepezil is that it only needs to be given once a day.
Tacrine
This was the first drug to be introduced and, in many ways, was the gold standard by which the others were measured. The drug has positive effects on cognitive function at dosages of 160 mg/day, and benefits have been seen in terms of ADL and global function.18,19 Unfortunately, almost half of all patients experience liver side effects, usually a rise in transaminases, and so a search began for an agent as effective as tacrine, but without side effects.
Donepezil
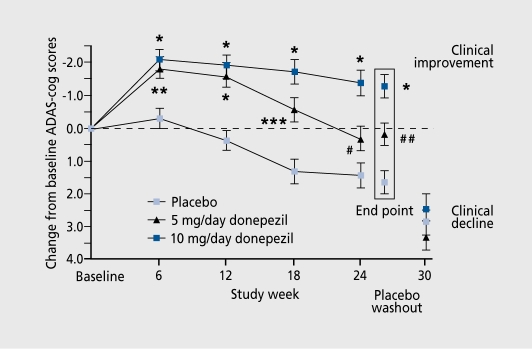
As a piperidinc-based compound, the introduction of donepezil was important because of its lack of liver side effects and the convenience of once-daily dosing. One multinational study20 involved patients in Australia, Belgium, Canada, France, Germany, Ireland, New Zealand, South Africa, and the UK. Fight hundred and eighteen (818) patients were randomized to receive placebo (n=274), 5 mg/day donepezil (n=271), or 10 mg/day donepezil (n=273).The mean age of patients was just over 70 and they all satisfied the NINCDS/ADRDA (National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer Disease and Related Disorders Association)21 criteria for probable Alzheimer's disease. Patients with mild-to-moderate impairment were included, as assessed by an MMSE score of between 10 and 26 and a CDR of 1 (mild) or 2 (moderate). The study lasted 30 weeks: 24 weeks with a double-blind, placebo-controlled phase followed by a single-blind placebo washout over 6 weeks. Patients started with 5 mg/day donepezil for 7 days followed by 10 mg/day. The positive effects on the ADAS-Cog arc shown in Figure 1. The percentage of patients rated as improved was 21 % for 5 mg/day, 25% for 10 mg/day, and 14% for placebo. The pattern of side effects (mostly related to the digestive system, eg, diarrhea, nausea, and vomiting, understandable in terms of the physiological effect of a cholinergic drug) was the same (10%) for placebo and 5 mg/day of the drug, and double that in those taking the higher dose. The IDDD was used to assess ADL and the drug showed a protective effect against the decline and activity that occurred with placebo. A similar USA-based study22 was in accordance with these findings and there was evidence that the 10 mg/day dosage was superior to the 5 mg/day dosage.
Figure 1. Effect of donepezil, 5 and 10 mg/day, and placebo on Alzheimer's Disease Assessment Schedule-Cognitive Section (ADAS-Cog) scores. Data are least square means±SE. *P<0.0001 ; **P=0.0005; ***P=0.0002; #P=0.031; ## P=0.0021.
Reproduced from reference 20: Burns A, Rossor M, Hecker J, et al. The effects of donepezil in Alzheimer's disease: results from a multinational trial. Dements Cognitive Geriatr Disord. 1999:10:237-244. Copyright © 1999, Karger.
The longer-term efficacy and safety of donepezil has been shown by an analysis of the continuation of the US study.23 In total, 133 patients completed the trial, which lasted nearly 5 years and showed that the rate of deterioration in those taking the active drug was less than that of placebo, that adverse events were mild and transient, and that there was no evidence of liver toxicity. Winblad et al reported a 12-month study in 286 patients in Nordic countries in Europe.24 Two thirds of the patients in the donepezil and placebo group completed the study (patients took 5 mg/day donepezil 28 days followed by 10 mg/day). Another study, also of a year's duration, examined the effects of donepezil in preserving function over time.25 A predetermined definition of a decline in functional status was operationalized and it was found that those on the active drug were 5 months slower at reaching this end point than those on placebo. This was quantified as showing that the drug reduced the risk of functional decline by 38% compared with placebo. The effects of the drug have also been examined in people with more severe Alzheimer's disease26 with 144 patients randomized to donepezil and 146 to placebo over 24 weeks. Despite the severity of the illness, benefits were seen in terms of global measures of change, cognitive function, ADL, and psychiatric symptoms; 86% of placebo patients completed the trial with 6% withdrawing because of adverse events, compared with 84% and 8%, respectively, in those on active drug.
