Abstract
Although tobacco use and smoking were introduced long ago, it was only recently that the nicotine contained in the tobacco leaves was recognized as an addictive substance acting on the central nervous system (CNS). However, even prior to this recognition, several studies have reported an association between smoking and psychiatric disorders. One of the many observations was that cessation is accompanied by a marked increase in the probability of major depression. In parallel with the discovery of the neuronal nicotinic acetylcholine receptors and their extensive expression in the CNS, this association sheds new light on the influence of cholinergic transmission in depression. In this article, we examine the various modes of action of nicotine in the CNS and discuss the mechanisms by which this alkaloid can prevent or precipitate mood disorders, and the possibility of discovering new therapeutic avenues for the treatment of depression.
Keywords: nicotine, depression, ligand-gated channel, acetylcholine
Abstract
Aunque el empleo del tabaco y el fumar fueron introducidos hace mucho tiempo, sólo recientemente se ha reconocido que la nicotina contenida en las hojas de tabaco constituye una sustancia adictiva que actúa en el sistema nervioso central (SNC). Sin embargo, aun previo a este reconocimiento diversos estudios habían demostrado una asociación entre el fumar y los trastornos psiquiátricos. Una de las muchas observaciones fue que el dejar de fumar se acompañaba de un marcado aumento en la probabilidad de presentar una depresión mayor. En paralelo con el descubrimiento de los receptores nicotínicos neuronales de acetilcolina y su extensa expresión en el SNC, esta asociación ha aportado nuevas luces acerca de la influencia de la transmisión colinérgica en la depresión. En este artículo se examinan varios modos de acción de la nicotina en el SNC y se discuten los mecanismos mediante los cuales este alcaloide puede prevenir o precipitar los trastornos del ánimo, y la posibilidad del descubrimiento de nuevas alternatives para el tratamiento de la depresión.
Abstract
Bien que la consommation de tabac soit entrée dans les mœurs de nos sociétés, il y a plusieurs centaines d'années, ce n'est que très récemment que la nicotine, qui est présente dans les feuilles du tabac, a été reconnue comme provoquant une dépendance au niveau du système nerveux central. De nombreuses études ont mis en évidence des associations entre certaines maladies psychiatriques et le comportement tabagique. L'une de ces associations est l'augmentation du risque de dépression majeure chez les fumeurs en sevrage. Les découvertes effectuées dans le domaine de la recherche fondamentale avec la mise en évidence des récepteurs de l'acétylcholine à haute affinité pour la nicotine dans le système nerveux central ont révêlé des mécanismes jusque-là insoupçonnés sur l'importance du système cholinergique dans les processus cognitifs et la dépression. Dans ce travail, nous examinons les modes d'action de la nicotine dans le système nerveux central et nous discutons des mécanismes par lesquels cet alcaloïde peut empêcher ou précipiter des troubles de l'humeur. Cette revue a également pour but de stimuler les réflexions sur de nouvelles voies thérapeutiques dans le traitement de la dépression.
The observation that a large majority of psychiatric patients smoke cigarettes leads to the question about the possible relationship between smoking, dependence, and neurological diseases.1,2 Several studies have reported an association between smoking and depression.3,4 This question takes on a new dimension when we consider that nicotine, the natural alkaloid contained in tobacco leaves, is a powerful and addictive compound acting on the central nervous system (CNS).
A pivotal point in this line of thinking is what we know about the mechanisms by which nicotine acts on the CNS and what we can gain from a better understanding of the intimate processes that drive to tobacco consumption.5,6 In this article, we will examine the basic functioning of the key players in nicotine addiction, ie, the neuronal nicotinic acetylcholine (ACh) receptors, and their possible role in depression.
The ACh receptors
First called Vagustoff by Loewi, due to its discovery in the heart muscle in 1921, the neurotransmitter ACh exerts many different actions. ACh is synthesized in the terminal bouton and stored in clear vesicles, and is released by nerve activity in the synaptic cleft. After a rapid increase, the ACh concentration quickly declines due to rapid hydrolysis (catalyzed by the enzyme acetylcholinesterase) and diffusion. The determinant role of this enzymatic activity in regulating the cholinergic tone and its brain function was revealed more than 150 years ago, well before the discovery of ACh, with the use of compounds that were later shown to be centrally active anticholinesterase agents.7,8 The observation that injection of physostigmine causes a rapid modification of mood and temporarily reverses acute mania suggested a possible hypersensitivite cholinergic equilibrium.9,10 Although these studies shed a new light on variations in cholinergic tone, they could not tell which receptors were stimulated by the sustained increase in ACh. With progress in molecular biology and genetics, we now know that ACh acts on two types of receptors: the muscarinic receptors and the nicotinic receptors.
The muscarinic ACh receptors
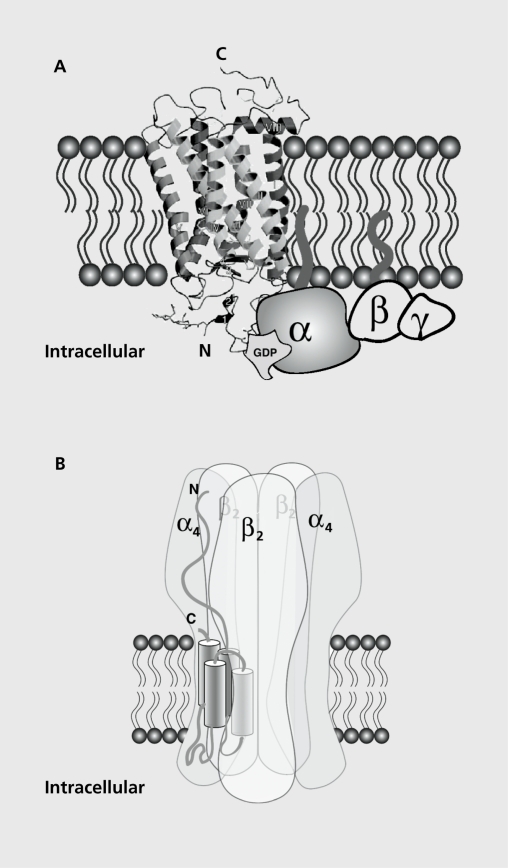
The muscarinic ACh receptors belong to the superfamily of G-coupled proteins, which display the structural characteristics of seven transmembrane proteins (Figure 1A).11 Five genes encoding muscarinic receptors have been identified to date, and their chromosomic localization determined. Binding of ACh stabilizes the receptor in a conformation that activates G-proteins present in their vicinity. A further subdivision of the muscarinic receptors in two groups can be made as a function of the second-messenger pathways activated: (i) M1 to M3, which stimulate the hydrolysis of phosphoinositol and trigger an increase in intracellular calcium concentration together with cyclic adenosine monophosphate (cAMP); and (ii) M4 to M5, which inhibit adenylyl cyclase.12
Figure 1. Schematic representation of the cholinergic receptors in the plasma membrane. A. Side view of the muscarinic receptor with a G-protein complex. Note the N- and C-terminal end of the protein with its seven transmembrane segments. The acetylcholine (ACh) binding site is thought to be in the center of the receptor complex. B. Side view of the nicotinic ACh receptor. The receptor represented corresponds to the α4β2 which is thought to be the major brain high-affinity nicotinic receptor. Note the subunit arrangement with the axis of symmetry and the ionic pore that is formed in the center of the assembly. The ACh binding site is at the interface between two adjacent α-β subunits.
