Abstract
The clinical observations of diurnal variation of mood and early morning awakening in depression have been incorporated into established diagnostic systems, as has the seasonal modifier defining winter depression (seasonal affective disorder, SAD). Many circadian rhythms measured in depressive patients are abnormal: earlier in timing, diminished in amplitude, or of greater variability. Whether these disturbances are of etiological significance for the role of circadian rhythms in mood disorders, or a consequence of altered behavior can only be dissected out with stringent protocols (eg, constant routine or forced desynchrony). These protocols quantify contributions of the circadian pacemaker and a homeostatic sleep process impacting on mood, energy, appetite, and sleep. Future studies will elucidate any allelic mutations in “circadian clock” –related or “sleep”-related genes in depression. With respect to treatment, antidepressants and mood stabilizers have no consistent effect on circadian rhythmicity. The most rapid antidepressant modality known so far is nonpharmacological: total or partial sleep deprivation in the second half of the night. The disadvantage of sleep deprivation, that most patients relapse after recovery sleep, can be prevented by coadministration of lithium, pindolol, serotonin (5-HT) reuptake inhibitors, bright light, or a subsequent phase-advance procedure. Phase advance of the sleep-wake cycle alone also has rapid effects on depressed mood, which lasts longer than sleep deprivation. Light is the treatment of choice for SAD and may prove to be useful for nonseasonal depression, alone or as an adjunct to medication. Chronobiological concepts emphasize the important role of zeitgebers to stabilize phase, light being the most important, but dark (and rest) periods, regularity of social schedules and meal times, and use of melatonin or its analogues should also be considered. Advances in chronobiology continue to contribute novel treatments for affective disorders.
Keywords: major depression, seasonal affective disorder, circadian rhythm, sleep deprivation, light therapy, melatonin
Abstract
Las observaciones clínicas de la variatión diurna del ánimo y el despertar precoz en la depresión se han incorporado a sistemas diagnósticos establecidos, como es el caso de la modificatión estacional que define la depresión invernal (trastorno afectivo estacional, TAE). Muchos ritmos circadianos medidos en pacientes depresivos son anormales: por ocurrir antes del tiempo que corresponde, tener una amplitud disminuida o una mayor variabilidad. Para precisar si estas alteraciones tienen un significado etiológico en el rol que cumplen los ritmos circadianos en los trastornos afectivos o si son una consecuencia de conductas alteradas se requiere de un análisis minucioso con protocolos muy estrictos (por ejemplo, rutina constante o desincronía forzada). Estos protocolos cuaniifican las contribuciones del marcapaso circadiano y del proceso de sueño homeostático que influyen en el ánimo, la energía, el apetito y el sueño. Estudios futuros aclararán algunas mutaciones alélicas de genes relacionados con el “reloj circadiano” o el “sueño” en la depresión. Respecto al tratamiento, los antidepresivos y los estabilizadores del ánimo no tienen efectos consistentes en la ritmicidad circadiana. La esirategia antidepresiva más rápida conocida hasta la fecha es de tipo no farmacológico: la privatión total o parcial de sueño durante la segunda mitad de la noche. La desventaja de la privatión de sueño es que la mayoría de los patientes recaen después de recuperar el sueño; esto puede prevenirse mediante la coadministratión de litio, pindolol, inhibidores de la recaptatión de serotonina (5-HT), luz brillante, o a través de un procedimiento posterior de avance de fase. El avance de fase del ciclo sueño vigilia en forma exclusiva tiene también rápidos efectos en el ánimo depresivo, lo que dura mayor tiempo que la privation de sueño. La luz es el tratamiento de electión para el TAE y puede resulter útil en la depresión no estacional al administrarla sola o en combinatión con medicamentos. Los conceptos cronobiológicos enfatizan el importante papel de los “zeitgebers” para estabilizar la fase, siendo la luz el más importante, pero también se deben considerar los períodos de oscuridad (y reposo), la regularidad de los horarios sociales y de las comidas y el empleo de melatonina o de sus análogos. Los avances en la cronobiologia continúan para contribuir a nuevos tratamientos para los trastornos afectivos.
Abstract
Les observations cliniques de variations diurnes de l'humeur et de réveil matinal précoce dans la dépression ont été intégrées dans des systèmes diagnostiques établis tel le facteur saisonnier qui définit la dépression hivernale (trouble affectif saisonnier, TAS). Beaucoup de rythmes circadiens mesurés chez les patients dépressifs sont anormaux: plus précoces, diminués en amplitude ou de plus grande variabilité. Seuls des protocoles rigoureux (par exemple, routine constante ou désynchronisation forcée) sont à même de déterminer si ces perturbations ont une signification étiologique quant au rôle des rythmes circadiens dans les troubles de l'humeur ou si elles sont la conséquence d'une modification comportementale. Ces protocoles quantifient les participations respectives de l'oscillateur circadien et d'un processus homéostatique lié au sommeil ayant des répercussions sur l'humeur, l'énergie, l'appétit et le sommeil. Les études à venir mettront en évidence, si tant est qu'elles existent, les mutations alléliques des gènes qui interviennent dans les phénomènes « d'horloge » ou de « sommeil » au cours de la dépression. En ce qui concerne le traitement, les antidépresseurs et les régulateurs de l'humeur n'ont pas d'effet constant sur le rythme circadien. L'effet antidépresseur le plus rapide connu à ce jour n'est pas pharmacologique: c'est la privation totale ou partielle de sommeil dans la seconde moitié de la nuit. L'inconvénient de la privation de sommeil, constitué par la rechute de la plupart des patients après le sommeil de récupération, peut être prévenu par l'administration concomitante de lithium, de pindolol, d'inhibiteurs de la recapture de la sérotonine (5-HT), de lumière vive ou par une procédure d'avance de phase. L'avance de phase dans les cycles veille-sommeil exerce par elle-même également des effets rapides sur l'humeur dépressive qui se maintiennent plus longtemps que ceux de la privation de sommeil, La photothérapie est le traitement de choix du TAS et pourra s'avérer utile dans la dépression non saisonnière, seule ou en association à un traitement médicamenteux. Les concepts chronobiologiques soulignent le rôle important des synchroniseurs dans la stabilisation de phase, la lumière étant le plus important. Cependant, les périodes d'obscurité (et de repos), la régularité des repas et des rythmes sociaux et l'utilisation de la mélatonine ou de ses analogues doivent être également considérées. Les avancées en chronobiologie continuent à contribuer au développement de médicaments nouveaux dans les troubles affectifs.
In order for Dialogues in Clinical Neuroscience to be truly designated “dialogues,” I will raise specific and critical questions about the putative circadian rhythm disturbances in depression, provide a model within which to understand them, and summarize the present status and application of chronobiological therapies. This short overview will not go into detail of the clinical and experimental findings related to biological rhythms in depression, which have been extensively reviewed elsewhere.1-9
Chronobiologists predicate their work on a primary axiom, that temporal order is essential for health. Psychological, behavioral, physiological, and hormonal rhythms are specifically and functionally timed (entrained or synchronized) with respect to sleep and the day-night cycle. The converse premise implies that temporal disorder must have clinical correlates. Rhythmic characteristics of mood disorders were precisely described as far back as ancient times. However, it is still unclear whether circadian rhythms are reliably linked with psychopathology, if they provide clues to underlying mechanisms, and how they can be understood with respect to the established neurotransmitter models of depression.
