Abstract
Schizophrenia is a chronic progressive disorder that has at its origin structural brain changes in both white and gray matter. It is likely that these changes begin prior to the onset of clinical symptoms in cortical regions, particularly those concerned with language processing. Later, they can be detected by progressive ventricular enlargement. Current magnetic resonance imaging (MRI) technology can provide a valuable tool for detecting early changes in cortical atrophy and anomalous language processing, which may be predictive of who will develop schizophrenia.
Keywords: schizophrenia, magnetic resonance imaging, brain, ventricular enlargement, cortical atrophy
Abstract
La esquizofrenia es una enfermedad progresiva, crónica, que se origina por cambios estructurales cerebrales, tanto de la sustancia blanca como de la sustancia gris. Es probable que estos cambios comiencen antes de la aparición de los síntomas clínicos en regiones corticales, especialmente aquéllas relacionadas con el procesamiento del lenguaje. Más tardíamente estos cambios pueden ser detectados por un progresivo crecimiento de los ventrículos. La tecnología actual de imágenes por resonancia magnética puede aportar una valiosa herramienta para detectar precozmente cambios atrofíeos corticales y anomalías en el procesamiento del lenguaje que podrían permitir de identificar personas susceptibles de desarrollar una esquizofrenia.
Abstract
La schizophrénie est une maladie progressive chronique pour laquelle on retrouve, à l'origine, des modifications cérébrales structurales des substances grise et blanche. Il est probable que ces modifications surviennent avant le début de l'apparition des symptômes cliniques dans les régions corticales, surtout celles concernées par le processus du langage. Plus tardivement elles peuvent être détectées par un élargissement ventriculaire progressif. L'IRM (Imagerie par résonance magnétique) actuelle peut être un outil précieux pour détecter les modifications précoces d'atrophie corticale et d'anomalies de processus du langage qui permettraient d'identifier les personnes susceptibles de développer une schizophrénie.
It has long been known that the disorder we currently call schizophrenia is characterized by progressive clinical and cognitive change, as well as structural brain anomalies. Kraepelin himself in his series of textbooks1 (particularly documented in 1919) illustrated his own views of what the cellular damage to the cortex must, look like, although there is no evidence that this was actually based on any research findings. However, as early as the late 1920s, a few fairly large pneumoencephalographic studies had been conducted, which showed on a more macroscopic level that large ventricles were characteristic of patients with chronic schizophrenia.2-7 At the time, this was assumed to represent, a degenerative process.
To date, numerous other structural brain differences between chronic patients with schizophrenia and controls have been reported from computed tomography (CT) and magnetic resonance imaging (MRI) studies. These include nonlocalizcd reduced gray-matter and white-matter changes, temporal lobe volume reductions, and, particularly, anomalies of the superior temporal gyrus and temporal and frontal lobe white-matter connections, ic, arcuate, uncinate, and fornix.8,9
Some of the early pneumoencephalographic studies repeated the evaluations of patients a few years later and clearly a showed progressive change that correlated with clinical deterioration, but only present in some patients. 3,4,6 It should be noted that, while there were certainly other treatments available at the time of these studies, neuroleptics had not, yet, been introduced. This is important, since recently there has been much interest, in the idea that neuroleptics might be responsible for certain progressive brain changes (see below), but clearly this cannot, be the complete explanation.
Beginning in the late 1980s, we conducted a longitudinal study of individuals who had a first psychotic episode and were admitted to hospital, and were then reevaluated in the community as part, of a 10-year longitudinal study of brain changes in schizophrenia.10-14 While Figure 1 illustrates an extreme example of what, was observed when subjects from this study were rescanned, it was clear from these longitudinal data that ventricular enlargement is progressive, and not a developmcntally fixed parameter as previously thought.15
Figure 1. Magnetic resonance imaging (MRI) of a female patient who initially was scanned at the time of hospitalization for a first episode of schizophrenia. At the tenth year of follow-up, at age 34, she was an outpatient with a diagnosis of chronic schizophrenia stabilized with predominantly negative symptoms. She also had a brother with chronic schizophrenia, but he did not participate in the longitudinal study.
