Abstract
Specifying the complex genetic architecture of the “fuzzy” clinical phenotype of schizophrenia is an imposing problem. Utilizing metabolic, neurocognitive, and neurophysiological “intermediate” endophenotypic measures offers significant advantages from a statistical genetics stand-point. Endophenotypic measures are amenable to quantitative genetic analyses, conferring upon them a major methodological advantage compared with largely qualitative diagnoses using the Diagnostic and Statistical Manual of Mental Health, 4th Edition (DSM-IV). Endophenotypic deficits occur across the schizophrenia spectrum in schizophrenia patients, schizotypal patients, and clinically unaffected relatives of schizophrenia patients, Neurophysiological measures, such as P50 event-related suppression and the prepulse inhibition (PPI) of the startle response, are endophenotypes that can be conceptualized as being impaired because of a single genetic abnormality in the functional cascade of DNA to RNA to protein. The “endophenotype approach” is also being used to understand other medical disorders, such as colon cancer, hemochromatosis, and hypertension, where there is interplay between genetically conferred vulnerability and nongenetic stressors. The power and utility of utilizing endophenotypes to understand the genetics of schizophrenia is discussed in detail in this article.
Keywords: schizophrenia, endophenotype, P50 suppression, prepulse inhibition, sensorimotor gating
Abstract
El poder precisar la compleja arquitectura genética del “enredado” fenotipo clíninico de la esquizofrenia constituye un problema gigantesco.El empleo de mediciones endofenotípicas metabólicas, neurocognitivas y neurofisiológicas “intermediaries” ofrece ventajas significativas desde un punto de vista genético estad í stico. Las mediciones endofenot í picas sustituyen a los an á lisis genéticos cuantitativos confiriéndoles a ellas una mayor ventaja metodol ó gica en comparaci ó n con los diagn ó sticos principalmente cualitativos qua amplea el Manual Diagnóstico y Estadístico de Salud Mental en su cuarta edición (DSM IV). Los déficits endofenotípicos apareeen en todo el espectro esquizofrénico: en pacientes con esquizofrenia, en pacientes esquizotípicos y en familiares de pacientes esquizofrénicos sin evidencias clínicas de la enfermedad. Las mediciones neurofisiológicas, como la supresión de los eventos relacionados con la onda P50 y la inhibición de la respuesta de sobresalto ante un astímulo, constituyen endofenotipos qua pueden ser conceptualizados como una alteratión debida a una anormalidad genética simple en la cascada funcional desde el ADN al ARN y a las proteínas. La “aproximación endofenotípica” también se está empleando para comprender otros cuadros médicos, como el cáncer de colon, la hemocromatosis y la hipertensión en los cuales hay una interacción entre los astresores no genéticos y la predisposición genética que confiere cierta vulnerabilidad. En este artículo se discutirá en detalle la capacidad y la utilidad del empleo de endofenotipos para una mejor comprensión de la genética de la esquizofrenia.
Abstract
Preciser l'architecture génétique complexe du phénotype clinique « enchevette » de la schizophrénie constitue un problème impressionnant. Utiliser des mesures endophénotypiques métaboliques, neurocognitives et neurophysiologiques « mterme-diaires » offre des avantages certains d'un point de vue génétique statistique. Les mesures endophénotypiques relèvent des analyses génétiques quantitatives, leur conférant un avantage méthodologique majeur comparé aux diagnostics en grande partie qualitatifs qui utilisent le DSM-IV (Diagnostic and Statistical Manual of Mental Health, 4e édition). Des déficits endophénotypiques se produisent au cours de la schizophrénie chez les patients schizophrènes, les patients schizotypiques et les parents cliniquement indemnes de patients schizophrènes. Les endophénotypes sont des moyens neurophysiologiques, tels que la suppression des événements liés au P50 et que l'inhibition de la réponse de sursaut avant un stimulus, que l'on peut imaginer être altérés par une anomalie génétique simple dans la cascade de fonctionnement ADN vers ARN vers protéine. L'« approche endophénotypique » est aussi utilisée pour comprendre d'autres pathologies, comme le cancer du colon, l'hémochromatose et l'hypertension dans lesquels il existe une interaction entre des agents stressants non génétiques et une prédisposition génétique conférant une certaine vulnérabilité. Dans cet article, nous allons parler en détail de la possibilité et de l'utilité d'utiliser des endophénotypes pour comprendre la génétique de la schizophrénie.
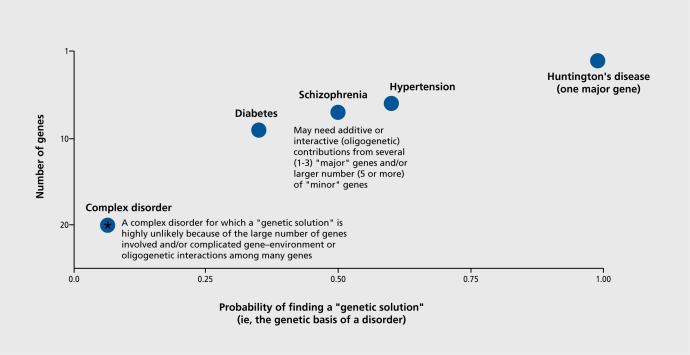
In this article, we review the utilization of endophenotypes in research into the genetics of schizophrenia, focusing on neurophysiological measures. Since Bleuler1 coined the term “schizophrenia,” this complex and devastating disorder has gone through significant iterations in terms of how the scientific community conceptualizes it. When Bleuler utilized his impressive clinical experience and intuition in describing schizophrenia, he recognized that schizophrenia represents a group of disorders that share important unifying underlying features. Initially, psychological developmental factors such as aberrant communication styles2,3 were felt to cause schizophrenia. Then, the seminal Danish studies of Kety et al4 revealed the clear genetic transmission of schizophrenia and schizotypy-related abnormalities of psychological functioning subsumed under the term “schizotaxa.”5 The seminal contributions of these and other family studies pointed the way for the current conceptualization of schizophrenia as one of the wide-ranging group of complex genetic disorders (Figure 1). Unlike the mendelian-dominant heritability pattern of Huntington's disease, schizophrenia may represent a group of related disorders with substantial heterogeneity6
Figure 1. Genetic architecture of complex disorders. This illustrates a major conundrum of research into complex human disorders. The Huntington's gene was identified 20 years ago, but has not yet led to a genetic “cure.” The profoundly important potential of genetic treatments for even more “complex genetic disorders” (eg, schizophrenia, hypertension, and diabetes) will depend on related scientific advances.