The nicotinic ACh receptors
Neuronal nicotinic ACh receptors (nAChRs), which will be discussed here, belong to the family of ligand-gated channels. These receptors constitute both the ligand-binding site and the ionic pore through which ions can flow when the receptor is stabilized in the open conformation. Historically, the existence of such receptors was first revealed in 1857 by Bernard, who showed that the poison curare blocks transmission at the neuromuscular junction, but does not prevent muscle contraction elicited by electrical stimulation. Since this observation, the neuromuscular junction has been used as a reference for synaptic transmission in physiology and pharmacology. It was also recognized a long time ago that ACh is the neurotransmitter that acts on the parasympathetic ganglia, but little was known about the precise mechanisms underlying this neurotransmission. Although electro-physiological experiments in the 1950s13 showed that the pharmacology of ganglionic receptors differs from that of the neuromuscular junction,14 a clear distinction between the structure and function of these receptor classes had to await progress in molecular biology. Cloning and sequencing of the genes that encode the neuromuscular junction receptors revealed that embryonic muscle receptors result from the assembly of five subunits in the stoichiometry α2, α, γ, and δ, while adult receptors are made from α2, β, ε, and δ.15 Sequence homologies and low-stringency hybridization soon led researchers to clone a series of genes encoding for proteins that resemble those of the neuromuscular junction receptors, but displaying significant differences. To date, 12 genes encoding for α2 to α10 and β2 to β4 have been isolated in vertebrates and their chromosomic localization identified. Following an international agreement, the nomenclature between α and β subunits was made according to specific sequences of these proteins, with a subunits showing the highest degree of homology with their muscle counterpart and the presence of two adjacent cysteines in the N-terminal extracellular domain.16
It is widely accepted that nAChRs result, as the muscle receptors, from the assembly of five subunits, each of which spans the membrane four times (Figure 1B).15,17,18 This basic structural feature is common to a series of ligand-gated channels, which include the serotonin receptor 5-HT3, zinc-activated protein (ZAC), the glycine receptors, and the γ-aminobutyric acid (GABA) receptors GABAA and GABAC. The large extracellular domain comprises the ligand-binding site and the ionic pore lies in the center of the assembly. Each of the five subunits lines the pore by its second transmembrane domain. Despite the lack of a full crystal structure of the nAChRs, it is believed that the natural ligand ACh or nicotine binds at the interface between two adjacent subunits in the extracellular domain. Thus, the contribution of two adjacent subunits to the formation of the ligand-binding domain implies that both subunits will define the physiological and pharmacological properties of the resulting receptor. Although some subunits such as α7 can assemble in a homomeric manner, most receptors result from the heteromeric assembly of at least two subunits, for instance α4β2. In the case of heteromeric receptors, a further complexity can arise for triplet combinations, such as α4α6β2, etc. This gives rise to a wider diversity of the receptor function, which correlates with the pattern of expression of the different subunits.
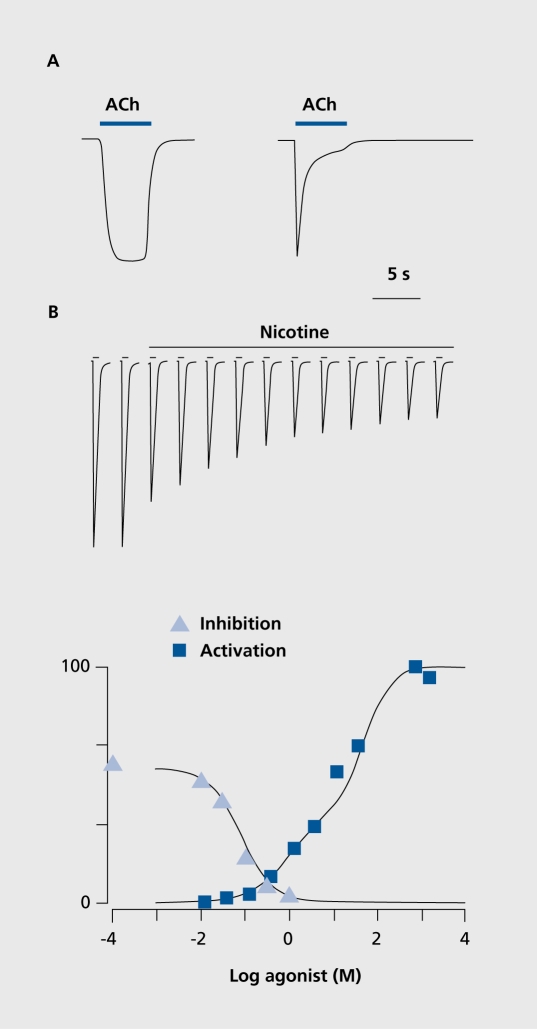
Binding of an agonist stabilizes the receptor in the active open state and causes cations to rapidly diffuse across the minute ionic pore. Significant differences in physiological properties, in terms of sensitivity to the agonist and time course of response, can be observed between different subtypes of nAChRs. One extreme is the α7 receptors, which have a low sensitivity to ACh, but a very fast response; the other extreme is receptors like the α4β2 receptor, which is highly sensitive to ACh and nicotine, but has a slow response (Figure 2A). As illustrated in Figure 2, α7 responses are phasic, while α4β2 responses are tonic. An additional and characteristic feature of α7 nAChRs is their high permeability to calcium ions.19,20 Since these divalent cations have been shown to play an important role as a second messenger, it can be expected that α7 activation could modify neuronal activity or gene expression.