Despite this, it is likely that the progression begins early and can be detected even before the onset of clinical symptoms. At the first hospitalization, we and others could already detect many differences, although not all differences were reported in chronic patients and, also, not, to the same extent, as they were seen in the chronic patients.8,10,11
Over the past, decade, there have been several short-term longitudinal studies. First, there are the studies beginning with an initial scan at the first episode (Table I) with varying results.10-14,16-26 In the studies from our own cohort, we found ventricular enlargement over time and whole hemispheric volume decreases over a 5- to 10-year period12-14 some independent investigative groups support, this as well (Table I), while other studies support variable regional changes. However, whether these progressive changes are correlated with outcome, and are thus clinically relevant, remains unclear.
Table I. Brain changes over time in first-episode schizophrenia.
| Study | Number of patients/ | Years of follow-up | Findings |
| number of controls | |||
| DeGreef et al16 1991 | 13/8 | 1-2 | No change in ventricles, change |
| Lieberman et al,17 2001 | 51/13 | 1-2 | associated with poor outcome |
| Desli et al,10-14 1991, 1992 | 50/20 | 4-5 | Decreased hemisphere, cerebellum, increased ventricles |
| 1995, 1997, 2004 | 26/10 | 10 | and associated with good outcome, no decrease in |
| superior temporal gyrus | |||
| Gur et al,18 1998 | 20/17 | 2-3 | Decreased frontal lobe, associated with good outcome |
| Kasai et al,19 2003 | 13/14 | 1.5 | Decreased left superior temporal gyrus and planum temporale |
| Jaskiw et al,20 1994 (CT) | 7/0 | 5-8 | No ventricle change |
| Sponheim et al,21 1991 (CT) | 15/0 | 1-3 | No ventricle change |
| Vita et al22 1994 (CT) | 9/0 | 2-4 | No ventricle change |
| Wood et al,23 2001 | 30/26 | 0.5-4.2 | Decreased whole brain |
| Cahn et al,24 2002 | 34/36 | 1 | Decreased gray metter, increased ventricle, associated with |
| poor outcome and medication | |||
| James et al,22 2002 | 16/16 | 2.7/1.7 | No change |
| Ho et al,26 2003 | 73/23 | 3.0 | Decreased frontal white matter and increased cerebrospinal fluid |
Interestingly, the studies of chronic patients more consistently show ventricular increases over time, particularly in the more severely ill patients (Table II).27-38 This discrepancy could be explained if ventricular enlargement is secondary to underlying changes in the cortex that may begin earlier (Table III) 39-42 and, when they are extensive enough, are detected indirectly by progressive ventricular enlargement. Thus, ventricular enlargement, would more consistently be seen later in the course of the illness. We further hypothesize that the cortical brain regions most, affected are those involved in language processing (ie, superior temporal gyrus and its connections) and that, the symptoms of schizophrenia develop on the basis that these pathways are anomalous.
Table II. Brain changes over time in chronic schizophrenia .
| Study | Number of patients/ | Years of follow-up | Findigs |
| number of controls | |||
| Davis et al,27 1998 (CT) | 53/13 | 5 | Increased ventricles, poor outcome only |
| Illowsky et al,28 1998 (CT) | 13/0 | 7-9 | No change in ventricles |
| Kermali et al,29 1989 (CT) | 18/8 | 3 | Increased ventricles (1/3 patients) |
| Mathalon et al,30 1998 | 24/25 | 0.7-7.5 | Decreased gray matter, increased cerebrospinal fluid, |
| decreased superior temporal gyrus | |||
| Nair et al,31 1997 | 18/5 | 1.1-3.8 | Increased ventricles, poor outcome only |
| Nasrallah et al,32 1986 (CT) | 11/0 | 3 | No change in ventricles |
| Rapoport et al,33 1997; | 16-24 | 1.5-4 | Increased ventricles, decreased hemispheres, |
| Jacobsen et al,34 1998; | 50/101 | temporal lobe, superior temporal gyrus, | |
| Thompson et al,35 2011 | hippocampus, thalamus, and striatum | ||
| Keller et al,36 2003 | |||
| Vita et al,37 1988 (CT) | 15/0 | 2-5 | No change in ventricles |
| Woods et al,38 1990 (CT) | 9/0 | 1-4.5 | Increased ventricles (8/9 patients) |
Table III. Studies of brain changes in prodromal patients.
