Abstract
The presence of neurological signs and disturbed psychomotor performance have been consistently confirmed by clinical studies in schizophrenic patients. These parameters are mainly assessed by using clinical rating scales. In recent years, new approaches such as ultrasonic movement analysis systems have been introduced in order to objectively evaluate motor disturbances in schizophrenic patients. Ultrasonic movement analysis systems calculate the three-dimensional positions of tiny markers, which are attached to moving body parts, with high spatial and temporal resolution. Thus, key parameters of gait and hand movements can be determined precisely. This article summarizes and discusses several studies using these new methods. Results indicate that schizophrenia causes a specific motor deficit pattern, with a predominant disturbance of spatial parameters. Conventional antipsychotic treatment usually worsens these deficits, whereas the effects of atypical antipsychotic treatments are less pronounced. Disturbed motor performance can be normalized by external sensory stimuli, but only when no major attentional processes are required, and it can be enhanced by an attentional strategy, but not to the extent that motor parameters are normalized.
Keywords: schizophrenia, motor disturbance, antipsychotic treatment, psychomotor disturbance, quantitative movement analysis
Abstract
Los estudios clínicos en pacientes esquizofrénicos han confirmado sólidamente la presencia de signos neurológicos y alteraciones en el rendimiento psicomotor. Estos parámetros se estudian utílizando principalmente escalas de evaluación clínica. Recientemente se ban introducido nuevas aproximaciones como los sistemas de análisis ultrasónico del movimiento con la intención de evaluar objetivamente las alteraciones motoras en los pacientes esquizofrénicos. Los sistemas de análisis ultrasónico del movimiento calculan las posiciones en tre dimensiones de diminutos sensores, los cuales están adosados a partes del cuerpo que se mueven, con una alta resolución espacial y temporal. De este modo se pueden determinar con precisión parámetros clave de la marcha y movimientos de la mano. Este artículo resume y discute algunos estudios que utilizan esta nueva metodología. Los resultados indican que la esquizofrenia genera un patrón específico de déficit motor, con una alteración predominante de los parámetros espaciales. Habitualmente el tratamiento antipsicótico convencional empeora estos déficits, mientras que los efectos de los tratamientos con antipsicóticos atípicos son menos pronunciados. La alteración del rendimiento motor puede ser normalizada por estímulos sensoriales externos, sólo cuando no se requieren importantes procesos de atención, y puede ser reforzada por una estrategia de atención, pero no en la cuantía en que son normalizados los parámetros motores.
Abstract
Des études cliniques chez des patients schizophrènes ont régulièrement confirmé la présence de signes neurologiques et un fonctionnement psychomoteur perturbé. Ces paramètres sont principalement évalués par des échelles d'évaluation cliniques. Ces dernières années, de nouvelles approches comme les systèmes d'analyse de mouvement par ultrasons ont été introduites afin d'évaluer objectivement les troubles moteurs chez les patients schizophrènes. Les systèmes d'analyse de mouvement par ultrasons calculent les positions en trois dimensions de minuscules marqueurs attachés aux parties mobiles du corps, avec une haute résolution spatiale et temporelle. Les paramètres clés des mouvements de la main et de la démarche peuvent être ainsi déterminés avec précision. L'article résume et commente plusieurs études ayant utilisé ces nouvelles méthodes. Les résultats montrent que la schizophrénie est responsable d'un schéma spécifique de déficit moteur avec une perturbation prédominante des paramètres spatiaux. Le traitement antipsychotique conventionnel aggrave habituellement ces déficits alors que les effets des traitements antipsychotiques atypiques sont moins prononcés. Un fonctionnement moteur perturbé peut être normalisé par des stimuli sensoriels externes mais uniquement en l'absence de besoin des processus d'attention majeure et il peut être augmenté mais pas normalisé par une stratégie attentionnelle.
Motor disturbances: symptoms or side effects?
Motor deficits arc common and disabling symptoms in schizophrenic patients, and have an enormous impact on the long-term outcome of the disease by affecting work performance and daily functioning. A major problem for the clinician is that motor disturbances in schizophrenic patients can be caused by dopamine-blocking antipsychotic medication, but they can also be a primary symptom of the disorder itself. This is quite obvious in catatonic schizophrenia, but, even in noncatatonic schizophrenic patients, subtle disturbances of psychomotor performance-the so-called neurological soft signs-can frequently be observed.