The first question is common to all clinical research: what do we mean by biologically homogeneous groups? Here too, diagnostic issues are the crux. In addition to the distinction unipolar, bipolar, or seasonal affective disorder (SAD), the stage of the illness may be important for chronobiological disturbances. Acute depression is probably different from chronic, and in rapid cyclers it is known that there is a continuous shift in circadian phase during depression and that this reverses during mania.1 Given that antidepressants act on neurotransmitter mechanisms also involved in circadian rhythm generation and entrainment, only untreated patients may reveal an “endogenous” rhythm disturbance, if present.
The second question regards conceptual clarity. What do we mean by a clock disturbance in depression? What one sees clinically may have its origins at a variety of different levels―not necessarily the hypothalamic biological clock itself, but epiphenomena related to altered rhythmic behavior, disturbed sleep, or abnormal environmental input.
The third question is whether the studies purporting to document circadian rhythm disturbances in depression have been adequately carried out. Alas, methodological issues characterize most investigations―not in terms of scientific caliber or intent, but because it was previously not sufficiently recognized how strongly “masking” (behavioral or environmental factors that modify the variable measured) obscures the underlying endogenous rhythms. This is a particular problem with measuring the core body temperature rhythm, since temperature is easily and rapidly masked by motor activity, postural change, meals, etc. Cortisol increases with stress, particularly at the evening nadir; thus, this circadian marker is also often masked by psychophysiological response. Melatonin, the pineal hormone considered to provide the best estimate of circadian rhythm phase, is suppressed by light, particularly in the evening: it is sensitive to masking by light as low as ca 100 lux.10 Thus, even indoor room light may delay the apparent onset of nocturnal secretion. Only in the last decade have controlled protocols using state-oft-he-art chronobiological techniques provided unequivocal circadian markers.
The fourth question concerns which models are useful. Concepts of an underlying genetic and stress-related vulnerability for depression can be discussed in terms of both neurotransmitter and circadian rhythm dysregulation. Here, I will draw on the two-process model of sleep-wake regulation11 as a way of understanding some aspects of depressive symptomatology.
Trie final question is whether we can find out about putative circadian mechanisms underlying affective disorder through understanding clinically successful chronobiological treatments. Circadian rhythm or sleep manipulations do improve depression and provide some fascinating clues.
Clinical observations
Periodicity in affective disorders (from seasonal recurrence to 48-h rapid cycling) is the clinical observation; diurnal variation of mood, early morning awakening, and sleep disturbances are the classical symptoms that have linked depression with circadian rhythm function. Many rhythms, such as core body temperature, Cortisol, monoamine metabolism, are different in depressive patients: phase advanced (timed earlier) with respect to the sleep-wake cycle, diminished in amplitude, and/or with day-to-day variability in their synchronization to social cues (entrainment).1 However, altered rhythmicity could be either a cause or an effect of altered affective state. Both could independently reflect abnormalities in a third system, such as psychomotor activity. Apparent lability may be caused solely by lack of appropriate feedback to the circadian system (eg, reduced activity). In addition, sleep disturbances are inextricably linked with depressive illness. These clinical observations can be formalized in terms of circadian and sleep physiology.
The neurobiology of circadian rhythms
Circadian rhythms arc generated by a master pacemaker located in the suprachiasmatic nuclei (SCN) of the anterior hypothalamus.12 Individual, genetically determined endogenous periodicity is slightly different from 24 h (usually longer) and requires daily synchronization to the 24-h day by “zeitgebers,” which are regularly recurring environmental signals. Light is the major zeitgeber for the SCN, transmitted by novel photoreceptors in retinal ganglion cells.13 This nonvisual, non–image -forming pathway via the retinohypothalamic tract counts photons, in particular the transitions at dawn and dusk, and is actively gated by a second clock in the eye.14 An indirect visual pathway reaches the SCN via the intergeniculate leaflet of the lateral geniculate complex. From the raphe nucleus, a serotonergic pathway provides nonphotic input to the SCN, and it is perhaps of some importance in the context of depression that concentrations of serotonin (5-HT) in the brain are highest in these nuclei. An important output leads from the SCN to the paraventricular nucleus (PVN) and via a multisynaptic pathway to the pineal gland, where melatonin is synthesized at night and suppressed by light during the day Melatonin transduces the night signal for the body as the nocturnal duration of hormone secretion (”the day within“).15 Melatonin onset in the early evening has proved to be the most reliable biological marker of circadian timing (provided samples are taken under dim light conditions).16 The PVN is also the site of corticotropinreleasing factor synthesis, ie, part of the hypothalamo-pituitary-adrenal (HPA) axis. The nadir of the Cortisol rhythm provides a reliable output of the SCN clock (whereas the maximum is influenced by environmental factors).17
Zeitgeber stimuli, of which light is the most important, can phase shift―and thus entrain―the SCN.18,19 Light during the early part of the night induces phase delays, whereas light given in the second half of the night (after the core body temperature minimum) induces phase advances.18,19 Administration of exogenous melatonin shows patterns nearly opposite to phase shifting to light/20 Other nonphotic zeitgebers (exercise, perhaps sleep or darkness, and nutrients) have been less well investigated and are probably weaker zeitgebers than light.21 Social zeitgebers (jobs, social demands or tasks, and personal relationships) may act directly or indirectly on the SCN, since they determine the timing of meals, sleep, physical exercise, and outdoor light exposure. TTttcsc social factors also have the potential to disrupt circadian rhythms.22 Some of the particular psychosocial precipitants of depressive disorder, such as life events, chronic stresses, or lack of appropriate social support systems, may act as precipitants by disrupting circadian rhythms.
Clocks everywhere
The concept of a master pacemaker driving all circadian rhythms has been very useful. It needs to be supplemented by the concept of peripheral clocks distributed in every organ and perhaps in every cell.23 Each organ has its own relevant and specifically timed circadian rhythms―of heart rate, liver metabolism, and kidney transport, and also of gene expression. Under normal conditions, all rhythms are synchronized by the SCN.23
The SCN signal is translated mainly by the PVN into a hormonal and autonomic signal to peripheral organs. Visceral, sensory, and hormonal information feeds back on the hypothalamus, providing fine-tuning to synchronize time-of-day input from the external light-dark cycle with metabolic information from the inside. The phase of each rhythm can be adjusted by differential responses of a given tissue's circadian clock to a signal from the SCN or from the environment. Such a system can adjust well to small, gradual changes in the input signal (such as seasonal changes in daylength), but may become temporarily and severely disorganized if the change in phase of this signal is abrupt and large (as is most obvious for rapid transmeridian travel and shift work). How could this system go wrong in affective disorders?