Rivastigmine
The effect, of rivastigmine has been described in a USbased study over 26 weeks in 699 patients with mild-tomodcrate Alzheimer's disease.27 Significant improvements on the ADAS-Cog compared with placebo were seen and these were particularly marked in those taking a higher dosage (6-12 mg/day) An analysis of patients with moderate and severe Alzheimer's disease has shown that the effects are as marked in this group of subjects and it. has been suggested that patients with comorbid vascular disease gain a particular benefit.28,29 Improvements have been seen in patients with advanced dementia and behavioral disturbances using the NPI with at least 50% of subjects improving by a third on the scale and 44% being able to reduce or stop concurrent psychotropic medication.
There were also significant, benefits in ADL. A European study assessed the safety and efficacy of two dosages of rivastigmine (up to 4 mg/day and up to 12 mg/day) over 26 weeks.30 In the rivastigmine group, 24% had improved compared with 16% in placebo by at least 4 points on the ADAS-Cog; 37% of people on rivastigmine compared with 20% on placebo showed evidence of a global improvement. Figure 2 shows these changes.
Figure 2. The effects of rivastigmine, 1 and 4 mg/day, and 6 and 12 mg/day compared with placebo on Alzheimer's Disease Assessment Schedule-Cognitive Section (ADAS-Cog). *P<0.05 compared with placebo.
Reproduced from reference 30: Rosier M, Anand R, Cicin-Sain A, et al. Efficacy and safety of rivastigmine in patients with Alzheimer's disease: international randomised controlled trial. BMJ. 1999:318:633-638. Copyright © 1999, BMJ.
The effects of rivastigmine have also been demonstrated in patients with dementia of the I ,ewy body type.31 Patients with this disorder appear to have a particularly profound deficit in cholinergic function and the symptoms are characterized by significant, psychiatric symptoms and behavioral disturbances. One hundred and twenty (120) patients who satisfied standard criteria for Lewy body dementia (the vast majority having fluctuating cognitive function and recurrent visual hallucinations) were recruited in the UK, Spain, and Italy (92 completed the study). Treatment started with 1.5 mg rivastigmine or placebo twice a day, increasing by 1.5 mg twice a day, for 2 weeks until 12 mg/day or a maximum well-tolerated maintenance dosage was reached. The primary efficacy measure was a reduction in scores on the NPI. Results showed significant improvement over the course of the study, with evidence of some benefit on the global functioning.
