Abstract
Therapeutic drug monitoring (TDM) of psychotropic drugs such as antidepressants has been widely introduced for optimization of pharmacotherapy in psychiatric patients. The interdisciplinary TDM group of the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie (AGNP) has worked out consensus guidelines with the aim of providing psychiatrists and TDM laboratories with a tool to optimize the use of TDM. Five research-based levels of recommendation were defined with regard to routine monitoring of drug plasma concentrations: (i) strongly recommended; (ii) recommended; (iii) useful; (iv) probably useful; and (v) not recommended. In addition, a list of indications that justify the use of TDM is presented, eg, control of compliance, lack of clinical response or adverse effects at recommended doses, drug interactions, pharmacovigilance programs, presence of a genetic particularity concerning drug metabolism, and children, adolescents, and elderly patients. For some drugs, studies on therapeutic ranges are lacking, but target ranges for clinically relevant plasma concentrations are presented for most drugs, based on pharmacokinetic studies reported in the literature. For many antidepressants, a thorough analysis of the literature on studies dealing with the plasma concentration–clinical effectiveness relationship allowed inclusion of therapeutic ranges of plasma concentrations. In addition, recommendations are made with regard to the combination of pharmacogenetic (phenotyping or genotyping) tests with TDM, Finally, practical instructions are given for the laboratory practitioners and the treating physicians how to use TDM: preparation of TDM, drug analysis, reporting and interpretation of results, and adequate use of information for patient treatment. TDM is a complex process that needs optimal interdisciplinary coordination of a procedure implicating patients, treating physicians, clinical pharmacologists, and clinical laboratory specialists. These consensus guidelines should be helpful for optimizing TDM of antidepressants.
Keywords: therapeutic drug monitoring, antidepressant, consensus guidelines, pharmacotherapy, psychiatry
Abstract
El monitoreo terapéutico de fármacos (TDM), que incluye los antidepresivos entre los psicofármacos, se ha introducido extensamente para optimizar la farmacoterapia en los pacientes psiquiátricos. El grupo interdisciplinario de TDM del Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie (AGNP) ha trabajado en pautas de consenso con el objetivo de entregar a los psiquiatras y a los laboratories de TDM una herramienta que permita optimizar el empleo del TDM. Se definieron cinco niveles de recomendación basados en la investigación relacionada con el monitoreo de rutina de concentraciones plasmáticas de fármacos: (1) altamente recomendado, (2) recomendado, (3) útil, (4) probablemente útil y (5) no recomendado, Además se presentó una lista de indicaciones que justifican el uso del TDM, como por ejemplo, el control de la adherencia, la falta de respuesta clínica o los efectos adversos a dosis recomendadas, las interacciones de fármacos, los programas de farmacovigilancia, la presencia de alguna particulandad genetica en relación con el metabolismo de los fármacos y los pacientes infanto-juweniles y ancianos. Para algunos fármacos faltan estudios en rangos terapéuticos. De acuerdo con estudios farmacocinéticos reportados en la literatura, se presentan los rangos objetivos para concentraciones plasmáticas clínicamente relevantes de la mayoría de los fármacos. Para muchos antidepresivos un completo análisis de la literatura de los estudios que abordan la relación concentración plasmática–eficacia clínica ha permitido la inclusión de rangos terapéuticos de concentraciones plasmáticas. Además, se han realizado recomendaciones relacionadas con la combinaaón de pruebas farmacogenéticas (fenotipo o genotipo) con el TDM. Finalmente, se han entregado instrucciones prácticas para los profesionales de los laboratorios y los psiquiatras tratantes de cómo utilizar el TDM: preparación del TDM, análisis de fármacos, informe e interpreiación de resultados y un adecuado uso de la información para el tratamiento del paciente. El TDM es un proceso complejo que requiere de una óptima coordinación interdisciplinaria de un procedimiento que involucra pacientes, psiquiatras tratantes, farmacólogos clínicos y especialistas en laboratorio clínico. Esta pauta de consenso debiera ser útil para optimizar el TDM de los antidepresivos.
Abstract
Le dosage plasmatique de médicaments psychotropes dans un but thérapeutique (therapeutic drug monitoring (TDM)) y compris des antidépresseurs a été largement introduit pour optimiser la pharmacothérapie de patients psychiatriques. Le groupe interdisciplinaire AGNP-TDM (Arbeitsgemeinschaft für Neuropsychopharmakologie und Phamakopsychiatrie; Association de Neuro-psycho-pharmacologie et de Pharmacopsychiatrie) a élaboré des recommandations dans le but de procurer aux psychiatres et aux laboratoires TDM un outil pour optimiser l'utilisation du TDM. Basés sur des résultats obtenus par la recherche, cinq niveaux de recommandations ont été définis par rapport au monitoring de routine des taux plasmatiques de médicaments: 1. fortement recommandé, 2. recommandé, 3. utile, 4. probablement utile, 5. pas recommandé. De plus une liste d'indications qui justifient l'utilisation des TDM est présentée, par exemple : contrôle de l'observance, absence de réponse clinique ou effets secondaires a des doses généralement recommandées, interactions médicamenteuses, programme de pharmacovigilance, présence d'une particularité génétique concernant le métabolisme de médicaments, enfants, adolescents et patients ages. Pour quelques médicaments, des études sur les marges thérapeutiques manquent, mais des marges cibles pour des concentrations plasmatiques cliniquement significatives sont présentées pour la plupart des médicaments, basées sur des études pharmacocinétiques rapportées dans la littérature. Pour beaucoup d'antidépresseurs, une analyse complète de la littérature sur les études qui traitent de la relation concentration plasmatique – efficacité clinique a permis de présenter des marges thérapeutiques de concentrations plasmatiques. En outre, des recommandations sont données par rapport a la combinaison de tests pharmacogénétiques (phénotypage ou génotypage) avec le TDM. Finalement, des instructions pratiques sont données aux techniciens responsables de laboratoires et aux médecins traitants qui utilisent le TDM: préparation du TDM, analyses de médicaments, communication et interprétation du résultat et utilisation adéquate de l'information pour le traitement du patient. Le TDM est un processus qui nécessite une coordination interdisciplinaire optimale d'une procédure qui implique des patients, des médecins traitants, des pharmacologues cliniques et des spécialistes du laboratoire clinique. Ce « Consensus guideline » (recommandations) devrait être utile pour optimiser le TDM d'antidépresseurs.
Pharmacopsychiatry and psychotherapy are beneficial for many patients with depression. Evidence-based and clinical experience collected during the past decades has allowed the introduction of guidelines and recommendations from experts in the field1-3 to optimize antidepressant pharmacotherapy. However, partial response and nonresponse are frequent,4 despite the introduction of new psychotropic agents, including ”third-generation antidepressants,“5 and amelioration and remission rates are still far from optimal. The efficacy of available drugs can be increased, not only by the use of augmentation strategies6,7 and other combination treatments,8,9 but also by analysis of antidepressant drug concentrations in blood plasma.10 Recently, a group of psychiatrists, clinical pharmacologists, biochemists, and clinical chemists, all members of the AGNP (Arbeitsgemeinschaft fur Neuropsychopharmakologie und Pharmakopsychiatrie; www.agnp.de), worked out consensus guidelines for therapeutic drug monitoring (TDM) in psychiatry, after they had compiled information from the literature.11 These guidelines were mainly based on the hypothesis that some inadequate or insufficient treatments of psychiatric patients can be explained by the fact that psychotropic drugs not only differ in their pharmacological profile, but also in their metabolism and pharmacokinetics in the individual patient. Treatment should therefore be adapted accord_ ing to this situation by using TDM and pharmacogenetic tests. This combined strategy takes into consideration the fact that the fate of the drug depends on both environmental (diet, smoking habits, comorbidities, and cornedications) and genetic factors.
Pioneering work in this field was mainly carried out in Sweden, where the first study on the plasma concentration–clinical effectiveness relationship of an antidepressant (nortriptyline)12 was performed. This was an outstanding demonstration of the usefulness of the combination of TDM and pharmacogenetic tests (CYP 2D6) in a pharmacovigilance case situation.13 Over the past 20 years, TDM for antidepressants has been widely introduced, but consensus guidelines published to date, or other state-of-the-art reports on the use of TDM for antidepressants concentrated primarily on tricyclic drugs.14-17 There is an increasing trend to recommend TDM in combination with pharmacogenetic tests.18,19
Aims of the consensus document
The present consensus guidelines were elaborated to assist psychiatrists, laboratory practitioners, and heads of laboratories involved in psychopharmacotherapy to optimise the use of TDM. Here we focus on antidepressants,* and give recommendations on how to use TDM and genotyping/phenotyping procedures.
Pharmacokinetics, metabolism, and pharmacogenetics of antidepressants
Antidepressants share many common features, such as high lipophilicity, a molecular weight between 200 and 500, and basicity. We therefore present a general summary of their pharmacokinetic properties in Table I, 20-26 though numerous compounds constitute exceptions: citalopram is known for its high bioavailability (about 90%) and relatively low binding to plasma proteins (80%); venlafaxine, trazodone, tranylcypromine, and moclobemide display a short (about 2-10 h) and fluoxetine a long plasma half -life (3-15 days, taking into account its active metabolite). It should also be considered that many antidepressants, such as venlafaxine, citalopram, and mirtazapine, are used as racemic compounds, the enantiomers of which differ in their pharmacological, metabolic, and pharmacokinetic properties.27,28
Table I. General pharmacokinetic properties (absorption, distribution, metabolism, and elimination [ADME]) of antidepressants.11,20 .
| Pharmacokinetic phase | Characteristics | |
| Absorption | A | Good absorption from gastrointestinal tract |
| Maximum plasma concentration within a short time after administration (tmax of about 0.5 to 4 h) | ||
| Distribution | D | High distribution volume |
| Fast distribution from plasma to the central nervous system | ||
| 10 to 40 times higher levels in brain than in blood | ||
| Possible regulation of transport intestine-blood and blood-brain by transport proteins (glycoprotein) | ||
| Low plasma concentrations in steady-state conditions (trough levels: 0.5-500 ng/mL) | ||
| High plasma protein binding (90% 99%) | ||
| Metabolism | M | Metabolism: a prerequisite for excretion |
| High first-pass metabolism (systemic availability: 10%-70%) | ||
| Main metabolic enzyme systems: cytochrome P-450, UDP-glucuronosyltransferases | ||
| Genetic polymorphisms for some enzymes (extensive, intermediate, poor; and ultrarapid metabolizers) | ||
| Inducibility of some enzymes by drugs or other xenobiotics | ||
| Generally formation of active, but more polar metabolites | ||
| Occurrence and relevance of metabolism in brain doubtful | ||
| Important effect of hepatic insufficiency on hepatic elimination | ||
| High risk for inhibition of drug metabolism by comedication, inhibitors of cytochrome P-450 | ||
| Elimination | E | Low renal excretion |
| Small effect of renal insufficiency on plasma kinetics of drug and its metabolites | ||
| Slow elimination from plasma (half life 12 36 h), mainly by hepatic metabolism | ||
| ADME | Linear pharmacokinetics at clinically relevant doses |
Most antidepressants undergo phase I metabolism by oxidation, such as aromatic ring and aliphatic hydroxylation, N- and Odealkylation, N- and O-oxidation to N-oxides, carbonyl reduction to secondary alcohols, and Soxidation to sulfoxides or sulfones, which results in an increase in polarity.29 The introduction of a functional group (eg, a hydroxy group) or the presence of a tertiary amine group may enable a phase II metabolic step, typically a glucuronidation.30-32 Metabolism occurs mainly in the liver and in the intestinal mucosa. It may be agedependent, and vary as a consequence of the influence of environmental factors, such as somatic diseases, comedication, food, and smoking. TDM should include the assay of active metabolites33-35 (eg, clomipramine [norclomipramine] and fluoxetine [norfluoxetine]), but the parent compound/inactive metabolite ratio may be helpful to evaluate the metabolic state or compliance of the patient.