Schizophrenia has undergone a transition from being viewed as a psychologically caused familial disorder to being understood as a complex genetic disorder in which multiple genes contribute in an additive or perhaps interactive, oligogenic fashion to yield a total risk or a vulnerability to developing the disorder. Interestingly, in a sort of figure ground reversal, the initial enthusiasm of seeing schizophrenia as an easy-to-dissect genetic disorder was eventually replaced by the understanding that schizophrenia is about 50% genetically mediated7 with the remainder of disease liability probably attributable to nongenetic factors.8-10
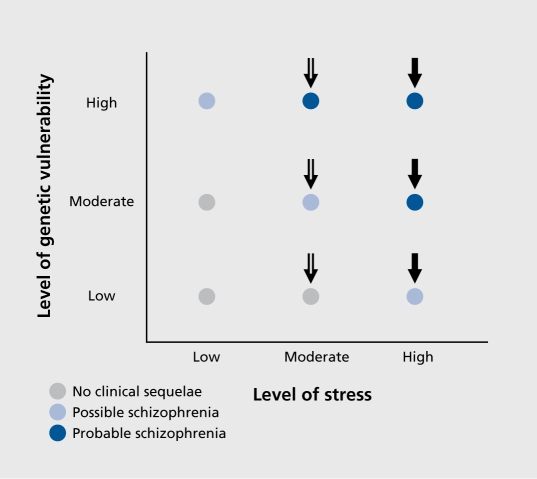
The evolution of our understanding of schizophrenia as a family of disorders that are mediated by complex genetic vulnerability and gene-environment interactions parallel the advances seen in the conceptualization of many other medical disorders, such as colon cancer, hemochromatosis, diabetes, and hypertension.7 Interestingly, all of these disorders are felt to be attributable to a complex interplay of vulnerability genes that predispose an individual to developing a disease and nongenetic “second hits” that precipitate the disorders (Figure 2). If the genetic loading or risk is strong enough (for example, as in multiplex families), even minor precipitants may result in the development of the disorder. On the other hand, if the cumulative genetic risk of developing schizophrenia is relatively mild, it may take a more profound nongenetic second hit (Figure 2) to start the cascade of events that ultimately result in the full expression of the disease.
Figure 2. The vulnerability-stress 2-hit model of schizophrenia. “High” levels of vulnerability interacting with high levels of stressors (eg, neonatal hypoxema or adolescent stimulant abuse) may “evoke” the emergence of schizophrenia. Despite the view of schizophrenia as a “genetic disorder,” 50% of causation is genetic and 50% or so of the disorder is caused by “nongenetic” second hits.
There is an interesting “natural history” in the schizophrenia literature itself. First, there were descriptions of the disorder and associated “deficits” in many domains. Second, studies of clinically unaffected relatives of schizophrenia patients pointed the way to an intermediate state of impairment (called endophenotypes) in each of these independently studied domains. These types of deficits occur across multiple domains such as metabolic functioning (catechol-O-methyltransferase [COMT]), neurophysiology (P50 event-related potential [ERP] suppression), and neurocognition (vigilance, as measured by the continuous performance task [CPT], and verbal memory, as measured by the California Verbal Learning Test [CVLT]).7 The “intermediate” or partial deficits found in clinically unaffected relatives of schizophrenia patients gave investigators the insight crucial for the development and understanding of “endophenotypes” or intermediate phenotypes. Whereas the “fuzzy” clinical phenotype of schizophrenia is quite difficult to characterize, laboratorybased endophenotypes may be more closely linked to specific genetic abnormalities that are partially or wholly expressed (ie, variable penetrance) in clinically unaffected relatives of schizophrenia patients. This variable expression makes these endophenotypes amenable to statistical approaches utilizing quantitative trait methodologies.11-14
Thus, the picture that was painted is that for many of these endophenotypes, there are: (i) findings of deficits in schizophrenia patients versus normal comparison subjects; and (ii) the deficits are identified across the schizophrenia spectrum (including schizotypal personality disordered patients and clinically unaffected family members of schizophrenia patients) (Table I)15-47 In addition, across the schizophrenia spectrum, it is observed that increasing deficits in these endophenotypes are noted with increasing genetic load or genetic risk. For example, groups of first-degree relatives of schizophrenia patients typically have a greater level of endophenotypic abnormality than groups of second-degree relatives, etc. Thus, there is the explicit assumption that “levels” of genetic relative risk48 act as powerful predisposing factors that make the individual vulnerable to developing schizophrenia (Figure 2). In parallel, as the power of “strong inference”49 in molecular biology became apparent, the understanding of the template of “DNA to RNA to protein” became very important across all species. In concert with these findings, the human genome project has identified the sequence of basepairs that characterize the human genome. The challenge for understanding the basis of mendelian-dominant genetic disorders (eg, Huntington's disease) and the many “partially” genetic disorders (eg, hypertension, diabetes, bipolar disorder, Tourette syndrome, and schizophrenia; (Figure 1) is to parse the clinical heterogeneity and complexities into understanding the genetic architecture and nongenetic contributions into quantitative measures that are amenable to analysis via advanced statistical quantitative trait analytic genetic methods.11-14 Thus, for a complex psychiatric illness like schizophrenia, the relationship between genes, gene products, and the disorder itself is hardly straightforward. Indeed, an understanding of the exact cascade of DNA to RNA to (abnormal) protein to endophenotypic dysfunction in schizophrenia has remained elusive, but is amenable to serious investigations and analyses.
Table I. Neurophysiological and neuropsychological endophenotypes: effect size difference between schizophrenia patients, normal comparison groups, and schizophrenia spectrum groups. Effect sizes in schizophrenia patients, clinically unaffected relatives of schizophrenia patients, and schizotypal personality disordered patients compared with those in normal subjects. These effect sizes were computed by using the mean and standard deviations for normal comparison subjects and the means of the patient groups. The range of values differs from study to study because different investigators used different patient populations taking different types and amounts of medications; also, multiple conditions were used, some of which were needed to establish the floor and ceiling effect. In these cases, we generally cited the most robust effect sizes. *Also, Cadenhead KS, unpublished data, 2000.
| Endophenotypes | Schizophrenia patients | Relatives of schizophrenia patients | Schizotypal personality disordered patients | References |
| Antisaccade | 4.88-6.38 | 1.38-3.75 | 0.75-1.36 | 15, 16 |
| Smooth-pursuit eye movement | 2.0-3.0 | 0.29-1.3 | 0.29 | 16-18 |
| Thought disorder | 1.56-2.98 | 0.34-0.83 | 1.04-1.28 | 16, 19-21 |
| Working memory | 1.42-2.2 | 0.42 | 0.73-1.04 | 22-24 |
| P50 suppression | 0.92-1.29 | 0.79 | 0.79 | 25, 27 |
| Reaction time | 0.59-1.05 | 0.44 | 0.79-0.99 | 28-30 |
| Prepulse inhibition | 0.51-0.85 | 1.0 | 1.45 | 31-33 |
| Span of apprehension | 0.5-2.5 | 0.6-1.5 | 34-39 | |
| Continuous performance test | 0.45-3.30 | 0.46-2.97 | 0.45-0.78 | 34, 40-42 |
| Executive functioning | 0.47-1.97 | 0.73-1.6 | 0.72 | 40, 43 |
| P300 event-related potential | 0.45-1.05 | 0.17 | 0.36 | 44,45 |
| Visual backward masking | 0.33-0.65 | 0.43-0.57 | 0.45-0.67 | 28, 46, 47* |
Because of the complexity of schizophrenia and the fact that it is a “fuzzy” diagnostic phenotype, a number of strategies have been utilized in order to understand the genetic underpinnings of the disorder. The most simple and most commonly used strategy of molecular genetics that is applied to complex psychiatric disorders first assumes the distribution of illness in a family represents the effect of a single gene and utilizes the techniques of genetic analysis that are commonly used to identify that single gene. This approach assumes that the signal of the disease attributable to a single gene can be identified in the very complex and relatively “noisy” genetic background of the disease.
