Abstract
Sleep disorders encompass a wide spectrum of diseases with significant individual health consequences and high economic costs to society. To facilitate the diagnosis and treatment of sleep disorders, this review provides a framework using the International Classification of Sleep Disorders, Primary and secondary insomnia are differentiated, and pharmacological and nonpharmacological treatments are discussed. Common circadian rhythm disorders are described in conjunction with interventions, including chronotherapy and light therapy. The diagnosis and treatment of restless legs syndrome/periodic limb movement disorder is addressed. Attention is focused on obstructive sleep apnea and upper airway resistance syndrome, and their treatment. The constellation of symptoms and findings in narcolepsy are reviewed together with diagnostic testing and therapy, Parasomnias, including sleep terrors, somnambulism, and rapid eye movement (REM) behavior sleep disorders are described, together with associated laboratory testing results and treatment.
Keywords: diagnosis, treatment, sleep disorder, insomnia, arcadian rhythm disorder, excessive somnolence, parasomnia
Abstract
Los trastornos del sueño incluyen un amplío espectro de enfermedades con consecuencias significativas para la salud individual y altos costos económicos para la sociedad. Para facilitar el diagnóstico y tratamiento de los trastornos del sueño esta revisión se estructuró utilizando la Clasificación internacional de Trastornos del Sueño, Se diferencia el insomnio primario y secundario, y se discuten los tratamientos farmacológicos y no farmacológicos. Se describen los trastornos comunes del ritmo círcadíano en conjunto con intervenciones que incluyen la cronoterapía y la fototerapia. También se revisa el diagnóstico y tratamiento del síndrome de las piernas inquietas o trastorno del movimiento periódico de las piernas. Además se centra la atención en la apnea obstructiva del sueño y el síndrome de resistencia de la vía aérea superior y su tratamiento. Se revisa la constelación de síntomas y hallazgos de la narcolepsia, junto con las pruebas diagnósticas y la terapia. Se describen las parasom-nias, incluyendo los terrores nocturnos, el sonambulismo y los trastornos de conducta asociados al sueño REM (movimiento rápido de ojos) junto con los resultados de las pruebas de laboratorio y el tratamiento.
Abstract
Les troubles du sommeil comprennent un large spectre de maladies avec des conséquences significatives individuelles en termes de santé et un coût économique élevé pour la société. Pour faciliter le diagnostic et le traitement des troubles du sommeil, cette revue fournit un cadre utilisant la classification internationale des troubles du sommeil. Les insomnies primaires et secondaires sont différenciées et les traitements pharmacologiques et non pharmacologiques sont discutés. Les troubles courants du rythme circadien sont décrits conjointement avec les interventions dont la chronothérapie et la luxihérapie. Le diagnostic et le traitement du syndrome des jambes sans repos/mouvements périodiques des membres sont abordés. Les apnées obstructives du sommeil et le syndrome de résistance des voies aériennes supérieures et leur traitement reçoivent une attention particulière. La constellation de symptômes et les acquisitions sur la narcolepsie sont passées en revue ainsi que les épreuves diagnostiques et le traitement. Les parasomnies dont les terreurs nocturnes, le somnambulisme et les perturbations des mouvements oculaires rapides (REM, rapid eye movement] sont décrits conjointement avec les résultats des tests de laboratoire et le traitement
Forty million Americans are afflicted with chronic disorders of sleep and wakefulness, which interfere with work, driving, and social activities. Sleep disorders cause 38 000 cardiovascular deaths and cost over 16 billion annually.1 Indirect costs of accidents, property destruction, litigation, hospitalization, and death add another 50 to $100 billion.1 The most common sleep disorders include insomnia, sleep apnea, restless legs syndrome, and narcolepsy.1-3
Classification of sleep disorders
The International Classification of Sleep Disorders diagnostic and coding manual 2000 lists four major categories of sleep disorders: dyssomnias; parasomnias; sleep disorders associated with mental, neurologic, or other medical disorders; and proposed sleep disorders (Table I) 4-7
Table I. Classification of sleep disorders4. NOS, not otherwise specified; REM, rapid eye movement.
| Dyssomnias | Parasomnias | Sleep disorders associated with mental, neurological, or other medical disorders | Proposed sleep disorders |
| • Intrinsic sleep disorders | • Arousal disorders | • Associated with mental disorders | - Short sleeper |
| - Psychophysiogical insomnia | - Confusional arousals | - Psychoses | - Long sleeper |
| - Sleep state misperception | - Sleepwalking | - Mood disorders | - Subwakefulness syndrome |
| - Idiopathic insomnia | - Sleep terrors | - Anxiety disorders | - Fragmentary myoclonus |
| - Narcolepsy | • Sleep-wake transition disorders | - Panic disorders | -Sleep hyperhidrosis |
| - Recurrent hyperomnia | Rhythmic starts | Alcoholism | - Menstrual-associated sleep disorder |
| - Idiopathic hypersomnia | - Sleep starts | • Associated with neurological disorder | - Pregnancy-associated sleep disorder |
| - Posttraumatic hypersomnia | - Sleep talking | - Cerebral degenerative disorders | - Terrifying hypnagogic hallucinations |
| - Central alveolar hypo-ventilation syndrome | - Nocturnal leg cramps | - Dementia | - Sleep-related neurogenic tachypnea |
| - Periodic limb movement disorder | • Parasomnias usually associated with REM sleep | - Parkinsonism | - Sleep-related laryngospasm |
| - Restless legs syndrome | - Nightmares | - Fatal familial insomnia | - Sleep-choking syndrome |
| - Intrinsic sleep disorder NOS | - Sleep paralysis | - Sleep-related epilepsy | |
| • Extrinsk sleep disorders | - Impaired sleep-related penile erections | - Electrical status epilepticus of sleep | |
| - Inadequate sleep hygiene | - REM sleep-related sinus arrest | - Sleep-related headaches | |
| - Environmental sleep disorder | - REM sleep behavior disorder | • Associated with other medical disorders | |
| - Altitude insomnia | • Other parasomnias | - Chronic obstructive pulmonary disease | |
| - Adjustment sleep disorder | - Sleep bruxism | - Sleep-related asthma | |
| - Insufficient sleep syndrome | - Sleep enuresis | - Sleep-related gastroesophageal reflux | |
| - Limit-setting sleep disorder | - Sleep-related abnormal swallowing syndrome | - Peptic ulcer disease | |
| - Sleep-onset association disorder | - Noctural paroxysmal dystonia | - Fibromyalgia | |
| - Food allergy insomnia | - Sudden unexplained nocturnal death syndrome | ||
| - Nocturnal eating (drinking) syndrome | - Primary snoring | ||
| - Hypnotic-dependent sleep disorder | - Infant sleep apnea | ||
| - Stimulant-dependent sleep disorder | - Congenital central hypo-ventilation syndrome | ||
| - Toxin-induced sleep disorder | - Sudden infant death syndrome | ||
| - Extrinsic sleep disorder NOS | - Benign neonatal sleep myoclonyus | ||
| • Circadian rhythm sleep disorders | - Other parasomnias NOS | ||
| - Jet lag syndrome | |||
| - Shift work sleep disorder | |||
| - Irregular sleep-wake pattern | |||
| - Delayed sleep-phase syndrome | |||
| - Advanced sleep-phase syndrome | |||
| - Non-24-h sleep-wake disorder | |||
| - Circadian rhythm sleep disorder NOS | |||
| - Circadian rhythm sleep disorder NOS |
Dyssomnias are disorders characterized by either excessive sleepiness or difficulty initiating or maintaining sleep4. On the basis of pathophysiological mechanisms, they can be subdivided into intrinsic, extrinsic, and circadian rhythm sleep disorders.4-9 Intrinsic sleep disorders are disorders that originate or develop within the body or that arise from causes within the body Common intrinsic sleep disorders include idiopathic and psychophysiological insomnia, narcolepsy, obstructive sleep apnea syndrome (OSAS), periodic limb movement disorder (PLMD), and restless legs syndrome (RLS)4-7. Sleep disorders caused by external factors are termed extrinsic sleep disorders and include inadequate sleep hygiene, environmental sleep disorder, adjustment sleep disorder, insufficient sleep syndrome, limit-setting sleep disorder, sleep-onset association disorder, and hypnotic-, stimulant-, or alcohol-dependent sleep disorder:4-7 Circadian rhythm sleep disorders share a common chronophysiological basis whereby there is a discordance between the patient's sleep pattern and the desired or societal sleep norm.4-9 Examples of circadian rhythm sleep disorders include shift work sleep disorder, delayed sleep phase syndrome, and advanced sleep phase syndrome.
Parasomnias are characterized by undesirable behavioral and physical phenomena that occur predominantly during sleep4-7. They include disorders of arousal, partial arousal, and sleep-stage transition.
Sleep disorders can also be associated with mental disorders, such as psychoses, mood disorders, anxiety disorders, panic disorders, and alcoholism. Neurological conditions associated with sleep disorders include cerebral degenerative disorders, dementia, parkinsonism, fatal familial insomnia, sleep-related epilepsy, electrical status cpilepticus of sleep, and sleep-related headaches.4-10 Sleep disorders can occur with medical disorders, such as sleeping sickness, nocturnal cardiac ischemia, chronic obstructive pulmonary disease, sleep-related asthma, sleeprelated gastroesophageal reflux, peptic ulcer disease, irritable bowel syndrome and fibromyalgia.-4,11-14
Proposed sleep disorders include short sleeper, long sleeper, subwakefulness syndrome, fragmentary myoclonus, sleep hyperhidrosis, menstrual-associated sleep disorder, pregnancy-associated sleep disorder, terrifying hypnagogic hallucinations, sleep-related neurogenic tachypnea, sleep-related laryngospasm, and sleep choking syndrome.4
Approach to sleep disorders
History and physical examination
An accurate and detailed history from the patient, bed partner, or family member combined with a sleep questionnaire can elicit critical information. Most sleep complaints fall into three categories: insomnia (sleep onset, maintenance, or early morning awakening); excessive sleepiness; or abnormal behaviors during sleep. The procedure is as follows.
Inquire into the chief complaint, when symptom(s) started, the pattern since onset, and associated factors (medical, environmental, occupational, psychological/stress, lifestyle choices) that may have predisposed to or precipitated the illness, perpetuated the condition, and improved or worsened symptoms.7
Assess the impact of the sleep complaint on the patient's life, and inquire about meal and sleep schedules, sleep hygiene, restless legs sensation, snoring, witnessed apneic episodes, sweating, coughing, gasping/ choking/snorting, dryness of the mouth, bruxism, excessive movements during sleep, periodic limb movements, any abnormal behaviors during sleep, daytime sleepiness, presence of cataplexy, sleep paralysis, and hypnagogic or hypnapompic hallucinations.
Ask about caffeine intake, alcohol and nicotine use, as well as use of illicit drugs.
Review the pertinent medical/surgical/psychiatric history and past treatments, and their efficacy or lack thereof.
Determine if there is any family history of sleep disorders (snoring, OSAS, narcolepsy, RLS).
A completed 2-week sleep log or sleep diary can be utilized to compute sleep efficiency, total sleep time, and number of awakenings during the night, and can be used to diagnose sleep disorders and monitor efficacy of treatment. On the basis of the information from questionnaires and sleep diary, the chief complaint, and the history, a working diagnosis is outlined.
