Abstract
Until recently, a review of nonpharmacological, somatic treatments of psychiatric disorders would have included only electroconvulsive therapy (ECT). This situation is now changing very substantially Although ECT remains the only modality in widespread clinical use, several new techniques are under investigation. Their principal indication in the psychiatric context is the treatment of major depression, but other applications are also being studied. All the novel treatments involve brain stimulation, which is achieved by different technological methods. The treatment closest to the threshold of clinical acceptability is transcranial magnetic stimulation (TMS). Although TMS is safe and relatively easy to administer, its efficacy has still to be definitively established. Other modalities, at various stages of research development, include magnetic seizure therapy (MST), deep brain stimulation (DBS), and vagus nerve stimulation (VNS). We briefly review the development and technical aspects of these treatments, their potential role in the treatment of major depression, adverse effects, and putative mechanism of action. As the only one of these treatment modalities that is in widespread clinical use, more extended consideration is given to ECT. Although more than half a century has elapsed since ECT was first introduced, it remains the most effective treatment for major depression, with efficacy in patients refractory to antidepressant drugs and an acceptable safety profile. Although they hold considerable promise, the novel brain stimulation techniques reviewed here will be need to be further developed before they achieve clinical acceptability.
Keywords: depression, electroconvulsive therapy, transcranial magnetic stimulation, deep brain stimulation, vagus nerve stimulation
Abstract
Hasta hace muy poco tiempo una revisión de los tratamientos somáticos, no farmacológicos de los trastornos psiquiátricos habría incluido sólo la terapia electrocovulsiva (TEC). Esta situación está cambiando significativamente en la actualidad. Aunque la TEC permanece como la única modalidad de amplio uso clínico, existen nuevas técnicas que están en investigación. La principal indicación en el ámbito psiquiátrico es el tratamiento de la depresión mayor, pero también se están estudiando otras aplicaciones. Todos los nuevos tratamientos incorporan la estimulación cerebral, la que se obtiene a través de diferentes métodos tecnológicos. El tratamiento más cercano al límite de la aceptación clínica es la estimulación magnética transcraneal (EMT). Aunque la EMT es segura y relativamente fácil de administrar, su eficacia aun no ha sido definitivamente establecida. Otras modalidades, en diversas etapas de investigación, incluyen la terapia magnética convulsiva (TMC), la estimulación cerebral profunda (ECP) y la estimulación del nervio vago (ENV). Se revisa brevemente el desarrollo y los aspectos técnicos de estos tratamientos, su potencial papel en el tratamiento de la depresión, los efectos adversos y los mecanismos de acción. Se revisa en mayor extensión la TEC por ser la única de estas modalidades terapéuticas que se utiliza ampliamente en clínica. Aunque ha transcurrido más de medio siglo desde que la TEC fue introducida por primera vez, ésta continúa siendo el tratamiento más efectivo para la depresión mayor, con eficacia en pacientes refractarios a los fármacos antidepresivos y con un aceptable perfil de seguridad. Aunque las nuevas técnicas de estimulación cerebral revisadas acá parecen promisorias, requerirán de un mayor desarrollo a futuro antes de que alcancen aceptación clínica.
Abstract
II y a peu de temps encore, une analyse des traitements somatiques non pharmacologiques des troubles psychiatriques n'aurait compris que l'électrochoc. Cette situation est en train de changer notablement. Bien que l'électrochoc reste la seule modalité en pratique clinique courante, plusieurs nouvelles techniques sont en expérimentation. Leur indication principale dans le contexte psychiatrique est le traitement de la dépression majeure, mais d'autres applications sont en cours d'étude. Tous les nouveaux traitements font intervenir la stimulation cérébrale, réalisée par différentes techniques. Le traitement le plus proche du seuil d'acceptabilité clinique est la stimulation magnétique transcrânienne (SMT). Bien que la SMT soit sûre et relativement facile à administrer, son efficacité doit encore être définitivement établie. D'autres modalités, à différents stades de recherche, comprennent le traitement d'attaque magnétique (TAM), la stimulation cérébrale profonde (SCP) et la stimulation du nerf vagal (SNV). Nous analysons brièvement le développement et les aspects techniques de ces traitements, leur rôle potentiel dans le traitement de la dépression majeure, leurs effets indésirables et leur mécanisme d'action reconnu. Une attention plus importante est portée à l'électrochoc, car c'est le seul de ces traitements qui est utilisé en pratique clinique courante. Bien que l'introduction de l'électrochoc remonte à plus d'un demi siècle, il reste le traitement le plus efficace de la dépression majeure, efficace chez les patients résistants aux antidépresseurs et doté d'un profil de sécurité acceptable. Bien que très prometteuses, les nouvelles techniques de stimulation cérébrale analysées ici devront faire l'objet d'un développement ultérieur avant d'obtenir une acceptabilité clinique.
For more than 50 years, electroconvulsive therapy (ECT) has been the only nonpharmacological, somatic treatment of psychiatric disorders in widespread clinical use. Other modalities, such as insulin coma therapy, were used for varying periods, but no longer have any place in clinical psychiatry. This situation is now changing. Brain stimulation techniques are rapidly becoming a highly promising novel avenue for treatment of psychiatric disorders in general, and major depression in particular. Research in this field is at a very important juncture, and there are signs that the first two decades of the current millennium could well be the decades of brain stimulation in psychiatry. Several different approaches are under study. Some have the potential to cross the threshold to clinical use, while others are still at a very limited stage of application in the research context only.
In this review, we will consider several novel brain stimulation techniques for the treatment of depression: transcranial magnetic stimulation (TMS), magnetic seizure therapy (MST), deep brain stimulation (DBS), and vagus nerve stimulation (VNS). A comprehensive evaluation of each modality is not possible in this context. We will provide an overview of key aspects of each treatment such as its development, technique, application in major depression, adverse effects, and putative mechanism(s) of action. The novel brain stimulation modalities will be discussed on the background of a wider consideration of ECT, which is used extensively and has been the focus of intensive basic and clinical research for several decades.
Electroconvulsive therapy
Development of ECT
The production of epileptiform convulsions as a treatment for psychiatric illness was introduced in 1934 by the Hungarian psychiatrist, Laszlo Meduna.1 The first treatments were drug-induced convulsions.2 A few years later, electrical seizure induction was introduced by Cerletti and Bini in Rome.3,4 The introduction of antidepressant drugs during the 1950s and 1960s reduced the use of ECT as a first-line therapy for depression. Nevertheless, ECT is still the treatment of choice in pharmacotherapy-resistant cases. Although ECT is considered effective and safe, it continues to be regarded with suspicion by much of the public and the medical profession. Hollywood films such as The Snakepit (1948) and One Flew Over the Cuckoo's Nest (1975) set an antipsychiatric and anti-ECT tone that has never completely abated.5 Unmodified ECT was indeed associated with serious complications such as extremity fractures and compressive spinal fractures. However, for more than 50 years ECT has been administered under general anesthesia with neuromuscular relaxants, and this has eliminated these serious complications.6 Interestingly, the fear of permanent braindamage caused by ECT was recently placed in a different perspective by reports that ECT might actually increase new neuron growth (neurogenesis) in the hippocampus.7
In order to minimize short- and longer-term memory deficits associated with ECT, major research efforts have been invested in trying to limit the stimulus path and to adapt the stimulus intensity to the seizure threshold of the individual patient.8 There are two main modalities of ECT, differentiated by electrode placement: bilateral and unilateral ECT In bilateral ECT the electrical stimulus traverses both cerebral hemispheres, while in unilateral ECT only the nondominant cerebral hemisphere is stimulated. In both cases, effective treatment requires that a generalized seizure be elicited. Although unilateral ECT results in fewer cognitive adverse effects, its efficacy relative to bilateral ECT was a source of controversy for many years.9 Recently, Sackeim and colleagues found that high-dosage unilateral ECT (electrical dosage 500% above seizure threshold) and moderately suprathreshold bilateral ECT (electrical dosage 150% above seizure threshold,) are equivalent in response rate.10 Importantly, high-dose unilateral ECT is not associated with increased cognitive adverse effects. These findings underscore an important basic concept in ECT: although the seizure may seem to be an all-or-none event, not every generalized seizure has antidepressant properties. Stimulus intensity relative to threshold is a major factor in the efficacy of the therapy.11,12
Technique of ECT administration
Pretreatment evaluation includes complete medical history, physical, neurological, and preanesthesia examinations, and relevant laboratory tests. Patients' concurrent medications should be noted, since they might affect the seizure threshold or interact with other medications used during ECT (Table I). Pretreatment preparations include 6- to 12-hour fasting, removal of dentures or other foreign objects from the patient's mouth, insertion of a bite block into the mouth, and preoxygenation (100% O2 at a rate of 5 L/min).
Table I. Drug coadministration with electroconvulsive therapy.
| Drug | Concomitant administration with ECT |
| Lithium13 | Discontinue before ECT. Lithium decreases the intracellular production of |
| acetylcholine, may prolong the action of succinylcholine, and may prolong | |
| seizure duration | |
| Tricyclic antidepressant, monoamine oxidase inhibitors | Safe |
| selective serotonin reuptake inhibitors14,15 | |
| Bupropion16 | Controversial. Bupropion may cause prolonged seizure |
| Benzodiazepines | Safe, but have an anticonvulsive activity, and might raise the seizure threshold |
| Antipsychotics17 | Safe |
| Clozapine18 | Controversial, might induce late seizures |
Anesthetic agents should induce rapid unconsciousness and recovery and minimally affect hemodynamic parameters or seizure threshold.18 The most commonly used anesthetic is methohexital (0.75 to 1.0 mg/kg), due to its rapid onset, short duration of action, minimal anticonvulsive effect, and rapid recovery.19 Other anesthetics include thiopental, propofol, and etomidate. A muscle relaxant agent is administered 1 to 2 minutes after the anesthetic agent. Muscle relaxation eliminates injuries resulting from motor activity during the treatment, and is of particular importance in patients who are at high risk for fractures or disk herniation. Succinylcholine, a short-acting depolarizing agent (0.5 to 1.0 mg/kg), is used in most patients. Before the muscle relaxant is administered, a blood pressure cuff is inflated above the systolic blood pressure at one ankle, to allow observation of the motor seizure. A peripherally acting anticholinergic such as glycopyrrolate may used to increase heart rate before treatment, especially if the patient is bradycardic.
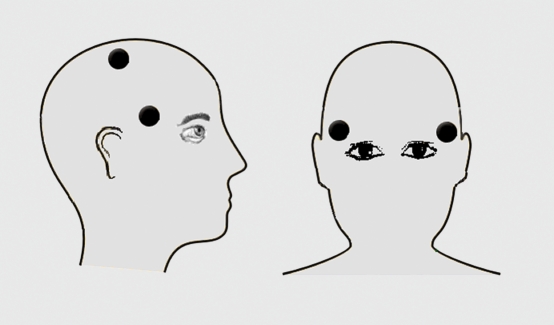
ECT is administered using two electrodes, located bilaterally or unilaterally, as illustrated in Figure 1. The electrical stimulus is a brief pulse waveform (bidirectional rectangular pulse). The intensity of the ECT stimulus is assessed in terms of the total delivered charge. This total charge (Q, measured using units of millicoulombs) can be defined as:
Figure 1. Electrode placement in ECT. In bilateral ECT, bifrontotemporal electrode placement is used: the electrodes are placed 5 cm above the midpoint of the distance between the auditory meatus and the external canthus. In unilateral ECT, the d'Elia positioning is used: one electrode is placed at the standard frontotemporal position, and the other electrode is placed near the vertex (3 cm down from the midpoint of a line joining the two auditory meati and the sagittal midline of the skull). ECT, electroconvulsive therapy.
Q = (1/1000) * PW * 2F * D
where I is current (milliamperes), PW is pulse width (milliseconds), F is frequency (hertz, cycle per second) and D is duration (seconds). The standard pulse width used in ECT is 1 millisecond or greater. Recently, it has been found that an ultrabrief stimulus, using 0.3 millisecond pulse width, requires less energy to produce a generalized seizure. This may be related to the fact that neuronal depolarization is 0.3 to 1.0 milliseconds, and long pulse width may result in excess stimulation after neurons have fired and are in a refractory or relative refractory phase. Although the amnesia and cognitive side effects following ECT are reduced with ultrabrief stimulation, data regarding its efficacy relative to the traditional stimulus are still insufficient.
The electrical path of the ECT stimulus includes the ECT output device, stimulus electrodes, scalp, skull, cerebrospinal fluid, and brain tissue. The most variable impediment is the patient impedance (mostly scalp and skull), measured in ohms. Energy is another unit that assesses the intensity of the total electrical stimulus. It is dependent on the impedance during stimulation, and can be calculated as
U = (Q/1000) * (1/1000) * R
where Q is charge (millicoulombs), I is current (milliamperes), and R is resistance (ohms).
