Abstract
The core hypothesis underlying pharmacogenetics is that genetic factors play a significant role in the well-recognized differences between individuals in response to medication and susceptibility to adverse effects. If these genetic factors can be identified and understood, they may serve as predictors to guide clinicians in tailoring medication to the individual patient. Recent developments in the field of antipsychotic drug treatment suggest that pharmacogenetics could play an important role, permitting the use of first-generation antipsychotics (FGAs) for patients in whom the use of second-generation antipsychotics (SGAs) is limited by efficacy considerations or adverse effects, in this paper, key issues that need to be taken into consideration in designing and interpreting pharmacogenetic studies of antipsychotic drugs are discussed against the background of data emanaling from studies on the genetics of tardive dyskinesia (TD), an important adverse effect of FGAs. The issues considered include the advantages and potential pitfalls of case-control association studies of pharmacogenetic traits, the role of demographic factors such as age and gender, additive effects of genes, and gene-gene and gene-environment interaction. The prospects for implementation of pharmacogenetic testing in the clinic are considered in the context of a preliminary model that has been tested for prediction of susceptibility to TD.
Keywords: pharmacogenetics, typical antipsychotic, atypical antipsychotic, tardive dyskinesia
Abstract
La hipótesis central en que se fundamenta la fármacogenética alude a que los factures genéticos tienen un papel significativo en las diferencias bien reconocidas entre los sujetos en la respuesta a los medicamentos y en la susceptibilidad a los efectos adversos. Si estos factores genéticos pueden ser identificados y comprendidos, ellos pueden servir como predictores para guiar a los clínicos en la adaptación de la medicación al paciente individual. Recientes desarrollos en el campo de la terapia antipsicótica sugieren que la farmacogenética podría tener un importante papel, permitiendo el empleo de antipsicóticos de primera generación (APG) en pacientes en quienes la utilización de antipsicóticos de segunda generación (ASG) está limitada por consideraciones acerca de la eficacia o los efectos adversos. En este artículo se discuten los temas clave que necesitan ser tomados en cuenta al diseñar e interpretar los estudios farmacogenéticos de los antipsicóticos, frente al respaldo de datos que provienen de estudios acerca de la genética de la disquinesia tardía (DT), un efecto adverso importante de los APG. Los temas considerados incluyen las ventajas y potenciales peligros de los estudios de asociación caso control de los rasgos farmacogenéticos, el papel de factores demográficos tales como edad y sexo, los efectos aditivos de los genes y las interacciones entre los genes (gen-gen) y de los genes con el ambiente. También se han considerado las proyecciones para la implementación de pruebas farmacogenéticas en la clínica, en el contexto de un modelo preliminar que ha sido probado para la predicción de susceptibilidad para la DT.
Abstract
Le rôle essentiel joué par le facteur génétique au niveau des différences observées entre les individus en réponse au traitement et à la susceptibilité vis-à-vis des événements indésirables est la principale hypothèse de la pharmacogénétique. Si ces facteurs génétiques peuvent être identifiés et compris, ils peuvent servir de guide aux médecins pour adapter un traitement à chaque patient. Les développements récents dans le domaine du traitement antipsychotique suggèrent que la pharmacogénétique pourrait jouer un rôle important, permettant l'utilisation des antipsychotiques de première génération (APG) pour les patients chez qui l'utilisation des antipsychotiques de seconde génération (ASG) est limitée pour des raisons d'efficacité et d'événements indésirables. Dans cet article, nous parierons des points-clés qui doivent être pris en considération pour concevoir et interpréter les études pharmacogénétiques des antipsychotiques par rapport à l'historique des données issues des études sur la génétique des dyskinésies tardives (DT), événement indésirable important des APG. Les questions étudiées comprennent les avantages et les embûches potentielles des études d'association cas-témoins des particularités pharmacogénétiques, le rôle des facteurs démographiques tels que l'âge et le sexe, les effets additifs des gènes et l'interaction gènegène et gène-environnement On envisage les perspectives de réalisation de l'expérimentation pharmacogénétique en clinique dans le contexte d'un modèle préliminaire déjà testé pour prévoir la susceptibilité aux DT.
Pharmacogenetics is one of the most exciting and clinically relevant applications of the enormous strides that have been made in defining the genetic basis of human variation. Completion of the human genome project. brought, with it the means to catalogue such variation on a genome -wide basis, and to apply this knowledge to the clinical context. The core hypothesis underlying pharmacogenetics is that genetic factors play a major role in the well-recognized differences between individuals in response to medication and susceptibility to adverse effects. If these genetic factors can be identified and understood, they may serve as predictors to guide clinicians in tailoring medication to the individual patient. The technological tools required in order to achieve this objective are readily available in the form of high-throughput genotyping systems that allow thousands of individual genotypes to be generated at costs that are dramatically declining. At the same time, great progress has been made in developing the information technology that, is needed in order to permit, efficient, access to the vast body of data that is being deposited in electronic databases on a daily basis.