Laboratory studies
Laboratory tests that arc performed to assess and therefore treat sleep disorders include the polysomnogram (PSG), multiple sleep latency test (MSLT), maintenance of wakefulness test (M'WT), actigraphy, video-PSG, nocturnal penile tumescence monitoring (NPT), and electroencephalography (EEG), including 24-h ambulatory EEG. PSG is a complete, nocturnal, laboratory-based monitoring, which simultaneously records numerous variables during sleep. It includes sleep staging (EEG), elcctro-oculogram (EOG), submental electromyogram (EMG), nasal or oral airflow, respiratory effort, oximetry, electrocardiogram (ECG), anterior tibialis EMG, and position monitoring. Depending upon the clinical diagnosis, additional parameters may be added: transcutaneous CO2 monitoring or end-tidal gas analysis; extremity muscle activity; motor activity movement; extended video-EEG; penile tumescence; esophageal pressure; gastroesophageal reflux; snoring; and continuous blood pressure recording.15-17
Modified forms of PSG include daytime nap PSG, splitnight studies, and portable recording studies.18-21 Daytime PSG is reported to have a high negative predictive value (95% when the apnea-hypopnea index AHI] ≥10) for OSAS, but results are inconsistent.18 Split-night studies may save time and money, but it is still controversial whether diagnosis and treatment are adequately established21,22. The American Academy of Sleep Medicine (AASM) has formulated guidelines for the use of PSGs, split-night studies, and portable recordings.15,16,19
The MSLT is used to confirm the diagnosis of narcolepsy; to assess complaints of moderate to severe sleepiness in patients with mild to moderate OSAS, idiopathic hypersomnia, PLMD, some circadian rhythm disorders, and unknown causes of excessive sleepiness; to evaluate the complaint of insomnia when moderate to severe excessive daytime sleepiness is suspected; and to assess response to treatment following therapy for disorders that cause sleepiness when an additional sleep disorder that produces sleepiness is suspected:23,24 The MWT is used less commonly than the MSLT mainly to assess improved alertness following therapeutic interventions.23-25
Actigraphy uses a small portable device that senses physical motion and stores the resulting information. Actigraphic studies need to be conducted for a minimum of three consecutive 24-h periods.26,27 The AASM Standards of Practice Committee recently updated practice parameters which state that actigraphy is not indicated for the routine diagnosis, assessment of severity, or management of any of the sleep disorders.28 However, it may be a useful adjunct that provides objective demonstration of multiday rest/activity patterns, which can be used to assist in the diagnosis, treatment, and/or assessment of treatment effects in various sleep disorders, including insomnia, circadian rhythm disorders, RLS/PLMD, and disorders of excessive sleepiness.26
Video-PSG may be helpful in the diagnosis of patients with arousal disorders or other sleep disruptions that are believed to be seizure-related.15,16 NPT for sleep-related erections (SRE) is an adjunct in the diagnosis of impotence.29 It usually requires 2 nights of PSG, although 1 night is sufficient if SRE is normal. Twenty-four hours of EEG is monitored for patients with suspected epilepsy.
Description of common sleep disorders
It is beyond the scope of this review to describe the entire gamut of sleep disorders. We will focus on the following common or severe sleep disorders: insomnia, circadian rhythm disorders, disorders of excessive somnolence (sleep apnea, narcolepsy, RLS/PLMD), and parasomnias.
Insomnia
Insomnia refers to almost nightly complaints of insufficient amounts of sleep or not feeling rested after the habitual sleep episode. As the most common sleepwake-related disorder, it is more common in women and has a prevalence ranging from 10% to 30%.23 It can be classified based on severity (mild, moderate, severe) or duration (acute, subacute, chronic).4 Transient insomnia can occur in adjustment sleep disorders triggered by acute stress, travel, or sleeping in an unfamiliar environment.7 Symptoms usually resolve once the stress is reduced or removed, or the individual's adaptation to the stressor increases. For transient insomnia, treatment consists of education and advice about healthy sleep practices. If these are insufficient, short-term treatment with hypnotics can be undertaken.
Chronic insomnia may be primary, or secondary to circadian rhythm, environmental, behavioral, medical, neurological, and psychiatric disorders. Vgontzas et al and Rodenback and Hajak reported nyctohemeral activation of the hypothalamic-pituitary-adrenal axis (HPA) in patients with chronic insomnia consistent with the arousal theory of insomnia.30,31 Vgontzas et al demonstrated a shift in interleukin-6 (IL-6) and tumor necrosis factor (TNF) secretion from nighttime to daytime in chronic insomniacs, and postulated that these could explain the daytime fatigue and performance decrements associated with insomnia.32,33
The diagnosis of primary insomnia requires exclusion of the direct physiological effects of a substance or general medical condition. It does not occur exclusively during the course of a mental disorder or other sleep disorder. Among the primary insomnias, idiopathic insomnia represents a lifelong sleep disturbance associated with reduction in daytime alertness and performance, increased sleep latency, and decreased sleep efficiency on PSG.4 Other primary insomnias include psychophysiological insomnia and sleep-state misperccption. Psychophysiological insomnia refers to maladaptive sleep-preventing behaviors, which perpetuate the sleep disturbance. Typically, these patients sleep better in any place other than their own bedroom. PSG shows increased sleep latency, increased number of awakenings, and poor sleep efficiency. Sleep-state misperccption refers to complaints of sleep difficulties with no PSG evidence of significant sleep disturbance; the sleep latency, quality, and architecture arc normal. Inadequate sleep hygiene and behavioral disorders can also produce chronic insomnia.4 Limit-setting disorder occurs in 5% to 10% of children and is characterized by refusal to go to sleep when asked to do so and delaying bedtime; the PSG is normal.4,7
Secondary insomnia can result from medical, neurological, environmental, drugs, or psychiatric causes. Medical causes include pain, thyroid disease, acid reflux, coronary artery disease, pulmonary disease (chronic obstructive pulmonary disease, asthma, sleep apnea, central alveolar hypoventilation syndrome), chronic renal insufficiency, eating disorders, thyroid dysfunction, fibromyalgia, menstrual-associated sleep disorder, and pregnancy.34-36
Neurological causes of insomnia include headaches, Parkinson's disease, and sleep-related movement disorders (nocturnal myoclonus, RLS). Environmental sleep disorders can be triggered by excessive noise, noxious odors, bright light, or extremes of ambient temperature. Alcohol-, hypnotic-, and stimulant-dependent sleep disorders also contribute to chronic insomnia.
Psychiatric disorders are characterized by sleep-onset difficulties, frequent arousals, sleep fragmentation, shortened total sleep time, and decreased sleep efficiency. These disorders include alcoholism, anxiety disorders, mood disorders, panic disorders, and psychoses. Preliminary data indicate that chronic insomnia may precede depressive episodes by several years, and the question of systematic treatment of chronic insomnia as a means of avoiding depression is being studied. Stressful life events can precipitate chronic insomnia in predisposed individuals with neurotic depression, rumination, chronic anxiety, inhibition of emotions, and inability to express anger.36 PSG in anxiety disorders shows increased sleep latency, decreased rapid eye movement (REM) sleep, and reduced sleep efficiency, while PSG in mood disorders demonstrates frequent arousals and awakenings, decreased slow-wave sleep (SWS), decreased REM latency, increased first REM period duration, and increased REM density.34 Insomnia assessment tools can utilize self-reporting methods (sleep diary and Pittsburgh Sleep Quality Index) and objective methods include actigraphy and PSG.26,37
Treatment for insomnia can be categorized into pharmacological and nonpharmacological treatments. Pharmacological strategies must achieve a balance between hypnotic and adverse effects. Hypnotics are indicated in psychophysiological insomnia for occasional intermittent use or short-term (2 weeks) administration. Benzodiazepine usage can result in impaired sleep quality, residual sedation, memory or functional impairment the day following drug administration, or rebound insomnia. Other problems may include increased rates of falls, drowsiness, dizziness, cognitive impairment, and automobile accidents.35,38-40 Nonbenzodiazepine hypnotics, type I selective γ-aminobutyric acid (GABA) receptor agents, such as Zolpidem (ti/2=2.4 h), zopiclone (tia=5 h), and zaleplon (ti/2=l h), have hypnoscdative action similar to the benzodiazepines and interact preferentially with δ1 receptors.41 Nonbenzodiazepines preserve psychomotor tasks and memory capacities better than benzodiazepines and do not possess respiratory depressive side effects.35 Petit and colleagues suggest that pharmacological therapy be limited to 4 weeks.35
Nonpharmacological treatments for chronic insomnia include stimulus control therapy, sleep restriction, sleep hygiene education, cognitive therapy, paradoxical intention, relaxation therapy and multicomponent therapy.34,35,42-48 Stimulus control therapy is based on the premise that insomnia is a conditioned response to temporal (bedtime) and environmental (bed/bedroom cues) that are typically associated with sleep.34 Interventions result in reduction of sleep-onset latency (SOL) and wake after sleep onset ( WASO) to 30 min or less, with total sleep time increased by 30 to 40 min.
Sleep restriction creates a mild state of sleep deprivation, decreases sleep latency, and promotes more efficient sleep, with less intcrnight variability.34 Interventions curtail the amount of time spent in bed to match sleep efficiency as determined through sleep diaries or actigraphy, with a caveat of a minimum of 5 h in bed. Adjustments are made weekly until optimal sleep duration is achieved. Sleep hygiene education promotes better sleep through awareness of environmental factors (light, noise, temperature, and mattress) and health practices (diet, exercise, and substance use) that may be beneficial or detrimental to sleep. Poor sleep hygiene complicates insomnia and hinders progress in therapy. Guilleminault et al reported statistically significant improvement at the end of 4 weeks in insomnia patients treated with sleep hygiene and light treatment.48
Cognitive therapy identifies patient-specific dysfunctional sleep cognition, challenges their validity, and replaces them with more adaptive substitutes using attention shifting, decatastrophizing, reappraisal, reattribution testing, and hypotheses testing.34,37,42,44,46
Paradoxical intention is a form of cognitive restructuring to alleviate performance anxiety and is based on the premise that performance anxiety hinders sleep onset.34 It is a method that consists of persuading a patient to engage in his most feared behavior, ie, staying awake.
Relaxation treatments include progressive muscle relaxation (PMR), imagery training, meditation, and biofeedback. Meta-analyses of PMR trials have demonstrated reduced SOI . and WASO by an average of 20 to 30 min from baseline to posttreatment with equivalent increases in total sleep time in addition to enhanced perception of sleep quality.34,35 Studies on imagery training have yielded variable results.34,35 Three studies on meditation demonstrated significant improvements in SOL or WASO.34 Biofeedback training reduced SOL with improvement rates similar to those obtained with standard relaxation procedures.35 Various nonpharmacological treatments may be combined as multicomponent therapy. Table II lists a combination of interventions derived from stimulus control therapy, sleep restriction therapy, sleep hygiene, and light therapy.
Table II. Multicomponent therapy instructions.
| Reduce and limit intake of caffeine, tobacco, and other stimulants to the earlier part of the day Discontinue nicotine and cafeine at least 4 to 6 h before bedtime |
| Avoid alcohol as a steeping aid |
| Regularize sleep-wake schedule, meal times, and exercise time |
| Exercise daily, but not closer than 3 h before bedtime |
| If racing thoughts predominate during bedtime, set aside 15- to 20-min “worry time” earlier during the day Use this to think about or list worries, problems, concerns, etc |
| Avoid work-related or strenuous activities dose to bedtime |
| Engage in relaxing, pleasant activities 1 to 2 h before bedtime to “'wind down” from the stresses of the day Focus on positive thoughts at bedtime |
| Minimize noise, light, and excessive temperature during sleep. If needed, use earplugs, eye shades, or an electric blanket/air conditioner |
| Go to bed only when sleepy |
| If sleep restriction is chosen as a treatment option, determine average estimated sleep time. Restrict the time in bed to the average estimated sleep time and continue with the weekly sleep diary. Using the sleep diary, determine the sleep efficiency (total sleep time/time m bed x100%) each week. Increase time in bed by 15 to 20 mm when sleep efficiency >90% %. Decrease time in bed by 15 to 20 min when sleep efficiency <80% Maintain time in bed if sleep efficiency is 80% to 90%. Adjust the time in bed each week until the ideal sleep duration is obtained. The minimum time in bed is 5 h per night. |
| Use the bedroom only for sleep and sex. Do not read or watch TV in bed |
| Get out of bed and go into another room when unable to fall asleep or return to sleep within 15 to 20 min. When out of bed, engage in relaxing and pleasant activities in a dimly lit room. Return to bed only when sleepy again. If still unable to sleep, repeat the same instructions |
| Maintain a regular arising time m the morning regardless of how much or how little sleep you got during the prior nights. |
| Expose yourself to outdoor light for 30 mm within Î5 mm of arising |
| Avoid daytime napping unless there are safety issues If so, take a short early afternoon nap (less than 1 h) |
Circadian rhythm disorders
Delayed and advanced sleep phase disorders
Disorders of circadian sleep-wake rhythms can present with complaints of chronic insomnia as well as excessive daytime somnolence:4-7-49-54 Delayed sleep phase syndrome sufferers report inability to fall asleep until the early morning hours and difficulty arising until late morning or early afternoon; sleep is normal after onset. PSG shows delayed sleep latency if the sufferer sleeps at the desired bedtime rather than the usual bedtime. In contrast, advanced sleep phase syndrome sufferers complain of severe inability to delay their bedtime (usually between 6 pm to 9 pm) and subsequent awakening earlier than desired (often between 1 am to 3 am).4,7,49,54 PSG performed at the person's desired bedtime reveals shortened sleep latency and early morning awakening.