Considerable and clinically relevant knowledge has been acquired during the past 30 years on the important role of cytochrome P-450 (CYP) isozymes, CYP 1A2, CYP 2D6, CYP 2C9, CYP 2C19, and CYP 3A4/5, in the biotransformation of antidepressants.36-42 The genetically determined polymorphism of CYP 2D6 is of high clinical relevance for antidepressants, which are substrates of this isozyme, including tricyclic antidepressants, some selective serotonin reuptake inhibitors (SSRls) (eg, paroxetine and fluoxetine), and “third-generation” antidepressants (eg, venlafaxine and mirtazapine). About 5% to 8% and 1% to 7% of the Caucasian population are considered as poor metabolizers (PMs) or ultrarapid metabolizers (UMs), respectively (Table I).22,43,44 In Caucasians, there is a lower proportion (3% -5%) of PMs of CYP 2C19, which is frequently involved in Ndemethylation of tertiary amines (amitriptyline and citalopram). CYP 3A4/5 shows wide interindividual variability in its activity. CYP 3A5 is expressed in only one-third of the Caucasian population.45 As regards CYP 1A2, only its inducibility (eg, by tobacco smoke) is genetically polymorphic.46,47 Clinically, a PM status may represent a higher risk for adverse effects in patients treated with antidepressants known to be substrates of the deficient enzyme, while UMs undergo a higher risk for nonresponse, due to subtherapeutic plasma concentrations.39,48-53 The clinical relevance of the genetic polymorphisms of UDP-glucuronosyltransf erases in pharmacopsychiatry is not clear.30,54
Genotyping, which represents a “trait marker,” is readily available and clinically recommended for CYP 1A2, CYP 2C9, CYP 2C19, CYP 2D6, and CYP 3A4/5; phenotyping, used as a “state-marker,” may be performed for the same enzymes. The result of genotyping is not influenced by environmental factors and has life-long validity Phenotyping requires the administration of drugs and is therefore a more invasive procedure. Therefore, indications for phenotyping and genotyping may differ.
As mentioned in Table I, transport proteins such as P-glycoprotein in the intestinal mucosa and in the blood–brain barrier may be implicated in the regulation of the availability of antidepressants for the brain, but there is still a lack of clinical data.55-57
Relationships between drug doses, plasma concentrations, and clinical variables
TDM is based on the hypothesis assumption that there is a well-defined relationship between the drug plasma concentration and its clinical effects (therapeutic effect, adverse effects, and toxicity). However, while such a relationship is generally well admitted for lithium and for the tricyclic antidepressants nortriptyline, amitriptyline, desipramine, and imipramine, inconsistent results were obtained in studies on other tricyclic or similarly structured antidepressants, SSRls, and other recently introduced antidepressants.20,58-62 Interestingly, systematic reviews and meta-analyses14,59 that were based on adequately designed studies yielded evidence of a relationship between clinical variables and plasma concentration for some tricyclic drugs. This suggests that numerous studies were poorly designed methodologically in order to demonstrate an evident relationship between concentration and effects or side effects. Recently, Ulrich and Läuter60 defined criteria for quality assessment of TDM studies, which include the use of valid chemical and analytical methods, adequate psychopathology rating scales, appropriate selection criteria for patients (eg, exclusion of known nonresponders), and reporting of comedication.
Analytical procedures
Plasma or serum samples are generally used for TDM. Concentrations of antidepressants are low, most often in the nmol/L (ng/mL) range. Therefore, highly sensitive and selective analytical methods are needed for accurate and precise quantification.63-66
Most laboratories use now gas chromatography (GC) or high-performance liquid chromatography (HPLC) for the assay of antidepressants for TDM purposes. For GC, the most recommended detection systems are mass spectrometry (GC-MS) or nitrogen phosphor detectors (GC-NPD). Ultraviolet (UV) detectors, fluorescence detectors, and mass spectrometry (LC-MS), in increasing order, are useful for a selective and sensitive drug assay. Clearly, the need for sample preparation before chromatographic separation represents a time-consuming step, and this procedure also implies a limited sample throughput, despite the availability of automated sample preparation prior to GC or HPLC.67 Direct injection (“column switching HPLC”) of plasma or serum into the HPLC system is now available for a number of antidepressants.68-70 LC-MS and LC-MS-MS (tandem mass spectrometry) will increasingly be the method of choice, as it may be applied to almost any psychotropic drug including metabolites, while GC-MS is applicable only for volatile compounds.
Economic aspects of TDM in psychiatry
TDM for a single psychoactive drug, including a metabolite, costs between 20 and 80 €, which includes costs for staff, instrumentation, chemicals, and other materials. In some countries, analyses may be billed according to the analytical technique used (higher rates for mass spectrometric quantification).
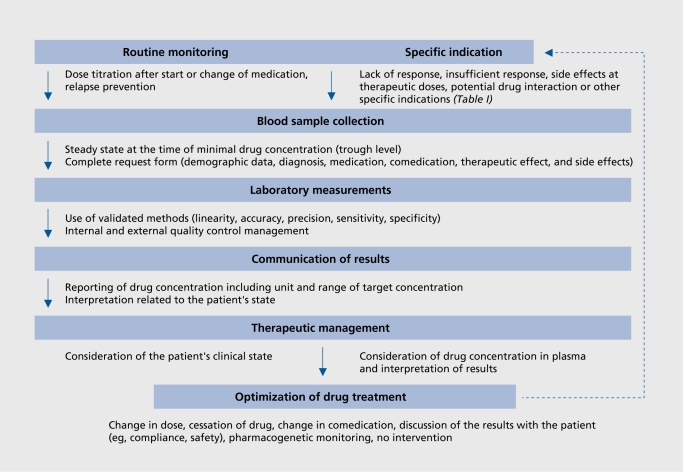
A proof of cost-effectiveness has been provided for only a few antidepressants.71,72 However, additional studies are required. They should be designed to take account of the complexity of the TDM process (Figure 1). For example, a recent prospective study carried out under naturalistic conditions showed that dose adjustment by the treating physician was frequently inappropriate, in that he or she neglected the results of the laboratory assays.73
Figure 1. Summary of the therapeutic drug monitoring (TDM) process for optimization of the pharmacotherpy of psychiatric patients. Routine monitoring should be restricted to psychoactive drugs with established therapeutic ranges and who levels of recommendation to use TDM are at least 2 (Table IV). Specific requests may be useful for any psychoactive drug and many indications (Table I), even without well-established therapeutic ranges.11 .
Table IV. Recommended target plasma concentration ranges for antidepressant drugs and levels of recommendation for routine monitoring.11 Therapeutic ranges indicate trough concentrations of drugs in serum or plasma of patients under steady-state medication. Level of recommendation: 1. Strongly recommended (for lithium TDM should be a standard of care): established therapeutic range; 2. Recommended: suggested therapeutic ranges obtained from plasma concentrations at therapeutically effective doses (fixed dose studies); 3. Useful: suggested therapeutic ranges are plasma concentrations at therapeutically effective doses obtained from steady-state pharmacokinetic studies; 4. Probably useful: suggested therapeutic ranges from steady-state pharmacokinetic studies at therapeutically effective doses; 5. Not recommended. SPC, Summary of Product Characteristics.
| Drug and active metabolite for antidepressants | Recommended therapeutic range (consensus) | Level of recommendation | References for reports on therapeutic ranges | Reference for reports on intoxications |
| Amitriptyline plus nortriptyline | 80-200 ng/mL | 1 | Ulrich and Läuter60 2002 | Preskorn and Jerkovich,124 1990 |
| Pedersen et al,123 1982 | ||||
| Citalopram | 30-130 ng/mL | 3 | Bjerkenstedt et al,125 1985 | Jonasson and Saldeen,127 2002 |
| Leinonen et al,126 1996 | ||||
| Clomipramine plus norclomipramine | 175-450 ng/mL | 1 | DUAG,85 1999 | Mclntyre et al,130 1994 |
| Gex-Fabry et al,128 1999 | ||||
| Mavissakalian et al,129 1990 | ||||
| Desipramine | 100-300 ng/mL | 2 | Perry et al,59 1994 | Preskorn and Jerkovich,124 1990 |
| Pedersen et al,123 1982 | ||||
| Doxepin plus nordoxepin | 50-150 ng/mL | 3 | Leucht et al,95 2001 | Preskorn and Fast, 1992 |
| Rodriguez de la Torre et al,131 2001 | ||||
| Escitalopram | 15-80 ng/mL | 4 | SPC | |
| Fluoxetine plus norfluoxetine | 120-300 ng/mL | 3 | Lundmark et al,98 2001 | |
| Amsterdam et al,99 1997 | ||||
| Fluvoxamine | 4 | 150-300 ng/mL | Gerstenberg et al,102 2003 | Kasper et al,101 1993 |
| Goodnick,133 1994 | ||||
| Imipramine plus desipramine | 175-300 ng/mL | 1 | Perry et al,59 1994 | Pedersen et al,123 1982 |
| Maprotilin | 125-200 ng/mL | 3 | SPC Kasper et al,101 1993 | Pedersen et al,123 1982 |
| Mianserin | 15-70 ng/mL | 3 | Montgomery et al,134 1978 | Isacsson et al,135 1997 |
| Mirtazapine | 40-80 ng/mL | 3 | Timmer et al,136 2000 | Velazquez et al,137 2001 |
| Moclobemide | 300-1000 ng/mL | 4 | Fritze et al,138 1989 | Hernandez et al,140 1995 |
| Gex4=abry et al,139 1995 | ||||
| Nortriptyline | 70-170 ng/mL | 1 | Perry et al,12 1994 | Asberg et al,141 1970 |
| Asberg et al,59 1971 | ||||
| Paroxetine | 70-120 ng/mL | 3 | Lundmark et al,114 2000 | |
| Tasker et al,142 1989 | ||||
| Reboxetine | 10-100 ng/mL | 4 | Ohman et al,143 2001 | |
| Sertraline | 10-50 ng/mL | 3 | Lundmark et al,114 2000 | Milner et al,144 1998 |
| Tranylcypromine | 0-50 ng/mL | 5 | Burke and Preskorn,145 1999 | Iwersen and Schmoldt,146 1996 |
| Trazodone | 650-1500 ng/mL | 3 | Monteleone et al,147 1939 | |
| Goeringer et al,148 2000 | ||||
| Trimipramine | 150-350 ng/mL | 3 | Cournoyer et al,118 1987 | |
| Isaccson et al,135 1997 | ||||
| Venlafaxine plus O-desmethylvenlafaxine | 195-400 ng/mL | 2 | Veefkind et al,149 2000 | |
| Levine et al,150 1998 | ||||
| Viloxazine | 20-500 ng/mL | 3 | Norman et al,151 1980 | Falcy et al,153 1983 |
| Altamura et al,152 1986 |
Preliminary data suggest that phenotyping or genotyping of patients may help decrease the cost of their treatment with substrates of CYP 2D6.74 The costs of treating patients who are either UMs or PMs (CYP 2D6) are seemingly thousands of US dollars per year higher than those for extensive metabolizers (EMs).75 However, the tools to assess the cost-effectiveness of pharmacogenetic tests are still insufficiently developed.76
Consensus
TDM should be limited to situations where it may be expected that the result will help to solve a therapeutic problem. There are many indications for using TDM (Table II) in antidepressant pharmacotherapy, such as suspicion of noncompliance or intoxication. In pharmacovigilance programs, TDM may be considered as a valid indication for all drugs and groups of patients. To recommend TDM as routine monitoring, it must be proven that TDM is of value. Five levels of recommendation for TDM were defined, which range from “strongly recommended” to “not recommended.” In a second step, a recommendation tailored to the individual drug was defined.