Figure 2. A. Schematic representation of typical acetylcholine (ACh) evoked currents recorded in cells expressing the α4β2 (left trace) or α7 (right trace) receptors. B. Upper panel. Typical protocol used to determine the inhibition caused by a sustained nicotine exposure. The cell is challenged at periodic time intervals with a brief test pulse of agonist at low concentration. Having established that the cell response is stable, a sustained concentration of nicotine concentration comparable to that found in the smoker's brain is applied to the bath. Note the progressive decline of the responses that stabilizes after about half an hour. Lower panel. Plot of the fraction of response as a function of the logarithm of the nicotine concentration yields a typical inhibition dose-response curve that can be compared to the activation profile.
While a brief agonist exposure activates nAChRs, a sustained exposure to an agonist provokes a slow desensitization and therefore inhibits subsequent agonist-evoked responses. Figure 2 illustrates the typical protocol used to assess desensitization to prolonged nicotine exposure together with the dose-response inhibition curve. Superposition of the desensitization and activation curves indicates that there is a small window in which a ligand such as nicotine can provoke sustained receptor activation. On the basis of the nicotine concentration determined in the cerebrospinal fluid (CSF) of smokers,21 which can reach 100 to 200 nM, it is possible to deduce that nicotine should cause a small but sustained receptor opening. The activation and desensitization profiles are specific for each nAChR subtype.
Receptor distribution
To understand the possible contribution of nAChRs in the CNS function, it is essential to know their precise brain and cellular distribution. Receptor labeling relies either on the use of specific ligands or antibodies.22,23 Alternatively, receptors can be labeled in vivo using brain imaging techniques, such as positron emission tomography (PET). PET studies in monkey and human using A-85380, a ligand that preferentially labels the α4β2 nAChRs, reported significant labeling in the thalamus and more diffuse labeling in the cortical areas.24-26 While these results demonstrate the importance of heteromeric receptors in human brain, it should also be noted that a significant labeling is observed when the toxin from the snake Bungarus multicintus (α-Bgt), which specifically binds to the muscle and the α7 receptors, is used.23,27,28 [125I]α-Bgt studies have shown that α7 is widely distributed in mammalian brain and that its area of expression differs from that of α4β2.23,28
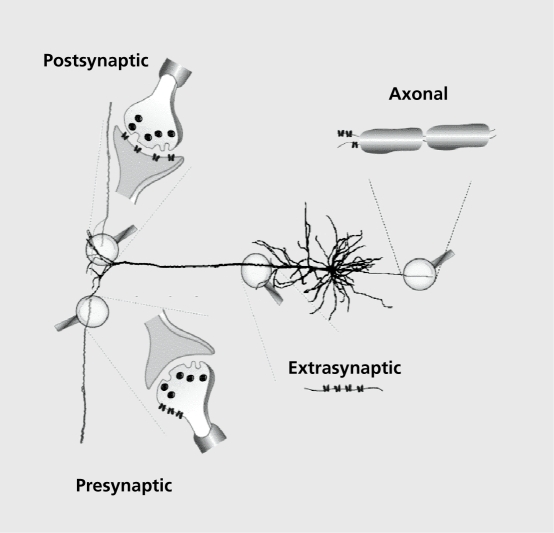
To better understand the function, however, we need to know the subcellular distribution of the receptors. While it is beyond the scope of this work to enter into details of receptor distribution, it is important to know that the expression of nAChRs is not restricted to the synaptic cleft and that a high density of receptors is observed on the cell body, as well as in the presynaptic and/or extrasynaptic areas Figure 3. Moreover, the presynaptic and extrasynaptic localization of the nAChRs has been shown to play an important role in the modulation of neurotransmitter release and neuronal activity.29-31
Figure 3. Schematic representation subcellular localization of neuronal nicotinic receptors with their postsynaptic, presynaptic, extrasynaptic, and axonal distributions.
Multiple functions of nAChRs
The broad distribution of nAChRs in the CNS suggests that these receptors play a major role in brain physiology. Surprisingly, however, bath application of nicotine to brain slices produces little or no effect, a result that was interpreted as the lack of action of nicotine in the CNS. However, if the neuronal properties are examined more closely, the action of nicotine can be detected in most brain areas. The reason for this discrepancy is mainly due to the method of drug application. When applied in the bath, nicotine provokes both a small activation of the receptors and their desensitization. Because bath application is rather slow, the short-lasting activation is essentially masked by the receptor desensitization. In contrast, when an agonist such as ACh or nicotine is briefly applied to neurons the physiological consequences of the nAChRs can be detected.
It has been shown that activation of nAChRs causes multiple effects according to the localization of the receptor. For example, somatic receptors will cause a depolarization of the neuron and therefore modulate its firing activity, while presynaptic receptors modulate the release of other neurotransmitter, such as dopamine, glutamate, and 5-HT.31 To understand the basis of these neurotransmitter interactions, it should be recalled that a significant fraction of the nAChRs are expressed on presynaptic boutons. Activation of the nAChRs causes two important mechanisms that are (i) depolarization of the synaptic bouton; and (ii) in the case of α7 nAChRs, a significant calcium influx. Both mechanisms provoke an increase in the intracellular calcium concentration, which is known to be the key step in the liberation of the neurotransmitter contained in the vesicle of the synaptic bouton by exocytosis.
While a few experiments have shown that nAChRs can participate in synaptic transmission, it is generally accepted that these receptors have more a modulatory role in neuronal function, rather than a determining role in the fast excitatory pathways. In agreement with these observations, knocking out a given nAChR subunit in mice is not lethal, but was shown to alter behavior.32
Nicotine and depression
In view of the modulatory role of nAChRs and their multiple interactions with various neurotransmitter systems, such as dopamine or 5-HT, it is not surprising that an association between nicotine and depression is often reported.3,4 The main pathways associating nicotine and depression are summarized in Table I. During the past 5 years, more than 250 scientific publications have discussed the interaction between nicotine and depression. The main question that remain open is by which mechanisms nicotine could act on the mood and/or depression. To examine this point further, we shall discuss a series of hypotheses that could explain the reported association between nicotine and depression.
