Abstract
Biological traits that are predictive of the later development of psychosis have not yet been identified. The complex, multidetermined nature of schizophrenia and other psychoses makes it unlikely that any single biomarker will be both sensitive and specific enough to unambiguously identify individuals who will later become psychotic. However, current genetic research has begun to identify genes associated with schizophrenia, some of which have phenotypes that appear early in life. While these phenotypes have low predictive power for identifying individuals who will become psychotic, they do serve as biomarkers for pathophysiological processes that can become the targets of prevention strategies. Examples are given from work on the role of the α7-nicotinic receptor and its gene CHRNA7 on chromosome 15 in the neurobiology and genetic transmission of schizophrenia.
Keywords: schizophrenia, development, genetics, chromosome 15, nicotinic receptor, hippocampus, inhibition, auditory evoked potentials, eye movement
Abstract
Aun no se han identificado los rasgos biológicos que predicen el desarrollo posterior de una psicosis. La naturaleza compleja y multideterminada de la esquizofrenia y de otras psicosis hace poco probable que algún biomarcador aislado sea lo suficientemente sensible y aspecífico para identificar con certeza sujetos que más tarde llegarán a ser psicóticos. Sin embargo, la investigación genética actual ha comenzado a identificar genes que se asocian con la esquizofrenia, algunos de los cuales tienen fenotipos qua aparacen precozmente en la vida. Aunque estos fenotipos tienen un bajo podar predictor para identificar individuos qua llegarán a ser psicóticos, ellos sirven como biomarcadores de procesos fisiopatológicos que pueden llegar a ser los blancos da las estrategias da prevención. Ejemplos de esto provienen del trabajo acerca del papel del receptor nicotínico α7 y su gen CHRNA 7 en el cromosoma 15 en la neurobiología y en la transmisión genética de la esquizofrenia.
Abstract
Les particularités biologiques prédictives du développement ultérieur d'une psychose n'ont pas encore été identifiées. La nature complexe, multi-factorielle de la schizophrénie et d'autres psychoses rend peu probable qu'un seul biomarqueur soit a la fois suffisamment sensible et spécifique pour permettre d'identifier sans ambiguïté les individus qui deviendront psychotiques. Cependant, la recherche génétique actuelle a commence a identifier des gènes associes a la schizophrénie, certains ayant des phénotypes qui apparaissent précocement dans le cours de la vie. Alors que ces phénotypes ont un faible pouvoir prédictif pour identifier les individus qui développeront une psychose, ils servent de biomarqueurs pour les processus physiopathologiques pouvant devenir les cibles des stratégies de prévention. Des exemples sont fournis par un travail sur le rôle du récepteur α7-nicotinique et de son gène CHRNA7 situe sur le chromosome 15, en neurobiologie et dans la transmission génétique de la schizophrénie.
Identification of the biological concomitants of psychiatric illnesses such as schizophrenia is a goal that is pursued for a variety of purposes. First is the hope that, as in other branches of medicine, biological measurements or markers will increase the precision of diagnosis. Second is the possible usefulness of the markers to subtype groups that may respond to particular medications and to monitor the progress of the treatment. A third hope, the subject of this essay, is the possibility that a biological process will be observable before the onset of illness, so that vulnerable individuals can be identified early and perhaps treated before the full development of psychopathology If there were markers that fulfilled the first two criteria, then it would be straightforward to consider their use for early identification and preventive treatment. However, no biological marker has yet been recognized that definitively fulfills any one of these criteria, so that the application to prevention has remained uncertain. This paper will suggest that the failure to find biological markers that fit the first two criteria is a consequence of the complex, multidetermined pathophysiology of major mental disorders, but that this failure does not preclude the possibility that biomarkers will be helpful in designing strategies for prevention of schizophrenia.
The use of a biological marker to identify someone as ill prior to the onset of clinically detectable symptoms carries enormous responsibility when the illness is expected to be serious and not amenable to a curative treatment. Even if the marker has high validity, examination of individual persons for its presence would be ethically problematic. When the marker has lower validity the ethical problem would seem to be increased. Specifically, labeling of a child as a future schizophrenic based on our present understanding of the biology of the illness seems unconscionable. Nevertheless, biological markers may indicate the presence of a pathophysiological process that can be addressed with a preventive treatment. Therefore, the identification of biomarkers prior to onset of psychosis has enormous potential importance for the design of future preventive strategies. The success of preventive treatments such as prenatal folic acid supplementation for a wide variety of conditions, including cleft palate and neural tube defects, suggests that early intervention may be surprisingly effective and often relatively benign, so that prevention could be applied to individuals for whom there is little certainty that they would have disease in the future. Thus, a paradox is that identification of biomarkers for predictive purposes, which may be unethical, does not preclude their ethical use for the design of prevention strategies.
Most cases of schizophrenia occur during late adolescence and early adulthood. Although there is often a prodrome during which signs of illness are present, most individuals who develop schizophrenia have had some period, generally from childhood through early adolescence, during which they did not have enough symptoms to be declared ill.1 The question relevant to the search for early biomarkers is whether the neurobiological substrates of illness are already present, perhaps from birth, and only awaiting adolescence to become manifest as a clinical behavioral syndrome or whether adolescence itself somehow causes the illness. Despite the profound biological change that accompanies adolescence, mental illness stands out as the only major category of illness that occurs during the transition into adulthood. Therefore, one goal for the investigation of biomarkers is to use them to establish when during development the pathophysiological defects associated with schizophrenia first occur, so that the appropriate window of time for intervention can be identified.
The emphasis on the genetic basis of schizophrenia and other major mental disorders suggests that a similar emphasis on genetic factors should influence the search for early biomarkers for psychosis. In contrast to the peak incidence of schizophrenia in late adolescence, genetic factors are obviously present from conception, and their neurobiological expression could therefore begin as early as the fetal period. In fact, more genes are expressed more robustly during fetal development than at any other time during the life cycle, so that a reasonable presumption is that their maximal effect would be expected in that period. Few of the currently investigated candidate genes for schizophrenia or bipolar disorder have expression patterns that suggest maximal influence at any other time during the life cycle. Therefore, it is reasonable to search for biological markers as early as during the period of fetal development.
Genetic risk schizophrenia
While genetic risk is certainly a reasonable clue to follow to search for the developmental biomarkers of schizophrenia, there are inherent difficulties in this strategy. Schizophrenia is a genetically complex illness. Although the heritability for schizophrenia is estimated to be as high as 70%, the illness clearly does not have a pattern of inheritance in any population or even in single families that is consistent with the effect of a single gene. Thus, like many common illnesses such as diabetes and hypertension, it is more likely that multiple genes are involved. Multigenic illnesses were once considered unanalyzable by genetic linkage techniques, but the use of large sample sizes, dense chromosomal maps, and improved statistical methodology has led to the detection of a number of genetic loci. For some of these loci, promising candidate genes have been identified and, for some of these genes, polymorphisms have been discovered that are associated with schizophrenia and would seem to alter gene function to produce a neurobiological effect. Most of the genes identified have some role in the development or function of neurotransmission.