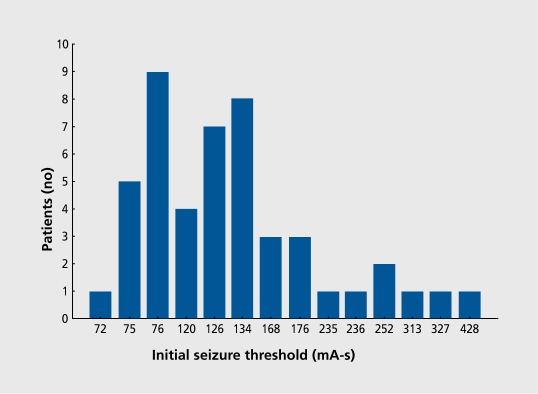
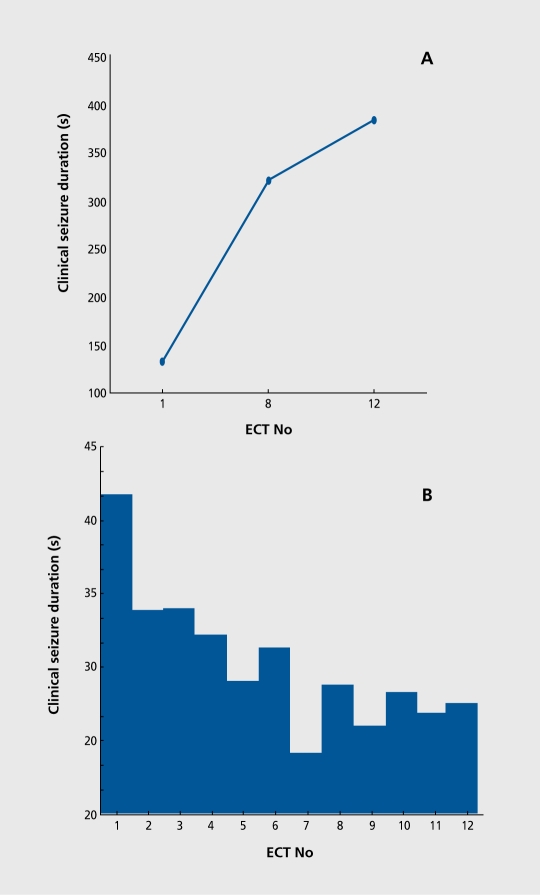
Seizure threshold, defined as the minimal stimulus intensity necessary to produce a seizure, differs up to 40-fold among patients (Figure 2). For example, seizure threshold is higher in men than in women and is higher in older than younger adults. Seizure threshold is also altered by mechanical factors that impede the path of the stimulus and increase resistance. Seizure threshold may change in the same patient during the ECT course, and tends to increase, as shown in Figure 3.
Figure 2. Initial seizure threshold in milliampere seconds (millicoulombs) as determined by a titration technique at the first treatment of a bilateral ECT course.20 ECT, electroconvulsive therapy.
Figure 3. Increase in seizure threshold measured by titration at treatments 1, 8, and 12 (A) and decrease in seizure duration measured clinically in a cuffed limb (B) in patients treated with bilateral ECT.20 ECT, electroconvulsive therapy.
Optimal stimulus intensity during ECT is important because excessive electrical stimulation increases cognitive deficits. On the other hand, insufficient electrical stimulation, barely above the seizure threshold, reduces efficacy In a study that compared bilateral and right unilateral ECT at different stimulus intensities, Sackeim and colleagues concluded that the optimal electrical dosage is 2.5 times the seizure threshold for bilateral ECT and 4 to 6 times the seizure threshold for unilateral ECT10 For ultrabrief stimulation (pulse width 0.3 millisecond), the electrical dosage might be higher.
Determining the stimulus energy requires a method to estimate the patient's seizure threshold in the first treatment session. Empirical titration involves administration of subconvulsive intensities in the first treatment and finding the stimulus energy that produces a seizure. In subsequent sessions, a fixed stimulus above the seizure threshold is administered. In every treatment session, up to three trials of stimulus may be conducted. Using empirical titration permits accurate determination of the seizure threshold. Another method to estimate seizure threshold, the preselected dosage method, involves use of known predictors of seizure threshold such as electrode placement, age, and gender. Based on these criteria, a suprathreshold dose is preselected and given at the first and subsequent treatments, unless severe cognitive side effects occur. This approach eliminates the need for subconvulsive stimulations in establishing seizure thresh-old, but is less accurate than the titration method.
The motor seizure consists of two phases. The tonic phase lasts 10 to 20 seconds and involves contraction of the jaw and facial muscles, plantar extension, and high-frequency sharp EEG activity. The second phase, the clonic phase, involves rhythmic contractions and bursts of polyspike EEG activity which persist for a few seconds after the clonic movements stop. A seizure is effective if it lasts at least 20 to 25 seconds. Prolonged seizures can be terminated with intravenous benzodiazepines.
In the United States, ECT is usually administered three times weekly. In other parts of the world, twice -weekly administration is more common. Twice-weekly administration has been shown experimentally to be an optimum schedule for bilateral ECT, considering maximal antidepressant effect and minimal cognitive impairment.21,22 If clinical indications require a more rapid antidepressant effect, three times weekly administration might be used.21,22 The number of treatments during an ECT course is usually 8 to 12; the treatment is terminated when a plateau of improvement is reached, usually when the patient does not continue to improve after two consecutive treatments.
ECT in the treatment of major depression
It is well established that ECT is an effective treatment for major depression, superior to placebo, simulated ECT (anesthesia only), and antidepressant medication.23-26 Of patients with major depression who receive ECT as a first-line treatment, 80% to 90% show significant improvement. Currently, most patients with major depression treated with ECT have failed two or more courses of antidepressant medication. ECT is effective in over half of these patients.10,27 ECT is indicated in patients intolerant of antidepressant medication and those with medical illnesses that contraindicate the use of antidepressants. ECT may be considered as a first-line treatment in severe depression or depression with specific features, such as psychosis,28,29 catatonia,30 melancholia (mainly food refusal leading to nutritional deficit),31 or suicidally32-34 ECT is also effective and safe in the elderly, among whom depressions tend to be persistent, and the patients suffer from other systemic disorders and consume many medications.35 During pregnancy, ECT is usually only considered if the fetus is at risk from the unstable psychiatric condition of the mother.36 ECT may also be considered for patients who have previously shown a positive response to ECT or patients who prefer this treatment.
Although it is difficult to predict response to ECT, there are factors associated with poorer response to ECT such as refractoriness to antidepressant medication, chronicity of the depression, and personality disorders.37,38 Relapse rate during the 6 months following ECT exceeds 50 %,39,40 with the bulk of the relapses occurring within 1 month of termination of the treatment course. Continuation therapy markedly reduces the relapse rate.41 Following ECT, continuation therapy might include pharmacotherapy,42 maintenance ECT,43 or a combination of maintenance ECT and an antidepressant agent. In a recent multicenter randomized study, the combination of lithium and nortriptyline was shown to reduce the relapse rate by 50 %.44
Adverse effects
The most important adverse effect of ECT is memory impairment. Concern about memory loss is intensified for the patient and family by the transient confusion that occurs after each seizure. High-dose unilateral ECT produces less severe and persistent cognitive adverse effects than bilateral ECT10 In the postictal period, bilateral ECT causes more prolonged disorientation and more severe retrograde amnesia than unilateral ECT. One week and 2 months after the course, bilateral ECT is associated with greater anterograde and retrograde memory deficits. During the first 2 months after ECT, bilateral ECT is associated with greater retrograde amnesia than unilateral ECT Most patients return to their cognitive baselines 6 months after ECT, although a few patients complain of permanent memory loss.
Other side effects of ECT include headache, nausea, vomiting, myalgia, back pain, or damage to teeth if appropriate precautions are not taken.
Patients with increased intracranial pressure (due to an intracranial mass or obstruction of cerebrospinal fluid flow) are at risk for brain edema or herniation after ECT Most clinicians regard increased intracranial pressure as an absolute contraindication to ECT In these patients, pretreatment with steroid, diuretic, or antihypertensive agents can reduce the risk. Several coexisting disease processes warrant special attention due to their potential for complications in the context of ECT The cardiovascular risk of ECT is a product of the stress of ECT itself, the severity and stability of coronary artery disease, and hemodynamic changes after the ECT (parasympathetic and then sympathetic response).45 Identifying and controlling risk factors such as hypertension, arrhythmias (especially tachycardia), angina, congestive heart failure, and diabetes mellitus can minimize the risk of post-ECT ischemia.18 Controlling hypertension is especially important since during ECT, systemic blood pressure increases acutely.
The estimated mortality rate with ECT is between two and ten per 100 000, about 0.002% per treatment, and 0.01% for each patient.46,47 This mortality rate is equivalent to the mortality rate with general anesthesia (1:50,000). 48
Mechanism of action
The mechanism of action of ECT has intrigued psychiatrists and neuroscientists since the treatment was first introduced. Laszlo Meduna,1 the inventor of convulsive therapy, suggested that chemically induced seizures were effective in the treatment of schizophrenia by “changing the chemical composition of the brain.” The first comprehensive book on ECT mechanisms was published in 1974.49 A second book on the topic appeared a decade later.50 Several dedicated review papers and book chapters have been published since. In the course of ECT an electrical current traverses brain tissue and a grand mal seizure ensues; it is inevitable that events such as these will have major physiological consequences. As noted by Seymour Kety,51 ECT “.. involves massive discharge over wide areas of the brain, activation of the peripheral autonomic nervous system, release of the secretion of many endocrine glands..” and as a result “.. the difficulty lies not in demonstrating such changes but in differentiating., which of the changes may be related to the important antidepressive and amnestic effects and which are quite irrelevant to these.”
An important implication of Kety's highly relevant observation is that research into the mechanism of action of ECT should take into account clinical aspects of the treatment that have a potentially important impact in the research context. The first consideration is that longstanding changes induced by repeated administrations of electroconvulsive shock (ECS) are much more likely to be relevant to the therapeutic mechanism of ECT than transient effects of a single ECS. The therapeutic spectrum of ECT is another highly relevant consideration. In addition to its antidepressant properties, ECT has antimanic, antipsychotic, anticatatonic, antiepileptic, and anticonvulsant effects, and is used clinically for all these indications. It is highly implausible that a single mechanism of action will explain all these varied, and in some cases opposite, clinical effects. In considering the antidepressant mechanism of ECT, it is also important to note that ECT is substantially more effective than antidepressants; more than 50% of patients who have not responded to at least two adequate trials of antidepressant medication will respond to ECT Furthermore, ECT is effective in patients with psychotic depression whereas antidepressant drugs are not, unless administered in conjunction with an antipsychotic. All these observations provide some explanation of why a definitive understanding of the mechanism of action of ECT has proved so elusive in spite of the enormous efforts that have been invested. A comprehensive analysis of the various theories of ECT action in depression and the evidence that has been gathered in support of them is beyond the scope of this paper. The overall focus of recent work may be summarized under a few general headings. Recent intriguing findings regarding the effect of ECT on synaptic plasticity and neurogenesis will be considered more extensively.