Psychiatry is a very important candidate area for the application of pharmacogenetics to clinical practice.1,2 Response rates to psychotropic drugs are highly variable, and this includes all the major classes such as antipsychotics, antidepressants, mood stabilizers, and antianxiety agents. In the field of antipsychotics, a major clinical dilemma is beginning to emerge, with growing recognition of the important limitations of the second-generation (SGA) or atypical antipsychotic drugs. Except, for clozapine, there is little evidence to indicate that, SGAs are more effective than the classical, first-generation antipsychotics (FGAs). This impression has been borne out by the initial results of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study which showed no therapeutic advantage of the SGAs risperidone, quetiapine, and ziprasidone over the lowpotency FGA, perphenazine.3 There was a limited advantage of olanzapine, but at the cost of a significantly greater incidence of weight, gain and adverse metabolic effects. The advantage of SGAs is thought, to lie in their superior side-effect profile, in particular the reduced incidence of extrapyramidal symptoms (EPS) such as dystonia, parkinsonism, and akathisia, and, in the long term, the substantially smaller risk of tardive dyskinesia (TD).
However, a recent population-based study suggests that, older individuals treated with high-dose SGAs may be at similar risk of EPS to patients treated with FGAs.4
The current trend in clinical practice is to eliminate FGAs as far as possible, and to employ SGAs as the first-line medication for the treatment, of acute schizophrenic psychosis. This trend has not been implemented worldwide because of economic considerations, given the major price differences between SGAs and FGAs, and FGAs are still widely prescribed. The results of recent, studies such as CATIE3 raise the important, consideration that, FGAs may have a place in the treatment of schizophrenia, subject to appropriate risk -benefit, considerations. Predictors of susceptibility to EPS and TD, in the case of the FGAs, and to weight gain and metabolic adverse effects, in the case of SGAs, could radically alter clinical practice, allowing FGAs or SGAs to be prescribed in accordance with the risk profile of the individual patient. The availability of predictors of therapeutic response to FGAs and SGAs would further improve the risk-benefit ratio. Genetic predictors are highly feasible in this context, and are the focus of intensive research in the field of psychiatric pharmacogenetics.
Genetic factors that, influence drug metabolism (pharmacokinetics) and molecular targets of drug action (pharmacodynamics) may be implicated separately or interactively in the pharmacogenetic profile of a patient in relation to a particular class of drugs. Extensive research is needed in order to identify the genes involved, and the precise variants within these genes that underlie interindividual variability. In the case of pharmacokinetic factors, the underlying genetic cause is often a mutation in a single gene, such as a member of the extended cytochrome P450 family, which is pivotally involved in the metabolism of psychotropic drugs.5 Pharmacodynamic targets include receptors or transporters to which the drugs bind. Variants in these genes are more likely to be of small effect with several different, loci being involved, each contributing to the phenotype to a small and variable degree. This type of polygenic effect is difficult, to define clinically, and is sensitive to spurious influences. Most, of the pharmacogenetic effects that are widely relevant are likely to be polygenic, requiring significant, research efforts to generate and replicate data that will ultimately be clinically useful.
In this paper, we will consider key issues that need to be taken into consideration in designing and interpreting pharmacogenetic studies of antipsychotic drugs. Examples will be given from a series of studies that has identified several genes involved in susceptibility to TD in patients treated with FGAs for an extended period.
Case-control association studies armacogenetics: advantages a tential pitfalls
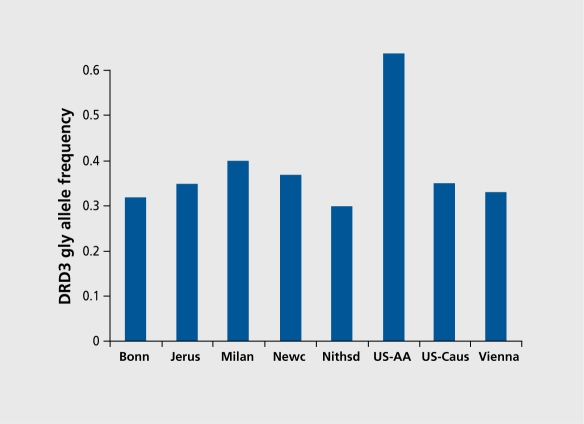
The classical strategies employed in the search for genes that predispose to complex phenotypes are linkage and association. linkage studies require recruitment, of family units of varying size, and are not, practically feasible in the context, of pharmacogenetics. Family-based association studies require smaller family units, but they too are not practical for pharmacogenetics, except, for studies conducted in pediatric populations. Case-control association studies are the most suitable strategy for pharmacogenetics. Their advantages are considerable, the most, striking being the possibility of recruiting large samples that have sufficient, statistical power to investigate genetic variants of relatively small effect. Large samples are particularly important if the effect of more than one gene is being studied in the same sample, and interactions among genes and between genes and the environment, are being sought. On the other hand, case -control designs are notoriously susceptible to the effects of ethnic stratification, which can lead to spurious results. These are due to a particular allele being enriched in a particular population. If this population is overrepresented in the case or control group, a spurious finding will result, in which the allele is erroneously associated with the phenotype. The nature of the problem is illustrated in Figure 1,6 which shows the frequency of the gly allele of the dopamine D3 receptor gene (DRD3) scr9gly polymorphism in samples from several populations. These samples were included in a pooled and meta-analytic study of the DRD3 ser9gly polymorphism as a risk factor for TD.6 Great variability in the frequency of the 9gly allele in the different samples included in the study is immediately evident, ranging from 30% to 40% among Caucasians from different countries, and reaching 80% among African-Americans.
