Abstract
Since the introduction of antidepressants to psychopharmacology in the 1960s, the Hamilton Depression Rating Scale (HAM-D) has been the most frequently used rating scale for depression. When used as a scale for prediction of outcome with antidepressants, the HAM-D, by its total score, has obtained limited use analogous to the Diagnostic and Statistical Manual of Mental Disorders. 4th ed (DSM-IV) diagnosis of major depression. Most research has been devoted to the use of the HAM-D to discriminate between placebo and active drugs or to show dose-response relationship in patients with major depression. An improvement in the total HAM-D score during a drug trial does not, however, in itself qualify the drug as an antidepressant, because the total score is not a sufficient statistic. The problem of statistical versus clinical significance when analyzing placebo-controlled trials, including dose-response relationship, is outlined, with the recommendation to use effect size statistics.
Keywords: depression rating scales, HAM-D, antidepressant, major depression, placebo-controlled trials
Abstract
Desde que se introdujeron los antidepresivos en la psico farmacología, en la década de 1960, la escala de depresión de Hamilton (HAM-D) ha sido la escala de evaluación para la depresión que se ha utilizado con mayor frecuencia. Cuando se emplea la HAM-D como escala para la predicción de la respuesta a los antidepresivos, por su puntuación total, su utilización es limitada tal como ocurre con el diagnóstico de depresión mediante el DSM-IV (Manual Diagnóstico y Estadístico de los Trastornos Mentales, Cuarta Edición). Se ha dedicado gran parte de la investigación al uso de la HAM-D para discriminar entre placebo y drogas activas o para mostrar la relación dosis-respuesta en pacientes con depresión mayor. Sin embargo, una mejoría en la puntuación total de la HAM-D durante un ensayo clínico no califica a la droga como antidepresivo, ya que la puntuación total no es un estadístico suficiente. Se comenta el problema de la significación estadística versus la clínica cuando se analizan los ensayos placebo controlados incluyendo la relación dosis-respuesta, con la recomendación de utilizar estadísticos con efecto sobre el tamaño.
Abstract
Depuis l'introduction des antidépresseurs dans la psychopharmacologie au cours des années 1960, l'échelle d'évaluation de la dépression de Hamilton (HAM-D) est celle la plus utilisée pour la dépression. L'HAM-D, lorsqu'elle est utilisée comme échelle de prévision de l'évolution avec les antidépresseurs, atteint ses limites en raison de son score total de même que pour le diagnostic de dépression majeure décrit dans le DSM-IV (Diagnostic and Statistical Manual of Mental Disorders 4e éd).
Une grande partie des recherches a été consacrée à l'utilisation de la HAM-D pour différencier le placebo du médicament actif ou pour montrer les relations dose-réponse chez les patients ayant une dépression majeure. Une amélioration du score total de la HAM-D au cours d'une étude ne qualifie pas, en soi, un médicament comme antidépresseur, le score total n'étant pas une statistique suffisante. Nous soulignons le problème de la signification statistique versus clinique en analysant des études contrôlées contre placebo qui comprennent des rapports dose-réponse, en recommandant l'utilisation de statistiques qui prennent en compte la taille de l'effet.
Depression rating scales were introduced into clinical psychiatry in the 1960s, with the advent of antidepressants such as imipramine and phenelzine.1-3 In the early trials, both global improvement scales and the Hamilton Depression Rating Scale (HAM-D) were used. As discussed by Lam et al,1 historically the use of depression symptom scales such as the HAM-D was not a routine aspect of patient care for frontline mental health clinicians. The present situation seems to be that we are facing two prototypes of clinicians, “Dr Gestalt,” who uses a global clinical impression scale, and “Dr Scales, ” who has incorporated the routine use of rating scales into daily clinical practice.1
When comparing Dr Gestalt with Dr Scales with respect to limitations and pitfalls in using depression rating scales, it seems appropriate to use the functional analysis proposed by Emmelkamp.2 According to this proposal, we can refer to macroanalysis and microanalysis of rating scales. Macroanalysis focuses on the diagnosis of depression and thereby the prediction of treatment response, while microanalysis focuses on outcome measures of treatment. At the macroanalytic level, it is appropriate to discuss depression rating scales such as the HAM-D in comparison with a diagnostic system of mental disorders such as the Diagnostic and Statistical Manual of Mental Disorders, 4th ed (DSM-IV),3 while at the microanalytic level a direct comparison between Dr Gestalt and Dr Scales is relevant.
Macroanalysis
Emmelkamp2 used the polythetic algorithms of the DSM-IV to illustrate the limitation of the clinical diagnosis of depression when developing treatment strategies for the patients. According to DSM-IV, in major depression five out of nine depression symptoms have to be present. This implies, as discussed by Emmelkamp, that totally different patients may fulfil these symptomatic requirements, because the fixed number of five items may refer to different items from patient to patient. Consequently, this heterogeneity has serious limitations for the predictive validity of the diagnosis concerning choice of treatment.