When treated with antipsychotic agents, a certain number of patients exhibit, extrapyramidal side effects, such as dystonia, akathisia, dyskinesia, and neurolepticinduced parkinsonism (NIP). Epidemiological studies have displayed extremely varying NIP prevalence rates in schizophrenic patients, ranging from 5% to 90%.1,3 On the other hand, studies in first-episode neuroleptic-naive patients have revealed that psychomotor disturbances are also present, at, the onset of illness, as well as in clinically unaffected relatives of schizophrenic patients.4 Psychomotor disturbances in unmedicated schizophrenics have been interpreted as manifestations of dysfunctional neural connections between subcortical and cortical areas, or of defective brain structures.5-7 Gupta et al made the point, that neurological abnormalities in schizophrenic patients may be present independently of side effects of medication, but that, antipsychotics do contribute to their prevalence.5
Motor disturbances and subjective well-being
In schizophrenia, the subjective well-being of the patients may not only be affected by the disabling symptoms of the disorder, but also by side effects of the antipsychotic treatment. Antipsychotic treatment has been associated with a variety of motor side effects, as well as affective, cognitive, and social impairments, which can reduce quality of life.8-12 Motor disturbances are associated with a substantial reduction in the patient's quality of life and in compliance with the treatment. Van Puttcn found a significant, relationship of noncompliance with motor side effects, particularly with akathisia.13
In this context, we assessed the correlations of subjective well-being with objectively measured gait, parameters, expert-rated motor disturbances, and psychopathological status in conventionally treated, atypically treated, and drug-naïve patients.14 The main variables were the SWN (Subjective Well-being under Neuroleptic Treatment Scale) scores,15 the ESRS (Extrapyramidal Rating Scale) scores,16 and the PANSS (Positive and Negative Syndrome Scale) scores.17 The SWN is a 20-item self-rating scale, consisting of five subscales: emotional regulation, self-control, mental functioning, social integration, and physical functioning. It does not require patients' distinction between pharmacogenic and morbogenic components. Spatial and temporal parameters of gait were measured by using an ultrasonic system for gait analysis.
The study revealed three major results: first, in conventionally treated patients, the SWN total score significantly correlated with stride length (R 2=0.39;P<0.01), whereas in atypically treated and drug-naïve patients it significantly correlated with the PANSS score (atypically treated: R 2=0.25,P<0.05; drug-naïve: R 2=0,64, P<0.01), mainly due to the correlations with the “negative symptoms” and the “general psychopathology” subscores. Second, correlations with stride length were significant, not, only in the “physical functioning” subscore of the SWN, but also in all other subscores. And third, correlations of the SWN scores with ESRS scores were weak.
Consequently, these results suggest that, under conventional antipsychotic treatment, subjective well-being particularly depends on motor side effects, whereas in atypically treated and drug-naïve schizophrenic patients it, is mainly influenced by the psychopathological status. Additionally, motor adverse effects of antipsychotic treatment can not be considered as isolated physical side effects, but have severe implications for other aspects of the patients' well-being. The results also suggest that objectively measured parameters of motor performance represent the influences of motor disturbances on subjective well-being much more closely than the expert rating, the ESRS. Thus, the use of quantitative methods in the assessment of motor disturbances of schizophrenicpatients might be very useful and promising.
How can motor disturbances be measured?
Most studies on psychomotor performance in schizophrenic patients have restricted their work to the assessment of motor disturbances by using clinical ratings, such as the ESRS,16 the Abnormal Involuntary Movement Scale (AIMS),18 the Barnes Akathisia Scale“ (BAS),19 and others. Almost, all clinical trials on antipsychotic treatment, have used one or more of these clinical ratings for the detection of motor side effects.
More specific studies on psychomotor disturbances in schizophrenic patients additionally used clinical observations and ratings of the performance of special motor tasks. For example, a common test for subtle psychomotor disturbances and disturbed motor coordination in schizophrenic patients is the performance of diadochokinetic movements. This is tested by asking the patient, to alternate between pronation and supination of the hand. Motor disturbances in schizophrenic patients can also be detected by analyzing the patient's handwriting, as shown by Haase as early as the 1950s in his tests of the effects of neuroleptic treatment, on writing.20 Some authors have observed clinical pictures of gait, disturbances in schizophrenic patients. However, despite the high frequency of dysfunctional motor performance in schizophrenic patients, very few studies have attempted to quantify these disturbances by using an objective method.21,22 The lack of studies on quantitatively measured spatial and temporal parameters of motor performance in schizophrenic patients is mirrored by the lack of knowledge on the pathogenesis of motor disturbances in psychiatric diseases in general. In this context, we introduced a three-dimensional ultrasonic movement, analysis system into our investigations on psychiatric disorders.