Figure 1. Frequency of the gly allele of the dopamine D3 receptor (DRD3) ser9gly polymorphism in eight samples of different ethnic origin, vhich were included in a pooled meta-analysis in which association of the DRD3 ser9gly polymorphism with susceptibility to tardive dyskinesia (TD) was examined.6 The samples from Bonn (n=240), Milan (n=93), Newcastle (n=69), Nithsdale (n=100), and Vienna (n=61 ) were all made up of Caucasians. The Jerusalem sample (n=113) was made up of subjects of Jewish Ashkenazi (n=66) or non-Ashkenazi (n=47) origin. There was no difference in gly allele frequency between the Ashkenazi and non-Ashkenazi subjects. US-Cauc, Caucasian subjects recruited in the US (n=81); US-AA, US African-Americans (n=23).
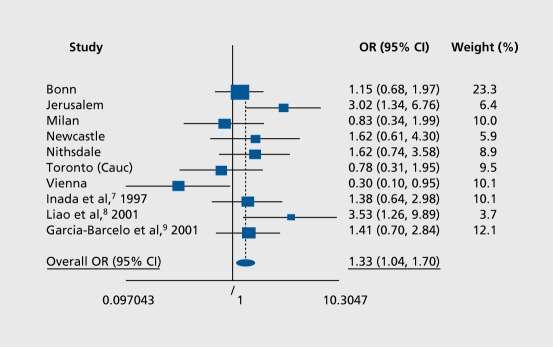
If the association of the DRD3 gly 9 allele with TD were tested without controlling for ethnicity in this pooled sample, made up of 317 patients with TD and 463 patients without TD, spurious results could easily arise. Therefore, a stepwise logistic regression was employed so as to allow the confounding effects of ethnicity and also age and gender to be taken into account. TD was significantly associated with DRD3 gly9 allele carrier status (X2=4.46, df=1, P=0.04) over and above the effect, of ethnicity. Similar positive effects were observed when controlling for age and gender (X2=5.02, df=1, P=0.02).6-9 A meta-analysis was performed, which included all the samples in the pooled analysis, as well as data from additional published studies. The Mantel-Haenszel pooled odds ratio (OR) for DRD3 gly9 allele carrier status increasing susceptibility to TD was 1.33 (95% confidence interval [CI] 1.04-1.70, P=0.02) (Figure 2). Meta-analyses are less sensitive to stratification and admixture (unless these are present in the samples included in the meta-analysis), since original data are not pooled and the unit, of analysis is studies and not individuals.
Figure 2. Meta-analysis of DRD3 risk allele (gly) among seven of the groups included in the pooled analysis reported by Lerer et al6 (the African-American group was excluded because of skewed distribution) and three other published studies.7-9 The size of each plot is proportional to the sample size of each group. The Mantel-Haenszel pooled odds ratio (OR) is =1.33 (95% confidence interval [CI] 1.04-1.70) and the associated test of OR=1 is significant (X2=5.12, df=1, P=0.02), while the cumulative pooled OR is 1.52 with z=11.78 and P<0.0001. The heterogeneity X2=16.97, df=9, P=0.049. Reproduced from reference 6: Lerer B, Segman RH, Fangerau, et al. Pharmacogenetics of tardive dyskinesia: Combined analysis of 780 patients supports association with dopamine D3receptor gene ser9gly polymorphism. Neuropsychopharmacology. 2001;27:109-119. Copyright © Nature Publishing Group 2001.
The findings of our studies of the DRD3 ser9gly polymorphism and TD indicate that, when pooled pharmacogenetic analyses arc conducted on well-characterized samples, it is possible to control for the potentially artifactual effects of ethnicity. This is important, because it is essential to gather large samples for pharmacogenetic studies so as to have sufficient statistical power. The same general strategy was implemented to examine the association of the serotonin 5-HT2A receptor gene and TD,10 and also to examine association of the 5-HT2C receptor gene with unipolar and bipolar affective disorder.11 When using ostensibly homogeneous samples, the genomic control method may be implemented to rule out population influences on the markers being studied.12 This involves genotyping additional markers that are putatively unrelated to the phenotype, and these are used to determine the degree of stratification that is present in the sample.
Demography matters: age-related effects of genetic variants
It is often not taken into account, that genetic variants may have differing functional importance depending on the stage of the life cycle. This is obvious in the case of genes that predispose to disorders of later life such as Alzheimer's disease (although the pathophysiological effects of variations in these genes may be manifested long before the clinical threshold is crossed). Similarly, genes that, influence neurodevelopment in utero may be associated with susceptibility to schizophrenia that only becomes clinically manifest, in late adolescence. Agerelatedness may also be important, in pharmacogenetic phenotypes. We have observed an age-related association of two serotonergic genes, the 5-HT2C (HTR2C) and the 5-HT2A receptor (HTR2A) with susceptibility to TD.13 Our finding with the 5-HT2A receptor has been replicated in a large multicenter study.10
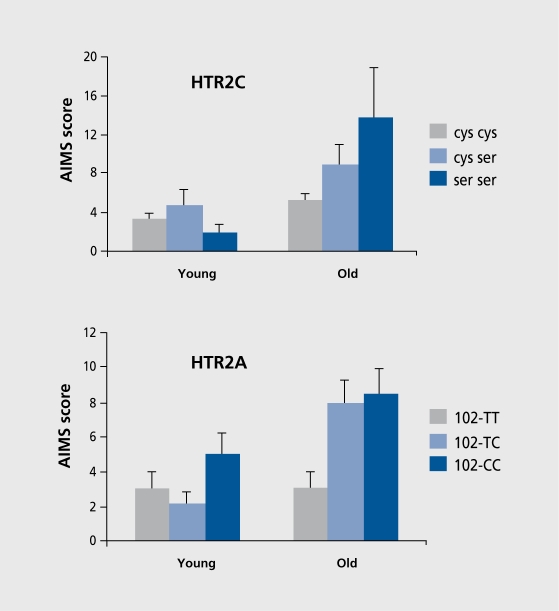
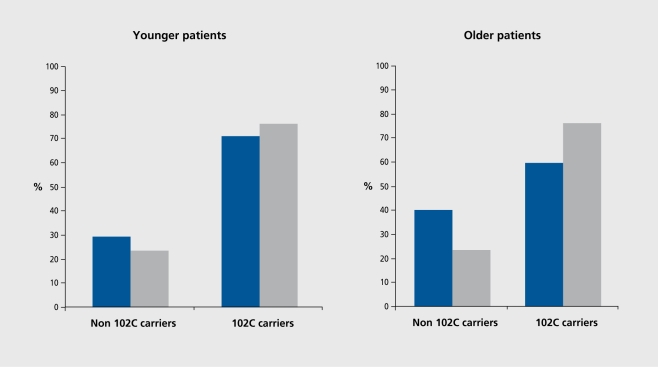