In 1979, the Montgomery-Asberg Depression Rating Scale (MADRS) was introduced into clinical psychiatry because the existing depression rating scales reflected “ ... diagnostic features rather than being sensitive to change ... .”4 Thus, the HAM-D was considered was considered by Montgomery and Asberg to be a diagnostic scale although Hamilton had designed it as a scale measuring the severity of depressive states and not the diagnosis.5
After 1980, with the introduction of the Diagnostic and Statistical Manual of Mental Disorders, 3rd ed (DSM-III)6 the diagnosis of depression was symptom-based, but, as illustrated by Emmelkamp,2 the algorithm of major depression is resistant to quantification.
Studies with the HAM-D have indicated that the HAMD is not a unidimensional scale,7 suggesting that the profile of factors, eg, suicidal behavior, anxiety-somatization, sleep, and appetite or weight loss should be used in a macroanalytic approach when developing a treatment strategy with antidepressants.
In the study by Montgomery and Asberg,4 the item most sensitive to change during treatment was the sleep item; this may be explained by the antidepressants used in the analysis (amitriptyline, clomipramine, maprotiline, and mianserin). One of the limitations of depression rating scales as claimed by Montgomery and Asberg4 was that they are only rarely consistent in finding differences between active drugs, even when the known mechanisms of action are different. However, in a judgment analysis it was found that clomipramine was superior to citalopram, but only on the item of sleep and not on the specific items of depression.8 We can thus differentiate between sedative antidepressants such as amitriptyline clomipramine, and mianserin (all antihistamines) and nonsedative antidepressants such as as citalopram or other selective serotonin reuptake inhibitors (SSRIs). In this context, the sleep and agitation factor on the HAMD might become predictive of choice of antidepressants. However, Katz et al9 have argued for also including factors such as somatization, hostility, and interpersonal sensitivity from the Symptom Checklist (SCL-90) when selecting the type of antidepressant. Likewise, the symptom of suicidal behavior should be analyzed separately when selecting the most appropriate treatment and care for the patient.
Macroanalyses of rating scales are rarely performed, but a multidimensional scale such as the HAM-D might give the clinician better information than the DSM-IV diagnosis of major depression when selecting the most appropriate antidepressant treatment for the individual patient. With the DSM-IV symptoms of depression it is possible to create a profile of a patient by the score on agitation versus retardation, suicidal behavior, sleep problems, and weight loss versus weight gain.
The only rating scales designed specifically to measure predictive validity of treatment by their total scores are the Newcastle Depression Scales (Newcastle 196510 and Newcastle 197111). With the introduction of DSM-III and DSM-IV, the subdivision of depression into endogenous and reactive depression was deleted, and research on the Newcastle scales, which had been based on this concept, became very limited.
The various guidelines on how to use the different antidepressants with reference to treatment-specific algorithms are typically based on the safety of the drugs and the patient-specific history of treatment resistance, rather than on the DSM-IV diagnosis of major depression or on a score on a depression rating scale.12
Research on how to uncover medication history to help with the treatment decision has been very limited. Posternak and Zimmerman13 have recently examined how accurately patients can recall prior treatments with antidepressants. The results showed that approximately 80% remembered monotherapy correctly, while only 25% recalled augmentation therapy correctly.
In the macroanalysis of the choice of treatment, it must therefore be concluded that rating scales with a factor profile such as the HAM-D seem to be superior to the DSM-IV diagnosis of major depression, but the DSM-IV depression symptoms individually can give important information about choice of treatment. However, when making decisions about individual patient-specific treatments, the tolerability of the antidepressant plays an important role, as does the history of previous outcome, especially in regard to treatment resistance.
Microanalysis
According to Emmelkamp,2 the microanalysis of a depression rating scale is mainly focused on the clinimetric analysis of outcome measurements of treatment. This type of analysis, as discussed by Faravelli14 is based on certain assumptions which often involve pitfalls to such a degree that they can lead to “evidence-biased” rather than “evidence-based” psychiatry. The assumptions listed by Faravelli are:
An illness is the sum of its symptoms;
The symptoms are represented by the numbers associated with specific behaviors;
Operations conducted statistically on these numbers reflect actual changes in the clinical reality;
The relationship among numbers is represented by simple additive effect, regardless of reciprocal interaction.
These assumptions are the focus of the dialogue between Dr Gestalt and Dr Scales.1 One of the aspects discussed by Lam et al1 is that Dr Gestalt in his treatment may focus only on one symptom which might be misleading, while Dr Scales has a fuller picture of the patient's current state. From Faravelli' s point of view, Dr Gestalt is a very experienced psychiatrist, while in Lam's discussion Dr Gestalt is as inexperienced as Dr Scales.