One important research direction has been the effect of ECT on neurotransmitters, receptors, and postreceptor signaling mechanisms in the brain, particularly those that are implicated in the mechanism of action of antidepressant drugs. The emphasis has been primarily on serotonergic, noradrenergic, and dopaminergic systems with some consideration of γ-aminobutyric acid (GAB A)-ergic and more recently glutamatergic mechanisms.52-55 Electrophysiological studies suggest that an important effect of ECS on brain serotonergic systems in rodent brain is sensitization of postsynaptic serotonin (5-HT)1A receptors and a consequent increase in serotonergic transmission.56 This may be reflected in patients in increased responsiveness to serotonergically mediated neuroendocrine challenges.57 There is great variability, however, in the overall effect of ECS on serotonin receptors as well as regional differences.54,58 In the noradrenergic system, the density of postsynaptic β-adrenergic receptors is reduced by ECS, while autoreceptors that modulate noradrenaline release are inhibited.52,53 The net effect may be an increase in postsynaptic signal transduction.55,58 Dopaminergic function is increased postsynaptically, a finding that is consistent with the antiparkinsonian effects of ECT but difficult to reconcile with its antipsychotic action.59
A second major research direction may be termed neurophysiological. It encompasses the extensive work that has been done to evaluate the effects of ECT on brain electrical activity and cerebral blood flow (CBF) and metabolism. There is considerable evidence that depressed patients have reduced CBF and metabolism compared with normal subjects, although in some brain areas it may be increased.60,61 Some studies suggest that reduced CBF in depression is reversed by ECT, but others report a further reduction.62,63 Reduced brain function as a consequence of ECT is consistent with the hypothesis that recruitment of endogenous inhibitory processes to terminate the seizure is important in the therapeutic action of ECT.59
A third research direction takes as its starting point the substantial endocrine effects of ECT and suggests that these effects are implicated in the therapeutic mechanism of the treatment.64 Plasma prolactin levels are acutely increased by ECT65,66 This is a consistent finding, but it has been difficult to explain how it might be related to the therapeutic action of ECT. Another focus has been on the effect of ECT on thyrotropin-releasing hormone (TRH) and TRH-receptor function.67
More recently there has been a great deal of emphasis on the effect of ECS on synaptic plasticity and neurogenesis. Adult neurogenesis, the lifelong addition of new neurons, was first documented in rat hippocampus.68 It is now well established that neurogenesis occurs in several different species, including humans.69 The newly generated cells mature into functional neurons.70 Neurogenesis is regulated by many factors. Upregulation of neurogenesis occurs in response to enriched environment,71 exercise,72,73 and learning. Downregulation of neurogenesis occurs in response to aging74,75 and stress (psychological or environmental).76 It is well established that the volume of hippocampus is decreased in patients suffering from depression.71,72 Repeated stress causes atrophy of dendrites in the CA3 region, and both acute and chronic stress suppresses neurogenesis of rat dentate gyrus granule neurons. The hippocampus is an especially plastic and vulnerable region, and a target of stress hormones (gonadal, thyroid, and adrenal hormones). This cell loss might explain the reduction in hippocampal volume observed in depression. Decreased neurogenesis might also explain some of the symptoms of depression, such as cognitive abnormalities and loss of inhibitory control of the hypothalamic-pituitary-adrenal (HPA) axis. Recently, it has been demonstrated that chronic administration of several classes of antidepressant treatment, such as serotonin or norepinephrine selective reuptake inhibitors, monoamine oxidase inhibitors, lithium, and ECS upregulates neurogenesis in adult rodent hippocampus.79-84 ECS influences some molecular markers of neuronal plasticity; for example, ECS decreases the level of phosphorylated heavy and light neurofilament subunit (NF-H and NF-L), that may be part of the cytoskeletal remodeling.85 ECS also influences several trophic factors that are related to neurogenesis, for example, brain-derived neurotrophic factor (BDNF), which increases the synaptic strength, survival, and growth of adult neurons. ECS prolongs the expression of BDNF and its receptor, trkB, and blocks the downregulation of BDNF mRNA in the hip pocampus in response to restraint stress.86 ECS has been demonstrated to change gene transcription in rat hippocampus, including genes that are related to neurogenesis, such as BDNF-MAP kinase-cAMP-cAMP response element-binding protein pathway and other immediate-early genes.87
Transcranial magnetic stimulation
Development of TMS
From the late 19th century many attempts were made to induce neural activity by magnetic stimulation until Barker and colleagues showed 20 years ago that magnetic stimulation of the human motor cortex produces depolarization of cortical areas.88 Transcranial magnetic stimulation has been found to be a noninvasive, easily tolerated method of probing cortical brain function. During the last decade, many studies have indicated that TMS has antidepressant properties,89,90 but its clinical effect is not yet clear.
Technique of TMS
In TMS, a magnetic field is generated by an electric current, and this magnetic field induces an electric current within the brain. The patient is awake, and sessions last 20 to 60 min. The treatment lasts a few weeks, since multiple sessions are indicated. An alternating electric current passes through a metal coil that is placed on the patient's scalp.91 The electric current induces an alternating magnetic field, perpendicular in orientation to the current flow. The magnetic field passes through the scalp and skull without impedance and causes depolarization of cortical brain cells. The electrical current is parallel and opposite in direction to the electrical current in the coil. The stimulated brain area depends on two major factors: the coil design92 and the coil orientation.93 The magnetic field depolarizes cells to a depth of 2 cm below the scalp, near the gray-white junction of the nervous tissue.94 Single-pulse TMS is generated by a single magnetic pulse, while repetitive TMS (rTMS) is generated by magnetic pulses given in a regular frequency The stimulation frequency might be fast (more than 1 Hz) or slow (1 Hz and less). The two frequencies of stimulation have opposite effects on brain excitability and metabolism. Fast rTMS and slow rTMS have been associated with increased and decreased cortical excitability and regional blood flow, respectively.95,96 Slow frequency stimulation has a lower risk of inducing seizures.97 The intensity of the magnetic pulse is measured relative to the motor threshold, which is the lowest intensity of stimulation that produces specific muscle contraction in at least 5 of 10 trials.98
Most studies uses fast rTMS over the left hemisphere and slow rTMS over the right hemisphere. Using fast rTMS over the left dorsolateral prefrontal cortex is based on the finding that functional activity in this area is low in patients suffering from major depression99 and assumes that fast rTMS will enhance activity in this brain area. Use of slow rTMS over the right dorsolateral prefrontal cortex is aimed at reducing overactivity in this brain area and thus resolving a suspected hemispheric imbalance.100
TMS in the treatment of major depression
Administering rTMS to healthy individuals has not been shown to induce significant mood changes,101 although left prefrontal rTMS is associated with transient decreased happiness and right prefrontal rTMS with transient decreased sadness.102,103 Compared with sham administration, slow and fast rTMS have been shown to have some antidepressant properties.104-109 However, analyzing these studies is difficult due to the different techniques used such as different frequencies, coil design, and positions.
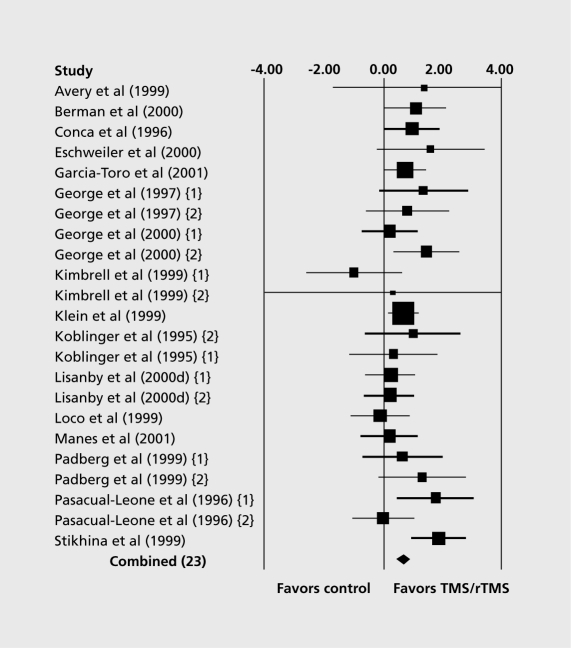
A systematic review by Burt et al evaluated the antidepressant effect of TMS.110 A meta-analysis of open and uncontrolled studies showed an antidepressant effect, but the clinical significance of this effect was uncertain, since most patients did not meet standard criteria for clinical response or remission. A meta-analysis of controlled studies showed that rTMS has superior antidepressant properties compared with sham administration (Figure 4). However, similarly to the uncontrolled studies, the therapeutic effect was of doubtful clinical significance due to modest average effect and small average difference in improvement between active and sham conditions. A subsequent systematic review and meta-analysis included 14 trials.111 Pooled analysis using the Hamilton Rating Scale for Depression showed an effect in favor of rTMS compared with sham after 2 weeks of treatment, but this was not significant at follow-up 2 weeks after the intervention period. The conclusion of this analysis was that “current trials are of low quality and provide insufficient evidence to support the use of rTMS in the treatment of depression.” This conclusion is shared by two other reviews112,113 but not by another meta-analysis of randomized sham-controlled trials of left prefrontal rTMS that found an “acute antidepressant treatment with statistically significant effect sizes and measurable clinical improvement.”114 It is clear that further controlled studies using standardized methodology are needed in order to establish the place of rTMS in the treatment of major depression.
Figure 4. Meta-analysis of controlled trials of TMS. Figure shows effect size (d) and 95% confidence intervals for randomized, controlled studies of TMS and rTMS in the treatment of depression. The size of the boxes is proportional to the sample size. The overall combined effect size is indicated by a diamond. See Burt et al104 for a review of the individual studies included in the meta-analysis. Reproduced from reference 104: Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. Int J Neuropsychopharmacol. 2002;5:73-103. Copyright © Cambridge University Press, 2002.
A few studies have compared the antidepressant effect of rTMS and ECT115-118 These suggest that the antidepressant effect of rTMS is similar or slightly inferior to the antidepressant effect of ECT; however, in these studies the average improvement with ECT was unusually low. Comparing psychotic and nonpsychotic patients, it has been reported that rTMS and ECT have a similar antidepressant effect in nonpsychotic major depressive disorder,116,117 but ECT has been found to be superior for psychotic major depressive disorder.117
Older age, treatment refractoriness, and psychotic depression have been found to be negative predictors of depression improvement with TMS.115,119 Pretreatment cerebral metabolism has been found to correlate with antidepressant response to TMS120; for example, hypometabolism in the temporal lobes, cerebellum, anterior and occipital cingulate regions has been associated with improvement with fast rTMS while hypermetabolism had been associated with improvement with slow rTMS.121
Some preliminary data suggest that TMS might be used as a maintenance treatment for patients with depression.122 TMS has recently been shown to accelerate the antidepressant effect of amitriptyline123; previously it had not been shown that concomitant use of antidepressant medication influences the therapeutic effect of TMS.110 Course duration of more than 10 days had been found to be associated with a better antidepressant effect,124 and treatment for at least 4 weeks is considered to have clinically meaningful benefits.125 More intense magnetic pulses (100% to 110% of motor threshold) have been shown to be more effective that less intense pulses (80% to 90% of motor threshold), and more pulses per day (1200 to 1600 pulses per day) has been shown to be more effective than fewer pulses per day (800 to 1000 pulses per day).124 High-frequency rTMS has not been shown to be superior to low-frequency rTMS.126-127 Low-frequency rTMS is considered safer, and its use is recommended.110
Adverse effects
TMS is considered a safe procedure, without clinically significant changes in cognitive parameters,128 hearing, or hormone levels.129 The major risk of TMS is seizure induction, associated primarily with high-frequency rTMS. Since the introduction of standards of safety for the administration of TMS,105 no TMS-induced seizure has been reported. Other adverse effects include headaches, scalp facial muscle twitching, and mild tinnitus, which usually respond to analgesics.