Table I. Main pathways influenced by nicotine believed to be associated.
| Cortex |
| Hippocampus |
| Thalamus |
| Hypothalamic-pituitary-adrenal (HPA) axis |
| Mesolimbic system |
Pharmacology of the receptor
Some compounds commonly used to treat depression, including tricyclic antidepressants such as imipramine and amitriptyline, have been shown to inhibit the reuptake of biogenic amine and thereby cause accumulation of neurotransmitters in the synaptic cleft. The relatively small molecular structure of these molecules and their tricyclic nature indicate that their effects may not be restricted to reuptake inhibition and that they may interact in other physiologically important ways.
Another structural feature of these molecules can be deduced from experiments with phenothiazine derivatives, such as chlorpromazine (a major antipsychotic used to treat schizophrenia). Earlier reports that chlorpromazine can enter the ionic pore of the muscle nicotinic receptor and be used to label amino acids that line that pore33 prompted further investigation on the possible action of tricyclic molecules on nAChRs. According to the muscle receptor data, imipramine and desipramine inhibit the nAChRs and concentrations as low as 0.17 μM are sufficient to halve the receptor activity.34 Similarly, it was shown that, in the micromolar range, clomipramine inhibits the α4β2 receptor subtype, which is known to be expressed in the CNS.35 Furthermore, it was also shown that buproprion and phencylidine inhibit nicotinic receptors in cell lines and in brain slices in a noncompetitive manner.36,37
Therefore, the first indication of the mode of action of tricyclic molecules was provided by the fact that chlorpromazine can be used to label amino acids supposed to be in the ionic pore.33 Detailed voltage clamp analysis of the mode of action of noncompetitive blockers, including tricyclic compounds, confirmed that these molecules can enter the ionic pore of the human neuronal nicotinic receptor α4β2 and block the channel conduction by steric hindrance.38 Additional experiments carried out in rat brain slices further confirmed the effects of monoamine blockers on rat brain slices, which revealed inhibitory mechanisms in the low micromolar range.39 Molecules structurally related to the classical tricyclic reuptake inhibitors also include the well-known antiepileptic drug carbamazepine. It was shown that carbamazepine blocks nAChRs at a concentration that is found in the CSF of patients being treated with this drug.40 Interestingly, it was found that some mutations of the α4 subunit of the nicotinic receptor found in patients suffering from genetically transmissible nocturnal epilepsy exhibit a higher sensitivity to carbamazepine.40 In addition, the more specific 5-HT reuptake inhibitor fluoxetine was also found to interact with nicotinic receptors at concentrations as low as 0.57 μM.41
Altogether these data suggest that inhibition of the nAChR might contribute to the antidepressant action of these particular agents. The question that remains is how blockade of these ligand-gated channels could account for a beneficial outcome on the depressive status of the patients. The use of a broad-spectrum blocker of the nicotinic receptors, mecamylamine, which is devoid of action on monoamine reuptake, may provide a first indication, especially since it was found to have a beneficial effect in the treatment of depression.42,43 The hypothesis is that depression is accompanied by hypercholinergic activity.42,43
Nicotinic receptors and the HPA axis
Mood disorders, which include depression, are often thought of as a dysfunction or imbalance of the hypothalamic-pituitary-adrenal (HPA) system. The two major contributions with opposing modulation of the HPA are the amygdala, with a positive action, and the hippocampus, with a negative feedback. We should therefore consider three features of the nicotinic receptors in the HPA system:
-
The high level and diversity of neuronal nicotinic receptor expression in both hippocampus27,28 and amygdala.44
The importance of nAChRs in the hypothalamus circuits.45
The fact that steroids and mineralocorticoids modulate the nAChRs function.46-48
While both amygdala and hippocampus have a large number of nicotinic receptors, more attention was paid to studies of nicotinic receptor function in the hippocampus.31,49 However, given the complexity of hippocampal circuits and the multiple effects of acute and chronic nicotine exposure, the main outcome of nAChR stimulation remains to be elucidated. Despite our incomplete understanding of nicotine's action on the amygdala and hippocampus circuitry, there is no doubt that exposure to this agent will alter the network activity and may cause an imbalance of the HPA.
Histological analysis of the hypothalamus revealed that this brain area has a high level of nAChR expression.28 Moreover, the functionality of these receptors in the paraventricular nucleus has been demonstrated by electrophysiology.50 Parvocellular and magnocellular neurons that project to the anterior and posterior areas of the pituitary, respectively, have been shown to respond to ACh or nicotine.50 In an attempt to study effects of nicotine withdrawal in an animal model, rats were implanted with minipumps dispensing nicotine. HPA activity was determined on the second day after withdrawal of nicotine using the stress-induced corticosterone response and the dexamethasone suppression test.51 The results obtained by these authors suggest that the lower sensitivity of the HPA axis to stress during nicotine with-drawal may trigger depression during smoking cessation, but glucocorticoid receptor and corticotropin-releasing hormone do not appear to play a significant role in the condition tested. Although our knowledge of the role of the nAChRs in the parvocellular neurons is far from complete, these data demonstrate unequivocally that nicotine can modify the activity of these neurons and could, thereby, change the HPA equilibrium.
The first evidence of a direct action of steroids on nAChRs came from the observation that ACh-evoked currents recorded in cells expressing the α4β2 nAChR are inhibited in a noncompetitive manner by progesterone.52 Following this initial observation, it was shown that this inhibition is mediated by an allosteric interaction of steroids with this subtype of nAChRs. It was also observed that steroids inhibit the function of the α7 receptors. Further studies revealed that, while progesterone inhibit the rat or human α4β2 nAChRs, the neurosteroid 17β-estradiol markedly enhances the response of these receptors.48 These workers also revealed the determinant contribution of the short peptide segment of the human α4 subunit C-terminal end.46,48
Steroids and neurosteroids have also been shown to modulate the inhibitory GABAA receptors and some act through comparable protein interactions.53 Altogether, these data therefore support the correlation reported between neurosteroids and psychopathology.54 When we examine the HPA system, it is important to recall that the adrenal medulla is part of the sympathetic division, but with the particularity that preganglionic fibers terminate directly in the gland. Thus, ACh is the principal neurotransmitter that mediates signaling between the nerve and the gland activity. Moreover, ACh released by the preganglionic fibers is known to activate neuronal nicotinic receptors that result from the assembly of the α3 and β4 subunits. In view of the rather high level of circulating nicotine and its multiple effects on both central and peripheral receptors, the modification of the regulatory circuits of the adrenal gland should not be ignored.