It is certainly a disadvantage to believe that the use of depression rating scales is an attempt to replace experienced psychiatrists by young and inexperienced clinicians in clinical trials. In this context it is important to be aware of the instructions for the Clinical Global Impression Scale (CGI) by Guy15 When using the CGI, the clinician has to make his or her assessment on the basis of previous experience with depressed patients. It is thus with reference to experience that the clinician should make the comparison with all the other severely depressed patients he or she has ever treated. In their daily routine, as stated by Hamilton,16 experienced clinicians always perform a global rating when assessing a depressed patient's need for hospitalization or when deciding whether to discharge an inpatient. The clinically most significant method for validating a depression symptom rating scale such as the HAM-D is to use experienced psychiatrists, both in the group of raters making the global assessment and in the group of raters making the rating scale assessment. This approach was analyzed by Bech et al17 and showed that both groups of experienced psychiatrists were able to obtain an adequate interobserver reliability on the global assessment as well as in HAM-D ratings. An item analysis showed that only six of the 17 HAM-D items validly reflected the global assessment.17 These six items (HAMD6) are shown in Table L The three items listed at the top of Table I are the specific items of depression in accordance with DSM-IV and the International Classification of Diseases, 10th revision (ICD-10).18 This was supported by Hamilton in his last study,19 in which he also demonstrated that the item of psychic anxiety is a specific item of depression. The remaining two items in Table I are “guilt feelings” and “psychomotor retardation.” Guilt feelings are the specific item of negative thoughts which, according to Beck's cognitive model, are a central feature of depressive states.20 Psychomotor retardation is the most specific observational symptom of depression, and in the Melancholia Scale (MES), which is a depression rating scale based on the HAM-D6, the item “psychomotor retardation” has been subdivided into motor, verbal, intellectual, and emotional retardation.21
Table I. Specific depression subscales derived from the HAM-D by the micro-analytic approach.
| HAM-D items | HAM-D subscales | ||
| HAM-D6 | Maier | Core factor | |
| (20) | subscale (31) | subscale (32) | |
| Depressed mood | + | + | + |
| Work and interests | + | + | + |
| General somatic | |||
| (tiredness) | + | ||
| Psychic anxiety | + | + | |
| Guilt feelings | + | + | + |
| Psychomotor retardation | + | + | + |
| Psychomotor agitation | + | ||
| Suicide | + | ||
| Clinimetric validity | |||
| a) global impression | |||
| by experienced | |||
| psychiatrist | + | - | - |
| b) Psychometric analysis | |||
| • Item reponse analysis | + | + | - |
| • Factor analysis | - | + | + |
As discussed by Frances et al,22 the items considered to be most specific for a disorder such as depression might have poor ability to discriminate this disorder from other disorders, and the items that are most dicriminating may not be close to the core symptoms. The HAM-D6 items in Table I are those that in the microanalytic sense are specific for antidepressant activity, while the items identified at the macroanalytic level to discriminate between treatments are, for instance, sleep, appetite, agitation, and suicidal behavior.
Table I shows the three most frequently used subscales for measuring antidepressant activity The HAM-D6 has been used in trials with fluoxetine,23 citalopram,24 escitalopram,25 paroxetine,26 and mirtazapine,27 while the Maier subscale28 and the core factor subscale29 have recently been included in the duloxetine program.30 The order of HAM-D items in Table I is listed according to their appearance in the depressive states when having taken into account the severity degrees of the individual items. To be additive in Farvelli's sense, the individual items of a rating scale must be consistently rankordered according to their relation to the severity of depressive illness. This implies that scoring of lowerpre valence items (low appearance) presupposes scorings on higher-prevalence items (high appearance). Thus, a score on guilt feelings or psychomotor retardation (which has low prevalence) has to be preceded by high scores on depressed mood and work and interests (which have the highest prevalence). The statistical analysis based on this criterion of additivity (ie, the total score being a sufficient statistic or unidimensionality of the scale items) is referred to as item response analysis.26 The item of psychomotor agitation was excluded from the HAMD6 development by both the experienced psychiatrists17 and by the item response theory model26 because of a reciprocal interaction with the other items.