Table II. General indications for therapeutic drug monitoring (TDM) of antidepressants.11 .
| Suspected noncompliance |
| Drugs, for which TDM is mandatory for safety reasons (eg, lithium) |
| Lack of clinical response, or insufficient response, even if dose is considered as adequate |
| Adverse effects, despite the use of generally recommended doses |
| Suspected drug interactions |
| TDM in pharmacovigilance programs |
| Combination treatment with a drug known for its interaction potential, in situations of comorbidities, “augmentation,” etc |
| Relapse prevention in long-term treatments, prophylactic: treatments |
| Recurrence despite good compliance and adequate doses |
| Presence of a genetic particularity concerning the drug metabolism (genetic: deficiency, gene multiplication) |
| Children and adolescents |
| Elderly patients (>65 years) |
| Patients with pharmacokinetically relevant comorbidities (hepatic or renal insufficiency, cardiovascular disease) |
| Forensic psychiatry |
| Problems occurring after switching from an original preparation to a generic form (and vice versa) |
Levels of recommendations to use TDM as routine monitoring
The therapeutic strategy will only be improved by the use of TDM, if the already mentioned criteria are fulfilled.60 There is sufficient evidence that TDM can be useful for patients treated with antidepressants, as concluded by the authors of this consensus guideline, after a careful examination of the literature: (i) guidelines; (ii) meta-analyses; (iii) prospective studies on the clinical effectiveness of drugs in which drug plasma concentrations were reported; and (iv) pharmacokinetic studies. However, the latter often do not allow definition of a therapeutic plasma concentration range, in the absence of clinical data. Five levels of recommendation to use TDM as routine monitoring were defined as follows, as reported earlier.10
1. Strongly recommended
Established therapeutic range
Level of evidence: Controlled clinical trials have shown benefit of TDM; reports on toxic effects at “supratherapeutlc” plasma concentrations.
Clinical consequences: At therapeutic plasma concentrations highest probability of response; at “subtherapeutic” plasma concentrations response rate similar to placebo; at plasma concentrations higher than therapeutlc concentrations increasing risk of adverse effects.
2. Recommended
Suggested therapeutic ranges obtained from plasma concentrations at therapeutically effective doses (fixed dose studies).
Level of evidence: At least one welldeslgned prospective study with well-defined outcome criteria reports intoxications at “suprather apeutlc” plasma concentrations.
Clinical consequences: TDM most probably will optimize response in nonresponders: at “subtherapeutic” plasma concentrations risk of poor response; at “supratherapeutlc” plasma concentrations risk of adverse effects and/or decreased response.
3. Useful
Suggested therapeutic ranges are plasma concentrations at effective doses obtained from steady-state pharmacokinetic studies.
Level of evidence: Clinical data from retrospective analysis of TDM data; single case reports; or nonsystematlc clinical experience.
Clinical consequences: TDM useful to control whether plasma concentrations are plausible for a given dose; optimizing of clinical response In nonresponders who display low concentrations Is possible.
4. Probably useful
Suggested therapeutic ranges from steady-state pharmacokinetic studies at therapeutically effective doses.
Level of evidence: Valid clinical data so far lacking or Inconsistent results.
Clinical consequences: TDM useful to control whether plasma concentrations are plausible for a given dose.
5. Not recommended
Unique pharmacology of the drug, eg, irreversible blockade of an enzyme or flexible dosing according to clinical symptoms.
Level of evidence: Textbook knowledge, basic pharmacology.
Clinical consequences: TDM should not be used.
Drug-specific TDM recommendations
The knowledge of plasma concentrations ranges observed after treatment of subjects at well-defined doses of the antidepressant (Table III) may efficiently help the clinician In some of the situations listed in Table II: suspicion of noncompliance, drug Interactions, problems occurring after switching from an original preparation to a generic form (and vice versa), or presence of a pharmacogenetic PM or UM status. The information available in Table III is also helpful In situations where the levels of recommendations 3 and 4 apply (le, TDM useful or probably useful).
Table III. Dose-related steady-state plasma concentrations of antidepressants.11 Generally, arithmetic means ± standarad deviations are given; numbers in parentheses indicate ranges. md# median value; gm, geometric mean; m, males; f, females. *Extensive metabolizers (CYP 2D6). †Doxepin + desmethyldoxepin. ‡Patients were treated with 20 mg/day citalopram, and S-citalopram and its metabolite were measured. §Nonsmokers. "Smokers. ¶Concentrations given in ng.kg/mL.mg, in extensive metabolizers (CYP 2D6). #Concentrations show very little differences when given 50 mg/day tid.
| Antidepressant | Active metabolite (or metabolite recommended for TDM) | Dose steady-state plasma concentrations* | |||
| Dose (mg/day) | Parent compound (ng/mL) | Metabolite (ng/mL) | References | ||
| Amitriptyline | Nortriptyline | 150 | 102±59(34-278) | 85±60 (16-326) | Baumann et al, 77 1986 |
| 150 | 122±62 | 84±48 | Jungkunz and Kuss, 78 1980 | ||
| 150 | 76±30 | 84±38 | Breyer-Pfaff et al, 79 1982 | ||
| 150 | 100±41 | 71±38 | Breyer-Pfaff et aI, 80 1982 | ||
| 200 | 146±21 (sem) | 129±23 (sem) | Kupfer et al, 81 1977 | ||
| Citalopram | Demethylcitalopram | 40 | 86±38 | 35±11 | Baumann et al, 82 1996 |
| 40 iv | 70±23 | 30±12 | Baumann et al, 83 1998 | ||
| Clomipramine | Demethylclomipramine | 75 bid | 63 md (22-230)* | 148 md ( 51-331)* | Kramer Nielsen et aI, 84 1992 |
| 50 | 24 md (5-69)* | 15 md (6-78)* | DUAG, 85 1999 | ||
| 75 | 38 md (9-78)* | 43 md (5-102)* | DUAG, 85 1999 | ||
| 125 | 83 md (31-224)* | 105 md (41-335)* | DUAG, 85 1999 | ||
| 200 | 202 md (50-340)* | 283 md (138-446)* | DUAG, 85 1999 | ||
| 100 iv | 122±73 | 145±118 | Müller-Oerlinghausen and Fähndrich, 86 1985 | ||
| 150 | 74-310 | 69-267 | Burch et at, 87 1982 | ||
| Desipramine | 200 | 173(28-882) | Friedel et al, 88 1979 | ||
| 186±24 | 188±152 | Amsterdam et al, 89 1985 | |||
| 75-250 | 16-502 | Nelson et aI, 90 1985 | |||
| Dothiepine | Dothiepine-SO | 150 | 95±67 | 323±191 | Maguire et al, 91 1982 |
| Northiaden | 150 | 16±12 | Maguire et al, 91 1982 | ||
| Dothiepine-SO | 3.22+0.99 mg/kg | 67 (4-258) | 352 (45-953) | llett et al, 92 1993 | |
| Northiaden | 3.22±0.99 mg/kg | 37 (0-230) | llett et al, 92 1993 | ||
| Doxepin (DOX) | Demethyldoxepin (DDOX) | 250 | 484±251 nmol/L† | Adler et al, 93 1997 | |
| Demethyldoxepin | 250 | 130±113 | 132±94 | Deuschle et al, 94 1997 | |
| trans-Demethyldoxepin | 250 | 72±80 | Deuschle et al, 94 1997 | ||
| cis-Demethyldoxepin | 250 | 60±45 | Deuschle et al, 94 1997 | ||
| 143±30 | 89±75† | Leucht et al, 95 2001 | |||
| Esdtalopram‡ | S-Demethylcitalopram | 10‡ | 27±14 | 14±5 | Bondolfi et al, 96 1998 |
| 10‡ | 28±9 | 11±3 | Bondolfi et al, 97 2000 | ||
| Fluoxetine | Norfluoxetine | 20 | 80 (9-265) md | 128 (30-300) md | Lundmark et al, 98 2001 |
| 40 | 195 (40-496) md | 221 (20-449) md | |||
| 20 | 97±51 | 128+49 | Amsterdam et aI, 99 1997 | ||
| Fluvoxamine | 100 | 90±29 (f) | Härtter et al, 100 1998 | ||
| 100 | 59±22 (m) | Härtter et al, 100 1998 | |||
| 200 | 274±73 (f) | Härtter et al, 100 1998 | |||
| 200 | 237±90 (m) | Härtter et al, 100 1998 | |||
| 229+47 | 142±108 (20-417) | Kasper et al, 101 1993 | |||
| 200 | 162±144 (13-333) | Gerstenberg et al, 102 2003 | |||
| Imipramine | Desipramine | 225 | (6-268) | (18-498) | Reisby et al, 103 1377 |
| Mapratiline | (Desmethylmaprotiline) | 150 | 116±47 | Gabris et al, 104 1985 | |
| 236±32 | 202±134 (12-428) | Kasper et al, 101 1993 | |||
| Mianserin | Demethylmianserin | 30 | 22(12-48) | 9(3-24) | Otani et al, 105 1991 |
| (MIA) | (DMIA) | 30 | 14 (6-37) (S-MIA) | Mihara et al, 106 1997 | |
| 30 | 9 (4-18) (R-MIA) | Mihara et al, 106 1997 | |||
| 60 | 37±19 (14-67) (S-MIA) | 10±5 (6-23) (S-DMIA) | Eap et al, 107 1999 | ||
| 60 | 19±11 (10-51) (R-MIA) | 21±15(1O-52)( R-DMIA) | Eap et al, 107 1999 | ||
| Mirtazapine | (Demethylmirtazapine) | 15 | 7.3±3.2 | Timmer et al, 108 1995 | |
| 30 | 18±7 | Timmer et al, 108 1995 | |||
| 45 | 28±12 | Timmer et al, 108 1995 | |||
| 60 | 38±16 | Timmer et al, 108 1995 | |||
| 70 | 46±16 | Timmer et al, 108 1995 | |||
| Moclobemide | 100 tid | 216±55 | Schoerlin et al, 109 1987 | ||
| Nortriptyline | 150 | 141±48(48-238) | |||
| 75-225 | 90±40 (32-164) | Asberg et al, 12 1971 | |||
| Paroxetine | 30 | 36.3 (1.7-60.8) | Lundmark et al, 110 1989 | ||
| 30 | 27 rnd (12-45)* | Sindrup et al, 111 1992 | |||
| 30 | 36(9-70) | Kaye et al, 112 1989 | |||
| Reboxetine | 4 | 50±20 | Pellizzoni et al, 113 1996 | ||
| Sertraline | (Norsertraline) | 50 | 12±17gm (3-134) | 30±24 gm (7-143) | Lundmark et al, 114 2000 |
| 100 | 19±18 gm (3-109) | 45±35 gm (10-273) | Lundmark et al, 114 2000 | ||
| 150 | 31±29 gm (8-145) | 65±47 gm (7-138) | Lundmark et al, 114 2000 | ||
| 200 | 29±18 gm (9-82) | 87±43 gm (40-189) | Lundmark et al, 114 2000 | ||
| 50 | 12±8 (4-32) | Axelson et al, 115 2002 | |||
| Trazodone | m-Chlorophenylpiperazine | 150 | 624(271-1062) | 65(34-108) | Otani et aI, 116 1998 |
| 150 | 680±257§ | 65±21§ | Mihara et al, 117 2001 | ||
| 150 | 541±277" | 56±21" | Mihara et al, 117 2001 | ||
| Trimipramine | Desmethyltrimipramine | 200 | 277±67 | 169±51 | Cournoyer et al, 118 1987 |
| (TRI) | (DTRI) | 21±11 (7-47) (L-TRI)¶ | 7±6 (1-23) (L-DTRI)¶ | Eap et al, 119 2000 | |
| 18±6 (8-32) (D-TRI)¶ | 10±7 (2-29) (D-DTRI)¶ | Eap et al, 119 2000 | |||
| Venlafaxine | O -Demethylvenlafaxine | 75 bid# | 56±31 | 194±75 | Troy et al, 120 1335 |
| 75 | 75±93 (5-427) | 116±65 (16-260) | Reis et al, 121 2002 | ||
| 150 | 109±232 (4-1903) | 186±94 (16-411) | Reis et al, 121 2002 | ||
| 225 | 178±283 (9-1421) | 232±132 (63-736) | Reis et al, 121 2002 | ||
| 300 | 155±109 (21-438) | 249±121 (104-516) | Reis et al, 121 2002 | ||
| Viloxazine | 300 | 1200 (ca 400-1600) | Müller-Oerlinghausen and Ruethe, 122 1979 |
However, the data presented In Table III are Insufficient to allow levels of recommendations 1 or 2, as It does not Include studies on the plasma concentration–clinical effectiveness relationship. Therefore, the literature had to be reexamined to define which antidepressants may get a level 3 or 4 of recommendation for their monitoring. By consensus, a therapeutic range was then also defined for their “main” (= depression) indication (Table IV), as data for other indications (eg, anxiety disorders) are most often lacking, and some studies suggest that optimal ranges may differ, depending on the pathology154 Antidepressants differ widely in their chemical structure and their pharmacological activity, even though most are serotonergic and/or noradrenergic. “Therapeutic windows” have been defined for most tricyclic antidepressants, and TDM is recommended to avoid intoxications, which may be lethal (Table IV),
As regards more recently introduced antidepressants, a clearcut plasma level–clinical effectiveness relationship was not demonstrated for tetracyclic antidepressants (maprotiline, mianserin, or mirtazapine), trazodone, reboxetine, the monoamine oxidase inhibitors mocloberoide and tranylcypromine,133 and SSRls.21,155,156 However, TDM of SSRls was shown to be cost-effective, as it helps to use minimum effective doses.114 Therefore, data on the plasma concentrations at therapeutic doses may be clinically useful for these drugs (Table III), in situations of noncompliance, nonresponse, adverse effects, or intoxication.