Mechanism of action
TMS causes functional changes in the brain. Performing magnetic resonance imaging (MRI) scans before and after rTMS in depressed patients did not reveal any structural difference, and volumetric analysis of the prefrontal lobe showed no changes.130 However, many studies have demonstrated that TMS changes cortical excitability131 and that higher intensity TMS causes greater activation than lower intensity TMS.132 These changes in cortical excitability occur at the primary site of excitation (neuronal activation in sites under the coil) as well as in distant brain areas.133
Clinical improvement in depression using rTMS has been associated with changes in cerebral blood flow in the prefrontal and paralimbic areas.134 SPECT scans that were obtained from patients with major depression resistant to medication before and after 10 days of rTMS, demonstrated that treatment responders had significantly less pretreatment blood flow in the left amygdala compared with nonresponders, and only the responders demonstrated two patterns of change in regional blood flow with treatment: a reduction in orbitofrontal blood flow and/or a reduction in anterior cingulate blood flow.135
Using animal models, rTMS-induced changes in neurotransmitters have been found. Some of these changes are similar to the effect of other antidepressant therapy (such as ECS).136-138 For example, a single rTMS session was associated with increased hippocampal dopamine and serotonin.136 Chronic rTMS was associated with upregulation of β-adrenergic and serotonin receptors in the frontal cortex, with downregulation of β-adrenergic receptors in the striatum137 and with subsensitivity of presynaptic serotonergic autoreceptors, an effect that is shared with antidepressant drugs.132
rTMS has been shown to have some metabolic and neuroendocrine effects. Using proton magnetic resonance spectroscopy following high-frequency rTMS in healthy volunteers, it was demonstrated that rTMS affects cortical glutamate/glutamine levels, both close to the stimulation site (left dorsolateral prefrontal cortex) and in remote brain regions (right dorsolateral prefrontal cortex, left cingulate cortex). These data indicate that rTMS may act via stimulation of glutamatergic prefrontal neurons.139 rTMS has been shown to increase thyroid-stimulating hormone (TSH) in healthy individuals140 and in patients with major depression.141 In patients with depression who remitted after rTMS, reversal of dexamethasone suppression test (DST) abnormality was demonstrated.142
rTMS has recently been associated with neuroplasticity and neurogenesis. For example, rTMS can modulate astroglial gene expression; following rTMS, an increased level of glial fibrillary acidic protein (GFAP) messenger ribonucleic acid (mRNA) was found in the hippocampal dentate gyrus.143 rTMS can also increase immediate early gene expression, such as c-fos and c-jun.144,145
It had been suggested that a change in local blood-brain barrier settings, allowing passage of peripheral substances directly into brain parenchyma, may be the mechanism of TMS. However, it has recently been demonstrated that TMS does not result in leakage of the blood-brain barrier in patients with depression.146
Magnetic seizure therapy
Magnetic seizure therapy (MST) is a novel brain stimulation method that uses transcranial magnetic stimulation at convulsive parameters in order to induce therapeutic seizures under general anesthesia, in the same setting used for ECT147 After its introduction in 2000, a few case reports described successful treatment of patients suffering from major depression using MST110,148 but it is not yet established that MST has antidepressant efficacy. In a recent study by Lisanby et al,110 10 patients with major depression received two treatments with MST followed by two treatments with ECT, in randomized order. MST seizures were found to have shorter duration, lower ictal EEG amplitude, and less postictal suppression than ECT seizures.149 MST might cause fewer cognitive side effects than ECT, by inducing more focused seizures and sparing cortical regions associated with memory loss. In a nonhuman primate model (Rhesus macaque monkeys), MST was shown to result in a more favorable acute cognitive side effect profile than ECT with regard to long-term memory of a constant target, short-term memory of a variable target, and recall of previously learned three-item lists.150,151 Preliminary clinical data are seen as suggesting that MST has antidepressant properties and fewer cognitive side effects than ECT152 For example, patients recover orientation more quickly and have fewer attention difficulties or less retrograde amnesia after MST compared with ECT153
Deep brain stimulation
Development of DBS
Deep brain stimulation (DBS) was introduced in the late 1980s by Benabid and colleagues, for the treatment of movement disorders.153 Their original assumption was that chronic high-frequency stimulation of the brain areas might be similar to surgical ablation of these areas.154 For example, thalamic stimulation for the treatment of intractable tremor was found to have clinical benefits similar to those achieved by surgical thalamotomy155 and stimulation of the subthalamic nucleus or globus pallidus internus for the treatment of Parkinson's disease could replace the traditional pallidotomy156 Over the last decade, DBS has become a popular treatment for movement disorders such as Parkinson's disease and essential tremor.157 During the last few years, DBS has been suggested as a treatment for psychiatric disorders, such as depression158 and obsessive-compulsive disorder.159
Technical aspects
The surgical procedure for the implantation of DBS electrodes is based on stereotactic techniques that include imaging modalities, physiological mapping, and surgical navigation computers.160 A stereotactic frame is fixed to the patient's head, and preoperative magnetic resonance images are obtained. Under local anesthesia, a burr hole is drilled, the underlying dura mater is opened, and microelectrodes are inserted using MRI guidance. The electrode location is confirmed by postoperative MRI. Right and left quadripolar electrodes are implanted. The electrodes remain externalized for a week for clinical testing, and then are connected to a pulse generator that is implanted in the infraclavicular region. The frequency, intensity, and pulse width of the stimulation are programmable, within safety limits. The physician sets the stimulus parameters, and the patient might also alter a few parameters by himor herself. Stimulation can be programmed to continuous or intermittent firing, or to on and off cycles during fixed time intervals. DBS is reversible, and the stimulation parameters can be changed according to patient's symptoms or disease progression.
DBS in the treatment of major depression
To date, a few case reports suggest that DBS might be a useful treatment for refractory depression. Recently, Mayberg and colleagues158 found that DBS of the white matter tracts adjacent to the subgenual cingulate gyrus was associated with improvement in depressive symptoms in 6 patients with refractory depression. By 1 and 6 months, two and four patients met criteria for clinical response, respectively Remission was achieved by three patients after 6 months.158 Deep brain stimulation of the ventral caudate nucleus improved anxiety, depressive, and compulsive symptoms in one patient who suffered from resistant obsessive-compulsive disorder and resistant major depression.159 Deep-brain stimulation of the inferior thalamic peduncle improved depressive symptoms in one patient suffering from recurrent unipolar depression and borderline personality disorders.161
Adverse effects
Major side effects of DBS are seizure (1% to 3%), hemorrhage (1% to 5%), infection (2% to 25%, usually superficial infections but rarely cerebritis or brain abscess) and hardware-related complications (about 25%) that include fracture of leads, disconnection, lead movement, and malfunction.162,163 Stimulation-induced adverse effects such as parasthesia, muscle contraction, dysarthria, or diplopia are usually reversible with changes in stimulation parameters.164
Mechanism of action
Investigating the mechanism of deep brain stimulation reveals a basic paradox: the clinical effect of deep brain stimulation, which is usually regarded as a method of activating neurons, is similar to the traditional ablation of specific brain areas.165 Studies aimed at resolving this paradox have led to the development of four major theories regarding the mechanism of action of DBS. The first theory suggests that irregular activity in neurons converging with other neurons can result in a loss of information transfer and thus cause clinical pathology. The therapeutic effect of DBS may be due to its driving neurons at regular frequencies, and thus modulating the pathological network activity and increasing neuronal activity in the output nuclei.166 Many studies using functional imaging demonstrate increased cortical activity during DBS treatment. For example, activation of the thalamus and basal ganglia was demonstrated by fMRI studies167 and activation of motor cortex and supplementary motor area was demonstrated by PET studies.168,169 The second theory suggests that excitation of axon terminals near the stimulation electrode releases inhibitory factors that cause synaptic inhibition of the neuron.170 The third theory suggests that high-frequency tetanus produces a blockade of the spontaneous activities of neurons as a result of a strong depression of intrinsic voltage-gated currents,171 and thus DBS causes a depolarization blockade. Theories of synaptic inhibition and depolarization blockade are both supported by the decreased recordings of somatic activity in the stimulated nuclei. The fourth theory suggests that the stimulation causes synaptic depression by transmitter depletion.172,173
Although depression is probably a disorder of multiple brain areas, neuronal pathways, neurotransmitters, and genomic systems, DBS requires stimulation of a single brain area. Many studies indicate that the limbic-cortical pathways and specifically the subgenual cingulate (Cg25) are involved in acute sadness and in the antidepressant effect of medications, electroconvulsive therapy, and transcranial magnetic stimulation.174-176 Therefore, this area was first tested by Mayberg and colleagues for the efficacy of DBS as a treatment for major depression. By using pretreatment and post-treatment PET scans, it was demonstrated that the cerebral blood flow abnormalities related to depression, such as decreased blood flow in the prefrontal area and increased blood flow in the subgenual cingulate (Cg25), were normalized after DBS in the treatment-responsive patients.158
Vagus nerwe stimulation
Development of VNS
The vagus nerve, the longest of the cranial nerves, is a mixed nerve, with 80% of the fibers carrying afferent information (to the brain) and 20% of the fibers carrying efferent information (from the brain). Afferent sensory fibers within the vagus nerve terminate in the nucleus tractus solitarius (NTS), which innervates many brain regions that are related to psychiatric disorders (for example, locus ceruleus, amygdala, and hypothalamus). The potential of vagal nerve stimulation to influence central nervous system function was demonstrated long before its use as a therapeutic intervention was considered.177,178 During the 1980s, Zabara showed that VNS has an anticonvulsant action in dogs179 and during the 1990s VNS became a treatment modality for epilepsy in humans.180,181 In 2000, VNS was found to be associated with mood improvements in patients with epilepsy.182,183 VNS has also been demonstrated to affect specific brain areas including the limbic system184 and to alter concentration of monoamines within the central nervous system (such as serotonin, norepinephrine, GABA, and glutamate).185,186 These clinical and laboratory findings, together with the efficacy of anticonvulsant medications as a treatment for depressive episodes, has led to the hypothesis that VNS might be an effective treatment for major depression.187
Technique of VNS
VNS is performed in humans by stimulation of the left cervical vagus nerve using a subcutaneous generator that sends an electrical signal to the nerve.188 The generator is implanted into the left chest wall Bipolar electrodes are wrapped around the left vagus in the neck through a special incision, and tunneled under the skin toward the chest.189 The stimulation parameters that can be adjusted by the physician include current intensity, pulse width, frequency, and duration of the on and off periods. The generator is designed to shut off in the presence of a magnetic field, and the patient is given a magnet that can turn off the stimulation when held over the generator.
VNS in the treatment of major depression
VNS was recently demonstrated to have an antidepressant effect in a rat model, significantly better than sham treatment, and similar to other antidepressant treatments (desipramine or ECS).190 During the past 5 years, Sackeim, Rush, and colleagues have published results of open and randomized controlled studies of VNS in the treatment of major depression. A preliminary open study of VNS in 60 patients with treatment-resistant nonpsychotic major depressive episode revealed a response rate of 30% to 38% by 10 weeks of treatment.191 A 2-year follow-up of this open study found a response rate of 40% to 44% after 1 year and 42% after 2 years, and a remission rate of 27% after 1 year and 22% after 2 years.192,193 A randomized controlled study of VNS in over 200 patients with treatment-resistant, nonpsychotic, major depressive episode showed that acute treatment (10 weeks) yielded a response rate of 15% that was similar to the response rate with sham treatment (10%). 194 After the acute treatment, all patients (VNS and sham groups) received long-term treatment with VNS for another 12 months. This was associated with a response rate of 27% and a remission rate of 16%. 195 The response rate in the group of patients who were receiving VNS plus medication or ECT for a year (27%) was significantly better than the response rate of a similar but nonrandomized group of patients with treatment-resistant depression who were receiving only medication or ECT for a year (response rate was only 13 %).196
Adverse effects
The most common side effects of VNS are voice alteration or hoarseness (55%), coughing (17%), shortness of breath (15%), headache (22%), neck pain (17%), dysphagia (20%), and pain (15%). Although most side effects usually resolve within a few weeks, voice alteration and dyspnea might persist for long periods. Reduction in current intensity decreases the severity of these symptoms.191
Mechanism of action
VNS most probably alters synaptic activities at vagal afferent terminations, stimulates deep brain areas, and thus modulates antidepressant neuronal circuits in multiple limbic system structures. Brain imaging studies reveal some of these suspected brain changes. PET measurements of cerebral blood flow in 10 patients with epilepsy before and during acute VNS treatment (both low- and high- stimulation VNS) demonstrated increased blood flow in the rostral, dorsal-central medulla, the right postcentral gyrus, bilaterally in the hypothalami, thalami, and insular cortices, and in the cerebellar hemispheres inferiorly Decreased blood flow was demonstrated bilaterally in hippocampus, amygdala, and posterior cingulate gyri.184 Similar changes in cerebral blood flow were also demonstrated during prolonged VNS treatment.197 Some of these findings share features with changes of regional cerebral blood flow previously associated with the administration of antidepressant drugs (such as selective serotonin reuptake inhibitors).198 fMRI studies confirmed that VNS induces changes in the orbitofrontal and parieto-occipital cortex bilaterally, left temporal cortex, hypothalamus, and left amygdala199 and suggested that VNS at different frequencies has frequency or dose-dependent modulatory effects on brain activities.200
In addition, VNS is associated with neurobiological changes that are related to the pathogenesis of depression:
VNS has been found to alter concentrations of neurotransmitters that are probably involved in the mechanism of depression. VNS was associated with increased GABA, 5-hydroxyindoleacetic acid and homovanillic acid levels and decreased aspartate and glutamate levels.186,201
VNS was associated with neuroimmunological changes such as a marked peripheral increase in pro- and anti-inflammatory circulating cytokines, such as IL-6,TNF-α, and TGF-β.202
A preliminary study suggests that VNS treatment changes the hypothalamic-pituitary-adrenal (HPA) axis stress system. In patients with chronic depression, corticotrophin-releasing hormone (CRH) challenge causes increased adrenocorticotrophic hormone (ACTH) levels. VNS treatment of depressed patients reversed this abnormally increased ACTH response to CRH challenge.203
VNS treatment was associated with improvement in abnormal sleep architecture in patients with depression.204
Conclusions
Several novel nonpharmacological, somatic treatments for major depression have been reviewed. All are based on the principle of brain stimulation. Other than ECT, TMS is the only one of these treatments that is relatively widely used. The clinical efficacy of TMS is not conclusively established, and its precise therapeutic niche still needs to be defined. TMS does not appear to be a viable alternative to ECT for treatment-refractory, depressed patients. There is very great interest in the potential of MST. If magnetically induced seizures are effective clinically but induce fewer cognitive adverse effects than ECT, this would be a very great advantage. Early studies suggest that this may be so, but the field is still at a very early stage of development and further research is needed. The last two modalities discussed, DBS and VNS, are both characterized by high cost and the potential for troublesome adverse effects. Both entail surgical procedures. Their indication, if efficacy is established and technical issues are resolved, would be for highly resistant patients where the complexity of the treatment and its expense are warranted. Some trials of VNS have been conducted. Their results suggest equivocal efficacy in the short term, but longer-term effects might be more promising. The clinical application of DBS is still at very early stage.
