Abstract
In contrast to the conventional view of dopamine involvement in schizophrenia, which posits hyperactive dopaminergic transmission, we propose that for unknown developmental andfor biochemical reasons, a primary defect occurs in efficient, tight dopaminergic synaptic transmission, triggering feedback activation and receptor upregulation, and resulting in the well-characterized increase in dopaminergic tone. This hypothesis is driven by suggestive evidence for subpopulations of dopamine D2 receptors delivering contrasting forms of dopaminergic transmission: synaptic receptors, responsible for basic dopaminergic function and subject to effective feedback control, and poorly controlled extrasynaptic receptors partly responsible for the positive symptoms of psychosis. Since the primary defect is dopamine deficiency, we term this theory the dopaminergic deficit hypothesis of schizophrenia, it is currently informing clinical studies with novel partial dopamine antagonists (dopamine stabilizers) such as ACR16, which preferentially target extrasynaptic receptors while leaving synaptic transmission and basic dopamine function intact.
Keywords: schizophrenia, dopamine
Abstract
Contrariamente a la tesis convencional de la participación de la dopamina en la esquizofrenia, que propone una transmisión hiperactiva por los receptores de dopamina D2, nosotros creemos que, por razones desconocidas con el desarrollo o la bioquímica, se produce un defecto primario de la transmisión sináptica dopaminérgica eficiente y rigurosa que pone en marcha una activación por retroacción y una regulación al alza de los receptores, cuyo resultado es el aumento bien conocido del tono dopaminérgico. Esta hipótesis se basa en la presencia de subpoblaciones de receptores dopaminérgicos D2 que transmiten la dopamina de forma opuesta: los receptores sinápticos se encargan de la función dopaminérgica básica y están sujetos a un control eficaz por retroacción y los receptores extrasinápticos, mal controlados, son parcialmente responsables de los síntomas positivos de la psicosis. Como el defecto primario consiste en una carencia de dopamina, hemos bautizado esta teoría como la hipótesis del déficit dopaminérgico en la esquizofrenia. En estos momentos se realizan estudios clínicos con nuevos antagonistas partiales de la dopamina o estabilizadores dopamínicos, como ACR16, que se dirigen preferentemente a los receptores extrasinápticos y dejan intacta la transmisión sináptica y la función dopamínica básica.
Abstract
Contrairement aux idées conventionnelles d'intervention dopaminergique dans la schizophrénie, qui postule pour une transmission dopaminergique hyperactive, nous posons l'hypothèse qu'une anomalie initiale de la transmission synaptique dopaminergique efficace et régulière, pour des raisons biochimiques ou de développement inconnues, déclenche une rétroactivation et une régulation positive du récepteur et résulte d'une augmentation caractérisée du tonus dopaminergique. Cette hypothèse de transmission dopaminergique sous des formes variées par des sous-populations de récepteurs dopaminergiques D2 est soutenue par l'existence de récepteurs synaptiques responsables de la fonction dopaminergique de base et sujets à un rétroconirôle efficace, et de récepteurs exirasynaptiques mal contrôlés responsables en partie des symptômes de la psychose. Puisque l'anomalie initiale est un déficit en dopamine, cette théorie est appelée « hypothèse du déficit dopaminergique de la schizophrénie ». Elle participe aux études cliniques actuelles sur les nouveaux antagonistes partiels de la dopamine, ou sur les stabilisants de la dopamine, tel que l'ACR16, qui ciblent préférentiellement les récepteurs exirasynaptiques tout en laissant intactes la transmission synaptique et la fonction dopaminergique de base.