In an earlier report13 (Figure 3), we showed that scores on the Abnormal Involuntary Movements Scale (AIMS, the hallmark of TD) were significantly related to the ser23 allele of the cys23ser polymorphism of the 5-HTC receptor gene and to the 102C allele of the T102C polymorphism in the 5-HT2A receptor gene, in older but, not, younger patients. We sought, to replicate this finding the context of a multicenter study involving 635 patients, 256 of whom manifested TD and 379 of whom did not.10 Our initial analysis employed stepwise logistic regression as for our DRD3 study6 and showed an overall association of the T102C polymorphism in the 5-HT1A receptor gene with TD. We then analyzed the younger and older patients separately. In keeping with our finding in the Jerusalem sample alone,13 the T102C polymorphism was significantly associated with TD in the older patients only (Figure 4). 10
Figure 3. Abnormal Involuntary Movements Scale (AIMS) scores according to 5-HT2C (HTR2C) receptor (upper panel) and 5-HT2A (HTR2A) receptor (lower panel) genotype. Scores in younger patients (<47 years) are shown on the left of each panel and in older patients (>47 years) on the right of each panel. For HTR2C: Old cys-cys versus Old cys-ser, P=0.02; versus Old serser, P=0.001. For HTR2A: Old 102-TT versus Old 102-TC, P=0.01; versus Old 102-CC, P=0.01. No differences were significant in the young groups. Reproduced from reference 13: Segman RH, Lerer B. Age and the relationship of dopamine D3, serotonin 2C and serotonin 2A receptor genes to abnormal involuntary movements in chronic schizophrenia. Mol Psychiatry. 2002;7:137-139. Copyright © Nature Publishing Group 2001.
Figure 4. Frequency of 102C allele carriers (TC heterozygotes and CC homozygotes) in younger patients (<47 years) with tardive dyskinesia (Y-TD) (n=96) and without TD (N-TD) (n=215) (left panel) and in older Y-TD (n=159) and N-TD patients (n=164) (right panel). Likelihood ratio test for TD status predicting T102 CC allele carrier status in younger patients (<47 years): X2 0.18, df==2, P=0.60; in older patients (>47 years): X2=10.16, df=1, P=0.001. Reproduced from reference 10: Lerer B, Segman RH, Tan E-C, et al. Combined analysis of 635 patients confirms an age-related association of the serotonin 2A receptor gene with tardive dyskinesia and specificity for the non-orofacial subtype. Int J Neuropsychopharmacol. 2005:8:411. Copyright © Cambridge University Press 2005.
These replicated findings accentuate the importance of taking into account the possibility that pharmacogenetic effects may be age-related. Thus, an association first, reported in an older sample may be missed if replication is sought, in a younger sample. This was the case with our initial report, of an association of the T102C polymorphism in the 5-HT2A receptor gene with TD,14 which was initially not, replicated by Basile et al15 who studied a sample more than two decades younger than ours. The age range of the pooled sample studied by Lerer et al7 was wide enough to allow replication of the finding in the older subjects.
Additive and interactive effects of genes
Pharmacogenetic phenotypes (such as treatment outcome, onset, of therapeutic effect, rapidity of response, and adverse effects) are complex traits to which background and demographic factors contribute, as well as factors related to the treatment. The genetic background is likely to be polygenic, with an as-yet unknown number of genes contributing a small proportion of the variance. These genes can be expected to act in different, ways. One model is additive, the assumption being that, each gene contributes cumulatively to the phenotype, and that, the overall risk is a sum of these individual contributions. A second, more complex model postulates that the effects of certain genes are conditional on others, implying gene-gene interaction or epistasis. Under this model, a specific allele of gene A will confer an effect, in the presence of a specific allele of gene B, but will not confer susceptibility in its absence. The situation is rendered more complex by the possibility that haplotypes made up of two or more variants may contribute additively or interactively to susceptibility. In addition, gene-environment interaction must also be taken into account.
These considerations are applicable to TD, which is a complex trait, related to drug treatment, to which several demographic and background factors contribute, most notably age, and with which several genes have been associated. As yet, there has been relatively little work in psychiatric pharmacogenetics on the combined effects of genes and on gene-gene interaction. These models have been tested on a limited basis in the context of susceptibility to TD in samples that we and other investigators have studied; the scope of such testing is constrained by the size of the samples.