Consideration of the finding of multigenic inheritance illuminates the problem of detecting biomarkers for schizophrenia. For example, if two genes on different chromosomes are hypothesized to be responsible for all cases of schizophrenia, then, for 1% of the population to have schizophrenia, the frequency of the allele associated with schizophrenia must be approximately 5% per chromosome for each gene. An individual would then have 5% chance of inheriting a disease allele for the first gene from mother and 5% from father, for a total of approximately 10%. For the second gene, a similar consideration applies. The combined probability of inheriting disease alleles in both genes would be 10% times 10%, or 1% total risk. If a parent had schizophrenia, so that he or she carried disease alleles for both genes, the probability of transmitting both would be 50% for each disease allele, since one of two chromosomes, one carrying the disease-associated allele and one not, is transmitted to an off-spring through the sperm or egg. The probability of transmitting disease alleles for both genes would be 50% times 50%, or 25%. Since the observed transmission of risk from parent to child is only about 10%, the actual genetic structure is more complicated than a simple two-gene model. However, the two-gene model comes closer than a one-gene model, for which parent–child transmission would be 50%. Additional complexities could include the involvement of more than two genes or environmental factors to produce the illness, which could range from perinatal developmental injury to psychosocial stressors.
The implication of this theoretical exercise for the early assessment of risk factors is that disease allele frequencies of 5% per chromosome (termed the allele frequency) or about 10% per individual, considering the possibility of the occurrence of a disease allele on either of the two chromosomes, mean that a biomarker associated with a specific gene would occur in about 10% of the population. Thus, even if we perfectly understood both the molecular biology and the associated neurobiological deficits for one of the genes that convey risk for schizophrenia, and if that gene were involved in all cases of schizophrenia, then we would still detect its presence in 10% of the population, which would include the 1% who would actually develop schizophrenia. Treatment of 10% of adolescents with neuroleptic drugs, to delay the onset of psychotic symptoms that might occur in only 1 out of 10 of the identified individuals would probably be viewed as medically unsound from the perspective of risk and benefit. Of course, if we could identify all the genes and all their interactions with each other and with environmental factors, then we might be able to restrict the identification to a subset of individuals who were more likely to develop schizophrenia, but such a complete understanding has not yet been achieved.
The actual findings with genetic linkages and candidate genes associated with risk for schizophrenia suggest that the theoretical two-gene exercise underestimates the prevalence of genetic risk in the population. For example, we have conducted genetic linkage analysis for schizophrenia in the National Institute of Mental Health (NIMH) Genetics Initiative families.2 The first 88 families were selected to have two ill individuals, generally a pair of siblings, who met the then current diagnostic criteria (Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised [DSM-III-R]) for schizophrenia or schizoaffective disorder, with at least one individual meeting criteria for schizophrenia. The families were collected by a consortium of investigators at Harvard University, Washington University, and Columbia University and the genotyping was performed by Millennium Pharmaceuticals. Several genetic analyses of the sample have been published, which point to loci on a number of chromosomes. A maximum likelihood statistical analysis was used to determine the association between genetic markers on each of the chromosomes and the inheritance pattern of the illness in each family. The analysis determined the common logarithm of the ratio of the odds that there is a true linkage between chromosomal markers and the illness, as opposed to a chance association, termed the LOD score. Scores above 3.3 are considered indicative of linkage, through lower LOD scores also considered worthy of further investigation. Chromosomes 15 and 10 contain two of the more significance loci (Table I).
Table I. Genetic linkage in the National Institute of Mental Health (NIMH) pedigrees. Autosomal dominant model f(A)=0.0045. The HLOD is the LOD score under the assumption of genetic heterogeneity, ie, different genetic loci responsible for transmission of schizophrenia in different subgroups of families. The scores were computed using pairs of adjacent genetic markers. α is the estimate from the heterogeneity analysis of the proportion of linked pedigrees at any given locus.
| Locus | HLOD score (α) |
| 6q13-21 | 1.11 (0.35) |
| 9q32 | 1.09 (0.40) |
| 10p13 | 2.40 (0.45) |
| 13q32 | 0.58 (0.25) |
| 15q14 | 3.97 (0.95) |
| 15q21 | 1.00 (0.50) |
| 17p13 | 0.93 (0.40) |
| 20q13 | 1.56 (0.45) |
Significance of a linkage finding is based on several factors, the most relevant of which is the pathophysiological significance of the underlying putative genetic variant that causes the illness. Genetic linkage does not directly identify the variant; rather, it finds genetic markers that are close to or linked to an undiscovered genetic variant that actually contributes to risk for the illness. Thus, other factors can equally influence the LOD score, such as genetic homogeneity of the population, so that the number of other possible genetic causes of schizophrenia in the population is limited. Accordingly, although findings with chromosomes 15 and 10 have been replicated in other populations, there are considerable differences in genetic findings for schizophrenia across different populations and studies. Thus, the selection of chromosomal loci in this example is only one of several strategies that could be used.
The more powerful genetic analytic strategies use likelihood analyses that assume a model of inheritance, which can be either dominant or recessive. Additional parameters that are specified are the allele frequency of each putative allele and the penetrances, which are the probability that each putative genotype will produce illness. Thus, if A and a are two alleles of a putative gene associated with risk for schizophrenia, and the A variant is associated with risk and the a variant is not, there would be four possible genotypes if we list the paternally inherited allele, followed by the maternally inherited allele: AA, aa, Aa, and aA. The frequency of each genotype is the product of the two allele frequencies. For a dominant genetic model with complete penetrance, the penetrance is 1.0 for any genotype containing A. For a gene with only two alleles, the two allele frequencies add to 1.0, and the sum of the products of the penetrances of each genotype and the frequency of each genotype is the frequency of the illness in the general population. The frequency of the illness in the general population (0.01) is a piece of real data that anchors the model in reality Thus, in the example in Table II, the sum of the gene frequencies, which are the products of the two allele frequencies, is 1, accounting for all genotypes. The penetrances of the three genotypes that contain A, either as the homozygote AA or the heterozygotes Aa and aA, are 1.0, because this is a completely dominant illness. The penetrance of aa, a genotype that does not convey risk, is 0.00103, which essentially accounts for the possibility of other genetic and nongenetic causes of illness that do not involve this gene. The sum of the product of the gene frequencies is 0.01, which is the frequency of schizophrenia in the population. These parameters were used in the initial analyses for a Canadian linkage study that identified a locus on chromosome 1 with the highest statistical confidence level yet reported for a genetic linkage finding in schizophrenia.3 The assumptions of the model, in addition to dominant transmission, is that the gene that conveys risk for schizophrenia is highly penetrant so that the genotypes containing A always produce illness and there are very few cases (1/10) that do not have this particular genetic cause. The model posits that schizophrenia is caused by a gene that has an extremely deleterious effect on brain function, resulting in a very severe illness, chronic schizophrenia. A similar model has been used to discover other major genetic influences in schizophrenia.4 In the Canadian study, it turned out that a similar recessive model better explained the data.