Consider the vegetative symptoms that are an integral part of the depressive syndrome, and often appear as forerunners. If sleep is no longer in correct alignment with the inner or outer clock, if food intake decreases, or if behavior turns inward so that motor activity declines and the amount of outdoor light exposure is reduced (as well as social contact), is it not conceivable that these behaviors each act on different clocks, shifting their timing with respect to each other and the day-night cycle to different degrees? This temporal cacophony could initiate an internal stress reaction. Given the concept of a final common neuroendocrine pathway of depression via hyperactivity of the HPA axis, this may be an important mediating system from physiology to psyche.
Clock genes, sleep genes
Individual preference in timing of the sleep-wake cycle (chronotype, ie, whether “larks” or “owls”)24 is determined by clock genes, of which 10 have been cloned so far.25 Individual sleep and wake duration (long sleepers versus short sleepers) is also probably programmed in certain sleep genes26). Since the timing of sleep appears to be rather important for mood, these genetic factors may be relevant to a chronobiological vulnerability for depression, in that wrong or poor alignment of internal phase with the outdoor world increases susceptibility to depressive mood swings. Although familial forms of circadian sleep disorders (such as advanced or delayed sleep phase syndrome) have been found, with allelic mutations on one or other of the clock genes,27-29 the first studies in depression have been negative (eg, the clock gene in major depression30 or the per2 gene in bipolar disorder31). Circadian clock-related polymorphisms seem to be related, interestingly enough, to susceptibility to SAD together with evening chronotype.32 This research is still in its infancy.
Circadian rhythm desynchronization
It is unlikely, however, that affective disorders will be characterized as simple clock gene mutations. Rather, internal desynchronization may be a major contributing factor to mood state. New findings on desynchronization in clock gene expression illustrate this vividly. The clock genes in the SCN gradually adapt to a phase shift of the light-dark cycle (as found in shift work and transmeridian travel), whereas clock genes in muscle, liver, and lung resynchronize at their own rates.33 This results in a double desynchronization, not only between internal (SCN) and external time, but also between different clocks and organs within the body itself. The temporal orchestra can quickly get out of tune. Moreover, the different organ clocks respond to different, specific zeitgebers; for example, food can shift the clock in the liver rather fast, but light does not affect it; the SCN clock reacts to light, but is not influenced by meals.34 Peripheral clocks in muscle may be synchronized by exercise.
This provides a new view on circadian rhythm disturbances in depression. Since peripheral clocks complement the central clock's function of maintaining temporal order, more clocks in body and brain only add to the possibilities of this organization going awry. There may be different patterns of desynchronization that result in similar physiological or psychological consequences. The classical idea of internal circadian phase disturbances in depression can be extended to zeitgeber phase disturbances.6 Even an apparently minor reduction in zeitgeber strength or diminished behavior can loosen temporal coordination, not only between internal rhythms, but also with respect to the social and physical clock, resulting in mood detriments, diurnal variation, and day-to-day mood variability. However, the precise neurobiological mechanisms by which altered circadian phase relationships lead to altered mood state remain unknown.
Bipolar disorder, in particular rapid cycling, is the most striking example of a mood disorder linked to abnormal or changing circadian rhythm phase.1 Here the environment (light or dark) as well as behavior (sleep or its deficit)35 strongly modulate affective state and, recently, these factors have begun to be used as treatments.36-39
Sleep regulation
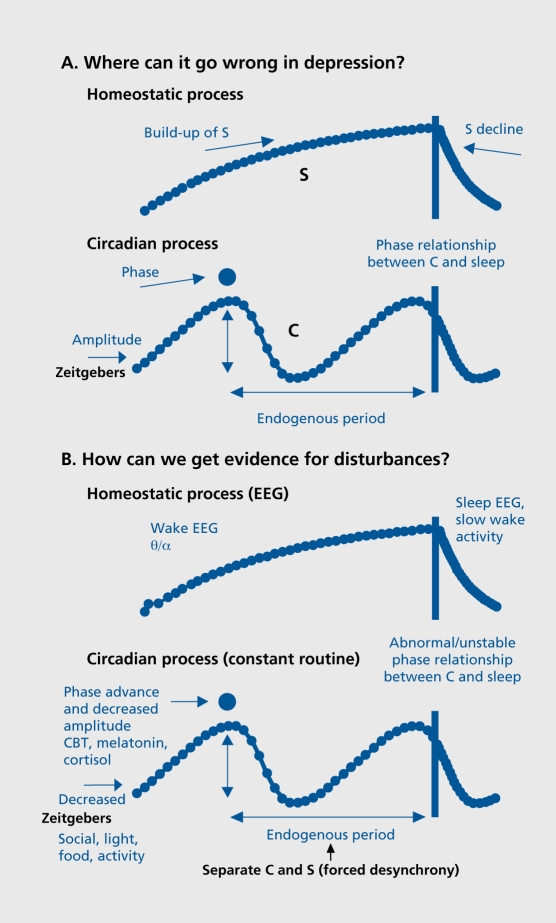
The sleep-wake cycle is the most obvious circadian rhythm in humans, and sleep disturbances are a prominent feature of depression. In the two-process model of sleep regulation, a homeostatic process S increases during waking and declines exponentially during sleep; it interacts with a circadian process C to determine the timing and architecture of sleep.11 This model can also be used to describe possible disturbances in either process during depression (Figure 1A). The clinical sleep disturbance with early morning awakening could arise from an impaired build-up of S during waking (diminished sleep pressure) or an earlier timing of process C. There are a number of sleep manipulations that improve clinical state (sec below and Table I).The rapid antidepressant effect of one night's sleep deprivation is proposed to act by a short-term increase in process S to normal levels.40 The slower antidepressant effect of a phase advance of the sleep-wake cycle8 may be related to more gradual shifts towards a correct phase relationship with respect to process C. Other possibile abnormalities could lie in the decline of S during sleep, or circadian period, phase, or amplitude (process C).
Figure 1. A. The two-process model of sleep regulation, considered in terms of what could go wrong in depression. The homeostatic component (process S) builds up during wakefulness and declines during sleep. The circadian pacemaker (process C) ticks along at its individual (genetically programmed) endogenous period. Decreased amplitude would increase variability of daily timing and it would be more vulnerable to phase shifts. If the rhythm was advanced or delayed in phase, the resultant altered phase relationships between process C and sleep timing could explain many depressive phenomena. B. Biological markers of process S and process C. The exponential rise in sleep pressure can be followed by theta-alpha (θ/α) power in the wake electroencephalogram (EEG). The exponential decline in sleep pressure is evident in slow-wave activity in the sleep EEG. In a constant routine protocol, the rhythms of core body temperature (CBT), melatonin, and Cortisol provide estimates of circadian phase and amplitude. In a forced desynchrony protocol, the endogenous period of the circadian pacemaker can be reduced as well as the relative contributions of process C and process S to any given measure, from psychological to physiological.
