Abstract
The identification of endophenotypes in the personality disorders may provide a basis for the identification of underlying genotypes that influence the traits and dimensions of the personality disorders, as well as susceptibility to major psychiatric illnesses. Clinical dimensions of personality disorders that lend themselves to the study of corresponding endophenotypes include affective instability impulsiwity aggression, emotional information processing, cognitive disorganization, social deficits, and psychosis. For example, the propensity to aggression can be evaluated by psychometric measures, interview, laboratory paradigms, neurochemical imaging, and pharmacological studies. These suggest that aggression is a measurable trait that may be related to reduced serotonergic activity. Hyperresponsiveness of amygdala and other limbic structures may be related to affective instability, while structural and functional brain alterations underlie the cognitive disorganization in psychoticlike symptoms of schizotypal personality disorder. Thus, an endophenotypic approach not only provides clues to underlying candidate genes contributing to these behavioral dimensions, but may also point the way to a better understanding of pathophysiological mechanisms.
Keywords: endophenotype, borderline personality disorder, schizotypal personality disorder, psychosis, candidate gene
Abstract
La identificación de endofenotipos en los trastornos de personalidad puede proporcionar las bases para la identificación de genotipos fundamentales que influyan sobre rasgos y dimensiones de los trastornos de personalidad, como también sobre la susceptibilidad a las principales enfermedades psiquiátricas. Las dimensiones clínicas de los trastornos de personalidad que se prestan para el estudio de los endofenotipos correspondientes incluyen la inestabilidad afectiva, la impulsividad, la agresividad, el procesamiento de la información emocional, la desorganización cognitiva, los déficits sociales y la psicosis. Por ejemplo, la tendencia a la agresividad puede ser evaluada mediante mediciones psicométricas, entrevistas, paradigmas de laboratorio, neuroimágenes funcionales y estudios farmacológicos. Estas evaluaciones sugieren que la agresividad es un rasgo medible que puede relacionarse con una disminución de la actividad serotoninérgica. La hiperrespuesta de la amígdala y de otras estructuras límbicas se puede relacionar con la inestabilidad afectiva, mientras que alteraciones cerebrales estructurales y funcionales subyacen a la desorganización cognitiva de los síntomas de tipo psicótico del trastorno de personalidad esquizotípico. Por lo tanto, una aproximación endofenotípica no sólo proporciona pistas para los genes candidato fundamentales que contribuyen a estas dimensiones conductuales, sino que también puede iluminar el camino para una mejor comprensión de los mecanismos fisiopatológicos.
Abstract
L'identification des endophénotypes dans les troubles de la personnalité peut fournir des bases à l'identification des génotypes sous-jacents qui influent sur les caractéristiques et l'étendue des troubles de la personnalité comme sur la susceptibilité aux maladies psychiatriques majeures. L'étendue clinique des troubles de la personnalité qui donnent matière à l'étude des endophénotypes correspondants comprend l'instabilité affective, l'impulsivité, l'agression, la transformation de l'information émotionnelle, la désorganisation cognitive, les inadaptations sociales et la psychose. Par exemple, la propension aux agressions peut être évaluée par des mesures psychométriques, des entretiens oraux, des modèles de laboratoire, de l'imagerie neurochimique et des études pharmacologiques. Ces éléments suggèrent que l'agression est une caractéristique mesurable qui peut être reliée à une activité sérotoninergique réduite, L'hyper réactivité de l'amygdale et des autres structures limbiques peut avoir un lien avec une instabilité affective, alors que les altérations cérébrales structurales et fonctionnelles laissent supposer une désorganisation cognitive dans les symptômes psychomimétiques des troubles de la personnalité schizotypiques. Ainsi, une approche endophénotypique ne fournit pas seulement des indices sur les gènes candidats sous-jacents qui participent à ces dimensions comportementales mais elle peut également indiquer la voie vers une meilleure compréhension des mécanismes physiopathologiques.
The classification of the personality disorders has posed a challenge to epidemiologists, clinicians, geneticists, and psychologists. Because of the varied academic perspectives on these disorders that range from behaviorist to interpersonal to psychodynamic to trait theory, the schemata that have evolved to categorize the personality disorders have been highly variable and controversial. The result has been a nomenclature for these disorders defined, for example, in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) in polythetic criteria that in some cases reflect an epidemiological and/or behavioral tradition, such as antisocial personality disorder, or in other cases, a psychoanalytically oriented tradition, such as in narcissistic personality disorder. An alternative approach to understanding and classifying psychiatric disorders, which has not been extensively investigated in the personality disorders, is reframing the diagnostic nomenclature in terms of specific and measurable biologically and/or genetically based endophenotypes, which open up the possibility of identifying genetic predisposing factors, as well as providing a possibly more rational classification schema. This approach, while not necessarily incompatible with other diagnostic approaches that are formulated from alternative perspectives such as behavioral or psychodynamic approaches, raises the possibility of generating an underlying “vocabulary” of personality disorders grounded in specific biologic substrates. Combinations of these endophenotypically based dimensions of personality disorders, such as affective instability or impulsivity/aggression, might then become the basis of more complex multifactorial personality disorders recognized by the clinician, such as borderline personality disorder (BPD) or schizotypal personality disorder (SPD). Furthermore, such an endophenotypic approach may help clarify the interaction of underlying genetic predispositions with environmental influences. By identifying measurable characteristics that reflect an underlying genotype or are more closely related to that genotype than to the diagnostic category itself, the opportunity to unravel pathophysiological pathways involving specific candidate genes as well as environmental influences on their expression becomes a more feasible possibility.
There are a variety of endophenotypic strategies, including identifying specific clinical characteristics of a disorder, for example, age of onset, positive family history, or suicide history.1 An endophenotypic strategy for personality disorders might also be based on an underlying dimensional structure of the personality disorders, which has gained increasing acceptance among investigators in the field.2,3 Thus, the strategy of identifying intermediate phenotypes for dimensions of impulsivity, aggression, affective instability, and emotional information processing in the cluster B disorders; dimensions of psychoticlike perceptual distortions, social deficits, and cognitive impairment in the cluster A disorders; and dimensions of anxiety and behavioral inhibition, and compulsivity in the cluster C personality disorders may be a promising one (Table I). While a dimensional approach is defined at the level of psychopathology, cognitive neuroscience can provide measurable characteristics of performance in domains such as sustained attention or working memory. At a more fundamental psychophysiological or neurophysiological level, characteristics such as P50 evoked potentials, eye movement dysfunction, or startle/blink paradigms can provide promising endophenotypes that have proved useful in the schizophrenia spectrum.3 At a more fundamental biological level, neurochemical parameters, including receptor binding or neuroimaging variables, may be useful as potential endophenotypes.
Table I. Dimensions of cluster A, B, and C disorders. DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, 4th Edition.
| DSM-IV cluster | Dimensions |
| Cluster A disorders |
|
|
|
|
|
| Cluster B disorders |
|
|
|
|
|
|
|
| Cluster C disorders |
|
|
Ideally, an endophenotype would have high concordance in twins with a high monozygotic-to-dyzygotic twin ratio, high correlation in sibpairs, longitudinal stability in patients and control cohorts, and high discriminability between patients with a specific diagnostic category in comparison to healthy or psychiatric comparison groups. It is desirable for an endophenotype to have a specific mode of inheritance as well, and it is particularly important for it to be relatively convenient and accessible to measure in order to feasibly evaluate the characteristic in large populations.4 Criteria have been developed for the identification of endophenotypes for use in psychiatric genetic studies and include:
An association with the illness in the general population.
Heritability and emergence before the onset of illness.
State independence.
Close segregation with the illness in families.
Higher prevalence in nonaffected family members than in the general population, although less than in affected family members.
Because the personality disorders, by definition, represent relatively enduring or persistent traits or coping styles, which may be in some cases related to the susceptibility to major Axis I disorders (eg, SPD to schizophrenia, avoidant personality disorder to social phobia or generalized anxiety disorder), they may lend themselves particularly well to endophenotypic approaches. In this overview, we focus on specific dimensions of personality disorder that may represent behavioral intermediate phenotypes and discuss more biologically based endophenotypes that may underlie these dimensions, with a particular focus on several prototypic personality disorders: BPD, SPD, and avoidant personality disorder. We start with a review of studies suggesting heritability for personality disorders and, for our prototypic disorders in particular, we follow this with a discussion of strategies for genetic studies of personality disorders, and then we discuss specific prototypical disorders and related dimensions.