| Study | No of subjects | Follow-up diagnosis | Initial findings | Changes in follow-up |
| Pantelis et al,39 2003 | 21 | 1 year | Decreased rihgt temporal, | Decreased left parahippocampal |
| 10 psychotic | right inferior frontal, | gyrus, left fusiform, left orbitofrontal, | ||
| 11 nonpsychotic | cingulate bilaterally | left cerebellum, cingulate bilaterally, | ||
| left temporal | ||||
| Wood (unpublished data) | 75 | 23 psychotic | ||
| 52 nonpsychotic | ||||
| Lawrie et al,40 2002 | 66 | 2 years; | Decreased left and right | Decreased right and left temporal, |
| Job et al,41 2005 | 19 psychotic | anterior cingulate, left | right and left superior temporal gyrus, | |
| 47 nonpsychotic | parahippocampal gyrus, | left cingulate, left and right uncinate, | ||
| left temporal lobe gray, | left fusiform, left uncus, left and right | |||
| right preffrontal, thalamus | parahippocampal gyrus, right amygdala; | |||
| Johnstone et al,42 2002 | 65 | 1.5 years: | no ventricle change | |
| 18 psychotic |
The questions that then remain are:
Is the progression is an artifact of neuroleptic medication or some other physiological process unrelated to the illness pathology; or is it central to the process and begin prior to the clinical syndrome?
Is the progression due to decreased myelination or a faulty pruning process during adolescence?
Is the progression sufficient to explain all the brain changes seen in schizophrenia?
Neuroleptics and progressive brain change
Lieberman and colleagues recently published a paper in the Archives in General. Psychiatry from a study comparing olanzapine with haloperidol in first-episode patients and comparing any brain changes to control changes over time.“13 They claim that, over a 2-year period, whole gray matter volume decreases significantly more in patients administered haloperidol than in controls or patients on olanzapine. However, the time of the follow-up MRI scans was short; there were many dropout subjects in this study and disproportionately among the groups; and some time periods were missing in one group entirely, thus hampering interpretation of these results.
There have now been several other studies attempting to examine the question of neuroleptic effects on brain structure. While it, appears consistently in most, but, not all, studies that the caudate enlarges with typical neuroleptics, the changes seen with respect, to other cortical regions and ventricular enlargement have yet to be shown to be due to medication (Table IV).“35
Table IV. Neuroleptics and brain morphology over time.
| Study | Patients | Treatment | Duration | Findings |
| Dazzan et al,44 2005 | 84 first-episode | Typical antipsychotic versus | 36 months | Increased thalamus (atyptcal), |
| atypical antipsychotic | increased right ventricle (typical), | |||
| versus no treatment | decreased frontal (typical) | |||
| Garver et al,45 2005 | 19 | Typical antipsychotic versus | 1 month | Increased cortical gray (atypical), |
| atypical antipsychotic | no change (typical) | |||
| versus no treatment | ||||
| Lieberman et al,43 2005 | 161 | Haloperidol versus olanzapine | Maximum 24 months | Decrease in gray matter, no change |
| with haloperidol or olanzapine | ||||
| Massana et al,46 2005 | 11 first-episode | Risperidone | 3 months | Increased caudate |
| Lang et al,47 2001 | 30 first-episode | Risperidone | 12 months | No change in caudate |
| Scheepers et al,48 2001 | 28 nonresponders | Clozapine | 5 months | Decreased left caudate in clozapine |
| responders only | ||||
| Corson et al,49 1999 | 23 male | Typical antipsychotic versus | 24 months | Increased caudate (typical), |
| atypical antisychotic | decreased caudate (atypical) | |||
| Chakos et al,50 1994 | 29 first-episode | Typical antipyschotic | 18 months | Increased caudate |
| Keshavan et al,51 1994 | ? | Typical antipyschotic | ? | Increased caudate |
How early do the brain changes begin?
There are two large and interesting independent, studies of people with a prodromal syndrome that, is high likely to lead to schizophrenia - one in Scotland-“ and another in Melbourne,39 Australia (Table III). Both these studies have performed very parallel investigations. Initially during the prodrome, a change in brain structure seems to be present in the temporal lobe volume and cingulated. On follow-up in those who have gone onto a psychotic episode, further changes can be seen in the cingulate, temporal lobe, and parahippocampal gyrus. These two independent studies have results that are not entirely consistent with each other, but it is interesting that neither show ventricular enlargement, or its progression at this stage. In general, while both research groups see initial changes in temporal and frontal lobes in people who later develop schizophrenia and progressive change in the time interval from prodrome to onset, of clinical illness, the specific changes that are clearly predictive of illness need to be further delineated.