Table II. An autosomal dominant model of schizophrenia for a single gene.
| Genotype | Allele frequency | Gene frequency | Penetrance | Proportion affected |
| AA | 0.0045x0.0045 | 0.00002025 | 1 | 0.00002 |
| Aa | 0.0045x0.9955 | 0.00447975 | 1 | 0.00448 |
| aA | 0.9955x0.0045 | 0.00447975 | 1 | 0.00448 |
| aa | 0.9955x0.9955 | 0.99102025 | 0.00103 | 0.00102 |
| 1.00000000 | 0.01000 |
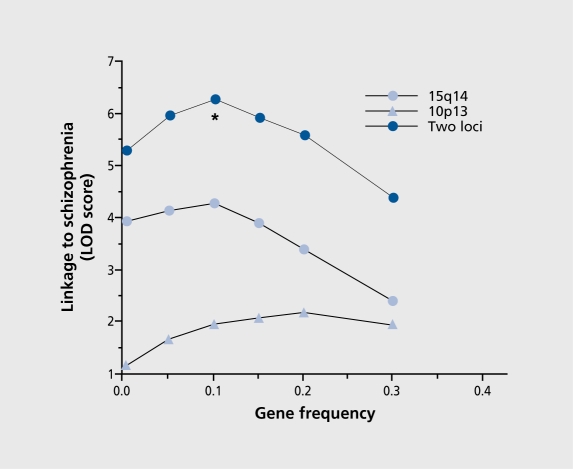
Returning to the NIMH families, the finding of multiple genetic signal calls this simple model into question. Considering only the two most positive linkages, chromosomes 15 and 10, there would seem to be two possibilities. One possibility is that there are two types of schizophrenia, a 15 type and a 10 type, where the data are best explained as two independent findings, with some families having one type and some having another, a heterogeneous model. A second possibility is that both genetic factors are active in the same families and that schizophrenia results from their additive effect. This model implies that neither gene by itself produces an effect that is completely devastating to normal brain function, but when deficits from the two genes occur together, a threshold is crossed, and a brain dysfunction occurs that results in psychosis. The genetic model becomes more complex, but the products of genotypes and penetrances must still add to 0.01, the population frequency of schizophrenia (Table III). For the additive model, if we allow the frequency of the putative disease variants in each of the two genes to vary we see that the maximum likelihood occurs with much higher allele frequencies than the 0.0045 frequency that we initially assumed (Figure 1). At this high frequency, penetrance is also reduced (Table III), indicating that individuals who carry the genotypes associated with illness now have only a 25% probability of having the illness. A comparison of this additive model with the heterogeneous model, by subtracting their respective maximum likelihood, reveals that the additive model is significantly more likely. Thus, genetic linkage data in the NIMH population supports the hypothesis that schizophrenia is caused by multiple genes acting together and the variants associated with the illness are common in the population.
Figure 1. Relationship of maximum LOD score to gene frequencies for two locus linkage to schizophrenia. This model produces a peak LOD score at allele frequencies of 0.1 , which is significantly greater than the score derived from the heterogeneous model (Chisquare=14.54; df=1; P=0.000069).
Reproduced from reference 2: Freedman R, Leonard S, Olincy A, et al. Evidence for the multigenic inheritance of schizophrenia. Am J Med Genet. 2001;105:794-800. Copyright © 2001, Wiley-Liss, Inc.
Table III. A model of schizophrenia requiring two genetic loci.
| Penetrances for multigenic threshold model (fA=0.1; fB=0.1) | ||||
| Trait locus 1 | ||||
| AA | Aa | aa | ||
| BB | 0.25 | 0.25 | 0.001 | |
| Trait locus 2 | Bb | 0.25 | 0.25 | 0.001 |
| bb | 0.001 | 0.001 | 0.001 |
This hypothesis is further supported by another type of genetic data. For many of the linkage signals, candidate genes are now being identified. At chromosomal region 15q14, the most extensively studied candidate gene is CHRNA7, the gene for the α7-nicotinic acetylcholine receptor. This gene was first identified by neurobiological research into a pathophysiological abnormality in schizophrenia, the failure to inhibit the P50 auditory evoked response to the second of paired stimuli.5 This inhibitory deficit, one of many such physiological sensory gating deficits characterized in schizophrenia, is related to patients’ inability to maintain sustained attention, one of the notable neuropsychological deficits of schizophrenia. These sensory gating deficits have been related clinically to patients' filtering deficits.6 They describe being overwhelmed or flooded by sensory stimuli in their environment that most normal subjects can ignore. Deficits in prepulse inhibition of startle, poor performance in the continuous performance test, and various smooth pursuit eye tracking abnormalities have all been characterized as failures in inhibitory and sensory gating function in schizophrenia. Deficits in inhibitory neurons, including failures of migration, diminished expression of inhibitory neurotransmitters, and loss of the neurons themselves, have all been found in postmortem studies of brain tissue for persons who had schizophrenia.7 Deficits in expression of α7-nicotinic receptors, which are highly expressed on many interneurons, are consistent with the other deficits found in these interneurons. Animal models with genetically diminished expression of the α7-nicotinic receptor have deficits in inhibition of auditory evoked responses that resemble the deficit in schizophrenia.8 These models suggest that nicotinic receptor activation of interneurons in the hippocampus is a critical mechanism in sensory inhibition. Thus, deficits in inhibition in general and diminished nicotinic receptor activation of inhibitory interneurons in particular appear to be two of the neurobiological features of schizophrenia (Figure 2).
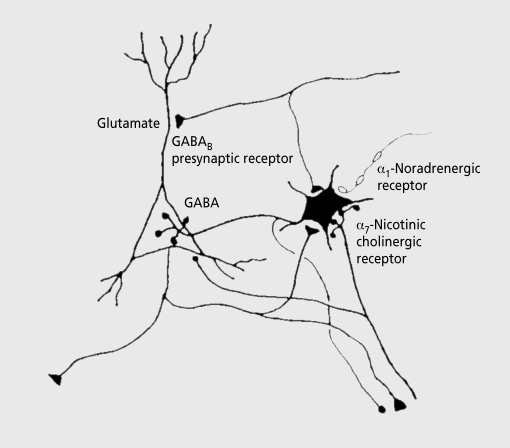
Figure 2. Neurophysiological studies of the P50 sensory gating phenomena have implicated the pyramidal neurons of the hippocampus as a source of the wave. Interneurons in the hippocampus are responsible for inhibition of pyramidal neuron response in the conditioning-testing or paired stimulus paradigm. These interneurons are excited by cholinergic innervation via α7-nicotinic cholinergic receptors and inhibited by adrenergic receptors. GABA, γ-aminobutyric acid.