Patients with delayed and advanced sleep phase insomnia can be treated with proper timing of bright light and behavioral changes.4,7,49 The goal of light therapy is to entrain the endogenous sleep-wake rhythm to coincide with the patient's social and occupational schedule. Melatonin administration can be utilized to entrain freerunning circadian rhythms and may be helpful in blind subjects.51
For delayed sleep phase syndrome patients, Dahl utilizes chronotherapy with cognitive behavioral therapy to advance the sleep phase, employing successive 3-h delays in bedtime for 6 days:47 To minimize school or work disruption, he prefers to start on a 'Ihursday (Table III).
Table III. Chronotherapy instructions to advance sleep phase.47 .
| Patient takes responsibility for the success of the treatment and reorganizes habits and associations to improve sleep hygiene |
| Cognitive behavioral therapy focuses on positive thoughts at bedtime |
| For adolescents, behavioral contract with parents/guardian specifies rewards and consequences |
| One-week induction phase Stay up the whole night on Wednesday Bedtime schedules are as follows. Thursday 6 AM to 3 AM, Friday 9 AM to 5 AM, Saturday 12 noon to 8 AM, Sunday 3 AM to 12 midnight, Monday 6 AM to 2 AM, Tuesday and thereafter 9 AM to,6 AM. Stay in bed between 8 5 and 9 h |
| Maintenance phase Adhere to the schedule rigidly for at least 1 month After this, allow minor changes during the weekend (wake-up time is still within 2 h of school or work time) Only permit one late night on the weekend, but impose strict wakeup within 2 h of school or work time. No napping is allowed |
To phase delay the circadian clock for advanced sleep phase syndrome patients, combine bright light exposure (10 000 lux for 30-45 min) between 7 and 9 pm together with a 15-min delay in bedtime every few days.7 Once the desired schedule is achieved for either phase delay or phase advance, it is crucial to lock in the wake-up time to maintain a stable sleep-wake rhythm. The benefit of light therapy is dependent upon the magnitude of light intensity and exposure time. Hither natural outdoors light or a light box (10 000 lux) or light visor (3000-5000 lux) can be utilized, with minimum exposure of at least 30 min.
Shift work sleep disorder
Shift work sleep disorder sufferers complain of difficulty initiating or maintaining sleep or poor quality sleep or excessive sleepiness that is temporally related to a work period that occurs during the habitual sleep phase.4,7,55
These patients are chronically fatigued and have an increased incidence of accidents at work. Shift workers have a higher incidence of chronic depression, emotional problems, family life dysfunction, excessive drug and alcohol use, ulcers, and myocardial infarction compared to the general population. Disturbances in circadian rhythms with internal desynchrony secondary to work shift time changes or sleep loss are postulated to cause this disorder. PSG shows increased sleep latency, numerous arousals during sleep, and early awakening, as well as sleep efficiency below 85%.4,7
A twofold approach to shift work problems involves treatment directed individually toward the patient, in addition to attempts to encourage the workplace (through occupational medicine and workers compensation programs) to adapt to the worker's needs and reduce the overall incidence of shift work-related sleep disorders.55-60 Treatment recommendations include the following: maintain a regular sleep and meal schedule; take naps to limit sleep loss; and practice good sleep hygiene. If sleep is necessary during daylight hours, optimize sleep by darkening the room and screening for noise and interruptions. Light environment is important - exposure to bright light during the first portion of the shift and protection from bright light after work (sunglasses) and before sleep may be beneficial. Short-halflife hypnotics can be used by those who only occasionally work shifts to help initiate sleep; chronic hypnotic use by long-term shift workers is not encouraged.7,55
Disorders of excessive somnolence
Sleep apnea, hypopnea, and upper airway resistance syndrome
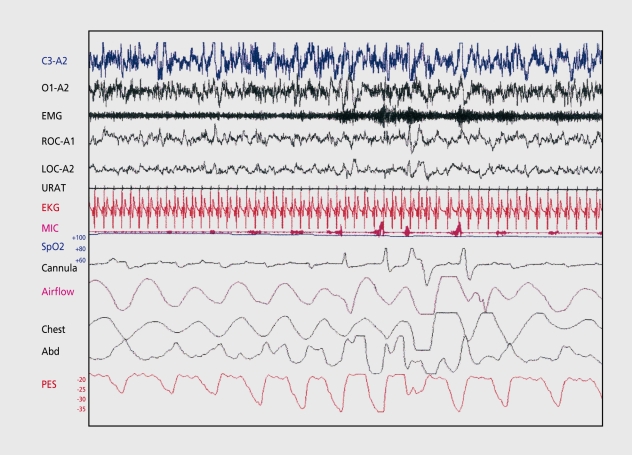
Apnea is defined as cessation in airflow for longer than 10 s. Hypopnea refers to an abnormal respiratory event lasting longer than 10 s associated with at least a 30% reduction in thoracoabdominal movement or airflow compared to baseline, associated with ≥4% oxygen desaturation.61 Figure 1 demonstrates hypopneas seen during PSG monitoring of a patient with sleep apnea. Apneas and hypopneas are combined to form the AHI (ratio of total apneas and hypopneas to the total sleep time in hours), also known as respiratory disturbance index (RDI). An AH1>5 in an adult is abnormal. Apneas and hypopneas can result from upper airway obstruction (obstructive), loss of ventilatory effort (central), or a mixture of both (mixed). OSAS is characterized by repetitive episodes of upper airway obstruction that occur during sleep, usually associated with oxygen desaturation.4 The clinical features of OSAS are listed in Table IV. Some patients have increased upper airway resistance without observed apneas or hypopneas and exhibit increased respiratory effort with Pes (esophageal pressure) crescendos and Pes reversals. Guilleminault et al described the upper airway resistance syndrome (UARS) in patients who had Pes-documented increased respiratory effort associated with increased arousals and daytime sleepiness.62-64
Table IV. Clinical features of obstructive sleep apnea syndrome.
| Ordinal symptoms | Other symptoms | Physical examination findings |
| - Excessive sleepiness or insomnia | - Nonrestorative sleep | - increased body mass index (BMI) |
| - Frequent episodes of obstructed breathing during sleep | - Gasping or choking at night | - Nasal obstruction |
| - Gastroesophageal reflux | - High-arched hard palate | |
| - Loud snoring | - Sexual dysfunction with decreased libido and impotence | - Low hanging soft palate |
| - Morning headaches | - Large or long uvula | |
| - Dryness of the mouth or sore throat on awakening | - Cognitive deficits with memory and intellectual impairment | - Crowded and small oropharynx with or without enlarged tonsils and adenoids |
| - Decreased vigilance | - Malocciusion of the jaw with overjet | |
| - Mood changes with either depression or anxiety or anxiety | - Micrognathia, retrognathia, mandibular hypoplasia | |
| - Macroglossia, scallopmg of the tongue | ||
| - Neck circumference >44 cm in men | ||
| - Brachycephalic head shape |
Figure 1. Hypopnea in a patient with obstructive sleep apnea syndrome. Note the low amplitude signals seen in the nasal cannula and airflow channels with increasing effort demonstrated on the chest and abdominal (Abd) channels. The Pes (esophageal pressure [PES]) channel shows crescendo increases in esophageal pressure with reversal.
Sleep-disordered breathing (OSAS and UARS) in children peaks between ages 2 to 5 with a second peak in middle to late adolescence. Continuous snoring, failure to thrive, mouth breathing, enlarged tonsils and adenoids, and predominance of hypopneas rather than apneas are common features in childhood OSAS.62,65-68 Children with sleep-disordered breathing have a threefold increase in behavioral and neurocognitive abnormalities. It has been estimated that 5% to 39% of attention-deficit/hypcractivity disorder (ADHD) could be attributed to sleep-disordered breathing.65-69
In OSAS, the PSG demonstrates more than five obstructive apneas per hour of sleep and one or more of the following: frequent arousals associated with the apneas; bradytachycardia; and arterial oxygen desaturation in association with the apneas. Sleep architecture in OSAS and UARS patients is abnormal with fragmented sleep (mainly during non-rapid eye movement [NREM] stages 1 and 11) and frequent arousals and awakenings. The amount of SWS (NREM stages III and IV) and REM sleep is decreased.4,7 MSLT performed the day after the PSG may or may not demonstrate sleepiness (ie, mean sleep latency <10 min).
Treatment for OSAS consists of nonsurgical as well as surgical treatments. Nonsurgical treatment encompasses general/behavioral measures, such as weight loss, body position during sleep (avoid supine position), and mechanical measures, which include continuous positive airway pressure (CPAP) or bilcvel positive airway pressure (BIPAP) and oral appliances.
A consensus statement by Loube and colleagues recommended CPAP treatment for all OSAS patients with RDI>30 regardless of symptoms and for patients with RDI=5 or 30 events per hour if accompanied by symptoms of excessive daytime somnolence, impaired cognition, mood disorders, insomnia, or documented cardiovascular diseases (ischemic heart disease, hypertension), or stroke.70 Improvement or elimination of apneas improves sleep architecture and reduces daytime sleepiness.71,72 Beneficial effects of CPAP or surgery reported in patients with frequent sleep apneas (>20) and patients with sleep-disordered breathing (RD1<20) without subjective pathological sleepiness include improvement in well-being, mood, functional status, breathing, oxygen saturation, and cardiac rhythm.71-76 CPAP has also been successfully utilized to treat OSAS in infants and children younger than 2 years of age.77,78 However, compliance with CPAP is problematic, with published rates ranging from 65% to 95% when assessed subjectively.79,87 Strollo and colleagues have recommended management strategies for common side effects of nasal CPAP.80
Autotitrating continuous positive airway pressure (APAP) can be used to treat many patients with OSAS or to identify an effective optimal fixed level of CPAP for treatment, but is not recommended for patients with congestive heart failure, chronic obstructive pulmonary disease, daytime hypoxemia and respiratory failure from any cause, or prominent nocturnal desaturation other than from OSAS.88,89 Indications for BIPAP include intolerance of CPAP, development of central apneas resulting in sleep fragmentation, mask leaks at a high CPAP pressure, and persistent alveolar hypoventilation.80 Patients with OSAS on CPAP or BIPAP should be reevaluated at regular intervals to assess compliance, address problems, and reinforce the importance of continued treatment.
Surgery is indicated for OSAS patients who have an underlying specific surgically correctable abnormality that is causing sleep apnea and may be indicated in patients who are not candidates for or have failed other noninvasive treatments, desire surgery, and are medically stable.90 Identification of the site(s) of obstruction is necessary in choosing the appropriate surgical intervention. Methods of localizing the site of obstruction include endoscopy, pressure catheters, fluoroscopy, computed tomography (CT) scan, or magnetic resonance imaging (MRI).91 Surgical procedures can be divided into phase I and phase II surgical procedures.92-96 Phase I involves palatal and lingual surgery: tonsillectomy, uvulopalatopharyngoplasty (LJPPP),uvulopalatal flap (UPF), modified UPPP, palatal advancement, genioglossus advancement, hyoid suspension, laser midline glossectomy, lingualoplasty, and radiofrequency of the soft palate and tongue base. Phase II procedures either advance the jaws (maxillomandibular osteotomy) or widen the jaws using distraction procedures.