Galantamine
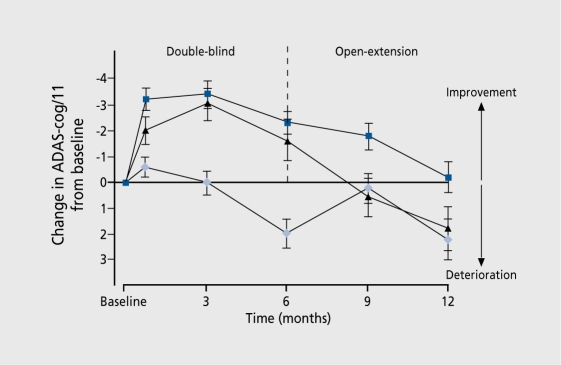
Galantamine has a somewhat novel, dual mode of action in that, in addition to its anticholinesterase activity, it has a modulating effect, on nicotinic acetylcholine receptors in the brain, which seem to have a role in potentiating the response to acetylcholine. In Europe and Canada, Wilcock32 reported a 6-month study of 653 patients with mild-to-moderate Alzheimer's disease, who were randomly assigned to either placebo or a maintenance dosage of galantamine of 24 or 32 mg/day. At. 6 months, improvements in ADL and on the CIBTC+ were recorded. Raskind et al33 reported on a 6-month, randomized, placebo-controlled trial followed by a 6-month extension. Patients with mild-to-moderate Alzheimer's disease (n=636) were assigned to either placebo or an escalating dosage of 24 or 32 mg/day galantamine, followed by a 6-month, open-label study with 24 mg/day. The conclusion was that at 24 mg/day, the drug is effective and safe in improving cognitive function and global function (Figure 3) over 6 months, and maintaining that improvement at 12 months. A total of 978 patients were enrolled in a relatively slow escalation study described by Tariot et al.34 A 4-week, placebo run-in was concluded with patients being randomized to receive placebo or 8, 16, or 24 mg/day galantamine. After 5 months, those on galantamine showed improvement on the ADAS-Cog, the CIBIC+, a number of psychiatric symptoms, and ADL. Adverse events resulting in discontinuation from the trial were found in 10% of the galantamine group and 7% of the placebo group. Coylc and Kershaw35 carried out. an analysis of the extension studies of galantamine and found that patients who had been treated with 24 mg/day throughout the trials had better cognitive function compared with those on placebo. The suggestion that stabilization occurs would be in keeping with the additional nicotinic receptor modulation activity of the drug.
Figure 3. The effects of galantamine and placebo on Alzheimer's Disease Assessment Schedule-Cognitive Section (ADAS-Cog) in the double-blind and extension study over 12 months. The blue squares represent 24 mg/day galantamine in the double-blind study followed by 24 mg/day galantamine in the open extension; the black triangles, 32 mg/day galantamine in the double-blind study followed by 24 mg/day galantamine in the open extension; and the light-blue diamonds placebo in the double-blind study followed by 24 mg/day galantamine in the open extension.
Reproduced from reference 34: Tariot PN, Solomon PR, Morris JC, et al. A 5-month randomized, placebo-controlled trial of galantamine in Alzheimer's disease. Neurohgy. 2000:54:2269-2276. Copyright © 2000, Lippincott, Williams and Wilkins.
What is the difference between the anticholinesterase drugs?
There are currently direct comparison (head-to-head) trials taking place to compare the three anticholinesterase drugs (tacrine is no longer marketed). Each drug has its own advantages and disadvantages, but. these are often only in terms of theoretical differences reflected in the marketing of individual drugs and represent a particular interest or scale that has been used by investigators. For example, because of its long half-life, donepezil can be prescribed once a day. It. has been suggested that, galantamine delays the onset, of behavioral problems and psychiatric symptoms in dementia. Rivastigmine seems to have fewer drug interactions36 and has been shown to be effective in dementia with Lewy bodies.
With regard to improvement, in cognitive function, comparison of the rates shows that the difference between the ADAS-Cog and placebo in the trials are 4.1 points for tacrine, 2.5 and 2.9 points for 5 and 10 mg/day donepezil, respectively, 4.9 points for rivastigmine (8.0 points for patients taking between 6 and 12 mg/day with moderately severe to severe Alzheimer's disease; 6.2 points for those with Alzheimer's disease and comorbid vascular risk factors), and 3.8 and 3.9 points, respectively, for 32 and 24 mg/day galantamine.
The commonest adverse events are nausea, vomiting, diarrhea, anorexia, and dizziness. Rates are between 5% and 15%. There is evidence to suggest, that rivastigmine and galantamine (particularly at higher doses) are more likely to induce nausea, vomiting, and diarrhea as well as dizziness, although generally speaking, the longer the titration time, the smaller the number of side effects (something that agrees with clinical practice37).