Several neurotransmitters interact in the pathogenesis of schizophrenia. The first, to be implicated, in 1956, was serotonin. This followed the discovery, in Bernard Brodie's Laboratory of Chemical Pharmacology at the National Heart. Institute, that reserpine depleted the body's stores of serotonin, including in the brain.1 A little later our own group found that reserpine had the same effect, on noradrenaline. This led us to dopamine. Eventually we understood that the effect, of reserpine could actually be accounted for in terms of dopamine. Rabbits treated with reserpine display catalcpsy-the maintenance of even abnormal body posture. Injection of the dopamine precursor, dopa, had a dramatic effect on both motor performance and wakefulness, proving beyond doubt that, dopamine was the main neurotransmitter involved.2-4
Early phase: dopamine agonists
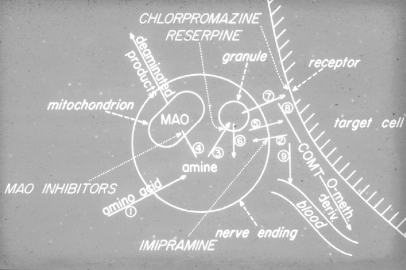
Reserpine acts by blocking neurotransmitter uptake into monoaminergic nerve terminal storage sites (Figure 1). A few years after this discovery, we discovered that chlorpromazine did not act on these stores but, on postsynaptic cell receptors-not only dopamine receptors (although it was here that the effect, was most, striking), but, also noradrenaline and serotonin receptors.6 Subsequent research in many different, laboratories turned increasingly to dopamine as the most important neurotransmitter mediating the effect, of chlorpromazine, haloperidol, and similar drugs. This led to the development of selective compounds acting on dopamine receptors. However, these agents did not have the dramatic increase in clinical effect which might, have been expected.
Figure 1. Cross-section through a monoaminergic nerve terminal.5 COMT, catechol-O-methyl transferase; MAO, monoamine oxidase. Reproduced from reference 5: Carlsson A. Physiological and pharmacological release of monoamines in the central nervous system. In: von Euler US, Rosell S, Uvnäs B, eds. Mechanisms of Release of Biogenic Amines. Oxford, UK: Pergamon Press; 1966;331-346. Copyright © Pergamon Press 1966.
Atypical antipsychotics
The discovery of the dibenzodiazepinc clozapine led to the identification of the atypical antipsychotics, which are mixed antagonists of all three receptors (dopamine, serotonin, and noradrenaline). Their advantage was that they displayed antipsychotic activity with fewer or no extrapyramidal side effects, which was quite a novelty at the time. It became well accepted that adding antagonists acting on these other neurotransmitters improved the therapeutic profile, by decreasing extrapyramidal side effects.
When considering benzamides, we faced a rather confusing situation. Benzamides as a family displayed high selectivity for dopamine D2 and D3 receptors, yet at, least two of them, amisulpride and remoxipride, were atypical. How could a dopamine receptor-selective compound be atypical? The answer could not lie in the benzamide structure, since a modification of the benzamide molecule yielded raclopride, a typical neuroleptic. A tentative explanation was that the D2 receptor population was heterogeneous, despite being one and the same molecule. How could that, come about?
D2 receptor heterogeneity
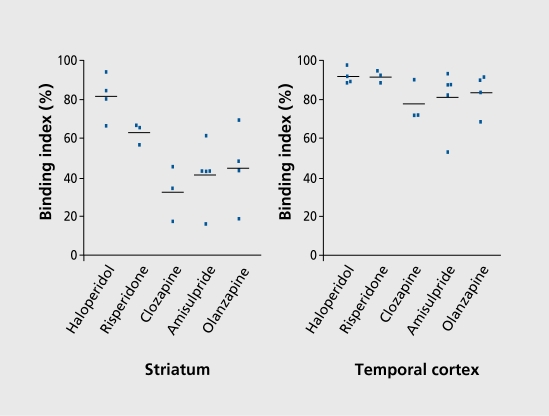
D2 receptor heterogeneity was confirmed using positron emission tomography (PET) in patients who first received conventional therapy for optimal antipsychotic effect, and were then given a highly selective Dopamine D2 antagonist, as a labeled ligand.8 Binding indices were determined in striatum and temporal cortex (Figure 2). The resulting profiles differed markedly. In striatum, the profile was as expected: high haloperidol, and low clozapine. In temporal cortex, all profiles were high and bunched. The striatal pattern could be viewed as predicting extrapyramidal side effects, and the temporal cortex pattern as predicting antipsychotic effects.
Figure 2. Binding index in striatum (left panel) and temporal cortex (right panel) of patients treated with typical and atypical antipsychotics.8 Reproduced from reference 8: Xiberas X, Martinot JL, Mallet L, et al. Extrastriatal and striatal D(2) dopamine receptor blockade with haloperidol or new antipsychotic drugs in patients with schizophrenia. Br J Psychiatry. 2001;179:503-508. Copyright © Royal College of Psychiatrists 2001.
This study is not unique. A similar study used a different ligand and a different, technique, but. had the same outcome,9 confirming the suspicion that dopamine D2 receptor populations in striatum and temporal cortex are not identical. There is more than one receptor subtype or subpopulation. The speculation is that, in striatum the population is dominated by synaptically located receptors whereas in temporal cortex the dominant, receptor subtype is extrasynaptic.