Central sleep apnea is characterized by either shallow or absent breathing during sleep associated with one of the following features: gasping, grunting, choking movements, frequent body movement, and cyanosis. The PSG shows central apneic pauses >10 s (20 s in infancy) in duration, with one or more of the following: bradytachycardia; frequent arousals from sleep; or oxygen desaturation associated with apneas4. MSLT may or may not demonstrate a mean sleep latency <10 min. Treatment of central sleep apnea involves treatment of comorbid medical conditions (congestive heart failure, nasal congestion, OSAS), consideration of supplemental oxygen (1-2 L/min via nasal cannula), or use of acetazolamide (125-250 mg, two to three times per day).7 Patients with central apneas before and after an arousal, without evidence of desaturation, may benefit from a trial of a hypnotic agent (Zolpidem, 5-10 mg at night).7
RLS and PLMD
RLS has a prevalence of 10% to 15% among patients between the ages of 27 to 41 years.97 It consists of unpleasant creeping or crawling sensations inside the calves and generalized aches and pains in the legs associated with a desire to move the extremities, motor restlessness, worsening of symptoms at rest with at least temporary relief by activity, nocturnal worsening of symptoms (circadian pattern), and difficulty initiating sleep in the absence of any medical, mental, or other sleep disorder that would account for the symptoms.97-99
RLS can be idiopathic or secondary to iron deficiency, peripheral neuropathies, or uremia. There are two recognized phenotypes in the idiopathic category: earlyonset RLS starts before age 45 and progresses slowly, demonstrating autosomal dominant inheritance, while late-onset RLS starts after age 45 and progresses rapidly, with limited familial aggregation.101 Increased cerebrospinal fluid (CSF) hypocrctin-1 levels are present in early-onset RLS patients, whereas levels in late-onset RLS patients are normal. Allen et al postulate that increased hypocretin levels may modulate or promote insomnia and increase motor activity101
RLS involves various areas in the nervous system from the spinal cord up to the basal ganglia.98,101,102 Using single photo emission computed tomography (SPECT) and positron emission tomography (PET), various researchers have demonstrated a decrease in dopamine D2 receptor binding in the striatum of RI-S patients, suggesting that RLS is related to a deficiency of dopaminergic function.97,100,102-104 Iron deficiency accompanying RLS may be associated with hypofunction of the D2 receptor.97,100
More than 80% of RLS patients manifest periodic limb movements (PLMs) during sleep.100 PLMs consist of four or more repetitive episodes of muscle contraction (0.55 s in duration) separated by an interval (≥5 s but <90 s), which may be associated with an arousal. A PI M index (events/hour) >5 is abnormal. Tricyclic antidepressants, lithium, and selective serotonin reuptake inhibitors (SSRIs) can increase PLMs.
Saletu and colleagues performed EEG mapping in RLS patients and demonstrated an increase in both absolute δ and absolute and relative α2 power, a decrease in absolute and relative α1 power, an acceleration of the dominant frequency and the a centroid, and a slowing of the δ/θ centroid, as well as a nonsignificant attenuation in total power.105 These findings arc characteristic of dissociated vigilance changes described in depression and correlated with higher depression and anxiety scores, lower quality of life, and deteriorated sleep quality despite normal Epworth Sleepiness scale scores.
Treatment options for RLS include dopaminergic agents (pramipexole, ropinirole, pergolide, levodopa/carbidopa), opioids (oxycodone, propoxyphene), benzodiazepines (clonazepam), anticonvulsants (gabapentin, carbamazepine), and clonidine.97,102,106-117 Patients with low serum ferritin levels may benefit from iron therapy.
Treatment with dopaminergic agents is complicated by rebound (worsening of symptoms at the end of the dosing period with late night or morning recurrence of symptoms and PLMs) and augmentation (worsening of symptoms seen with long-term use, particularly with higher doses, presenting with earlier time-of-day onset of symptoms and expansion of symptoms beyond the legs). With levodopa, rebound occurs in 20% of RLS patients, while augmentation affects 82% of patients; augmentation is increased in patients with more severe RLS and in those receiving higher doses.7,106,117 Dopamine agonists are useful in treating patients with RLS.108 Pergolide therapy reduced PLMs and increased total sleep time in 83% of RLS patients, but mild augmentation also occurred. Augmentation may be managed through a combination of behavioral strategies (walking and other physical activities) and medication-timing strategies.7,102
Narcolepsy
Westphal described the first unequivocal case of narcolepsy in 1877, and Gelineau coined the term narcolepsy in 1880. The prevalence of narcolepsy in the United States is 1/2000.3,118 Narcolepsy is a neurological disorder that affects men and women equally, with usual age of onset between 15 and 30 years. It is characterized by the following tetrad of symptoms: excessive daytime somnolence (EDS), which can be a continuous feeling of sleepiness or “sleep attacks,” cataplexy, hypnagogic or hypnapompic hallucinations, and sleep paralysis.4,118-125 Guilleminault et al reported that EDS alone or in combination with sleep paralysis or hypnagogic hallucinations is the initial symptom in 90% of patients and that 5% to 8% of patients present with cataplexy.126,127 Only 10% of patients experience the full tetrad.119
After onset, EDS persists daily, although it can fluctuate during the day in a stereotyped individual pattern. Attention fluctuates modulated by situational circumstances. The attack usually starts with drowsiness associated with blurry or double vision and usually lasts for less than 20 min. Sleepiness is often relieved by a sleep attack, but the relief lasts for only several hours.
Cataplexy involves sudden bilateral atonia of striated muscles with partial or complete weakness that is brought on by emotion or excitement. Laughter is the most typical trigger and, less frequently, anger or surprise. Other triggers include anticipation of something special or hilarious, attempts at bantering, feeling amused, or immobility in response to a call for immediate action. The patient's “state” and circumstances also influence whether an attack occurs: sleep deprivation or strong feeling of sleepiness can lower the attack threshold. The attacks start abruptly, but take several seconds to reach their maximum, with most attacks lasting less than a minute. During partial attacks, the knees may give way and there may be sagging of the jaw, inclination of the head, and weakness of the muscles responsible for speech so that the patient is either unable to speak or has slurred speech. Even with severe attacks, eye movements and respiration are spared. Neurological examination during the attack shows atonia, loss of tendon reflexes, and extensor plantar responses. Prolonged episodes may be associated with hallucinations and rarely, “status cataplexies.” Video-polygraphic analysis of cataplectic attacks demonstrate three phases: (i) initial phase, consisting of arrest of eye movements and phasic, massive, inhibitory muscular events; (ii) falling phase, characterized by a rhythmic pattern of suppressions and enhancements of muscular activity leading to the fall; and (iii) atonic phase, associated with complete muscle atonia.128
Injuiry is uncommon because most people are able to find support or sit down at the onset of the attack. A consistent individual pattern is seen. Attacks vary in frequency from more than 10 per day to less than 1 per month.
Hypnagogic hallucinations (at sleep onset) or hypnapompic hallucinations (on waking) represent vivid dreamlike experiences of visual imagery (constant or changing colored forms), auditory hallucinations, or tactile sensations. Smell and taste are rarely affected. Some patients describe out-of-body experiences at sleep onset. Attacks usually last less than 10 min, and the frequency varies from less than once a month to more than once a day.
Sleep paralysis represents inability to move either at sleep onset or upon awakening; the episode can last up to 10 min. Patients can be frightened because they are unable to open their eyes or move their fingers and feel they have to struggle to move.
Disturbed nocturnal sleep is the fifth component of the “tetrad” and is due to frequent awakenings. Although patients typically have short SOL, they may have trouble returning to sleep once awakened. Other reported symptoms include automatic behavior (episodes of amnesia associated with semipurposeful activity), subjective memory impairment that is not validated during standard memory testing, tiredness or fatigue, blurry or double vision, and sexual dysfunction (which may be related to drug therapy).124
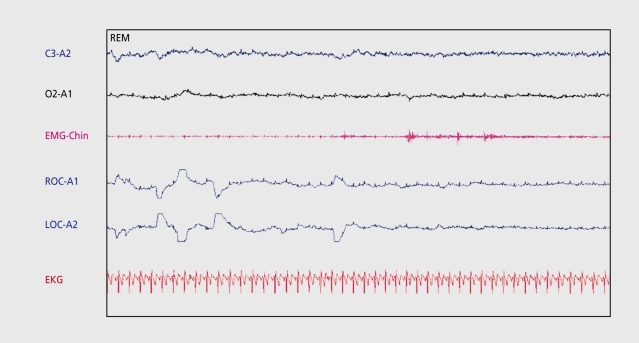
The PSG demonstrates SOL less than 10 min and REM sleep latency less than 20 min.4 An MSLT demonstrates a mean sleep latency of less than 5 min with two or more sleep-onset REM (SOREM) episodes.4 Figure 2 depicts SOREM during an MSLT nap.
Figure 2. Sleep-onset rapid eye movement (REM) during an mean sleep latency test (MSLT) nap in a patient with narcolepsy. Electroencephalogram (EEG) leads (C3-A2 and 02-A1) demonstrate low voltage mixed frequency theta activity. EMG-Chin shows atonia with phasic events. Electrooculography (EOG) demonstrates REM.
HLA typing demonstrates an increased frequency of DQB1 *0602 or DR2 in patients with narcolepsy, especially with cataplexy. Low CSF levels of hypocretin-1 are highly associated with narcolepsy with cataplexy (89.5%), particularly in patients with cataplexy who are HLA DQBl*0602-positive (95.7%).129-132
Stimulant medications are the mainstay of treatment of EDS, with the objective of allowing the fullest possible return of normal function for patients at work, home, and school.118,122,123,125,133-135 The most common stimulants used, listed in incrementing order of relative efficacy are: pemoline, modafinil, dextroamphetamine, mcthamphetamine, and methylphenidate.133,135 The maximum recommended daily dosages of stimulants in adults are: dextroamphetamine sulfate, 100 mg; methamphetamine hydrochloride, 80 mg; and methylphenidate, 100 mg.133 Pemoline was utilized in the past, but is not currently recommended due to concerns about the risk of acute hepatic failure.7 Apart from modafinil, all stimulants are centrally acting sympathomimetic agents that enhance the release of monoamines in the synaptic cleft and block their reuptake.133,134 Modafinil is a novel stimulant with an uncertain mechanism of action that may increase dopamine signaling.136 For newly diagnosed narcoleptics, modafinil may represent a reasonable initial choice because of its long duration of action, low frequency and severity of side effects, and low potential for dependence or tolerance. However, patients should be cautioned about drug interference with other medications, such as oral contraceptives. There are no well-controlled studies of pregnant women using stimulants. The benefits for the patient have to be weighed against the potential risks for the fetus. Mitlcr and colleagues recommend dosage reduction or discontinuation of stimulants during attempts at conception and during pregnancy.133
REM -suppressant drugs are utilized in the treatment of cataplexy, hypnagogic hallucinations, and sleep paralysis. Drugs that block norepinephrine reuptake, such as the tricyclic antidepressants, protriptyline, clomipramine, and imipramine, have been effective, but are frequently associated with tolerance and anticholinergic side effects. Tricyclics should not be discontinued abruptly because of the risk of severe aggravation of cataplexy, including status cataplecticus.136 SSRIs such as fluoxetine, paroxetine, and citalopram are also effective. Vcnlafaxine, a norepinephrine/serotonin reuptake inhibitor, is highly effective and well tolerated.
γ-Hydroxybutyrate (GHB),a short-acting putative neurotransmitter that acts as a hypnotic, reduces cataplexy, hypnagogic hallucinations, and subjective sleepiness. Three to nine grams of GHB is administered in bed with half of the dose at bedtime and the remainder 2.5 to 3 h later. Nausea, dizziness, and incontinence have been reported with high doses. Due to the risk of precipitating confusional arousals and even coma, doses >9 g should never be prescribed. Triazolam may be useful in treating insomnia in narcoleptics by increasing total sleep time and sleep efficiency without affecting alertness the following day.137
Nonpharmacological therapy includes regular sleep and wake times, short scheduled naps, prevention of sleep deprivation, avoidance of shift work, and working in a stimulating environment. Narcoleptic patients need to be cautioned about driving risks when undertreated.