As an alternative approach, one could make an assumption about the biology or endophenotypes expressed in an illness and then search for candidate genes that underlie those functions to see if they are mutated. It is important to note that both of these approaches have been successful to some degree in schizophrenia studies. For example, whole genome scans have revealed replicated linkage findings for schizophrenia obtained at locations on chromosomes 1, 6, 8, 13, 15, and 22 (see, for example, references 7 and 48). The problem with these whole genome linkage studies is that the functional correlates of these linkages are unclear. Conversely, DNA mutations have also been found in “candidate genes” such as NURRI,50 a gene that codes for the receptor for retinoic acid and mediates critical pathways in neuronal development. A limiting consequence of dealing with a group of disorders is that these neurobiologically significant and face-valid abnormalities in NURRI candidate genes are mutated in only a small number of patients with a diagnosis of schizophrenia.51
A third approach uses endophenotypes to sharpen the clinical phenotype, in order to understand the genetic basis of specific schizophrenia-linked abnormalities. This approach assumes that a specific genetic abnormality causes a specific protein change leading to a specific quantitative functional abnormality. Thus, the wide array of possible genetically mediated domains that could be examined in schizophrenia include metabolic functions, brain structure and functional imaging, neurophysiology, neuropsychology, and other endophenotypic abnormalities that run in families.7,9,51 The relationship between these endophenotypic abnormalities and genes can also be discovered and evaluated via the use of linkage or candidate gene analysis. Hence, levels of association of specific quantitative traits and their related genetic abnormalities would be stronger than the relationship of specific genetic abnormalities to the clinical endophenotype of a heterogeneous population of schizophrenia patients. This approach is hardly unique since it is clear in other medical conditions that the search for endophenotypes and their genetic determinants can be more “focused” when looking through an “endophenotypic lens” rather than looking at the genetic basis of the complex disorders themselves. For example, in hemochromatosis, it is not the clinical illness, but rather a high serum level of iron that is the most clearly identifiable and penetrant heritable trait.52 Likewise, gene discovery in colon cancer has revealed that it is not the cancer itself, but rather familial polyposis that is the crucial,53 genetically heritable disease vector. In the case of colon cancer, the assumption is that there is a genetically mediated vulnerability resulting in polyp formation, which converts to cancer via the influence of other genetic or nongenetic factors (ie, diet, environmental toxin exposure). Thus, these polyps will often convert to colon cancer in “high-risk” individuals.
The assessment of endophenotypes has come to be increasingly important in our attempts to understand schizophrenia. Of course, when one considers that there are about 16 000 genes expressed in the brain and, of these, about 6000 to 8000 are expressed only in the brain,54 searching for causative genes associated with the clinical entity of schizophrenia per se is a daunting task. In dealing with quantitative endophenotypic markers and the probability of causal genetic heterogeneity where multiple mutations may induce endophenotypic abnormalities, we face a difficult challenge. Also, in analyzing endophenotypic abnormalities, the fact that many brain-based genes are expressed in multiple areas, under varying promoting or disease-inducing nongenetic conditions and across critical neurodevelopmental epochs in the life of the individual, the search for endophenotype–genetic “connections” requires us to sharpen our focus when searching for the vulnerability gene(s) in schizophrenia. According to Mendel's second law that genetic traits segregate independently in the family, some siblings will express specific endophenotypes independently of others and may be better subjects for characterizing endophenotypic abnormities than the patients themselves. The patients themselves have multiple abnormalities relating to the scope and severity of their disease, the treatments used for the disease, and the psychosocial, medical, nutritional, and many factors associated with schizophrenia. The voyage that has been undertaken in searching for endophenotypes in schizophrenia has taken advantage of a generation of important scientific findings. First among these, of course, is the fulcrum finding of the double helix structure of DNA.55 Second, after the structure of DNA was identified, the advances in the understanding of the transformation of DNA to RNA to proteins to function have taken place over the last 50 years in a rapidly accelerated fashion that has enabled us to come within “hailing distance” of truly understanding the relationship of DNA mutations to clinical and endophenotypic abnormalities.
Genetic studies of endophenotypes in schizophrenia
The candidate endophenotypes that have been examined in schizophrenia range from metabolic and developmental measures to brain structural and functional traits, as well as neuropsychological and neurophysiological indices. The neurodevelopmental endophenotype candidates include mutations in candidate genes such as NURRI. Clearly, this pathway is critical in neuronal development and, although the mutations in this gene are found in a minority of schizophrenia patients, one cannot help but be impressed by the fact that this endophenotypic trait aligns with a neurodevelopmental hypothesis. Likewise, Harrison and Weinberger51 have pointed out that “schizophrenia genes” and their expression may converge on critical neuronal synaptic and glial populations in crucial brain areas, such as the hippocampus, dorsal thalamus, and dorsolateral prefrontal cortex. These structures are all part of the cortico-striato-pallido-thalamic (CSPT) circuitry. This CSPT circuitry involves complex loops and connections that are derived from Penney and Young's56 examination of the neural substrate of motor functions. The finding of distributed neural network abnormalities in the CSPT circuitry was described in psychiatric populations in a definitive manner by Swerdlow and Koob57 and has led to many neurophysiological and “brain connectivity” hypotheses. These hypotheses include Andreason's concept of cognitive dysmetria,58 which attempts to “connect the dots” of brain dysfunction in schizophrenia patients,59 and the evolution of corticocortical coherence measures to assess functional connectivity deficits to probe the multiple cognitive deficits of schizophrenia patients.60 As Harrison and Weinberger point out, “a way forward is provided by the recent identification of several putative susceptibility genes including neuroregulin, dysdindin, COMT, DISCI, RGS4, GRN3, G72.”51 These authors discuss the evidence for these and other genes as vulnerability vectors along dimensions of their expression profiles and neurobiological roles. While the evidence for genetic abnormalities in these critical genes with their important integrative functions is attractive, the causative allele or mechanism that results in the development of schizophrenia is unknown. Harrison and Weinberger51 also point out that COMT may be an exception where a causative allele may have been identified. Nevertheless, in the area of brain connectivity and synaptic plasticity, they have proposed that the genes cited above may all converge functionally via an influence upon synaptic plasticity and the development and stabilization of functionally important cortical microcircuitry.51 Thus, at the most basic level, these neurodevelopmental genes may characterize a molecular biological basis for a genetic cytoarchitecture that has the potential to incrementally advance our understanding of schizophrenia.
Neurophysiological endophenotypes: gating abnormalities
Many neurophysiological endophenotypes have undergone extensive study and analysis (Table I).15-47 These endophenotypic measures include antisaccade oculomotor functioning, smooth pursuit eye movement, P50 suppression, prepulse inhibition (PPI) of the startle response, P300 ERPs, and visual backward masking. Each of these endophenotypes has undergone a logical, natural history of progression in scientific analyses: schizophrenia patients, their clinically unaffected family members, and schizotypal personality disordered subjects have deficits compared with normal subjects. Exemplars of neurophysiological endophenotypes in schizophrenia are the gating abnormalities reflected by PPI and P50 suppression deficits.