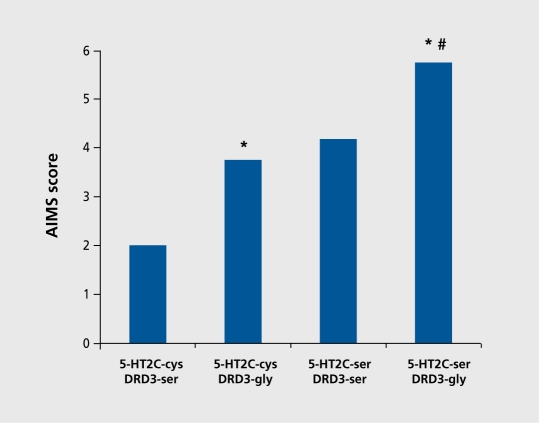
The additive effects of two genetic variants on susceptibility to TD were first reported by Scgman et al.16 Patients who carried both the gly9 allele of the DRD3 ser9gly polymorphism and the ser23 allele of the 5-HT2C cys23ser polymorphism showed evidence of an additive effect of the two risk alleles, both of which had been shown to separately increase risk for TD. As shown in Figure 5,16 the highest, AIMS orofacial dyskinesia scores were observed in patients who carried both mutant risk alleles.
Figure 5. Abnormal Involuntary Movements Scale (AIMS) orofacial dyskinesia scores among patients carrying: 5-HT2C-cys and DRD3-ser alleles (both wild type); 5-HT2C-cys and DRD3-gly (wild type + mutant); 5-HT2C-ser and DRD3-ser (mutant +wild type); or, 5-HT2C-ser and DRD3-gly (mutant + mutant). #P<0.05 versus 5-HT2C-cys and DRD3-ser (both wild type). #P<0.05 versus 5-HT2-cys and DRD3gly (wild type + mutant). Reproduced from reference 16: Segman RH, Heresco-Levy U, Finkel B, et al. Association between the serotonin 2C receptor gene and tardive dyskinesia in chronic schizophrenia; additive contribution of 5-HT2Cser and DRD3gly alleles to susceptibility. Psychopharmacology. 2000;152:408-413. Copyright © Springer 2000.
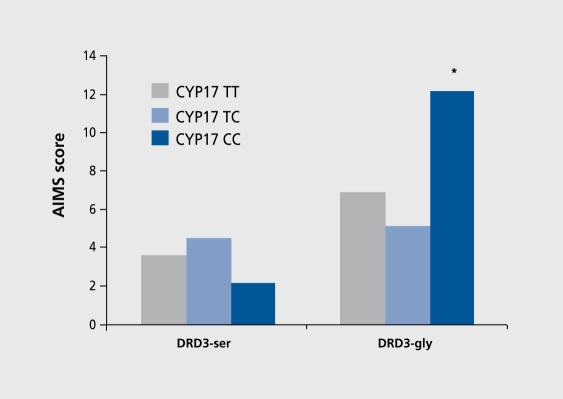
Studying gene-gene interaction and risk for TD, Segman et al17 examined the interactive contribution of a T→C polymorphism in the promoter region of the cytochrome P450 17α-hydroxylase gene (CYP17) and the ser9gly polymorphism of DRD3 that was previously shown to be associated with TD. CYP17 variability could influence susceptibility to TD because of effects on neuroprotective capacity or dopamine output through its role in the conversion of the neurosteroid, pregnanolone, to dehydroepiandrosterone (DHEA). A T→C transition (A2 allele) in the 5' promoter region of the CYP17 gene creates an additional Spl type (CCACC box) promoter site, resulting in a functionally relevant, increase in transcription rate.18 Segman et al17 found that the T→C polymorphism was not in itself associated with TD. However, there was a significant excess of DRD3 gly allele carriers among carriers of the CYP17 CC genotype with TD compared with those without TD (75.0% vs 14.3%; X 2=6.0, df=1, P=0.01). Significant differences were not observed among carriers of the CYP17TT genotype or CYP17TC genotypes with TD and carriers of these genotypes without TD. Two way analysis of variance (ANOVA) with age at first, antipsychotic treatment as covariate showed a significant main effect of DRD3 genotype on AIMS total score (F=14.9, df=1, 106, P=0.0002). There was no main effect, of CYP17 genotype on the AIMS score. There was a significant, interaction between DRD3 and CYP17 genotype on AIMS total score (F=4.2, df=2, 106, P=0.02). Figure 6 shows adjusted mean AIMS scores (derived from analysis of covariance [ANCOVA]) for the patients grouped according to DRD3 and CYP17 genotype. Posthoc Newman-Keuls tests showed that the highest AIMS total (P<0.03) was in patients carrying the DRD3 gly9 allele and the CYP17 CC genotype.
Figure 6. Interactive effect of DRD3 and CYP17 genes on Abnormal Involuntary Movements Scale (AIMS) scores according to DRD3 ser9gly and CYP17 genotype. Left: patients homozygous for the DRD3 ser allele; right: carriers of the DRD3 gly allele (hétérozygotes and homozygotes). There is a significant main effect of DRD3 genotype on AIMS total score (F=14.9, df=1.106, P=0.0002) and a significant interaction between DRD3 and CYP17 genotype (F=4.2, df=2,.106, P=0.02). *P=0.03, post-hoc Newman-Keuls test.