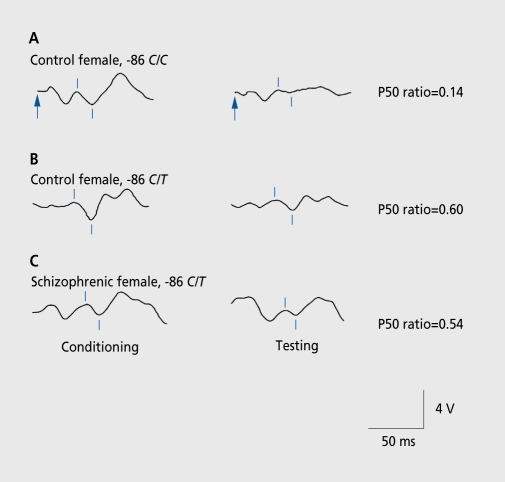
Genome-wide linkage studies initially related the loss of P50 inhibition in schizophrenia to the chromosomal region 15q14 locus of CHRNA7. Mutating screening in the CHRNA7 gene resulted in the discovery of single nucleotide polymorphisms in the promoter and other abnormalities that have been associated with schizophrenia itself and also with the failure to inhibit P50 responses in schizophrenia (Figure 3). While the significance of all of these polymorphisms is still being sorted out, the allele frequency of these polymorphisms is relatively high, as was predicted from the linkage data.9 Similarly high frequencies have been reported for most other putative candidate genes for schizophrenia.10 Taking all these genes into account, it would seem that the majority of the population has at least one of the genetic risk factors for schizophrenia.
Figure 3. Effects of a single nucleotide polymorphism in the promoter of CHRNA7 on P50 sensory gating. The normal subject with more common alleles (-86 C/C) has normal suppression of P50, measured as a low P50 test to conditioning amplitude ratio. The subjects with the polymorphism (-86 C/T) have higher, more abnormal ratios. The physiological abnormality is expressed regardless of whether the subject is normal or has schizophrenia.
Reproduced from reference 9: Leonard S, Gault J, Hopkins J, et al. Association of promoter variants in the α7-nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry. 2002;59:1085-1096. Copyright © 2002, American Medical Association.
Thus, the somewhat unexpected result of genetic research to date is that genetic risk is much more wide-spread than initially posited. Identification of individuals at risk by genetic means alone is not likely to select individuals who have a high probability of actually developing schizophrenia. However, the situation is not as hopeless from the perspective of prevention. First, for CHRNA7 and for many of the other genes being discovered for schizophrenia, the neurobiological phenotypes are being elucidated. Most of the genes are associated with dysfunction in the mechanisms of neurotransmission. For CHRNA7, there is an apparent link to an inhibitory dysfunction that can be measured physiologically, in both animal models and humans. To the extent that this dysfunction and similar dysfunctions can be traced through development, a biological developmental course of schizophrenia can be ascertained, so that the window for possible prevention can be determined. While this time course could have been established without the genetic information, the genetic associations and linkages relate the physiological dysfunction, eg, diminished sensory gating, to a well-identified biological element, eg, diminished activation of α7-nicotinic receptors. The dominant model with complete penetrance posited a model of pathophysiology in which a single genetic deficit produces a near catastrophic effect on brain function that results in schizophrenia. The more complex model posited here suggests that schizophrenia may be the coincidence in a single individual of multiple deficits, none of which in themselves are particularly problematic. Small changes in the pathophysiological effect of any one of these deficits could have significant effects on the development of illness.
The developmental time course of physiological dysfunction in schizophrenia
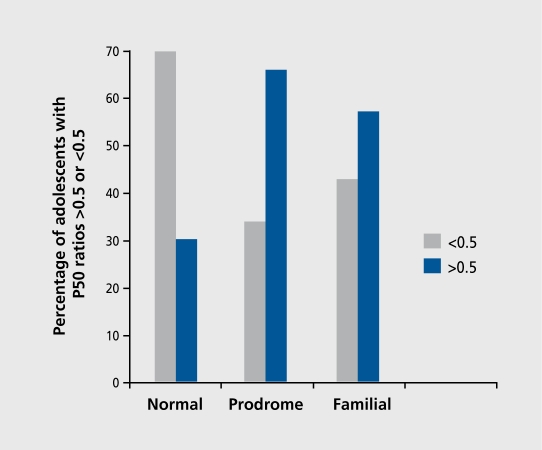
In adolescence, persons at risk for schizophrenia can be identified in two ways―by the individual having a parent who has schizophrenia, particularly in a family that already has other cases of schizophrenia, or by the individual displaying prodromal features of schizophrenia. Palau is a small island nation in Micronesia in which many individuals with schizophrenia have been found in large, multiply affected families. Adolescents in these families have an elevated prevalence of diminished P50 inhibition. Adolescents identified by self-report and follow-up interview as displaying prodromal symptoms also have an elevated prevalence of diminished P50 inhibition.11 Thus, diminished P50 inhibition is present in adolescents at risk for schizophrenia before the onset of psychotic symptoms (Figure 4).
Figure 4. Percentages of P50 ratios in the abnormal range (amplitude of test wave/amplitude of conditioning wave>0.5) is elevated in adolescents with prodromal symptoms of schizophrenia or with first-degree relatives who have schizophrenia. Data are from a study conducted on the island Palau by Myles-Worsley etal.11 .
Attempts to measure P50 in younger children have shown that normal adult levels of P50 inhibition are present in only about half of preadolescent children. In these younger children, diminished levels of inhibition do not correlate with any obvious psychopathology or familial risk. Rather, the children with diminished P50 inhibition tend to display increased levels of activity that are well within the normal range for children of their age. P50 inhibition is not solely determined by linkage to CHRNA7. Adrenergic activity, for example, diminishes inhibitory interneuron response and is correlated with diminished P50 inhibition in normals. While it is not known whether normal children with diminished P50 inhibition have increased adrenergic activity it is known that children are much better able than adults to tolerate psychotomimetic drugs, such as amphetamine and ketamine, both of which increase catecholaminergic activity. Whether the apparent maturation of P50 inhibition at the end of adolescence and the loss of relative protection against psychotomimetic effects of drugs and the peak incidence of development of schizophrenia during this transition to adulthood have a common neurobiological basis is a question that has yet to be addressed.
Adrenergic activity itself varies considerably with the state of alertness and stress. Experimental elimination of adrenergic activity by pharmacological means in animals results in the normalization of the inhibition of auditory responses to repeated stimuli. Similar experiments in normal children would be informative about the neurobiology of P50 inhibition, but exposure of these children to medication is problematic. However, loss of adrenergic activity is one of the physiological concomitants of rapid eye movement (REM) sleep. Therefore, a natural state exists in which P50 inhibition can be measured without the interference of adrenergic activity. In adults, recordings during REM show the same differences in P50 inhibition between normals and schizophrenics that have been observed during the waking state.12 For schoolaged children, the REM state would also be achieved after sleep deprivation or by all-night recording, but for newborns, REM is a frequently achieved state during the day. Therefore, we have recorded P50 inhibition during the first 3 months of life.