As indicated in Table I, the clinimetric background for the Maier subscale is an item response analysis which was performed in a study showing that the HAM-D6, in contrast to the MADRS, was a unidimensional scale, and where the Maier subscale emerged as a byproduct of the statistical analysis.28 The core factor subscale was identified by an exploratory factor analysis by Cleary,29 but has never been confirmed by other factor analyses. A recent comparison between HAM-D6 and the Maier subscale31 has shown that both scales were valid, while the CGI was unreliable. Although the theoretical score range of the HAM-D6 goes from 0 to 22 and that of the Maier subscale from 0 to 24, the standardization of the two scales showed identical cutoff scores. Thus, a score above 10 on the Maier subscale indicates 18 on the HAM-D17 (moderate depression) and a score above 12 indicates 25 on the HAM-D17 (severe depression), while a score below 5 indicates 7 on the HAM-D17 (remission). As no patient can have a maximum score on both psychomotor retardation and psychomotor agitation, the Maier subscale should be considered having a practical score range corresponding to the HAM-D6.
Neither in the MADRS nor in the Melancholia Scale (MES) is the item of psychomotor agitation included. Therefore, to develop a MADRS6 subscale to cover the specific depression items according to Table I, only the HAM-D6 is available.24 The psychometrically most significant method for analyzing Faravelli' s assumptions is the use of item response theory models.26 By use of the nonparametric model of Mokken it has been shown that the MADRS6 is also a unidimensional depression scale.24 The MADRS6 includes the corresponding HAM-D6 items.
A major pitfall in a microanalysis of the HAM-D is the use of factor analysis to test Faravelli' s assumptions. A comprehensive review by Bagby et al7 has shown that factor analysis as used from 1980 to 2003 in many psychometric analyses of the HAM-D has identified quite different factor scores. As discussed elsewhere,32 the clinimetric analysis of a rating scale should indicate to what extent the total score is a sufficient statistic by considering both the individual items of the scale and the population under examination.
When trying to define the antidepressant effect of a drug, Prien and Le vine33 concluded that a greater improvement in total HAM-D scores does not necessarily indicate antidepressant action (“... assume that a group treated with an experimental drug shows significantly more improvement than a group treated with placebo on the factors of anxiety, somatization or sleep disturbances and no significant change on other factors. These changes, by themselves, should not qualify the drug as an antidepressant...”33).
Another major pitfall to be considered is the use of several depression scales in the same trial without clearly indicating a priori which of them has been determined to be the the primary measure of antidepressant effect. To avoid this problem, a researcher should always use the specific items of depression, eg, the HAM-D6 or the MADRS6, as the primary efficacy measure. When determining clinically significant antidepressant effect, it is recommended to use standardized effect size statistics.34 These statistics examine the reduction of rating scale scores from baseline to end point (mean scores) for both active drug and placebo in relation to the pooled standard deviation of the two treatments. Thus, if the baseline score is 24 for both treatments, but the change score is 14 for the active drug while it is 10 for the placebo, and if the pooled standard deviation is 8, then the effect size is 4/8 or 0.50. In clinical trials with antidepressants an effect size of 0.40 or higher is considered a clinically significant response criterion.35 This equals a 20% advantage of the active drug over placebo by using either a global impression score of very much and much response36 or a 50% reduction in baseline rating scores on the HAM-D.23
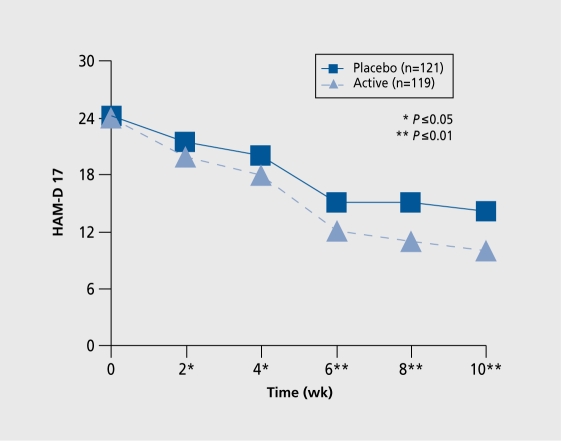
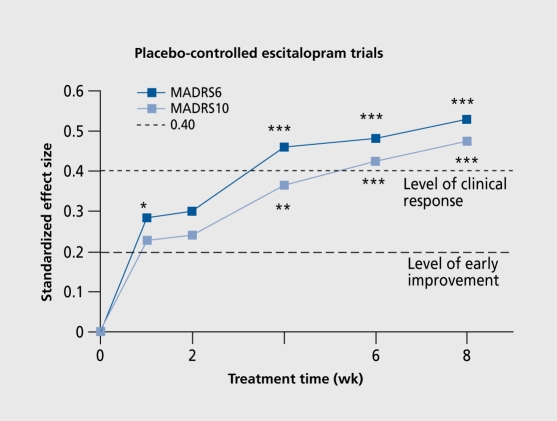
Illustrating antidepressant effect, as shown in (Figure 1)., is yet another difficult area. Because both groups of patients, ie, on active drug treatment as well as on placebo treatment, exceed 100 subjects, a small statistically significant difference will be found. In the example illustrated in (Figure 2)., it is obvious that the effect of escitalopram is of clinical significance (effect size >0.40) in depressed patients after only 4 weeks.