Specific indications for TDM in psychiatry
Therapeutic windows should be interpreted in the context of the clinical situation, before the decision to change treatment strategy is taken. As an example, low levels may be sufficient for the antidepressant doxepin, if it is used to obtain sedation.95
Interestingly, despite the increasing use of generics, there are few data available that demonstrate unambiguously the occurrence of pharmacokinetic problems after switching from an original preparation to a generic form (and vice versa).157-160 TDM is a general indication for the administration of psychotropic drugs in children and adolescents because psychopharmacotherapy of children and adolescents differs from that of adults (Gerlach et al, in press): (i) There are differences in the pharmacokinetic behavior of drugs used in dependence on the stage of development; it is therefore not appropriate to use dosages recommended for adults, (ii) Many drugs are not approved for use in children and adolescents; the consequence is that the criteria for efficacy and safety, guaranteed for the use in adults, are not given for administration in children and adolescents. There is, however, a need to carry out standardized studies to find therapeutic ranges of plasma concentrations for children and adolescents.
In these patients, but also in elderly subjects, TDM may help distinguish between pharmacokinetic and pharmacodynamic factors in the occurrence of adverse effects. Consequently, TDM also represents a useful tool in situations of pharmaco vigilance programs.
Antidepressants should be monitored in the blood of pregnant or lactating women in order to minimize drug exposure of the fetus or newborn infant.161-165
Investigations on the “therapeutic window” of patients should not only be included in phase IV studies. If possible, they should also be carried out in phase III studies, in relationship with clinical ratings, in order to propose TDM with the introduction of the new drug. As stated in the doc? ument published by the European Agency for the Evaluation of Medicinal Products,166 an established concentration-response relationship is the basis to forecast the chance of toxicity due to pharmacokinetic differences, drug-disease, or drug-drug interactions.
Pharmacogenetic tests in addition to TDM
There is increasing evidence for an advantage to combine pharmacogenetic tests with TDM.18,39,44,167 However, pharmacogenetic tests alone have limited value, as environmental factors also regulate drug metabolism.168 Some of the most important indications for phenotyping and/or genotyping (in combination with TDM) are the following.51,168
The metabolism of the medication (or its active metabolite) is governed to a significant extent by the enzyme, which is considered to be phenotyped or genotyped.
The patient is treated with a substrate whose metabolism shows a wide interindividual variability, as demonstrated by TDM.
A drug is characterized by a low therapeutic index, ie, risk of toxicity in the case of a genetically impaired metabolism or, on the other hand, risk of nonresponse due to an ultrarapid metabolism and the inability to reach therapeutic drug levels.
The patient presents unusual plasma concentrations of the drug or its metabolite(s), and genetic factors are suspected to be responsible.
The patient suffers from a chronic illness, which requires life-long treatment.
As outlined above, both phenotyping and genotyping are recommended in some circumstances, as a “traitmarker” and a “state-marker.” Currently, data obtained by TDM represent a “state-marker.”
Practical aspects of TDM
Previous studies suggest that the “compliance” of the treating physician needs to be improved, as many requests or indications for TDM were inappropriate.169
Moreover, clinicians frequently do not follow the recommendations given by the laboratory to adjust the treatment.73 Therefore, some practical recommendations are summarized (see reference 11 for a comprehensive presentation) for the optimal use of TDM, as illustrated in Figure 1.
Recommendations for the treating physician
Preparation of TDM
Some patients may particularly benefit from TDM: an antidepressant drug should then be recommended for which TDM is available, either to minimize adverse effects or optimize its clinical efficacy. A well-defined “therapeutic window” for this drug (Table IV) or at least known plasma concentration ranges for clinical doses (Table II) should be available.
Blood should be collected for TDM in steady-state conditions, ie, at least 5 drug half-lives after changes in dose and during the terminal β -elimination phase. Generally, the appropriate sampling time for most antidepressants (except for fluoxetine) is 1 week after stable daily dosing and immediately before ingestion of the morning dose, ie, about 12 to 16 h (or 24 h if the drug is given once daily) after the last medication. It should be considered that both after a modification of the dose and after prescription of a comedication, which may inhibit or enhance the metabolism of the drug to be measured, steady-state conditions are reached again only after a few days. TDM should then be delayed, in case unexpected side effects are observed.
Most antidepressants are stable in serum or plasma for at least 24 h170 and can therefore be sent to the laboratory at room temperature. It is mandatory to consider technical recommendations given by the laboratory: choice of anticoagulant (plasma, serum), sample volume and its labeling, conditions for mailing, influence of light, and temperature. Information on comedication may help the laboratory to avoid analytical problems (interferences with other drugs). It is strongly recommended to fill out the request forms adequately and completely (diagnosis, comorbidities, comedications, treatment duration, doses, sex and age of the patient, and reasons for the request), in order to allow interpretation of the result by clinical pharmacologists. Some of these data may also represent important information for the laboratory to judge plausibility of the result.
Critical appreciation of the results
A pharmacological treatment should be guided by sound clinical judgment. TDM has to be considered as an additional and useful tool for optimizing therapy.
Analytical methods used in the laboratories may differ in their quality. The physician should be aware that some drug levels are not accurately measured, even though most laboratories have introduced a program to measure quality. Indeed, worldwide external quality-control programs show considerable variability between laboratories in the results of analysis of control samples. The physician may obtain discrepant results when a drug was monitored several times in a patient, but analyzed in different laboratories. When comparisons of TDM values obtained from different laboratories are carried out, the clinician should take into account the units (ng/mL, μg/L, μmol/L, nmol/L) in which the results of the analysis are expressed.
Low plasma drug concentrations suggest either irregular intake of the drug or ultrarapid metabolism, and in this situation, a pharmacogenetic test may be indicated. In the first case, TDM should be repeated in order to verify compliance. These examples show that it may be advantageous for the clinician to collaborate with a TDM laboratory that offers pharmacological consultation.
TDM interpretation and treatment of patients
A TDM result represents a guide to adjust the treatment of the individual patient, but expert interpretation and adequate use of this pharmacokinetic data are mandatory for an optimal clinical benefit. Reporting of results and inclusion of dose recommendations and other comments by the laboratory must be guided by the best available evidence. However, the laboratory has only limited knowledge of the clinical context. The physician should also take into consideration whether the “reference plasma concentrations range” reflects only “drug plasma concentrations at clinically relevant doses” (Table III) or whether they are “therapeutic ranges” (Table IV). Information on the level of recommendation for TDM of the particular drug may also help evaluate the clinical significance of the result (Table IV). If the plasma concentration of the drug is within the therapeutic range, an adaptation of the dose is, of course, only recommended when clinical reasons, such as adverse effects or nonresponse, clearly justify such a decision. When the advice given on the TDM report is not followed, the reason for such a decision should be carefully documented.
Recommendations for the laboratory
Analytical procedures
The concentrations of antidepressants are generally low, in the ng/mL range, and many patients are comedicated with various, potentially interfering drugs. The methods should be adapted to this situation by precision (coefficient of variation <f 5%), accurateness (deviation from nominal value <15%), and robustness.5 Each assay needs to be validated, documented, and regularly assessed for linearity, selectivity, accuracy, precision, recovery, and sensitivity (limits of detection [LOD] and quantification [LOQ]). Internal and external quality control procedures are mandatory to ensure maximal quality of TDM. If quality controls are outside the expected range, the reason underlying the outlier needs to be clarified and documented.64-66
Where indicated the laboratory should analyze both the drug and its active metabolite(s) (Tables II and III). Moreover, the analysis of (active and inactive) metabolites represents an additional tool to verify compliance of patients.
Reporting of results
In addition to the result, the appropriate target range should be communicated to the physician (Tables II and III), using, of course, the same units (either mass or molar units). The LOD, or preferentially the LOQ, should be indicated in situations when plasma drug concentrations are below these values. The results should be available for clinical interpretation within a clinically meaningful time, especially in case of suspected intoxications. An interpretation and clinical and pharmacological advice should be provided with every report. Therefore, it is advantageous for the clinician to choose a laboratory that offers this service.
Plasma concentrations must be interpreted in the light of sound clinical judgment. Most frequently, recommendations on dose changes are given, and in a situation of drug concentrations above the recommended range, rapidity of communication may enhance successful intervention in patients at risk of toxicity. The physician will also appreciate comments related to genetic polymorphisms, risk for pharmacokinetic interactions in situations, and pharmacokinetic properties of the drug when given to elderly patients or patients with hepatic or renal insufficiency.
In situations where drug concentrations are particularly low, it is often not clear whether the patient is an UM or whether he or she is noncompliant in that the drug intake is irregular. The analysis of a second plasma sample may help verify compliance but, depending on the result, a pharmacogenetic test should be carried out.
Clearly a PM (CYP 2D6) status should not automatically result in interruption of a treatment,18,171 but the dose should be adapted using clinical judgment and TDM.
Conclusion
TDM is a valuable approach to optimize both shortterm and lifelong treatment of psychiatric patients with antidepressants,172 and a combination of TDM with pharmacogenetic tests will be increasingly useful, particularly because in near future, pharmacogenetic tests regarding pharmacodynamic parameters will also be clinically relevant.173 Many data on plasma concentrations of psychotropic drugs and the plasma concentration–clinical effectiveness relationship have accumulated over the past few years, and encouraged this interdisciplinary collaboration of specialists who brought about this consensus on TDM.11 Hopefully, it will help to use TDM optimally from a scientific, clinical, and economic point of view.
Selected abbrewiations and acronyms
- CYP
cytochrome P-450
- GC
gas chromatography
- HPLC
high-performance liquid chromatography
- LOD
limit of detection
- LOQ
limit of quantification
- PM
poor metabolizer
- SSRI
selective serotonin reuptake inhibitor
- TDM
therapeutic drug monitoring
- UM
ultrarapid metabolizer
*This review takes into consideration antidepressant agents currently available in Switzerland and Germany, and therefore does not claim to be exhaustive.
This article is a modified version of an article published in the journal Pharmacopsychiatry in December 2004: Baumann P, Hiemke C, Ulrich S, et al. The AGNP-TDM expert group consensus guidelines: therapeutic drug monitoring in psychiatry. Pharmacopsychiatry. 2004;37:243-265. It is published here with the kind permission of the publishers Georg Thieme Verlag KG, Stuttgart, Germany.
Contributor Information
Pierre Baumann, Department of Psychiatry, University of Lausanne, Prilly-Lausanne, Switzerland.
Sven Ulrich, Department of Clinical Pharmacology, University of Magdeburg, Magdeburg, Germany.
Gabriel Eckermann, Bezirkskrankenhaus Kaufbeuren, Kaufbeuren, Germany.
Manfred Gerlach, Department of Child and Adolescent Psychiatry, University of Wuerzburg, Würzburg, Germany.
Hans-Joachim Kuss, Department of Psychiatry, University of Munich, Munich, Germany.
Gerd Laux, Bezirksklinikum Gabersee, Wasserburg/Inn, Germany.
Bruno Müller-Oerlinghausen, Drug Commission of the German Medical Association, Berlin and Cologne, Germany.