What is the cause?
The underlying basis for the changes detected by imaging could be related to abnormalities in axonal integrity and organization that begin to take place during the normal adolescent neuronal pruning and reorganizational process, and continue through out, the lifetime of the individual during aging and brain response to normal stresses.52,53 In some individuals, it, may even begin prenatally,54 but last, a lifetime. Perhaps examining white matter integrity will give clues. We now have the techniques in MRI, ie, diffusion tensor imaging (DTI) and magnetization transfer (MT). DTI55 focuses on the diffusion of water in the brain. Two measurements based on DTI images are the apparent, diffusion coefficient (ADC), which measures the water content and reflects the amount of cerebrospinal fluid (CSF),56 and fractional anisotropy (FA), which measures the direction of flow or, indirectly, the lining up of fibers. The FA is high when fibers are orientated in one direction and low when there is diffusion and the fibers are more disorganized. The ADC is high when the water content, is high and low when the water content, is low. Magnetization transfer (MT) is a proton-weighted MRI image that can give information about the integrity of myelin, in particular with the quantification of the magnetization transfer ratio (MTR).57
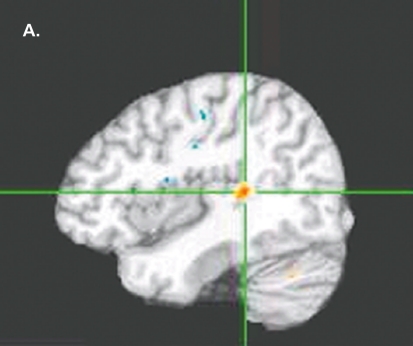
The most, recent, focus of our research group has been to extend the previous longitudinal studies back in time from the first, episode to the study of individuals at high genetic risk for schizophrenia who arc in the age range for peak incidence of developing the disorder. Current preliminary data are illustrated on 15 such adolescents, 15 controls, and 15 of their siblings with chronic schizophrenia (Figures 2 to 6). Figure 2 shows a DTI comparison of FA in high-risk subjects with controls illustrating evidence of reduced FA (or directional axonal organization) already taking place in the left, posterior superior temporal gyrus. Figure 3 shows evidence of higher ADC (or water content, ic, CSF) already evident in the left parahippocampal gyrus and right, superior temporal gyrus in the high-risk patients. This is more widespread in those with schizophrenia, suggesting that atrophic changes occur early and could be progressing into later stages of illness. Figure 4andFigure 5 show that MT changes are also present, ie, changes in fiber membranes in the superior frontal gyrus and posterior cingulate. In additionne have been performing functional MRI (fMRI) lexical decision task, as previously developed,58 which has the ability to show lateralized activation in the superior temporal gyrus in normal individuals. In our preliminary analyses, less lateralized activation is seen in the individuals at, high-risk for schizophrenia than controls, similar but to a lesser extent, than what, is seen in the patients with chronic schizophrenia (Figure 6). These studies taken together indicate that changes are occurring early in the brains of people who are likely to later develop schizophrenia, and that these changes are relevant to those regions of the brain that are involved in language processing.
Figure 2. Diffusion tensor imaging (DTI). Fractional anisotropy (FA) of 15 subjects at high genetic risk for schizophrenia. Sagittal view showing FA reduced in the left posterior superior temporal gyrus in high-risk subjects compared with controls (P<0.01 , minimum cluster size =100). Talairach coordinates of cluster peaks: x=-41 , y=-36, z=9.
Figure 3. Sagittal, coronal, and axial views of the region in the vicinity of the left parahippocampal gyrus and right superior frontal gyrus, where the apparent diffusion coefficient (ADC) was higher both in (A, C) subjects at high genetic risk for schizophrenia and (B, D) the patients with schizophrenia P<0.01 , cluster size >200 mm3 as compared with controls. Sagittal, coronal, and axial views of the region in the left superior frontal gyrus and left middle frontal gyrus shows that subjects at high genetic risk for schizophrenia (E, G) and patients with schizophrenia (F, H) had higher ADC compared with controls: P<0.01, cluster size >200 mm3 in these regions as well.