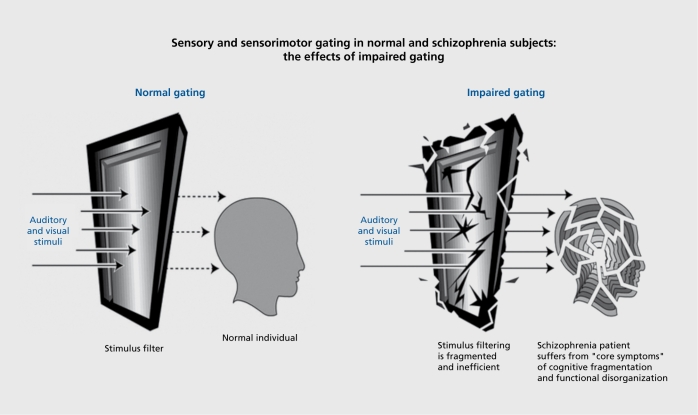
Abnormalities of sensorimotor (PPI) and sensory (P50 suppression) gating are thought to reflect an inability to screen out trivial stimuli in order to focus on important and information laden aspects of the environment.61-64
Normal sensory gating (Figure 3) allows individuals to navigate through a stimulus-laden world and to apportion attentional resources to salient stimuli. PPI of the startle reflex measures sensorimotor gating with a clear understanding of the underlying CSPT circuitry defined from decades of animal model studies. While PPI and P50 suppression measures of “gating” are often conceptually linked, there is evidence that they actually diverge in nonpsychiatric and clinical populations.65-68 Thus, these two different, but seemingly closely related gating abnormalities, may be characteristic of different subgroups of patients.
Figure 3. The effects of a loss of normal gating. In the left panel, an individual with intact filtering and inhibition filters out irrelevant sensory stimuli. In the right panel, impaired gating leads to a sequence of sensory inundation, cognitive fragmentation, and disorganized thinking.
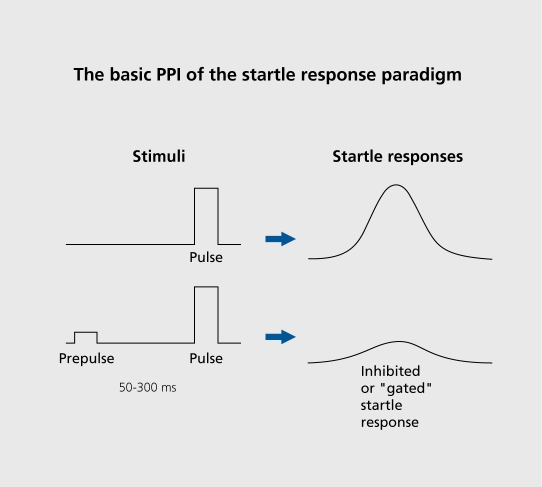
PPI normally occurs when a weak prestimulus precedes a strong “startling” stimulus by 50 to 300 ms (Figure 4); the weak prestimulus inhibits the startle response. PPI deficits in schizophrenia patients31,61,69 extend to clinically unaffected relatives of schizophrenia patients as well as schizotypal patients.33,70 Impaired gating function putatively results in the devastating consequences of cognitive fragmentation (Figure 3).
Figure 4. Prepulse inhibition (PPI) is a profound decrease in startle response magnitude when the startling pulse is preceded by a weak prepulse. PPI is an operational measure of sensorimotor gating.
One of the major advantages of endophenotypes, such as PPI, is that there are animal models that point to genetically mediated strain-related differences71 and to specific quantitative trait loci (Schork et al, 1995, unpublished manuscript). In addition to strain-related differences in baseline PPI, pharmacological regulation of PPI is also strain-related.71-77 In addition, PPI deficits induced by the dopamine agonist apomorphine are reversed in a very lawful manner by antipsychotic medications with “dose–response” characteristics that parallel the efficacy of these antipsychotic medications in schizophrenia patients.78 Therefore, although PPI is not as advanced in terms of its genetic analysis as P50 suppression (see below), it offers an important window on endophenotype (dys)function in schizophrenia. The CSPT circuitry is crucial for understanding cognitive integration and inhibitory functions. In animal model studies, this circuitry is impaired by lesions or neurotransmitter manipulations at multiple loci in the CSPT circuitry that induce PPI deficits.79,80 Thus, infusion and lesion studies along nodes within the CSPT axes can disrupt PPL It is not too big a “leap of faith” to believe that specific single nucleotide polymorphisms (SNPs) might affect these loci and reflect multiple genetic mutations that contribute to the vulnerability to developing schizophrenia. Of course, these hypotheses can only be worked out in humans in largescale studies such as the National Institute of Mental Health Consortium of Genetics of Schizophrenia,81 where endophenotype deficits found in schizophrenia are being tracked in a large cohort of families in order to understand both patterns of heritability and the specific genetic abnormalities associated with these endophenotypic deficits in schizophrenia.
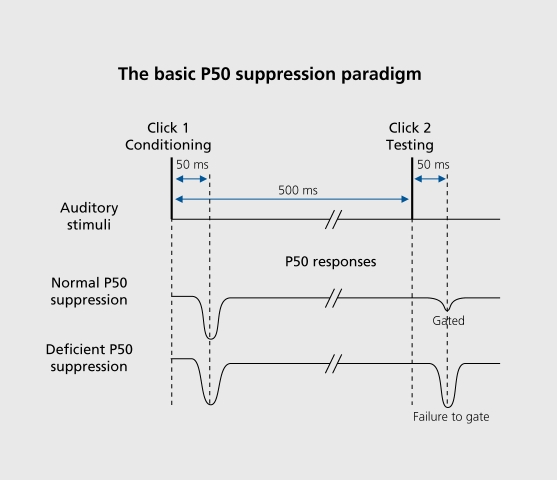
P50 suppression is an ERP measure of inhibitory function that has been studied in a variety of patient and nonpsychiatric populations. An electroencephalogram (EEG) is recorded in response to click pairs presented 500 ms apart. The EEG responses to each of the clicks are separately averaged. The P50 component of the auditory ERP is a positive-polarity waveform that occurs approximately 50 ms after each click is presented. In normal subjects, the P50 response to the second click is typically reduced in amplitude relative to the response to the first click.25,26,82-84 P50 suppression is the percentage of P50 response amplitude reduction from the first to the second click (Figure 5). Over the past 20 years, many studies have demonstrated that schizophrenia patients have P50 suppression deficits (Table I) and that these deficits extend to clinically unaffected relatives of schizophrenia patients.26,85-87 Individuals with schizotypal personality disorder also have P50 suppression deficits,27,88 indicating that these deficits are present across the schizophrenia spectrum.
Figure 5. Zuditory click pairs are presented to subjects; the electroencephalogram (EEG) is averaged across trials. The P50 component of the averaged event-related potential (ERP) is measured in response to the first and second clicks. The percentage of P50 amplitude reduction from the first to the second click is referred to as P50 suppression or P50 gating, and is considered an operational measure of sensory gating. Deficient P50 suppression is often seen in patients with schizophrenia, their clinically unaffected family members, and individuals with schizotypal personality disorder.
Although it is clear that, much like PPI, P50 suppression is almost inevitably the function of a wide-ranging neural circuitry involving multiple brain structures and complex brain circuits, the utilization of P50 suppression as a “candidate endophenotype” advanced rapidly for two reasons. First, there was the critical findings of nicotine transiently improving P50 suppression deficits in both schizophrenia patients89 and their clinically unaffected family members.90 Second, an animal model of P50 suppression was developed, similar to that seen in PPI. This allows specific neurochemical manipulations to be made and it was found that P50 suppression deficits in DBA/2 mice could be restored to normal levels by α7-agonists.91 Then, a relatively unique element in the ideal progression of identifying endophenotypes in schizophrenia was made: it was found that there is a genetic marker at the locus of the α7-subunit of the nicotinic receptor gene linking a candidate endophenotype of information-processing deficits in schizophrenia to a specific chromosomal region.84 Leonard et al92 found a specific SNP genetic mutation in the promoter region of the α7-subunit of the nicotinic gene, which seems to account for the P50 findings listed above. Thus, P50 suppression represents the most “complete” “DNA to RNA to protein” story of an endophenotype–genetic abnormality linkage.