Heritability of personality disorders
Both twin and family studies, including adoptive studies, strongly suggest a genetic component for personality and personality disorder diagnosis. These are strongest when the personality or personality disorder phenotype is formulated in terms of continuous dimensions. Thus, twin studies, including monozygotic twins reared together and apart, support a robust genetic influence on personality dimensions such as neuroticism and extraversion.5,6 Twin studies have also suggested a genetic substrate for two of the prototypic disorders we addressed: SPD7 and BPD.8 Both twin9 and family studies10-12 suggest that specific dimensions or traits of the personality disorders, such as impulsivity or affective instability, may be more heritable than the disorder itself. For example, the dimension of impulsive aggression, which has been hypothesized to be a central dimension of BPD,13 has been shown to have substantial heritability in at least two twin populations.14 Thus, these criteria are supported for personality disorders, such as BPD, by numerous family and adoption studies.10
A variety of genetic studies, both twin and adoptive, have also established a genetic basis for schizophrenia spectrum that includes both schizophrenia and SPD.15 Both family and adoptive studies provide evidence for a greater prevalence of schizophrenia-related personality disorders in relatives of schizophrenic subjects,16 but genetic loading for schizophrenia in families of schizotypal probands may be less robust because schizophrenia is not as common or consistent in schizotypal probands as in family members of schizophrenic patients.3 Twin studies suggest that differential heritable factors may in fact be identified within the schizophrenia spectrum or SPD: one reflecting more psychotic-like symptoms and the other reflecting more deficit or negative symptoms.17,18
Anxiety is increased in relatives of patients with cluster C diagnosis, the “anxious cluster”19 including dependent personality, and continuity of social anxiety has been documented in twin and longitudinal studies.20 Complex personality disorders like borderline and SPD may emerge from substrates for more than one dimension. We will review dimensions related to these and other specific personality disorders.
Strategy for genetic studies of prototypic personality disorders
A variety of complementary approaches to identifying endophenotypes in the personality disorders may provide convergent validity for the most promising endophenotypes. Many of these strategies follow directly from the criteria proposed by Gottesman and Gould21 and Leboyer et al.1 Heritability could be established most definitively in large samples of twins in an epidemiologically ascertained sample that could provide enough variance for the major dimensions of these personality disorders. Subjects would be evaluated for clinical phenotypic measures by diagnostic interview, self-report measures, and mental status evaluations that reflect specific dimensions of psychopathology. Laboratory measures including neuropsychological, psychophysiological, or laboratory behavioral tests could then be measured in this population to define potentially heritable endophenotypes. A complementary approach is to identify such endophenotypes in the personality disorder in question, such as BPD or SPD, and demonstrate a specific increase in these endophenotypes compared with normal control or psychiatric comparison groups. State independence or longitudinal stability could be established in longitudinal studies with repeated measures of the endophenotypic tests of interest. Finally, genetic studies of clinically identified samples could be used to determine whether the endophenotypic measure cosegregates with the illness or personality disorder in family members, and is also found in nonaffected family members at a higher rate than in the general population. These strategies are already being applied to the study of BPD and, to a lesser extent, SPD.
Endophenotypes in BPD
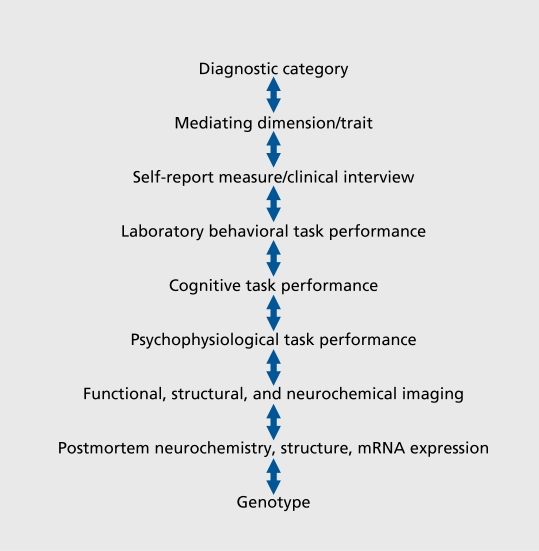
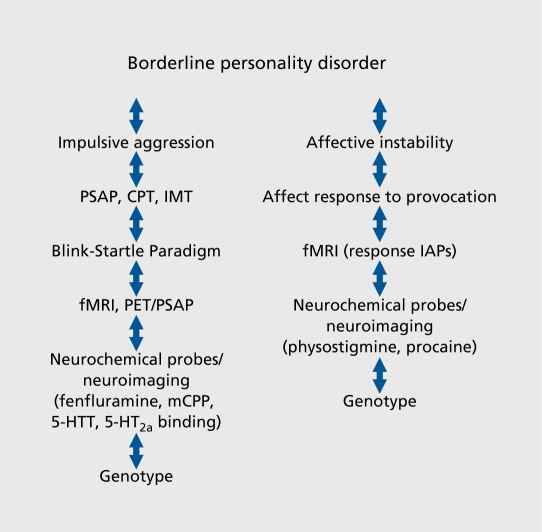
BPD has been formulated as an emergent personality disorder grounded in the interaction of underlying genetically based dimensions including impulsive aggression, affective instability, and altered emotional information processing. Identifying endophenotypes for these partially discriminable dimensions may thus represent a more achievable goal than identifying endophenotypes for the more complex parent disorder. For each dimension, diagnostic interview criteria, psychometric variables from self-report measures, laboratory behavioral tests, neurochemical variables and neuroimaging paradigms, postmortem neurochemistry and molecular biology techniques, as well as brain structural techniques, also represent potential endophenotypes that may identify promising genotypes (Figure 1 and 2).
Figure 1. Identifying promising genotypes from a diagnostic category.
Figure 2. Identifying promising genotypes in borderline personality disorder. PSAP, Point Subtraction Aggression Paradigm; CPT, Continuous Performance Task; IMT, Immediate Memory Task; fMRI, functional magnetic resonance imaging; PET, positron emission tomography; mCPP, meta-chlorophenylpiperazine; 5-HTT, serotonin transporter; 5-HT2a, 5-hydroxytryptamine (serotonin) 2a receptor; IAP, International Affective Picture slide.
Impulsivity
Impulsivity is a central characteristic of many of the cluster B personality disorders and, as noted above, most aggressive acts committed by personality-disordered patients represent impulsive rather than planned aggression. Impulsive aggression may also be directed toward the subject himself or herself as in self -injurious behavior. Other forms of impulsive behavior, such as binge eating, reckless driving, or gambling, may also be observed in personality-disordered patients.
Impulsivity is thus defined as a propensity or readiness to act without reflection or appropriate constraint, often resulting in behaviors that bring on negative consequences such as aggression; it is a critical dimension of BPD12 and, as discussed above, appears to be heritable, relatively stable in longitudinal studies, and a potential target for both pharmacological and psychosocial treatment. While impulsivity is often expressed in the domain of aggression in BPD, the two dimensions may be partially discriminable and will be treated separately. Psychometric measures that might be used for assessments of impulsive tendencies include the Barrett Impulsivity Scale (BIS-11)22,23 and interviews that evaluate life history of actual impulsive behaviors, such as the Life History of Impulsive Behavior.24 These psychometric measures may be complemented by laboratory assessments that identify critical components of impulsivity. For example, the Immediate Memory Task (IMT) reflects “attentional impulsivity,” while go/stop tasks or go/no go Continuous Performance Tasks (CPTs) reflect a disinhibition or “motor impulsivity.” The Single Key Impulsivity Paradigm (SKIP) reflects “nonplanning impulsivity.” Multifactorial analyses of self-report psychometric data support the characterization on these subtypes of impulsivity (Flory et al, personal communication). Both preclinical studies25 and clinical studies suggest that a more fine-grained multidimensional approach to impulsivity may be warranted and that nonplanning impulsivity may be a key ingredient of BPD.