Table I. Chronobiological therapies of major depression. Therapies in italics are for one or two studies only. TSD, total sleep deprivation; PSD, partial sleep deprivation; rTMS, repetitive transcranial magnetic stimulation; SSRI, selective serotonin reuptake inhibitor; SAD, seasonal affective disorder; MD, major depression.
| Steep manipulations | Zeitgabers |
| TSD | Light therapy (SAD) |
| PSD (second half of the night) | Light therapy (nonseasonal MD) |
| Phase actance of the sleep-wake cycle | Light therapy as adjuwant to SSRls (nonseasonal MD) |
| TSD followed by phase advance | Dark or rest therapy (rapid-cyclers) |
| Repeated TSD or PSD | Dark therapy (mania) |
| Repeated TSD or PSD with antidepressants | |
| Single or repeated TSD or PSD plus: | |
| • Light therapy | |
| • Light therapy and phase advance | |
| • rTMS | |
| Single or repeated TSD or PSD plus | |
| • Lithium | |
| • SSRls | |
| • Pindoiot |
How to measure process C and S
The model helps clarify which biological markers could be measured to test these hypotheses (Figure 1B). Correct methodology is important to define experimental conditions where masking is reduced. There are two major approaches, both requiring subjects to undergo demanding and highly controlled protocols. The first protocol is the “constant routine,” in which subjects remain awake during an entire 24-h cycle or longer, with external and behavioral conditions constant (very low light levels not to affect the circadian pacemaker, supine posture in bed, and regular small isocaloric meals). The constant routine provides information about process C: amplitude and phase estimates of rhythms in, for example, melatonin, Cortisol, and core body temperature.18 Only such parameters that are little affected by sleep deprivation are valid as circadian markers. The second protocol is “forced desynchrony,” in which subjects live on very long or very short sleep-wake cycles, while the clock remains at its endogenous period, somewhat longer than 24 h. This protocol allows quantification of many measures with respect to either time of day (process C) or to duration of prior wakefulness (process S).18
Process C and S in SAD
Both the constant routine and forced desynchrony protocols have been employed in patients with SAD, both when depressed and euthymic, in winter and summer. The endogenous period appears normal.41 A phase delay in process C (as measured by core body temperature or melatonin rhythms in constant routine) has been found,42 but not in all studies or all markers.41,43 The decline in process S (as measured by spectral analyses of the sleep electroencephalogram [EEG]) was no different in SAD patients compared with controls.44,45 However, the rise in process S (as measured by spectral analyses of the wake EEG) was different, indicating a factor related to daytime vigilance.46,47 Wake EEG patterns in evening chronotypes are similar to this,48 which may mean that the above finding is not pathogenetic for SAD, since the patient chronotype is skewed towards ”owls,“ shows the above tendency to phase delay, and has common clock-related polymorphisms.32
War of the zeitgebers?
What is fascinating is that both circadian and wake-dependent factors contribute to a subjective measure such as mood. This has been demonstrated in healthy subjects in both protocols.6,41,49,50 The day-to-day change in patterns of diurnal mood variation in a forced desynchrony protocol has remarkable similarities to the day-to-day variability in diurnal mood variation found in depressive patients, and even more similarity to the mood patterns following a phase advance of the sleep-wake cycle.8 Thus, mood fluctuations can indeed be understood in terms of abnormal or changing phase relationships.
Mood-related cognitive and attributional disturbances have been postulated to be sequelae of shifting circadian rhythms.5 'Ihis is an important point for the above findings. If SAD patients are vulnerable to short winter days, is this an abnormality of the biological clock, or is it rather a subjective interpretation of internal temporal disorder? The following findings are perhaps relevant to this argument. Some subjects in experiments where they live free of time cues manifest spontaneous internal desynchronization, in that their sleep-wake cycle desynchronizes from circadian rhythms such as core body temperature. They do not notice that this phenomenon has occurred, nor do they show any decrement in mood or performance―on the contrary, they feel rather well.51 This is in marked contrast to the situation resulting from external desynchronization, when sleep timing is shifted by shift work or transmeridian travel. Here the internal desynchronization between sleep and the clock is additionally in conflict with light and social zeitgebers in the outer world; and it is postulated that this aspect may underlie the often-associated depressive disturbances.5,52
It may not only be phase relationships that are important, but perhaps also the light-dark ratio (daylength or photoperiod). Some of the evidence for SAD suggests that the duration of nocturnal melatonin secretion is important for triggering psychopathology in winter.53 Conversely, in a study of healthy subjects kept on long winter nights, one volunteer became severely suicidal, even though all the others felt remarkably well on this protocol.54
Diurnal variation or instability of mood can thus be quite well explained by considering changing phase relationships between processes C and S. Even in healthy subjects, some phase relationships are favorable, others unfavorable. Modest but reliable mood decrements occur after a phase delay of the sleep-wake cycle55 (reviewed in reference 5). Sudden delays (as induced by night shift or westwards flights across time zones) can even precipitate depressive symptoms in predisposed individuals with a history of affective illness.56,57 This points to a particular vulnerability of mood state when sleep is shifted later with respect to circadian rhythms. Such an association also appears to be valid for the circadian sleep disorder of delayed sleep phase syndrome (inappropriately late sleep timing with respect to the endogenous circadian clock). In these persons there is a high comorbidity of depressive symptoms.58 Conversely, flying east may be more correlated with hypomanic or manic states.56,57
Psychopharmacology and circadian rhythms
The earliest link between psychopharmacology and circadian rhythms came from the observation that lithium slows down circadian periodicity in plants.59 These effects of lithium are consistent across species, including humans,60 and are measurable even at the level of individual SCN neurones.61 However, attempts to generalize across various classes of antidepressant drugs have not been successful7: even though the monoamine oxidase inhibitor (MAOI) clorgyline lengthened circadian period,62 the MAOI moclobemide shortened it,63 and selective serotonin reuptake inhibitors (SSRIs) had no effect.63 When considering the model (Figure 1A), it is clear that drugs could act not only on circadian period but may also change phase position or phase relationships with the sleep-wake cycle, to enhance circadian amplitude or sensitivity to zeitgebers. Evidence that imipramine and lithium modify the phase angle between the circadian temperature rhythm and the rest-activity cycle is interesting,64 as is the concept that stabilization of circadian rhythms may be a key action of clinically effective mood-stabilizing drugs.65 In addition, sensitivity to light could be affected, as is the case with chronic clorgyline and lithium treatments.66
Nonpharmacological therapies
Sleep deprivation
Well documented is the rapid, usually short-lasting improvement following total sleep deprivation and the rapid return of depressive symptoms after subsequent recovery sleep, indicating that the depressive process is strongly sleep dependent.8 Additionally, sleep deprivation needs to coincide with an early morning circadian phase for optimal antidepressant response. Partial sleep deprivation in the second half of the night or phase-advance of the sleep-wake cycle are equally efficacious (see Table I for a list of therapeutic modalities). The spontaneous switch out of depression (and into hypomania and mania) often occurs after a “natural” sleep deprivation.
This remarkable and immediate antidepressant modality has been recognized for 30 years, but is little used in everyday clinical practice. Perhaps it is the paradox of taking sleep away from the depressive insomniac that has a negative connotation for both patient and psychiatrist (“wake therapy” would be a more positive alternative name). Perhaps it is also the short-term nature of the response that has hindered its use, though the magnitude of the clinical changes brought about by sleep deprivation still remain highly intriguing and may provide clues for understanding the pathophysiology of depression.