The P50 suppression findings represent an example of how endophenotypes can be utilized as neurobiologically meaningful markers that contribute to our understanding of the genetics and potentially the treatment of schizophrenia. Importantly, these types of studies do not merely identify a “schizophrenia endophenotype,” but rather the linkage of deficits (P50 suppression) in schizophrenia patients to a specific chromosomal region.
Conundrums and caveats, and the use of endophenotypes in the genetics of schizophrenia
Although there are many candidate endophenotypes in schizophrenia, imposing challenges still exist. First, since some endophenotypes are at least partially normalized by current second-generation antipsychotic medications, the statistical genetic approach to these data sets presents many daunting challenges. For example, the fact that clozapine improves P50 suppression deficits93-95 suggests that patients on clozapine cannot be utilized in studies of P50 suppression as a candidate endophenotype. It would be optimal to use never-medicated schizophrenia patients in studies of endophenotypes in schizophrenia. Unfortunately, given the power demands of such studies, finding enough never-medicated patients, even in a multisite study such as the Consortium on the Genetics of Schizophrenia (COGS) would seem to be virtually impossible. Family studies that rely on identifying probands with endophenotypic deficits then become difficult to interpret. Where significant endophenotypic normalization occurs with antipsychotic treatment, statistical strategies will have to be utilized that allow us to “exclude” or “account for” the (partially) “normalized” schizophrenia patients or to utilize only clinically unaffected family members in genetic studies. This reliance on clinically unaffected family members is what Braff and Freedman7 referred to as the “null-proband” strategy. Medicated probands must either be excluded from analyses or a complex “adjustment” on a phenotypic value must be made in order to utilize them in the genetic analysis. One could posit that a temporary withdrawal of antipsychotic medication would allow us to identify these trait-related endophenotypic markers, but this is ethically and practically unfeasible. Fortunately, almost all of the large effect sizes reported for endophenotypic deficits in schizophrenia in Table I are derived from medicated patients, so that it appears that fairly straightforward statistical analyses can be utilized with most available patients.
Summary
Genetic studies in schizophrenia are on the cusp of an exciting new era of utilizing specific laboratory-based endophenotypes to parse the complex genetic architecture of the “groups of schizophrenia.” The template described above for P50 suppression studies has already yielded a sequence of findings leading to the identification of a specific abnormality that accounts for P50 suppression deficits in schizophrenia patients and their clinically unaffected relatives. In addition to this, there are many studies examining heritability of other strong candidate endophenotypes, as listed in Table I Other study strategies are now being utilized in endophenotypic research in schizophrenia. Investigations are underway in a number of settings to identify genes that convey a risk for schizophrenia. For example, whole genome linkage studies have revealed loci that might be of functional importance. In addition, the endophenotypic strategy, however, allows us to understand the underlying neurobiology and neural substrates of these genetic abnormalities. Many conundrums and obstacles must be overcome in this endeavor. For example, the improvement of endophenotypic abnormalities via the use of second-generation96 antipsychotic medications may (or may not) impede our ability to carefully conduct family heritability studies, which will allow us to ultimately identify genetic abnormalities characteristic of schizophrenia. With the use of statistical genetics methods, unmedicated patients, animal model identification of quantitative trait loci, and specific genetic abnormalities, the exciting possibility exists for matching endophenotypes with their underlying genetic abnormalities and then constructing “composite endophenotypes” consisting of neurobiologically coherent combinations of more than one of the identified biomarkers. It is very important to identify the convergence and divergence of these endophenotype–gene abnormality linkages in schizophrenia patients in order to see whether a single genetic abnormality is likely to induce the multiple observed deficits of schizophrenia patients. Ultimately, the specification of how different gene–environment interactions contribute to neuronal pathology associated with psychosis may enable us to further clarify the nosology of schizophrenia. Quantitative endophenotype-based strategies play an important role that will help elucidate the genetic basis of schizophrenia and point the way toward molecularly derived strategies for the treatment of important subgroups of patients with this complex disorder.
Selected abbreviations and acronyms
- COMT
catechol-O-methyltransferase
- CSPT
cortico-striato-pallido-thalamic (circuitry)
- ERP
event-related potential
- PPI
prepulse inhibition
- SNP
single nucleotide polymorphism
Grants from the National Institute of Mental Health: MH042228 and MH065571 (the Consortium on the Genetics of Schizophrenia [COGS]), and the Department of Veteran Affairs through the VISN 22 MIRECC (Mental Illness Research, Education and Clinical Center). This work was also supported in part by the Bowman Family Foundation partnership with the National Alliance for Research on Schizophrenia and Depression (NARSAD).
Contributor Information
David L. Braff, Department of Psychiatry, University of California, San Diego, Calif, USA.
Gregory A. Light, Department of Psychiatry, University of California, San Diego, Calif, USA.