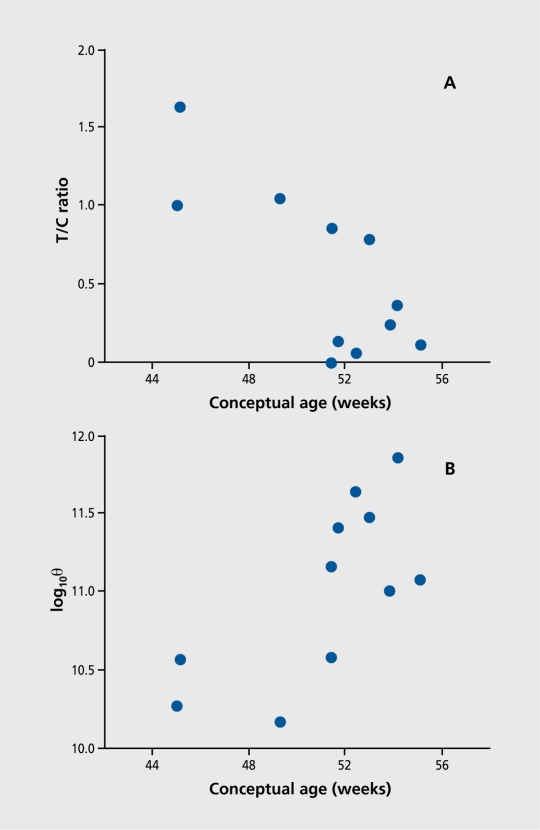
In initial experiments, infants could indeed be recorded during REM sleep and P50 responses were elicited.13 The degree of inhibition was correlated with gestational age, calculated from conception. This method is used to avoid the confound of premature birth in the calculation of perinatal development. By the first 3 months of life, most infants have developed near-adult levels of P50 inhibition (Figure 5). Recording of infants at risk for schizophrenia is a logical next step in determining whether or not the neurobiological processes that result in abnormal P50 inhibition in schizophrenia are present as early as the neonatal period.
Figure 5. Relationship between age and selected electrophysiological variables. A. P50 T/C ratio generally decreases (ie, sensory gating improves) with advancing age. B. Electroencephalogram (EEG) spectral power in the θ band (4-8 Hz), associated with rapid-eye movement (REM) sleep in the hippocampus, also increases in the same time period.
Reproduced from reference 12: Kisley MA, Olincy A, Robbins E, et al. Sensory gating impairment associated with schizophrenia persists into REM sleep. Psychophysiology. 2003;40:29-38. Copyright © 2003, Blackwell Publishing.
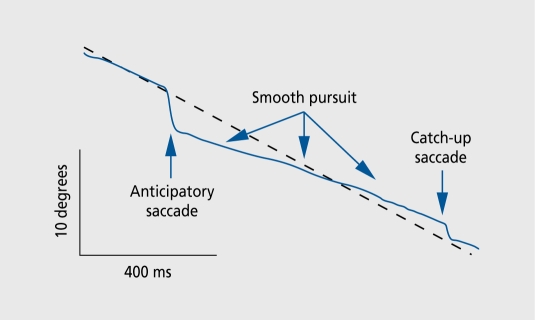
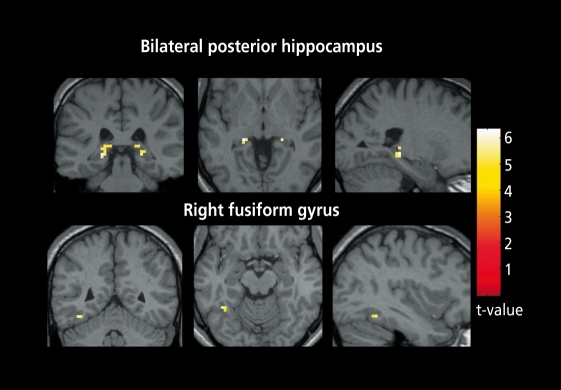
Other inhibitory dysfunctions associated with schizophrenia are also present during childhood. Persons with schizophrenia have abnormal smooth pursuit eye movement tracking. The task consists of following a slowly moving target, which the subject must follow with his or her eyes, while eye movement is monitored using infrared reflectometry. Normal persons are able to move their eyes precisely, so that the image of the target always remains in the small foveal region of the retina. Persons with schizophrenia and some of their relatives have diminished performance of the task. One of the elements of this abnormality is the inability to inhibit saccadic eye movements, so that their eyes jump ahead of the target and then wait for the target to catch up (Figure 6). Functional magnetic resonance imaging during the task reveals increased hemodynamic activity in the hippocampus (Figure 7). 14 This increased activity is consistent with the putative decreased hippocampal inhibition that has also been proposed as a mechanism for the diminished inhibitory gating of the P50 response.15
Figure 6. In schizophrenia, there are several abnormalities in smooth pursuit eye movements. One of these is elevated frequency of anticipatory saccades, the failure to inhibit fast or saccadic eye movements that cause the eyes to jump ahead of the slowly moving target. Broken line, target position; unbroken line, eye position.
Figure 7. Functional magnetic resonance imaging of 14 schizophrenics and 14 normals performing smooth pursuit eye movements. The images are comparisons between the two groups. The schizophrenics have increased hemodynamic activity in the hippocampus, consistent with loss of inhibition.
Reproduced from reference 14: Tregellas JR, Tanabe JL, Miller DE, Ross RG, Olincy A, Freedman R. Neurobiology of smooth pursuit eye movement deficits in schizophrenia: an fMRI study. Am J Psychiatry. 2004;161:315-321 . Copyright © 2004, American Psychiatric Association.
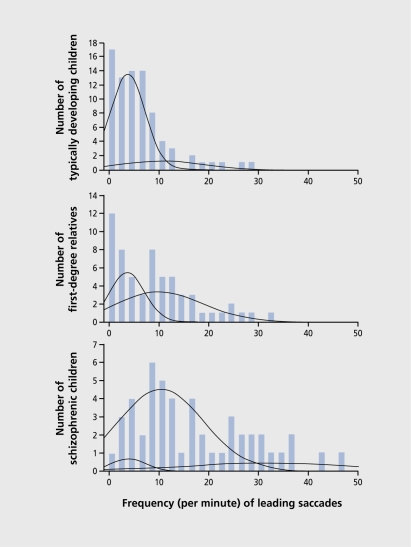
About half of children with a parent who has schizophrenia also have abnormal smooth pursuit eye movements with the intrusion of saccades, which can be detected as early as age 6.16 This abnormality is thus the earliest studied physiological dysfunction associated with the eventual appearance of schizophrenia (Figure 8). The abnormality is associated with neuropsychological evidence of attention dysfunction, one of the cognitive abnormalities found in children at risk for schizophrenia.17 Thus, the use of physiological dysfunctions associated with the genetic risk for schizophrenia to identify putative windows during which preventive efforts might be possible points to the expression of these dysfunctions before the onset of schizophrenia. Dysfunction is certainly present in adolescents at risk and in school-aged children, but the development of inhibitory function in the early perinatal period suggests that it is reasonable to look even earlier.
Figure 8. Admixture analysis of the frequency (per minute) of leading saccades during a smooth-pursuit eye movement task in 189 children, aged 6 to 15 years. For the typically developing children, 81% perform in the lowest (best-performing) mode and 19% in the moderately elevated mode. First-degree relatives of persons with schizophrenia are equally distributed, with 50% in the lowest mode and 50% in the moderately elevated mode. For children who have schizophrenia, 6% have leading saccade frequencies in the lowest mode, 86% in the moderately elevated mode, and 8% in the severely elevated mode.
Reproduced from reference 16: Ross RG. Early expression of a pathophysiological feature of schizophrenia: saccadic intrusions into smooth-pursuit eye movements in school-age children vulnerable to schizophrenia. J Am Acad Child Adolescent Psychiatry. 2003;42:468-476. Copyright © 2003, Lippincott, Williams and Wilkins.