Stress and dramatic changes in hormone levels in postpartum women are often thought to be at the origin of what is now called “postpartum depression.” Despite some association between postpartum depression and smoking, the evidence remains weak, and more thorough studies are needed to reach what is an otherwise tempting conclusion. Although progress has been made in our understanding of the endocrinology leading to hormone therapy and introduction of a wider set of drugs available to a larger fraction of the population, hormone substitution or addition continues to raise a number of concerns.55
Smoking and depression
The association between smoking and depression has been reported in many studies.3,4 Moreover, the relationship between smoking and depression is bidirectional and genetic factors may account up to 67 % for smoking initiation, maintenance, and dependence. Notwithstanding, the mechanisms that link smoking and depression are still poorly understood as several factors are acting concomitantly. One hypothesis is that, while, at first, nicotine may exert an anxiolytic effect, its prolonged consumption may switch its action to an anxiogenic effect. To understand how the same compound may act differently as a function of time, it is necessary to examine effects of prolonged nicotine exposure. Postmortem studies in human brain from smokers and nonsmokers revealed a surprising result.56 Namely, a marked difference in the amount of nicotine binding was observed, with an increased binding in smokers’ brains versus nonsmokers’. This observation contradicted the initial theory that a progressive increase in tobacco consumption could be attributed to a reduction in the receptor number, such as that observed in other drugs of abuse and the accompanying so-called downregulation. These studies triggered a renewal of interest in the effects of prolonged nicotine exposure. Chronic exposure to nicotine has also been shown to cause a differential upregulation of the specific receptor subtypes accompanied by selective expression of receptor subtypes in different areas.57,58 Although nicotinic ACh receptor upregulation is a well-accepted phenomenon, debate still exists about the molecular mechanisms that cause such upregulation.
To better understand the outcome of chronic nicotine exposure, it may be necessary to understand the functional status of receptors that are chronically exposed to low agonist concentrations. Use of cells that stably express the human α4β2 nAChRs provided a first set of clues.59 The functional properties were investigated using intracellular recordings and fast agonist application. This revealed that, while the responses of receptors are reduced when recorded in the presence of nicotine, there was a significant increase in cell response and an increase in receptor sensitivity to ACh. These results indicate that, if such mechanisms exist in vivo, chronic exposure to nicotine should cause a dual modification of the physiological properties of nAChRs with, on the one hand, an inhibition when the receptor is exposed to nicotine and, on the other, an increase in response upon removal of the drug. In support of the importance of nicotine effects, a single injection of nicotine in the rat was shown to cause a modification of the physiological properties of nicotinic receptors expressed in hippocampus within a few hours.36 Taken together these data indicate that chronic nicotine exposure triggers a number of cellular processes that induce physiological changes, the outcome of which is specific to the particular subtypes of nicotinic receptors expressed in a given brain area. In addition, prenatal exposure to nicotine transmitted by the mother in the fetal circulation was shown to be sufficient to cause detectable changes in rats.60 Low concentration of this alkaloid in milk was also found to be sufficient to trigger detectable changes in the level and pattern of receptor distribution in the brains of babies.61 This suggests that long-term memory of drug exposure can significantly modify brain function and must be taken into account when analyzing nicotine's effects.
A further complexity in the effects caused by chronic nicotine exposure is the modification of gene expression and alteration of other cellular functions. For example, it was reported that nicotine causes an increase in the number of 5-HT transporters in prefrontal cortex and hippocampus.62 This observation could explain the loss of 5-HT observed in the dorsal hippocampus following chronic nicotine infusion. Since it is known that the dorsal hippocampus may be associated with anxiogenic effects, a reduction in 5-HT in this brain area would be expected to cause an anxiolytic effect.63
Nicotinic receptors and sleep disorders
Another association between nicotine and depression is provided by the examination of circadian rhythms. Major depressive disorders are typically characterized by the alteration of sleep, which is thought to further imbalance the patient's equilibrium. On the basis of the observation that the frequency of major depression is increased during nicotine cessation, it was inferred that nicotine could protect against depression.64 In support of this hypothesis, it was reported that administration of nicotine might have beneficial effects on both sleep and depression.65 Together, the high level of expression of nicotinic receptors in the thalamus and the determinant role of thalamocortical loops during sleep further underline the relevance of the nicotinic receptors in sleep regulation. In addition, it was recently shown that nicotine inhibits sleep-promoting neuron activity in rat brain slices.66 These data illustrate the importance of neurotransmitters in the regulation of sleep and suggest that presence of nicotine in the brain may modify sleep control.
The importance of nAChRs function during sleep in humans has been further illustrated by recent genetic analysis. The finding in humans of an association between a genetically transmissible form of nocturnal frontal lobe epilepsy with mutations in the genes that encode for either the α4 or the β2 subunits of the nAChRs shed a new light on the contribution of this family of ligandgated channels to neurological and sleep disorders.67 To date, all mutations analyzed in functional studies have shown an increase in ACh sensitivity.49,68,69 This suggests that this specific form of epilepsy, which is caused by an imbalance between inhibition and excitation, is due to an increase in neuronal nicotinic receptor function.
While providing preliminary evidences of the determining contribution of neuronal nicotinic receptors in neurological disorders, these findings also indicate that mutations in the genes that encode for neuronal nicotinic receptors could play a role in other brain dysfunctions, such as depression.
Conclusions
The state “smoking is dangerous for your health” is mainly identified by the general population as meaning that smoking causes cardiovascular and respiratory impairments, while its relevance for psychiatric and mood disorders is generally not considered. The association between smoking cessation and depression is, however, well recognized by specialists and well documented in many clinical studies.
An important step in the understanding of nicotine dependence and the multiple effects caused by chronic exposure of our brain to this alkaloid was made with the discovery of an entire family of genes that encode for ligand-gated channels, which display a high affinity for nicotine and that are widely expressed in the human brain. Since then, numerous studies have addressed the role of nAChRs in mammalian brain and they were found to play an important role in the modulation of neuronal activity and release of neurotransmitters such as dopamine, glutamate, or 5-HT
The identification of interactions between nicotine and compounds typically used in the treatment of depression, such as monoamine reuptake inhibitors, sheds new light on our understanding of the brain pharmacology and opens up new avenues for research into treatments. Finally, polymorphisms and mutations identified in genes encoding for the nAChRs and their association with sleep and neurological disorders provide compelling evidence for the fast-evolving field of pharmacogenomics, and reveals individual differences, comparable to the well-known example of blue or brown eye color, that must be taken into account in the diagnosis and treatment of the multiple forms of depression.