Noncholinergic approaches
Glutamatergic antagonists
Glutamate is a hitherto relatively neglected excitatory neurotransmitter in the brain and is probably present in 70% of neurones. A number of different receptor types are involved, one of particular relevance to Alzheimer's disease being the N-methyl-D-aspartate (NMDA) receptor. These receptors appear to have a specific role in the plasticity of neurones and therefore a specific function in terms of the formation of memories and learning. In excess, glutamate is excitotoxic and activates NMDA receptors. There is evidence that glutamate may be involved in the pathological process of Alzheimer's disease and its presence seems to stimulate the deposition of β-amyloid. Drugs that, have a high affinity for NMDA produce side effects including schizophreniform psychoses, but those that have lower receptor antagonist affinity seem only to have an influence in pathological conditions.
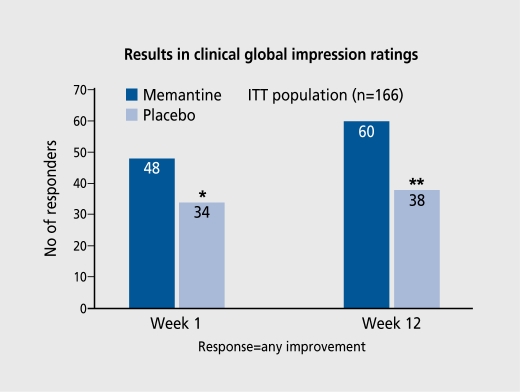
The most widely studied of these drugs is memantine. Several double-blind studies in the early 1990s suggested a role for the drug in dementia, but more recently a European-based study and a USA-based study have shown the drug to be effective in people with moderately severe to severe Alzheimer's disease. Two studies suggest that it is effective in people with vascular dementia. The drug currently has a license under European regulations for the treatment of moderately severe to severe Alzheimer's disease, making it stand apart from the cholincstcrase drugs. Significant improvements in global ratings of dementia, ADL, and cognitive function (as assessed by the Severe Impairment. Battery) have been demonstrated for dosages of 10 or 20 mg/day (escalating from 5 mg/day over 1 week). The results of the clinical global impression ratings appear in Figure 4. 38 Open-label studies at the end of the double-blind phases have demonstrated that improvements can still occur when there is a delay to the initiation of treatment. The side effects of the drug tend to be quite minor, the commonest being dizziness, but. confusion and hallucinations are commoner in the group taking the active drug. Agitation is much commoner in people on placebo. Memantine has been used in Germany for many years and so a significant body of safety data is available.38 Whether the drug will be suitable for people with mild-to-moderate dementia, whether it. will have a significant action against, vascular dementia, and whether treatment in combination with cholinesterase drugs are effective strategies remain to be evaluated.
Figure 4. Results of global rating of change in patients on memantime.38 *P=0.001; **P=0.006. ITT, intention-to-treat.
Estrogen
Estrogen has positive and beneficial effects on the brain in a number of areas.39 There is good evidence from epidemiological work that postmenopausal women are protected against the development of Alzheimer's disease if they are taking estrogen. The evidence so far that estrogen itself helps the symptoms of Alzheimer's disease is less clear cut. The results from different studies appear to be contradictory: while some studies suggest that there is no benefit,40-42 Asthana et al43 have reported that estradiol may produce improvements. In a prospective study, Zandi et al44 found that women who used hormone replacement, therapy (HRT) had a lower incidence of Alzheimer's disease over 3 years' follow-up than nonusers.The distinct, relationship between Alzheimer's disease risk and duration of HRT observed in this study highlights the need for continued research into the optimal regimen, dosage, and timing of HRT for possible neuroprotection. Although the combined estrogen-progestin arm of the Women's Health Initiative randomized trial was terminated due to a specific risk-benefit profile for a specific therapeutic regimen, the risk-benefit profile may well change if new studies confirmed these results.