Figure 1. A typical illustration from a placebo-controlled trial with a new potential antidepressant. Previously presented in a poster at: 4th annual meeting of the Scandinavian College of Neuropsychopharmacology: Elsinore, Denmark; 2005.
Figure 2. MADRS6 and MADRS10 showing the antidepressant activity of 20 mg escitalopram in a placebo-controlled trial in patients with severe major depression.
In dose-response trials, the HAM-D6 and the MADRS6 were much more sensitive than the full versions of the respective scales, ie, HAM-D17 and MADRS10.23,37 Both the HAM-D6 and the Maier subscale obtained an effect size of approximately 0.50 for venlafaxine and 0.40 for fluoxetine in placebo-controlled trials in patients with major depression, while the HAM-D17 even for venlafaxine, obtained an effect size of below 0.40.38
In a comparison of most of the placebo-controlled trials of SSRIs in patients with major depression39 it was found that the HAM-D17 was used more frequently than the MADRS10. As no difference was seen between the two scales in differentiating between active drug and placebo, only the HAM-D17 results were considered.39
The correct use of depression rating scales in clinical trials of antidepressants is, as illustrated in Figure 2, to indicate the effect size of the specific items of depression and to accept an effect size of 0.40 or higher as being the clinically significant effect. The current tradition of including at least two depression rating scales without focusing on the specific items of depression seems to constitute a “scientific wrapping” with which the companies decorate their antidepressants, eg, in a figure analogous to Figure I. This industry habit of “dressing” antidepressant activity does now also include the use of the Hamilton Anxiety Scale (HAM- A) to show the antianxiety activity of an SSRI. The 14-item version of the HAMA40 includes an item of depressed mood. However, when using the HAM-A to indicate an effect on generalized anxiety, only its specific items should be used.41 The HAM-A subscale with the six specific items of generalized anxiety is shown in Table II42
When evaluating the antidepressant activity of new drugs in placebo-controlled trials, it has been customary to use clinicianrated scales to demonstrate efficacy, ie, the balance between the specific antidepressant effect and the safety of the drug in terms of adverse drug effects. However, the measure of patient-rated quality of life domains43 has implied that patient-rated depression rating scales or questionnaires should also be used in placebo-controlled trials. In general, self-rating depression scales such as the Beck Depression Inventory (BDI) or the Zung Depression Scale (SDS) have very rarely been used to demonstrate the clinical effect of SSRIs.39 Because the classical self-rating scales for depression (BDI, SDS) cover many items, but not all specific items it- of depression (Table I), it might be appropriate to include in the selfrating scale of the HAM-D as released by Bent-Hansen et al.44 The self-rating version of the HAM-D6 is shown in Table III. Studies are ongoing to evaluate the >ly sensitivity of HAM-D6 in placebo-controlled trials.
The use of a self-rating version of HAM-D has focused on translation procedures when preparing non-English versions of the scale. This has also implied that the pit-falls of using nonauthorized versions of the HAM-D have been discussed. Even in the most recently published book on assessment scales,1 the HAM-D17 version that is shown is not the original English HAM-D version, although the authors refer to Hamilton's first work with his scale.45 In the first version of the HAM-D, the item of agitation was measured from 0 to 3, but in the second version, Hamilton changed the scoring to 0-5.5 The version published by Lam et al1 is an American version which was not accepted by Hamilton himself,46 in contrast to the HAM-D6 version.47 Hamilton's criticism of the American version included the following: “... A further deficiency was that it regarded the spontaneous mention of a symptom as indicating greater severity than if it had been elicited by questioning. There are many reasons why patients may not mention a symptom at an interview. For example, they may not think it relevant (eg, feelings of guilt), they may be embarrassed (eg, loss of libido) or they may be too polite to mention to the interviewer that they believe they are suffering from a physical illness ...”
Table II. The specific items of generalized anxiety in HAM-A6.
| Psychic anxiety (worrying) |
| Tension (psychic) |
| Fears |
| Difficulty in concentration |
| Muscular tension |
| Behavior during interview |
Table III. The HAM-D6 Questionnaire.