Sleep deprivation is the paradigm par excellence for depression research: rapid, nonpharmacological, and short lasting. It may be the nonpharmacological nature of sleep deprivation (it cannot be patented) that has contributed to its status as an “orphan drug.”67 It is surprising that no pharmaceutical company has focused on this model to search for that much-needed rapid-acting antidepressant.8 This lack may be remedied in the future; new research reveals that, whereas sleep induces very few genes, wakefulness increases expression of several groups of genes,68 and here comparisons with the effects of antidepressant drug treatment may narrow down the candidates.
Some committed proponents of sleep deprivation have recognized its clinical usefulness to initiate rapid improvement, particularly in the most severely depressed patients in whom time is of the essence. Sleep deprivation is effective in all diagnostic subgroups of depression. The problem is the relapse after recovery sleep, and new strategies have sought treatments to prevent this. Response appears to be well maintained by treatment with lithium, antidepressants (in particular SSRIs), or the 5-HT1A receptor antagonist pindolol, as well as nonpharmacological adjuvants such as repetitive transcranial magnetic stimulation (rTMS),69 light therapy, or phase advance of the sleep-wake cycle, or various combinations thereof (see, for example, reference 36 and 70, reviewed in reference 8; Table I).
Light therapy
Light therapy can be considered to be the most successful clinical application of circadian rhythm concepts in psychiatry to date. Light is the treatment of choice for SAD.71 The quality of recent SAD studies has been exemplary, and the response rate is well above placebo (in fact, superior to analogous trials with antidepressant drugs).72 The success of this nonpharmacological treatment has been astonishing, but it has taken rather long for light therapy to be accepted by establishment psychiatry,72 and trials of other indications are still in the research phase. Its very success in SAD has limited use in other forms of depression (characterized as “it's a chronobiological treatment for a chronobiological subset of depressive patients”). However, light acts on the same neurotransmitters, in particular serotonin, as the major antidepressant drugs.71 This has been shown with tryptophan deletion tests, where relapse after successful light therapy is induced, as well as the successful treatment of SAD patients by SSRIs.71 More direct evidence of the immediate effects of light on serotonin turnover in the brain has come from an in vivo study in healthy subjects: not only is serotonin turnover high in spring and summer and low in autumn and winter (the pattern following the hours of available sunshine), but serotonin turnover increases immediately after light exposure.73 Assuming that mood state is at least partially linked to serotonin turnover, the conclusions are obvious: more light, better mood.
The serotonin connection suggests that a broader use of light therapy is indicated. A rapid response within a week in SAD does not mean that other major depressive disorders will improve so fast: trials of light therapy over at least 4 to 6 weeks, as would be standard for a drug treatment trial, are required. There is already good evidence for efficacy in bulimia, preliminary evidence for usefulness in prepartum and postpartum depression (clinical indications where new nondrug therapies are sorely needed),74 and promising findings in major depression, particularly as an adjuvant (Table I). 74 Light is being recognized not only as a major zeitgeber necessary for our daily well-being (with applications in the work place and in architecture), but also as a ”drug“ that can be prescribed in dose, timing, and duration for specific diagnoses.71
An important step forward for the clinician has been that all available randomized studies of light therapy for both SAD and nonseasonal depression are being analyzed for efficacy, and will soon be published in the Cochrane Library (www.cochrane.de).
“Dark” therapy
Single case studies of rapidly cycling bipolars have shown that extending darkness (or rest, or sleep) immediately stops the recurring pattern, which is a rather astonishing result in these therapy -resistant patients.38,39 Further support comes from recent findings that extended darkness (not rest and not sleep) in manic bipolar patients can control their symptoms within days (B. Barbini, personal communication).
The pineal hormone melatonin is designated the “hormone of darkness.” Physiologically, it is important for timing the cascade of events initiating sleep in humans.20 The nocturnal onset of melatonin secretion opens the gateway for sleep propensity, involving peripheral thermoregulatory mechanisms.75 The “warm feet effect” underlies its soporific action and use in a variety of sleep disorders.20 The few studies administering melatonin to depressed patients have indeed found improvements in sleep, but not in mood.76,77
Emerging therapies
New drugs, such as agomelatine (a melatonin agonist and 5-HT2c antagonist), with a core action on circadian rhythms, are currently in development for the treatment of mood disorders.
A large multicenter study investigating agomelatine in major depression has yielded an excellent antidepressant response,78 which has been linked to the action of the compound on the melatonergic and serotonergic systems. Moreover, the 5-HT2c receptor subtype is considered to be relevant to the therapeutic properties of SSRIs, and―to link this to chronobiology―5-HT2c receptor agonists, which mimic the effects of light in rat CNS.79
Sleep shifts and zeitgebers as therapy
The above concepts point toward a multimodal approach to using chronobiological therapies in major depression. “Wake therapy” (increasing the level of process S) induces rapid clinical improvement in all diagnostic subgroups; phase advance (changing the timing of sleep) maintains the response, as does light, drugs acting on the serotonergic system, or rTMS (which acts on the SCN80). Increasing zeitgeber strength improves the consistency of entrainment and circadian amplitude: this may be one mechanism underlying the therapeutic efficacy of bright light and the melatonin agonist. There is evidence that depressed patients, including those with SAD, have greater day-today and within-day mood variability than controls.81,82 In SAD patients, it has been shown that increasing zeitgeber strength with light therapy reduced or eliminated both group differences in mean level and variability of mood.82 Other zeitgebers (social cues, activity, and food) are important for improving behavioral feedback from peripheral clocks to overall cntrainment stability. This is extremely important in bipolar patients.37 The combination needed by the clinician for the sought-after rapid and long-lasting antidepressant, might well be an eclectic mix of these nonpharmacological modalities with antidepressant drugs.
Conclusion
We live in a 24-h society that is no longer strongly synchronized to the change in daylength or temperature across the seasons. A permanent “summer day” is the result of artificial lighting, yet it is of insufficient intensity for stable entrainment. Too little is known of the sequelae of irregular patterns of light exposure on a vulnerable circadian system, and how light could trigger or alleviate a depressive phase. Could part of the increase in prevalence of depression in modern society be related to such factors? Genetic predisposition, hormonal fluctuations, environmental stress, and altered light-dark cycles could all induce rhythm disturbances. Conversely, altered sleep patterns, hyperarousal, eating behavior, and mood state could feed back onto the circadian system via hormones and effects on peripheral oscillators. These new insights provide us with useful strategies and a variety of methods to improve robustness of the circadian pacemaker and better synchronize its timing with respect to the day-night cycle. It is interesting to reconsider those empirically developed 19th century psychiatric treatments, which consisted of establishing regularity in social schedules and meal times, and manipulating sleep (albeit with “cures”) and temperature (with cold baths), in terms of modern chronobiology and the importance of correctly timed zeitgebers.