Figure 4. Magnetisation transfer (MT): Coronal (A and C) and sagittal (B) views showing a greater magnetisation transfer ratio (MTR) in controls compared with subjects at high genetic risk for schizophrenia bilaterally in the superior frontal gyrus (P<0.05, minimum cluster size =100). Talairach coordinates of cluster peaks: A and B, x=-10,y=14, z=52; C, x=10, y=1 5, z=51.
Figure 5. Magnetization transfer (MT). Greater magnetization transfer ratio (MTR) is shown in controls versus subjects at high genetic risk for schizophrenia in the posterior cingulate gyrus (P<0.05, minimum cluster size =100). Talairach coordinates of cluster peaks: A, x=-0, y=-36, z=27; B, x=8, y=-45, z=22.
Figure 6. Functional magnetic resonance imaging (fMRI) showing brain activation during a lexical decision task (no REST contrast) in 11 controls (A), 9 subjects at high risk for schizophrenia (B), and 11 patients with chronic schizophrenia (C). Lateralization of activation is reduced in the schizophrenic patients compared to controls (P<0.01) as well as the subjects at high risk, but to a lesser extent (P<0.01).
Conclusion
It appears that brain structural change is detectable in both gray and white matter prior to illness onset, that, active progression of the changes may also begin prior to the onset of clinical symptoms, that progressive brain changes may account, for the brain structural anomalies seen in chronic schizophrenia, and that the structures involved in language processing are affected. White-matter anomalies in the anatomical connections relevant to language and/or myelination of these connections could be involved. The ability to have specific MRI predictors of who will develop schizophrenia among those at high risk appears hopeful for the near future. Having the ability to predict, the development, of illness will then lead to studies to determine whether early pharmacological treatment, will prevent, the cortical progressive brain cortical change and, in doing so, have a significant effect, on clinical outcome.
This work was supported by R21 MH071720-01 from the National Institute of Mental Health. The co-authors wish to thank the following investigators from the Center for Advanced Brain Imaging at the Nathan S. Kline Institute for assistance in developing and implementing the new MRI protocol as well as image analysis for preliminary pilot data shown here: Babak Ardekani, Craig Branch, Matthew Hoptman, and Raj Sangoi.
Contributor Information
Lynn E. DeLisi, The Nathan S. Kline Institute for Psychiatric Research, 140 Old Orangeburg Road, Orangeburg, New York, NY, USA.
Kamila U. Szulc, The Nathan S. Kline Institute for Psychiatric Research, 140 Old Orangeburg Road, Orangeburg, New York, NY, USA.
Hilary C. Bertisch, The Nathan S. Kline Institute for Psychiatric Research, 140 Old Orangeburg Road, Orangeburg, New York, NY, USA.
Magda Majcher, The Nathan S. Kline Institute for Psychiatric Research, 140 Old Orangeburg Road, Orangeburg, New York, NY, USA.
Kyle Brown, The Nathan S. Kline Institute for Psychiatric Research, 140 Old Orangeburg Road, Orangeburg, New York, NY, USA.
REFERENCES
- 1.Kraepelin E. Dementia Praecox and Paraphrenia. Barclay RM, trans. New York, NY: Krieger; 1971 [Google Scholar]
- 2.DeLisi LE. Defining the course of brain structural growth and plasticity in schizophrenia. Psychiatry Res-Neuroimag. 1999;92:1–9. doi: 10.1016/s0925-4927(99)00033-5. [DOI] [PubMed] [Google Scholar]
- 3.Moore MD., Nathan AR., Elliot G., et al. Encéphalographie studies in mental disease. Am J Psychiatry. 1935;92:43–67. [Google Scholar]
- 4.Haug JO. Pneumoencephalographic studies in mental disease. Acta Psychol Neurol ScandSuppl. 1963;165:11–104. [PubMed] [Google Scholar]
- 5.Haug JO. Pneumoencephalographic evidence of brain atrophy in acute and chronic schizophrenic patients. Acta Psychiatr Scand. 1982;66:374–383. doi: 10.1111/j.1600-0447.1982.tb06719.x. [DOI] [PubMed] [Google Scholar]