| In this questionnaire you will find six groups of statements. Please choose the one statement in each group that best describes how you have been feeling over the past three days, including today, and mark it with an X in the corresponding box. | |||
| (1) During the past three days | (4) During the past three days | ||
| I have been in my usual good mood | 0 | I have felt neither restless nor slowed down | 0 |
| I have felt a little more sad than usual | 1 | I have felt a little slowed down | 1 |
| I have been clearly more sad than usual but haven't | I have felt rather slowed down or have been talking | ||
| felt helpless or hopeless | 2 | a little less than usual | 2 |
| I have been so gloomy that I briefly have felt | I have felt clearly slowed down or subdued or have | ||
| overpowered by hopelessness | 3 | talked much less than usual | 3 |
| I have been so low in my moods that everything | I have hardly been talking at all or felt extremely | ||
| seems dark and hopeless | 4 | slowed down all the time | 4 |
| (2) During the past three days | (5) During the past three days | ||
| I have been quite satisfied with myself | 0 | I have been calm and relaxed | 0 |
| I have been a little more self-critical than usual with | I have felt a little more tense or insecure than usual | 1 | |
| a tendency to feel less worthy than others | 1 | I have been clearly more worried or tense than usual | |
| I have been brooding over my failures in the past | 2 | but have not felt that I lost control | 2 |
| I have been plagued with distressing guilt feelings | 3 | I have been so tense or worried that I have briefly | |
| I have been convinced that my current condition | felt close to panic | 3 | |
| is a punishment | 4 | I have had episodes where I was overwhelmed by panic | 4 |
| (3) During the past three days | (6) During the past three days | ||
| My daily activities have been as usual | 0 | I have been as active and have had as much energy | |
| I have been less interested in my usual activities | 1 | as usual | 0 |
| I have felt that I have had difficulty performing my | I have felt rather low in energy or physically unwell | ||
| daily activities, but I was still able to perform them | with some bodily pains | 1 | |
| with great effort | 2 | I have felt very low in energy or had bodily pains | 2 |
| I have had difficulty performing even simple routine activities | 3 | ||
| I have not been able to do any of the most simple | |||
| day-to-day activities without help | 4 |
Conclusion
Since the introduction of antidepressants into psychopharmacology in the 1960s, the HAM-D has been the most frequently used rating scale for depresssion. When used as a scale for prediction of outcome with antidepressants, the HAM-D by its total score has obtained limited use analogous to the DSM-IV diagnosis of major depression. Among the individual HAM-D items or factors, sleep and agitation are associated with the sedative antidepressants.
Most research has been devoted to the use of HAM-D to discriminate between placebo and active drugs or to show dose-response relationship in patients with major depression. An improvement in the total HAM-D score during a drug trial can, however, not in itself qualify the drug as an antidepressant because the total score is not a sufficient statistic. This implies that the improvement may be found in nonspecific HAM-D factors such as sleep, anxiety, or appetite. To overcome this major pitfall, the specific HAM-D subscales, eg, HAM-D6 have been discussed with reference also to the analogous subscale from the MADRS6.
The problem of statistical versus clinical significance when analyzing placebo-controlled trials including dose-response relationship has been outlined, with the recommendation to use effect size statistics.
Finally, the pitfall of using unauthorized scale versions has been discussed with reference to self-rating depression scales.
Selected abbreviations and acronyms
- CGI
Clinical Global Impression Scale
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders. 4th ed
- HAM-D
Hamilton Depression Rating Scale
- MADRS
Montgomery-Asberg Depression Rating Scale
- SSRI
selective serotonin reuptake inhibitor
REFERENCES
- 1.Lam RW., Michalak EE., Swinson RP. Assessment Scales in Depression, Mania and Anxiety. London, UK: Taylor and Francis; 2005 [Google Scholar]
- 2.Emmelkamp PMG. The additional value of clinimetrics needs to be established rather than assumed. . Psychother Psychosom. 2004;73:142–144. doi: 10.1159/000076450. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and Statistical Manual Of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association. 1994 [Google Scholar]