Selected abbreviations and acronyms
- ACh
acetylcholine
- cAMP
cyclic adenosine monophosphate
- GABA
γ-aminobutyric acid
- HPA
hypothalamic-pituitary-adrenal (axis)
- 5-HT
5-hydroxytryptamine (serotonin)
- nAChR
neuronal nicotinic acetylcholine receptor
The author wishes to thank Dr P. Schulz for fruitful discussion and suggestions. This work was supported by the Swiss National Science Foundation.
REFERENCES
- 1.Hughes JR., Hatsukami DK., Mitchell JE., Dahlgren LA. Prevalence of smoking among psychiatric outpatients. Am J Psychiatry. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- 2.Leonard S., Adler LE., Benhammou K., et al. Smoking and mental illness. Pharmacol Biochem Behav. 2001;70:561–570. doi: 10.1016/s0091-3057(01)00677-3. [DOI] [PubMed] [Google Scholar]
- 3.Laje RP., Berman JA., Glassman AH. Depression and nicotine: preclinical and clinical evidence for common mechanisms. Curr Psychiatry Rep. 2001;3:470–474. doi: 10.1007/s11920-001-0040-z. [DOI] [PubMed] [Google Scholar]
- 4.Paperwalla KN., Levin TT., Weiner J., Saravay SM. Smoking and depression. Med Clin North Am. 2004;88:1483–1494. doi: 10.1016/j.mcna.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Laviolette SR., van der Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci. 2004;5:55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- 6.Wonnacott S., Sidhpura N., Balfour DJ. Nicotine: from molecular mechanisms to behaviour. Curr Opin Pharmacol. 2005;5:53–59. doi: 10.1016/j.coph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Daniell WF. On the natives of Old Calabar, west coast Africa. Edinburgh New Philosophical J. 1846;40:318–319. [Google Scholar]
- 8.Koelle GB. Anticholinesterase agents. In: Goodman LS, Gilman A, eds. The Pharmacological Basis of Therapeutics. New York, NY: MacMillan. 1970:442–465. [Google Scholar]
- 9.Janowsky DS., Davis JM., EI-Youssef MK., Sekerke HJ. Acetylcholine and depression. Psychosom Med. 1973;35:568. doi: 10.1097/00006842-197405000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Janowsky DS., Risch C., Parker D., Huey L., Judd L. Increased vulnerability to cholinergic stimulation in affect disorder patients. Psychopharmacol Bull. 1980;16:29. [PubMed] [Google Scholar]
- 11.Lu ZL., Saldanha JW., Hulme EC. Seven-transmembrane receptors: crystals clarify. Trends Pharmacol Sci. 2002;23:140–146. doi: 10.1016/S0165-6147(00)01973-8. [DOI] [PubMed] [Google Scholar]
- 12.Wess J. Novel insights into muscarinic acetylcholine receptor function using gene targeting technology. Trends Pharmacol Sci. 2003;24:414–420. doi: 10.1016/S0165-6147(03)00195-0. [DOI] [PubMed] [Google Scholar]
- 13.Rosenblueth A. The autonomic nervous system. Ann Rev Physiol. 1940;2:236–286. [Google Scholar]
- 14.Ascher P., Large WA., Rang HP. The action of ganglion-blocking drugs studied by voltage clamp. J Physiol. 1978;280:17P. [PubMed] [Google Scholar]
- 15.Changeux JP., Benoit P., Bessis A., et al. The acetylcholine receptor: functional architecture and regulation. Adv Second Messenger Phosphoprotein Res. 1990;24:15–19. [PubMed] [Google Scholar]
- 16.Lukas RJ., Changeux JP., Le Novere N., et al. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol Rev. 1999;51:397–401. [PubMed] [Google Scholar]
- 17.Devillers-Thiery A., Galzi JL., Eisele JL., Bertrand S., Bertrand D., Changeux JP. Functional architecture of the nicotinic acetylcholine receptor: a prototype of ligand-gated ion channels. J Membr Biol. 1993;136:97–112. doi: 10.1007/BF02505755. [DOI] [PubMed] [Google Scholar]
- 18.Miyazawa A., Fujiyoshi Y., Stowell M., Unwin N. Nicotinic acetylcholine receptor at 4.6 Å resolution: transverse tunnels in the channel wall. J Mol Biol. 1999;288:765–786. doi: 10.1006/jmbi.1999.2721. [DOI] [PubMed] [Google Scholar]
- 19.Bertrand D., Galzi JL., Devillers-Thiery A., Bertrand S., Changeux JP. Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal α7 nicotinic receptor. Proc Natl Acad Sci U S A. 1993;90:6971–6975. doi: 10.1073/pnas.90.15.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seguela P., Wadiche J., Dineley-Miller K., Dani JA., Patrick JW. Molecular cloning, functional properties, and distribution of rat brain α7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henningfield JE., Stapleton JM., Benowitz NL., Grayson RF., London ED. Higher levels of nicotine in arterial than in venous blood after cigarette smoking. Drug Alcohol Depend. 1993;33:23–29. doi: 10.1016/0376-8716(93)90030-t. [DOI] [PubMed] [Google Scholar]
- 22.Fabian-Fine R., Skehel P., Errington ML., et al. Ultrastructural distribution of the α7 nicotinic acetylcholine receptor subunit in rat hippocampus. J Neurosci. 2001;21:7993–8003. doi: 10.1523/JNEUROSCI.21-20-07993.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spurden DP., Court JA., Lloyd S., et al. Nicotinic receptor distribution in the human thalamus: autoradiographical localization of [3H]nicotine and [125I]α-bungarotoxin binding. J Chem Neuroanat. 1997;13:105–113. doi: 10.1016/s0891-0618(97)00038-0. [DOI] [PubMed] [Google Scholar]
- 24.Gallezot JD., Bottlaender M., Gregoire MC., et al. In vivo imaging of human cerebral nicotinic acetylcholine receptors with 2-18F-fIuoro-A-85380 and PET. J Nucl Med. 2005;46:240–247. [PubMed] [Google Scholar]