- 6.Huber G. Pneumoencephalographische und psychopathologische biider bei endogen psychosen. Berlin, Germany: Springer; 1957 [Google Scholar]
- 7.Jacobi W., Winkler H. Encephalographische studien an chronische schizophrenen. Archiv Psychiatr Nervenkrankheiten. 1927;81:299–332. [Google Scholar]
- 8.Shenton ME., Dickey CC., Frumin M., McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubicki M., McCarley RW., Shenton ME. Evidence for white matter abnormalities in schizophrenia. CurrOpin Psychiatry. 2005;18:121–134. doi: 10.1097/00001504-200503000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeLisi LE., Hoff A., Schwartz J., et al. Brain morphology in first-episode schizophrenic-like psychotic patients: a quantitative magnetic resonance imaging study. Biol Psychiatry. 1991;29:159–175. doi: 10.1016/0006-3223(91)90044-m. [DOI] [PubMed] [Google Scholar]
- 11.DeLisi LE., Stritzke P., Riordan H., et al. The timing of brain morphological changes in schizophrenia and their relationship to clinical outcome. Biol Psychiatry. 1992;31:241–254. doi: 10.1016/0006-3223(92)90047-4. [DOI] [PubMed] [Google Scholar]
- 12.DeLisi LE., Tew W., Xie SH., et al. A prospective follow-up study of brain morphology and cognition in first episode schizophrenic patients. Biol Psychiatry. 1995;38:349–360. doi: 10.1016/0006-3223(94)00376-e. [DOI] [PubMed] [Google Scholar]
- 13.DeLisi LE., Grimson R., Sakuma M., Tew W., Kushner M., Hoff AL. Schizophrenia as a chronic active brain process: a study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry ResNeuroimag. 1997;74:129–140. doi: 10.1016/s0925-4927(97)00012-7. [DOI] [PubMed] [Google Scholar]
- 14.DeLisi LE., Sakuma M., Maurizio A., Hoff AL. Ten-year follow-up of ventricular enlargement in first-episode patients with schizophrenia. Psychiatry Res-Neuroimag. 2004;130:57–70. doi: 10.1016/j.pscychresns.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 16.DeGreef G., Ashtari M., Wu H., Borenstein M., Geisler S., Lieberman J. Follow-up MRI study in first-episode schizophrenia. Schizophr Res. 1991;5:204–206. doi: 10.1016/0920-9964(91)90075-3. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman J., Chakos M., Wu H., et al. Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry. 2001;49:487–499. doi: 10.1016/s0006-3223(01)01067-8. [DOI] [PubMed] [Google Scholar]
- 18.Gur RE., Cowell P., Turetsky Bl., et al. A follow-up magnetic resonance imaging study of schizophrenia. Relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry. 1998;55:145–1 52. doi: 10.1001/archpsyc.55.2.145. [DOI] [PubMed] [Google Scholar]
- 19.Kasai K., Shenton ME., Salisbury DF., et al. Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am J Psychiatry. 2003;160:1 56–164. doi: 10.1176/appi.ajp.160.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaskiw GE., Juliano DM., Goldberg TE., Hertzman M., Urow-Hamell E., Weinberger DR. Cerebral ventricular enlargement in schizophreniform disorder does not progress: a seven-year follow-up study. Schizophr Res. 1994;14:23–28. doi: 10.1016/0920-9964(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 21.Sponheim SR., lacono WG., Beiser M. Stability of ventricular size after the onset of psychosis in schizophrenia. Psychiatry Res-Neuroimag. 1991;40:21–29. doi: 10.1016/0925-4927(91)90026-m. [DOI] [PubMed] [Google Scholar]
- 22.Vita A., Giobbio GM., Died M., et al. Stability of cerebral ventricular size from the appearance of the first psychotic symptoms to the later diagnosis of schizophrenia. Biol Psychiatry. 1994;35:960–962. doi: 10.1016/0006-3223(94)91243-2. [DOI] [PubMed] [Google Scholar]
- 23.Wood SJ., Velakoulis D., Smith DJ., et al. A longitudinal study of hippocampal volume in first episode psychosis and chronic schizophrenia. Schizophr Res. 2001;52:37–46. doi: 10.1016/s0920-9964(01)00175-x. [DOI] [PubMed] [Google Scholar]