Three-dimensional ultrasonic movement analysis
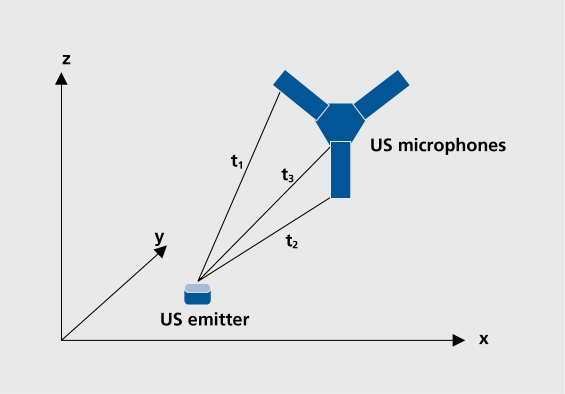
In our studies, spatial and temporal parameters were assessed with the Zebris CMS70P; MA70P3 system (Zebris Medical Systems, Tubingen, Germany). This three-dimensional movement analysis system can be flexibly used in the assessment, of spatial and temporal parameters of various movements. We used it, mainly for the analysis of gait and hand movements. The system is based on an ultrasonic movement analysis system that continuously calculates the three-dimensional spatial positions of tiny markers (diameter 0.8 mm) which are attached to precisely defined positions on moving body parts. The ultrasonic system evaluates transmission times of ultrasound impulses (40 kHz), emitted from these markers and received by three microphones mounted on a stationary frame. Thus, a local coordinate system is determined, and three-dimensional coordinates of the markers can be derived by triangulation (Figure 1. ). By this technique, the spatial positions of the markers are sampled at a frequency of up to 100 Hz, which corresponds a temporal resolution of 10 ms. The spatial resolution of the system is less than 0.6 mm. Many spatial and temporal motor parameters can be computed by using special software packages. Excellent, reproducibility and accuracy of the device has been demonstrated in several studies.23,24
Figure 1. Ultrasonic movement analysis: calculation of the three-dimensional spatial position (coordinates: x, y, z) of ultrasonic emitters on the different transmission times (t1-3) of the ultrasound signals to the three microphones. US, ultrasonic.
Analysis of gait disturbances in patients
By using this system, we assessed the locomotor patterns of gait in schizophrenic patients and differentiated intrinsic effects of the illness from those caused by conventional and atypical neuroleptic treatment.25 Gait parameters of 16 drug-nai've, 25 conventionally treated (halopcridol: n=17, mean dose 6.4 +/- standard deviation [SD] 3.4 mg/day; fluphenazine: n=5, 11.2 +/- SD 7.7 mg/day; flupentixol: n=3, 6.7 +/- SD 2.9 mg/day) and 25 atypically treated patients (olanzapine: n=20, mean dose 16.2 +/- SD 6.3 mg/day; amisulpridc: n=5; 560 +/- SD 89 mg/day), as well as 25 control subjects, were evaluated. Differences in gait, velocity and in stride length between the four investigated groups were highly significant, (analysis of variance [ANOVA]: P<0.001). Mean gait velocities of all patient groups were significantly slower than those of controls, with the most, striking difference observed between the control group and patients treated with conventional neuroleptics (P<0.001). Amongst the patient, groups, significant differences were detected between patients treated with conventional neuroleptics, and both patients treated with atypical neuroleptics and drug-nai've patients (P<0.05), but not between untreated and atypically treated patients.
In all patient, groups the reduction in gait velocity was due to a smaller mean stride length, while the cadence (steps per minute) was not, changed.
These results indicate that schizophrenia causes a primary disturbance of stride length regulation. Conventional antipsychotic treatment, intensifies this deficit, whereas atypical antipsychotic treatment docs not cause any additional gait, disturbances. In contrast, to the spatial parameters, the temporal structure of schizophrenic gait, is not affected either by antipsychotic treatment, or schizophrenia itself.
Effect of external sensory stimulation by treadmill walking
In this study, gait parameters of 14 drug-naive schizophrenic patients, 14 patients treated with conventional antipsychotics (haloperidol: n=10, mean dose 6.7 +/- SD 3.5 mg/day; fluphcnazine: n=4, 13.0 +/- SD 6.78 mg/day) 14 patients treated with olanzapine (17.7 +/- SD 5.7 mg/day), as well as 14 matched controls, were assessed on a walkway and on a treadmill at three different, velocities (very slow, intermediately slow, and comfortable).26
In Parkinson's disease (PD) studies indicate that, conditions involving the use of exteroceptive information may facilitate movement. Although PD patients typically walk with smaller steps, they achieved the desired stride amplitude when provided with external cues while walking on a treadmill.27 External stimulation is supposed to lead to an activation of a correct, stepping response by triggering the use of nonaffected brain areas.