Idiopathic hypersomnia
Idiopathic hypersomnia is a clinically heterogeneous disorder of chronic sleepiness without cataplexy that has a prevalence of 2 to 5/100 000.138,139 Symptoms present between ages 15 to 30 years and include variable daytime drowsiness (nonimperative versus irresistible), naps that range from short and refreshing to long and unrefreshing, prolonged nighttime sleep >12 h or restless sleep with frequent arousals, sleep “drunkenness,” and automatic behavior associated with blank stares and microsleep episodes.4,138,139
Three subgroups of patients are recognized. Subgroup 1 consists of patients with HLA Cw2 antigen and a positive family history of EDS associated with autonomic dysfunction (syncope, orthostatic hypotension, Raynaudtypc phenomena). Subgroup 2 consists of individuals who had a viral illness (Guillain-Barre, mononucleosis, hepatitis, atypical viral pneumonia) followed by persistent EDS. Subgroup 3 includes patients with no family history or viral infection prior to onset of EDS. The PSG demonstrates a combination of normal or long nocturnal sleep, and the MSLT performed the day after the PSG shows short SOL without sleep-onset REM periods.139 Pharmacological treatment involves use of stimulants, starting with either modafinil or methylphenidate and switching to dexedrine spansules if initial treatment is ineffective. Nonpharmacological treatment includes one scheduled daily nap (noon or late afternoon) no longer than 45 min; avoidance of alcohol, sleep deprivation, heavy meals and shift work; and observance of regular sleep (at least 8.5 h per night) and wake schedules.
Parasomnias
Parasomnias are characterized by undesirable physical phenomena or behaviors that occur predominantly during sleep. Skeletal muscle activity and autonomic nervous system changes are prominent. Parasomnias are composed of disorders of arousal, partial arousal, and sleepstage transition (Table I).
Disorders of arousal are the most common form of parasomnia.They usually occur during SWS (NREM stages III and IV), and symptoms typically present in the first third of the night. Studies of twin cohorts and families with sleep terror and sleepwalking suggest that genetic factors may be involved, and there may be a family history of the same or other NREM arousal parasomnia.140-142 Factors that increase SWS, such as young age, natural deep sleeper, recoverd from sleep deprivation, central nervous system (CNS) depressant medications (sedatives, hypnotics, alcohol), fever, and the hypersomniac period in Klcine-Levin syndrome, may aggravate the arousal disorder. Factors that lead to sleep fragmentation, including stress, environmental stimuli, endogenous stimuli, pain, pregnancy, stimulants, thyroxine taken in the evening, migraine headaches, or Tourcttc's syndrome, may trigger the parasomnia.
Confusional arousals (nocturnal sleep drunkenness)
This disorder is more common in children younger than 5 years of age, becomes less frequent during adolescence, and is rare in adulthood. The patient partially awakens from a deep sleep during the first third of the night, is confused and slow in mentation, disoriented to time and space, poorly/partially responsive to external stimuli, manifests automatic behavior (picking at bedclothes), and moans and mumbles incomprehensibly. Attacks last from 30 s to 10 min, and the patient is amnesic for the behavior and for any dream-like or thought-like mentation. PSG shows movement arousal in SWS followed by decreased amplitude of the EEG and the appearance during the period of mental confusion of either NREM stage I or a diffuse a that is slower by 1 to 2 Hz compared to that of wakefulness. Confusional arousals in children do not necessarily warrant treatment. In adults who exhibit aggression towards others or self-injury, room safety precautions need to be implemented and conditions facilitating or triggering attacks need to be avoided. The attacks should be allowed to terminate spontaneously. Benzodiazepines or tricyclic medications may be useful as short-term therapy for a few days or weeks during periods when attacks are more common.
Sleep terrors
The peak prevalence of sleep terrors is between 5 and 7 years of age. By age 8, half of the children are attack-free, while 36% continue to have attacks until adolescence. Episodes of sleep terror occur during the first third of the night and also during daytime naps. The child sits up, emits a piercing scream, and appears frightened, with increased pulse and respiratory rates and profuse sweating. The episodes last from 30 s to 5 min, and the child is amnesic for the events during the episode. PSG shows explosive arousal with marked increases in muscle tone, heart rate, and respiratory rate, and a rapid decrease in skin resistance. Facilitating and precipitating factors need to be avoided. Treatment may include either a short-acting benzodiazepine, such as midazolam (10-20 mg), oxazepam (1020 mg), or clonazepam (0.5-2 mg). Patients unresponsive to benzodiazepines may benefit from tricyclic antidepressants such as clomipramine, desipramine, or imipramine (10-50 mg at hour of sleep). If total control of the episodes occurs and is sustained over several months, a slow and progressive withdrawal of medication may be performed.
Sleepwalking (somnambulism)
The patient ambulates during sleep, is difficult to arouse during an episode, and is usually amnesic following the episode. Guilleminault et al indicated that children over the age of 4 reported vague memories of having to act, run away, escape or defend themselves against monsters, animals, snakes, spiders, ants, intruders, or other threats, and that they felt completely isolated and fearful.143,144
Episodes usually occur in the first third of the night during SWS.4,143,144 This disorder has a peak age of onset at 5 years of age and peak prevalence at about 12 years. Most children outgrow the episodes by age 15. PSG recordings demonstrate 2 abnormalities during the first sleep cycle: frequent, brief, nonbehavioral EEG-defined arousals prior to the somnambulistic episode and abnormally low 8 (0.75-2.0 Hz) EEG power on spectral analysis, correlating with high-voltage “hypersynchronic δ” waves lasting 10 to 15 s occurring just prior to the movement.140,142-145 This is followed by stage I NREM sleep, and there is no evidence of complete awakening.
REM behavior sleep disorder
In REM behavior sleep disorder (RBD), the patient complains of violent or injurious behavior during sleep with disruption of sleep continuity and excessive motor activity during dreaming, accompanied by loss of REM sleep EMG atonia.4,145-151 The frequency of nocturnal events varies from several times a night to once every 3 months. The most common behaviors consist of arm flailing and punching, kicking, and vocalizations; these behaviors occur in bed or result in falling out of bed. About 32% of patients report self-injury ranging from falling out of bed to striking or bumping into the furniture or walls. Olson reported one patient attempted to fire an unloaded gun, while another attempted to set fire to his bed.147 Sixty-four percent of spouses report being assaulted during sleep.147 Dream content in RBD has aggressive themes in about 89% of patients, with the most common one being defense of the sleeper against attack. Although RBD is usually idiopathic, it can occur secondarily on a transient or chronic basis. Acute RBD can result from drug withdrawal (meprobamate, pentazocine, nitrazepam, and butalbital)152 or intoxication (bipcriden, tricyclic antidepressants, monoamine oxidase [MAO] inhibitors, or caffeine).149,153 Chronic RBD can be produced by drugs (tricyclic antidepressants, fluoxetine, venlafaxine, mirtazapine, selegeline, and anticholinergic medications), vascular problems (subarachnoid hemorrhage, vasculitis), tumors (pontine neoplasms, acoustic tumors), infectious/postinfectious diseases (Guillain-Barre), degenerative or demyelinating conditions (amyotrophic lateral sclerosis, fatal familial insomnia, dementia, Parkinson's disease, multiple sclerosis, olivopontocerebellar degeneration, Shy-Drager syndrome, multiple system atrophy), and developmental, congenital, or familial diseases (narcolepsy, Tourctte's syndrome, Group A xeroderma pigmentosum, mitochondrial encephalomyopathy).147,149,153-155 Because of the overwhelming male preponderance (90%), questions of relationships between sexual hormones, aggression, and violence have been raised.148,149 Diffuse lesions of the hemispheres, bilateral thalamic abnormalities, or primary brain stem lesions may result in RBD.150
The PSG shows at least one of the following: excessive augmentation of chin-EMG tone or excessive chin/limb phasic EM'G twitching associated with one or more of the following: excessive limb or body jerking, complex vigorous/violent behaviors, and absence of epileptic activity in association with the disorder. Shirakawa and colleagues performed M'RI and SPECT imaging on 20 patients with RBD and reported decreased blood flow in the upper portions of the frontal lobe and pons.156 Albin and colleagues found decreased striatal dopaminergic innervation in RBD patients.157
Treatment of RBD has been effective in 90% of patients using clonazepam starting at 0.5 mg at bedtime and gradually incrementing the dose until control is effected. Other drugs, such as gabapentin, clonidine, carbamazepine, donezepil, levodopa, and melatonin have been anecdotally reported to be useful.149,158-162
Environmental safety measures are very important. Potentially dangerous objects should be removed from the bedroom, weapons (if any) should be stored and locked away safely outside the bedroom with the key entrusted to another person, the corners around the bed should be padded or cushioned, the mattress may be placed on the floor, and window protection should be considered.
Conclusions
Sleep disorders constitute a ubiquitous group of diseases that have important consequences for individual health as well as economic costs to society. The diagnosis of sleep disorders requires careful history taking, examination , and laboratory testing. Although general guidelines in management for the more common and important sleep disorders have been discussed, treatment needs to be tailored to the individual patient.
Selected abbreviations and acronyms
- AHI
apnea-hypopnea index
- BIPAP
bilevel positive airway pressure
- CPAP
continuous positive airway pressure
- EDS
excessive daytime somnolence
- EMG
electrornyograrn
- EOG
electro-oculogram
- MSLT
mean sleep latency test
- MWT
maintenance of wakefulness test
- NPT
nocturnal penile tumescence
- NREM
non-rapid eye movement
- OSAS
obstructive sleep apnea syndrome
- PLMD
periodic limb movement disorder
- PMR
progressive muscle relaxation
- PSG
polysomnogram
- RBD
REM behavior sleep disorder
- RDI
respiratory disturbance index
- REM
rapid eye movement
- RLS
restless legs syndrome
- SOL
sleep-onset latency
- SWS
slow-wave sleep
- UARS
upper airway resistance syndrome
- WASO
wake after sleep onset
Contributor Information
Vivien C. Abad, Stanford University Sleep Disorders Clinic and Research Center, Stanford University, School of Medicine, Stanford, Calif, USA.
Christian Guilleminault, Stanford University Sleep Disorders Clinic and Research Center, Stanford University, School of Medicine, Stanford, Calif, USA.