Statins
Epidemiological studies have suggested that people on statins have a lower rate of Alzheimer's disease compared with those not taking the drugs.45 There is good biochemical evidence to postulate why statins may be of benefit, not least in their role in reducing the influence of vascular risk factors on the degree of cognitive impairment. It may be that, statins are of some benefit even if the serum cholesterol is normal.46,47 There is insufficient, evidence at. this stage for the prescription of these drugs solely for an anti-Alzheimer's effect.
Ginkgo biloba
One published study48 suggested a beneficial effect of Ginkgo biloba over placebo in people with dementia. However, the effects, while significant, are marginal and are not. as persuasive as for the anticholinesterase drugs. Because Ginkgo biloba can be bought over the counter, it remains something that patients, sometimes encouraged by their carers, will take to alleviate the symptoms of dementia. Individuals often report a beneficial effect.
Other approaches
There is good evidence that oxidative damage occurs in Alzheimer's disease and so intervention with an antioxidant, may prove to be of benefit in people with Alzheimer's disease. One study has suggested that vitamin E delays the progression of Alzheimer's disease49 and several reports have now documented that high levels of homocysteine (reflecting, probably poor intake of vitamins B12 and folate) are associated with Alzheimer's disease.50,51 Vitamin C may also have some benefit in protection against Alzheimer's disease. However, the antioxidant vitamin taken seems to reduce the incidence of the disease, particularly those including vitamin E.52,53 There was much publicity recently when a vaccine was introduced for Alzheimer's disease, which potentially had an antiamyloid effect.54 However, clinical studies have been suspended because some patients in these two trials developed inflammation of the central nervous system. Recent negative publicity in the UK surrounding the combined measles, mumps, and rubella (MMR) vaccine has probably had the effect of directing public enthusiasm away from vaccinations.
As case-control studies become more popular and epidemiological databases, which document risk factors, can easily be interrogated, the number of other risk - or protective - factors for Alzheimer's disease being described has increased. Mental and physical exercise is protective,55 red wine is protective,56 moderate alcohol intake of any type seems to be of benefit, and, most, recently, drinking coffee appears to reduce the rate of Alzheimer's disease.57 One specific study looked at the control of blood pressure and showed that rates of dementia can be significantly reduced in this way.58 Chelation of metals may also have a beneficial effect. Translating the epidemiological findings into things that will change people's lifestyles, or even suggest a treatment strategy, is a long way off.
Conclusion
A number of drugs are now available that, can improve the symptoms of Alzeheimer's disease. The most, consistent benefits have been demonstrated with the anticholinesterase drugs. Their efficacy in mild-to-modcrate Alzheimer's disease has been documented, but their effects on more severe dementia and other dementias such as vascular dementia and Lewy body dementia are being tried. Memantine is a new agent that holds promise, not least because it. acts on a different neurotransmitter system. A trial of adding drugs together has yet. to be tried, but no doubt will be. The armamentarium with which the clinician has to fight. Alzheimer's disease has never been greater.