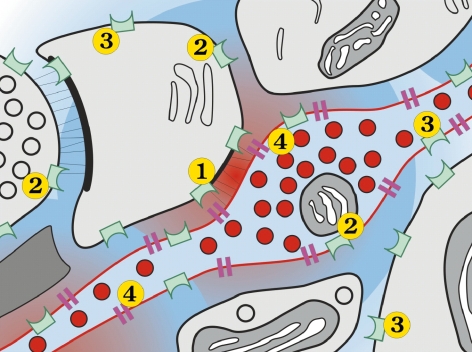
Dopamine D2 receptors are located at dopaminergic synapses, as well as extrasynaptically (Figure 3). Dopamine concentrations are proportional to their proximity to the synapse, highest, being closest. Some receptors-the autore ceptors, which have long been recognized-are extrasy naptic and located on the dopaminergic neuron itself. All have a tremendous capacity for up and downregulation according to the variations in dopamine concentration at. the different, sites. Extrasynaptic receptors are known to be much more responsive than postsynaptic receptors. Thus, receptor heterogeneity is reflected by synaptic versus extrasynaptic receptors. Yet despite the dichotomy, a form of continuity prevails.
Figure 3. Electron micrograph of a dopaminergic nerve terminal. (1) Synaptic receptors; (2) and (3) extrasynaptic receptors; (4) dopamine transporter. Red: highest dopamine concentration (near synapse).10 Reproduced from reference 10: Sesack SR. Synaptology of dopamine neurons. In: Di Chiara G, ed. Dopamine in the CNS. Vol 1. Berlin: Springer-Verlag; 2002:63-120. Copyright © Springer-Verlag 2002.
Partial dopamine agonists
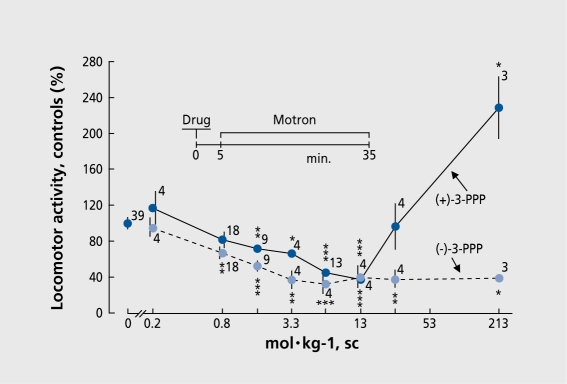
In the 1980s we became interested in (-)-3-(3-hydroxyphcnyl)-N-n-propylpipcridine (3-PPP, prcclamol) which has an asymmetric carbon: (+)-3-PPP behaves as a full agonist, displaying a biphasic curve, first inhibiting then stimulating animal locomotor activity, much like apomorphinc (Figure 4). This is due at least in part to the greater sensitivity of autoreceptors: when autoreceptors are stimulated, the dopaminergic system is inhibited by negative feedback. As the dose increases, postsynaptic receptors take over and the system becomes stimulated. The (-)enantiomer, on the other hand, is only a partial agonist, meaning that it. has an agonist effect on the highly responsive autoreceptors but an antagonistic effect on postsynaptic receptors (although achieving some stimulation in the absence of dopamine). This intrinsic activity explains why 3-PPP exhibits not, only antipsychotic12 but, also antiparkinsonian properties.13
Figure 4. Effect of (+)- and (-)-3-(3-hydroxyphenyl)-N-n-propylpiperidine (3-PPP) on rat locomotor activity, sc, subcutaneous.