Selected abbreviations and acronyms
- HPA
hypothalamo-pituixary -adrenal (axis)
- 5-HT
serotonin (5-hydroxytryptamine)
- PVN
paraventricular nucleus
- rTMS
repetitive transcranial magnetic stimulation
- SAD
seasonal affective disorder
- SCN
suprachiasmatic nucleus
- SSRl
selective serotonin reuptake inhibitor
REFERENCES
- 1.Wehr TA., Goodwin FK. Biological rhythms in manic-depressive illness. In: Wehr TA, Goodwin FK, eds. Circadian Rhythms in Psychiatry. Pacific Grove, Calif: The Boxwood Press; 1983:129–184. [Google Scholar]
- 2.Wu JC., Bunney WE. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psychiatry. 1990;147:14–21. doi: 10.1176/ajp.147.1.14. [DOI] [PubMed] [Google Scholar]
- 3.Kuhs H., Tölle R. Sleep deprivation therapy. Biol Psychiatry. 1991;29:1129–1148. doi: 10.1016/0006-3223(91)90255-k. [DOI] [PubMed] [Google Scholar]
- 4.Leibenluft E., Wehr TA. Is sleep deprivation useful in the treatment of depression? Am J Psychiatry. 1992;149:159–168. doi: 10.1176/ajp.149.2.159. [DOI] [PubMed] [Google Scholar]
- 5.Healy D., Waterhouse JM. The circadian system and the therapeutics of the affective disorders. Pharmacol Ther. 1995;65:241–263. doi: 10.1016/0163-7258(94)00077-g. [DOI] [PubMed] [Google Scholar]
- 6.Wirz-Justice A. Biological rhythms in mood disorders. In: Bloom FE, Kupfer DJ, eds. Psychopharmacology: The Fourth Generation of Progress. New York, NY: Raven Press; 1995:999–1017. [Google Scholar]
- 7.Rosenwasser AM., Wirz-Justice A. Circadian rhythms and depression: clinical and experimental models. In: Redfern PH, Lemmer B, eds. Physiology and Pharmacology of Biological Rhythms. Berlin, Germany: Springer Verlag; 1997:457–486. [Google Scholar]
- 8.Wirz-Justice A., Van den Hoofdakker RH. Sleep deprivation in depression: what do we know, where do we go? Biol Psychiatry. 1999;46:445–453. doi: 10.1016/s0006-3223(99)00125-0. [DOI] [PubMed] [Google Scholar]
- 9.Boivin DB. Influence of sleep-wake and circadian rhythm disturbances in psychiatric disorders. J Psychiatry Neurosci. 2000;25:446–458. [PMC free article] [PubMed] [Google Scholar]
- 10.Zeitzer JM., Dijk DJ., Kronauer RE., Brown EN., Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daan S., Beersma DGM., Borbély AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol. 1984;246:R161–R183. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- 12.Klein DC., Moore RY., Reppert SM. Suprachiasmatic Nucleus: The Mind's Clock. New York, NY: Oxford University Press; 1991 [Google Scholar]
- 13.Berson DM., Dunn FA., Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 14.Remé CE., Wirz-Justice A., Terman M. The visual input stage of the mammalian circadian pacemaking system: I. Is there a clock in the mammalian eye? J Biol Rhythms. 1991;6:5–29. doi: 10.1177/074873049100600104. [DOI] [PubMed] [Google Scholar]
- 15.Wehr TA. Photoperiodism in humans and other primates: evidence and implications. J Biol Rhythms. 2001;16:348–364. doi: 10.1177/074873001129002060. [DOI] [PubMed] [Google Scholar]
- 16.ewy AJ. The dim light melatonin onset, melatonin assays and biological rhythm research in humans. Biol Signals Recept. 1999;8:79–83. doi: 10.1159/000014573. [DOI] [PubMed] [Google Scholar]
- 17.Linkowski P., Van Onderbergen A., Kerkhofs M., Bosson D., Mendlewicz J., Van Cauter E. Twin study of the 24-h Cortisol profile: evidence for genetic control of the human circadian clock. Am J Physiol. 1993;264:E173–E181. doi: 10.1152/ajpendo.1993.264.2.E173. [DOI] [PubMed] [Google Scholar]
- 18.Czeisler CA., Khalsa SBS. The human circadian timing system and sleep-wake regulation. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 3rd ed. Philadelphia, Pa: WB Saunders Company; 2000:353–375. [Google Scholar]
- 19.Honma Kl., Hashimoto S., Nakao M., Honma S. Period and phase adjustments of human circadian rhythms in the real world. J Biol Rhythms. 2003;18:261–270. doi: 10.1177/0748730403018003008. [DOI] [PubMed] [Google Scholar]
- 20.Czeisler CA., Cajochen C., Turek FW. Melatonin in the regulation of sleep and circadian rhythms. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 3rd ed. Philadelphia, Pa: WB Saunders Company; 2000:400–406. [Google Scholar]
- 21.anilenko KV., Cajochen C., Wirz-Justice A. Is sleep per se a zeitgeber in humans? J Biol Rhythms. 2003;18:170–178. doi: 10.1177/0748730403251732. [DOI] [PubMed] [Google Scholar]
- 22.Monk TH., Kupfer DJ., Frank E., Ritenour AM. The Social Rhythm Metric (SRM): measuring daily social rhythms over 12 weeks. Psychiatry Res. 1991;36:195–207. doi: 10.1016/0165-1781(91)90131-8. [DOI] [PubMed] [Google Scholar]
- 23.Buijs RM., Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nature Rev Neurosci. 2001;2:521–526. doi: 10.1038/35081582. [DOI] [PubMed] [Google Scholar]
- 24.Roenneberg T., Wirz-Justice A., Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 25.Roenneberg T., Merrow M. The network of time: understanding the molecular circadian system. Curr Biol. 2003;13:R198–R207. doi: 10.1016/s0960-9822(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 26.Franken P., Chollet D., Tafti M. The homeostatic regulation of sleep need is under genetic control. J Neurosci. 2001;21:2610–2621. doi: 10.1523/JNEUROSCI.21-08-02610.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones CR., Campbell SS., Zone SE., et al. Familial advanced sleep-phase syndrome: a short-period circadian rhythm variant in humans. Nat Med. 1999;5:1062–1065. doi: 10.1038/12502. [DOI] [PubMed] [Google Scholar]
- 28.Ebisawa T., Uchiyama M., Kajimura N., et al. Association of structural polymorphisms in the human period 3 gene with delayed sleep phase syndrome. EMBO Rep. 2001;2:342–346. doi: 10.1093/embo-reports/kve070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwase T., Kajimura N., Uchiyama M., et al. Mutation screening of the human Clock gene in circadian rhythm sleep disorders. Psychiatry Res. 2002;109:121–128. doi: 10.1016/s0165-1781(02)00006-9. [DOI] [PubMed] [Google Scholar]