Marie Luise Rao, Department of Psychiatry, University of Bonn, Bonn, Germany.
Peter Riederer, Department of Psychiatry, University of Wuerzburg, Würzburg, Germany.
Gerald Zernig, Department of Psychiatry, University of Innsbruck, Innsbruck, Austria.
Christoph Hiemke, Department of Psychiatry, University of Mainz, Germany.
REFERENCES
- 1.American Psychiatrie Association Practice Guidelines. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry. 2000;157(suppl 4):1–45. [PubMed] [Google Scholar]
- 2.Bauer M., Whybrow PC., Angst J., Versiani M., MöIIer HJ. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders. Part 1: acute and continuation treatment of major depressive disorder. World J Biol Psychiatry. 2002;3:5–43. doi: 10.3109/15622970209150599. [DOI] [PubMed] [Google Scholar]
- 3.Donoghue J., Taylor DM. Suboptimal use of antidepressants in the treatment of depression. CNS Drugs. 2000;13:365–383. [Google Scholar]
- 4.Hirschfeld RMA., Montgomery SA., Aguglia E., et al. Partial response and nonresponse to antidepressant therapy: current approaches and treatment options. J Clin Psychiatry. 2002;63:826–837. doi: 10.4088/jcp.v63n0913. [DOI] [PubMed] [Google Scholar]
- 5.Olver JS., Burrows GD., Norman TR. Third-generation antidepressants―do they offer advantages over the SSRls? CNS Drugs. 2001;15:941–954. doi: 10.2165/00023210-200115120-00004. [DOI] [PubMed] [Google Scholar]
- 6.Nelson JC. Augmentation strategies in depression 2000. J Clin Psychiatry. 2000;61 (suppl2):13–19. [PubMed] [Google Scholar]
- 7.Zullino D., Baumann P. Lithium augmentation in depressive patients not responding to selective serotonin reuptake inhibitors. Pharmacopsychiatry. 2001;34:119–127. doi: 10.1055/s-2001-15873. [DOI] [PubMed] [Google Scholar]
- 8.Stimpson N., Agrawal N., Lewis G. Randomised controlled trials investigating pharmacological and psychological interventions for treatmentrefractory depression. Br J Psychiatry. 2002;181:284–294. doi: 10.1192/bjp.181.4.284. [DOI] [PubMed] [Google Scholar]
- 9.Thase ME. The need for clinically relevant research on treatment-resistant depression. J Clin Psychiatry. 2001;62:221–224. doi: 10.4088/jcp.v62n0401. [DOI] [PubMed] [Google Scholar]
- 10.Michelson D., Allen AJ., Busner J., et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry. 2002;159:1896–1901. doi: 10.1176/appi.ajp.159.11.1896. [DOI] [PubMed] [Google Scholar]
- 11.Baumann P., Hiemke C., Ulrich S., et al. The AGNP-TDM expert group consensus guidelines: therapeutic drug monitoring in psychiatry. Pharmacopsychiatry. 2004;37:243–265. doi: 10.1055/s-2004-832687. [DOI] [PubMed] [Google Scholar]
- 12.Åsberg M., Cronholm B., Sjöqvist F., Tuck D. Relationship between plasma level and therapeutic effect of nortriptyline. BMJ. 1971;3:331–334. doi: 10.1136/bmj.3.5770.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertilsson L., Mellström B., Sjöqvist F., Mårtensson B., Åsberg M. Slow hydroxylation of nortriptyline and concomitant poor debrisoquine hydroxylation: clinical implications. Lancet. 1981;i:560–561. doi: 10.1016/s0140-6736(81)92894-4. [DOI] [PubMed] [Google Scholar]
- 14.Task Force. Tricyclic antidepressants―blood level measurements and clinical outcome: an APATask Force report. Am J Psychiatry. 1985;142:155–162. doi: 10.1176/ajp.142.2.155. [DOI] [PubMed] [Google Scholar]
- 15.Orsulak PJ. Therapeutic monitoring of antidepressant drugs―guidelines updated. Ther Drug Monit. 1989;11:497–507. [PubMed] [Google Scholar]
- 16.Linder MW., Keck PE., Jr. Standards of laboratory practice: antidepressant drug monitoring. Clin Chem. 1998;44:1073–1084. [PubMed] [Google Scholar]
- 17.Laux G., Riederer P (eds). XX. Plasmaspiegel-bestimmung von Psychopharmaka: Therapeutisches Drug―Monitoring Versuch einer ersten Standortbestimmung. Stuttgart, Germany: Wissenschaftliche Verlagsgesellschaft mbH; 1992;XX:XX–XX. [Google Scholar]
- 18.Kirchheiner J., Brøsen K., Dahl ML., et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand. 2001;104:173–192. doi: 10.1034/j.1600-0447.2001.00299.x. [DOI] [PubMed] [Google Scholar]
- 19.Dahl ML. Cytochrome P450 phenotyping/genotyping in patients receiving antipsychotics: useful aid to prescribing? Clin Pharmacokinet. 2002;41:453–470. doi: 10.2165/00003088-200241070-00001. [DOI] [PubMed] [Google Scholar]
- 20.Ulrich S., Wurthmann C., Brosz M., Meyer FP. The relationship between serum concentration and therapeutic effect of haloperidol in patients with acute schizophrenia. Clin Pharmacokinet. 1998;34:227–263. doi: 10.2165/00003088-199834030-00005. [DOI] [PubMed] [Google Scholar]
- 21.Hiemke C., Härtter S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol Ther. 2000;85:11–28. doi: 10.1016/s0163-7258(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 22.Greenblatt DJ., Von Moltke LL., Harmatz JS., Shader Rl. Human cytochromes and some newer antidepressants: kinetics, metabolism, and drug interactions. J Clin Psychopharmacol. 1999;19(suppl 1):23S–35S. doi: 10.1097/00004714-199910001-00003. [DOI] [PubMed] [Google Scholar]
- 23.Nemeroff CB., DeVane CL., Pollock BG. Newer antidepressants and the cytochrome P450 system. Am J Psychiatry. 1996;153:311–320. doi: 10.1176/ajp.153.3.311. [DOI] [PubMed] [Google Scholar]
- 24.Meyer UA., Amrein R., Balant LP., et al. Antidepressants and drug-metabolizing enzymes―expert group report. Acta Psychiatr Scand. 1996;93:71–79. doi: 10.1111/j.1600-0447.1996.tb09805.x. [DOI] [PubMed] [Google Scholar]
- 25.Rudorfer MV., Potter WZ. Metabolism of tricyclic antidepressants. Cell Mol Neurobiol. 1999;19:373–409. doi: 10.1023/A:1006949816036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breyer-Pfaff U., Gaertner HJ., Baumann P. XX. Antidepressiva―Pharmakologie, therapeutischer Einsatz und Klinik der Depressionen. 2nd ed. Stuttgart, Germany: Wissenschaftliche Verlagsgesellschaft mbH; 2005;XX:XX–XX. [Google Scholar]
- 27.Marzo A., Balant LP. Investigation of xenobiotic metabolism by CYP2D6 and CYP2C19: importance of enantioselective analytical methods. J Chromatogr B Biomed Appl. 1996;678:73–92. doi: 10.1016/0378-4347(95)00229-4. [DOI] [PubMed] [Google Scholar]
- 28.Baumann P., Zullino DF., Eap CB. Enantiomers' potential in psychopharmacology―a critical analysis with special emphasis on the antidepressant escitalopram. Eur Neuropsychopharmacol. 2002;12:433–444. doi: 10.1016/s0924-977x(02)00051-2. [DOI] [PubMed] [Google Scholar]
- 29.Testa B. Drug metabolism. In: Wolff ME, ed. Burger's Medicinal Chemistry and Drug Discovery. 5th ed. New York, NY: John Wiley and Sons; 1995;XX:129–180. [Google Scholar]
- 30.Liston HL., Markowitz JS., DeVane CL. Drug glucuronidation in clinical psychopharmacology. J Clin Psychopharmacol. 2001;21:500–515. doi: 10.1097/00004714-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Burchell B. Transformation reactions: glucuronidation. In: Woolf TF, ed. Handbook of Drug Metabolism. New York, NY: Marcel Dekker; 1999;XX:153–173. [Google Scholar]
- 32.Penzak SR., Hon YY., Lawhorn WD., Shirley KL., Spratlin V., Jann MW. Influence of ritonavir on olanzapine pharmacokinetics in healthy volunteers. J Clin Psychopharmacol. 2002;22:366–370. doi: 10.1097/00004714-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Caccia S., Garattini S. Formation of active metabolites of psychotropic drugs: an updated review of their significance. Clin Pharmacokinet. 1990;18:434–459. doi: 10.2165/00003088-199018060-00002. [DOI] [PubMed] [Google Scholar]
- 34.Eadie MJ. Formation of active metabolites of anticonvulsant drugs―a review of their pharmacokinetic and therapeutic significance. Clin Pharmacokinet. 1991;21:27–41. doi: 10.2165/00003088-199121010-00003. [DOI] [PubMed] [Google Scholar]
- 35.Rudorfer MV., Potter WZ. The role of metabolites of antidepressants in the treatment of depression. CNS Drugs. 1997;7:273–312. doi: 10.2165/00023210-199707040-00003. [DOI] [PubMed] [Google Scholar]
- 36.Bertilsson L., Dahl ML., Dalen P., AI Shurbaji A. Molecular genetics of CYP 2D6: clinical relevance with focus on psychotropic drugs. Br J Clin Pharmacol. 2002;53:111–122. doi: 10.1046/j.0306-5251.2001.01548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cascorbi I. Pharmacogenetics of cytochrome P4502D6: genetic background and clinical implication. Eur J Clin invest. 2003;33(suppl 2):17–22. doi: 10.1046/j.1365-2362.33.s2.3.x. [DOI] [PubMed] [Google Scholar]
- 38.Murray M. P450 Enzymes. Inhibition mechanisms, genetic regulation and effects of liver disease. Clin Pharmacokinet. 1992;23:132–146. doi: 10.2165/00003088-199223020-00005. [DOI] [PubMed] [Google Scholar]
- 39.Dahl ML., Sjöqvist F. Pharmacogenetic methods as a complement to therapeutic monitoring of antidepressants and neuroleptics. Ther Drug Monit. 2000;22:114–117. doi: 10.1097/00007691-200002000-00024. [DOI] [PubMed] [Google Scholar]
- 40.Kirchheiner J., Nickchen K., Bauer M., et al. Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatry. 2004;9:442–473. doi: 10.1038/sj.mp.4001494. [DOI] [PubMed] [Google Scholar]
- 41.Vasiliou V., Pappa A., Petersen DR. Role of aldehyde dehydrogenases in endogenous and xenobiotic metabolism. Chem Biol Interact. 2000;129:1–19. doi: 10.1016/s0009-2797(00)00211-8. [DOI] [PubMed] [Google Scholar]
- 42.Coutts RT., Urichuk LJ. Polymorphic cytochromes P450 and drugs used in psychiatry. Cell Mol Neurobiol. 1999;19:325–354. doi: 10.1023/A:1006945715127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benet LZ., Kroetz DL., Sheiner LB. Pharmacokinetics the dynamics of drug absorption, distribution, and elimination. In: Hardman JG, Limbird LE, eds. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill; 1996;XX:3–27. [Google Scholar]
- 44.Scordo MG., Spina E. Cytochrome P450 polymorphisms and response to antipsychotic therapy. Pharmacogenomics. 2002;3:201–218. doi: 10.1517/14622416.3.2.201. [DOI] [PubMed] [Google Scholar]
- 45.Kuehl P., Zhang J., Lin Y., et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27:383–391. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 46.Nakajima M., Yokoi T., Mizutani M., Kinoshita M., Funayama M., Kamataki T. Genetic polymorphism in the 5'-fIanking region of human CYP1A2 gene: effect on the CYP1A2 inducibility in humans. J Biochem. 1999;125:803–808. doi: 10.1093/oxfordjournals.jbchem.a022352. [DOI] [PubMed] [Google Scholar]