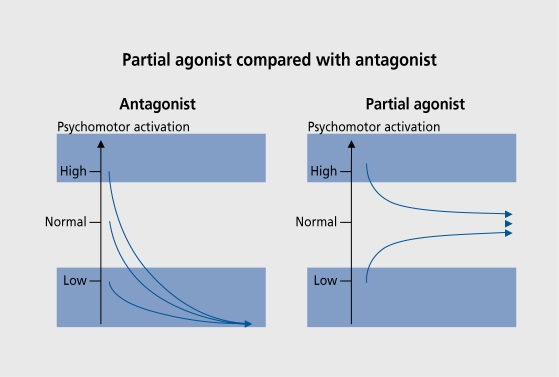
Figure 5 illustrates the differences between the effect, of antagonists and partial agonists on motor activation. A partial agonist will act, as an inhibitor in the presence of high dopamine activity and as a stimulant in the presence of ver}' low dopamine activity. The two activities meet at a level which represents the drug's intrinsic activity. A partial agonist can thus be described as a “dopamine stabilizer.” In contrast, a pure antagonist, will inhibit to zero irrespective of the initial activity level, leading to catalepsy. A partial agonist, can thus act as an antipsychotic if it, has sufficiently low intrinsic activity. In schizophrenics (-)-3-PPP was an active antipsychotic but became ineffective after more than 1 week of treatment.13 The suggested explanation was that, its intrinsic activity was too high, making the autoreceptors subsensitive. It, was therefore predicted that agents with lower intrinsic activity would have sustained antipsychotic activity. This proved to be precisely the case with aripiprazole, which was shown to be an effective antipsychotic with almost no extrapyramidal side effects. It also induced a slight but, significant decrease in prolactin. This is the profile expected of a partial dopamine receptor agonist.
Figure 5. Effect on psychomotor activation of partial agonists (right panel) versus pure antagonists (left panel).11 Reproduced from reference 11: Hjorth S, Carlsson A, Clark D, et al. Central dopamine receptor agonist and antagonist actions of the enantiomers of 3-PPP. Psychopharmacology. 1983;81:89-99. Copyright © Springer-Verlag 1983.
Dopamine stabilizers (or partial antagonists)
Surprisingly, we found dopamine stabilizers that were similar to partial agonists, yet which proved to have zero intrinsic activity when tested on dopamine receptors in vivo (for review, see ref 14). Their zero intrinsic activity was particularly puzzling. One such compound, the substituted phenylpiperidine (-)-OSU6162,has a somewhat, similar pattern of psychomotor activation to the partial agonist (-)-3-PPP (Figure 5). It, is also quite similar in structure. Yet it is not, a partial agonist. This and a related compound, ACR16, bind to the receptors but, show low affinity for dopamine D2 receptors in vitro.
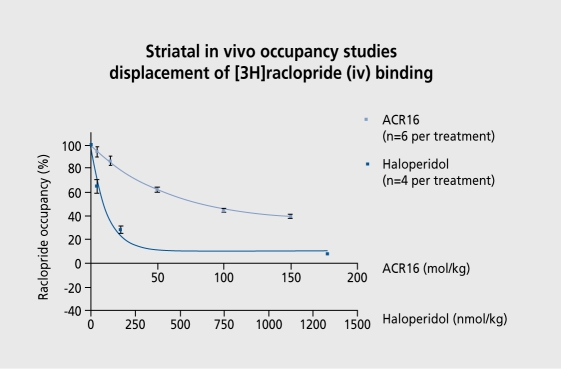
The effect of ACR16 was compared with that, of haloperidol on in vivo displacement in rats of raclopride, a dopamine D2 receptor antagonist, (Figure 6). The doseresponse curve with ACR16 was much shallower, and it was impossible to determine when it. would have reached zero. This points to a subpopulation of dopamine D2 receptors that is available to haloperidol but less avail ablc-and perhaps not available at all-to dopamine stabilizers. These compounds are dopamine receptor antagonists, able to displace at dopamine receptors, but. not to the same extent as haloperidol.
Figure 6. Striatal in vivo occupancy studies: displacement of 3Hradopride binding by ACR16 and haloperidol. (Reprinted with the permission of M. Rigby, Merck Pharmaceuticals, West Dayton, Middlesex, UK).
We suggest, that it is the extrasynaptic subpopulation of dopamine receptors that is available to these compounds, and that the synaptic subpopulation is less readily available. Insofar as synaptic function is responsible for basic dopamine activity, stabilizers have an insufficient impact on the dopamine system to produce extrapyramidal side effects and the cognitive repercussions of hypodopaminergia.The receptors that gear up dopamine function to an extent sufficient, to produce psychosis are proposed to be predominantly extrasynaptic. Because partial dopamine antagonists can reach extrasynaptic receptors, including the autoreceptors but, not synaptic receptors, they can exert antipsychotic activity while simultaneously protecting the synapse. In summary, the hypothetical mechanism of action of dopamine stabilizers or partial antagonists in psychosis is that they preferentially inhibit extrasynaptic dopaminergic transmission while leaving synaptic transmission and basic dopamine function essentially intact.