Selected abbreviations and acronyms
- ADAS-Cog
Alzheimer's Disease Assessment Schedule-Cognitive Section
- ADL
activities of daily living
- CDR
Clinical Dementia Rating
- CIBIC+
Clinicians' Interview-based Impression of Change-plus
- IDDD
Interview for Deterioration in Daily living activities in Dementia
- MMSE
Mini-Mental State Examination
- NMDA
N-methyl-D-aspartate
- NPI
Neuropsychiatric Inventory
REFERENCES
- 1.Cummings J., Mega M., Gray K., et al. The Neuropsychiatrie Inventory: comprehensive assessment of psychopathology of dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 2.Finkel S., Burns A (eds). Behavioral and psychological symptoms of dementia (BPSD): a clinical and research update. Int Psychogeriatr. 2000;12(suppl 1) [Google Scholar]
- 3.Skoog I. Vascular aspects in Alzheimer's disease. J Neural Transm Suppl. 2000;59:37–43. doi: 10.1007/978-3-7091-6781-6_6. [DOI] [PubMed] [Google Scholar]
- 4.Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Pathological correlates of lateonset dementia in a multicentre, community-based population in England and Wales. Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 5.Rosen W., Mohs R., Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 6.Blessed G., Tomlinson B., Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 7.Saxton J., McGonigle-Gibson K., et al. Assessment of severely impaired patients: description and validation of a new neuropsychological test battery. Psychol Assess. 1990;2:298–303. [Google Scholar]
- 8.Reisberg B., Finkel S., Overall J., et al. The Alzheimer's disease Activities of Daily Living International Scale (ADL-IS). Int Psychogeriatr. 2001;13:163–181 . doi: 10.1017/s1041610201007566. [DOI] [PubMed] [Google Scholar]
- 9.Folstein M., Folstein S., McHugh P. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 10.DeJong R., Ostgerlund OW., Roy GW. Measurement of quality of life changes in patients with Alzheimer's disease. Clin Ther. 1989;11:545–554. [PubMed] [Google Scholar]
- 11.Teunisse S., Derix MM. Measurement of activities of daily living in patients with dementia living at home: development of a questionnaire. Tijdschr Gerontol Geriatr. 1991;22:53–59. [PubMed] [Google Scholar]
- 12.Galasko D., Bennett D., Sano M., et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S33–S39. [PubMed] [Google Scholar]
- 13.Hughes C., Berg L., Dantziger WL., Coben L., Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 14.Berg L. Clinical Dementia Rating. Br J Psychiatry. 1984;145:339. [PubMed] [Google Scholar]
- 15.Reisberg B., Ferris SH., de Leon MJ., Crook T. The Global Deterioration Scale (GDS) for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 16.Guy W (ed). Clinical Global Impressions. In: ECDEU Assessment Manual for Psychopharmacology. X Rockville, Md: US Dept of Health and Human services, Public Health Service, Alcohol Drug Abuse and Mental Health Administration, NIMH Psychopharmacology Research Branch 218-222. ADCS-CGIC; 1976 [Google Scholar]
- 17.Schneider LS., Olin JT., Doody RT., et al. Validity and reliability of the Alzheimer's Disease Cooperative Study - Clinical Global Impression of Change. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S22–S32. doi: 10.1097/00002093-199700112-00004. [DOI] [PubMed] [Google Scholar]
- 18.Knopman D., Schneider L., David K., et al. Long-term tacrine (Conex) treatment effects on nursing home placement and mortality. Neurology. 1996;47:166–177. doi: 10.1212/wnl.47.1.166. [DOI] [PubMed] [Google Scholar]
- 19.Knapp MJ., Knopman DS., Solomon PR., et al. A 30-week randomized controlled trial of high-dose tacrine in patients with Alzheimer's disease. JAMA. 1994;271:985–991. [PubMed] [Google Scholar]
- 20.Burns A., Rossor M., Hecker J., et al. The effects of donepezil in Alzheimer's disease: results from a multinational trial. Dementia Cognitive Geriatr Disord. 1999;10:237–244. doi: 10.1159/000017126. [DOI] [PubMed] [Google Scholar]