REFERENCES
- 1.Understanding Sleep: Brain Basics. Office of Communications and Public Liaison, National Institute of Neurological Disorders and Stroke, Bethesda, Md. Available at: http//www.ninds.nih.gov/index.htm. Accessed 7 August 2003. [Google Scholar]
- 2.Ohayon M., Guilleminault C. Epidemiology of Sleep Disorders In: Chokroverty S, ed. Sleep Disorders Medicine. Woburn, Mass: Butterworth Heinemann. 1999:301–316. [Google Scholar]
- 3.Partinen M., Hublin C. Epidemiology of Sleep Disorders In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. Philadelphia, Pa: WB Saunders. 1979:558–579. [Google Scholar]
- 4.American Academy of Sleep Medicine. The International Classification of Sleep Disorders, revised: Diagnostic and Coding Manual. Rochester, Minn: American Academy of Sleep Medicine. 2000 [Google Scholar]
- 5.Thorpy M. Classification of Sleep Disorders. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 3rd ed. Philadelphia, Pa: WB Saunders. 2000:547–557. [Google Scholar]
- 6.Thorpy M. Classification of Sleep Disorders. In: Chokroverty S, ed. Sleep Disorders Medicine. Woburn, Mass: Butterworth Heinemann. 1999:287–300. [Google Scholar]
- 7.Reite M., Ruddy J., Nagel K. Concise Guide to Evaluation and Management of Sleep Disorders. Washington, DC: American Psychiatric Publishing. 2002:1–273. [Google Scholar]
- 8.Borbely A. Sleep: circadian rhythm vs recovery process. In: Koukou-Lehman M, ed. Functional States of the Brain: their Determinants. Amsterdam: Elsevier/North Holland. 1980:151–161. [Google Scholar]
- 9.Zee P., Harsanyi K. Highlights of sleep neuroscience. In: Bowman T, ed. Review of Sleep Medicine. Burlington, Mass: Butterworth Heinemann. 2003:19–39. [Google Scholar]
- 10.Silber MH. Neurologic treatment sleep disorders. Neurol Clin. 2001;19:173–186. doi: 10.1016/s0733-8619(05)70011-6. [DOI] [PubMed] [Google Scholar]
- 11.Elsenbruch S., Thompson JJ., Hamish MJ., Exton MS., Orr WC. Behavioral and physiological sleep characteristics in women with irritable bowel syndrome. Am J Gastroenterol. 2002;97:2306–2314. doi: 10.1111/j.1572-0241.2002.05984.x. [DOI] [PubMed] [Google Scholar]
- 12.Moldofsky H. Management of sleep disorders in fibromyalgia. Rheum Dis Clin North Am. 2002;28:173–186. doi: 10.1016/s0889-857x(01)00012-6. [DOI] [PubMed] [Google Scholar]
- 13.Chokroverty S. Diagnosis and treatment of sleep disorders caused by comorbid disease. Neurology. 2000;54(5 suppl 1):S8–S15. [PubMed] [Google Scholar]
- 14.Neubauer D. Sleep problems in the elderly. Am Fam Physician. 1999;59:2551–2560. [PubMed] [Google Scholar]
- 15.Chesson, AL Jr, Ferber RA., Fry JM., et al. Practice parameters for the indications for polysomnography and related procedures. Sleep. 1997:406–422. doi: 10.1093/sleep/20.6.423. [DOI] [PubMed] [Google Scholar]
- 16.Chesson AL Jr., Ferber RA., Fry JM., et al. The indications for polysomnography and related procedures. Sleep. 1997;20:423–487. doi: 10.1093/sleep/20.6.423. [DOI] [PubMed] [Google Scholar]
- 17.Keenan S. Polysomnography technique: an overview. In: Bowman, ed. Review of Sleep Medicine. Burlington, Mass: Butterworth Heinemann. 2003:107–132. [Google Scholar]
- 18.Chervin R. Use of clinical tools and tests in sleep medicine. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 3rd ed. Philadelphia, Pa: WB Saunders. 2000:537–543. [Google Scholar]
- 19.Thorpy M., Chesson A., Ferber R., et al. Practice parameters for the use of portable recording in the assessment of obstructive sleep apnea. Sleep. 1994;17:372–377. [PubMed] [Google Scholar]
- 20.Van Kempelma AR., Rutgers SR., Strijers RL. The value of 1-hour daytime sleep recording in the diagnosis of the sleep apnea syndrome. J Sleep Res. 1993;2:257–259. doi: 10.1111/j.1365-2869.1993.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 21.Coleman J. Sleep Studies. Current Techniques and Future Trends. Otolaryngol Clin North Am. 1999;32:195–210. doi: 10.1016/s0030-6665(05)70124-9. [DOI] [PubMed] [Google Scholar]
- 22.Strohl KP. Timing, number and complexities of sleep studies. Sleep Breathing. 1997;2:45–49. doi: 10.1007/BF03038972. [DOI] [PubMed] [Google Scholar]
- 23.Thorpy M. The Clinical Use of the Multiple Sleep Latency Test. The Standards of Practice Committee of the American Sleep Disorders Association. Sleep. 1992;15:268–276. doi: 10.1093/sleep/15.3.268. [DOI] [PubMed] [Google Scholar]
- 24.Mitler M., Carskadon M., Hirshkowitz M. Evaluating sleepiness. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 3rd ed. Philadelphia, Pa: WB Saunders. 2000:1251–1257. [Google Scholar]
- 25.Doghramji K., Mitler MM., Sangal RB., et al. A normative study of the maintenance of wakefulness test (MWT). Electroencephalogr Clin Neurophysiol. 1997;103:554–562. doi: 10.1016/s0013-4694(97)00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Littner M., Kushida C., Anderson W., et al. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26:337–341. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- 27.Thorpy M., Chesson A., Derderian S. Practice parameters for the use of actigraphy in the clinical assessment of sleep disorders. Sleep. 1995;18:285–287. doi: 10.1093/sleep/18.4.285. [DOI] [PubMed] [Google Scholar]
- 28.Littner M., Kushida CA., Anderson WM., et al. practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26:337–341. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- 29.Ware J., Hirshkowitz M. Assessment of sleep-related erections. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 3rd ed. Philadelphia, Pa: WB Saunders. 2000:1231–1237. [Google Scholar]
- 30.Vgontzas AN., Bixler EO., Lin HM., et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86:3787–3794. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 31.Rodenbeck A., Hajak G. Neuroendocrine dysregulation in primary insomnia. Rev Neurol (Paris). 2001;157:S57–S61. [PubMed] [Google Scholar]
- 32.Vgontzas AN., Zoumakis M., Papanicolaou DA., et al. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism. 2002;51:887–892. doi: 10.1053/meta.2002.33357. [DOI] [PubMed] [Google Scholar]
- 33.Vgontzas AN., Chrousos GP. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin. 2002;31:15–36. doi: 10.1016/s0889-8529(01)00005-6. [DOI] [PubMed] [Google Scholar]
- 34.Morin C., Hauri P., Espie C., et al. Non-pharmacologic treatment of chronic insomnia. Sleep. 1999;22:1134–1156. doi: 10.1093/sleep/22.8.1134. [DOI] [PubMed] [Google Scholar]
- 35.Petit L., Azad N., Byszewski A., et al. Non-pharmacological management of primary and secondary insomnia among older people: review of assessment tools and treatments. Age Ageing. 2003;32:19–25. doi: 10.1093/ageing/32.1.19. [DOI] [PubMed] [Google Scholar]
- 36.Kales A., Kales R. Evaluation and Treatment of Insomnia. New York, NY: Oxford Press. 1984:2–320. [Google Scholar]
- 37.Buysee DJ., Reynolds CF 3rd., Monk TH., et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatr Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 38.Holbrook A., Crowther R., Lotter A., et al. Meta-analysis of benzodiazepine use in the treatment of insomnia. Can Med Assoc J. 2000;162:225–233. [PMC free article] [PubMed] [Google Scholar]
- 39.Shorr Rl., Robin DW. Rational use of benzodiazepines in the elderly. Drugs Aging. 1994;4:9–20. doi: 10.2165/00002512-199404010-00002. [DOI] [PubMed] [Google Scholar]
- 40.Vgontzas AN., Kales A., Bixler EO. Benzodiazepine side effects: role of pharmacokinetics and pharmacodynamics. Pharmacology. 1995;51:205–223. doi: 10.1159/000139363. [DOI] [PubMed] [Google Scholar]
- 41.Terzano MG., Rossi M., Palomba V., et al. New drugs for insomnia: comparative tolerability of zopiclone, Zolpidem, and zaleplon. Drug Saf. 2003;26:261–282. doi: 10.2165/00002018-200326040-00004. [DOI] [PubMed] [Google Scholar]
- 42.Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40:869–893. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 43.Johnson LC., Spinweber CL. Quality of sleep and performance in the Navy: a longitudinal study of good and poor sleepers. In: Guilleminault C, Lugaresi E, eds. Sleep/wake Disorders: Natural History, Epidemiology and Long-term: Evolution. New York, NY: Raven Press. 1983 [Google Scholar]
- 44.Backhaus J., Hohagen F., Voderholzer U., Riemann D. Long-term effectiveness of a short-term cognitive-behavioral group treatment for primary insomnia. Eur Arch Psychiatry Clin Neurosci. 2001;251:35–41. doi: 10.1007/s004060170066. [DOI] [PubMed] [Google Scholar]
- 45.Kales JD., Kales A., Bixler EO., et al. Biopsychobehavioral correlates of insomnia, V. Clinical and behavioral correlates. Am J Psychiatry. 1984;141:1371–1376. doi: 10.1176/ajp.141.11.1371. [DOI] [PubMed] [Google Scholar]
- 46.Mamber R., Kuo T. Cognitive-behavioral therapies for insomnia. In: Chiong, Sateia, Carskadon, eds. Sleep Medicine. Philadelphia, Pa: Hanley & Belfus. 2002:177–185. [Google Scholar]
- 47.Dahl R. The Interface between Sleep Disorders and Behavioral/ Emotional Disturbances Cases. Proceedings in the 9th Annual Course in Pediatric Sleep Medicine by the School of Sleep Medicine, Palo Alto, Calif, March 22-26. x. 2003:x. [Google Scholar]
- 48.Guilleminault C., Clerk A., Black J., et al. Nondrug treatment trials in psychophysiologic insomnia. Arch Intern Med. 1995;155:838–844. [PubMed] [Google Scholar]
- 49.Baker S., Zee P. Circadian disorders of the sleep-wake cycle. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 3rd ed. Philadelphia, Pa: WB Saunders. 2000:606–614. [Google Scholar]
- 50.Turek F., Dugovic C., Zee P. Current Understanding of the circadian clock and the clinical implications for neurological disorders. Arch Neurol. 2001;58:1781–1787. doi: 10.1001/archneur.58.11.1781. [DOI] [PubMed] [Google Scholar]
- 51.Skene DJ. Optimization of light and melatonin to phase-shift human circadian rhythms. J Neuroendocrinol. 2003;15:438–441. doi: 10.1046/j.1365-2826.2003.01006.x. [DOI] [PubMed] [Google Scholar]
- 52.Rivkees S. Mechanisms and clinical significance of circadian rhythms in children. CurrOpin Pediatr. 2001;13:352–357. doi: 10.1097/00008480-200108000-00012. [DOI] [PubMed] [Google Scholar]
- 53.Cardinali DP., Brusco LI., Lloret SP., Furio AM. Melatonin in sleep disorders and jet-lag. Neuroendocrinol Lett. 2002;23(suppl 1):9–13. [PubMed] [Google Scholar]
- 54.Ondze B., Espa F., Ming LC., et al. Advanced sleep phase syndrome. Rev Neurol (Paris). 2001;157(11 Pt 2):S130–S134. [PubMed] [Google Scholar]
- 55.Monk T. Shift work. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 3rd ed. Philadelphia, Pa: WB Saunders. 2000:600–605. [Google Scholar]
- 56.Burgess HJ., Sharkey KM., Eastman CI. Bright light, dark and melatonin can promote circadian adaptation in night shift workers. Sleep Med Rev. 2002;6:407–420. [PubMed] [Google Scholar]
- 57.Quera-Salva MA. Rapid shift in sleep time and acrophase of melatonin secretion in short shift work schedule. Sleep. 1996;19:539–543. [PubMed] [Google Scholar]