The developmental effects of gene dysfunction
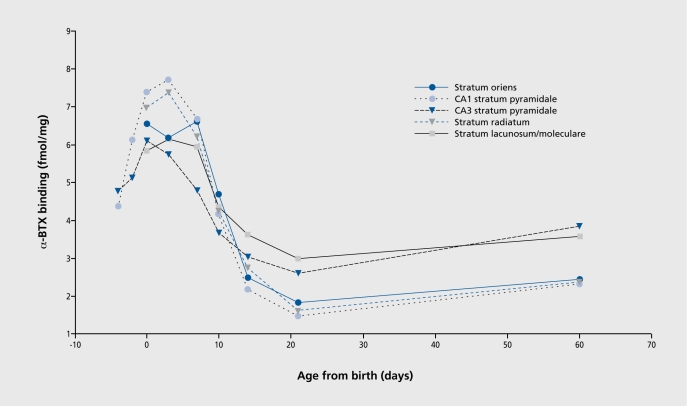
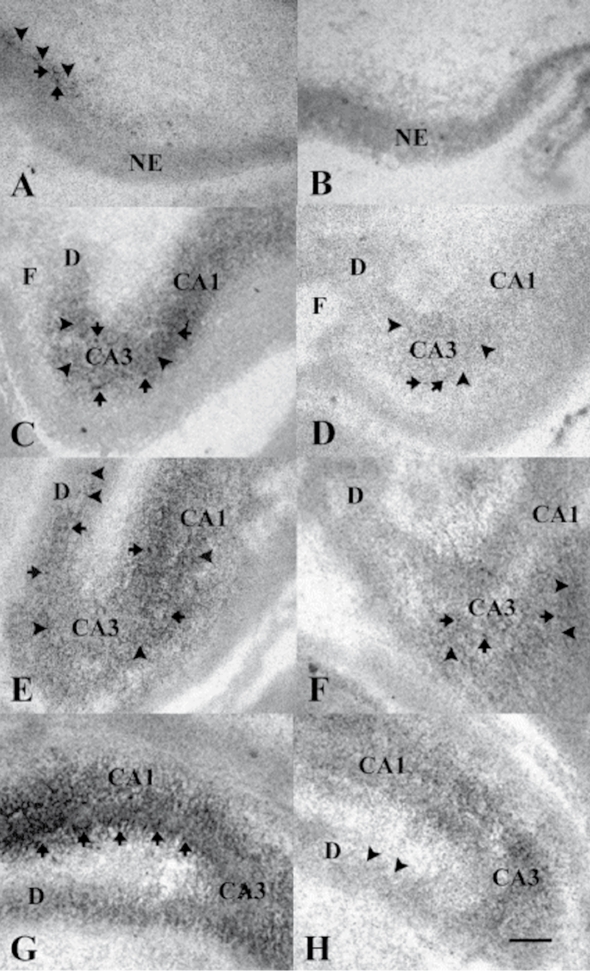
The identification of a window for a developmental effect is a major clue to the mechanism of developmental abnormality in schizophrenia, but it does not immediately identify possible mechanisms. An advantage of a genetically associated pathophysiological feature is that the cellular mechanism can be immediately deduced from the gene's product. Most of this work is necessarily performed in animal models, because neurobiological investigation at the cellular level cannot generally be performed in human beings. As in the previous section, the example will come from our work on CHRNA7 and inhibitory brain mechanisms, but similar examples are possible with many of the other genes currently being investigated for schizophrenia. α7-Nicotinic receptors are formed early in development, when neurons first differentiate from the neuroepithelium. During adult life in rodents, the expression is particularly marked in the hippocampus.18,19 In primates including humans, there is prominent expression in the hippocampus as well, but also in the cingulate cortex and in the nucleus reticularis thalami, which is a thin sheet of inhibitory neurons that surrounds the thalamus.20 In rodents and in humans, hippocampal pyramidal neurons have diminished response to repeated stimuli, making the hippocampus is a putative source of the diminished P50 response to repeated stimuli that can be modeled in rodents.15 α7-Nicotinic receptors are found both presynaptically and postsynaptically throughout the hippocampus, the area studied most thoroughly at the ultrastructural level using electron microscopy α7-Nicotinic receptors are found within glutamate synapses, where they anchor to common postsynaptic densities.21 However, the most prominent expression is postsynaptic on interneurons throughout the hippocampus (Figure 9). During perinatal development there is also prominent expression on pyramidal neurons in the CA1 region, but this expression soon disappears early in postnatal development.22 Indeed, the perinatal expression of α7-nicotinic receptors is greater than at any other time during the life cycle (Figure 10). The DBA/2 inbred mouse strain has lower levels of α7-receptors in the hippocampus and diminished inhibition of the hippocampal evoked response to the repeated auditory stimuli. Although P50 has several sources in human brain, it can be recorded from the hippocampus. The DBA/2 mouse also has polymorphisms in the CHRNA7 gene, outside the amino acid coding region, which correlate with diminished expression of the gene. Thus, the DBA/2 mouse mimics pathophysiological features found in many persons with schizophrenia, ie, diminished expression of α7-nicotinic receptors and polymorphisms in CHRNA7, as well as diminished inhibition of the P50-type response to repeated stimuli.8 In DBA/2 mice, the expression of α7-nicotinic receptors is diminished in the CA3 region of the hippocampus, but is relatively abundant in some areas of CA1. These differences are maintained when the region of mouse chromosome 7 that contains CHRNA7 is selectively bred onto the genetic backgrounds of other inbred strains of mice.23 During development, DBA/2 mice lag behind comparison strains in the development of an adult pattern of α7-nicotinic receptor expression (Figure 11). 24
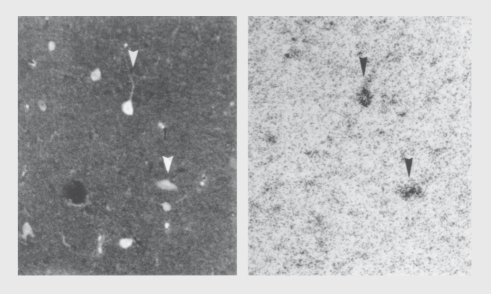
Figure 9. Dual labeling with an antibody for the inhibitory neurotransmitter GABA (left) and radioactive α-bungarotoxin (right), which labels α7-nicotinic receptors, demonstrates expression of these receptors on inhibitory interneurons in the rat hippocampus. For other examples, see reference 1. GABA, γ-aminobutyric acid.
Figure 10. Expression of α7-nicotinic receptors in the hippocampus, measured by labeling with α-bungarotoxin (α-BTX), in rats during early development and adulthood.
Reproduced from reference 23: Adams CE, Stitzel JA, Collins AC, Freedman R. α7-Nicotinic receptor expression and the anatomical organization of hippocampal interneurons. Brain Res. 2001;922:180-190. Copyright © 2001, Elsevier.