REFERENCES
- 1.Bleuler E. Dementia Praecox. New York: International Universities Press; 1911/1950 [Google Scholar]
- 2.Singer MT. The consensus Rorschach and family transaction. J Proj Tech Pers Assess. 1968;32:348–351. doi: 10.1080/0091651X.1968.10120496. [DOI] [PubMed] [Google Scholar]
- 3.Singer MT., Wynne LC. Differentiating characteristics of parents of childhood schizophrenics, childhood neurotics, and young adult schizophrenics. Am J Psychiatry. 1963;120:234–243. doi: 10.1176/ajp.120.3.234. [DOI] [PubMed] [Google Scholar]
- 4.Kety SS., Rosenthal D., Wender PH., Schulsinger F. The types and prevalence of mental illness in the biological and adoptive families of adopted schizophrenics. In: Rosenthal D, Kety S, eds. The Transmission of Schizophrenia. New York, NY: Pergamon Press; 1968:345–362. [Google Scholar]
- 5.Meehl PE. Schizotaxia revisited. Arch Gen Psychiatry. 1989;46:935–944. doi: 10.1001/archpsyc.1989.01810100077015. [DOI] [PubMed] [Google Scholar]
- 6.Faraone SV., Tsuang D., Tsuang MT. Genetics of Mental Disorders: A Guide for Students, Clinicians, and Researchers. New York, NY: Guilford; 1999 [Google Scholar]
- 7.Braff DL., Freedman R. Endophenotypes in studies of the genetics of schizophrenia. In: Davis KL, Charney DS, Coyle JT, Nemeroff C, eds. Neuropsychopharmacology: The Fifth Generation of Progress. Philadelphia, PA: Lippincott, Williams & Wilkins; 2002:703–716. [Google Scholar]
- 8.McDonald C., Murray RM. Early and late environmental risk factors for schizophrenia. Brain Res Brain Res Rev. 2000;31:130–137. doi: 10.1016/s0165-0173(99)00030-2. [DOI] [PubMed] [Google Scholar]
- 9.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 10.Lipska BK., Swerdlow NR., Geyer MA., Jaskiw GE., Braff DL., Weinberger DR. Neonatal excitotoxic hippocampal damage in rats causes post-pubertal changes in prepulse inhibition of startle and its disruption by apomorphine. Psychopharmacology (Berl). 1995;122:35–43. doi: 10.1007/BF02246439. [DOI] [PubMed] [Google Scholar]
- 11.Schork NJ. Extended multipoint identity-by-descent analysis of human quantitative traits: efficiency, power, and modeling considerations. Am J Hum Genet. 1993;53:1306–1319. [PMC free article] [PubMed] [Google Scholar]
- 12.Schork NJ. Genome partitioning and whole-genome analysis. Adv Genet. 2001;42:299–322. doi: 10.1016/s0065-2660(01)42030-x. [DOI] [PubMed] [Google Scholar]
- 13.Schork NJ., Nath SK., Fallin D., Chakravarti A. Linkage disequilibrium analysis of biallelic DNA markers, human quantitative trait loci, and threshold-defined case and control subjects. Am J Hum Genet. 2000;67:1208–1218. doi: 10.1086/321201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fallin D., Schork NJ. Accuracy of haplotype frequency estimation for biallelic loci, via the expectation-maximization algorithm for unphased diploid genotype data. Am J Hum Genet. 2000;67:947–959. doi: 10.1086/303069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDowell JE., Myles-Worsley M., Coon H., et al. Measuring liability for schizophrenia using optimized antisaccade stimulus parameters. Psychophysiology. 1999;36:138–141. doi: 10.1017/s0048577299980836. [DOI] [PubMed] [Google Scholar]
- 16.Holzman PS., Coleman M., Lenzenweger MF., et al. Working memory deficits, antisaccades, and thought disorder in relation to perceptual aberration. In: Raine A, Lencz T, et al, eds. Schizotypal Personality. New York, NY: Cambridge University Press; 1995:353–381. [Google Scholar]
- 17.Clementz BA., Grove WM., lacono WG., et al. Smooth-pursuit eye movement dysfunction and liability for schizophrenia: implications for genetic modeling. J Abnorm Psychol. 1992;101:117–129. doi: 10.1037//0021-843x.101.1.117. [DOI] [PubMed] [Google Scholar]
- 18.Thaker GK., Cassady S., Adami H., et al. Eye movements in spectrum personality disorders: Comparison of community subjects and relatives of schizophrenic patients. Am J Psychiatry. 1996;153:362–368. doi: 10.1176/ajp.153.3.362. [DOI] [PubMed] [Google Scholar]
- 19.Kinney DK., Holzman PS., Jacobson B., et al. Thought disorder in schizophrenic and control adoptees and their relatives. Arch Gen Psychiatry. 1997;54:475–479. doi: 10.1001/archpsyc.1997.01830170101013. [DOI] [PubMed] [Google Scholar]
- 20.Perry W., Braff DL. Thought disorder and the group of schizophrenia. Schizophr Res. 1993;9:107. [Google Scholar]
- 21.Perry W., Cadenhead KS., Braff DL. Thought disorder in the group of schizophrenias. Biol Psychiatry. 1992;31:117–118A. [Google Scholar]
- 22.Cadenhead KS., Perry W., Shafer K., et al. Cognitive functions in schizotypal personality disorder. Schizophr Res. 1999;37:123–132. doi: 10.1016/s0920-9964(98)00147-9. [DOI] [PubMed] [Google Scholar]
- 23.Perry W., Heaton RK., Potterat E., et al. Working memory in schizophrenia: transient “on line” storage versus executive functioning. Schizophr Bull. 2005. In press. doi: 10.1093/oxfordjournals.schbul.a006854. [DOI] [PubMed] [Google Scholar]
- 24.Cadenhead KS., Shafer K., Perry W., et al. Working memory in the schizophrenia spectrum. Biol Psychiatry. 1998;43:127S. [Google Scholar]
- 25.Clementz BA., Geyer MA., Braff DL. P50 suppression among schizophrenia and normal comparison subjects: a methodological analysis. Biol Psychiatry. 1997;41:1035–1044. doi: 10.1016/S0006-3223(96)00208-9. [DOI] [PubMed] [Google Scholar]
- 26.Clementz BA., Geyer MA., Braff DL. Poor P50 suppression among schizophrenia patients and their first-degree biological relatives. Am J Psychiatry. 1998;155:1691–1694. doi: 10.1176/ajp.155.12.1691. [DOI] [PubMed] [Google Scholar]
- 27.Cadenhead KS., Light GA., Geyer MA., Braff DL. Sensory gating deficits assessed by the P50 event-related potential in subjects with schizotypal personality disorder. Am J Psychiatry. 2000;157:55–59. doi: 10.1176/ajp.157.1.55. [DOI] [PubMed] [Google Scholar]
- 28.Cadenhead KS., Geyer MA., Butler RW., et al. Information processing deficits of schizophrenia patients: Relationship to clinical ratings, gender and medication status. Schizophr Res. 1997;28:51–62. doi: 10.1016/s0920-9964(97)00085-6. [DOI] [PubMed] [Google Scholar]
- 29.Maier W., Franke P., Kopp B., et al. Reaction time paradigms in subjects at risk for schizophrenia. Schizophr Res. 1994;13:35–43. doi: 10.1016/0920-9964(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 30.Sarkin AJ., Dionisio DP., Hillix WA., et al. Positive and negative schizotypal symptoms relate to different aspects of crossover reaction time task performance. Psychiatry Res. 1998;81:241–249. doi: 10.1016/s0165-1781(98)00101-2. [DOI] [PubMed] [Google Scholar]
- 31.Braff DL., Grillon C., Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- 32.Braff DL., Swerdlow NR., Geyer MA. Symptom correlates of prepulse inhibition deficits in male schizophrenic patients. Am J Psychiatry. 1999;156:596–602. doi: 10.1176/ajp.156.4.596. [DOI] [PubMed] [Google Scholar]
- 33.Cadenhead KS., Swerdlow NR., Shafer KM., et al. Modulation of the startle response and startle laterality in relatives of schizophrenic patients and schizotypal personality disordered subjects: evidence of inhibitory deficits. Am J Psychiatry. 2000;157:1660–1668. doi: 10.1176/appi.ajp.157.10.1660. [DOI] [PubMed] [Google Scholar]