- 58.Van Reeth O., Mennuni G. Fatigue and sleep: the point of view of the chronobiologist. Rev Med Brux. 2002;23:A288–A293. [PubMed] [Google Scholar]
- 59.Horowitz TS., Tanigawa T. Circadian-based new technologies for night workers. Ind Health. 2002;40:223–236. doi: 10.2486/indhealth.40.223. [DOI] [PubMed] [Google Scholar]
- 60.Purnell MT., Feyer AM., Herbison GP. The impact of a nap opportunity during the night shift on the performance and alertness of 1 2-h shift workers. J Sleep Res. 2002;11:219–227. doi: 10.1046/j.1365-2869.2002.00309.x. [DOI] [PubMed] [Google Scholar]
- 61.Meoli A., Casey K., Clark R., et al. Hypopnea in sleep-disordered breathing in adults. Sleep. 2001;24:469–470. [PubMed] [Google Scholar]
- 62.Guilleminault C., Stoohs R., Clerk A., Cetel M., Maistros P. A cause of excessive daytime sleepiness. The upper airway resistance syndrome. Chest. 1993;104:781–787. doi: 10.1378/chest.104.3.781. [DOI] [PubMed] [Google Scholar]
- 63.Black JE., Guilleminault C., Colrain IM., Carrillo O. Upper airway resistance syndrome. Central electroencephalograph ic power and changes in breathing effort. Am J Respir Crit Care Med. 2000;162:406–411. doi: 10.1164/ajrccm.162.2.9901026. [DOI] [PubMed] [Google Scholar]
- 64.Guilleminault C., Kim YD., Palombini L., Li K., Powell N. Upper airway resistance syndrome and its treatment. Sleep. 2000;23(suppl 4):S197–S200. [PubMed] [Google Scholar]
- 65.Schecter M and the Section on Pediatric Pulmonology Subcommittee on Obstructive Sleep Apnea Syndrome Technical report: diagnosis and management of childhood obstructive sleep apnea. Pediatrics. 2002;109:e69. doi: 10.1542/peds.109.4.e69. [DOI] [PubMed] [Google Scholar]
- 66.Bower C., Gungor A. Update on the pediatric airway. Pediatric obstructive sleep apnea. Otolaryngol Clin North Am. 2000;33:49–75. doi: 10.1016/s0030-6665(05)70207-3. [DOI] [PubMed] [Google Scholar]
- 67.Guilleminault C., Pelayo R., Léger D., Clerk A., Bocian R. Recognition of sleepdisordered breathing in children. Pediatrics. 1996;98:871–882. [PubMed] [Google Scholar]
- 68.Chervin RD., Archbold KH., Dillon JE., et al. Inattention, hyperactivity, and symptoms of sleep-disordered breathing. Pediatrics. 2002;109:449–456. doi: 10.1542/peds.109.3.449. [DOI] [PubMed] [Google Scholar]
- 69.Corkum P., Muldofsky H., Hogg-Johnson S., Humphries T., Tannock R. Sleep problems in children with attention deficit/hyperactivity disorder: impact of subtype, comorbidity, and stimulant medication. J Am Acad Child Adoles Psychiatry. 1999;38:1285–1293. doi: 10.1097/00004583-199910000-00018. [DOI] [PubMed] [Google Scholar]
- 70.Loube Dl., Gay PC., Strohl KP., et al. Indications for positive airway pressure treatment of adult obstructive sleep apnea patients. A consensus statement. Chest. 1999;115:863–866. doi: 10.1378/chest.115.3.863. [DOI] [PubMed] [Google Scholar]
- 71.Mosk S. Self-reported depressive symptomatology, mood ratings, and treatment outcome in sleep disorder patients. J Clin Psychol. 1989;45:51–60. doi: 10.1002/1097-4679(198901)45:1<51::aid-jclp2270450107>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 72.Sforza E., Lugaresi E. Daytime sleepiness and nasal continuous positive airway pressure therapy in obstructive sleep apnea syndrome patients; effects of chronic treatment and 1-night therapy withdrawal. Sleep. 1995;18:195–201. [PubMed] [Google Scholar]
- 73.Redline S., Adams N., Strauss ME., et al. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med. 1998;157:858–865. doi: 10.1164/ajrccm.157.3.9709042. [DOI] [PubMed] [Google Scholar]
- 74.Kribbs NB., Pack AI., Kline LR., et al. Effects of one night without nasal CPAP treatment on sleep and sleepiness in patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;14:1162–1168. doi: 10.1164/ajrccm/147.5.1162. [DOI] [PubMed] [Google Scholar]
- 75.Rajagopal KR., Bennett LL., Dillard TA., Tellis CJ., Tenholder MF. Overnight nasal CPAP improves hypersomnolence in sleep apnea. Chest. 1986;90:172–176. doi: 10.1378/chest.90.2.172. [DOI] [PubMed] [Google Scholar]
- 76.Engleman HM., Martin SE., Deary IJ., Douglas NJ. Effects of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet. 1994;343:572–575. doi: 10.1016/s0140-6736(94)91522-9. [DOI] [PubMed] [Google Scholar]
- 77.Downey R 3rd., Perkin RM., MacQuarrie J. Nasal continuous positive airway pressure use in children with obstructive sleep apnea younger than 2 years of age. Chest. x. 2000;117:1608–1612. doi: 10.1378/chest.117.6.1608. [DOI] [PubMed] [Google Scholar]
- 78.McNamara F., Sullivan C. Obstructive sleep apnea in infants and its management with nasal continuous positive airway pressure. Chest. 1999;116:1016. doi: 10.1378/chest.116.1.10. [DOI] [PubMed] [Google Scholar]
- 79.Anstead M., Phillips B., Buch K. Tolerance and intolerance to continuous positive airway pressure. Curr Opin Pulm Med. 1998;4:351–354. doi: 10.1097/00063198-199811000-00008. [DOI] [PubMed] [Google Scholar]
- 80.Strollo PJ Jr., Sanders MH., Atwood CW. Positive pressure therapy. Clin Chest Med. 1998;19:55–68. doi: 10.1016/s0272-5231(05)70431-7. [DOI] [PubMed] [Google Scholar]
- 81.Hoffstein V., Viner S., Mateika S., Conway J. Treatment of obstructive sleep apnea with nasal continuous positive airway pressure patient compliance, perception of benefits, and side effects. Am. Rev Respir Dis. 1992;145:841–845. doi: 10.1164/ajrccm/145.4_Pt_1.841. [DOI] [PubMed] [Google Scholar]
- 82.Issa FG., Sullivan CE. Reversal of central sleep apnea using nasal CPAP. Chest. 1984;90:165–171. doi: 10.1378/chest.90.2.165. [DOI] [PubMed] [Google Scholar]
- 83.Rauscher H., Popp W., Wanke T., Zwick H. Acceptance of CPAP therapy for sleep apnea. Chest. 1991;100:1019–1023. doi: 10.1378/chest.100.4.1019. [DOI] [PubMed] [Google Scholar]
- 84.Rolfe I., Olson LG., Saunders NA. Long-term acceptance of continuous positive airway pressure in obstructive sleep apnea. Am Rev Respir Dis. 1991;144:1130–1133. doi: 10.1164/ajrccm/144.5.1130. [DOI] [PubMed] [Google Scholar]
- 85.Nino-Murcia G., McCann CC., Bliwise DL., Guilleminault C., Dement WC. Compliance and side effects in sleep apnea patients treated with nasal continuous positive airway pressure. West J Med. 1989;150:165–169. [PMC free article] [PubMed] [Google Scholar]
- 86.Sanders MH., Gruendl CA., Rogers RM. Patient compliance with nasal CPAP therapy for sleep apnea. Chest. 1986;90:330–333. doi: 10.1378/chest.90.3.330. [DOI] [PubMed] [Google Scholar]
- 87.Waldhorn RE., Herrick TW., Nguyen MC., O'Donnell AE., Sodero J., Potolicchio SJ. Long-term compliance with nasal continuous positive airway pressure therapy of obstructive sleep apnea. Chest. 1990;97:33–38. doi: 10.1378/chest.97.1.33. [DOI] [PubMed] [Google Scholar]
- 88.Littner M., Hirshkowitz M., Davila D., et al. Practice parameters of the use of auto-titrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome. Sleep. 2002;25:143–147. doi: 10.1093/sleep/25.2.143. [DOI] [PubMed] [Google Scholar]
- 89.Berry RB. The use of auto-titrating continuous positive airway pressure for treatment of adult obstructive sleep apnea. Sleep. 2002;25:148–173. [PubMed] [Google Scholar]
- 90.American Sleep Disorders Association Practice parameters for the treatment of obstructive sleep apnea in adults: the efficacy of surgical modifications of the upper airway. Sleep. 1996;19:152–155. [PubMed] [Google Scholar]
- 91.Rama AN., Tekwani SH., Kushida CA. Sites of obstruction in obstructive sleep apnea. Chest. 2002;122:1139–1147. doi: 10.1378/chest.122.4.1139. [DOI] [PubMed] [Google Scholar]
- 92.Sher A., Schechtman K., Piccirillo J. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19:156–177. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
- 93.Loube D. Technologic advances in the treatment of obstructive sleep apnea syndrome. Chest. 1999;116:1426–1433. doi: 10.1378/chest.116.5.1426. [DOI] [PubMed] [Google Scholar]
- 94.Riley R., Powell N., Li K., Guilleminault C. Surgical therapy for obstructive sleep apnea-hypopnea syndrome. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 3rd ed. Philadelphia, Pa: WB Saunders. 2000:913–929. [Google Scholar]
- 95.Powell N., Riley R., Guilleminault C. Radiofrequency tongue base reduction in sleep-disordered breathing. Otolaryngol Head Neck Surg. 1999;120:656–664. doi: 10.1053/hn.1999.v120.a96956. [DOI] [PubMed] [Google Scholar]
- 96.Riley R. Powell-Riley Stanford surgical protocol. Proceedings of the Conference on Contemporary Surgical Concepts and Techniques in Snoring and Sleep Disordered Breathing. Burlingame, Calif. April 11-12. 2003 [Google Scholar]
- 97.Montplaisir J., Nicolas A., Godbout R., Walters A. Restless legs syndrome and periodic limb movement disorder. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 3rd ed. Philadelphia, Pa: WB Saunders. 2000:742–752. [Google Scholar]
- 98.Hening WA. Restless legs syndrome: a sensorimotor disorder of sleep/wake motor regulation. Curr Neurol Neurosci Rep. 2002;2:186–196. doi: 10.1007/s11910-002-0029-y. [DOI] [PubMed] [Google Scholar]
- 99.Stiasny K., Oertel WH., Trenkwalder C. Clinical symptomatology and treatment of restless legs syndrome and periodic limb movement disorder. Sleep Med Rev. 2002;6:247–251. doi: 10.1053/smrv.2001.0193. [DOI] [PubMed] [Google Scholar]
- 100.Montplaisir J., Nicolas A., Denesle R., Gomez-Mancilla B. Restless legs syndrome improved by pramipexole. Neurology. 1999;52:938–943. doi: 10.1212/wnl.52.5.938. [DOI] [PubMed] [Google Scholar]
- 101.Allen RP., Mignot E., Ripley B., Nishino S., Earley CJ. Increased CSF hypocretin-1 (orexin-A) in restless legs syndrome. Neurology. 2002;59:639–641. doi: 10.1212/wnl.59.4.639. [DOI] [PubMed] [Google Scholar]
- 102.Chokroverty S., Jankovic J. Restless legs syndrome. Neurology. 1999;52:907. doi: 10.1212/wnl.52.5.907. [DOI] [PubMed] [Google Scholar]
- 103.Turjanski N., Lees AJ., Brooks DJ. Striatal dopaminergic function in restless legs syndrome: ,8F-dopa and 11C-raclopride PET studies. Neurology. 1999;52:932–937. doi: 10.1212/wnl.52.5.932. [DOI] [PubMed] [Google Scholar]
- 104.Staedt J., Stoppe G., Kogler A., et al. Nocturnal myoclonus syndrome (periodic movements in sleep) related to central dopamine D2-receptor alteration. Eur Arch Psychiatry Clin Neurosci. 1995;245:8–10. doi: 10.1007/BF02191538. [DOI] [PubMed] [Google Scholar]
- 105.Saletu M., Anderer P., Saletu B., et al. EEG mapping in patients with restless legs syndrome as compared with normal controls. Psychiatry Res. 2002;115:49–61. doi: 10.1016/s0925-4927(02)00023-9. [DOI] [PubMed] [Google Scholar]
- 106.Hening W., Allen R., Earley C., et al. The treatment of restless legs syndrome and periodic limb movement disorder. Sleep. 1999;22:970–999. [PubMed] [Google Scholar]
- 107.Chesson A., Wise M., Davila D., et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep. 1999;22:961–968. doi: 10.1093/sleep/22.7.961. [DOI] [PubMed] [Google Scholar]
- 108.Cornelia C. Restless legs syndrome. Treatment with dopaminergic agents. Neurology. 2002;58:S87–S92. doi: 10.1212/wnl.58.suppl_1.s87. [DOI] [PubMed] [Google Scholar]