Figure 11. Developmental time course of α7-nicotinic receptors in DBA/2 mice (right) and a control strain, C3H (left). Prenatal days 13, 16, 17, and 18 are shown from top to bottom.24 A. Binding for α-bungarotoxin (α-BTX) is initially observed in the dorsal portion of the hippocampal anlage of C3H mice on embryonic day 13 (E13). Binding is both diffuse (arrowheads) and clumped (arrows) in appearance. No α-BTX binding is observed in the neuroepithelium (NE). B. Binding of α-BTX is not detectable in the developing hippocampus of DBA/2 mice on E13. C. Binding of α-BTX is increased in density and is present in both hippocampal areas CA1 and CA3 of the C3H mouse strain on E16, but not in the immature dentate gyrus (D). The pattern of hippocampal α-BTX binding is again comprised of a mixture of diffuse (arrowheads) and clumped (arrows) silver grains. D. Binding of α-BTX is initially detected in the hippocampal formation of DBA/2 mice on E16. The level of binding is higher in area CA3 than in area CA1 , while no binding is observed in the dentate gyrus. Scattered clumps of silver grains (arrows) are seen within a more diffuse background (arrowheads). Overall, the level of α-BTX binding in DBA/2 mouse hippocampus on E16 is much lower than that observed in C3H mouse hippocampus at the same age. E. Binding of α-BTX is increased in density in hippocampal areas CA1 and CA3 and is initially detected in the dentate gyrus (D) on E17 in C3H mice (arrowheads). F. The level of α-BTX binding is also increased in hippocampal areas CA1 and CA3 in DBA/2 mice on E17, but is not detectable in the dentate gyrus. G. A marked increase in the density of α-BTX binding is seen in hippocampal area CA1 of C3H mice on E18, especially at the border between stratum pyramidale and stratum radiatum of area CA1 (arrows). α-BTX binding density is also increased in hippocampal area CA3 and in the dentate gyrus of C3H mice at this stage of development. H. Binding of α-BTX is initially observed (arrowheads) in the dentate gyrus (D) of DBA/2 mice on E18. An increase in the density of α-BTX binding is also seen in both hippocampal areas CA1 and CA3 of DBA/2 mice at this age, although binding levels are still greater in area CA3 than in area CA1 . Calibration bar =120 mm.
Reproduced from reference 24: Adams CE. Comparison of α7-nicotinic acetylcholine receptor development in the hippocampal formation of C3H and DBA/2 mice. Brain Res Dev Brain Res. 2003;143:137-149. Copyright © 2003, Elsevier.
Like N-methyl-D-aspartate (NMDA) –type glutamate receptors, activation of α7-receptors admits calcium ions, as well as other cations, into neurons and thereby activates nitric oxide synthetase, to produce the second messenger nitric oxide.25 Presumably this second messenger system is part of the mechanism of the development of neuronal circuitry that underlies reciprocal excitation and inhibition in the hippocampus. Activation of the receptors may be part of a complex maturation of glutamatergic synapses. Early in development, cholinergic receptors can depolarize neurons before they receive glutamatergic synapses, which eventually will become the primary depolarizing or excitatory receptors of the central nervous system. The depolarization produced by α7-receptors may thus be critical to early circuit formation at about the time of birth, in both rodents and primates. Indeed, DBA/2 mice, in addition to their deficiencies in α7-nicotinic receptors, have alterations in the expression of glutamate receptors, eg, decreased AMPA (α-amino3-hydroxy-5-methyl-4-isoxazole proprionic acid) and kainate receptors and increased NMDA receptors, a pathological change that has also been found in the hippocampus of schizophrenics.26,27
Thus, developmental abnormalities in α7-nicotinic receptors may have effects beyond cholinergic neurotransmission. These nicotinic developmental abnormalities may affect the development of the major glutamatergic pathways that support most information-processing mechanisms in the brain. Several of the genes associated with schizophrenia are involved in the formation of glutamatergic synapses as well, suggesting that the development of these synapses may be a final common pathway for several genetic risk factors.10 Prom the adult perspective, CHRNA7 deficiencies appear as elementary problems in inhibition, which have neurocognitive significance for the individual. However, from the neurodevelopmental perspective, CHRNA7 deficiencies could have a profound effect on the development of the excitatory and inhibitory synapses. Thus, remediation of CHRNA7 deficiencies in adult life by pharmacological means might not sufficiently reverse their developmental insult.
Significance for possible interventions
The significance of developmental pathophysiology for the prevention of schizophrenia is the possibility that an intervention can be performed at some time in the life cycle before the onset of schizophrenia, possibly in the perinatal period. Whether a strategy to activate nicotinic cholinergic receptors can be formulated and whether it would be effective in all cases or only in individuals who have polymorphisms in CHRNA7 receptors is unknown. Biomarkers such as diminished P50 inhibition or saccadic intrusions into smooth pursuit eye movements could be helpful to establish the timing and effectiveness of the intervention. More generally, the identification of the time course and cellular mechanism of developmental abnormalities associated with schizophrenia thus has the potential to identify possible strategies for reversal of these developmental abnormalities. Pharmacological strategies to activate deficient receptors or to replace deficient growth factors are within the range of current biological possibilities. As genetic information about schizophrenia and other mental disorders increases, the possibilities for such interventions are likely to increase. In some cases, the genetic information may indicate unique mechanisms, but in other cases the mechanisms may converge to common targets, such as the development of excitatory and inhibitory synapses.
The use of biomarkers for studies of the development of schizophrenia can thus have implications for treatment discovery even in the absence of the marker's sensitivity and specificity at the levels necessary for diagnostic purposes. More extensive animal model studies are necessary to design such interventions, but mouse strains with similar genetic and neurobiological features would seem to be useful for such work. Eventually, safe treatments that can be applied population wide may result from the consideration of developmental processes that produce biomarkers related to schizophrenia.
Supported by the VA Medical Research Service and VISN19 MIRECC, MH68582, MH38321, and the Institute for Children’s Mental Disorders.
Contributor Information
Robert Freedman, Departments of Psychiatry; Pharmacology.
Randal Ross, Departments of Psychiatry.
Sherry Leonard, Departments of Psychiatry; Pharmacology.
Catherine E. Adams, Departments of Psychiatry.
Merilyne Waldo, Departments of Psychiatry; Denver VAMC and University of Colorado, Denver, Co; and Department of Psychiatry, University of Utah School of Medicine, Utah, USA.
Jason Tregellas, Departments of Psychiatry.
Laura Martin, Departments of Psychiatry.
Ann Olincy, Departments of Psychiatry.
Jody Tanabe, Radiology.
Michael A. Kisley, Departments of Psychiatry.
Sharon Hunter, Departments of Psychiatry.
Karen E. Stevens, Departments of Psychiatry.