In practical terms ECT remains the only widely available, nonpharmacological, somatic treatment of depression that is effective, safe, and relatively inexpensive. This is likely to remain the situation in the short term. In the longer term, other approaches to brain stimulation may become clinically viable and eventually replace ECT Such a projection must be made with due caution. The epitaph of ECT has been written repeatedly over the past 50 years. Nevertheless, it remains one of the longest-standing, continuously used treatments in medicine. Research efforts over the next decade in the field of brain stimulation will be crucial in establishing how long ECT will continue to occupy this unique position.
Selected abbreviations and acronyms
- DBS
deep brain stimulation
- ECT
electroconvulsive therapy
- MST
magnetic seizure therapy
- TMS
transcranial magnetic stimulation
- VNS
vagus nerve stimulation
Contributor Information
Renana Eitan, Biological Psychiatry Laboratory, Department of Psychiatry, Hadassah-Hebrew University Medical Center, Jerusalem, Israel.
Bernard Lerer, Biological Psychiatry Laboratory, Department of Psychiatry, Hadassah-Hebrew University Medical Center, Jerusalem, Israel.
REFERENCES
- 1.Meduna L. New methods of medical treatment of schizophrenia. Arch Neurol Psychiatry. 1936;35:361–363. [Google Scholar]
- 2.Bennett AE. Convulsive (pentamethylenetetrazol) shock therapy in depressive psychoses. Am J Med Sci. 1938;196:420–428. [Google Scholar]
- 3.Bini L. Experimental researches on epileptic attacks induced by electric current. Am J Psychiatry. 1938;94(suppl):172–174. [Google Scholar]
- 4.Cerletti U. Old and new information about electroshock. Am J Psychiatry. 1950;107:87–94. doi: 10.1176/ajp.107.2.87. [DOI] [PubMed] [Google Scholar]
- 5.Fink M. Convulsive therapy: a review of the first 55 years. J Affect Disord. 2001;63:1–15. doi: 10.1016/s0165-0327(00)00367-0. [DOI] [PubMed] [Google Scholar]
- 6.Bennett AE. Curare: a preventive in traumatic convulsive shock therapy. Am J Psychiatry. 1941;97:1040. doi: 10.1176/ajp.151.6.248. [DOI] [PubMed] [Google Scholar]
- 7.Vaidya VA., Siuciak JA., Du F., Duman RS. Hippocampal mossy fiber sprouting induced by chronic electroconvulsive seizures. Neuroscience. 1999;89:157–166. doi: 10.1016/s0306-4522(98)00289-9. [DOI] [PubMed] [Google Scholar]
- 8.Rasmussen KG. Clinical applications of recent research on electroconvulsive therapy. Bull Menninger Clin. 2003;67:18–31. doi: 10.1521/bumc.67.1.18.23449. [DOI] [PubMed] [Google Scholar]
- 9.Abrams R., Taylor MA. Diencephalic stimulation and the effects of ECT in endogenous depression. Br J Psychiatry. 1976;126:482–485. doi: 10.1192/bjp.129.5.482. [DOI] [PubMed] [Google Scholar]
- 10.Sackeim HA., Prudic J., Devanand DP., et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57:425–434. doi: 10.1001/archpsyc.57.5.425. [DOI] [PubMed] [Google Scholar]
- 11.Sackeim HA., Decina P., Kanzler M., Kerr B., Malitz S. Effects of electrode placement on the efficacy of titrated, low-dose ECT. Am J Psychiatry. 1987;144:1449–1455. doi: 10.1176/ajp.144.11.1449. [DOI] [PubMed] [Google Scholar]
- 12.Sackeim HA., Prudic J., Devanand DP., et al. Effect of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. N Engl J Med. 1993;328:839–846. doi: 10.1056/NEJM199303253281204. [DOI] [PubMed] [Google Scholar]
- 13.Borden MD., Clarke MT., Katz H. The use of pancuronium bromide in patients receiving lithium carbonate. Can Anaesth Soc J. 1974;21:79–82. doi: 10.1007/BF03004581. [DOI] [PubMed] [Google Scholar]
- 14.Harsh HH., Haddox JD. Electroconvulsive therapy and fluoxetine. Convulsive Ther. 1990;6:250–251. [PubMed] [Google Scholar]
- 15.Dursun SM., Patel JK., Drybala T., Shinkwin R., Drybala G., Reveley MA. Effects of antidepressant treatments on first-ECT seizure duration in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:437–443. doi: 10.1016/s0278-5846(00)00173-1. [DOI] [PubMed] [Google Scholar]
- 16.Conway CR., Nelson LA. The combined use of bupropion, lithium, and venlafaxine during ECT: a case of prolonged seizure activity. J ECT. 2001;17:216–218. doi: 10.1097/00124509-200109000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Braga RG., Petrides G. The combined use of electroconvulsive therapy and antipsychotics in patients with schizophrenia. J ECT. 2005;21:75–83. doi: 10.1097/01.yct.0000165500.60784.05. [DOI] [PubMed] [Google Scholar]
- 18.Folk JW., Kellner CH., Beale MD., Conroy JM., Duc TA. Anesthesia for electroconvulsive therapy: a review. J ECT. 2000;16:157–170. doi: 10.1097/00124509-200006000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Dew RE. Seizure length and clinical outcome in electroconvulsive therapy using methohexital or thiopental. J ECT. 2005;21:16–8. doi: 10.1097/01.yct.0000154052.80893.f7. [DOI] [PubMed] [Google Scholar]
- 20.Shapira B., Lidsky D., Gorfine M., Lerer B. Electroconvulsive therapy and resistant depression: clinical implications of seizure threshold. J Clin Psychiatry. 1996;57:32–38. [PubMed] [Google Scholar]
- 21.Lerer B., Shapira B., Calev A., et al. Antidepressant and cognitive effects of twice- versus three-times-weekly ECT. Am J Psychiatry. 1995;152:564–70. doi: 10.1176/ajp.152.4.564. [DOI] [PubMed] [Google Scholar]
- 22.Shapira B., Tubi N., Drexler H., Lidsky D., Calev A., Lerer B. Cost and benefit in the choice of ECT schedule. Twice versus three times weekly ECT. Br J Psychiatry. 1998;172:44–48. doi: 10.1192/bjp.172.1.44. [DOI] [PubMed] [Google Scholar]
- 23.Avery D., Winokur G. The efficacy of electroconvulsive therapy and antidepressants in depression. Biol Psychiatry. 1977;12:507–523. [PubMed] [Google Scholar]
- 24.Janicak PG., Davis JM., Gibbons RD., Ericksen S., Chang S., Gallagher P. Efficacy of ECT: a meta-analysis. Am J Psychiatry. 1985;142:297–302. doi: 10.1176/ajp.142.3.297. [DOI] [PubMed] [Google Scholar]
- 25.UK ECT review group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361:799–808. doi: 10.1016/S0140-6736(03)12705-5. [DOI] [PubMed] [Google Scholar]
- 26.Pagnin D., de Queiroz V., Pini S., Cassano GB. Efficacy of ECT in depression: a meta-analytic review. J ECT. 2004;20:13–20. doi: 10.1097/00124509-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Devanand DP., Sackeim HA., Prudic J. Electroconvulsive therapy in the treatment-resistant patient. Psychiatr Clin North Am. 1991;14:905–923. [PubMed] [Google Scholar]
- 28.GIassman AH., Kantor SJ., Shostak M. Depression, delusions, and drug response. Am J Psychiatry. 1975;132:716–719. doi: 10.1176/ajp.132.7.716. [DOI] [PubMed] [Google Scholar]
- 29.Rothschild AJ. Challenges in the treatment of depression with psychotic features. Biol Psychiatry. 2003;53:680–690. doi: 10.1016/s0006-3223(02)01747-x. [DOI] [PubMed] [Google Scholar]
- 30.Taylor MA., Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160:1233–1241. doi: 10.1176/appi.ajp.160.7.1233. [DOI] [PubMed] [Google Scholar]
- 31.Hickie I., Mason C., Parker G., Brodaty H. Prediction of ECT response: validation of a refined sign-based (CORE) system for defining melancholia. Br J Psychiatry. 1996;169:68–74. doi: 10.1192/bjp.169.1.68. [DOI] [PubMed] [Google Scholar]
- 32.Prudic J., Sackeim HA. Electroconvulsive therapy and suicide risk. J Clin Psychiatry. discussion 111-116. 1999;60 (suppl 2):104–10. [PubMed] [Google Scholar]
- 33.Nemeroff CB., Compton MT., Berger J. The depressed suicidal patient. Assessment and treatment. Ann N Y Acad Sci. 2001;932:1–23. doi: 10.1111/j.1749-6632.2001.tb05795.x. [DOI] [PubMed] [Google Scholar]
- 34.KeIIner CH. Relief of expressed suicidal intent by ECT: a consortium for research in ECT study. Am J Psychiatry. 2005;162:977–982. doi: 10.1176/appi.ajp.162.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Wurff FB., Stek ML., Hoogendijk WJ., Beekman AT. The efficacy and safety of ECT in depressed older adults: a literature review. Int J Geriatr Psychiatry. 2003;18:894–904. doi: 10.1002/gps.944. [DOI] [PubMed] [Google Scholar]
- 36.Ferrill MJ., Kehoe WA., Jacisin JJ. ECT during pregnancy: physiologic and pharmacologic considerations. Convuls Ther. 1992;8:186–200. [PubMed] [Google Scholar]
- 37.KindIer S., Shapira B., Hadjez J., Abramowitz M., Brom D., Lerer B. Factors influencing response to bilateral electroconvulsive therapy in major depression. Convuls Ther. 1991;7:245–254. [PubMed] [Google Scholar]
- 38.de Vreede IM., Burger H., van Vliet IM. Prediction of response to ECT with routinely collected data in major depression. J Affect Disord. 2005;86(23):323–327. doi: 10.1016/j.jad.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Aronson TA., Shkla LI., Hoff A. Continuation therapy after ECT for delusional depression: a naturalistic study of prophylactic treatments and relapse. Convuls Ther. 1987;3:251–259. [PubMed] [Google Scholar]
- 40.O'Leary A., Leentjens AF. Seven year prognosis in depression. Mortality and readmission risk in the Nottingham ECT cohort. Br J Psychiatry. 1996;169:423–429. doi: 10.1192/bjp.169.4.423. [DOI] [PubMed] [Google Scholar]
- 41.Kay DW., Fahy T., Garside RF. A seven-month double-blind trial of amitriptyline and diazepam in ECT-treated depressed patients. Br J Psychiatry. 1970;117:667–671. doi: 10.1192/bjp.117.541.667. [DOI] [PubMed] [Google Scholar]
- 42.Shapira B., Gorfine M., Lerer B. A prospective study of lithium continuation therapy in depressed patients who have responded to electroconvulsive therapy. Convuls Ther. 1995;11:80–85. [PubMed] [Google Scholar]
- 43.Russell JC., Rasmussen KG., O'Connor MK., Copeman CA., Ryan DA., Rummans TA. Long-term maintenance ECT: a retrospective review of efficacy and cognitive outcome. J ECT. 2003;19:4–9. doi: 10.1097/00124509-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Sackeim HA., Haskett RF., Mulsant BH., et al. Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: a randomized controlled trial. JAMA. 2001;285:1299–1307. doi: 10.1001/jama.285.10.1299. [DOI] [PubMed] [Google Scholar]
- 45.AppIegate RJ. Diagnosis and management of ischemic heart disease in the patient scheduled to undergo electroconvulsive therapy. Convuls Ther. 1997;13:128–144. [PubMed] [Google Scholar]
- 46.Shiawch R., Reid W., Carmody T. An analysis of reported deaths following electroconvulsive therapy in Texas. 1993-1998. Psychiatr Serv. 2001;52:1095–1097. doi: 10.1176/appi.ps.52.8.1095. [DOI] [PubMed] [Google Scholar]
- 47.Nuttall GA., Bowersox MR., Douglass SB., et al. Morbidity and mortality in the use of electroconvulsive therapy. J ECT. 2004;20:237–241. doi: 10.1097/00124509-200412000-00009. [DOI] [PubMed] [Google Scholar]
- 48.Gibbs N., Rodoreda P. Anaesthetic mortality rates in Western Australia 1980-2002. Anaesth Intensive Care. 2005;33:616–622. doi: 10.1177/0310057X0503300511. [DOI] [PubMed] [Google Scholar]
- 49.Fink M., Kety S., McGaugh JWTA. eds. Psychobiology of Convulsive Therapy. Washington, DC: Winston and Sons; 1974 [Google Scholar]
- 50.Lerer B., Weiner RD., Belmaker RH. eds. ECT: Basic Mechanisms. London: John Libbey; 1984. (Republished by American Psychiatric Press, Washington, DC; 1986.) [Google Scholar]
- 51.Kety S. Effects of repeated electroconvulsive shock on brain catecholamines. In: Fink M, Kety S, McGaugh JWTA, eds. Psychobiology of Convulsive Therapy. Washington, DC: Winston and Sons. 1974 [Google Scholar]