Parallel to the results of the abovementioned study on free gait, in this study all patients showed a significantly decreased gait, velocity, predominantly due to a shorter stride length, when compared with the controls. The most striking difference could be observed between the patients treated with conventional neuroleptics and the controls (ANOVA: P< 0.001). Cadence (steps per second) did not differ between the investigated groups. When gait was evaluated on the treadmill, differences in stride length and cadence were significant, only at the very slow treadmill velocity (ANOVA: P< 0.05). In all patient, groups, mean stride length was decreased and cadence compensationally increased. Significant differences between the patient, groups were no longer detectable. With increasing treadmill velocities, gait, parameters of all patient groups were totally normalized.
The results show that, as in patients with PD, impaired gait parameters can also be normalized in schizophrenic patients by external stimulation via treadmill walking, but, only at normal gait, velocities, not, at slow gait, velocities.
Analysis of diadochokinetic hand movements
This study assessed the impact, of schizophrenia and of antipsychotic treatment on diadochokinesia in 20 drugnai've patients, 20 patients conventionally treated with antipsychotics (haloperidol: n=13, mean dose 7.6 +/- SD 3.6 mg/day; fluphcnazine: n=7, 6.7 +/- SD 3.2 mg/day), and 20 atypically treated (olanzapine: 16.8 +/- SD 6.4 mg/day) patients, as well as in 20 healthy controls.28
The study revealed that amplitude and peak velocity of diadochokinetic hand movements were significantly reduced in all patient, groups compared with the controls, while frequency of the repetitive movement remained unaffected. The reduction was most pronounced in the conventionally treated patients. In addition, peak acceleration and movement automation were impaired, but only on conventional antipsychotic treatment.
Effects of an attentional strategy
The study also tested the influences of disorder and medication on motor enhancement, as induced by an attentional strategy. First, the patients were asked to perform the movement, “as quickly as possible.” In this instruction there was no reference to the amplitude. Patients were then asked to perform the movement “as quickly as possible, but now with the widest, possible amplitude.” Thus, the patient's attention was drawn to the production of a maximal amplitude. The background for this experiment is that in patients with Parkinson's disease the use of such an attentional strategy leads to a normalization of the disturbed movement parameters.
The analysis of the enhancing effect, of an attentional strategy on diadochokinesia showed that patients in all groups were able to increase the amplitude, if they were instructed to do so. Yet, the degree to which the amplitude was increased was much smaller in the two patient groups under antipsychotic treatment, compared with both the drug-naïve patients and the controls. This result reveals a different, enhancing effect, under neuroleptic treatment, but does not confirm the hypothesis that an attentional strategy can normalize disturbed motor performance in schizophrenic patients, as has been derived from the observations in PD patients.
Discussion of the results and underlying pathophysiological mechanisms
Summarizing the results of these studies on motor disturbances in schizophrenic patients, we can state that:
Motor disturbances severely impair the patient's wellbeing, and the degree of impairment is represented much more closely by objectively measured parameters than by expert ratings of motor performance.
Drug-naive patients suffer from a primary motor deficit with predominantly disturbed spatial parameters: gait velocity is reduced by a decrease in stride length, whereas cadence (frequency) is normal, diadochokinetic amplitude is decreased, and peak velocity and regularity are hampered, whilst frequency again is not influenced.
Conventional antipsychotic treatment regularly worsens these specific primary deficits, whereas the effects of atypical antipsychotic treatment, are less pronounced.
Disturbed motor performance can be normalized by external sensory stimuli, but-in contrast to PD patients-only when no major attentional processes are required.
Disturbed motor performance can be enhanced by an attentional strategy, but-again in contrast to PD paticnts-not to the extent that motor parameters are normalized. The enhancement of movement amplitudes is much less pronounced in patients receiving antipsychotic medication.