REFERENCES
- 1.Freedman R. N Engl J Med. 2003;349:1738–1749. doi: 10.1056/NEJMra035458. [DOI] [PubMed] [Google Scholar]
- 2.Freedman R., Leonard S., Olincy A., et al. Evidence for the multigenic inheritance of schizophrenia. Am J Med Genet. 2001;105:794–800. doi: 10.1002/ajmg.10100. [DOI] [PubMed] [Google Scholar]
- 3.Brzustowicz LM., Hodgkinson KA., Chow EW., Honer WG., Bassett AS. Location of a major susceptibility locus for familial schizophrenia on chromosome 1q21-q22. Science. 2000;288:678–682. doi: 10.1126/science.288.5466.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pulver AE., Lasseter VK., Kasch L., et al. Schizophrenia: a genome scan targets chromosomes 3p and 8p as potential sites of susceptibility genes. Am J Med Genet. 1995;60:252–260. doi: 10.1002/ajmg.1320600316. [DOI] [PubMed] [Google Scholar]
- 5.Freedman R., Coon H., Myles-Worsley M., et al. Linkage of a neurophysiology deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci USA. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freedman R., Adler LE., Olincy A., et al. Input dysfunction, schizotypy, and genetic models of schizophrenia. Schizophr Res. 2002;54:25–32. doi: 10.1016/s0920-9964(01)00348-6. [DOI] [PubMed] [Google Scholar]
- 7.Freedman R., Adams CE., Leonard S. The α7-nicotinic acetylcholine receptor and the pathology of hippocampal interneurons in schizophrenia. J Chem Neuroanatomy. 2000;20:299–306. doi: 10.1016/s0891-0618(00)00109-5. [DOI] [PubMed] [Google Scholar]
- 8.Stevens KE., Freedman R., Collins AC., et al. Genetic correlation of inhibitory gating of hippocampal auditory evoked response and α-bungarotoxin-binding nicotinic cholinergic receptors in inbred mouse strains. Neuropsychopharmacology. 1996;15:152–162. doi: 10.1016/0893-133X(95)00178-G. [DOI] [PubMed] [Google Scholar]
- 9.Leonard S., Gault J., Hopkins J., et al. Association of promoter variants in the α7-nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry. 2002;59:1085–1096. doi: 10.1001/archpsyc.59.12.1085. [DOI] [PubMed] [Google Scholar]
- 10.Harrison PJ., Owen MJ. Genes for schizophrenia? Recent findings and their pathophysiological implications. Lancet. 2003;361:417–419. doi: 10.1016/S0140-6736(03)12379-3. [DOI] [PubMed] [Google Scholar]
- 11.Myles-Worsley M., Ord L., Blailes F., Ngiralmau H., Freedman R. P50 sensory gating in adolescents from a Pacific island isolate with elevated risk for schizophrenia. Biol Psychiatry. 2004;55:663–667. doi: 10.1016/j.biopsych.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Kisley MA., Olincy A., Robbins E., et al. Sensory gating impairment associated with schizophrenia persists into REM sleep. Psychophysiology. 2003;40:29–38. doi: 10.1111/1469-8986.00004. [DOI] [PubMed] [Google Scholar]
- 13.Kisley MA., Polk SD., Ross RG., Levisohn PM., Freedman R. Early postnatal development of sensory gating. Neuroreport. 2003;14:693–697. doi: 10.1097/00001756-200304150-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tregellas JR., Tanabe JL., Miller DE., Ross RG., Olincy A., Freedman R. Neurobiology of smooth pursuit eye movement deficits in schizophrenia: an fMRI study. Am J Psychiatry. 2004;161:315–321. doi: 10.1176/appi.ajp.161.2.315. [DOI] [PubMed] [Google Scholar]
- 15.Hershman KM., Freedman R., Bickford PC. GABAB antagonists diminish the inhibitory gating of auditory response in the rat hippocampus. Neurosci Lett. 1995;190:133–136. doi: 10.1016/0304-3940(95)11523-y. [DOI] [PubMed] [Google Scholar]
- 16.Ross RG. Early expression of a pathophysiological feature of schizophrenia: saccadic intrusions into smooth-pursuit eye movements in schoolage children vulnerable to schizophrenia. J Am Acad Child Adolescent Psychiatry. 2003;42:468–476. doi: 10.1097/01.CHI.0000046818.95464.42. [DOI] [PubMed] [Google Scholar]
- 17.Davalos DB., Compagnon N., Heinlein S., Ross RG. Neuropsychological deficits in children associated with increased familial risk for schizophrenia. Schizophr Res. 2004;67:123–130. doi: 10.1016/S0920-9964(03)00187-7. [DOI] [PubMed] [Google Scholar]
- 18.Freedman R., Wetmore C., Stromberg I., Leonard S., Olson L. α-Bungarotoxin binding to hippocampal interneurons: immunocytochemical characterization and effects on growth factor expression. J Neuroscience. 1993;13:1965–1975. doi: 10.1523/JNEUROSCI.13-05-01965.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawai H., Zago W., Berg DK. Nicotinic α7-receptor clusters on hippocampal GABAergic neurons: regulation by synaptic activity and neurotrophins. J Neurosci. 2002;22:7903–7912. doi: 10.1523/JNEUROSCI.22-18-07903.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breese CR., Adams C., Logel J., et al. Comparison of the regional expression of nicotinic acetylcholine receptor α7 mRNA and [125l]-α-bungarotoxin binding in human postmortem brain. J Comp Neurol. 1997;387:385–398. doi: 10.1002/(sici)1096-9861(19971027)387:3<385::aid-cne5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 21.Levy RB., Aoki C. α7-Nicotinic acetylcholine receptors occur at postsynaptic densities of AMPA receptor–positive and –negative excitatory synapses in rat sensory cortex. J Neuroscience. 2002;22:5001–5015. doi: 10.1523/JNEUROSCI.22-12-05001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams CE., Broide RS., Chen Y., et al. Development of the α7-nicotinic cholinergic receptor in rat hippocampal formation. Brain Res Dev Brain Res. 2002;139:175–187. doi: 10.1016/s0165-3806(02)00547-3. [DOI] [PubMed] [Google Scholar]
- 23.Adams CE., Stitzel JA., Collins AC., Freedman R. α7-Nicotinic receptor expression and the anatomical organization of hippocampal interneurons. Brain Res. 2001;922:180–190. doi: 10.1016/s0006-8993(01)03115-8. [DOI] [PubMed] [Google Scholar]
- 24.Adams CE. Comparison of α7-nicotinic acetylcholine receptor development in the hippocampal formation of C3H and DBA/2 mice. Brain Res Dev Brain Res. 2003;143:137–149. doi: 10.1016/s0165-3806(03)00106-8. [DOI] [PubMed] [Google Scholar]
- 25.Adams CE., Stevens KE., Kem WR., Freedman R. Inhibition of nitric oxide synthase prevents α7-nicotinic receptor–mediated restoration of inhibitory auditory gating in rat hippocampus. Brain Res. 2000;877:235–244. doi: 10.1016/s0006-8993(00)02677-9. [DOI] [PubMed] [Google Scholar]
- 26.Zilles K., Wu J., Crusio WE., Schwegler H. Water maze and radial maze learning and the density of binding sites of glutamate, GABA, and serotonin receptors in the hippocampus of inbred mouse strains. Hippocampus. 2000;10:213–225. doi: 10.1002/1098-1063(2000)10:3<213::AID-HIPO2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 27.Porter RH., Eastwood SL., Harrison PJ. Distribution of kainate receptor subunit mRNAs in human hippocampus, neocortex and cerebellum, and bilateral reduction of hippocampal GluR6 and KA2 transcripts in schizophrenia. Brain Res. 1997;751:217–231. doi: 10.1016/s0006-8993(96)01404-7. [DOI] [PubMed] [Google Scholar]