- 109.Walters AS., Wagner ML., Hening WA., et al. Successful treatment of the idiopathic restless legs syndrome in a randomized double-blind trial of oxycodone versus placebo. Sleep. 1993;16:327–332. doi: 10.1093/sleep/16.4.327. [DOI] [PubMed] [Google Scholar]
- 110.Kaplan PW., Allen RP., Buchholz DW., Walters JK. A double-blind placebocontrolled study of the treatment of periodic limb movements in sleep using carbidopa/levodopa and propoxyphene. Sleep. 1993;16:717–723. doi: 10.1093/sleep/16.8.717. [DOI] [PubMed] [Google Scholar]
- 111.Montagna P., Sassoli de Bianchi L., Zucconi M., et al. Clonazepam and vibration in restless legs syndrome. Acta Neurol Scand. 1984;69:428–430. doi: 10.1111/j.1600-0404.1984.tb07826.x. [DOI] [PubMed] [Google Scholar]
- 112.Boghen D., Lamothe L., Elie R., et al. The treatment of the restless legs syndrome with clonazepam: a prospective controlled study. Can J Neurol Sci. 1986;13:245–247. doi: 10.1017/s0317167100036350. [DOI] [PubMed] [Google Scholar]
- 113.Thorp ML., Morris CD., Bagby SP. A crossover study of gabapentin in treatment of restless legs syndrome among hemodialysis patients. Am J Kidney Dis. 2001;38:104–108. doi: 10.1053/ajkd.2001.25202. [DOI] [PubMed] [Google Scholar]
- 114.Garcia-Borreguero D., Larrosa O., de la Llave Y., et al. Treatment of restless legs syndrome with gabapentin. A double-blind, cross-over study. Neurology. 2002;59:1573–1579. doi: 10.1212/wnl.59.10.1573. [DOI] [PubMed] [Google Scholar]
- 115.Telstaad W., Sorensen O., Larsen S., et al. Treatment of the restless legs syndrome with carbamazepine: a double-blind study. BMJ (Clin Res Ed). 1984;288:444–446. doi: 10.1136/bmj.288.6415.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wagner ML., Walters AS., Coleman RG., Hening WA., Grasing K., Chokroverty S. Randomized, double-blind placebo-controlled study of clonidine in restless legs syndrome. Sleep. 1996;19:52–58. doi: 10.1093/sleep/19.1.52. [DOI] [PubMed] [Google Scholar]
- 117.Guilleminault C., Cetel M., Philip P. Dopaminergic treatment of restless legs and rebound phenomenon. Neurology. 1993;43:443–445. doi: 10.1212/wnl.43.2.445. [DOI] [PubMed] [Google Scholar]
- 118.Guilleminault C., Anagnos A. Narcolepsy. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 3rd ed. Philadelphia, Pa: WB Saunders. 2000:676–686. [Google Scholar]
- 119.Overeem S., Mignot E., van Dijk JG., Lammers GJ. Narcolepsy: clinical features, new pathophysiologic insights, and future perspectives. J Clin Neurophysiol. 2001;18:78–105. doi: 10.1097/00004691-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 120.Stores G. Recognition and management of narcolepsy. Arch Dis Child. 1999;81:519–524. doi: 10.1136/adc.81.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gerhardstein R., Day R., Rosenthal L. Narcolepsy and other causes of excessive daytime sleepiness. Respir Care Clin N Am. 1999;5:427–446. [PubMed] [Google Scholar]
- 122.Guilleminault C., Pelayo R. Narcolepsy in children: a practical guide to its diagnosis, treatment, and follow-up. Paediatr Drugs. 2000;2:1–9. doi: 10.2165/00148581-200002010-00001. [DOI] [PubMed] [Google Scholar]
- 123.Krahn LE., Black JL., Silber MH. Krahn, L. Narcolepsy: new understanding of irresistible sleep. Mayo Clinic Proc. 2001;76:185–194. doi: 10.1016/S0025-6196(11)63126-1. [DOI] [PubMed] [Google Scholar]
- 124.Aldrich M. Narcolepsy. N Engl J Med. 1990;323:389–394. doi: 10.1056/NEJM199008093230606. [DOI] [PubMed] [Google Scholar]
- 125.Black J. Narcolepsy: evaluation and management. CNS News Special Ed. 2001:25–29. [Google Scholar]
- 126.Guilleminault C., Wilson RA., Dement WC. A study on cataplexy. Arch Neurol. 1974;31:255–261. doi: 10.1001/archneur.1974.00490400069008. [DOI] [PubMed] [Google Scholar]
- 127.Guilleminault C., Anders TF. The pathophysiology of sleep disorders in pediatrics. Part II. Sleep disorders in children. AdvPediatr. 1976;22:151–174. [PubMed] [Google Scholar]
- 128.Rubboli G., d'Orsi G., Zaniboni A., et al. A video-polygraphic analysis of the cataplectic attack. Clin Neurophysiol. 2000;111 (suppl 2):S120–S128. doi: 10.1016/s1388-2457(00)00412-0. [DOI] [PubMed] [Google Scholar]
- 129.Overeem S., Scammell T., Lammers G. Hypocretin/orexin and sleep: implications for the pathophysiology and diagnosis of narcolepsy. Curr Opin Neurol. 2002;15:739–745. doi: 10.1097/01.wco.0000044800.53746.5a. [DOI] [PubMed] [Google Scholar]
- 130.Mignot E., Lammers GJ., Ripley B., et al. The role for cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–1562. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 131.Krahn LE., Pankratz VS., Oliver L., Boeve BF., Silber MH. Hypocretin (orexin) levels in cerebrospinal fluid of patients with narcolepsy: relationship to cataplexy and HLA DQB1*0602 status. Sleep. 2002;25:733–736. doi: 10.1093/sleep/25.7.733. [DOI] [PubMed] [Google Scholar]
- 132.Kanbayashi T., Inoue Y., Chiba S., et al. CSF hypocretin-1 (orexin-A) concentrations in narcolepsy with and without cataplexy and idiopathic hypersomnia. J Sleep Res. 2002;11:91–93. doi: 10.1046/j.1365-2869.2002.00284.x. [DOI] [PubMed] [Google Scholar]
- 133.Mitler MM., Aldrich MS., Koob GF., Zarcone VP. Narcolepsy and its treatment with stimulants: ÂSDA standards of practice. Sleep. 1994;17:352–371. [PubMed] [Google Scholar]
- 134.Parkes D. Introduction to the mechanism of action of different treatments of narcolepsy. Sleep. 1994;17(8 suppl):S93–S96. doi: 10.1093/sleep/17.suppl_8.s93. [DOI] [PubMed] [Google Scholar]
- 135.Littner M., Johnson SF., McCall VW., et al. Standards of Practice Committee. Practice parameters for the treatment of narcolepsy: an update for 2000. Sleep. 2001;24:451–466. [PubMed] [Google Scholar]
- 136.Scamell TE. The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol. 2003;53:154–166. doi: 10.1002/ana.10444. [DOI] [PubMed] [Google Scholar]
- 137.Thorpy MJ., Snyder M., Aloe FS., Ledereich PS., Starz KE. Short-term triazolam use improves nocturnal sleep of narcoleptics. Sleep. 1992;15:212–216. doi: 10.1093/sleep/15.3.212. [DOI] [PubMed] [Google Scholar]
- 138.Bassetti C., Aldrich M. Idiopathic hypersomnia. A series of 42 patients. Brain. 1997;120:1423–1435. doi: 10.1093/brain/120.8.1423. [DOI] [PubMed] [Google Scholar]
- 139.Guilleminault C., Brooks S. Idiopathic hypersomnia: a neurological dilemma. Sleep Med Rev. 2001;5:347–349. doi: 10.1053/smrv.2001.0210. [DOI] [PubMed] [Google Scholar]
- 140.Broughton R. NREM arousal parasomnias. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 3rd ed. Philadelphia, Pa: WB Saunders. 2000:687–692. [Google Scholar]
- 141.Kales A., Soldâtes CR., Bixler EO., et al. Hereditary factors in sleepwalking and night terrors. Br J Psychiatry. 1980;137:111–118. doi: 10.1192/bjp.137.2.111. [DOI] [PubMed] [Google Scholar]
- 142.Abe K., Amatomi M., Oda N. Sleepwalking and recurrent sleeptalking in children of childhood sleepwalkers. Ami J Psychiatry. 1984;141:800–801. doi: 10.1176/ajp.141.6.800. [DOI] [PubMed] [Google Scholar]
- 143.Guilleminault C., Poyares D., Abat F., Palombini L. Sleep and wakefulness in somnambulism. A spectral analysis study. J Psychosom Res. 2001;51:411–416. doi: 10.1016/s0022-3999(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 144.Guilleminault C., Palombini L., Pelayo R., Chervin R. Sleepwalking and sleep terrors in prepubertal children: what triggers them? Pediatrics. 2003;111:e17–e25. doi: 10.1542/peds.111.1.e17. [DOI] [PubMed] [Google Scholar]
- 145.Guilleminault C., Moscovich A., Léger D. Forensic sleep medicine and nocturnal wandering and violence. Sleep. 1995;15:173–184. doi: 10.1093/sleep/18.9.740. [DOI] [PubMed] [Google Scholar]
- 146.Broughton RJ., Shimuzu T. Sleep-related violence: a medical and forensic challenge. Sleep. 1995;18:727–730. doi: 10.1093/sleep/18.9.727. [DOI] [PubMed] [Google Scholar]
- 147.Olson E., Boeve B., Silbert M. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000;123:331–339. doi: 10.1093/brain/123.2.331. [DOI] [PubMed] [Google Scholar]
- 148.Mahowald M., Schenk C. Medical-legal aspects of sleep medicine. Neurol Clin. 1999;17:215–234. doi: 10.1016/s0733-8619(05)70126-2. [DOI] [PubMed] [Google Scholar]
- 149.Mahowald M., Schenk C. REM sleep parasomnias. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 3rd ed. Philadelphia, Pa: WB Saunders. 2000:724–741. [Google Scholar]
- 150.Ferini-Strambi L., Zucconi M. REM sleep behavior disorder. Clin Neurophysiol. 2000;111 (suppl 2):S136–S140. doi: 10.1016/s1388-2457(00)00414-4. [DOI] [PubMed] [Google Scholar]
- 151.Schenck C., Bundlie S., Ettinger M., Mahowald M. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9:293–308. doi: 10.1093/sleep/9.2.293. [DOI] [PubMed] [Google Scholar]
- 152.Silber M. REM sleep behavior disorder associated with barbiturate withdrawal. APSS Abstract. Sleep Res. 1996;25:371. [Google Scholar]
- 153.Husain A., Miller P., Carwile S. REM sleep behavior disorder: potential relationship to post-traumatic stress disorder. J Clin Neurophysiol. 2001;18:148–157. doi: 10.1097/00004691-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 154.Onofrj M., Luciano AL., Thomas A., et al. Mirtazapine induces REM sleep behavior disorder (RBD) in parkinsonism. Neurology. 2003;60:113–115. doi: 10.1212/01.wnl.0000042084.03066.c0. [DOI] [PubMed] [Google Scholar]
- 155.Schutte S., Doghramji K. REM behavior disorder seen with venlafaxine (Effexor). APSS Abstract. Sleep Res. 1996;25:364. [Google Scholar]
- 156.Shirakawa S., Takeuchi N., Uchimura N., et al. Study of image findings in rapid eye movement sleep behavioural disorder. Psychiatry Clin Neurosci. 2002;56:291–292. doi: 10.1046/j.1440-1819.2002.00961.x. [DOI] [PubMed] [Google Scholar]
- 157.Albin RL., Koeppe RA., Chervin RD., et al. Decreased striatal dopaminergic innervation in REM sleep behavior disorder. Neurology. 2000;55:1410–1412. doi: 10.1212/wnl.55.9.1410. [DOI] [PubMed] [Google Scholar]
- 158.Ringman JM., Simmons JH. Treatment of REM sleep behavior disorder with donezepil: a report of three cases. Neurology. 2000;55:870–871. doi: 10.1212/wnl.55.6.870. [DOI] [PubMed] [Google Scholar]
- 159.Bamford C. Carbamazepine in REM sleep behavior disorder. Sleep. 1993;16:33. [PubMed] [Google Scholar]
- 160.Mike ME., Kranz AJ. MAOI suppression of RBD refractory to clonazepam and other agents. Sleep Res. 1996;25:63. [Google Scholar]
- 161.Takeuchi N., Uchimura N., Hashizume Y., et al. Melatonin therapy for REM sleep behavior disorder. Psychiatry Clin Neurosci. 2001;55:267–279. doi: 10.1046/j.1440-1819.2001.00854.x. [DOI] [PubMed] [Google Scholar]
- 162.Kunz D., Bes F. Melatonin as a therapy in REM sleep behavior patients: an open- labeled pilot study on the possible influence of melatonin on REM sleep regulation. Mov Disord. 1999;14:507–511. doi: 10.1002/1531-8257(199905)14:3<507::aid-mds1021>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]