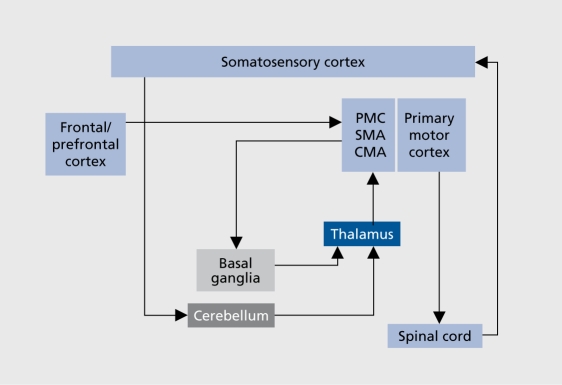
The pathophysiological mechanism underlying the decrease in movement, amplitude has not yet been elucidated, either in PD or in schizophrenia. A fundamental hypothesis explaining hypokinesia in PD states that, due to overactivity of inhibitory projections from the basal ganglia to the thalamus, thalamocortical projections fail to facilitate the motor-related cortical areas such as the supplementary motor area (SMA), cingulate motor areas (CM A), and premotor cortex (PMC) (Figure 2).29 Hypofunctioning of the SMA in PD patients during movements has been demonstrated by studies using functional magnetic resonance imaging (fMRI).30 The basal ganglia-SMA motor system, which regulates the elaboration of internally driven motor sequences, may be responsible for the adequate scaling of motor activity in normal movement. PD patients fail in this process because of the basal ganglia-SMA dysfunction. 31
Figure 2. Scheme of motor circuits involved in the pathophysiology of movement disturbances in schizophrenic patients. PMC, premotor cortex; SMA, supplementary motor area; CMA, angular motor area.
fMRI studies on motor activation in schizophrenic patients have also revealed decreased activation of the SMA.7,32,33 Thus, reduction in SMA activity during motor tasks seems to be a common characteristic in PD and schizophrenia. Reduced SMA activity could be caused by disturbed functioning, either of the cortico-cortical circuit, via the basal ganglia and the thalamus, or of the cortico-cerebellar-thalamic-cortical circuit (CCTCC). Both defects would lead to a deficient, thalamic output toward the SMA.
Evidence from various research methodologies supports the suggestion that a disordered function of neural circuits containing the basal ganglia and the thalamus has a role in the pathophysiology of schizophrenia.34,35 Studies have revealed a disturbed basal ganglia output in schizophrenic patients who were treated with antipsychotic medication.36-38 Additionally, dysfunction of the CCTCC leading to poorly coordinated mental activity and altered excitability of the motor cortex have been demonstrated in untreated schizophrenic patients.39,40.
Problems with the regularity and smoothness of the movement might, also be caused by basal ganglia dysfunction, but they are more likely to be caused by deficiencies in monitoring and optimization of movement by the use of sensory feedback information. As the cerebellum is mainly involved in these processes, cerebellar dysfunctions in schizophrenic patients could also contribute to the observed disturbances in regularity and smoothness.
In PD, hypokinesia may improve under exposure to sensory or emotional stimuli. Many studies have demonstrated that PD patients can use diverse external cues or attentional strategies to enhance motor performance.27,41
Our results indicate that the normalizing effect, of external sensory stimuli on motor parameters in schizophrenic patients is similar to that, in PD patients, whereas the effect, of attentional stimuli is much less pronounced. The pathophysiological basis for this finding remains unclear. According to cerebral blood flow studies, activity in the PMC and SMA is elevated in patients with PD when external cues or attentional strategies are used to enhance motor performance.42 It is hypothesized that in PD patients external cues and attentional strategies enable the PMC and SMA to better compensate for defective motor control mechanisms in the basal ganglia. The lateral PMC is preferentially active during externally cued movements, as opposed to non-cued movements,43 and PMC and parietal overactivity has been reported in PD patients during the performance of sensory-cued motor tasks.44
Hanakawa et al showed enhanced activation in the right lateral PMC in PD patients while walking on a treadmill. They concluded that a brain circuit including posterior parietal cortex, cerebellum, and lateral PMC plays a key role in the development of the paradoxically enhanced gait induced by external stimuli in PD patients. The authors suggested that, utilization of nonaffected brain areas is a compensatory mechanism for basal ganglia dysfunction in movement activation.45
In our study, we also observed a compensation for the impairment of stride-length regulation under external stimulation via treadmill walking in all patient groups. As in PD, external stimuli could enable the PMC and SMA to better compensate for deficiencies in thalamo-cortical output, caused either by antidopaminergic effects of antipsychotic treatment or by a primary pathophysiological condition of schizophrenia. In contrast to the effects of external sensory stimuli on gait, we could not demonstrate a normalization of diadochokinetic movements under the use of an attentional strategy. This contrasts with the findings in PD patients.