- 47.Sachse C., BrockmöIIer J., Bauer S., Roots I. Functional significance of a C_A polymorphism in intron I of the cytochrome P450 CYP 1A2 gene tested with caffeine. Br J Clin Pharmacol. 1999;47:445–449. doi: 10.1046/j.1365-2125.1999.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brøsen K. Drug-metabolizing enzymes and therapeutic drug monitoring in psychiatry. Ther Drug Monit. 1996;18:393–396. doi: 10.1097/00007691-199608000-00014. [DOI] [PubMed] [Google Scholar]
- 49.DeVane CL. Pharmacogenetics and drug metabolism of newer antidepressant agents. J Clin Psychia try. 1994;55(suppl 12):38–45. [PubMed] [Google Scholar]
- 50.Otani K., Aoshima T. Pharmacogenetics of classical and new antipsychotic drugs. Ther Drug Monit. 2000;22:118–121. doi: 10.1097/00007691-200002000-00025. [DOI] [PubMed] [Google Scholar]
- 51.Steimer W., Potter JM. Pharmacogenetic screening and therapeutic drugs. Clin ChimActa. 2002;315:137–155. doi: 10.1016/s0009-8981(01)00713-6. [DOI] [PubMed] [Google Scholar]
- 52.Kirchheiner J., Bertilsson L., Bruus H., Wolff A., Roots I., Bauer M. Individualized medicine―implementation of pharmacogenetic diagnostics in antidepressant drug treatment of major depressive disorders. Pharmacopsychiatry. 2003;36(suppl 3):S235–S243. doi: 10.1055/s-2003-45136. [DOI] [PubMed] [Google Scholar]
- 53.Eap CB., Jaquenoud Sirot E., Baumann P. Therapeutic monitoring of antidepressants in era of pharmacogenetics studies. Ther Drug Monit. 2004;26:152–155. doi: 10.1097/00007691-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 54.De Leon J. Glucuronidation enzymes, genes and psychiatry. Int J Neuropsychopharmacol. 2003;6:57–72. doi: 10.1017/S1461145703003249. [DOI] [PubMed] [Google Scholar]
- 55.Marzolini C., Paus E., Buclin T., Kim RB. Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin Pharmacol Ther. 2004;75:13–33. doi: 10.1016/j.clpt.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 56.Uhr M., Grauer MT. abcb1ab P-glycoprotein is involved in the uptake of citalopram and trimipramine into the brain of mice. J Psychiatr Res. 2003;37:179–185. doi: 10.1016/s0022-3956(03)00022-0. [DOI] [PubMed] [Google Scholar]
- 57.Uhr M., Steckler T., Yassouridis A., Holsboer F. Penetration of amitriptyline, but not of fluoxetine, into brain is enhanced in mice with blood-brain barrier deficiency due to Mdr1a P-glycoprotein gene disruption. Neuropsychopharmacology. 2000;22:380–387. doi: 10.1016/S0893-133X(99)00095-0. [DOI] [PubMed] [Google Scholar]
- 58.Perry PJ. The relationship between antidepressant response and tricyclic antidepressant plasma concentrations. A retrospective analysis of the literature using logistic regression analysis. Clin Pharmacokinet. 1987;13:381–392. doi: 10.2165/00003088-198713060-00003. [DOI] [PubMed] [Google Scholar]
- 59.Perry PJ., Zeilmann C., Arndt S. Tricyclic antidepressant concentrations in plasma: an estimate of their sensitivity and specificity as a predictor of response. J Clin Psychopharmacol. 1994;14:230–240. [PubMed] [Google Scholar]
- 60.Ulrich S., Läuter J. Comprehensive survey of the relationship between serum concentration and therapeutic effect of amitriptyline in depression. Clin Pharmacokinet. 2002;41:853–876. doi: 10.2165/00003088-200241110-00004. [DOI] [PubMed] [Google Scholar]
- 61.Balant-Gorgia EA., Balant LP. Therapeutic drug monitoring―relevance during the drug treatment of psychiatric disorders. CNS Drugs. 1995;4:432–453. [Google Scholar]
- 62.Eilers R. Therapeutic drug monitoring for the treatment of psychiatric disorders―clinical use and cost effectiveness. Clin Pharmacokinet. 1995;29:442–450. doi: 10.2165/00003088-199529060-00005. [DOI] [PubMed] [Google Scholar]
- 63.Green M. A practical guide to analytical method validation. Anal Chem News Features. 1996;XX:305–309. [Google Scholar]
- 64.Buick AR., Doig MV., Jeal SC., Land GS., Mcdowall RD. Method validation in the bioanalytical laboratory. J Pharm Biomed Anal. 1990;8:629–637. doi: 10.1016/0731-7085(90)80093-5. [DOI] [PubMed] [Google Scholar]
- 65.Shah VP., Midha KK., Dighe S., et al. Analytical methods validation―bioavailability, bioequivalence and pharmacokinetic studies. Pharm Res. 1992;9:588–592. doi: 10.1007/BF03189968. [DOI] [PubMed] [Google Scholar]
- 66.Causon R. Validation of chromatographic methods in biomedical analysis viewpoint and discussion. J Chromatogr B. 1997;689:175–180. doi: 10.1016/s0378-4347(96)00297-6. [DOI] [PubMed] [Google Scholar]
- 67.de la Torre R., Ortuño J., Pascual JA., Gonzalez S., Ballesta J. Quantitative determination of tricyclic antidepressants and their metabolites in plasma by solid-phase extraction (bond-elute TCA) and separation by capillary gas chromatography with nitrogen-phosphorus detection. Ther Drug Monit. 1998;20:340–346. doi: 10.1097/00007691-199806000-00017. [DOI] [PubMed] [Google Scholar]
- 68.Banger M., Hermes B., Härtter S., Hiemke C. Monitoring serum concentrations of clomipramine and metabolites: fluorescence polarization immunoassay versus high performance liquid chromatography. Pharmacopsychiatry. 1997;30:128–132. doi: 10.1055/s-2007-979498. [DOI] [PubMed] [Google Scholar]
- 69.KoIIroser M., Schober C. Simultaneous determination of seven tricyclic antidepressant drugs in human plasma by direct-injection HPLC-APCI-MSMS with an ion trap detector. Ther Drug Monit. 2002;24:537–544. doi: 10.1097/00007691-200208000-00013. [DOI] [PubMed] [Google Scholar]
- 70.Veuthey JL., Souverain S., Rudaz S. Column-switching procedures for the analysis of drugs in biologic samples. Ther Drug Monit. 2004;26:161–166. doi: 10.1097/00007691-200404000-00013. [DOI] [PubMed] [Google Scholar]
- 71.Simmons SA., Perry PJ., Rickert ED., Browne JL. Cost-benefit analysis of prospective pharmocokinetic dosing of nortriptyline in depressed inpatients. J Affect Disord. 1985;8:47–53. doi: 10.1016/0165-0327(85)90071-0. [DOI] [PubMed] [Google Scholar]
- 72.Lundmark J., Bengtsson F., Nordin C., Reis M., Walinder J. Therapeutic drug monitoring of selective serotonin reuptake inhibitors influences clinical dosing strategies and reduces drug costs in depressed elderly patients. Acta Psychiatr Scand. 2000;101:354–359. doi: 10.1034/j.1600-0447.2000.101005354.x. [DOI] [PubMed] [Google Scholar]
- 73.Müller MJ., Dragicevic A., Fric M., et al. Therapeutic drug monitoring of tricyclic antidepressants: how does it work under clinical conditions? Pharmacopsychiatry. 2003;36:98–104. doi: 10.1055/s-2003-39983. [DOI] [PubMed] [Google Scholar]
- 74.Chen S., Chou WH., Blouin RA., et al. The cytochrome P450 2D6 (CYP2D6) enzyme polymorphism: screening costs and influence on clinical outcomes in psychiatry. Clin Pharmacol Ther. 1996;60:522–534. doi: 10.1016/S0009-9236(96)90148-4. [DOI] [PubMed] [Google Scholar]
- 75.Chou WH., Yan FX., Barnhill J., et al. Extension of a pilot study: impact from the cytochrome P450 2D6 polymorphism on outcome and costs associated with severe mental illness. J Clin Psychopharmacol. 2000;20:246–251. doi: 10.1097/00004714-200004000-00019. [DOI] [PubMed] [Google Scholar]
- 76.Veenstra DL., Higashi MK. Assessing the cost-effectiveness of pharmacogenomics. AAPS Pharmsci. 2000;2:1–11. doi: 10.1208/ps020329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baumann P., Jonzier-Perey M., Koeb L., Le PK., Tinguely D., Schöpf J. Amitriptyline pharmacokinetics and clinical response. I. Free and total plasma amitriptyline and nortriptyline. Int Clin Psychopharmacol. 1986;1:89–101. doi: 10.1097/00004850-198604000-00001. [DOI] [PubMed] [Google Scholar]
- 78.Jungkunz G., Kuss HJ. On the relationship of nortriptyline: amitriptyline ratio to clinical improvement of amitriptyline-treated depressive patients. Pharmakopsychiatr Neuropsychopharmakol. 1980;13:111–116. doi: 10.1055/s-2007-1019620. [DOI] [PubMed] [Google Scholar]
- 79.Breyer-Pfaff U., Gaertner HJ., Kreuter F., Scharek G., Brinkschulte M., Wiatr R. Antidepressive effect and pharmacokinetics of amitriptyline with consideration of unbound drug and 10-hydroxynortriptyline plasma levels. Psychopharmacology. 1982;76:240–244. doi: 10.1007/BF00432553. [DOI] [PubMed] [Google Scholar]
- 80.Breyer-Pfaff U., Gaertner HJ., Giedke H. Plasma levels, psychophysiological variables, and clinical response to amitriptyline. Psychiatry Res. 1982;6:223–234. doi: 10.1016/0165-1781(82)90010-5. [DOI] [PubMed] [Google Scholar]
- 81.Kupfer DJ., Hanin I., Spiker DG., Grau T., Coble P. Amitriptyline plasma levels and clinical response in primary depression. Clin Pharmacol Ther. 1977;22:904–911. doi: 10.1002/cpt1977226904. [DOI] [PubMed] [Google Scholar]
- 82.Baumann P., Nil R., Souche A., et al. A double-blind, placebo-controlled study of citalopram with and without lithium in the treatment of therapy-resistant depressive patients: a clinical, pharmacokinetic, and pharmacogenetic investigation. J Clin Psychopharmacol. 1996;16:307–314. doi: 10.1097/00004714-199608000-00006. [DOI] [PubMed] [Google Scholar]
- 83.Baumann P., Nil R., Bertschy G., et al. A double-blind double-dummy study of citalopram comparing infusion versus oral administration. J Affect Disord. 1998;49:203–210. doi: 10.1016/s0165-0327(98)00024-x. [DOI] [PubMed] [Google Scholar]
- 84.Kramer Nielsen K., Brøsen K., Gram LF., et al. Steady-state plasma levels of clomipramine and its metabolites: impact of the sparteine/debrisoquine oxidation polymorphism. Eur J Clin Pharmacol. 1992;43:405–411. doi: 10.1007/BF02220617. [DOI] [PubMed] [Google Scholar]
- 85.Danish University Antidepressant Group. Clomipramine dose-effect study in patients with depression: clinical end points and pharmacokinetics. Clin Pharmacol Ther. 1999;66:152–165. doi: 10.1016/S0009-9236(99)90053-X. [DOI] [PubMed] [Google Scholar]
- 86.Müller-Oerlinghausen B., Fahndrich E. The relationship between pharmacokinetic data and the clinical response in patients treated with maprotiline or clomipramine by intravenous infusion. Pharmacopsychiatry. 2002;18:100–101. [Google Scholar]
- 87.Burch JE., Raddats MA. Time course of plasma drug levels during once-daily oral administration of clomipramine. Psychopharmacology. 1982;77:344–347. doi: 10.1007/BF00432768. [DOI] [PubMed] [Google Scholar]