Aggression
One of the more common impulsive behaviors evidenced by people with BPD are expressions of anger or reactive aggression. Thus, the kind of anger that is observed in BPD patients is an impulsive type of aggression, but the aggressive components may be analyzed by somewhat different measures than the impulsive components. For example, psychometric measures designed to measure aggression include the Buss-Perry Aggression Questionnaire (BPAQ),26 as well as measures of life history of overt aggressive behaviors (life history of aggression [LHA]). Both measures have well-established psychometric properties and heritability has been established in twin studies using the Buss Durkee Hostility Interview (BDHI),27 a precursor of the BPAQ. Preliminary data also suggest that life history of aggression may be heritable.
Laboratory paradigms that assess aggression behaviorally are available, including the Point Subtraction Aggression Paradigm (PSAP).28 In the PSAP, an experimental subject is instructed to accumulate “points” that can be exchanged for money and is told that they are playing in conjunction with a “confederate subject,” while in reality responses are generated by computer. Aggressive responses are often retaliatory to provocations from the “confederate” and do not net the subject of the study actual “points,” but may be initiated as an aggressive response to the perceived aggression of the confederate. The PSAP has been externally validated in violent and nonviolent male parolees and responses to this laboratory test have been correlated with other psychometric measures of aggression.29 The heritability of this laboratory measure has not been definitively established, but is being systematically assessed in studies of twins (Coccaro et al, personal communication). Another laboratory test for evaluating the propensity for aggression in response to provocation is the Taylor Aggression Paradigm,30 in which aggressive responses to mild electric shocks are administered to the subject, ostensibly by a fictitious opponent. Aggressive behavior is evaluated as a function of the shock intensities administered by the subject to this fictitious opponent. This paradigm has been used extensively in the evaluation of alcoholinduced aggression31 and has been applied to studies of reactive aggression in BPD (Coccaro et al, personal communication). Aggressive responding in the PSAP paradigm is a stable trait that can distinguish between aggressive and nonaggressive subjects, but, for both of these measures, the precise prevalence in specific personality disorders, such as BPD, and the degree of genetic influence on the PSAP has not been determined. Preliminary data from our laboratory, however, suggest that more aggressive responses at lower provocation-free interval on the PSAP can distinguish impulsive aggressive patients with BPD from patients with other personality disorders not characterized by aggression or controls (New et al, unpublished data). These data suggest that a polymorphism of catecholamine O-methyltransferase (COMT) is associated with aggressive responses on the PSAP (Flory et al, unpublished data).
There are also a variety of neuroendocrine/neurochemical as well as neuroimaging paradigms that suggest possible endophenotypic measures in the realm of aggression. The serotonin (5-hydroxytrypamine, 5-HT) system is the neurotransmitter system most consistently implicated in the pathogenesis of aggression. Fenfluramine, which releases serotonin and acts directly on serotonin receptors, stimulates prolactin release, probably by a 5-HT2c receptor-mediated mechanism. The prolactin responsiveness to fenfluramine administration thus provides an indirect reflection of the capacity of the serotonergic system, which depends on available serotonin for release, reuptake capacity, and receptor sensitivity. Patients with BPD demonstrate reduced prolactin responses to fenfluramine compared with controls,32 and the degree of response is highly significantly inversely correlated with scores on the Buss-Durkee “Assault” and “Irritability” subscales of the Hostility Inventory. Furthermore, reduced prolactin responses to fenfluramine are particularly associated with criteria of impulsivity, intense anger, and selfdamaging acts, but not to other criteria that reflect affective instability or identity/relational problems.32 As neuroendocrine paradigms cannot assess brain responsiveness in critical cortical inhibitory regions, serotonergic probe studies have shifted to assessment of cortical responses to these probes as assessed by fluorodeoxy glucose positron emission tomography (FDG-PET). Aggressive patients with BPD in one cohort33 and BPD patients in another cohort34 demonstrated reduced responses in prefrontal cortex to the administration of fenfluramine compared with placebo. These reductions were particularly pronounced in regions of orbital and ventral medial prefrontal cortex, while other more posterior regions did not necessarily differ between the two groups or were indeed enhanced in the impulsive patient populations. Furthermore, in the initial fenfluramine study, the areas of significant correlation of activation in response to fenfluramine in orbital frontal cortex with amygdalar activity suggesting an interactive circuit were more extensive in normal controls than they were in impulsive aggressive subjects, where areas with significant correlations with amygdala were more limited. Another serotonergic probe that has been used to evaluate cortical activation in relation to aggression that might serve as an endophenotype is the metabolite of trazadone, meta-chlorophenylpiperazine (mCPP). Baseline activity is reduced in male BPD patients with physical aggression, and mCPP responses compared with placebo responses are reduced in anterior cingular gyrus, particularly on the left, in BPD patients with any type of aggression.35 Furthermore, in this study as well, correlations between amygdala and anterior cingulate were disrupted in the aggressive BPD patients compared with controls.36 Finally, reductions in serotonin transporter (5-HTT) binding appear to be associated with impulsive aggression in BPD patients.37
These data are consistent with reduced serotonergic facilitation via 5-HT2a receptors of prefrontal cortical inhibitory regions, particularly anterior cingulate and orbital frontal cortex, which serve to “brake” the amygdala. Thus, reduced serotonergic activity may result in disinhibited aggression generated in response to negatively evaluated stimuli. This pathophysiological model could in part emerge from alterations in serotonergic activity, primarily reduced integrity of prefrontal inhibitory centers, or exaggerated responsiveness of amygdala and related limbic structures. Endophenotypes that reflect reduced serotonergic activity, altered frontal activation, or enhanced limbic reactivity thus might serve to characterize specific vulnerabilities of this functional circuitry in aggressive personality disorder patients. They also may be used in family studies to characterize relatives or in conjunction with candidate genes, for example, in the serotonergic system, in association studies. Thus, for example, a polymorphism in the serotonin transporter that determines the amount of transporter expressed has been associated with neuroticism,38 and aggression in some studies,39,40 but not in others.41 The s allele is associated with impulsivity and BPD in bulimic patients,42 aggression and violent suicide attempts in schizophrenic patients,43,44 aggression in cocaine abusers,45 and aggression and suicide in alcoholics,46,47 and also with a potential intermediate phenotype of aggression, the blunted prolactin response to fenfluramine.48 The polymorphism for tryptophan hydroxylase was reported to be associated with aggression in one pilot study,40 and with self -harm in another.49 A more recent study suggested association between the 5-HTR1B receptor in suicide history50 and recent data suggest the possibility of a 5-HT2a receptor polymorphism being associated with self -injurious behavior (New, personal communication). These studies illustrate how a dimensional approach might generate intermediate clinical variables or phenotypes to identify candidate genes of interest. Studies are underway to evaluate more objective laboratory evaluations in relation to these genetypes, such as the PSAP and potentially more biologically based “endo”-phenotypes based on neuroimaging studies.