- 52.Mann JJ. Neurobiological correlates of the antidepressant action of electroconvulsive therapy. J ECT. 1998;14:172–180. [PubMed] [Google Scholar]
- 53.Newman ME., Shapira B., Lerer B. Evaluation of central serotonergic function in affective and related disorders by the fenfluramine challenge test: a critical review. Int J Neuropsychopharmacol. 1998;1:49–70. doi: 10.1017/S1461145798001072. [DOI] [PubMed] [Google Scholar]
- 54.Dremencov E., Gur E., Lerer B., Newman ME. Effects of chronic antidepressants and electroconvulsive shock on serotonergic neurotransmission in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:729–739. doi: 10.1016/S0278-5846(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 55.Donati RJ., Rasenick MM. G protein signaling and the molecular basis of antidepressant action. Life Sci. 2003;73:1–17. doi: 10.1016/s0024-3205(03)00249-2. [DOI] [PubMed] [Google Scholar]
- 56.Dremencov E., Gur E., Lerer B., Newman ME. Effects of chronic antidepressants and electroconvulsive shock on serotonergic neurotransmission in the rat hypothalamus. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1029–1034. doi: 10.1016/s0278-5846(02)00227-0. [DOI] [PubMed] [Google Scholar]
- 57.Newman ME., Gur E., Shapira B., Lerer B. Neurochemical mechanisms of action of ECS: evidence from in vivo studies. J ECT. 1998;14:153–171. [PubMed] [Google Scholar]
- 58.Coyle JT., Duman RS. Finding the intracellular signaling pathways affected by mood disorder treatments. Neuron. 2003;38:157–160. doi: 10.1016/s0896-6273(03)00195-8. [DOI] [PubMed] [Google Scholar]
- 59.Sackeim HA., Devanand DP., Nobler MS. Electroconvulsive therapy. In: Bloom FE, Kupfer DJ, eds. Psychopharmacology - The Fourth Generation of Progress. New York, NY: Raven Press; 1995 [Google Scholar]
- 60.Moretti A., Gorini A., Villa RF. Affective disorders, antidepressant drugs and brain metabolism. Mol Psychiatry. 2003;8:773–785. doi: 10.1038/sj.mp.4001353. [DOI] [PubMed] [Google Scholar]
- 61.Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- 62.Bonne O., Krausz Y., Shapira B., et al. Increased brain Tc-99m HMPAO uptake in depressed patients who have responded to electroconvulsive therapy. J Nucl Med. 1996;37:1075–1080. [PubMed] [Google Scholar]
- 63.Nobler MS., Teneback CC., Nahas Z., et al. Structural and functional neuroimaging of electroconvulsive therapy and transcranial magnetic stimulation. Depress Anxiety. 2000;12:144–156. doi: 10.1002/1520-6394(2000)12:3<144::AID-DA6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 64.Szuba MP., O'Reardon JP., Evans DL. Physiological effects of electroconvulsive therapy and transcranial magnetic stimulation in major depression. Depress Anxiety. 2000;12:170–177. doi: 10.1002/1520-6394(2000)12:3<170::AID-DA9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 65.Swartz CM. Related Neuroendocrine effects of electroconvulsive therapy (ECT). Psychopharmacol Bull. 1997;33:265–71. [PubMed] [Google Scholar]
- 66.Markianos M., Hatzimanolis J., Lykouras L. Relationship between prolactin responses to ECT and dopaminergic and serotonergic responsivity in depressed patients. Eur Arch Psychiatry Clin Neurosci. 2002;252:166–171. doi: 10.1007/s00406-002-0377-2. [DOI] [PubMed] [Google Scholar]
- 67.Sattin A. The role of TRH and related peptides in the mechanism of action of ECT. J ECT. 1999;15:76–92. [PubMed] [Google Scholar]
- 68.AItman J., Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 69.Eriksson PS., Perfilieva E., Bjork-Eriksson T., et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 70.van Praag H., Schinder AF., Christie BR., Toni N., Palmer TD., Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kempermann G., Kuhn HG., Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 72.van Praag H., Kempermann G., Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 73.Farmer J., Zhao X., van Praag H., Wodtke K., Gage FH., Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 74.Kuhn HG., Dickinson-Anson H., Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cameron HA., McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- 76.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 77.Sheline Yl., Wang PW., Gado MH., Csernansky JG., Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bremner JD., Narayan M., Anderson ER., Staib LH., Miller HL., Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 79.Malberg JE., Eisch AJ., Nestler EJ., Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Duman RS., Nakagawa S., Malberg J. Regulation of adult neurogenesis by antidepressant treatment. Neuropsychopharmacology. 2001;25:836–844. doi: 10.1016/S0893-133X(01)00358-X. [DOI] [PubMed] [Google Scholar]
- 81.Duman RS., Malberg J., Nakagawa S. Regulation of adult neurogenesis by psychotropic drugs and stress. J Pharmacol Exp Ther. 2001;299:401–407. [PubMed] [Google Scholar]
- 82.Vaidya VA., Siuciak JA., Du F., Duman RS. Hippocampal mossy fiber sprouting induced by chronic electroconvulsive seizures. Neuroscience. 1999;89:157–166. doi: 10.1016/s0306-4522(98)00289-9. [DOI] [PubMed] [Google Scholar]
- 83.Lamont SR., PauIIs A., Stewart CA. Repeated electroconvulsive stimulation, but not antidepressant drugs, induces mossy fibre sprouting in the rat hippocampus. Brain Res. 2001;893:53–58. doi: 10.1016/s0006-8993(00)03287-x. [DOI] [PubMed] [Google Scholar]
- 84.Madsen TM., Treschow A., Bengzon J., Bolwig TG., Lindvall O., Tingstrom A. Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- 85.Vaidya VA., TerwiIIiger RZ., Duman RS. Alterations in heavy and light neurofilament proteins in hippocampus following chronic ECS administration. Synapse. 2000;35:137–143. doi: 10.1002/(SICI)1098-2396(200002)35:2<137::AID-SYN6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 86.Nibuya M., Morinobu S., Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Altar CA., Laeng P., Jurata LW., et al. Electroconvulsive seizures regulate gene expression of distinct neurotrophic signaling pathways. J Neurosci. 2004;24:2667–2677. doi: 10.1523/JNEUROSCI.5377-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barker AT., Jalinous R., Freeston IL. Non-invasive magnetic stimulation of human motor cortex, lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 89.Hoflich G., Kasper S., Hufnagel A., Ruhrmann S., MoIIer HJ. Application of transcranial magnetic stimulation in treatment of drug-resistant major depression - a report of two cases. Human Psychopharmacology. 1993;8:361–365. [Google Scholar]
- 90.Figiel GS., Epstein C., McDonald WM., et al. The use of rapid-rate transcranial magnetic stimulation (rTMS) in refractory depressed patients. J Neuropsychiatry Clin Neurosci. 1998;10:20–25. doi: 10.1176/jnp.10.1.20. [DOI] [PubMed] [Google Scholar]
- 91.Herwig U., Schonfeldt-Lecuona C., Wunderlich AP., et al. The navigation of transcranial magnetic stimulation. Psychiatry Res. 2001;108:123–131. doi: 10.1016/s0925-4927(01)00121-4. [DOI] [PubMed] [Google Scholar]
- 92.Rosier KM., Hess CW., Heckmann R., Ludin HP. Significance of shape and size of the stimulating coil in magnetic stimulation of the human motor cortex. Neurosci Lett. 1989;100:347–352. doi: 10.1016/0304-3940(89)90711-8. [DOI] [PubMed] [Google Scholar]
- 93.Mills KR., Boniface SJ., Schubert M. Magnetic brain stimulation with a double coil: the importance of coil orientation. Electroencephalogr Clin Neurophysiol. 1992;85:17–21. doi: 10.1016/0168-5597(92)90096-t. [DOI] [PubMed] [Google Scholar]
- 94.Epstein CM., Schwartzberg DG., Davey KR., Sudderth DB. Localizing the site of magnetic brain stimulation in humans. Neurology. 1990;40:666–670. doi: 10.1212/wnl.40.4.666. [DOI] [PubMed] [Google Scholar]
- 95.Pascual-Leone A., Valls-Sole J., Wassermann EM., Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- 96.Speer AM., Kimbrell TA., Wassermann EM., et al. Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol Psychiatry. 2000;48:1133–1141. doi: 10.1016/s0006-3223(00)01065-9. [DOI] [PubMed] [Google Scholar]
- 97.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5-7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 98.Kammer T. Motor thresholds in humans: a transcranial magnetic stimulation study comparing different pulse waveforms, current directions and stimulator types. Clin Neurophysiol. 2001;112:250–258. doi: 10.1016/s1388-2457(00)00513-7. [DOI] [PubMed] [Google Scholar]
- 99.Baxter LR., Schwartz JM., Phelps ME., et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- 100.Sackeim HA., Greenberg MS., Weiman AL., Gur RC., Hungerbuhler JP., Geschwind N. Hemispheric asymmetry in the expression of positive and negative emotions. Neurologic evidence. Arch Neurol. 1982;39:210–218. doi: 10.1001/archneur.1982.00510160016003. [DOI] [PubMed] [Google Scholar]
- 101.Grisaru N., Bruno R., Pridmore S. Effect on the emotions of healthy individuals of slow repetitive transcranial magnetic stimulation applied to the prefrontal cortex. J ECT. 2001;17:184–189. doi: 10.1097/00124509-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 102.George MS., Wassermann EM., Williams WA., et al. Changes in mood and hormone levels after rapid-rate transcranial magnetic stimulation (rTMS) of the prefrontal cortex. J Neuropsychiatry Clin Neurosci. 1996;8:172–180. doi: 10.1176/jnp.8.2.172. [DOI] [PubMed] [Google Scholar]
- 103.Pascual-Leone A., Catala MD., Pascual-Leone Pascual A. Lateralized effect of rapid-rate transcranial magnetic stimulation of the prefrontal cortex on mood. Neurology. 1996;46:499–502. doi: 10.1212/wnl.46.2.499. [DOI] [PubMed] [Google Scholar]
- 104.Pascual-Leone A., Rubio B., Pallardo F., Catala MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348:233–237. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- 105.Berman RM., Narasimhan M., Sanacora G., et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in the treatment of major depression. Biol Psychiatry. 2000;47:332–337. doi: 10.1016/s0006-3223(99)00243-7. [DOI] [PubMed] [Google Scholar]
- 106.George MS., Wassermann EM., Kimbrell TA., et al. Mood improvement following daily left prefrontal repetitive transcranial magnetic stimulation in patients with depression: a placebo-controlled crossover trial. Am J Psychiatry. 1997;154:1752–1756. doi: 10.1176/ajp.154.12.1752. [DOI] [PubMed] [Google Scholar]
- 107.Klein E., Kreinin I., Chistyakov A., et al. Therapeutic efficacy of right prefrontal slow repetitive transcranial magnetic stimulation in major depression: a double-blind controlled study. Arch Gen Psychiatry. 1999;56:315–320. doi: 10.1001/archpsyc.56.4.315. [DOI] [PubMed] [Google Scholar]
- 108.Lisanby SH., Pascual-Leone A., Sampson SM., et al. Augmentation of sertraline antidepressant treatment with transcranial magnetic stimulation. Biol Psychiatry. 2001;49:81S. [Google Scholar]
- 109.Avery DH., Holtzheimer PE., Fawaz W., et al. A controlled study of repetitive transcranial magnetic stimulation in medication-resistant major depression. Biol Psychiatry. 2005;59:187–194. doi: 10.1016/j.biopsych.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 110.Burt T., Lisanby SH., Sackeim HA. Neuropsychiatrie applications of transcranial magnetic stimulation: a meta analysis. IntJ Neuropsychopharmacol. 2002;5:73–103. doi: 10.1017/S1461145702002791. [DOI] [PubMed] [Google Scholar]