- 25.Gundisch D., Koren AO., Horti AG., et al. In vitro characterization of 6-[18F]fluoro-A-85380, a high-affinity ligand for α4β2* nicotinic acetylcholine receptors. Synapse. 2005;55:89–97. doi: 10.1002/syn.20096. [DOI] [PubMed] [Google Scholar]
- 26.Kimes AS., Horti AG., London ED., et al. 2-[18F]F-A-85380: PET imaging of brain nicotinic acetylcholine receptors and whole body distribution in humans. Faseb J. 2003;17:1331–1333. doi: 10.1096/fj.02-0492fje. [DOI] [PubMed] [Google Scholar]
- 27.Clarke PB., Pert A. Autoradiographic evidence for nicotine receptors on nigrostriatal and mesolimbic dopaminergic neurons. Brain Res. 1985;348:355–358. doi: 10.1016/0006-8993(85)90456-1. [DOI] [PubMed] [Google Scholar]
- 28.Tribollet E., Bertrand D., Marguerat A., Raggenbass M. Comparative distribution of nicotinic receptor subtypes during development, adulthood and aging: an autoradiographic study in the rat brain. Neuroscience. 2004;124:405–420. doi: 10.1016/j.neuroscience.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 29.Wonnacott S., Drasdo A., Sanderson E., Rowell P. Presynaptic nicotinic receptors and the modulation of transmitter release. Ciba Found Symp. 1990;152:87–101. doi: 10.1002/9780470513965.ch6. [DOI] [PubMed] [Google Scholar]
- 30.Bertrand D., Changeux JP. Nicotinic receptor: an allosteric protein specialized for intercellular communication. Seminar Neurosci. 1995;7:75–90. [Google Scholar]
- 31.Dani JA. Overview of nicotinic receptors and their roles in the central nervous system. Biol Psychiatry. 2001;49:166–174. doi: 10.1016/s0006-3223(00)01011-8. [DOI] [PubMed] [Google Scholar]
- 32.Picciotto MR., Zoli M., Changeux JP. Use of knock-out mice to determine the molecular basis for the actions of nicotine. Nicotine Tob Res. 1999;1(suppl 2):S121–S125 Discussion S139-S140. doi: 10.1080/14622299050011931. [DOI] [PubMed] [Google Scholar]
- 33.Giraudat J., Dennis M., Heidmann T., Haumont PY., Lederer F., Changeux JP. Structure of the high-affinity binding site for noncompetitive blockers of the acetylcholine receptor: [3H]chlorpromazine labels homologous residues in the beta and delta chains. Biochemistry. 1987;26:2410–2418. doi: 10.1021/bi00383a003. [DOI] [PubMed] [Google Scholar]
- 34.Rana B., McMorn SO., Reeve HL., Wyatt CN., Vaughan PF., Peers C. Inhibition of neuronal nicotinic acetylcholine receptors by imipramine and desipramine. Eur J Pharmacol. 1993;250:247–251. doi: 10.1016/0014-2999(93)90388-x. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Valdes H., Garcia-Colunga J., Miledi R. Effects of clomipramine on neuronal nicotinic acetylcholine receptors. Eur J Pharmacol. 2002;444:13–19. doi: 10.1016/s0014-2999(02)01556-x. [DOI] [PubMed] [Google Scholar]
- 36.Alkondon M., Albuquerque EX. Nicotinic receptor subtypes in rat hippocampal slices are differentially sensitive to desensitization and early in vivo functional upregulation by nicotine and to block by bupropion. J Pharmacol Exp Ther. 2005;313:740–750. doi: 10.1124/jpet.104.081232. [DOI] [PubMed] [Google Scholar]
- 37.Fryer JD., Lukas RJ. Noncompetitive functional inhibition at diverse, human nicotinic acetylcholine receptor subtypes by bupropion, phencyclidine, and ibogaine. J Pharmacol Exp Ther. 1999;288:88–92. [PubMed] [Google Scholar]
- 38.Buisson B., Bertrand D. Open-channel blockers at the human α4β2 neuronal nicotinic acetylcholine receptor. Mol Pharmacol. 1998;53:555–563. doi: 10.1124/mol.53.3.555. [DOI] [PubMed] [Google Scholar]
- 39.Hennings EC., Kiss JP., De Oliveira K., Toth PT., Vizi ES. Nicotinic acetylcholine receptor antagonistic activity of monoamine uptake blockers in rat hippocampal slices. J Neurochem. 1999;73:1043–1050. doi: 10.1046/j.1471-4159.1999.0731043.x. [DOI] [PubMed] [Google Scholar]
- 40.Picard F., Bertrand S., Steinlein OK., Bertrand D. Mutated nicotinic receptors responsible for autosomal dominant nocturnal frontal lobe epilepsy are more sensitive to carbamazepine. Epilepsia. 1999;40:1198–1209. doi: 10.1111/j.1528-1157.1999.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 41.Hennings EC., Kiss JP., Vizi ES. Nicotinic acetylcholine receptor antagonist effect of fluoxetine in rat hippocampal slices. Brain Res. 1997;759:292–294. doi: 10.1016/s0006-8993(97)00343-0. [DOI] [PubMed] [Google Scholar]
- 42.Shytle RD., Silver AA., Lukas RJ., Newman MB., Sheehan DV., Sanberg PR. Nicotinic acetylcholine receptors as targets for antidepressants. Mol Psychiatry. 2002;7:525–535. doi: 10.1038/sj.mp.4001035. [DOI] [PubMed] [Google Scholar]
- 43.Shytle RD., Silver AA., Sheehan KH., Sheehan DV., Sanberg PR. Neuronal nicotinic receptor inhibition for treating mood disorders: preliminary controlled evidence with mecamylamine. Depress Anxiety. 2002;16:89–92. doi: 10.1002/da.10035. [DOI] [PubMed] [Google Scholar]
- 44.Levin ED. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 2002;53:633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- 45.Zaninetti M., Raggenbass M. Oxytocin receptor agonists enhance inhibitory synaptic transmission in the rat hippocampus by activating interneurons in stratum pyramidale. Eur J Neurosci. 2000;12:3975–3984. doi: 10.1046/j.1460-9568.2000.00290.x. [DOI] [PubMed] [Google Scholar]