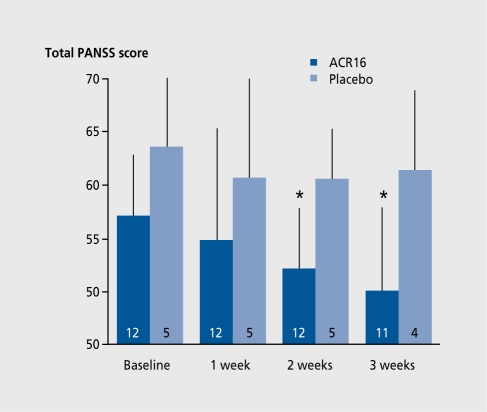
Clinical deployment, of these compounds remains largely experimental. Both have displayed documented antipsychotic activity and have been studied to similar degrees in small clinical groups. Short-term studies have shown (-)-OSU6162 to be an effective antipsychotic and antidyskinetic. Long-term studies remain to be performed. ACR16 has been found to be safe in phase I studies in healthy volunteers and has shown promising results in early phase II studies in patients with schizophrenia, Parkinson's disease, and Huntington's disease. In schizophrenic patients, add-on ACR16 significantly decreased Positive and Negative Syndrome Scale (PANSS) ratings after 2 weeks versus no effect with placebo (Figure 7). Dyskinesia was significantly reduced with both compounds. In advanced Parkinson's disease, (-)-OSU6162 decreased dyskinesia without, impairing voluntary movement (which actually improved). A similar result, was obtained with ACR16. (See ref 14 for references of preliminary reports).
Figure 7. ACR16 add-on trial in schizophrenia. Valid rating total Positive and Negative Syndrome Scale (PNSS) scores (means and SD). The bars show the number of patients. (Data from a Swedish multicenter study under the direction of Professor Leif Lindström, Psychiatric Research Unit, Västerås Hospital, Västerås, Sweden).
Our hypothesis is that, dopaminergic receptor heterogeneity accounts for the mechanism of antipsychotic action:
Typical antipsychotics inhibit both extrasynaptic and synaptic transmission, thereby exerting antipsychotic activity, but. also worsening primary negative symptoms and cognitive dysfunction, not to mention the extrapyramidal syndrome.
Dopamine stabilizers inhibit, extrasynaptic transmission, but actually bolster synaptic transmission by negative feedback, leading to antipsychotic activity and improvement in primary negative symptoms and cognition.
Atypical antipsychotics, eg, clozapine, occupy an intermediate position, with a profile that can be explained possibly not only in terms of serotonin or noradrenaline receptors, but, still in terms of dopamine receptors.
A dopaminergic deficit hypothesis of schizophrenia
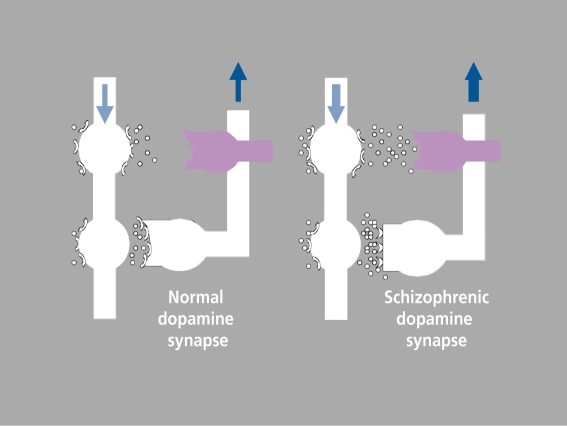
The concept of extrasynaptic versus synaptic dopaminergic transmission provides the basis for turning the previous hypothesis of dopamine involvement in schizophrenia on its head, into a dopaminergic deficit hypothesis. We suggest that, for unelucidated developmental and/or biochemical reason(s), dopaminergic synapses are defective in schizophrenia, leading to feedback activation and the resulting observed increase in dopaminergic tone (Figure 8). Hypertonicity spills over onto extrasynaptic transmission, to compensate for the deficiency in synaptic transmission (which could, if not remedied, produce negative symptoms). We propose that it, is this compensatory, poorly controlled increase in extrasynaptic transmission that is partly responsible for positive psychotic symptoms, since feedback is only effective and well-controlled where synaptic transmission is concerned. As for negative symptoms and cognitive deficit, we believe that, these result, from poor compensation for the synaptic defect.