- 21.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 22.Rogers SL., Farlow MR., Doody RS., et al. A 24-week double-blind placebo-controlled trial of donepezil in patients with Alzheimer's disease. Neurology. 1998;50:136–145. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 23.Rogers SL., Doody RS., Pratt Rd., leni JR. Long-term efficacy and safety of donepezil in the treatment of Alzheimer's disease: final analysis of a US multicentre open-label study. Eur Neuropharmacol. 2000;10:195–203. doi: 10.1016/s0924-977x(00)00067-5. [DOI] [PubMed] [Google Scholar]
- 24.Winblad B., Engedal K., Soininen H., et al. A one-year randomised placebo-controlled study of donepezil in patients with mild to moderate Alzheimer's disease. Neurology. 2001;57:489–495. doi: 10.1212/wnl.57.3.489. [DOI] [PubMed] [Google Scholar]
- 25.Mohs R., Doody RS., Morris J., et al. A one-year, placebo-controlled preservation of function survival study of donepezil in Alzheimer's disease patients. Neurology. 2001;57:481–488. doi: 10.1212/wnl.57.3.481. [DOI] [PubMed] [Google Scholar]
- 26.Feldman H., Gauthier S., Hecker J., et al. A 24-week randomised doubleblind study of donepezil in moderate and severe Alzheimer's disease. Neurology. 2001;57:613–620. doi: 10.1212/wnl.57.4.613. [DOI] [PubMed] [Google Scholar]
- 27.Corey-Bloom J., Anand R., Veach J., et al. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate) a new acetylcholinesterase inhibitor in patients with mild to moderately severe Alzheimer's disease. Int J Geriatr Psychopharmacol. 1998;1:55–65. [Google Scholar]
- 28.Doriswamy M., Anand R., Hartman R., et al. Cognitive effects of rivastigmine, a new generation cholinesterase inhibitor in moderately severe to advanced Alzheimer's disease. Paper presented at the American Association of Geriatric Psychiatry Meeting. March 12-15, 2000. Miami, Fla. [Google Scholar]
- 29.Kumar V., Anand R., Messina J., et al. An efficacy and safety analysis of Exelon in Alzheimer's disease patients with concurrent vascular risk factors. Eur J Neurol. 2000;7:159–169. doi: 10.1046/j.1468-1331.2000.00046.x. [DOI] [PubMed] [Google Scholar]
- 30.Rosier M., Anand R., Cicin-Sain A., et al. Efficacy and safety of rivastigmine in patients with Alzheimer's disease: international randomised controlled trial. BMJ. 1999;318:633–638. doi: 10.1136/bmj.318.7184.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKeith I., Del Ser T., Spano PF., et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356:2032–2036. doi: 10.1016/S0140-6736(00)03399-7. [DOI] [PubMed] [Google Scholar]
- 32.Wilcock GK., Lillienfeld S., Gaens E. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer's disease: a multicentre randomised controlled trial. BMJ. 2000;231:1445–1449. doi: 10.1136/bmj.321.7274.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raskind MA., Pesking ER., Wessel T., et al. A 6-month, randomised, placebocontrolled trial with a 6-month extension. Neurology. 2000;54:2269–2276. doi: 10.1212/wnl.54.12.2261. [DOI] [PubMed] [Google Scholar]
- 34.Tariot PN., Solomon PR., Morris JC., et al. A 5-month randomized, placebo-controlled trial of galantamine in Alzheimer's disease. Neurology. 2000;54:2269–2276. doi: 10.1212/wnl.54.12.2269. [DOI] [PubMed] [Google Scholar]
- 35.Coyle J., Kershaw P. Galantamine, the cholinesterase inhibitor that alosterically modulated nicotinic receptors. Biol Psychiatry. 2001;49:289–299. doi: 10.1016/s0006-3223(00)01101-x. [DOI] [PubMed] [Google Scholar]
- 36.Grossberg G., Stahelin HB., Messina JC., et al. Lack of adverse pharmacodynamic drug interactions with rivastigmine and 22 classes of medications. Int J Geriatr Psychiatry. 2000;15:242–247. doi: 10.1002/(sici)1099-1166(200003)15:3<242::aid-gps110>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 37.Zurad E. New treatments in Alzheimer's disease: a review. Drug Benefits Trends. July 2002 [Google Scholar]