- 88.Friedel RO., Veith RC., Bloom V., Bielski RJ. Desipramine plasma levels and cliical response in depressed outpatients. Commun Psychopharmacol. 1979;3:81–87. [PubMed] [Google Scholar]
- 89.Amsterdam JD., Brunswick DJ., Winokur A., Rickels K. Desipramine and 2-hydroxydesipramine plasma levels in endogenous depressed patients. Arch Gen Psychiatry. 1985;42:361–364. doi: 10.1001/archpsyc.1985.01790270051005. [DOI] [PubMed] [Google Scholar]
- 90.Nelson JC., Jatlow PI., Mazure C. Desipramine plasma levels and response in elderly melancholic patients. J Clin Psychopharmacol. 1985;5:217–220. [PubMed] [Google Scholar]
- 91.Maguire KP., Norman TR., Burrows GD., Davies B. Blood and plasma concentrations of dothiepin and its major metabolites and clinical response. J Affect Disord. 1982;4:41–48. doi: 10.1016/0165-0327(82)90018-0. [DOI] [PubMed] [Google Scholar]
- 92.Ilett KF., BIythe TH., Hackett LP., Ong RTT., Tannenbaum DA., Clarke TMF. Plasma concentrations of dothiepin and its metabolites are not correlated with clinical efficacy in major depressive illness. Ther Drug Monit. 1993;15:351–357. doi: 10.1097/00007691-199310000-00001. [DOI] [PubMed] [Google Scholar]
- 93.Adler L., Hajak G., Lehmann K., et al. On the problems of switching from intravenous to oral administration in drug treatment of endogenous depression―a placebo-controlled double-blind trial with doxepin. Pharmacopsychiatry. 1997;30:62–69. doi: 10.1055/s-2007-979484. [DOI] [PubMed] [Google Scholar]
- 94.Deuschle M., Härtter S., Hiemke C., Standhardt H., Heuser I. Doxepin and its metabolites in plasma and cerebrospinal fluid in depressed patients. Psychopharmacology. 1997;131:19–22. doi: 10.1007/s002130050260. [DOI] [PubMed] [Google Scholar]
- 95.Leucht S., Steimer W., Kreuz S., Abraham D., Orsuiak PJ., Kissling W. Doxepin plasma concentrations: is there really a therapeutic range? J Clin Psychopharmacol. 2001;21:432–439. doi: 10.1097/00004714-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 96.Bondolfï G., Chautems C., Rochat B., Bertschy G., Baumann P. Non-response to citalopram in depressive patients: pharmacokinetic and clinical consequences of a fluvoxamine augmentation. Psychopharmacology. 1996;128:421–425. doi: 10.1007/s002130050152. [DOI] [PubMed] [Google Scholar]
- 97.Bondolfï G., Lissner C., Kosel M., Eap CB., Baumann P. Fluoxetine augmentation in citalopram non-responders: pharmacokinetic and clinical consequences. Int J Neuropsychopharmacol. 2000;3:55–60. doi: 10.1017/S1461145799001686. [DOI] [PubMed] [Google Scholar]
- 98.Lundmark J., Reis M., Bengtsson F. Serum concentrations of fluoxetine in the clinical treatment setting. Ther Drug Monit. 2001;23:139–147. doi: 10.1097/00007691-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 99.Amsterdam JD., Fawcett J., Quitkin FM., et al. Fluoxetine and norfluoxetine plasma concentrations in major depression: a multicenter study. Am J Psychiatry. 1997;154:963–969. doi: 10.1176/ajp.154.7.963. [DOI] [PubMed] [Google Scholar]
- 100.Härtter S., Wetzel H., Hammes E., Torkzadeh M., Hiemke C. Nonlinear pharmacokinetics of fluvoxamine and gender differences. Ther Drug Monit. 1998;20:446–449. doi: 10.1097/00007691-199808000-00016. [DOI] [PubMed] [Google Scholar]
- 101.Kasper S., Dötsch M., Kick H., Vieira A., MoIIer HJ. Plasma concentrations of fluvoxamine and maprotiline in major depression: implications on therapeutic efficacy and side effects. Eur Neuropsychopharmacol. 1993;3:13–21. doi: 10.1016/0924-977x(93)90290-3. [DOI] [PubMed] [Google Scholar]
- 102.Gerstenberg G., Aoshima T., Fukasawa T., et al. Relationship between clinical effects of fluvoxamine and the steady-state plasma concentrations of fluvoxamine and its major metabolite fluvoxamino acid in Japanese depressed patients. Psychopharmacology. 2003;167:443–448. doi: 10.1007/s00213-003-1430-1. [DOI] [PubMed] [Google Scholar]
- 103.Reisby N., Gram LF., Bech P., et al. Imipramine: clinical effects and pharmacokinetic variability. Psychopharmacology. 1977;54:263–272. doi: 10.1007/BF00426574. [DOI] [PubMed] [Google Scholar]
- 104.Gabris G., Baumann P., Jonzier-Perey M., Bosshart P., Woggon B., Küpfer A. N-MethyIation of maprotiline in debrisoquine/mephenitoin-phenotyped depressive patients. Biochem Pharmacol. 1985;34:409–410. [Google Scholar]
- 105.Otani K., Kaneko S., Sasa H., Kondo T., Fukushima Y. Is there a therapeutic window for plasma concentration of mianserin plus desmethylmianserin? Hum Psychopharmacol Clin Exp. 1991;6:243–248. [Google Scholar]
- 106.Mihara K., Otani K., Tybring G., Dahl ML., Bertilsson L., Kaneko S. The CYP 2D6 genotype and plasma concentrations of mianserin enantiomers in relation to therapeutic response to mianserin in depressed Japanese patients. J Clin Psychopharmacol. 1997;17:467–471. doi: 10.1097/00004714-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 107.Eap CB., Yasui N., Kaneko S., Baumann P., Powell K., Otani K. Effects of carbamazepine coadministration on plasma concentrations of the enantiomers of mianserin and of its metabolites. Ther Drug Monit. 1999;21:166–170. doi: 10.1097/00007691-199904000-00005. [DOI] [PubMed] [Google Scholar]
- 108.Timmer CJ., Lohmann AAM., Mink CPA. Pharmacokinetic dose-proportionality study at steady state of mirtazapine from remeron tablets. Hum Psychopharmacol. 1995;10:S97–S106. [Google Scholar]
- 109.Schoerlin MP., Mayersohn M., Korn A., Eggers H. Disposition kinetics of moclobemide, a monoamine oxidase A enzyme inhibitor: single and multiple dosing in normal subjects. Clin Pharmacol Ther. 1987;42:395–404. doi: 10.1038/clpt.1987.169. [DOI] [PubMed] [Google Scholar]
- 110.Lundmark J., Thomsen IS., Fjord-Larsen T., et al. Paroxetine: pharmacokinetic and antidepressant effect in the elderly. Acta Psychiatr Scand. 1989;80(suppI350):76–80. doi: 10.1111/j.1600-0447.1989.tb07177.x. [DOI] [PubMed] [Google Scholar]
- 111.Sindrup SH., Bresen K., Gram LF., et al. The relationship between paroxetine and the sparteine oxidation polymorphism. Clin Pharmacol Ther. 1992;51:278–287. doi: 10.1038/clpt.1992.23. [DOI] [PubMed] [Google Scholar]
- 112.Kaye CM., Haddock RE., Langley PF., et al. A review of the metabolism and pharmacokinetics of paroxetine in man. Acta Psychiatr Scand. 1989;80(suppI350):60–75. doi: 10.1111/j.1600-0447.1989.tb07176.x. [DOI] [PubMed] [Google Scholar]
- 113.Pellizzoni C., Poggesi I., Jørgensen NP., Edwards DMF., Paus E., Strolin Benedetti M. Pharmacokinetics of reboxetine in healthy volunteers. Single against repeated oral doses and lack of enzymatic alterations. Biopharm Drug Dispos. 1996;17:623–633. doi: 10.1002/(SICI)1099-081X(199610)17:7<623::AID-BDD978>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 114.Lundmark J., Reis M., Bengtsson F. Therapeutic drug monitoring of sertraline: variability factors as displayed in a clinical setting. Ther Drug Monit. 2000;22:446–454. doi: 10.1097/00007691-200008000-00014. [DOI] [PubMed] [Google Scholar]
- 115.Axelson DA., Perel JM., Birmaher B., et al. Sertraline pharmacokinetics and dynamics in adolescents. J Am Acad Child Adolesc Psychiatry. 2002;41:1037–1044. doi: 10.1097/00004583-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 116.Otani K., Tybring G., Mihara K., et al. Correlation between steady-state plasma concentrations of mianserin and trazodone in depressed patients. Eur J Clin Pharmacol. 1998;53:347–349. doi: 10.1007/s002280050391. [DOI] [PubMed] [Google Scholar]
- 117.Mihara K., Kondo T., Suzuki A., et al. Effects of genetic polymorphism of CYP 1A2 inducibility on the steady-state plasma concentrations of trazodone and its active metabolite m-chlorophenylpiperazine in depressed Japanese patients. Pharmacol Toxicol. 2001;88:267–270. doi: 10.1034/j.1600-0773.2001.d01-115.x. [DOI] [PubMed] [Google Scholar]
- 118.Cournoyer G., De Montigny C., Ouellette J., et al. A comparative doubleblind controlled study of trimipramine and amitriptyline in major depression: lack of correlation with 5-hydroxytryptamine reuptake blockade. J Clin Psychopharmacol. 1987;7:385–393. [PubMed] [Google Scholar]
- 119.Eap CB., Bender S., Gastpar M., et al. Steady state plasma levels of the enantiomers of trimipramine and of its metabolites in CYP 2D6-, CYP 2C19- and CYP 3A4/5-phenotyped patients. Ther Drug Monit. 2000;22:209–214. doi: 10.1097/00007691-200004000-00012. [DOI] [PubMed] [Google Scholar]
- 120.Troy SM., Parker VD., FruncïIIo RJ., Chiang ST. The pharmacokinetics of venlafaxine when given in a twice-daily regimen. J Clin Pharmacol. 1995;35:404–409. doi: 10.1002/j.1552-4604.1995.tb04081.x. [DOI] [PubMed] [Google Scholar]
- 121.Reis M., Lundmark J., Bjork H., Bengtsson F. Therapeutic drug monitoring of racemic venlafaxine and its main metabolites in an everyday clinical setting. Ther Drug Monit. 2002;24:545–553. doi: 10.1097/00007691-200208000-00014. [DOI] [PubMed] [Google Scholar]
- 122.Müller-Oerlinghausen B., Rüther E. Clinical profile and serum concentration of viloxazine as compared to amitriptyline. Pharmakopsychiatr Neuropsychopharmakol. 1979;12:321–337. doi: 10.1055/s-0028-1094627. [DOI] [PubMed] [Google Scholar]
- 123.Pedersen OL., Gram LF., Kristensen CB., et al. Overdosage of antidepressants: clinical and pharmacokinetic aspects. Eur J Clin Pharmacol. 1982;23:513–521. doi: 10.1007/BF00637499. [DOI] [PubMed] [Google Scholar]
- 124.Preskorn S., Jerkovich GS. Central nervous system toxicity of tricyclic antidepressants: phenomenology, course, risk factors, and role of therapeutic drug monitoring. J Clin Psychopharmacol. 1990;10:88–95. doi: 10.1097/00004714-199004000-00003. [DOI] [PubMed] [Google Scholar]
- 125.Bjerkenstedt L., Flyckt L., Overø KF., Lingjaerde O. Relationship between clinical effects, serum drug concentration and serotonin uptake inhibition in depressed patients treated with citalopram. A double-blind comparison of three dose levels. Eur J Clin Pharmacol. 1985;28:553–557. doi: 10.1007/BF00544066. [DOI] [PubMed] [Google Scholar]
- 126.Leinonen E., Lepola U., Koponen H., Kinnunen I. The effect of age and concomitant treatment with other psychoactive drugs on serum concentrations of citalopram measured with a nonenantioselective method. Ther Drug Monit. 1996;18:111–117. doi: 10.1097/00007691-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 127.Jonasson B., Saldeen T. Citalopram in fatal poisoning cases. Forensic Sci int. 2002;126:1–6. doi: 10.1016/s0379-0738(01)00632-6. [DOI] [PubMed] [Google Scholar]