The reason for the different enhancing effects of sensory stimuli and attentional strategies in schizophrenic patients is unclear. One possible explanation for the variation of the enhancing effects of treadmill walking at the various velocities could be found in the varying degrees of gait, automation at the three tested gait velocities. Slow and very slow gaits are poorly automated-especially when performed on the treadmill-and require marked cognitive processes, whereas gait, at, normal velocity is highly automated. Thus, cognitive deficits in schizophrenic patients could lead to additional deficits in the generation of optimal gait, patterns. These cognitive deficits could also be the reason for the failure of attentional strategies to normalize disturbed motor parameters in schizophrenic patients, as has been observed in our study on diadochokinetic movements. This suggests that, the pathophysiological processes underlying motor disturbances in schizophrenic patients arc much more widespread than in PD patients, and also involve-in addition to the basal ganglia-cerebellar, frontal, and prefrontal structures.
In conclusion, the studies show that quantitative analyses of motor disturbances can provide objective data on primary motor disturbances in schizophrenic patients, as well as on motor side effects of various antipsychotic treatment options. Thus, they can provide further insight in the pathophysiological conditions of schizophrenia and of adverse effects of antipsychotic treatment. Investigations of the effects of enhancing strategies and the combination of quantitative movement, analysis with other methods, such as genetic or functional imaging studies, could further contribute to the identification of mechanisms underlying motor disturbances in schizophrenia.
Selected abbreviations and acronyms
- ESRS
Extrapyramidal Symptom Rating Scale
- PD
Parkinson 's disease
- PMC
premotor cortex
- SMA
supplementary motor area
- SWN
Subjective Well-being under Neuroleptic treatment scale
Contributor Information
Albert Putzhammer, Department of Psychiatry, University of Regensburg, Regensburg, Germany.
Helmfried E. Klein, Department of Psychiatry, University of Regensburg, Regensburg, Germany.
REFERENCES
- 1.Kennedy PF., Hershon HI., McGuire RJ. Extrapyramidal disorders after prolonged phenothiazine therapy. Br J Psychiatry. 1971;118:509–518. doi: 10.1192/bjp.118.546.509. [DOI] [PubMed] [Google Scholar]
- 2.Korczyn AD., Goldberg GJ. Extrapyramidal effects of neuroleptics. J Neurol Neurosurg Psychiatry. 1976;39:866–869. doi: 10.1136/jnnp.39.9.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephen PJ., Williamson J. Drug-induced parkinsonism in the elderly. Lancet. 1984;2:1082–1083. doi: 10.1016/s0140-6736(84)91516-2. [DOI] [PubMed] [Google Scholar]
- 4.Flyckt L., Sydow O., Bjerkenstedt L., Edman G., Rydin E., Wiesel FA. Neurological signs and psychomotor performance in patients with schizophrenia, their relatives and healthy controls. Psychiatry Res. 1999;86:113–129. doi: 10.1016/s0165-1781(99)00027-x. [DOI] [PubMed] [Google Scholar]
- 5.Gupta S., Andreasen NC., Arndt S., et al. Neurological soft signs in neuroleptic-naive and neuroleptic-treatecl schizophrenic patients and in normal comparison subjects. Am J Psychiatry. 1995;152:191–196. doi: 10.1176/ajp.152.2.191. [DOI] [PubMed] [Google Scholar]
- 6.Mueller JL., Roder CH., Schuierer G., Klein H. Motor-induced brain activation in cortical, subcortical and cerebellar regions in schizophrenic inpatients. A whole brain fMRI f ingertapping study. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:421–426. doi: 10.1016/s0278-5846(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 7.Schroder J., Wenz F., Schad LR., Baudendistel K., Knopp MV. Sensorimotor cortex and supplementary motor area changes in schizophrenia. A study with functional magnetic resonance imaging. Br J Psychiatry. 1995;167:197–201. doi: 10.1192/bjp.167.2.197. [DOI] [PubMed] [Google Scholar]
- 8.Browne S., Roe M., Lane A., et al. Quality of life in schizophrenia: relationship to sociodemographic factors, symptomatology and tardive dyskinesia. Acta PsychiatrScand. 1996;94:118–124. doi: 10.1111/j.1600-0447.1996.tb09835.x. [DOI] [PubMed] [Google Scholar]