- 38.Winblad, Poritis Memantine in severe dementia. Int J Geriatr Psychiatry. 1999;14:135–146. doi: 10.1002/(sici)1099-1166(199902)14:2<135::aid-gps906>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 39.Burns A., Murphy D. Protection against Alzheimer's disease. Lancet. 1996;348:420–421. doi: 10.1016/S0140-6736(05)64533-3. [DOI] [PubMed] [Google Scholar]
- 40.Wang PN., Liao SQ., Liu RS., et al. Effects of estrogen on cognition, mood, and cerebral blood flow in AD. A controlled study. Neurology. 2000;54:2061–2066. doi: 10.1212/wnl.54.11.2061. [DOI] [PubMed] [Google Scholar]
- 41.Mulnard R., Cotman CW., Kawa C., et al. Estrogen replacement therapy for treatment of mild to moderate Alzheimer's disease. JAMA. 2000;283:1007–1015. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- 42.Henderson VW., Paganini-Hill A., Miller BL., et al. Estrogen for Alzheimer's disease in women: randomized, double-blind, placebo-controlled trial. Neurology. 2000;54:295–301. doi: 10.1212/wnl.54.2.295. [DOI] [PubMed] [Google Scholar]
- 43.Asthana S., Craft S., Baker LD., et al. Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with Alzheimer's disease: results of a placebo-controlled, double-blind, pilot study. Psychoneuroendocrinology. 1999;24:657–677. doi: 10.1016/s0306-4530(99)00020-7. [DOI] [PubMed] [Google Scholar]
- 44.Zandi PP., Carlson MC., Plassman BL., et al. Hormone replacement therapy and incidence of Alzheimer disease in older women. The Cache County Study. JAMA. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- 45.Wolozin B., Kellman W., Ruosseau P., Celesia GG., Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- 46.Jick H., Zornberg GL., Jick SS., Seshadri S., Drachman DA. Statins and the risk of dementia. Lancet. 2001;357:1627–1631 . doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 47.Rockwood K., Kirkland S., Hogan DB., et al. Use of lipid-lowering agents, indication bias, and the risk of dementia in community-dwelling elderly people. Arch Neurol. 2002;59:223–227. doi: 10.1001/archneur.59.2.223. [DOI] [PubMed] [Google Scholar]
- 48.Le Bars PL., Katz MM., Berman N., Itil TM., Freedman AM., Schatzberg AF. A placebo-controlled, double-blind randomized trial of an extract of Ginkgo biloba for dementia. JAMA. 1997;278:1327–1332. doi: 10.1001/jama.278.16.1327. [DOI] [PubMed] [Google Scholar]
- 49.Sano M., Ernesto C., Thomas RG., et al. A controlled trial of selegiline, alpha-tocopherol or both as treatment for Alzheimer's disease. The Alzheimer's Disease Cooperative Study. N Engl J Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 50.Seshadri S., Beiser A., Selhub J., et al. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 51.Clarke R., Smith D., Jobst KA., Refsum H., Sutton L., Ueland PM. Folate, vitamin B12 and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol. 1998;55:1449–1455. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- 52.Engelhart MJ., Geerlings M., Ruitenberg A., et al. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287:3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 53.Morris MC., Evans DA., Bienias JL., et al. Dietary intake of antioxidant nutrients and the risk of Alzheimer disease in a biracial community study. JAMA. 2002;287:3230–3237. doi: 10.1001/jama.287.24.3230. [DOI] [PubMed] [Google Scholar]
- 54.Schenk D., Barbour R., Dunn W., et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 55.Wilson RS., Mendes de Leon CF., et al. Participation in cognitively stimulating activities and risk of incident Alzheimer's disease. JAMA. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 56.Orgogozo JM., DArtigues JF., Lafont S., et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris). 1997;153:185–192. [PubMed] [Google Scholar]
- 57.Maia L., De Mendonca A. Does caffeine intake protect from Alzheimer's disease? Eur J Neurol. 2002;4:377–382. doi: 10.1046/j.1468-1331.2002.00421.x. [DOI] [PubMed] [Google Scholar]
- 58.Forette F., Seux M., Staesson JA., et al. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 1998;352:1347–1351. doi: 10.1016/s0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]