Another gene-gene interaction reported in relation to TD involved DRD3 and cytochrome P450 1A2 (CYP1A2). Both genes had been found to be independently associated with schizophrenia.19 Basile et al19 further found that, those patients who exhibited the risk genotype at, both DRD3 (gly9/gly9) and at CYP1A2 (CC) had the most, severe TD, whereas those who had only one risk genotype (gly9/gly9 or CC) demonstrated intermediate severity of TD. Those patients who did not, have any risk genotypes at either locus demonstrated the lowest mean TD severity scores on the AIM'S scale (P<0.00007).
A more recent report of gene-gene interaction conferring susceptibility to TD concerns the functional ala9val polymorphism in exon 2 of the magnesium superoxide dismutase gene (MnSOD). MnSOD is one of three isoforms of superoxide dismutase (SOD) that are important, as antioxidants in protecting cells from the deleterious effects of free radicals. The ala9 variant, is associated with less efficient, functional activity of the enzyme. Hori et al20 found a small but, significant increase in the wild type val allele in Japanese schizophrenia patients with TD, suggesting that the ala allele may be protective against, free radical damage in TD. This finding was not, replicated by Zhang et al21 in Chinese schizophrenia patients with TD. However, in a subsequent report Zhang et al22 found that the val-val genotype was in fact associated with TD, but. only in those patients who were carriers of the gly allele of the DRD3 scr9gly polymorphism.
We examined association of the ala9val polymorphism of MnSOD with TD in Israeli schizophrenia patients and also tested for an interaction between this polymorphism and the DRD3 ser9gly polymorphism (Segman et al, unpublished data). Patients were grouped according to whether they were carriers of the ala allele of MnSOD, the gly allele of DRD3, both, or neither. This grouping takes into account an effect, of DRD3 gly to increase susceptibility and a protective effect, of MnSOD ala and anticipates that patients carrying DRD3 gly and lacking MnSOD-ala will be most, susceptible to TD and will have the most severe abnormal involuntary movements. This is indeed the case. The MnSOD-val DRD3-gly genotype combination was the most, frequent, among patients with TD (64%) and the MnSOD-ala DRD3-ser genotype (24%) was the least frequent. Among patients without, TD the pattern was reversed. The most frequent genotype combination was Mn.SOD-ala\ DRD3-ser (76%) and the least frequent, combination was MnSOD-val DRD3-gly (38%, X 2=10.5, df=3, P=0.01). Table I shows that patients with the MnSOD-val\ DRD3-gly genotype combination have the highest, AIM'S total scores and patients with the MnSOD-ala DRD3-ser genotype combination have the lowest (F=6.16; df=3, 102; P=0.0007).
Table I. Abnormal involuntary movements assessed by the Abnormal Involuntary Movements Scale (AIMS) in patients with chronic schizophrenia, grouped according to magnesium superoxide dismutase (MnSOD) and dopamine D3, receptor (DRD3) genotype combinations. MnSOD-alalDRD3-ser. MnSOD ala9 allele carriers (ala-ala homozygotes and ala-val hétérozygotes), DRD3 ser9 homozygotes; MnSOD-alalDRD3-gly: MnSOD ala9 allele carriers, DRD3 gly9 carriers (ser-gly hétérozygotes and gly-gly homozygotes); MnSOD val/DRD3-ser: MnSOD val9 homozygotes, DRD3 ser9 homozygotes; MnSOD va/DRD3-gly: MnSOD val9 homozygotes, DRD3 gly9 carriers. By analysis of variance (ANOVA): F=6.16; df=3,102; P=0.0007 *P=0.0004 vs MnSOD-ala/DRD3-ser, P=0.046 vs MnSOD-ala/DRD3 gly (post-hoc Newman-Keuls test) SD, standard deviation.
| Genotype combination | N | AIMS total score | SD |
| MnSOD-ala/DRD3-ser | 29 | 2.6 | 2.7 |
| MnSOD-ala/DRD3-gly | 47 | 5.7 | 5.4 |
| MnSOD-val/DRD3-ser | 9 | 7.0 | 6.6 |
| MnSOD-val/DRD3-gly | 21 | 9.2 | 7.9* |
Prospects for pharmacogenetic testing in the clinic
The aim of pharmacogenetic research is to develop clinically useful tests that will allow potential responders to a particular psychotropic agent to be identified prospectively, as well as those individuals likely to develop adverse effects. This is not an easy task in the case of psychotropic drugs. Some of the reasons for this difficulty are applicable to drugs used to treat other complex disorders; others are specific to psychotropic agents. Several have been discussed in this paper.
The polygenic basis of pharmacogenetic traits is an issue of major importance. For most traits it, is unclear how many genes arc involved, and genes that have been implicated thus far in well-studied phenotypes such as TD are of small effect. The OR observed rarely exceed 2.0, and for the most part, are less than 1.5. It requires large samples to explore such small gene effects in the definitive fashion required for their inclusion in a pharmacogenetic test. Large samples are also needed in order to tease apart, gene-gene and gene-environment interactions. Recruitment, of large samples inevitably increases the likelihood of stratification, which can lead to spurious results and must be taken into account. A further consideration is that, background and demographic variables must be considered (as pointed out in this paper for the role of age in the manifestation of certain gene effects in TD) as well as treatment-related variables such as medication dose, duration of treatment, and age at onset of treatment. Furthermore, treatment outcome is frequently related to severity of illness, and this must, also be taken into consideration. Thus, it, is clear that a model used to predict treatment outcome or susceptibility to adverse effects will be unavoidably complex, given the number of background and potential predicting variables that will need to be taken into account including gene-gene and gene-environment interactions.