- 30.Desan PH., Oren DA., Malison R., et al. Genetic polymorphism at the CLOCK gene locus and major depression. Am J Med Genet. 2000;96:418–421. doi: 10.1002/1096-8628(20000612)96:3<418::aid-ajmg34>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 31.hiino Y., Nakajima S., Ozeki Y., Isono T., Yamada N. Mutation screening of the human period 2 gene in bipolar disorder. Neurosci Lett. 2003;338:82–84. doi: 10.1016/s0304-3940(02)01290-9. [DOI] [PubMed] [Google Scholar]
- 32.Johansson C., Willeit M., Smedh C., et al. Circadian clock-related polymorphisms in seasonal affective disorder and their relevance to diurnal preference. Neuropsychopharmacology: 2003;28:734–739. doi: 10.1038/sj.npp.1300121. [DOI] [PubMed] [Google Scholar]
- 33.Yamazaki S., Numano R., Abe M., et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 34.chibler U., Ripperger J., Brown SA. Peripheral circadian oscillators in mammals: time and food, J Biol Rhythms. 2003;18:250–260. doi: 10.1177/0748730403018003007. [DOI] [PubMed] [Google Scholar]
- 35.Wehr TA., Sack DA., Rosenthal N. Sleep reduction as a final common pathway in the genesis of mania. Am J Psychiatry. 1987;144:201–204. doi: 10.1176/ajp.144.2.201. [DOI] [PubMed] [Google Scholar]
- 36.Benedetti F., Barbini B., Campori E., Fulgosi MC., Pontiggia A., Colombo C. Sleep phase advance and lithium to sustain the antidepressant effect of total sleep deprivation in bipolar depression: new findings supporting the internal coincidence model? J Psychiatr Res. 2001;35:323–329. doi: 10.1016/s0022-3956(01)00034-6. [DOI] [PubMed] [Google Scholar]
- 37.Frank E., Swartz HA., Kupfer DJ. Interpersonal and social rhythm therapy: managing the chaos of bipolar disorder. Biol Psychiatry. 2000;48:593–604. doi: 10.1016/s0006-3223(00)00969-0. [DOI] [PubMed] [Google Scholar]
- 38.Wehr TA., Turner EH., Shimada JM., Lowe CH., Barker C., Leibenluft E. Treatment of rapidly cycling bipolar patient by using extended bed rest and darkness to stabilize the timing and duration of sleep. Biol Psychiatry. 1998;43:822–828. doi: 10.1016/s0006-3223(97)00542-8. [DOI] [PubMed] [Google Scholar]
- 39.Wirz-Justice A., Quinto C., Cajochen C., Werth E., Hock C. A rapid-cycling bipolar patient treated with long nights, bedrest, and light. Biol Psychiatry. 1999;45:1075–1077. doi: 10.1016/s0006-3223(98)00289-3. [DOI] [PubMed] [Google Scholar]
- 40.Borbély AA., Wirz-Justice A. Sleep, sleep deprivation and depression. Hum Neurobiol. 1982;1:205–210. [PubMed] [Google Scholar]
- 41.oorengevel KM., Beersma DGM., den Boer JA., Van den Hoofdakker RH. A forced desynchrony study of circadian pacemaker characteristics in seasonal affective disorder. J Biol Rhythms. 2002;17:463–475. doi: 10.1177/074873002237140. [DOI] [PubMed] [Google Scholar]
- 42.Avery DH., Dahl K., Savage MV., et al. Circadian temperature and Cortisol rhythms during a constant routine are phase-delayed in hypersomnic winter depression. Biol Psychiatry. 1997;41:1109–1123. doi: 10.1016/S0006-3223(96)00210-7. [DOI] [PubMed] [Google Scholar]
- 43.Wirz-Justice A., Kräuchi K., Brunner DP., et al. Circadian rhythms and sleep regulation in seasonal affective disorder. Acta Neuropsychiatry. 1995;7:41–43. doi: 10.1017/S0924270800037522. [DOI] [PubMed] [Google Scholar]
- 44.Brunner DP., Kräuchi K., Dijk DJ., Leonhardt G., Haug HJ., Wirz-Justice A. Sleep electroencephalogram in seasonal affective disorder and in control women: effects of midday light treatment and sleep deprivation. Biol Psychiatry. 1996;40:485–496. doi: 10.1016/0006-3223(95)00656-7. [DOI] [PubMed] [Google Scholar]
- 45.Koorengevel K., Beersma D., Den Boer J., van den Hoofdakker R. Sleep in seasonal affective disorder patients in forced desynchrony: an explorative study. J Sleep Res. 2002;11:347–356. doi: 10.1046/j.1365-2869.2002.00319.x. [DOI] [PubMed] [Google Scholar]
- 46.Cajochen C., Brunner DP., Kräuchi K., Graw P., Wirz-Justice A. EEG and subjective sleepiness during extended wakefulness in seasonal affective disorder: circadian and homeostatic influences. Biol Psychiatry. 2000;47:610–617. doi: 10.1016/s0006-3223(99)00242-5. [DOI] [PubMed] [Google Scholar]
- 47.Putilov A., Donskaya OG., Jafarova OA., Danilenko KV. Waking EEG power density in hypersomnic winter depression. 12th Annual Meeting of the Society for Light Treatment and Biological Rhythms. 2000. Evanston, III. Abstracts p24. May 7-9; [Google Scholar]
- 48.Taillard J., Philip P., Coste O., Sagspe P., Bioulac B. Circadian and homeostatic buildup of sleep pressure during extended wakefulness in morning and evening chronotypes. J Sleep Res. 2003. In press. doi: 10.1046/j.0962-1105.2003.00369.x. [DOI] [PubMed] [Google Scholar]
- 49.Boivin DB., Czeisler CA., Dijk DJ., et al. Complex interaction of the sleepwake cycle and circadian phase modulates mood in healthy subjects. Arch Gen Psychiatry. 1997;54:145–152. doi: 10.1001/archpsyc.1997.01830140055010. [DOI] [PubMed] [Google Scholar]
- 50.Schröder C., Knoblauch V., Renz C., Kräuchi K., Wirz-Justice A., Cajochen C. Circadian modulation of mood under differential sleep pressure conditions. Sleep. 2003;26(suppl):A101. [Google Scholar]
- 51.Wever RA. The Circadian System of Man: Results of Experiments under Temporal Isolation. New York, NY: Springer Verlag; 1979 [Google Scholar]
- 52.Healy D., Minors DS., Waterhouse JM. Shiftwork, helplessness and depression. J Affect Disord. 1993;29:17–25. doi: 10.1016/0165-0327(93)90114-y. [DOI] [PubMed] [Google Scholar]
- 53.Wehr TA., Duncan WCJ., Sher L., et al. A circadian signal of change of season in patients with seasonal affective disorder. Arch Gen Psychiatry. 2001;58:1108–1114. doi: 10.1001/archpsyc.58.12.1108. [DOI] [PubMed] [Google Scholar]
- 54.Wehr TA., Moul DE., Barbato G., et al. Conservation of photoperiodresponsive mechanisms in humans. Am J Physiology. 1993;265:R846–R857. doi: 10.1152/ajpregu.1993.265.4.R846. [DOI] [PubMed] [Google Scholar]