- 111.Martin JL., Barbanoj MJ., Schlaepfer TE., Thompson E., Perez V., Kulisevsky J. Repetitive transcranial magnetic stimulation for the treatment of depression. Systematic review and meta-analysis. Br J Psychiatry. 2003;182:480–491. doi: 10.1192/bjp.182.6.480. [DOI] [PubMed] [Google Scholar]
- 112.Aerre TF., Dahl AA., Johansen JB., Kjonniksen I., Neckelmann D. Efficacy of repetitive transcranial magnetic stimulation in depression: a review of the evidence. Nord J Psychiatry. 2003;57:227–232. doi: 10.1080/08039480310001409. [DOI] [PubMed] [Google Scholar]
- 113.Simons W., Dierick M. Transcranial magnetic stimulation as a therapeutic tool in psychiatry. World J Biol Psychiatry. 2005;6:6–25. doi: 10.1080/15622970510029812. [DOI] [PubMed] [Google Scholar]
- 114.Kozel FA., George MS. Meta-analysis of left prefrontal repetitive transcranial magnetic stimulation (rTMS) to treat depression. J Psychiatr Pract. 2002;8:270–275. doi: 10.1097/00131746-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 115.Grunhaus L., Dannon PN., Schreiber S., et al. Repetitive transcranial magnetic stimulation is as effective as electroconvulsive therapy in the treatment of nondelusional major depressive disorder: an open study. Biol Psychiatry. 2000;47:314–324. doi: 10.1016/s0006-3223(99)00254-1. [DOI] [PubMed] [Google Scholar]
- 116.Grunhaus L., Schreiber S., Dolberg OT., Polak D., Dannon PN. A randomized controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depression. Biol Psychiatry. 2003;53:324–331. doi: 10.1016/s0006-3223(02)01499-3. [DOI] [PubMed] [Google Scholar]
- 117.Janicak PG., Dowd SM., Martis B., et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: preliminary results of a randomized trial. Biol Psychiatry. 2002;51:659–667. doi: 10.1016/s0006-3223(01)01354-3. [DOI] [PubMed] [Google Scholar]
- 118.Pridmore S., Bruno R., Turnier-Shea Y., Reid P., Rybak M. Comparison of unlimited numbers of rapid transcranial magnetic stimulation (rTMS) and ECT treatment sessions in major depressive episode. Int J Neuropsychopharmacol. 2000;3:129–134. doi: 10.1017/S1461145700001784. [DOI] [PubMed] [Google Scholar]
- 119.Fregni F., Marcolin M., Myczkowski M., et al. Predictors of antidepressant response in clinical trials of transcranial magnetic stimulation. Int J Neuropsychopharmacol. In press doi: 10.1017/S1461145705006280. [DOI] [PubMed] [Google Scholar]
- 120.Teneback CC., Nahas Z., Speer AM., et al. Changes in prefrontal cortex and paralimbic activity in depression following two weeks of daily left prefrontal TMS. J Neuropsychiatry Clin Neurosci. 1999;11:426–435. doi: 10.1176/jnp.11.4.426. [DOI] [PubMed] [Google Scholar]
- 121.Kimbrell TA., Little JT., Dunn RT., et al. Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. Biol Psychiatry. 1999;46:1603–1613. doi: 10.1016/s0006-3223(99)00195-x. [DOI] [PubMed] [Google Scholar]
- 122.Li X., Nahas Z., Anderson B., Kozel FA., George MS. Can left prefrontal rTMS be used as a maintenance treatment for bipolar depression? Depress Anxiety. 2004;20:98–100. doi: 10.1002/da.20027. [DOI] [PubMed] [Google Scholar]
- 123.Rumi DO., Gattaz WF., Rigonatti SP., et al. Transcranial magnetic stimulation accelerates the antidepressant effect of amitriptyline in severe depression: a double-blind placebo-controlled study. Biol Psychiatry. 2005;57:162–166. doi: 10.1016/j.biopsych.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 124.Gershon AA., Dannon PN., Grunhaus L. Transcranial magnetic stimulation in the treatment of depression. Am J Psychiatry. 2003;160:835–845. doi: 10.1176/appi.ajp.160.5.835. [DOI] [PubMed] [Google Scholar]
- 125.Fitzgerald PB., Brown TL., Marston NA., Daskalakis ZJ., De Castella A., Kulkarni J. Transcranial magnetic stimulation in the treatment of depression: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 2003;60:1002–1008. doi: 10.1001/archpsyc.60.9.1002. [DOI] [PubMed] [Google Scholar]
- 126.George MS., Nahas Z., MoIIoy M., et al. A controlled trial of daily left prefrontal cortex TMS for treating depression. Biol Psychiatry. 2000;48:962–970. doi: 10.1016/s0006-3223(00)01048-9. [DOI] [PubMed] [Google Scholar]
- 127.Miniussi C., Bonato C., Bignotti S., et al. Repetitive transcranial magnetic stimulation (rTMS) at high and low frequency: an efficacious therapy for major drug-resistant depression? Clin Neurophysiol. 2005;116:1062–1071. doi: 10.1016/j.clinph.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 128.Hufnagel A., Claus D., Brunhoelzl C., Sudhop T. Short-term memory: no evidence of effect of rapid-repetitive transcranial magnetic stimulation in healthy individuals. J Neurol. 1993;240:373–376. doi: 10.1007/BF00839970. [DOI] [PubMed] [Google Scholar]
- 129.Pascual-Leone A., Houser CM., Reese K., et al. Safety of rapid-rate transcranial magnetic stimulation in normal volunteers. Electroencephalogr Clin Neurophysiol. 1993;89:120–130. doi: 10.1016/0168-5597(93)90094-6. [DOI] [PubMed] [Google Scholar]
- 130.Nahas Z., DeBrux C., Chandler V., et al. Lack of significant changes on magnetic resonance scans before and after 2 weeks of daily left prefrontal repetitive transcranial magnetic stimulation for depression. J ECT. 2000;16:380–390. doi: 10.1097/00124509-200012000-00008. [DOI] [PubMed] [Google Scholar]
- 131.Chen R., Classen J., Gerloff C., et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- 132.Bohning DE., Shastri A., Wassermann EM., et al. BOLD-f MRI response to single-pulse transcranial magnetic stimulation (TMS). J Magn Reson Imaging. 2000;11:569–574. doi: 10.1002/1522-2586(200006)11:6<569::aid-jmri1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 133.Wassermann EM., Wedegaertner FR., Ziemann U., George MS., Chen R. Crossed reduction of human motor cortex excitability by 1-Hz transcranial magnetic stimulation. Neurosci Lett. 1998;250:141–144. doi: 10.1016/s0304-3940(98)00437-6. [DOI] [PubMed] [Google Scholar]
- 134.Teneback CC., Nahas Z., Speer AM., et al. Changes in prefrontal cortex and paralimbic activity in depression following two weeks of daily left prefrontal TMS. J Neuropsychiatry Clin Neurosci. 1999;11:426–435. doi: 10.1176/jnp.11.4.426. [DOI] [PubMed] [Google Scholar]
- 135.Nadeau SE., McCoy KJ., Crucian GP., et al. Cerebral blood flow changes in depressed patients after treatment with repetitive transcranial magnetic stimulation: evidence of individual variability. Neuropsychiatry Neu ropsychol Behav Neurol. 2002;15:159–175. [PubMed] [Google Scholar]
- 136.Ben-Shachar D., Belmaker RH., Grisaru N., Klein E. Transcranial magnetic stimulation induces alterations in brain monoamines. J Neural Transm. 1997;104:191–197. doi: 10.1007/BF01273180. [DOI] [PubMed] [Google Scholar]
- 137.Ben-Shachar D., Gazawi H., Riboyad-Levin J., Klein E. Chronic repetitive transcranial magnetic stimulation alters beta-adrenergic and 5-HT2 receptor characteristics in rat brain. Brain Res. 1999;816:78–83. doi: 10.1016/s0006-8993(98)01119-6. [DOI] [PubMed] [Google Scholar]
- 138.Gur E., Lerer B., Dremencov E., Newman ME. Chronic repetitive transcranial magnetic stimulation induces subsensitivity of presynaptic serotonergic autoreceptor activity in rat brain. Neuroreport. 2000;11:2925–2929. doi: 10.1097/00001756-200009110-00019. [DOI] [PubMed] [Google Scholar]
- 139.Michael N., Gosling M., Reutemann M., et al. Metabolic changes after repetitive transcranial magnetic stimulation (rTMS) of the left prefrontal cortex: a sham-controlled proton magnetic resonance spectroscopy (1H MRS) study of healthy brain. Eur J Neurosci. 2003;17:2462–2468. doi: 10.1046/j.1460-9568.2003.02683.x. [DOI] [PubMed] [Google Scholar]
- 140.George MS., Wassermann EM., Williams WA., et al. Changes in mood and hormone levels after rapid-rate transcranial magnetic stimulation (rTMS) of the prefrontal cortex. J Neuropsych Clin Neurosci. 1996;8:172–180. doi: 10.1176/jnp.8.2.172. [DOI] [PubMed] [Google Scholar]
- 141.Szuba MP., O'Reardon JP., Rai AS., et al. Acute mood and thyroid stimulating hormone effects of transcranial magnetic stimulation in major depression. Biol Psychiatry. 2001;50:22–27. doi: 10.1016/s0006-3223(00)01118-5. [DOI] [PubMed] [Google Scholar]
- 142.Reid PD., Pridmore S. Dexamethasone suppression test reversal in rapid transcranial magnetic stimulation-treated depression. Aust N Z J Psychiatry. 1999;33:274–277. doi: 10.1046/j.1440-1614.1999.00550.x. [DOI] [PubMed] [Google Scholar]
- 143.Fujiki M., Steward O. High frequency transcranial magnetic stimulation mimics the effects of ECS in upregulating astroglial gene expression in the murine CNS. Brain Res Mol Brain Res. 1997;44:301–308. doi: 10.1016/s0169-328x(96)00232-x. [DOI] [PubMed] [Google Scholar]
- 144.Hausmann A., Weis C., Marksteiner J., Hinterhuber H., Humpel C. Chronic repetitive transcranial magnetic stimulation enhances c-fos in the parietal cortex and hippocampus. Brain Res Mol Brain Res. 2000;76:355–362. doi: 10.1016/s0169-328x(00)00024-3. [DOI] [PubMed] [Google Scholar]
- 145.Ji R-R., Schlaepfer TE., Aizenman CD., et al. Repetitive transcranial magnetic stimulation activates specific regions in rat brain. Proc Natl Acad Sci U S A. 1998;95:15635–15640. doi: 10.1073/pnas.95.26.15635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li X., Nahas Z., Lomarev M., et al. Prefrontal cortex transcranial magnetic stimulation does not change local diffusion: a magnetic resonance imaging study in patients with depression. Cogn Behav Neurol. 2003;16:128–135. doi: 10.1097/00146965-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 147.Lisanby SH., Schlaepfer TE., Fisch HU., Sackeim HA. Magnetic seizure therapy of major depression. Arch Gen Psychiatry. 2001;58:303–305. doi: 10.1001/archpsyc.58.3.303. [DOI] [PubMed] [Google Scholar]
- 148.Kosel M., Frick C., Lisanby SH., Fisch HU., Schlaepfer TE. Magnetic seizure therapy improves mood in refractory major depression. Neuropsychopharmacology. 2003;28:2045–2048. doi: 10.1038/sj.npp.1300293. [DOI] [PubMed] [Google Scholar]
- 149.Lisanby SH., Luber B., Schlaepfer TE., Sackeim HA. Safety and feasibility of magnetic seizure therapy (MST) in major depression: randomized withinsubject comparison with electroconvulsive therapy. Neuropsychopharmacology. 2003;28:1852–1865. doi: 10.1038/sj.npp.1300229. [DOI] [PubMed] [Google Scholar]
- 150.Lisanby SH., Moscrip T., Morales O., Luber B., Schroeder C., Sackeim HA. Neurophysiological characterization of magnetic seizure therapy (MST) in non-human primates. Suppl Clin Neurophysiol. 2003;56:81–99. doi: 10.1016/s1567-424x(09)70212-0. [DOI] [PubMed] [Google Scholar]
- 151.Moscrip TD., Terrace HS., Sackeim HA., Lisanby SH. Randomized controlled trial of the cognitive side-effects of magnetic seizure therapy (MST) and electroconvulsive shock (ECS). Int J Neuropsychopharmacol. 2006;9:1–11. doi: 10.1017/S146114570500578X. [DOI] [PubMed] [Google Scholar]
- 152.Lisanby SH., Morales O., Payne N., et al. New developments in electroconvulsive therapy and magnetic seizure therapy. CNS Spectr. 2003;8:529–536. doi: 10.1017/s1092852900019003. [DOI] [PubMed] [Google Scholar]
- 153.Benabid AL., Pollak P., Hommel M., Gaio JM., de Rougemont J., Perret J. Treatment of Parkinson tremor by chronic stimulation of the ventral intermediate nucleus of the thalamus. Rev Neurol (Paris). In French. 1989;145:320–323. [PubMed] [Google Scholar]