The catecholaminergic system has also been implicated in aggression with reduced concentrations of the norepinephrine metabolite, MHPG (3 -methoxy-4-hydroxy glycol),51 and enhanced growth hormone responses to clonidine52 reported to be associated with aggression in personality-disordered patients. Impulsivity, particularly in relation to hyperactivity and substance abuse, has been associated with allelic variation in dopaminergic genes including the dopamine DRD4 receptor,53 dopamine transporter,54 D2 receptor,55 and D3 receptor.56 Monoamine oxidase A (MAOA), which produces both dopamine and serotonin as metabolites, has been associated with impulsivity57-59 Genetic variation of the α2a-adrenergic receptor has also been associated with impulsiveness and hostility in normal subjects.60 These studies did not in general use laboratory intermediate phenotype measures. A polymorphism near the val/met allele of COMT has been associated with self-reports of aggression as well as aggressive responses on the PSAP and impulsive errors on the CPT (Flory et al, unpublished data).61
Affect regulation
Psychometric measures of affect regulation that could be used for intermediate phenotypes are the Affective Lability Scale (ALS),62 which measures an individual's propensity to shift between affects of anger, depression, elation, and anxiety. The ALS has sound psychometric properties and good dimensional and diagnostic specificity. Another measure, the Affective Intensity Measure (AIM),63 measures the intensity of the experience of affect and it has been found to have some modest heritability as well (Coccaro et al, personal communication). A variety of laboratory and imaging paradigms may provide potential phenotypes for the affective instability of BPD, including startle eye blink paradigms which measure the magnitude of an eye blink in response to negative (enhancing) eye blink stimuli and positive (reducing) eye blink stimuli. This test has good test-retest reliability and response may be heritable, as suggested by family studies of schizophrenic patients. Skin conductance response has also been used to measure emotional arousal, and has good stability and test-retest reliability. Finally, corrugated muscle electromyography (EMG) activity is associated with the valence of affective stimuli and may differentiate externalizing from internalizing personalities.64 These paradigms have not been studied extensively in BPD. Imaging paradigms evaluating functional brain activity in response to emotionally provocative stimuli may also provide phenotypes for this dimension. For example, increased amygdala activity has been reported following emotionally provocative stimuli,65 and increased activation of lateral regions of prefrontal cortex, areas implicated in voluntary or effortful control of behavior, and increased activation of medial superior frontal cortex, implicated in self-referential perception, have been demonstrated in BPD patients compared with controls.66 Furthermore, in the latter study, the degree of activation of amygdala correlated with the degree of negative affective arousal. These psychophysiological and neuroimaging paradigms can then be studied in relation to promising candidate genes to identify underlying genotypes that may contribute to the excessive emotional lability and reactivity characteristic of a number of personality disorders in cluster B, particularly BPD. While a repeat polymorphism, the DRD4 gene has been associated with a related dimension, novelty-seeking,53 there have been few explicit studies of the genetics of affective instability and none in personality disorders.
Emotional information processing
While emotional lability and reactivity, discussed in the previous section, generally refer to alterations in the threshold of emotional reactivity, the processing and recognition of emotional information may be a partially discriminable dimension that enables appropriate social interaction. Impairment in neuronal circuits involving orbital frontal ventral medial prefrontal cortex and anterior cingulate may mediate the abnormal emotional information processing.
Psychometric measures that might be used to identify intermediate phenotypes of this dimension include the Emotion Attribution Questionnaire (EAQ) (Coccaro et al, personal communication) identifying a subject's ability to identify the emotion of another person in a vignette such as anger, sadness, fear, and disgust. These are similar to the emotions expressed in the Ekman Facial Emotional Recognition Task (EFERT). Indeed, the EFERT can be used to directly assess emotional information processing in the laboratory67 The Emotional Stroop Task68 may also be useful in assessing emotional information processing, as may be the Bechara Gambling Task (BGT) where subjects need to discriminate advantageous from disadvantageous decks of card.69 The EFERT is sensitive to ventral medial prefrontal cortical dysfunction. The Emotional Stroop Task test asks subjects to name the color of a word presented and BPD subjects exhibit a delay in naming the color when emotionally charged words are presented. The stability of these measures, their discriminability for specific personality disorders such as BPD, and their underlying genetics are not yet clear, but are a focus of current studies.
The schizophrenia spectrum personality disorders (SPD)
A number of critical dimensions underlie the schizophrenia spectrum or cluster A personality disorders. Cognitive disorganization, as exemplified in disturbed thinking patterns, odd speech or language, and even eccentric appearance, is a hallmark of SPD and may parallel the more massive cognitive disorganization observed in schizophrenia. Deficit-like or negative symptoms are prominent in SPD and to a lesser degree in schizoid personality disorder, and seem to represent attenuated versions of the more severe deficit symptoms of schizophrenia. The cognitive/perceptual distortions of SPD represent psychotic-like or positive symptoms analogous to the positive symptoms of schizophrenia and, while not meeting criteria for actual hallucinations and delusions, may reflect the same underlying dimension. Paranoia, which is characteristic of SPD and paranoid personality disorder and may reflect an underlying cognitive disorganization, can represent attempt to reorganize perceptions attributing to external sources the underlying chaos and fragmentation in thinking processes the individual with SPD experiences. These dimensions may be more effectively disentangled in SPD, where their severity is not as marked as in schizophrenia, and thus provide potential tools to identify genotypes not only for the spectrum personality disorders but for schizophrenia itself. For example, positive and negative symptoms are highly correlated with each other and with severity in schizophrenic patients, but may be more feasibly partially isolated as dimensions in SPD.
Cognitive disorganization
Cognitive disorganization may be evaluated psychometrically as a part of the Schizotypal Personality Questionnaire (SPQ).70 A number of cognitive domains are specifically impaired in SPD, including sustained attention as measured by the CPT,71 working memory as measured by auditory and visual working memory tasks,72 and verbal learning as measured by verbal learning and memory tasks, such as the California Verbal Learning Task.72,73 While these cognitive domains are more severely impaired in schizophrenia, they are part of a more generalized deterioration in cognitive function with deficits in general intelligence and motor capacity, which are not necessarily observed in SPD. Thus, the study of SPD may enable identification of these underlying endophenotypes for specific cognitive dysfunctions, which may apply to schizophrenia as well. A number of psychophysiological endophenotypes, which are being currently applied to studies of schizophrenic patients and their relatives, have also been usefully applied to SPD and may help to clarify underlying genetic substrates for this dimension. For example, most schizophrenic patients and their relatives show deficits in the P50 evoked potential paradigm, which has also been associated with an altered polymorphism in the α7-nicotinic receptor.74 This promising endophenotype has also been demonstrated to be impaired in schizotypal relatives of schizophrenic patients75 and in schizotypal subjects identified clinically through advertisements.76 A startle-blink paradigm, in which a prepulse stimuli results in a inhibition of the postpulse stimuli, has also been found to be abnormal not only in schizophrenic subjects and their family members, but also in schizotypal subjects.77 Other psychophysiological endophenotypes include impairment in backward masking performance, antisaccade generation, and smooth pursuit eye movement impairments.78 Imaging paradigms may be used to identify structural and functional brain correlates of these altered cognitive and psychophysiological functions. For example, reduced prefrontal volumes may be associated with cognitive impairment and deficit symptoms of SPD and anomalous temporal and other lateral cortical region activation during cognitive tasks may be associated with cognitive disorganization.
A polymorphism of COMT (Val/Met), which has been associated with working memory and other cognitive impairments in schizophrenia,79 also appears to be modestly associated with cognitive, particularly working memory impairment, in schizotypal subjects (Minzenberg et al, unpublished data).80 Other candidate genes that have been associated with cognitive impairment, such as dysbindin or GRM3, have yet to be tested in schizotypal subjects.81
Deficit symptoms
Deficit symptoms may also be assessed as part of the schizotypal personality questionnaire. Laboratory tests assessing social and information processing tasks may also be of use here, in this case, to identify misperceptions and distortions in information processing that reflect deficits in social perception (rather than the abnormal emotional biasing associated with the cluster B disorders). Tests assessing “theory of mind” have been employed in this regard in subjects with autism. Cognitive tasks addressing executive function are most likely to be associated with deficit symptoms. Reduced volume of frontal cortex has been associated with increases in deficit symptoms and executive dysfunction in schizotypal subjects.82,83 Structural magnetic resonance imaging (MRI) studies suggest that frontal lobe is relatively preserved as compared to temporal lobe in schizotypal subjects, while reductions in both are prominent in subjects with schizophrenia. FDG-PET studies suggest that schizotypal subjects show modest reductions in frontal activation during verbal learning tasks, although the deficits are not nearly as pervasive or severe as those in schizophrenic patients.3 In many regions, activation is comparable to that observed in normal volunteers and, in some, there may actually be compensatory activation (as can also be observed in schizophrenic subjects), in regions such as Brodmann Area (BA) 10, which may function as a super executive area in frontal pole.84 A working memory functional MRI (fMRI) study also suggests compensatory activation in BA10 and reduced activation in area BA46 compared with normal controls.85 While neuroimaging protocols may not be used routinely for endophenotypes in large-scale genetic studies, they may be useful in defining candidate genes such as the COMT polymorphism in more intensively studied selected clinical samples.