- 34.Nuechterlein KH., Asarnow RF., Subotnik KL., et al. Neurocognitive vulnerability factors for schizophrenia: Convergence across genetic risk studies and longitudinal trait/state studies. In: Lenzenweger MF, Dworkin RH, eds. Origins and Development of Schizophrenia: Advances in Experimental Psychopathology. Washington, DC: American Psychological Association; 1998:299–327. [Google Scholar]
- 35.D’Amato T., Saoud M., Triboulet P., et al. Vulnerability to schizophrenia. I: Familial nature of neuropsychologic indicators. Encephale. 1998;24:442–448. [PubMed] [Google Scholar]
- 36.Ito M., Kanno M., Mori Y., et al. Attention deficits assessed by Continuous Performance Test and Span of Apprehension Test in Japanese schizophrenic patients. Schizophr Res. 1997;23:205–211. doi: 10.1016/s0920-9964(96)00108-9. [DOI] [PubMed] [Google Scholar]
- 37.Buchanan RW., Strauss ME., Breier A., et al. Attentional impairments in deficit and nondeficit forms of schizophrenia. Am J Psychiatry. 1997;154:363–370. doi: 10.1176/ajp.154.3.363. [DOI] [PubMed] [Google Scholar]
- 38.Miller MB., Chapman LJ., Chapman JP., et al. Schizophrenic deficit in span of apprehension. J Abnorm Psychol. 1990;99:313–316. doi: 10.1037//0021-843x.99.3.313. [DOI] [PubMed] [Google Scholar]
- 39.Elkins IJ., Cromwell RL., Asarnow RF. Span of apprehension in schizophrenic patients as a function of distractor masking and laterality. J Abnorm Psychol. 1992;101:53–60. doi: 10.1037//0021-843x.101.1.53. [DOI] [PubMed] [Google Scholar]
- 40.Toomey R., Faraone SV., Seidman LJ., et al. Association of neuropsychological vulnerability markers in relatives of schizophrenic patients. Schizophr Res. 1998;31:89–98. doi: 10.1016/s0920-9964(98)00025-5. [DOI] [PubMed] [Google Scholar]
- 41.Franke P., Maier W., Hardt J., et al. Attentional abilities and measures of schizotypy: their variation and covariation in schizophrenic patients, their siblings, and normal control subjects. Psychiatry Res. 1994;54:259–272. doi: 10.1016/0165-1781(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 42.Roitman SE., Cornblatt BA., Bergman A., et al. Attentional functioning in schizotypal personality disorder. Am J Psychia try. 1997;154:655–660. doi: 10.1176/ajp.154.5.655. [DOI] [PubMed] [Google Scholar]
- 43.Goldberg TE., Torrey EF., Gold JM., et al. Genetic risk of neuropsychological impairment in schizophrenia: a study of monozygotic twins discordant and concordant for the disorder. Schizophr Res. 1995;17:77–84. doi: 10.1016/0920-9964(95)00032-h. [DOI] [PubMed] [Google Scholar]
- 44.Trestman RL., Horvath T., Kalus O., et al. Event-related potentials in schizotypal personality disorder. J Neuropsychiatry Clin Neurosci. 1996;8:33–40. doi: 10.1176/jnp.8.1.33. [DOI] [PubMed] [Google Scholar]
- 45.Frangou S., Sharma T., Alarcon G., et al. The Maudsley Family Study, II: Endogenous event-related potentials in familial schizophrenia. Schizophr Res. 1997;23:45–53. doi: 10.1016/S0920-9964(96)00089-8. [DOI] [PubMed] [Google Scholar]
- 46.Cadenhead KS., Perry W., Braff DL. The relationship of information-processing deficits and clinical symptoms in schizotypal personality disorder. Biol Psychiatry. 1996;40:853–858. doi: 10.1016/0006-3223(95)00547-1. [DOI] [PubMed] [Google Scholar]
- 47.Saccuzzo DS., Cadenhead KS., Braff DL. Backward versus forward visual masking deficits in schizophrenic patients: centrally, not peripherally, mediated. Am J Psychiatry. 1996;153:1564–1570. doi: 10.1176/ajp.153.12.1564. [DOI] [PubMed] [Google Scholar]
- 48.Gottesman II., Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 49.Piatt JR. Stong inference: certain systematic methods of scientific thinking may produce much more rapid progress than others. Science. 1964;146:347–352. doi: 10.1126/science.146.3642.347. [DOI] [PubMed] [Google Scholar]
- 50.Buervenich S., Carmine A., Arvidsson M., et al. NURR1 mutations in cases of schizophrenia and manic-depressive disorder. Am J Med Genet. 2000;96:808–813. doi: 10.1002/1096-8628(20001204)96:6<808::aid-ajmg23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 51.Harrison PJ., Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 52.Lalouel JM., Le Mignon L., Simon M., et al. Genetic analysis of idiopathic hemochromatosis using both qualitative (disease status) and quantitative (serum iron) information. Am J Hum Genet. 1985;37:700–718. [PMC free article] [PubMed] [Google Scholar]
- 53.Leppert M., Burt R., Hughes JP., et al. Genetic analysis of an inherited predisposition to colon cancer in a family with a variable number of adenomatous polyps. N Engl J Med. 1990;322:904–908. doi: 10.1056/NEJM199003293221306. [DOI] [PubMed] [Google Scholar]
- 54.Insel TR., Collins FS. Psychiatry in the genomics era. Am J Psychiatry. 2003;160:616–620. doi: 10.1176/appi.ajp.160.4.616. [DOI] [PubMed] [Google Scholar]
- 55.Watson JD., Crick FH. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature. 1953;171:737–738. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- 56.Penney JB., Jr., Young AB. Speculations on the functional anatomy of basal ganglia disorders. Annu Rev Neurosci. 1983;6:73–94. doi: 10.1146/annurev.ne.06.030183.000445. [DOI] [PubMed] [Google Scholar]
- 57.Swerdlow NR., Koob GF. Dopamine, schizophrenia, mania, and depression: Toward a unified hypothesis of cortico-stratio-pallido-thalamic function. Behav Brain Sci. 1987;10:197–245. [Google Scholar]
- 58.Andreasen NC. A unitary model of schizophrenia: Bleuler's “fragmented phrene” as schizencephaly. Arch Gen Psychiatry. 1999;56:781–787. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- 59.Braff DL. Connecting the “dots” of brain dysfunction in schizophrenia: what does the picture look like? Arch Gen Psychiatry. 1999;56:791–793. doi: 10.1001/archpsyc.56.9.791. [DOI] [PubMed] [Google Scholar]
- 60.Light GA. Probing cortico-cortical interactions that underlie the multiple sensory, cognitive, and “real-world” functional deficits in schizophrenia patients. Behav Brain Sci. 2005. In press. [Google Scholar]
- 61.Braff DL., Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- 62.Light GA., Braff DL. Human and animal studies of schizophrenia-related gating deficits. Curr Psychiatry Rep. 1999;1:31–40. doi: 10.1007/s11920-999-0008-y. [DOI] [PubMed] [Google Scholar]
- 63.Light GA., Braff DL. Do self-reports of perceptual anomalies reflect gating deficits in schizophrenia patients? Biol Psychiatry. 2000;47:463–467. doi: 10.1016/s0006-3223(99)00280-2. [DOI] [PubMed] [Google Scholar]
- 64.Light GA., Braff DL. Sensory gating deficits in schizophrenia: can we parse the effects of medication, nicotine use, and changes in clinical status? Clin Neurosci Res. 2003:47–54. [Google Scholar]
- 65.Schwarzkopf SB., Lamberti JS., Smith DA. Concurrent assessment of acoustic startle and auditory P50 evoked potential measures of sensory inhibition. Biol Psychiatry. 1993;33:815–828. doi: 10.1016/0006-3223(93)90023-7. [DOI] [PubMed] [Google Scholar]