- 55.Surridge-David M., MacLean A., Coulter ME., Knowles JB. Mood change following an acute delay of sleep. Psychiatry Res. 1987;22:149–158. doi: 10.1016/0165-1781(87)90102-8. [DOI] [PubMed] [Google Scholar]
- 56.Jauhar P., Weller MP. Psychiatric morbidity and time zone changes: a study of patients from Heathrow airport. Br J Psychiatry. 1982;140:231–235. doi: 10.1192/bjp.140.3.231. [DOI] [PubMed] [Google Scholar]
- 57.Young DM. Psychiatric morbidity in travelers to Honolulu, Hawaii. Compr Psychiatry. 1995;36:224–228. doi: 10.1016/0010-440x(95)90086-b. [DOI] [PubMed] [Google Scholar]
- 58.Regestein QR., Monk TH. Delayed sleep phase syndrome: a review of its clinical aspects. Am J Psychiatry. 1995;152:602–608. doi: 10.1176/ajp.152.4.602. [DOI] [PubMed] [Google Scholar]
- 59.Engelmann W. Lithium slows down the Kalanchoe clock. Z Naturforsch [B]. 1972;27:477. doi: 10.1515/znb-1972-0431. [DOI] [PubMed] [Google Scholar]
- 60.Johnsson A., Engelmann W., Pflug B., Klemke W. Influence of lithium ions on human circadian rhythms. Z Naturforsch [C]. 1980;35:503–507. doi: 10.1515/znc-1980-5-623. [DOI] [PubMed] [Google Scholar]
- 61.Abe M., Herzog ED., Block GD. Lithium lengthens the arcadian period of individual suprachiasmatic nucleus neurons. Neuroreport. 2000;11:3261–3264. doi: 10.1097/00001756-200009280-00042. [DOI] [PubMed] [Google Scholar]
- 62.Wirz-Justice A., Campbell IC. Antidepressant drugs can slow or dissociate circadian rhythms. Experientia. 1982;38:1301–1309. doi: 10.1007/BF01954918. [DOI] [PubMed] [Google Scholar]
- 63.Wollnik F. Effects of chronic administration and withdrawal of antidepressant agents on circadian activity rhythms in rats. Pharmacol Biochem Behav. 1992;43:549–561. doi: 10.1016/0091-3057(92)90190-q. [DOI] [PubMed] [Google Scholar]
- 64.Nagayama H. Chronic administration of imipramine and lithium changes the phase-angle relationship between the activity and core body temperature circadian rhythms in rats. Chronobiol Int. 1996;13:251–259. doi: 10.3109/07420529609020905. [DOI] [PubMed] [Google Scholar]
- 65.Klemfuss H., Kripke DF. Antimanic drugs stabilize hamster circadian rhythms. Psychiatry Res. 1995;57:215–222. doi: 10.1016/0165-1781(95)02687-r. [DOI] [PubMed] [Google Scholar]
- 66.Duncan WC., Johnson KA., Wehr TA. Decreased sensitivity to light of the photic entrainment pathway during chronic clorgyline and lithium treatments. J Biol Rhythms. 1998;13:330–346. doi: 10.1177/074873098129000165. [DOI] [PubMed] [Google Scholar]
- 67.Wirz-Justice A. Why is sleep deprivation an orphan drug? Psychiatry Res. 1998;81:281–282. doi: 10.1016/s0165-1781(98)00097-3. [DOI] [PubMed] [Google Scholar]
- 68.Cirelli C. How sleep deprivation affects gene expression in the brain: a review of recent findings. J Appl Physiol. 2002;92:394–400. doi: 10.1152/jappl.2002.92.1.394. [DOI] [PubMed] [Google Scholar]
- 69.Eichhammer P., Kharraz A., Wiegand R., et al. Sleep deprivation in depression stabilizing antidepressant effects by repetitive transcranial magnetic stimulation. Life So. 2002;70:1741–1749. doi: 10.1016/s0024-3205(02)01479-0. [DOI] [PubMed] [Google Scholar]
- 70.Colombo C., Lucca A., Benedetti F., Barbini B., Campori E., Smeraldi E. Total sleep deprivation combined with lithium and light therapy in the treatment of bipolar depression: replication of main effects and interaction. Psychiatry Res. 2000;95:43–53. doi: 10.1016/s0165-1781(00)00164-5. [DOI] [PubMed] [Google Scholar]
- 71.Lam RW., Levitt AJ. Canadian Consensus Guidelines for the Treatment of Seasonal Affective Disorder. Canada: Clinical & Academic Publishing; 1999 [Google Scholar]
- 72.Wirz-Justice A. Beginning to see the light. Arch Gen Psychiatry. 1998;55:861–862. doi: 10.1001/archpsyc.55.10.861. [DOI] [PubMed] [Google Scholar]
- 73.Lambert GW., Reid C., Kaye DM., Jennings GL., Esler MD. Effect of sunlight and season on serotonin turnover in the brain. Lancet. 2002;360:1840–1842. doi: 10.1016/s0140-6736(02)11737-5. [DOI] [PubMed] [Google Scholar]
- 74.Lam RW. Seasonal Affective Disorder and Beyond. Light Treatment for SAD and Non-SAD Conditions. Washington DC: American Psychiatric Press; 1998 [Google Scholar]
- 75.Kräuchi K., Wirz-Justice A. Circadian clues to sleep onset mechanisms. Neuropsychopharmacology. 2001;25:S92–S96. doi: 10.1016/S0893-133X(01)00315-3. [DOI] [PubMed] [Google Scholar]
- 76.deVries MW., Peelers FP. Melatonin as a therapeutic agent in the treatment of sleep disturbance in depression. J Nerv Ment Dis. 1997;185:201–202. doi: 10.1097/00005053-199703000-00010. [DOI] [PubMed] [Google Scholar]
- 77.Dolberg OT., Hirschmann S., Grunhaus L. Melatonin for the treatment of sleep disturbances in major depressive disorder. Am J Psychiatry. 1998;155:1119–1121. doi: 10.1176/ajp.155.8.1119. [DOI] [PubMed] [Google Scholar]
- 78.Lôo H., Dalery J., Macher JP., Payen A. Pilot study comparing in blind the therapeutic effect of two doses of agomelatine, melatoninergic agonist and selective 5-HT2C receptors antagonist, in the treatment of major depressive disorders. Encephale. 2003;28:356–362. [PubMed] [Google Scholar]
- 79.Kennaway DJ. Light, neurotransmitters and the suprachiasmatic nucleus control of pineal melatonin production in the rat. Biol Signals Recept. 1997;6:247–254. doi: 10.1159/000109135. [DOI] [PubMed] [Google Scholar]
- 80.Ji R., Schlaepfer T., Aizenman C., et al. Repetitive transcranial magnetic stimulation activates specific regions in rat brain. Proc Natl Acad Sci U S A. 1998;95:15635–15640. doi: 10.1073/pnas.95.26.15635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hall DP., Sing HC., Romanoski AJ. Identification and characterization of greater mood variance in depression. Am J Psychiatry. 1991;148:418–419. doi: 10.1176/ajp.148.10.1341. [DOI] [PubMed] [Google Scholar]
- 82.Krauss SS., Depue RA., Arbisi PA., Spoont M. Behavioral engagement level, variability, and diurnal rhythm as a function of bright light in bipolar II seasonal affective disorder: an exploratory study. Psychiatry Res. 1992;43:147–160. doi: 10.1016/0165-1781(92)90129-q. [DOI] [PubMed] [Google Scholar]