- 24.Cahn W., Hulshoff P., Hilleke E., et al. Brain volume changes in firstepisode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59:1002–1010. doi: 10.1001/archpsyc.59.11.1002. [DOI] [PubMed] [Google Scholar]
- 25.James AC., Javaloyes A., James S., Smith DM. Evidence for non-progressive changes in adolescent-onset schizophrenia: follow-up magnetic resonance imaging study. Br J Psychiatry. 2002;180:339–344. doi: 10.1192/bjp.180.4.339. [DOI] [PubMed] [Google Scholar]
- 26.Ho BC., Andreasen NC., Nopoulos P., et al. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry. 2003;60:585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- 27.Davis KL., Buchsbaum MS., Shihabuddin L., et al. Ventricular enlargement in poor-outcome schizophrenia. Biol Psychiatry. 1998;43:783–793. doi: 10.1016/s0006-3223(97)00553-2. [DOI] [PubMed] [Google Scholar]
- 28.Illowsky BP., Juliano DM., Bigelow LBG., Weinberger DR. Stability of CT scan findings in schizophrenia: results of an 8-year follow-up study. J Neurol Neurosurg Psychiatry. 1998;51:209–213. doi: 10.1136/jnnp.51.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kemali D., Maj M., Galderisi S., Milici N., Salvati A. Ventricle-to-brain ratio in schizophrenia: a controlled follow-up study. Biol Psychiatry. 1989;26:753–756. doi: 10.1016/0006-3223(89)90115-7. [DOI] [PubMed] [Google Scholar]
- 30.Mathalon DH., Sullivan EV., Lim KO., Pfefferbaum A. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2001 ;58:148–157. doi: 10.1001/archpsyc.58.2.148. [DOI] [PubMed] [Google Scholar]
- 31.Nair TR., Christensen JD., Kingsbury SJ., Kumar NG., Terry WM., Garver DL. Progression of cerebral ventricular enlargement and the subtyping of schizophrenia. Psychiatry Res. 1997;74:141–1 50. doi: 10.1016/s0925-4927(97)00013-9. [DOI] [PubMed] [Google Scholar]
- 32.Nasrallah HA., Olson SC., McCalley-Whitters M., Chapman S., Jacoby CG. Cerebral ventricular enlargement in schizophrenia: a preliminary follow-up study. Arch Gen Psychiatry. 1986;43:157–159. doi: 10.1001/archpsyc.1986.01800020067008. [DOI] [PubMed] [Google Scholar]
- 33.Rapoport JL., Giedd JN., Kumra S., et al. Childhood schizophrenia: progressive ventricular change during adolescence. Arch Gen Psychiatry. 1997;54:897–903. doi: 10.1001/archpsyc.1997.01830220013002. [DOI] [PubMed] [Google Scholar]
- 34.Jacobsen LK., Giedd JN., Castellanos X., et al. Progressive reduction of temporal lobe structures in childhood-onset schizophrenia. Am J Psychiatry. 1998;155:678–685. doi: 10.1176/ajp.155.5.678. [DOI] [PubMed] [Google Scholar]
- 35.Thompson PM., Vidal C., Giedd JN., et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very earlyonset schizophrenia. Proc Natl Acad Sci USA. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keller A., Castellanos FX., Vaituzis AC., Jeffries NO., Giedd JN., Rapoport JL. Progressive loss of cerebellar volume in childhood-onset schizophrenia. Am J Psychiatry. 2003;160:128–133. doi: 10.1176/appi.ajp.160.1.128. [DOI] [PubMed] [Google Scholar]
- 37.Vita A., Sacchetti E., Valvassori G., Cazullo CL. Brain morphology in schizophrenia: a 2-5 year CT scan follow-up study. Acta Psychiatr Scand. 1988;78:618–621. doi: 10.1111/j.1600-0447.1988.tb06394.x. [DOI] [PubMed] [Google Scholar]
- 38.Woods BT., Yurgelun-Todd D., Benes FM., Frankenburg FR., Pope HC., McSparren J. Progressive ventricular enlargement in schizophrenia: comparison to bipolar affective disorder and correlation to clinical course. Biol Psychiatry. 1990;27:341–352. doi: 10.1016/0006-3223(90)90008-p. [DOI] [PubMed] [Google Scholar]
- 39.Pantelis C., Velakoulis D., McGorry PD., et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 40.Lawrie SM., Whalley HC., Âbukmeil SS., et al. Temporal lobe volume changes in people at high risk of schizophrenia with psychotic symptoms. Br J Psychiatry. 2002;181:138–143. doi: 10.1017/s0007125000161860. [DOI] [PubMed] [Google Scholar]