Based on our previous work on the genetics of TD, we have developed a preliminary model that takes into account the various, background, clinical and genetic factors that, we have studied (Scgman et al, unpublished data). We employed logistic regression and entered background and clinical variables known to influence susceptibility to TD such as age, sex, cigarette-smoking, age at first, antipsychotic treatment, duration of antipsychotic treatment, antipsychotic dose in chlorpromazine units, and total score on the Positive And Negative Syndrome Scale (PANSS). The genetic variables included were DRD3 gly9 allele carrier status, MsSOD ala9 allele carrier status, HTR2C ser23 allele carrier status, CYP17 genotype and HTR2A genotype. Interactions were also tested for inclusion, but none were retained in the final model. The overall model (Table II) was significant (r2=0.32; P=0.0005).The variables that, significantly predicted TD within the model were age (OR=1.04, P=0.047), PANSS total score (OR=1.02, P=0.014),. DRD3 gly9 allele carrier status (OR=4.39, P=0.006), and HTR2A 102CC genotype (OR=4.18, P=0.02). MNSOD ala9 allele carrier status and HTR2C ser23 allele carrier status were retained in the overall model, but were not, significant. In this manner, 70.3% of cases could be correctly classified, compared with 60.2% prior to entry of the variables.
Table II. Logistic regression predicting tardive dyskinesia including background, clinical, and genetic variables. PANSS, Positive and Negative Syndrome Scale; CI, confidence interval. *102TT genotype is reference category. Significant values highlighted in bold font.
| Variable | β | SE | Wald | df | P | Odds ratio | 95.0% CI | |
| Lower | Upper | |||||||
| Background variables | ||||||||
| Age | 0.04 | 0.02 | 3.93 | 1 | 0.047 | 1.04 | 1.00 | 1.08 |
| Sex | 0.20 | 0.49 | 0.16 | 1 | 0.686 | 1.22 | 0.47 | 3015 |
| Cigarette-smoking | 0.14 | 0.46 | 0.09 | 1 | 0.766 | 1.15 | 0.47 | 2.83 |
| Clinical variables | ||||||||
| PANSS total score | 0.02 | 0.01 | 6.00 | 1 | 0.014 | 1.02 | 1.00 | 1.03 |
| Age at first antipsychotic treatment | 0.05 | 0.03 | 2.37 | 1 | 0.124 | 1.05 | 0.99 | 1.11 |
| Genetic variables | ||||||||
| DRDS gly9 allele | 1.48 | 0.54 | 7.61 | 1 | 0.006 | 439 | 1.54 | 12.56 |
| HTR2A 102C genotype | 8.80 | 2 | 0.012 | |||||
| HTR2A 102CC genotype* | 1.43 | 0.63 | 5.20 | 1 | 0.023 | 4.18 | 1.22 | 14.26 |
| HTR2A 1027TC genotype* | -020 | 0.54 | 0.14 | 1 | 0.71 | 0.82 | 0.28 | 2.37 |
| MNSOD ala9 allele | -0.39 | 0.51 | 0.58 | 1 | 0.445 | 0.68 | 0.25 | 1.83 |
| HTR2A ser23 allele | -0.94 | 0.53 | 3.13 | 1 | 0.077 | 0.39 | 0.14 | 1 11 |
| Constant | -5.61 | 1.53 | 13.41 | 1 | 0 | 0 |
The model described here is not, sensitive enough to have clinical utility. It is based on a single small sample of subjects, and may not, be generalizable to other samples and populations. While recognizing these limitations, the model does support the concept that a combination of background, clinical, and genetic variables could potentially be used to evaluate a priori the risk for TD in patients treated with antipsychotic drugs that have the potential to induce this adverse effect. This approach could be extended to other pharmacogenetic phenotypes, and ultimately allow the development, of clinically viable pharmacogenetic tests that, will serve as the basis for a rational assessment of cost-benefit ratios in the choice of treatment with antipsychotics and other antipsychotic drugs.
Selected abbreviations and acronyms
- AIMS
Abnormal Involuntary Movements Scale
- EPS
extrapyramidal symptoms
- FGA
first-generation antipsychotic
- 5-HT
serotonin
- PANSS
Positive And Negative Syndrome Scale
- SGA
second-generation antipsychotic
- TD
tardive dyskinesia
Supported in part by grants from the Israel Ministry of Science (Indian-Israeli Human Genome Cooperation) and the Office of the Chief Scientist, Israel Ministry of Health. The authors thank Kyra Kanyas, MA, for assistance with statistical analysis.