- 128.Gex-Fabry M., Balant-Gorgia AE., Balant LP. Clomipramine concentration as a predictor of delayed response: a naturalistic study. Eur J Clin Pharmacol. 1999;54:895–902. doi: 10.1007/s002280050572. [DOI] [PubMed] [Google Scholar]
- 129.Mavissakalian MR., Jones B., Olson S., Perel JM. Clomipramine in obsessive-compulsive disorder: clinical response and plasma levels. J Clin Psychopharmacol. 1990;10:261–268. [PubMed] [Google Scholar]
- 130.Mclntyre IM., King CV., Cordner SM., Drummer OH. Postmortem clomipramine: therapeutic or toxic concentrations? J Forensic Sci. 1994;39:486–493. [PubMed] [Google Scholar]
- 131.Rodriguez de la Torre B., Dreher J., Malevany l., et al. Serum levels and cardiovascular effects of tricyclic antidepressants and selective serotonin reuptake inhibitors in depressed patients. Ther Drug Monit. 2001;23:435–440. doi: 10.1097/00007691-200108000-00019. [DOI] [PubMed] [Google Scholar]
- 132.Preskorn SH., Fast GA. Tricyclic antidepressant-induced seizures and plasma drug concentration. J Clin Psychiatry. 1992;53:160–162. [PubMed] [Google Scholar]
- 133.Goodnick PJ. Pharmacokinetic optimisation of therapy with newer antidepressants. Clin Pharmacokinet. 1994;27:307–330. doi: 10.2165/00003088-199427040-00005. [DOI] [PubMed] [Google Scholar]
- 134.Montgomery SA., Montgomery DB., McAuley R., Rani SJ. Mianserin plasma levels and differential clinical response in endogenous and reactive depression. Acta Psychiatr Beg. 1978;78:798–812. [PubMed] [Google Scholar]
- 135.Isacsson G., Holmgren P., Druid H., Bergman U. The utilization of antidepressants―a key issue in the prevention of suicide: an analysis of 5281 suicides in Sweden during the period 1992-1994. Acta Psychiatr Scand. 1997;96:94–100. doi: 10.1111/j.1600-0447.1997.tb09912.x. [DOI] [PubMed] [Google Scholar]
- 136.Tïmmer CJ., Sitsen JMA., Delbressine LP. Clinical pharmacokinetics of mirtazapine. Clin Pharmacokinet. 2000;38:461–474. doi: 10.2165/00003088-200038060-00001. [DOI] [PubMed] [Google Scholar]
- 137.Velazquez C., Carlson A., Stokes KA., Leikin JB. Relative safety of mirtazapine overdose. Vet Hum Toxicol. 2001;43:342–344. [PubMed] [Google Scholar]
- 138.Fritze J., Laux G., Sofic E., et al. Plasma moclobemide and metabolites: lack of correlation with clinical response and biogenic amines. Psychopharmacology. 1989;99:252–256. doi: 10.1007/BF00442818. [DOI] [PubMed] [Google Scholar]
- 139.Gex-Fabry M., Balant-Gorgia AE., Balant LP. Potential of concentration monitoring data for a short half-life drug: analysis of pharmacokinetic variability for moclobemide. Ther Drug Monit. 1995;17:39–46. doi: 10.1097/00007691-199502000-00007. [DOI] [PubMed] [Google Scholar]
- 140.Baptista T., Teneud L., Contreras Q., et al. Lithium and body weight gain. Pharmacopsychiatry. 1995;28:35–44. doi: 10.1055/s-2007-979586. [DOI] [PubMed] [Google Scholar]
- 141.Åsberg M., Cronholm B., Sjöqvist F., Tuck D. Correlation of subjective side effects with plasma concentrations of nortriptyline. BMJ. 1970;4:18–21. doi: 10.1136/bmj.4.5726.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tasker TCG., Kaye CM., Zussman BD., Link CGG. Paroxetine plasma levels: lack of correlation with efficacy or adverse events. Acta Psychiatr Scand. 1989;80(suppI350):152–155. doi: 10.1111/j.1600-0447.1989.tb07201.x. [DOI] [PubMed] [Google Scholar]
- 143.Ohman D., Norlander B., Peterson C., Bengtsson F. Bioanalysis of racemic reboxetine and its desethylated metabolite in a therapeutic drug monitoring setting using solid phase extraction and HPLC. Ther Drug Monit. 2001;23:27–34. doi: 10.1097/00007691-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 144.Milner DA., Hall M., Davis GG., Brissie RM., Robinson CA. Fatal multiple drug intoxication following acute sertraline use. J Anal Toxicol. 1998;22:545–548. doi: 10.1093/jat/22.6.545. [DOI] [PubMed] [Google Scholar]
- 145.Burke MJ., Preskorn SH. Therapeutic drug monitoring of antidepressants―cost implications and relevance to clinical practice. Clin Pharmacokinet. 1999;37:147–165. doi: 10.2165/00003088-199937020-00004. [DOI] [PubMed] [Google Scholar]
- 146.Iwersen S., Schmoldt A. One fatal and one nonfatal intoxication with tranylcypromine. Absence of amphetamines as metabolites. J Anal Toxicol. 1996;20:301–304. doi: 10.1093/jat/20.5.301. [DOI] [PubMed] [Google Scholar]
- 147.Monteleone P., Gnocchi G., Delrio G. Plasma trazodone concentrations and clinical response in elderly depressed patients: a preliminary study. J Clin Psychopharmacol. 1989;9:284–287. [PubMed] [Google Scholar]
- 148.Goeringer KE., Raymon L., Christian GD., Logan BK. Postmortem forensic toxicology of selective serotonin reuptake inhibitors: a review of pharmacology and report of 168 cases. J Forensic Sci. 2000;45:633–648. [PubMed] [Google Scholar]
- 149.Veefkind AH., Haffmans PMJ., Hoencamp E. Venlafaxine serum levels and CYP 2D6 genotype. Ther Drug Monit. 2000;22:202–208. doi: 10.1097/00007691-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 150.Levine B., Jenkins AJ., Queen M., Jufer R., Smialek JE. Distribution of venlafaxine in three postmortem cases. J Anal Toxicol. 1996;20:502–505. doi: 10.1093/jat/20.6.502. [DOI] [PubMed] [Google Scholar]
- 151.Norman TR., Burrows GD., Davies BM., Maguire KP., Wurm JM. Viloxazine plasma concentrations and clinical response. J Affect Disord. 1980;2:157–164. doi: 10.1016/0165-0327(80)90002-6. [DOI] [PubMed] [Google Scholar]
- 152.Altamura AC., Mauri MC., Guercetti G. Age, therapeutic “milieu” and clinical outcome in depressive patients treated with viloxazine: a study with plasma levels. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10:67–75. doi: 10.1016/0278-5846(86)90045-x. [DOI] [PubMed] [Google Scholar]
- 153.Falcy M., Riboulet-Delmas G., Efthymiou ML. [Acute poisoning by viloxazine chlorhydrate taken by itself]. Encéphale. 1983;9:137–144. [PubMed] [Google Scholar]
- 154.McLeod DR., Hoehn-Saric R., Porges SW., Kowalski PA., Clark CM. Therapeutic effects of imipramine are counteracted by its metabolite, desipramine, in patients with generalized anxiety disorder. J Clin Psychopharmacol. 2000;20:615–621. doi: 10.1097/00004714-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 155.Baumann P. Pharmacokinetic-pharmacodynamic relationship of the selective serotonin reuptake inhibitors. Clin Pharmacokinet. 1996;31:444–469. doi: 10.2165/00003088-199631060-00004. [DOI] [PubMed] [Google Scholar]
- 156.Rasmussen BB., Bresen K. Is therapeutic drug monitoring a case for optimizing clinical outcome and avoiding interactions of the selective serotonin reuptake inhibitors? Ther Drug Monit. 2000;22:143–154. doi: 10.1097/00007691-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 157.Baumann P., Kahn JM. Les médicaments génériques: quels sont les problèmes et d'où viennent-ils? Med Hyg. 2003;61:879–884. [Google Scholar]
- 158.Sajbel TA., Carter GW., Wiley RB. Converting patients from brand-name clozapine to generic clozapine. Ann Pharmacother. 2001;35:281–284. doi: 10.1345/aph.10183. [DOI] [PubMed] [Google Scholar]
- 159.Meredith PA. Generic drugs―therapeutic equivalence. Drug Safety. 1996;15:233–242. doi: 10.2165/00002018-199615040-00001. [DOI] [PubMed] [Google Scholar]
- 160.Tse G., Thompson D., Procyshyn RM. Generic clozapine: a cost-saving alternative to brand name clozapine? PharmacoEconomics. 2003;21:1–11. doi: 10.2165/00019053-200321010-00001. [DOI] [PubMed] [Google Scholar]
- 161.American Academy of Pediatrics. Use of psychoactive medication during pregnancy and possible effects on the fetus and newborn. Pediatrics. 2000;105:880–887. doi: 10.1542/peds.105.4.880. [DOI] [PubMed] [Google Scholar]
- 162.Burt VK., Suri R., Altshuler L., Stowe Z., Hendrick VC., Muntean E. The use of psychotropic medications during breast-feeding. Am J Psychiatry. 2001;158:1001–1009. doi: 10.1176/appi.ajp.158.7.1001. [DOI] [PubMed] [Google Scholar]
- 163.Chaudron LH., Jefferson JW. Mood stabilizers during breastfeeding. A review. J Clin Psychiatry. 2001;61:79–90. doi: 10.4088/jcp.v61n0202. [DOI] [PubMed] [Google Scholar]
- 164.Llewellyn A., Stowe ZN. Psychotropic medications in lactation. J Clin Psychiatry. 1998;59(suppl 2):41–52. [PubMed] [Google Scholar]
- 165.Ernst CL., Goldberg JF. The reproductive safety profile of mood stabilizers, atypical antipsychotics, and broad-spectrum psychotropics. J Clin Psychiatry. 2002;63(suppl 4):42–55. [PubMed] [Google Scholar]
- 166.European Agency for the Evaluation of Medicinal Products (EMEA). Note for guidance on dose response information to support drug registration. 1995. Available at: www.emea.eu.int/pdfs/human/ich/037895en.pdf. XX. 2005 Jun Accessed 16;XX:XX–XX. [Google Scholar]
- 167.Bertilsson L., Dahl ML. Polymorphic drug oxidationXX.relevance to the treatment of psychiatric disorders. CNS Drugs. 1996;5:200–223. [Google Scholar]
- 168.Ensom MHH., Chang TKH., Patel P. Pharmacogenetics―the therapeutic drug monitoring of the future? Clin Pharmacokinet. 2001;40:783–802. doi: 10.2165/00003088-200140110-00001. [DOI] [PubMed] [Google Scholar]
- 169.Vuille F., Amey M., Baumann P. Use of plasma level monitoring of antidepressants in clinical practice―towards an analysis of clinical utility. Pharmacopsychiatry. 1991;24:190–195. doi: 10.1055/s-2007-1014468. [DOI] [PubMed] [Google Scholar]
- 170.Koutsilieri E., Scheller C., ter Meulen V., Riederer P. Monoamine oxidase inhibition and CNS immunodeficiency infection. Neurotoxicology. 2004;25:267–270. doi: 10.1016/S0161-813X(03)00105-0. [DOI] [PubMed] [Google Scholar]
- 171.Brockmoller J., Kirchheiner J., Schmider J., et al. The impact of the CYP 2D6 polymorphism on haloperidol pharmacokinetics and on the outcome of haloperidol treatment. Clin Pharmacol Ther. 2002;72:438–452. doi: 10.1067/mcp.2002.127494. [DOI] [PubMed] [Google Scholar]
- 172.Bengtsson F. Therapeutic drug monitoring of psychotropic drugs TDM “nouveau.”. Ther Drug Monit. 2004;26:145–151. doi: 10.1097/00007691-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 173.Bondy B., Zill P. Psychopharmacogenetics―a challenge for pharmacotherapy in psychiatry. World J Biol Psychiatry. 2001;2:178–183. doi: 10.3109/15622970109026806. [DOI] [PubMed] [Google Scholar]