- 41.Job DE., Whalley HC., Johnstone EC., Lawrie SM. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005;25:1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Johnstone EC., Lawrie SM., Cosway R. What does the Edinburgh high-risk study tell us about schizophrenia? Am J Med Genet (Neuropsychiatr Genet). 2002;114:906–912. doi: 10.1002/ajmg.b.10304. [DOI] [PubMed] [Google Scholar]
- 43.Lieberman JA., Tollefson GD., Charles C., et al. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62:361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- 44.Dazzan P., Morgan KD., Orr K., et al. Different effects of typical and atypical antipsychotics on grey matter in first episode psychosis: the AESOP study. Neuropsychopharmacology. 2005;30:765–774. doi: 10.1038/sj.npp.1300603. [DOI] [PubMed] [Google Scholar]
- 45.Garver DL., Holcomb JA., Christensen JD. Cerebral cortical gray expansion associated with two second-generation antipsychotics. Biol Psychiatry. 2005;58:62–66. doi: 10.1016/j.biopsych.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Massana G., Salgado-Pineda P., Junque C., et al. Volume changes in gray matter in first-episode neuroleptic-naive schizophrenic patients treated with risperidone. J. Clin Psychopharmacol. 2005;25:111–117. doi: 10.1097/01.jcp.0000155818.29091.53. [DOI] [PubMed] [Google Scholar]
- 47.Lang DJ., Kopala LC., Vandorpe RA., et al. An MRI study of basal ganglia volumes in first-episode schizophrenia patients treated with risperidone. Am J Psychiatry. 2001;158:625–631. doi: 10.1176/appi.ajp.158.4.625. [DOI] [PubMed] [Google Scholar]
- 48.Scheepers FE., Gispen de Wied CC., Hulshoff Pol HE., Kahn RS. Effect of clozapine on caudate nucleus volume in relation to symptoms of schizophrenia. Am J Psychiatry. 2001;1 58:644–646. doi: 10.1176/appi.ajp.158.4.644. [DOI] [PubMed] [Google Scholar]
- 49.Corson PW., Nopoulos P., Miller DD., et al. Change in basal ganglia volume over 2 years in patients with schizophrenia: typical versus atypical neuroleptics. Am J Psychiatry. 1999;156:1200–1204. doi: 10.1176/ajp.156.8.1200. [DOI] [PubMed] [Google Scholar]
- 50.Chakos MH., Lieberman JA., Bilder RM., et al. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry. 1994;1 51:1430–1436. doi: 10.1176/ajp.151.10.1430. [DOI] [PubMed] [Google Scholar]
- 51.Keshavan MS., Bagwell WW., Haas GL., et al. Changes in caudate volume with neuroleptic treatment. Lancet. 1994;344:1434. doi: 10.1016/s0140-6736(94)90599-1. [DOI] [PubMed] [Google Scholar]
- 52.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982-1983;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 53.DeLisi LE. Is schizophrenia a lifetime disorder of brain plasticity, growth and aging? Schizophr Res. 1997;23:119–129. doi: 10.1016/S0920-9964(96)00079-5. [DOI] [PubMed] [Google Scholar]
- 54.Murray RM., Jones P., O'Callaghan E. Fetal brain development and later schizophrenia. Ciba Foundation Symposium. 1991;156:155–163. doi: 10.1002/9780470514047.ch10. [DOI] [PubMed] [Google Scholar]
- 55.Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- 56.Ardekani BA., Bappal A., D'Angelo D., et al. Brain morphomety using diffusion weighted MRI: application to schizophrenia. Neuroreport. 2005;16:1455–1459. doi: 10.1097/01.wnr.0000177001.27569.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henkelman RM., Stanisz GJ., Graham SJ. Magnetization transfer in MRI: A review. NMR Biomed. 2001;14:57–64. doi: 10.1002/nbm.683. [DOI] [PubMed] [Google Scholar]
- 58.Pexman PM., Lupker SJ., Reggin LD. Phonological effects in visual word recognition: investigating the impact of feedback activation. J Exp Psychol Learning Memory Cogn. 2002;28:572–584. doi: 10.1037//0278-7393.28.3.572. [DOI] [PubMed] [Google Scholar]