- 4.Montgomery SA., Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 6.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. Washington, DC: American Psychiatric Association. 1980 [Google Scholar]
- 7.Bagby RM., Ryder AG., SchuIIer DR., Marshall MB. The Hamilton Depression Rating Scale: has the gold standard become a lead weight? Am J Psychiatry. 2004;161:2163–2177. doi: 10.1176/appi.ajp.161.12.2163. [DOI] [PubMed] [Google Scholar]
- 8.Bech P., Haaber A., Joyce CRB. Experiments on clinical observation and judgement in the assessment of depression: profiled videotapes and judgement analysis. Psychol Med. 1986;16:873–883. doi: 10.1017/s0033291700011880. [DOI] [PubMed] [Google Scholar]
- 9.Katz MM., Koslow SH., Berman N., et al. A multï-vantaged approach to measurement of behavioral and affect states for clinical and psychobiological research. Psychol Rev. 1984;55:619–671. doi: 10.2466/pr0.1984.55.2.619. [DOI] [PubMed] [Google Scholar]
- 10.Carney MWP., Roth M., Garside RF. The diagnosis of depressive syndromes and the prediction of ECT response. Br J Psychiatry. 1965;111:659–674. doi: 10.1192/bjp.111.477.659. [DOI] [PubMed] [Google Scholar]
- 11.Gurney C. Diagnostic scales for affective disorders. Proceedings of the Fifth World Conference of Psychiatry. Mexico City, Mexico. 1971;330 [Google Scholar]
- 12.Bauer M., Whybrow PC., Angst A., et al. Guidelines for the treatment of unipolar depressive disorders. World J Psychiatry. 2002;3:3–43. [Google Scholar]
- 13.Posternak MA., Zimmerman M. How accurate are patients in reporting their antidepressant treatment history? J Affect Disord. 2003;75:115–124. doi: 10.1016/s0165-0327(02)00049-6. [DOI] [PubMed] [Google Scholar]
- 14.Faravelli C. Assessment of psychopathology. Psychother Psychosom. 2004;73:139–141. doi: 10.1159/000076449. [DOI] [PubMed] [Google Scholar]
- 15.Guy W. Early Clinical Drug Evaluation (ECDEU) Assessment Manual for Psychopharmacology. Publication No. 76-338. Rockville, Md: National Institute of Mental Health; 1976 [Google Scholar]
- 16.Hamilton M. Methodology of Clinical Research. Edinburgh, UK: Churchill Livingstone. 1974 [Google Scholar]
- 17.Bech P., Gram LF., Dein E., Jacobsen O., Vitger J., Bolwig TG. Quantitative rating of depressive states. Acta Psychiatr Scand. 1975;51:161–170. doi: 10.1111/j.1600-0447.1975.tb00002.x. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. International Classification of Diseases. 10th revision. Geneva, Switzerland: World Health Organization, 1993 [Google Scholar]
- 19.Hamilton M. The effect of treatment on the melancholias (depressions). Br J Psychiatry. 1982;140:223–230. doi: 10.1192/bjp.140.3.223. [DOI] [PubMed] [Google Scholar]
- 20.Beck AT. Depression: Clinical Experimental, and Theoretical aspects Philadelphia, Pa: University of Pennsylvania Press. 1967 [Google Scholar]
- 21.Bech P. The Bech-Rafaelsen Melancholia Scale (MES) in clinical trials of therapies in depressive disorders: a 20-year review of its use as outcome measure. Acta Psychiatr Scand. 2002;106:252–264. doi: 10.1034/j.1600-0447.2002.01404.x. [DOI] [PubMed] [Google Scholar]
- 22.Frances A., Pincus HA., Widiger TA., Davis WW., First MB. DSM-IV: work in progress. Am J Psychiatry. 1990;147:1439–1448. doi: 10.1176/ajp.147.11.1439. [DOI] [PubMed] [Google Scholar]
- 23.Bech P., Cialdella P., Haugh M., et al. A meta-analysis of randomised controlled trials of fluoxetine versus placebo and tricyclic antidepressants in the short-term treatment of major depression. Br J Psychiatry. 2000;176:421–428. doi: 10.1192/bjp.176.5.421. [DOI] [PubMed] [Google Scholar]
- 24.Bech P., TanghØj P., Andersen HF., OverØ K. Citalopram dose-response revisited using an alternative psychometric approach to evaluate clinical effects of four fixed citalopram doses compared to placebo in patients with major depression. Psychopharmacology. 2002;163:20–25. doi: 10.1007/s00213-002-1147-6. [DOI] [PubMed] [Google Scholar]
- 25.Bech P., TanghØj P., Cialdella P., Friis Andersen H., Pedersen AG. Escitalopram dose-response revisited: an alternative psychometric approach to evaluate clinical effects of escitalopram compared to citalopram and placebo in patients with major depression. Int J Neuropsychopharmacol. 2004;7:283–290. doi: 10.1017/S1461145704004365. [DOI] [PubMed] [Google Scholar]