- 9.Casey DE. Motor and mental aspects of extrapyramidal syndromes. IntClin Psychopharmacol. 1995;10(suppl 3):105-14–105-114. [PubMed] [Google Scholar]
- 10.Gerlach J., Larsen EB. Subjective experience and mental side-effects of antipsychotic treatment. Acta Psychiatr Scand. 1999;395(suppl):113–117. doi: 10.1111/j.1600-0447.1999.tb05990.x. [DOI] [PubMed] [Google Scholar]
- 11.Larsen EB., Gerlach J. Subjective experience of treatment, side-effects, mental state and quality of life in chronic schizophrenic out-patients treated with depot neuroleptics. Acta PsychiatrScand. 1996;93:381–388. doi: 10.1111/j.1600-0447.1996.tb10664.x. [DOI] [PubMed] [Google Scholar]
- 12.Lewander T. Neuroleptics and the neuroleptic-induced deficit syndrome. Acta PsychiatrScand. 1994;380(suppl):8–13. doi: 10.1111/j.1600-0447.1994.tb05825.x. [DOI] [PubMed] [Google Scholar]
- 13.Van Putten T. Why do schizophrenic patients refuse to take their drugs?. Arch Gen Psychiatry. 1974;31:67–72. doi: 10.1001/archpsyc.1974.01760130049008. [DOI] [PubMed] [Google Scholar]
- 14.Putzharnrner A., Perfahl M., Pfeiff L., Hajak G. Correlation of subjective well-being in schizophrenic patients with gait parameters, expert-rated motor disturbances, and psychopathological status. Pharmacopsychiatry:. 2005;38:132–138. doi: 10.1055/s-2005-864125. [DOI] [PubMed] [Google Scholar]
- 15.Naber D. A self-rating to measure subjective effects of neuroleptic drugs, relationships to objective psychopathology, quality of life, compliance and other clinical variables. IntClin Psychopharmacol. 1995;10(suppl 3):133–138. [PubMed] [Google Scholar]
- 16.Chouinard G., Ross-Chouinard A., Annable L., Jones BD. The Extrapyramidal Symptoms Rating Scale. Can J Neurol Sci. 1980;7:233–244. [Google Scholar]
- 17.Kay SR., Fiszbein A., Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 18.Guy W. Abnormal Involuntary Movement Scale (AIMS). In:. ECDEU Assessment Manual for Psychopharmacology. Washington DC: Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration;. 1976 [Google Scholar]
- 19.Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- 20.Haase HJ. Ober Vorkommen und Deutung des psychomotorischen Parkinson-Syndroms bei Megaphen- bzw. Largactil-Dauerbehandlung. Nervenarzt. 1954;25:486–492. [PubMed] [Google Scholar]
- 21.Jahn T., Cohen R., Mai N., et al. Untersuchung der fein- und grobmotorischen Dysdiadochokinese schizophrener Patienten: Methodenentwicklung und erste Ergebnisse einer computergestutzten Mikroanalyse. Z Klin Psychol. 1995;24:300–315. [Google Scholar]
- 22.Tigges P., Mergl R., Frodl T., et al. Digitized analysis of abnormal hand-motor performance in schizophrenic patients. Schizophr Res. 2000;45:133–143. doi: 10.1016/s0920-9964(99)00185-1. [DOI] [PubMed] [Google Scholar]
- 23.Obens T., Becker NL., Hirning P. “Akustische” Laufanalyse. Orthopàdie Schuhtechnik. 1996;4:34–39. [Google Scholar]
- 24.Putzharnrner A., Heindl B., Muller J., et al. Three-dimensional ultrasonic gait analysis in schizophrenic patients. Psychiatr Prax. 2003;30:110–114. doi: 10.1055/s-2003-39759. [DOI] [PubMed] [Google Scholar]
- 25.Putzharnrner A., Heindl B., Broil K., Pfeiff L., Perfahl M., Hajak G. Spatial and temporal parameters of gait disturbances in schizophrenic patients. Schizophr Res. 2004;69:159–166. doi: 10.1016/s0920-9964(03)00090-2. [DOI] [PubMed] [Google Scholar]
- 26.Putzharnrner A., Perfahl M., Pfeiff L., Hajak G. Gait disturbances in schizophrenic patients and adaptation to treadmill walking. Psychiatry Clin Neurosci. 2005;59:303–310. doi: 10.1111/j.1440-1819.2005.01375.x. [DOI] [PubMed] [Google Scholar]
- 27.Morris M., lansek R., Matyas T., Summers J. Abnormalities in the stride length-cadence relation in parkinsonian gait. Mov Disord. 1998;13:61–69. doi: 10.1002/mds.870130115. [DOI] [PubMed] [Google Scholar]
- 28.Putzharnrner A. Perfahl M, Pfeiff L, et al. Performance of diadochokinetic movements in schizophrenic patients. Schizophr Res. 2005;79:271–280. doi: 10.1016/j.schres.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 29.Delong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 30.Rascol O., Sabatini U., Chollet F., et al. Supplementary and primary sensory motor area activity in Parkinson's disease. Regional cerebral blood flow changes during finger movements and effects of apomorphine. Arch Neurol. 1992;49:144–148. doi: 10.1001/archneur.1992.00530260044017. [DOI] [PubMed] [Google Scholar]