REFERENCES
- 1.Lerer B. éd. Pharmacogenetics of Psychotropic Drugs. Cambridge, UK: Cambridge University Press; 2002 [Google Scholar]
- 2.Pickar D., Rubinow K. Pharmacogenetics of psychiatric disorders. Trends Pharmacol Sci. 2001;22:75–83. doi: 10.1016/s0165-6147(00)01603-5. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman JA., Stroup TS., McEvoy JP., et al. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 4.Rochon PA., Stukel TA., Sykora K., et al. Atypical antipsychotics and parkinsonism. Arch Intern Med. 2005;165:1882–1888. doi: 10.1001/archinte.165.16.1882. [DOI] [PubMed] [Google Scholar]
- 5.Poolsup N., Li Wan Po A., Knight TL. Pharmacogenetics and psychopharmacotherapy. J Clin Pharm Ther. 2000;25:197–220. doi: 10.1046/j.1365-2710.2000.00281.x. [DOI] [PubMed] [Google Scholar]
- 6.Lerer B., Segman RH., Fangerau, et al. Pharmacogenetics of tardive dyskinesia: Combined analysis of 780 patients supports association with dopamine D3 receptor gene ser9gly polymorphism. Neuropsychopharmacology. 2001;27:109–119. doi: 10.1016/S0893-133X(02)00293-2. [DOI] [PubMed] [Google Scholar]
- 7.Inada T., Dobashi I., Sugita T., et al. Search for a susceptibility locus to tardive dyskinesia. Hum Psychopharmacol. 1997;12:35–39. [Google Scholar]
- 8.Liao DL., Yeh YC., Chen HM., Chen H., Hong CJ., Tsai SJ. Association between the Ser6Gly polymorphism of the dopamine D3 receptor gene and tardive dyskinesia in Chinese schizophrenic patients. Neuropsychobiology. 2001;44:95–98. doi: 10.1159/000054924. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Barcelo MM., Lam LC., Ungvari GS., Lam VK., Tang WK. Dopamine D3 receptor gene and tardive dyskinesia in Chinese schizophrenic patients. J Neural Transrn. 2001;108:671–677. doi: 10.1007/s007020170044. [DOI] [PubMed] [Google Scholar]
- 10.Lerer B., Segman RH., Tan E-C., et al. Combined analysis of 635 patients confirms an age-related association of the serotonin 2A receptor gene with tardive dyskinesia and specificity for the non-orofacial subtype. Int J Neuropsychopharmacol. 2005;8:411–425. doi: 10.1017/S1461145705005389. [DOI] [PubMed] [Google Scholar]
- 11.Lerer B., Macciardi F., Segman RH., et al. Variability of 5-HT2c receptor cys23ser polymorphism among European populations and vulnerability to affective disorder. Mol Psychiatry. 2001;6:579–585. doi: 10.1038/sj.mp.4000883. [DOI] [PubMed] [Google Scholar]
- 12.Pritchard JK., Rosenberg NA. Use of unlinked genetic markers to detect population stratification in association studies. Am J Hum Genet. 1999;65:220–228. doi: 10.1086/302449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segman RH., Lerer B. Age and the relationship of dopamine D3, serotonin 2C and serotonin 2A receptor genes to abnormal involuntary movements in chronic schizophrenia. Mol Psychiatry. 2002;7:137–139. doi: 10.1038/sj.mp.4000960. [DOI] [PubMed] [Google Scholar]
- 14.Segman RH., Heresco-Levy U., Finkel B., et al. Association between the serotonin 2A receptor gene and tardive dyskinesia in chronic schizophrenia. Mol Psychiatry. 2001;6:225–229. doi: 10.1038/sj.mp.4000842. [DOI] [PubMed] [Google Scholar]
- 15.Basile VS., Masellis M., Potkin SG., Kennedy JL. Pharmacogenomics in schizophrenia: the quest for individualized therapy. Hum Mol Gen. 2002;11:2517–2530. doi: 10.1093/hmg/11.20.2517. [DOI] [PubMed] [Google Scholar]
- 16.Segman RH., Heresco-Levy U., Finkel B., et al. Association between the serotonin 2C receptor gene and tardive dyskinesia in chronic schizophrenia; additive contribution of 5-HT2Cser and DRD3gly alleles to susceptibility. Psychopharrnacology. 2000;152:408–413. doi: 10.1007/s002130000521. [DOI] [PubMed] [Google Scholar]
- 17.Segman RH., Heresco-Levy U., et al. Interactive effect of cytochrome P450 17α-hydroxylase and dopamine D3 receptor gene polymorphisms on abnormal involuntary movements in chronic schizophrenia. Biol Psychiatry. 2002;51:261–263. doi: 10.1016/s0006-3223(01)01302-6. [DOI] [PubMed] [Google Scholar]
- 18.Feigelson IS., Shames IS., Pike MC., Coerzec GA., Stanozyk PZ., Henderson BE. Cytochrome P450c17alpha gene polymorphism is associated with elevated estrogen and progesterone concentrations. Cancer Res. 1998;58:585–587. [PubMed] [Google Scholar]
- 19.Basile VS., Ozdemir V., Masellis M., et al. Lack of association between serotonin-2A receptor gene (HTR2A) polymorphisms and tardive dyskinesia in schizophrenia. Mol Psychiatry. 2001;6:230–234. doi: 10.1038/sj.mp.4000847. [DOI] [PubMed] [Google Scholar]
- 20.Hori H., Ohmori O., Shinkai T. Manganese superoxide dismutase dene polymorphism and schizophrenia: relation to tardive dyskinesia. Neuropsychopharmacology. 2000;23:170–177. doi: 10.1016/S0893-133X(99)00156-6. [DOI] [PubMed] [Google Scholar]
- 21.Zhang ZJ., Zhang XB., Hou G., Sha WW., Reynolds GP. The increased activity of plasma manganese superoxide dismutase in tardive dyskinesia is unrelated to the Ala-9Val polymorphism. J Psychiatry Res. 2002;36:317–324. doi: 10.1016/s0022-3956(02)00007-9. [DOI] [PubMed] [Google Scholar]
- 22.Zhang ZJ., Zhang XB., Hou G., Yao H., Reynolds GP. Interaction between polymorphisms of the dopamine D3 receptor and manganese superoxide dismutase genes in susceptibility to tardive dyskinesia. Psychiatr Genet. 2003;13:187–192. doi: 10.1097/00041444-200309000-00010. [DOI] [PubMed] [Google Scholar]