Psychosis
The dimension of psychosis is a critical part of the symptomatology of the schizophrenic disorders and, while overt psychosis is an exclusion criteria for schizotypal and other schizophrenia-related personality disorders, psychotic-like symptoms are characteristic of people with SPD, representing attenuated symptoms on this dimension. Psychotic-like symptoms can be assessed both by interviewers as part of the schizotypal personality questionnaire86 or in the perceptual aberration or Per/Mag subscales of the Chapman Scales.87 This dimension or phenotype has been linked with indices of dopaminergic activity, and with higher dopaminergic activity associated with greater psychotic-like symptoms in the schizophrenic disorders. In SPDs, plasma homovanillic acid (HVA) was found to be higher in patients with SPD compared with normal controls83 and the plasma HVA concentrations were correlated with degree of psychotic-like symptomatology.83 In this study, group differences were abolished after covarying for psychotic-like symptoms. An identical configuration of results was found in another partially overlapping cohort of patients using cerebrospinal fluid (CSF) HVA.83 Another potential index of subcortical dopaminergic responsivity can be assessed by measuring plasma HVA responses to the glycopyruvic stressor, 2-deoxyglucose (2-DG). By blocking glucose absorption into brain cells of frontal lobe, 2-DG induces stress responses including plasma Cortisol and HVA increases following 2-DG administration. Patients with schizophrenia demonstrate elevated HVA responses to 2-DG compared to controls; schizotypal patients have normal responses and, compared with normal controls, reduced Cortisol responses.
Functional and structural imaging studies of striatum point to the possibility of increased dopaminergic activity. Thus, patients with SPD show increased striatal volume, particularly in ventral putamen, which may reflect dendritic proliferation secondary to increased dopaminergic activity. Increased metabolic activation of ventral putamen in SPD compared with schizophrenia is also consistent with reduced dopaminergic inhibitory tone and may also be a promising imaging endophenotype for candidate gene studies. Finally, amphetamine, by releasing dopamine, will displace radiotracers that bind the D2 receptor, which can be evaluated using IBZM ([123I]iodobenzamide) as a radioligand in single-photon emission computed tomography (SPECT) studies or in PET studies using raclopride as a radioligand. A SPECT study utilizing IBZM as a ligand indicated that SPD subjects have displacement of IBZM following amphetamine, which is intermediate between the markedly increased displacement values found in acute schizophrenic patients and normal controls.
Raclopride displacement studies using PET scanning following amphetamine also suggest significant increases in raclopride displacement in schizophrenic patients88 and such studies are underway in SPD patients. These studies suggest that dopaminergic activity in subcortex is normal to modestly increased, but consistently less than that observed in acutely schizophrenic patients. Thus, the dopamine system may be better buffered in schizotypal patients, and dopaminergic indices may provide promising endophenotypes for a dimension of psychosis in schizophrenia. Candidate genes related to dopamine activity, such as polymorphisms or haplotypes in the dopamine β-hydroxylase gene89,90 or the dopamine D4 receptor,3,91,92 have been found to be associated with psychosis-related symptomatology, but have not yet been assessed in SPD or in relation to these endophenotypic neurochemical and imaging endophenotypes.
Avoidant personality disorder
Avoidant personality disorder is prototypical of the anxious or cluster C personality disorders. The anxious cluster personality disorders are characterized by a susceptibility to marked anxiety as well as behavioral patterns designed to ward off potential future précipitants of anxiety. There have been few studies of the neurobiology and genetics of these disorders to point towards potentially promising endophenotypes. There is a high comorbidity between avoidant personality disorder and social phobia.93 Heritability of social anxiety has been established in twin studies and longitudinal studies of traits of inhibition, closely related to anxiety, show the stability of these traits94 and a familial relationship to major anxiety disorders.95 Limited studies suggest alterations in serotonergic receptors in some studies. For example, patients with social phobia have an enhanced fenfluramineinduced rise in Cortisol compared with normal controls, but responses to adrenergic probes have been variable.96 There are also indications of altered dopaminergic activity in social phobia.97 However, none of these studies were performed in patients with personality disorders such as avoidant personality disorder, and results have not been compelling or consistent. High, less variable heart rate has been consistently documented in individuals with behavioral inhibition94 and, while this has not been directly evaluated in avoidant personality disorder, it might be a promising endophenotype for some of the anxious cluster personality disorders or at least a dimension of behavioral inhibition, which could be evaluated in these disorders. There have been few genetic studies using endophenotypes in anxiety-related personality disorders, although the serotonin transporter has been associated with neuroticism and harm avoidance in normal volunteers and substance abusers.38,98 An allelic form of the DRD4 receptor has been associated with avoidant personality disorder symptoms99 as well as with the cluster C personality disorders.100
Conclusions
Personality disorders lend themselves particularly well to endophenotype studies, as they represent relatively stable traits that can be formulated in terms of underlying and interactive dimensions. These in turn may be associated with laboratory, behavioral, or neurobiological “endophenotypes” that bring our understanding closer to underlying genotypes. A few studies have already linked clinical dimensions and, in some cases, neurobiological measures to candidate genes (eg, COMT polymorphisms with cognitive impairment, 5-HTR1B polymorphisms with suicidal behavior). However, such approaches have only recently been applied to the Axis I disorders and have been initiated in relation to the personality disorders, which may represent the most prevalent phenotypes for underlying genotypes in susceptibility to major psychiatric disorders.
Selected abbreviations and acronyms
- BPD
borderline personality disorder
- COMT
catecholamine O-methyltransferase
- CPT
Continuous Performance Task
- FDG-PET
fluorodeoxyglucose positron emission tomography
- 5-HT
5-hydroxytryptamine (serotonin)
- HVA
homovanillic acid
- PSAP
Point Subtraction Aggression Paradigm
- SPD
schizotypal personality disorder
REFERENCES
- 1.Leboyer M., Bellivier F., Nosten-Bertrand M., Jouvent R., Pauls D., Mallet J. Psychiatric genetics: search for phenotypes. Trends Neurosci. 1998;21:102–105. doi: 10.1016/s0166-2236(97)01187-9. [DOI] [PubMed] [Google Scholar]
- 2.Widiger TA., Trull TJ., Hurt SW., Clarkin J., Frances A. A multidimensional scaling of the DSM-III personality disorders. Arch Gen psychiatry. 1987;44:557–563. doi: 10.1001/archpsyc.1987.01800180077012. [DOI] [PubMed] [Google Scholar]