- 31.Deiber MP., Passingham RE., Colebatch JG., Friston KJ., Nixon PD., Frackowiak RS. Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res. 1991;84:393–402. doi: 10.1007/BF00231461. [DOI] [PubMed] [Google Scholar]
- 32.Braus DF., Ende G., Weber-Fahr W., et al. Antipsychotic drug effects on motor activation measured by functional magnetic resonance imaging in schizophrenic patients. Schizophr Res. 1999;39:19–29. doi: 10.1016/s0920-9964(99)00032-8. [DOI] [PubMed] [Google Scholar]
- 33.Mueller J., Roeder C., Schuirer G., Klein HE. Subcortical overactivation in untreated schizophrenic patients: a functional magnetic resonance image finger-tapping study. Psychiatr Clin Neurosci. 2002;56:77–84. doi: 10.1046/j.1440-1819.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- 34.Clinton SM., Meador-Woodruff JH. Thalamic dysfunction in schizophrenia: neurochemical, neuropathological, and in vivo imaging abnormalities. Schizophr Res. 2004;69:237–253. doi: 10.1016/j.schres.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 35.Ring HA., Serra-Mestres J. Neuropsychiatry of the basal ganglia. J Neurol Neurosurg Psychiatry. 2002;72:12–21. doi: 10.1136/jnnp.72.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menon V., Anagnoson RT., Glover GH., Pfefferbaum A. Functional magnetic resonance imaging evidence for disrupted basal ganglia function in schizophrenia. Am J Psychiatry. 2001;158:646–649. doi: 10.1176/appi.ajp.158.4.646. [DOI] [PubMed] [Google Scholar]
- 37.Mueller JL., Klein HE. Neuroleptic therapy influences basal ganglia activation: a functional magnetic resonance imaging study comparing controls to haloperidol- and olanzapine-treated inpatients. Psychiatry Clin Neurosci. 2000;54:653–658. doi: 10.1046/j.1440-1819.2000.00766.x. [DOI] [PubMed] [Google Scholar]
- 38.Mueller JL., Deuticke C., Putzharnrner A., Roder CH., Hajak G., Winkler J. Schizophrenia and Parkinson's disease lead to equal motor-related changes in cortical and subcortical brain activation: an fMRI fingertapping study. Psychiatry Clin Neurosci. 2003;57:562–568. doi: 10.1046/j.1440-1819.2003.01168.x. [DOI] [PubMed] [Google Scholar]
- 39.Eichhammer P., Wiegand R., Kharraz A., Langguth B., Binder H., Hajak G. Cortical excitability in neuroleptic-naive first-episode schizophrenic patients. Schizophr Res. 2004;67:253–259. doi: 10.1016/S0920-9964(03)00223-8. [DOI] [PubMed] [Google Scholar]
- 40.Wiser AK., Andreasen NC., O'Leary DS., Watkins GL., Boles Ponto LL., Hichwa RD. Dysfunctional cortico-cerebellar circuits cause 'cognitive dysmetria' in schizophrenia. Neuroreport. 1998;9:1895–1899. doi: 10.1097/00001756-199806010-00042. [DOI] [PubMed] [Google Scholar]
- 41.Behrman, Teitelbaum P., Cauraugh JH. Verbal instructional sets to normalise the temporal and spatial gait variables in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1998;65:580–582. doi: 10.1136/jnnp.65.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jueptner M., Weiller C. A review of differences between basal ganglia and cerebellar control of movements as revealed by functional imaging studies. Brain. 1998;121:1437–1449. doi: 10.1093/brain/121.8.1437. [DOI] [PubMed] [Google Scholar]
- 43.Halsband U., Matsuzaka Y., Tanji J. Neuronal activity in the primate supplementary, pre-supplementary and premotor cortex during externally and internally instructed sequential movements. Neurosci Res. 1994;20:149–155. doi: 10.1016/0168-0102(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 44.Samuel M., Ceballos-Baumann AO., Blin J., et al. Evidence for lateral premotor and parietal overactivity in Parkinson's disease during sequential and bimanual movements. A PET study. Brain. 1997;120:963–976. doi: 10.1093/brain/120.6.963. [DOI] [PubMed] [Google Scholar]
- 45.Hanakawa T., Fukuyama H., Katsumi Y., Honda M., Shibasaki H. Enhanced lateral premotor activity during paradoxical gait in Parkinson's disease. Ann Neurol. 1999;45:329–336. doi: 10.1002/1531-8249(199903)45:3<329::aid-ana8>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]