- 46.Paradiso K., Zhang J., Steinbach JH. The C terminus of the human nicotinic α4β2 receptor forms a binding site required for potentiation by an estrogenic steroid. J Neurosci. 2001;21:6561–6568. doi: 10.1523/JNEUROSCI.21-17-06561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valera S., Ballivet M., Bertrand D. Progesterone modulates a neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1992;89:9949–9953. doi: 10.1073/pnas.89.20.9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Curtis L., Buisson B., Bertrand S., Bertrand D. Potentiation of human α4β2 neuronal nicotinic acetylcholine receptor by estradiol. Mol Pharmacol. 2002;61:127–135. doi: 10.1124/mol.61.1.127. [DOI] [PubMed] [Google Scholar]
- 49.Hogg RC., Raggenbass M., Bertrand D. Nicotinic acetylcholine receptors: from structure to brain function. Rev Physiol Biochem Pharmacol. 2003;147:1–46. doi: 10.1007/s10254-003-0005-1. [DOI] [PubMed] [Google Scholar]
- 50.Zaninetti M., Tribollet E., Bertrand D., Raggenbass M. Nicotinic cholinergic activation of magnocellular neurons of the hypothalamic paraventricular nucleus. Neuroscience. 2002;110:287–299. doi: 10.1016/s0306-4522(01)00536-x. [DOI] [PubMed] [Google Scholar]
- 51.Semba J., Wakuta M., Maeda J., Suhara T. Nicotine withdrawal induces subsensitivity of hypothalamic-pituitary-adrenal axis to stress in rats: implications for precipitation of depression during smoking cessation. Psychoneuroendocrinology. 2004;29:215–226. doi: 10.1016/s0306-4530(03)00024-6. [DOI] [PubMed] [Google Scholar]
- 52.Bertrand D., Valera S., Bertrand S., Ballivet M., Rungger D. Steroids inhibit nicotinic acetylcholine receptors. Neuroreport. 1991;2:277–280. doi: 10.1097/00001756-199105000-00016. [DOI] [PubMed] [Google Scholar]
- 53.Lambert JJ., Belelli D., Peden DR., Vardy AW., Peters JA. Neurosteroid modulation of GABAA receptors. Prog Neurobiol. 2003;71:67–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Dubrovsky BO. Steroids, neuroactive steroids and neurosteroids in psychopathology. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:169–192. doi: 10.1016/j.pnpbp.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Pearson H. Hormone therapy: a dangerous elixir? Nature. 2004;431:500–501. doi: 10.1038/431500a. [DOI] [PubMed] [Google Scholar]
- 56.Perry DC., Davila-Garcia Ml., Stockmeier CA., Kellar KJ. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exp Ther. 1999;289:1545–1552. [PubMed] [Google Scholar]
- 57.Teaktong T., Graham AJ., Johnson M., Court JA., Perry EK. Selective changes in nicotinic acetylcholine receptor subtypes related to tobacco smoking: an immunohistochemical study. Neuropathol Appl Neurobiol. 2004;30:243–254. doi: 10.1046/j.0305-1846.2003.00528.x. [DOI] [PubMed] [Google Scholar]
- 58.Kassiou M., Eberl S., Meikle SR., et al. In vivo imaging of nicotinic receptor upregulation following chronic (-)-nicotine treatment in baboon using SPECT. Nucl Med Biol. 2001;28:165–175. doi: 10.1016/s0969-8051(00)00206-7. [DOI] [PubMed] [Google Scholar]
- 59.Buisson B., Bertrand D. Chronic exposure to nicotine upregulates the human (α)4(β)2 nicotinic acetylcholine receptor function. J Neurosci. 2001;21:1819–1829. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abreu-Villaca Y., Seidler FJ., Tate CA., Cousins MM., Slotkin TA. Prenatal nicotine exposure alters the response to nicotine administration in adolescence: effects on cholinergic systems during exposure and withdrawal. Neuropsychopharmacology. 2004;29:879–890. doi: 10.1038/sj.npp.1300401. [DOI] [PubMed] [Google Scholar]
- 61.Narayanan U., Birru S., Vaglenova J., Breese CR. Nicotinic receptor expression following nicotine exposure via maternal milk. Neuroreport. 2002;13:961–963. doi: 10.1097/00001756-200205240-00012. [DOI] [PubMed] [Google Scholar]
- 62.Awtry TL., Werling LL. Acute and chronic effects of nicotine on serotonin uptake in prefrontal cortex and hippocampus of rats. Synapse. 2003;50:206–211. doi: 10.1002/syn.10259. [DOI] [PubMed] [Google Scholar]
- 63.Balfour DJ., Ridley DL. The effects of nicotine on neural pathways implicated in depression: a factor in nicotine addiction? Pharmacol Biochem Behav. 2000;66:79–85. doi: 10.1016/s0091-3057(00)00205-7. [DOI] [PubMed] [Google Scholar]
- 64.Killen JD., Fortmann SP., Schatzberg AF., Hayward C., Varady A. Onset of major depression during treatment for nicotine dependence. Addict Behav. 2003;28:461–470. doi: 10.1016/s0306-4603(01)00266-0. [DOI] [PubMed] [Google Scholar]
- 65.Haro R., Drucker-Colin R. A 2-year study on the effects of nicotine and its withdrawal on mood and sleep. Pharmacopsychiatry. 2004;37:221–227. doi: 10.1055/s-2004-832596. [DOI] [PubMed] [Google Scholar]
- 66.Saint-Mieux B., Eggermann E., Bisetti A., et al. Nicotinic enhancement of the noradrenergic inhibition of sleep-promoting neurons in the ventrolateral preoptic area. J Neurosci. 2004;24:63–67. doi: 10.1523/JNEUROSCI.0232-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steinlein OK. Genetic mechanisms that underlie epilepsy. Nat Rev Neurosci. 2004;5:400–408. doi: 10.1038/nrn1388. [DOI] [PubMed] [Google Scholar]
- 68.Leniger T., Kananura C., Hufnagel A., Bertrand S., Bertrand D., Steinlein OK. A new Chrna4 mutation with low penetrance in nocturnal frontal lobe epilepsy. Epilepsia. 2003;44:981–985. doi: 10.1046/j.1528-1157.2003.61102.x. [DOI] [PubMed] [Google Scholar]
- 69.Phillips HA., Favre I., Kirkpatrick M., et al. CHRNB2 is the second acetylcholine receptor subunit associated with autosomal dominant nocturnal frontal lobe epilepsy. Am J Hum Genet. 2001;68:225–231. doi: 10.1086/316946. [DOI] [PMC free article] [PubMed] [Google Scholar]