- 3.Siever LJ., Davis KL. The pathophysiology of schizophrenia disorders: perspectives from the spectrum. Am J Psychiatry. 2004;161:398–413. doi: 10.1176/appi.ajp.161.3.398. [DOI] [PubMed] [Google Scholar]
- 4.Leboyer M. Searching for alternative phenotypes in psychiatric genetics. Methods Mol Med. 2003;77:145–161. doi: 10.1385/1-59259-348-8:145. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen NL., McClearn GE., Plomin R., Nesselroade JR., Berg S., DeFaire The Swedish Adoption/Twin Study of Aging: an update. Arch Gen Med. 1991;40:7–20. doi: 10.1017/s0001566000006681. [DOI] [PubMed] [Google Scholar]
- 6.Tellegen A., Lykken TD., Bouchard TJ., Wilcox KJ., Segal NL., Rich S. Personality similarity in twins reared apart and together. J Pers Soc Psychol. 1988;54:1031–1039. doi: 10.1037//0022-3514.54.6.1031. [DOI] [PubMed] [Google Scholar]
- 7.Torgersen S., Skre I., Onstad S., Edvardsen J., Kringlen E. “True” schizotypal personality disorder: a study of co-twins and relatives of schizophrenic probands. Am J Psychiatry. 1993;150:1661–1667. doi: 10.1176/ajp.150.11.1661. [DOI] [PubMed] [Google Scholar]
- 8.Torgersen S., Lygren S., Oien PA., et al. A twin study of personality disorders. Compr Psychiatry. 2000;41:416–425. doi: 10.1053/comp.2000.16560. [DOI] [PubMed] [Google Scholar]
- 9.Torgersen S. Genetic and nosological aspects of schizotypal and borderline personality disorders. Arch Gen Psychiatry. 1984;41:546–554. doi: 10.1001/archpsyc.1984.01790170020003. [DOI] [PubMed] [Google Scholar]
- 10.Siever LJ., Torgersen S., Gunderson JG., Livesley WJ., Kendler KS. The borderline diagnosis III: identifying endophenotypes for genetic studies. Biol Psychiatry. 2002;51:964–968. doi: 10.1016/s0006-3223(02)01326-4. [DOI] [PubMed] [Google Scholar]
- 11.Silverman JM., Pinkham L., Horvath TB., et al. Affective and impulsive personality disorder traits in the relatives of patients with borderline personality disorder. Am J Psychiatry. 1991;148:1378–1385. doi: 10.1176/ajp.148.10.1378. [DOI] [PubMed] [Google Scholar]
- 12.Zanarini MC., Frankenburg FR., Yong L., et al. Borderline psychopathology in the first-degree relatives of borderline and axis II comparison probands. J Personal Disord. 2004;18:439–447. doi: 10.1521/pedi.18.5.439.51327. [DOI] [PubMed] [Google Scholar]
- 13.Siever LJ., Davis KL. A psychobiological perspective on the personality disorders. Am J Psychiatry. 1991;148:1647–1658. doi: 10.1176/ajp.148.12.1647. [DOI] [PubMed] [Google Scholar]
- 14.Coccaro EF., Bergman CS., Kavoussi RJ., Seroczynski AD. Heritability of aggression and irritability: a twin study of the Buss-Durkee Aggression Scales in adult male subjects. Biol Psychiatry. 1997;41:273–284. doi: 10.1016/s0006-3223(96)00257-0. [DOI] [PubMed] [Google Scholar]
- 15.Tsuang MT., Stone WS., Faraone SV. Schizophrenia: a review of genetic studies. Harv Rev Psychiatry. 1999;7:185–207. [PubMed] [Google Scholar]
- 16.Kendler KS., McGuire M., Gruenberg AM., O'Hare A., Spellman M., Walsh D. The roscommon family study I. Methods, diagnosis of probands and risk of schizophrenia in relatives. Arch Gen Psychiatry. 1993;50:527–540. doi: 10.1001/archpsyc.1993.01820190029004. [DOI] [PubMed] [Google Scholar]
- 17.Kendler KS., Ochs AL., Gorman AM., et al. The structure of schizotypy: a pilot multitrait twin study. Psychiatry Res. 1991;36:19–36. doi: 10.1016/0165-1781(91)90114-5. [DOI] [PubMed] [Google Scholar]
- 18.Fanous A., Gardner C., Walsh D., Kendler KS. Relationship between positive and negative symptoms of schizophrenia and schizotypal symptoms nonpsychotic relatives. Arch Gen Psychiatry. 2001;58:669–673. doi: 10.1001/archpsyc.58.7.669. [DOI] [PubMed] [Google Scholar]
- 19.Reich J. Avoidant and dependent personality traits in relatives of patients with panic disorder, patients with dependent personality disorder and normal controls. Psychiatry Res. 1991;39:89–98. doi: 10.1016/0165-1781(91)90011-d. [DOI] [PubMed] [Google Scholar]
- 20.Kagan J. The meanings of personality predicates. Am Psychol. 1988;43:614–620. doi: 10.1037//0003-066x.43.8.614. [DOI] [PubMed] [Google Scholar]
- 21.Gottesman II., Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 22.Barratt ES. Impulsiveness subtraits: arousal and information processing. In: Spence JT, Izard CE, eds. Motivation, Emotion and Personality. Amsterdam, The Netherlands: Elsevier Science Publishers; 1985:137–146. [Google Scholar]
- 23.Barratt ES. Impulsiveness and aggression. In: Monahan J, Steadman HJ, eds. Violence and Mental Disorder. Developments in Risk Assessment. Chicago, III: University of Chicago Press; 1994:61–79. [Google Scholar]
- 24.Schmidt CA., Fallon AE., Coccaro EF. Assessment of behavioral and cognitive impulsivity: development and validation of the Lifetime History of Impulsive Behaviors Interview. Psychiatry Res. 2004;126:107–121. doi: 10.1016/j.psychres.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 25.Winstanley CA., Dalley JW., Theobald DE., Robbins TW. Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology. 2004;29:1331–1343. doi: 10.1038/sj.npp.1300434. [DOI] [PubMed] [Google Scholar]
- 26.Buss AH., Perry M. The aggression questionnaire. J Personal Soc Psychol. 1992;63:452–459. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- 27.Buss AH., Durkee A. An inventory for assessing different kinds of hostility. J Consulting Psychol. 1957;21:343–348. doi: 10.1037/h0046900. [DOI] [PubMed] [Google Scholar]
- 28.Cherek DR. Effects of smoking different doses of nicotine on human aggressive behavior. Psychopharmacology. 1981;75:339–349. doi: 10.1007/BF00435849. [DOI] [PubMed] [Google Scholar]
- 29.Cherek DR., Moeller FG., Schnapp W., Dougherty DM. Studies of violent and nonviolent male parolees: I. Laboratory and psychometric measurements of aggression. Biol Psychiatry. 1997;41:514–522. doi: 10.1016/s0006-3223(96)00059-5. [DOI] [PubMed] [Google Scholar]
- 30.Taylor S. Aggressive behavior and physiological arousal as a function of provocation and the tendency to inhibit aggression. J Personal. 1967;35:297–310. doi: 10.1111/j.1467-6494.1967.tb01430.x. [DOI] [PubMed] [Google Scholar]
- 31.Giancola PR. Executive functioning and alcohol-related aggression. J Abnorm Psychol. 2004;113:541–555. doi: 10.1037/0021-843X.113.4.541. [DOI] [PubMed] [Google Scholar]
- 32.Coccaro EF., Siever LJ., Lkar H., et al. Serotonergic studies in patients with affective and personality disorders: correlates with suicidal and impulsive aggressive behavior. Arch Gen Psychiatry. 1989;46:587–599. doi: 10.1001/archpsyc.1989.01810070013002. [DOI] [PubMed] [Google Scholar]
- 33.Siever LJ., Buchsbaum MS., New AS., et al. d, 1-Fenfluramine response in impulsive personality disorder assessed with [18F]flurodeoxyglucose positron emission tomography. Neuropsychopharmacology. 1999;20:413–423. doi: 10.1016/S0893-133X(98)00111-0. [DOI] [PubMed] [Google Scholar]
- 34.Soloff PH., Meltzer CC., Greer PJ., Constantine D., Kelly TM. A fenfluramine-activated FDG-PET study of borderline personality disorder. Biol Psychiatry. 2000;47:540–547. doi: 10.1016/s0006-3223(99)00202-4. [DOI] [PubMed] [Google Scholar]
- 35.New AS., Goodman M., Buchsbaum M., et al. Neural circuitry dysfunction in BPD. 158th Annual Meeting of the American Psychiatric Association, Atlanta, Ga. 2005:24–25 Poster 17D. [Google Scholar]
- 36.New AS., Hazlett EA., Buchsbaum MS., et al. Blunted prefrontal cortical 18F-fluorodeoxyglucose positron emission tomography response to meta-chlorophenylpiperazine in impulsive aggression. Arch Gen Psychiatry. 2002;59:621–629. doi: 10.1001/archpsyc.59.7.621. [DOI] [PubMed] [Google Scholar]