- 154.Benabid AL., Pollak P., Gervason C., et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337:403–406. doi: 10.1016/0140-6736(91)91175-t. [DOI] [PubMed] [Google Scholar]
- 155.Benabid AL., Pollak P., Gao D., et al. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg. 1996;84:203–214. doi: 10.3171/jns.1996.84.2.0203. [DOI] [PubMed] [Google Scholar]
- 156.The Deep-Brain Stimulation for Parkinson's Disease Study Group. Deepbrain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson's disease. N Engl J Med. 2001;345:956–963. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- 157.Mogilner AY., Rezai AR. Brain stimulation: history, current clinical application, and future prospects. Acta Neurochir Suppl. 2003;87:115–20. doi: 10.1007/978-3-7091-6081-7_24. [DOI] [PubMed] [Google Scholar]
- 158.Mayberg HS., Lozano AM., Voon V., et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 159.Aouizerate B., Cuny E., Martin-Guehl C., et al. Deep brain stimulation of the ventral caudate nucleus in the treatment of obsessive-compulsive disorder and major depression. Case report. J Neurosurg. 2004;101:682–686. doi: 10.3171/jns.2004.101.4.0682. [DOI] [PubMed] [Google Scholar]
- 160.Abosch A., Lozano AM. Stereotactic neurosurgery for movement disorders. Can J Neurol Sci. 2003;30(suppl 1):S72–S82. doi: 10.1017/s0317167100003279. [DOI] [PubMed] [Google Scholar]
- 161.Jimenez F., Velasco F., Salin-Pascual R., et al. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57:585–593. doi: 10.1227/01.neu.0000170434.44335.19. [DOI] [PubMed] [Google Scholar]
- 162.Schuurman PR., Bosch DA., Bossuyt PM., et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med. 2000;342:461–468. doi: 10.1056/NEJM200002173420703. [DOI] [PubMed] [Google Scholar]
- 163.Oh MY., Hodaie M., Kim SH., Alkhani A., Lang AE., Lozano AM. Long-term hardware-related complications of deep brain stimulation. Neurosurgery. 2002;50:1268–1276. doi: 10.1097/00006123-200206000-00017. [DOI] [PubMed] [Google Scholar]
- 164.Greenberg BD., Rezai AR. Mechanisms and the current state of deep brain stimulation in neuropsychiatry. CNS Spectr. 2003;8:522–526. doi: 10.1017/s109285290001899x. [DOI] [PubMed] [Google Scholar]
- 165.Mclntyre CC., Savasta M., Kerkerian-Le Goff L., Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol. 2004;115:1239–1248. doi: 10.1016/j.clinph.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 166.Montgomery EB., Baker KB. Mechanisms of deep brain stimulation and future technical developments. Neurol Res. 2000;22:259–266. doi: 10.1080/01616412.2000.11740668. [DOI] [PubMed] [Google Scholar]
- 167.Rezai AR., Lozano AM., Crawley AP., et al. Thalamic stimulation and functional magnetic resonance imaging: localization of cortical and subcortical activation with implanted electrodes. Technical note. J Neurosurg. 1999;90:583–590. doi: 10.3171/jns.1999.90.3.0583. [DOI] [PubMed] [Google Scholar]
- 168.Ceballos-Baumann AO., Boecker H., Fogel W., et al. Thalamic stimulation for essential tremor activates motor and deactivates vestibular cortex. Neurology. 2001;56:1347–1354. doi: 10.1212/wnl.56.10.1347. [DOI] [PubMed] [Google Scholar]
- 169.Perlmutter JS., Mink JW., Bastian AJ., et al. Blood flow responses to deep brain stimulation of thalamus. Neurology. 2002;58:1388–1394. doi: 10.1212/wnl.58.9.1388. [DOI] [PubMed] [Google Scholar]
- 170.Dostrovsky JO., Levy R., Wu JP., Hutchison WD., Tasker RR., Lozano AM. Microstimulation-induced inhibition of neuronal firing in human globus pallidus. J Neurophysiol. 2000;84:570–574. doi: 10.1152/jn.2000.84.1.570. [DOI] [PubMed] [Google Scholar]
- 171.Beurrier C., Bioulac B., Audin J., Hammond C. High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J Neurophysiol. 2001;85:1351–1356. doi: 10.1152/jn.2001.85.4.1351. [DOI] [PubMed] [Google Scholar]
- 172.Llinas RR., Leznik E., Urbano FJ. Temporal binding via cortical coincidence detection of specific and nonspecific thalamocortical inputs: a voltage-dependent dye-imaging study in mouse brain slices. Proc Natl Acad Sci USA. 2002;99:449–454. doi: 10.1073/pnas.012604899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Urbano FJ., Leznik E., Llinas RR. Cortical activation patterns evoked by afferent axons stimuli at different frequencies: an in vitro voltage-sensitive dye imaging study. Thalamus Rel Syst. 2002;1:371–378. [Google Scholar]
- 174.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 175.Seminowicz DA., Mayberg HS., Mcintosh AR., et al. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 176.Barbas H., Saha S., Rempel-CIower N., Ghashghaei T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci. 2003;4:25. doi: 10.1186/1471-2202-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bailey P., Bremer F. A sensory cortical representation of the vagus nerve. J Neurophysiol. 1938;1:405–412. [Google Scholar]
- 178.Hallowitz RA., MacLean PD. Effects of vagal volleys on units of intralaminar and juxtalaminar thalamic nuclei in monkeys. Bra in Res. 1977;130:271–286. doi: 10.1016/0006-8993(77)90275-x. [DOI] [PubMed] [Google Scholar]
- 179.Zabara J. Peripheral control of hypersynchronous discharge in epilepsy. Electroencephalogr Clin Neurophysiol. 1985:S162. [Google Scholar]
- 180.Uthman BM., Wilder BJ., Penry JK., et al. Treatment of epilepsy by stimulation of the vagus nerve. Neurology. 1993;43:1338–1345. doi: 10.1212/wnl.43.7.1338. [DOI] [PubMed] [Google Scholar]
- 181.Ben-Menachem E., Manon-Espaillat R., Ristanovic R., et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35:616–626. doi: 10.1111/j.1528-1157.1994.tb02482.x. [DOI] [PubMed] [Google Scholar]
- 182.Elger G., Hoppe C., Falkai P., Rush AJ., Elger CE. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res. 2000;42:203–210. doi: 10.1016/s0920-1211(00)00181-9. [DOI] [PubMed] [Google Scholar]
- 183.Hoppe C., Helmstaedter C., Scherrmann J., Elger CE. Self-reported mood changes following 6 months of vagus nerve stimulation in epilepsy patients. Epilepsy Behav. 2001;2:335–342. doi: 10.1006/ebeh.2001.0194. [DOI] [PubMed] [Google Scholar]
- 184.Henry TR., Bakay RA., Votaw JR., et al. Brain blood flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: I. Acute effects at high and low levels of stimulation. Epilepsia. 1998;39:983–990. doi: 10.1111/j.1528-1157.1998.tb01448.x. [DOI] [PubMed] [Google Scholar]
- 185.Ben-Menachem E., Hamberger A., Hedner T., et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 1995;20:221–227. doi: 10.1016/0920-1211(94)00083-9. [DOI] [PubMed] [Google Scholar]
- 186.Carpenter LL., Moreno FA., Kling MA., et al. Effect of vagus nerve stimulation on cerebrospinal fluid monoamine metabolites, norepinephrine, and gamma-aminobutyric acid concentrations in depressed patients. Biol Psychiatry. 2004;56:418–426. doi: 10.1016/j.biopsych.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 187.Rush AJ., George MS., Sackeim HA., et al. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatry. 2000;47:276–286. doi: 10.1016/s0006-3223(99)00304-2. [DOI] [PubMed] [Google Scholar]
- 188.Amar AP., Heck CN., Levy ML., et al. An institutional experience with cervical vagus nerve trunk stimulation for medically refractory epilepsy: rationale, technique, and outcome. Neurosurgery. 1998;43:1265–1280. doi: 10.1097/00006123-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 189.George MS., Rush AJ., Sackeim HA., Marangell LB. Vagus nerve stimulation (VNS): utility in neuropsychiatrie disorders. Int J Neuropsychopharmacol. 2003;6:73–83. doi: 10.1017/S1461145703003250. [DOI] [PubMed] [Google Scholar]
- 190.Krahl SE., Senanayake SS., Pekary AE., Sattin A. Vagus nerve stimulation (VNS) is effective in a rat model of antidepressant action. J Psychiatr Res. 2004;38:237–240. doi: 10.1016/j.jpsychires.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 191.Sackeim HA., Rush AJ., George MS., et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25:713–728. doi: 10.1016/S0893-133X(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 192.Marangell LB., Rush AJ., George MS., et al. Vagus nerve stimulation (VNS) for major depressive episodes: one year outcomes. Biol Psychiatry. 2002;51:280–287. doi: 10.1016/s0006-3223(01)01343-9. [DOI] [PubMed] [Google Scholar]
- 193.Nahas Z., Marangell LB., Husain MM., et al. Two-year outcome of vagus nerve stimulation (VNS) for treatment of major depressive episodes. J Clin Psychiatry. 2005;66:1097–1104. doi: 10.4088/jcp.v66n0902. [DOI] [PubMed] [Google Scholar]
- 194.Rush AJ., Marangell LB., Sackeim HA., et al. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry. 2005;58:347–354. doi: 10.1016/j.biopsych.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 195.Rush AJ., Sackeim HA., Marangell LB., et al. Effects of 12 months of vagus nerve stimulation in treatment-resistant depression: a naturalistic study. Biol Psychiatry. 2005;58:355–363. doi: 10.1016/j.biopsych.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 196.George MS., Rush AJ., Marangell LB., et al. A one-year comparison of vagus nerve stimulation with treatment as usual for treatment-resistant depression. Biol Psychiatry. 2005;58:364–373. doi: 10.1016/j.biopsych.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 197.Henry TR., Bakay RA., Penneil PB., Epstein CM., Votaw JR. Brain bloodflow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: II. prolonged effects at high and low levels of stimulation. Epilepsia. 2004;45:1064–1070. doi: 10.1111/j.0013-9580.2004.03104.x. [DOI] [PubMed] [Google Scholar]
- 198.Zobel A., Joe A., Freymann N., et al. Changes in regional cerebral blood flow by therapeutic vagus nerve stimulation in depression: an exploratory approach. Psychiatry Res. 2005;139:165–179. doi: 10.1016/j.pscychresns.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 199.Bohning DE., Lomarev MP., Denslow S., Nahas Z., Shastri A., George MS. Feasibility of vagus nerve stimulation-synchronized blood oxygenation leveldependent functional MRI. Invest Radiol. 2001;36:470–479. doi: 10.1097/00004424-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 200.Lomarev M. Vagus nerve stimulation (VNS) synchronized BOLD fMRI suggests that VNS in depressed adults has frequency/dose dependent effects. J Psychiatr Res. 2002;36:219–227. doi: 10.1016/s0022-3956(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 201.Ben-Menachem E., Hamberger A., Hedner T., et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 1995;20:221–227. doi: 10.1016/0920-1211(94)00083-9. [DOI] [PubMed] [Google Scholar]
- 202.Corcoran C., Connor TJ., O'Keane V., Garland MR. The effects of vagus nerve stimulation on pro- and anti-inflammatory cytokines in humans: a preliminary report. Neuroimmunomodulation. 2005;12:307–309. doi: 10.1159/000087109. [DOI] [PubMed] [Google Scholar]
- 203.O'Keane V., Dinan TG., Scott L., Corcoran C. Changes in hypothalamicpituitary-adrenal axis measures after vagus nerve stimulation therapy in chronic depression. Biol Psychiatry. 2005;58:963–938. doi: 10.1016/j.biopsych.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 204.Armitage R., Husain M., Hoffmann R., Rush AJ. The effects of vagus nerve stimulation on sleep EEG in depression: a preliminary report. J Psychosom Res. 2003;54:475–482. doi: 10.1016/s0022-3999(02)00476-2. [DOI] [PubMed] [Google Scholar]