Figure 8. Updated dopaminergic deficit hypothesis: dopamine synapse in normal subjects (left) and schizophrenics (right), showing synaptic (white) and extrasynaptic (violet) transmission and feedback loop.15 Reproduced from reference 15: Carlsson A, Carlsson ML. Dopaminergic stabilisers. Adv Schizophrenia and Clin Psychiatry. 2005;1:118-128. Copyright © Remedica 2005.
This hypothesis would not, replace, but, would rather add to, the already existing hypothesis for the pathophysiology of schizophrenia, given the probable heterogeneity of this disorder.
Contributor Information
Arvid Carlsson, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
Maria L. Carlsson, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
REFERENCES
- 1.Pletscher A., Shore PA., Brodie BB. Serotonin release as a possible mechanism of reserpine action. Science. 1955;122:374–375. doi: 10.1126/science.122.3165.374. [DOI] [PubMed] [Google Scholar]
- 2.Carlsson A., Lindqvist M., Magnusson T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature (Lond). 1957;180:1200. doi: 10.1038/1801200a0. [DOI] [PubMed] [Google Scholar]
- 3.Carlsson A. The occurrence, distribution and physiological role of catecholamines in the nervous system. Pharmacol Rev. 1959;11:490–493. [PubMed] [Google Scholar]
- 4.Carlsson A. On the occurrence, distribution, and physiological role of catecholamines in the nervous system. Pharmacol Rev. 1959;11:490–493. [PubMed] [Google Scholar]
- 5.Carlsson A. Physiological and pharmacological release of monoamines in the central nervous system. In: von Euler US, Rosell S, Uvnäs B, eds. Mechanisms of Release of Biogenic Amines. Oxford, UK: Pergamon Press. 1966:331–346. [Google Scholar]
- 6.Carlsson A., Lindqvist M. Effect of chlorpromazine or haloperidol on the formation of 3-methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol. 1963;20:140–144. doi: 10.1111/j.1600-0773.1963.tb01730.x. [DOI] [PubMed] [Google Scholar]
- 7.Lecrubier Y. Amisulpride as a modehclinical effects of a pure dopaminergic agent. In: Kapur S, Lecrubier Y, eds. Dopamine in the Pathophysiology and Treatment of Schizophrenia. London, UK: Martin Dunitz. 2003:69–92. [Google Scholar]
- 8.Xiberas X., Martinot JL., Mallet L., et al. Extrastriatal and striatal D(2) dopamine receptor blockade with haloperidol or new antipsychotic drugs in patients with schizophrenia. Br J Psychiatry. 2001;179:503–508. doi: 10.1192/bjp.179.6.503. [DOI] [PubMed] [Google Scholar]
- 9.Bigliani V., Mulligan RS., Acton PD., et al. Striatal and temporal cortical D2/D3 receptor occupancy by olanzapine and sertindole in vivo. Psychopharmacology. 2000;150:132–140. doi: 10.1007/s002130000435. [DOI] [PubMed] [Google Scholar]
- 10.Sesack SR. Synaptology of dopamine neurons. In: Di Chiara G, ed. Dopamine in the CMS. Vol 1. Berlin, Germany: Springer. 2002:63–120. [Google Scholar]
- 11.Hjorth S., Carlsson A., Clark D., et al. Central dopamine receptor agonist and antagonist actions of the enantiomers of 3-PPP. Psychopharmacology. 1983;81:89–99. doi: 10.1007/BF00428999. [DOI] [PubMed] [Google Scholar]
- 12.Lahti AC., Weiler MA., Corey PK., Lahti RA., Carlsson A., Tamminga A. Antipsychotic properteis of the partial dopamine receptor agonist (3)-(3hydroxyphenyl)-N-n-propylpiperidine (preclamol). Biol Psychiatry. 1998;43:2–11. doi: 10.1016/s0006-3223(97)00030-9. [DOI] [PubMed] [Google Scholar]
- 13.Pirtosek Z., Merello M., Carlsson A., Stern G. Preclamol and parkinsonian fluctuations. Clin Neuropharmacol. 1993;16:550–554. [PubMed] [Google Scholar]
- 14.Carlsson ML., Carlsson A., Nilsson M. Schizophrenia. From dopamine to glutamate and back. Curr Medicinal Chem. 2004;11:267–277. doi: 10.2174/0929867043456034. [DOI] [PubMed] [Google Scholar]
- 15.Carlsson A., Carlsson ML. Dopaminergic stabilisers. Adv Schizophrenia and Clin Psychiatry. 2005;1:118–128. [Google Scholar]