- 37.Frankle WG., Lombardo I., New AS., et al. Brain serotonin transporters distribution in subjects with in impulsive aggression: a positron emission study with [11C]McN5652. Am J Psychiatry. 2005;162:915–923. doi: 10.1176/appi.ajp.162.5.915. [DOI] [PubMed] [Google Scholar]
- 38.Lesch KP., Bengel D., Heils A., et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 39.Nielson DA., Goldman D., Virkkunen M., Tokola R., Rawlings R., Linnoila M. Suicidality and 5-hydroxyindoleacetic acid concentration associated with a tryptophan hydroxylase polymorphism. Arch Gen Psychiatry. 1994;51:34–38. doi: 10.1001/archpsyc.1994.03950010034005. [DOI] [PubMed] [Google Scholar]
- 40.New AS., Gelernter J., Yovell Y., et al. Tryptophan hydroxylase genotype is associated with impulsive-aggression measures: a preliminary study. Am J Med Genet. 1998;81:13–17. doi: 10.1002/(sici)1096-8628(19980207)81:1<13::aid-ajmg3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 41.Gelernter J., Kranzler H., Coccaro EF., Siever LJ., New AS. Serotonin transporter protein gene polymorphism and personality measures in African American and European American subjects. Am J Psychiatry. 1998;155:1332–1338. doi: 10.1176/ajp.155.10.1332. [DOI] [PubMed] [Google Scholar]
- 42.Steiger H., Joober R., Israel M., et al. The 5HTTLPR polymorphism, psychopathologic symptom and platelet [3H]paroxetine binding in bulimic syndromes. Int J Eat Disord. 2004;37:57–60. doi: 10.1002/eat.20073. [DOI] [PubMed] [Google Scholar]
- 43.Bayle FJ., Leroy S., Gourion D., et al. 5HTTLPR polymorphism in schizophrenic patients: further support for association with violent suicide attempts. Am J Med Genet B Neuropsychiatr Genet. 2003;119:13–17. doi: 10.1002/ajmg.b.10037. [DOI] [PubMed] [Google Scholar]
- 44.Nolan KA., Volavka J., Lachman HM., Saito T. An association between a polymorphism of the tryptophan hydroxylase gene and aggression in schizophrenia and schizoaffective disorder. Psychiatr Genet. 2000;10:109–15. doi: 10.1097/00041444-200010030-00002. [DOI] [PubMed] [Google Scholar]
- 45.Patkar AA., Berrettini WH., Hoehe M., et al. Serotonin transporter polymorphisms and measures of impulsivity, aggression, and sensation-seeking among African-American cocaine-dependent individuals. Psychiatry Res. 2002;110:103–115. doi: 10.1016/s0165-1781(02)00098-7. [DOI] [PubMed] [Google Scholar]
- 46.Preuss UW., Soyka M., Bahlmann M., Wenzel K., Behrens S. de Jonge S, Kruger M, Bondy B. Serotonin transporter gene regulatory region polymorphism (5-HTTLPR), [3H]paroxetine binding in healthy control subjects and alcohol-dependent patients and their relationships to impulsivity. Psychiatry Res. 2000;96:51–61. doi: 10.1016/s0165-1781(00)00190-6. [DOI] [PubMed] [Google Scholar]
- 47.Gorwood P., Batel P., Ades J., Hamon M., Boni C. Serotonin transporter gene polymorphisms, alcoholism, and suicidal behavior. Biol Psychiatry. 2000;48:259–264. doi: 10.1016/s0006-3223(00)00840-4. [DOI] [PubMed] [Google Scholar]
- 48.Reist C., Mazzanti C., Vu R., Tran D., Goldman D. Serotonin transporter promoter polymorphism is associated with attenuated prolactin response to fenfluramine. Am J Med Genet. 2001;105:363–368. doi: 10.1002/ajmg.1360. [DOI] [PubMed] [Google Scholar]
- 49.Pooley EC., Houston K., Hawton K., Harrison PJ. Deliberate self-harm is associated with allelic variation in the tryptophan hydroxylase gene (TPH A779C), but not with polymorphisms in five other serotonergic genes. Psychol Med. 2003;33:775–783. doi: 10.1017/s0033291703007463. [DOI] [PubMed] [Google Scholar]
- 50.New AS., Gelernter J., Goodman M., et al. Suicide, impulsive aggression and the HTR1B genotype. Biol Psychiatry. 2001;50:62–65. doi: 10.1016/s0006-3223(01)01108-8. [DOI] [PubMed] [Google Scholar]
- 51.Roy A., Adinoff B., Linnoila M. Acting out hostility in normal volunteers: negative correlation with levels of 5HIAA in cerebrospinal fluid. Psychiatry Res. 1988;24:187–194. doi: 10.1016/0165-1781(88)90061-3. [DOI] [PubMed] [Google Scholar]
- 52.Coccaro EF., Lawrence T., Trestman RL., Gabriel S., Klar HM., Siever LJ. Growth hormone resonses to intravenous clonidine challenge correlates with behavioral irritability in psychiatric patients and in healthy volunteers. Psychiatry Res. 1991;39:129–139. doi: 10.1016/0165-1781(91)90082-z. [DOI] [PubMed] [Google Scholar]
- 53.Rogers G., Joyce P., Mulder R., et al. Association of a duplicated repeat polymorphism in the 5'-untranslated region of the DRD4 gene with novelty seeking. Am J Med Genet B Neuropsychiatr Genet. 2004;126:95–98. doi: 10.1002/ajmg.b.20133. [DOI] [PubMed] [Google Scholar]
- 54.Kahn RS., Khoury J., Nichols WC., Lanphear BP. Role of dopamine transporter genotype and maternal prenatal smoking in childhood hyperactive-impulsive, inattentive, and oppositional behaviors. J Pediatr. 2003;143:104–110. doi: 10.1016/S0022-3476(03)00208-7. [DOI] [PubMed] [Google Scholar]
- 55.Limosin F., Loze JY., Dubertret C., et al. Impulsiveness as the intermediate link between the dopamine receptor D2 gene and alcohol dependence. Psychiatr Genet. 2003;13:127–129. doi: 10.1097/01.ypg.0000066963.66429.00. [DOI] [PubMed] [Google Scholar]
- 56.Retz W., Rosier M., Supprian T., Retz-Junginger P., Thome J. Dopamine D3 receptor gene polymorphism and violent behavior: relation to impulsiveness and ADHD-related psychopathology. J Neural Transm. 2003;110:561–572. doi: 10.1007/s00702-002-0805-5. [DOI] [PubMed] [Google Scholar]
- 57.Manor I., Tyano S., Mel E., et al. Family-based and association studies of monoamine oxidase A and attention deficit hyperactivity disorder (ADHD): preferential transmission of the long promoter-region repeat and its association with impaired performance on a continuous performance test (TOVA). Mol Psychiatry. 2002;7:626–632. doi: 10.1038/sj.mp.4001037. [DOI] [PubMed] [Google Scholar]
- 58.Manuck SB., Flory JD., Ferrell RE., Mann JJ., Muldoon MF. A regulatory polymorphism of the monoamine oxidase-A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Res. 2000;95:9–23. doi: 10.1016/s0165-1781(00)00162-1. [DOI] [PubMed] [Google Scholar]
- 59.Brunner HG., Nelen M., Breakefield XO., Ropers HH., van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- 60.Comings DE., Johnson JP., Gonzalez NS., et al. Association between the adrenergic alpha 2A receptor gene (ADRA2A) and measures of irritability, hostility, impulsivity and memory in normal subjects. Psychiatr Genet. 2000;10:39–42. doi: 10.1097/00041444-200010010-00007. [DOI] [PubMed] [Google Scholar]
- 61.Siever LJ., New AS., Goodman M., et al. Behavioral, cognitive, and neuroimaging intermediate phenotypes in impulsive personality disorders. Biol Psychiatry. 2004;55:192S. [Google Scholar]
- 62.Harvey PD., Greenberg BR., Serper MR. The affective lability scales: development reliability and validity. J Clin Psychol. 1989;45:786–793. doi: 10.1002/1097-4679(198909)45:5<786::aid-jclp2270450515>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 63.Larsen RJ., Diener E., Emmons RA. Affect intensity and reactions to daily life events. J Person Soc Psychol. 1986;51:803–815. [Google Scholar]
- 64.Lang PJ., Greenwald MK., Bradley MM., Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- 65.Herpertz SC., Dietrich TM., et al. Evidence of abnormal amygdale functioning in borderline personality disorder: a functional MRI study. Biol Psychiatry. 2001;50:292–298. doi: 10.1016/s0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- 66.Koenigsberg HW., Siever LJ., Guo X., et al. Emotion processing in borderline personality disorder: a functional MRI perspective. Neuropsychopharmacology. 2004;29(suppl 1):S84. [Google Scholar]