- 66.Cadenhead KS., Light GA., Geyer MA., McDowell JE., Braff DL. Neurobiological measures of schizotypal personality disorder: defining an inhibitory endophenotype? Am J Psychiatry. 2002;159:869–871. doi: 10.1176/appi.ajp.159.5.869. [DOI] [PubMed] [Google Scholar]
- 67.Light GA., Braff DL. Measuring P50 suppression and prepulse inhibition in a single recording session. Am J Psychiatry. 2001;158:2066–2068. doi: 10.1176/appi.ajp.158.12.2066. [DOI] [PubMed] [Google Scholar]
- 68.Brenner CA., Edwards CR., Carroll CA., Kieffaber PD., Hetrick WP. P50 and acoustic startle gating are not related in healthy participants. Psychophysiology. 2004;41:702–78. doi: 10.1111/j.1469-8986.2004.00206.x. [DOI] [PubMed] [Google Scholar]
- 69.Braff D., Stone C., Callaway E., Geyer M., Glick I., Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- 70.Cadenhead KS., Geyer MA., Braff DL. Impaired startle prepulse inhibition and habituation in patients with schizotypal personality disorder. Am J Psychiatry. 1993;150:1862–1867. doi: 10.1176/ajp.150.12.1862. [DOI] [PubMed] [Google Scholar]
- 71.Swerdlow NR., Martinez ZA., Hanlon FM., et al. Toward understanding the biology of a complex phenotype: rat strain and substrain differences in the sensorimotor gating-disruptive effects of dopamine agonists. J Neurosci. 2000;20:4325–4336. doi: 10.1523/JNEUROSCI.20-11-04325.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Swerdlow NR., Platten A., Kim YK., et al. Sensitivity to the dopaminergic regulation of prepulse inhibition in rats: evidence for genetic, but not environmental determinants. Pharmacol Biochem Behav. 2001;70:219–226. doi: 10.1016/s0091-3057(01)00598-6. [DOI] [PubMed] [Google Scholar]
- 73.Swerdlow NR., Shoemaker JM., Pitcher L., et al. Genetic differences in startle gating-disruptive effects of apomorphine: evidence for central mediation. Behav Neurosci. 2002;116:682–690. doi: 10.1037//0735-7044.116.4.682. [DOI] [PubMed] [Google Scholar]
- 74.Swerdlow NR., Shoemaker JM., Platten A., Pitcher L., Goins J., Auerbach PP. Heritable differences in the dopaminergic regulation of sensorimotor gating. I. Apomorphine effects on startle gating in albino and hooded outbred rat strains and their F1 and N2 progeny. Psychopharmacology (Berl). 2004;174:441–451. doi: 10.1007/s00213-003-1481-3. [DOI] [PubMed] [Google Scholar]
- 75.Swerdlow NR., Shoemaker JM., Auerbach PP., Pitcher L., Goins J., Platten A. Heritable differences in the dopaminergic regulation of sensorimotor gating. II. Temporal, pharmacologic and generational analyses of apomorphine effects on prepulse inhibition. Psychopharmacology (Berl). 2004;174:452–462. doi: 10.1007/s00213-003-1480-4. [DOI] [PubMed] [Google Scholar]
- 76.Swerdlow NR., Kuczenski R., Goins JC., et al. Neurochemical analysis of rat strain differences in the startle gating-disruptive effects of dopamine agonists. Pharmacol Biochem Behav. 2005;80:203–211. doi: 10.1016/j.pbb.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 77.Ellenbroek BA., van Luijtelaar G., Frenken M., Cools AR. Sensory gating in rats: lack of correlation between auditory evoked potential gating and prepulse inhibition. Schizophr Bull. 1999;25:777–788. doi: 10.1093/oxfordjournals.schbul.a033418. [DOI] [PubMed] [Google Scholar]
- 78.Swerdlow NR., Braff DL., Taaid N., Geyer MA. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psychiatry. 1994;51:139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- 79.Geyer MA., Krebs-Thomson K., Braff DL., Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl). 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- 80.Swerdlow NR., Geyer MA., Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl). 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- 81.National Institute of Mental Health Consortium of Genetics of Schizophrenia. The genetics of endophenotypes and schizophrenia. Available at: https://npistat.com/cogs. 2005 Mar Accessed 22; [Google Scholar]
- 82.Adler LE., Pachtman E., Franks RD., Pecevich M., Waldo MC., Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry. 1982;17:639–654. [PubMed] [Google Scholar]
- 83.Freedman R., Coon H., Myles-Worsley M., et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci U S A. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Judd LL., McAdams L., Budnick B., Braff DL. Sensory gating deficits in schizophrenia: new results. Am J Psychiatry. 1992;149:488–493. doi: 10.1176/ajp.149.4.488. [DOI] [PubMed] [Google Scholar]
- 85.Waldo M., Myles-Worsley M., Madison A., Byerley W., Freedman R. Sensory gating deficits in parents of schizophrenics. Am J Med Genet. 1995;60:506–511. doi: 10.1002/ajmg.1320600605. [DOI] [PubMed] [Google Scholar]
- 86.Siegel C., Waldo M., Mizner G., Adler LE., Freedman R. Deficits in sensory gating in schizophrenic patients and their relatives. Evidence obtained with auditory evoked responses. Arch Gen Psychiatry. 1984;41:607–612. doi: 10.1001/archpsyc.1984.01790170081009. [DOI] [PubMed] [Google Scholar]
- 87.Waldo MC., Adler LE., Freedman R. Defects in auditory sensory gating and their apparent compensation in relatives of schizophrenics. Schizophr Res. 1988;1:19–24. doi: 10.1016/0920-9964(88)90035-7. [DOI] [PubMed] [Google Scholar]
- 88.Cadenhead KS., Shafer K., Light GA., et al. Vulnerability markers in schizotypal personality disorder: defining a phenotype. Biol Psychiatry. 2000;47:37S. [Google Scholar]
- 89.Adler LE., Hoffer LD., Wiser A., Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- 90.Adler LE., Hoffer LJ., Griffith J., Waldo MC., Freedman R. Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biol Psychiatry. 1992;32:607–616. doi: 10.1016/0006-3223(92)90073-9. [DOI] [PubMed] [Google Scholar]
- 91.Stevens KE., Kern WR., Mahnir VM., Freedman Selective α7-nicotinic agonists normalize inhibition of auditory response in DBA mice. Psychopharmacology. 1998;136:320–327. doi: 10.1007/s002130050573. [DOI] [PubMed] [Google Scholar]
- 92.Leonard S., Gault J., Hopkins J., et al. Association of promoter variants in the aynicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry. 2002;59:1085–1096. doi: 10.1001/archpsyc.59.12.1085. [DOI] [PubMed] [Google Scholar]
- 93.Nagamoto HT., Adler LE., Hea RA., Griffith JM., McRae KA., Freedman R. Gating of auditory P50 in schizophrenics: unique effects of clozapine. Biol Psychiatry. 1996;40:181–188. doi: 10.1016/0006-3223(95)00371-1. [DOI] [PubMed] [Google Scholar]
- 94.Nagamoto HT., Adler LE., McRae KA., et al. Auditory P50 in schizophrenics on clozapine: improved gating parallels clinical improvement and changes in plasma 3-methoxy-4-hydroxyphenylglycol. Neuropsychobiology. 1999;39:10–17. doi: 10.1159/000026553. [DOI] [PubMed] [Google Scholar]
- 95.Light GA., Geyer MA., Clementz BA., Cadenhead KS., Braff DL. Normal P50 suppression in schizophrenia patients treated with atypical antipsychotic medications. Am J Psychiatry. 2000;157:767–771. doi: 10.1176/appi.ajp.157.5.767. [DOI] [PubMed] [Google Scholar]
- 96.Lohr JB., Braff DL. The value of referring to recently introduced antipsychotics as “second generation.”. Am J Psychiatry. 2003;160:1371–1372. doi: 10.1176/appi.ajp.160.8.1371. [DOI] [PubMed] [Google Scholar]