- 26.Licht RW., Qvitzau S., AHerup P., Bech P. Validation of the Bech-Rafaelsen Melancholia Scale and the Hamilton Depression Scale in patients with major depression: is the total score a valid measure of illness severity. Acta Psychiatr Scand. 2005;111:144–149. doi: 10.1111/j.1600-0447.2004.00440.x. [DOI] [PubMed] [Google Scholar]
- 27.Bech P. Meta-analysis of placebo-controlled trials with mirtazapine using the core items of the Hamilton Depression Scale as evidence of a pure antidepressive effect in the short-term treatment of major depression. Int J Neuropsychopharmacol. 2001;4:337–345. doi: 10.1017/S1461145701002565. [DOI] [PubMed] [Google Scholar]
- 28.Maier W., Philipp M. Improving the assessment of severity of depressive states: a reduction of the Hamilton Depression Scale. Pharmacopsychiatry. 1985;18:114–115. [Google Scholar]
- 29.Geary PJ. Problems of internal consistency and scaling in life event schedules. J Psychosom Res. 1981;25:309–320. doi: 10.1016/0022-3999(81)90008-8. [DOI] [PubMed] [Google Scholar]
- 30.Detke MJ., Lu Y., Goldstein DJ., McNamara RK., Demitrack MA. Duloxetine 60 mg once daily dosing versus placebo in the acute treatment of major depression. J Psych Research. 2002;36:383–390. doi: 10.1016/s0022-3956(02)00060-2. [DOI] [PubMed] [Google Scholar]
- 31.uhe H., Dekker JJ., Peen J., Holman R., de Jonghe F. Clinical use of the Hamilton Depression Scale: is increased efficiency possible? A post hoc comparison of the Hamilton Depression Rating Scale, Maier and Bech subscales, Clinical Global Impression and Symptom CheckIist-90 scores. Comprehens Psychiatry. 2005;46:417–427. doi: 10.1016/j.comppsych.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Bech P. Modem psychometrics in clinimetrics: Impact on clinical trials of antidepressants. Psychother Psychosom. 2004;73:134–138. doi: 10.1159/000076448. [DOI] [PubMed] [Google Scholar]
- 33.Prien RF., Levine J. Research and methodological issues for evaluating the therapeutic effectiveness of antidepressant drugs. Psychopharmacol Bull. 1984;20:250–257. [PubMed] [Google Scholar]
- 34.Hedges LV., Olkin I. Statistical Methods for Meta-Analysis. New York, NY: Academic Press. 1985 [Google Scholar]
- 35.Faries D., Herrera J., Rayamajhi J., DeBrota D., Demitrack M., Potter WZ. The responsiveness of the Hamilton Depression Rating Scale. J Psychiatr Res. 2000;34:3–10. doi: 10.1016/s0022-3956(99)00037-0. [DOI] [PubMed] [Google Scholar]
- 36.Bech P. Clinical effects of selective serotonin reuptake inhibitors. In: Dahl SG, Gram LF, eds. Clinical Pharmacology in Psychiatry. Springer: Berlin; 1989:81–93. doi: 10.1007/978-3-642-74430-3_9. [DOI] [PubMed] [Google Scholar]
- 37.Bech P., Andersen HF. Effective dose of escitalopram in major depressive disorder. Nord J Pychiatry. 2005;59:406–407. [Google Scholar]
- 38.Entsuah R., Shaffer M., Zhang J. A critical examination of the sensitivity of unidimensional scales derived from the Hamilton Depression Rating Scale of antidepressant drug effects. J Psychiatr Res. 2002;36:437–448. doi: 10.1016/s0022-3956(02)00024-9. [DOI] [PubMed] [Google Scholar]
- 39.Bech P. Pharmacological treatment of depressive disorders: a review. In: Maj, M, Sartorius N, eds. Depressive Disorders. WPA Series Evidence and Experience in Psychiatry. 2nd ed. Chichester, UK: John Wiley & Sons; 2002:89–127. [Google Scholar]
- 40.Hamilton M. Diagnosis and rating of anxiety. Br J Psychiatry. 1969;special issue:76–79. [Google Scholar]
- 41.Bech P., Lunde M., Undén M. An inventory for the measurement of generalised anxiety distress symptoms, the GAD-10 Inventory. Acta Psychiatr Belg. 2005;105:111–118. [Google Scholar]
- 42.Meoni P., Salinas E., Brault Y., Hackett D. Pattern of symptom improvement following treatment with venlafaxine XR in patients with generalized anxiety disorder. Clin Psychiatry. 2001;62:888–893. doi: 10.4088/jcp.v62n1109. [DOI] [PubMed] [Google Scholar]
- 43.Bech P. Quality of Life in the Psychiatric Patient. London, UK; Mosby-Wolfe. 1998 [Google Scholar]
- 44.Bent-Hansen J., Lauritzen L., Clemmensen L., Lunde M., Kerner A. A definite and semi-definite questionnaire version of the Hamilton/ Melancholia Scale. J Affect Disord. 1995;33:143–150. doi: 10.1016/0165-0327(94)00057-g. [DOI] [PubMed] [Google Scholar]
- 45.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.HamiIton M., Shapiro CM. Depression. In: Peck DF, Shapiro CM, eds. Measuring Human Problems. Chichester, UK: John Wiley; 1990:25–65. [Google Scholar]
- 47.Bech P., Kastrup M., Rafaelsen OJ. Mini-compendium of rating scales for states of anxiety, depression, mania, schizophrenia with corresponding DSM-III syndromes. Acta Psychiatr Scand. 1986;73 (suppl 326):7–37. [PubMed] [Google Scholar]