- 67.Ekman P., Friesen WV. Measuring facial movement. Environ Psychol Nonverbal Behav. 1976;1:56–75. [Google Scholar]
- 68.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 69.Bechara A., Damasio AR., Damasio H., Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 70.Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17:555–564. doi: 10.1093/schbul/17.4.555. [DOI] [PubMed] [Google Scholar]
- 71.Lees-Roitman SE., Mitropoulou V., Keefe RSE., et al. Visuospatial working memory in schizotypal personality disorder patients. Schizophr Res. 2000;41:447–455. doi: 10.1016/s0920-9964(99)00085-7. [DOI] [PubMed] [Google Scholar]
- 72.Mitropoulou V., Harvey PD., Maldari LA., et al. Neuropsychological performance in schizotypal personality disorder: evidence regarding diagnostic specificity. Biol Psychiatry. 2002;52:1175–1182. doi: 10.1016/s0006-3223(02)01426-9. [DOI] [PubMed] [Google Scholar]
- 73.Bergman A., Harvey P., Lees-Roitman S., Mitropoulou V., Marder D., Siever LJ. Verbal learning and memory in schizotypal personality disorder. Schizophr Bull. 1998;24:635–641. doi: 10.1093/oxfordjournals.schbul.a033355. [DOI] [PubMed] [Google Scholar]
- 74.Freedman R., Coon H., Myles-Worsley M., et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci US A. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adler LE., Hoffer LJ., Griffith J., Waldo MC., Freedman R. Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biol Psychiatry. 1992;32:607–616. doi: 10.1016/0006-3223(92)90073-9. [DOI] [PubMed] [Google Scholar]
- 76.Cadenhead KS., Light GA., Geyer MA., Braff DL. Sensory gating deficits assessed by the P50 event-related potential in subjects with schizotypal personality disorder. Am J Psychiatry. 2000;157:55–59. doi: 10.1176/ajp.157.1.55. [DOI] [PubMed] [Google Scholar]
- 77.Cadenhead KS., Swerdlow NR., Shafer KM., Diaz M., Braff DL. Modulation of the startle response and startle laterality in relatives of schizophrenic patients and in subjects with schizotypal personality disorder: evidence of inhibitory deficits. Am J Psychiatry. 2000;157:1660-1668. Correction: 2000;157:1904. doi: 10.1176/appi.ajp.157.10.1660. [DOI] [PubMed] [Google Scholar]
- 78.Siever LJ., Davis KL. The pathophysiology of the schizophrenic disorders: perspective from the spectrum. Am J Psychiatry. 2004;161:398–413. doi: 10.1176/appi.ajp.161.3.398. [DOI] [PubMed] [Google Scholar]
- 79.Egan MF., Goldberg TE., Kolachana BS., et al. Effect of COMT Val108/158Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stefanis NC., Van OS J., Avramopoulos D., et al. Variation in catechol-O-methyltransferase val58met genotype associated with schizotypy but not cognition: a population study in 543 young men. Biol Psychiatry. 2004;56:510–515. doi: 10.1016/j.biopsych.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 81.Harrison PJ., Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 82.Raine A., Lencz T., Yaralian P., et al. Prefrontal structural and functional deficits in schizotypal personality disorder. Schizophr Bull. 2002;28:501–513. doi: 10.1093/oxfordjournals.schbul.a006957. [DOI] [PubMed] [Google Scholar]
- 83.Siever LJ., Amin F., Coccaro EF., et al. CSF homovanillic acid in schizotypal personality disorder. Am J Psychiatry. 1993;150:149–151. doi: 10.1176/ajp.150.1.149. [DOI] [PubMed] [Google Scholar]
- 84.Buchsbaum MS., Nenadic I., Hazlett EA., et al. Differential metabolic rates in prefrontal and temporal Brodmann areas in schizophrenia and schizotypal personality disorder. Schizophr Res. 2002;54:141–50. doi: 10.1016/s0920-9964(01)00361-9. [DOI] [PubMed] [Google Scholar]
- 85.Koenigsberg HW., Harvey PD., Mitropoulou V., et al. Characterizing affective instability in borderline and other personality disorders. Am J Psychiatry. 2002;159:784–788. doi: 10.1176/appi.ajp.159.5.784. [DOI] [PubMed] [Google Scholar]
- 86.Raine A., Sheard C., Reynolds GP., Lencz T. Prefrontal structural and functional deficits associated with individual differences in schizotypal personality. Schizophr Res. 1992;7:237–247. doi: 10.1016/0920-9964(92)90018-z. [DOI] [PubMed] [Google Scholar]
- 87.Chapman LJ., Edell WS., Chapman JP. Physical anhedonia, perceptual aberration, and psychosis proneness. Schizophr Bull. 1980;6:639–653. doi: 10.1093/schbul/6.4.639. [DOI] [PubMed] [Google Scholar]
- 88.Breier A., Su TP., Saunders R., et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission topography method. Proc Natl Acad Sci USA. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cubells JF., Kranzler HR., McCance-Katz E., et al. A haplotype at the DBH locus, associated with low plasma dopamine p-hydroxylase activity, also associates with cocaine-induced paranoia. Mol Psychiatry. 2000;5:56–63. doi: 10.1038/sj.mp.4000657. [DOI] [PubMed] [Google Scholar]
- 90.Wood JG., Joyce PR., Miller AL., Mulder RT., Kennedy MA. A polymorphism in the dopamine beta-hydroxylase gene is associated with “paranoid ideation” patients with major depression. Biol Psychiatry. 2002;51:365–369. doi: 10.1016/s0006-3223(01)01367-1. [DOI] [PubMed] [Google Scholar]
- 91.Rinetti G., Camarena B., Cruz C., et al. Dopamine D4 receptor (DRD4) gene polymorphism in the first psychotic episode. Arch Med Res. 2001;32:35–38. doi: 10.1016/s0188-4409(00)00257-5. [DOI] [PubMed] [Google Scholar]
- 92.Serrati A., Lilli R., Lorenzi C., Lattuada E., Smeraldi E. DRD4 exon variants associated with delusional symptomatology in major psychoses: a study on 2011 affected subjects. Am J Med Genet. 2001;105:283–290. doi: 10.1002/ajmg.1321. [DOI] [PubMed] [Google Scholar]
- 93.Schneier FR., Spitzer RL., Gibbon M., et al. The relationship of social phobia subtypes and avoidant personality disorder. Compr Psychiatry. 1991;32:496–502. doi: 10.1016/0010-440x(91)90028-b. [DOI] [PubMed] [Google Scholar]
- 94.Kagan J., Reznick S., Snidman N., et al. Childhood derivatives of inhibition and lack of inhibition to the unfamiliar. Child Dev. 1988;59:1580–1589. doi: 10.1111/j.1467-8624.1988.tb03685.x. [DOI] [PubMed] [Google Scholar]
- 95.Hirshfeld DR., Rosenbaum JF., Biederman J., et al. Stable behavioral inhibition and its association with anxiety disorder. J Am Acad Child Adolesc Psychiatry. 1992;31:103–111. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- 96.Uhde TW. Anxiety and growth disturbance: is there a connection? A review of biological studies in social phobia. J Clin Psychiatry. 1994;55 (suppl 6):17–27. [PubMed] [Google Scholar]
- 97.Potts NL., Davidson JR. Social phobia: biological aspects and pharmacotherapy. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16:635–646. doi: 10.1016/0278-5846(92)90020-f. [DOI] [PubMed] [Google Scholar]
- 98.Wiesbeck GA., Weijers HG., Wodarz N., et al. Serotonin transporter gene polymorphism and personality traits in primary alcohol dependence. World J Biol Psychiatry. 2004; 5:45–48. doi: 10.1080/15622970410029907. [DOI] [PubMed] [Google Scholar]
- 99.Joyce PR., Rogers GR., Miller AL., Mulder RT., Luty SE., Kennedy MA. Polymorphisms of DRD4 and DRD3 and risk of avoidant and obsessive personality traits and disorders. Psychiatry Res. 2003;119:1–10. doi: 10.1016/s0165-1781(03)00124-0. [DOI] [PubMed] [Google Scholar]
- 100.Jacob CP., Strobel A., Hohenberger K., et al. Association between allelic variation of serotonin transporter function and neuroticism in anxious cluster C personality disorders. Am J Psychiatry. 2004;161:569–572. doi: 10.1176/appi.ajp.161.3.569. [DOI] [PubMed] [Google Scholar]