Abstract
Biological rhythms and their temporal organization are adaptive phenomena to periodic changes in environmental factors linked to the earth's rotation on its axis and around the sun. Experimental data from the plant and animal kingdoms have led to many models and concepts related to biological clocks that help describe and understand the mechanisms of these changes. Many of the prevailing concepts apply to all organisms, but most of the experimental data are insufficient to explain the dynamics of human biological clocks. This review presents phenomena thai are mainly characteristic ofand unique to - human chronobiology, and which cannot be fully explained by concepts and models drawn from laboratory experiments. We deal with the functional advantages of the human temporal organization and the problem of desynchronization, with special reference to the period (τ) of the circadian rhythm and its interindividual and intraindividual variability. We describe the differences between right- and left-hand rhythms suggesting the existence of different biological clocks in the right and left cortices, Desynchronization of rhythms is rather frequent (one example is night shift workers). In some individuals, desynchronization causes no clinical symptoms and we propose the concept of “allochronism” to designate a variant of the human temporal organization with no pathological implications. We restrict the term “dyschronism” to changes or alterations in temporal organization associated with a set of symptoms similar to those observed in subjects intolerant to shift work, eg, persisting fatigue and mood and sleep alterations. Many diseases involve chronic deprivation of sleep at night and constitute conditions mimicking thai of night shift workers who are intolerant to desynchronization. We also present a genetic model (the dian-circadian model) to explain interindividual differences in the period of biological rhythms in certain conditions.
Keywords: biological rhythm, temporal organization, desynchronization, allochronism, dyschronism, shift work, affective disorder
Abstract
Los ritmos biológicos y su organización temporal son fenómenos de adaptación a las variaciones periódicas del ambiente relacionadas con la rotación de la tierra sobre su eje y alrededor del sol. Los datos experimentales provenientes de los reinos vegetal y animal han dado origen a muchos modelos y conceptos relacionados con relojes biológicos que ayudan a describir y comprender los mecanismos de estos cambios. Muchos de los conceptos comunes se aplican a todos los organismos, pero la mayoría de los datos experimentales son insuficientes para explicar la dinámica de los relojes biológicos humanos. Esta revisión presenta los fenómenos que caracterizan la cronobiología humana de manera única y que no pueden ser totalmente explicados a través de conceptos y modelos propuestos a partir de experimentos de laboratorio. Ella está centrada en las ventajas funcionales de la organización temporal humana y el problema de la desincronización, con especial atención al período (τ) del ritmo circadiano y su variabilidad interindividual e intraindividual, También se describen las diferencias entre los ritmos de los diestros y los zurdos, lo que sugiere la existencia de diferentes relojes biológicos en las cortezas derecha e izquierda. La desincronización de los ritmos es un fenómeno relativamente frecuente (por ejemplo, el trabajo con turnos nocturnos). En algunos sujetos la desincronización no se acompaña de ningún síntoma, por lo que se propone el concepto de “alosincronía” (alo = diferente), para designar una variedad de la organización temporal humana que no tiene repercusiones patológicas. Se réserva el término “discronía” (dis = alteración, perturbación) para cambios o alteraciones asociadas con un conjunto de síntomas similares a los observados en sujetos que no toleran los trabajos con turnos, como fatiga persistante y trastornos del ánimo y del sueño. Muchas enfermedades incluyen una privación crónica del sueño nocturno y originan una sintomatología similar a la de los trabajadores con turnos nocturnos que no toleran la desincronización, También se présenta un modelo genético (dian - circadiano) para explicar las diferencias interindividuales en el período de los ritmos biológicos en determinadas condiciones.
Abstract
Les rythmes biologiques et leur organisation temporelle sont des phénomènes d'adaptation aux variations périodiques de l'environnement liées aux rotations de la terre sur son axe et autour du soleil. Des données expérimentales issues des règnes végétal et animal ont fourni de nombreux modèles et concepts concernant les horloges biologiques. Ils permettent de décrire et de mieux comprendre les mécanismes de ces variations. Plusieurs des concepts majeurs s'appliquent à tous les organismes mais ces données expérimentales ne suffisent pas à expliquer la dynamique des horloges biologiques humaines. Cette revue présente des phénomènes qui caractérisent la chronobiologie humaine de manière unique et qui ne peuvent être entièrement expliqués par les concepts et les modèles expérimentaux. Elle est centrée sur l'avantage fonctionnel de l'organisation temporelle humaine et les problèmes de désynchronisation, la référence critique étant la période τ des rythmes circadiens et sa variabilité intra- et interindividuelle. Ainsi, nous avons décrit des différences entre les rythmes des mains droite et gauche qui donnent à penser que des horloges biologiques fonctionnelles différentes existent dans les cortex droit et gauche, La désynchronisation des rythmes est un phénomène relativement fréquent (par exemple: le travail posté). Chez certains sujets la désynchronisation ne s'accompagne d'aucun symptôme, ce qui conduit au concept d'«allosynchronisme» (allo = différent) qui désigne une variante de l'organisation temporelle humaine, sans implication pathologique. Nous réservons le terme «dyschronisme» (dys = altération, perturbation) aux changements ou aux altérations de l'organisation temporelle associés à des symptômes s'observant chez les sujets qui ne tolèrent pas le travail posté: fatigue persistante, troubles de l'humeur et du sommeil. De nombreuses maladies induisent une privation chronique du sommeil nocturne et sont à l'origine d'une symptomatologie similaire à celle des travailleurs postés intolérants à la désynchronisation. Nous présentons également un modèle génétique «dien-circadien» pour expliquer les différences interindividuelles de périodes de rythmes biologiques dans certaines circonstances.
The rhythmic (as opposed to linear) expression of biological variables and the temporal organization of these rhythms represent an adaptation of organisms to the rhythmic changes in the external environment. Periodic oscillations (rhythms) have been documented in biological variables in a whole spectrum of living organisms (from unicellular to multicellular).1, 2 However, this phenomenon is not merely a reaction to environmental changes; it is generally held that the rhythms are governed by an active system capable of self-sustained oscillations (endogenous rhythms).1 Consequently, the shape of rhythms and the temporal order are products of the interaction between endogenous (genetically controlled) oscillators and the phases (synchronizing, entraining) of external cues.
Features of biological rhythm
The parameters of a biological rhythm are as follows1-6 :
The period τ (τ=24 h in circadian rhythm; and τ<20 h in ultradian rhythm).
The acrophase (Φ, the peak time of the rhythm). This parameter usually includes a phase reference within the time axis of the rhythm (eg, for the circadian rhythm the acrophase relates to a phase reference like midnight, local time, or mid-sleep).
The amplitude (A), the pcak-to-trough difference.
The mean level, or mesor (M).
Rhythms that follow a cosine curve can be characterized by all four of these parameters, and rhythms that do not follow cosine shape are mostly characterized by M and τ. The majority of the rhythms studied in nature, and especially in humans, exhibit circadian periodicity, and this review will focus mainly on these (though most of discussions herein also apply to rhythms with other periodicities). Circadian rhythms have the following properties1-8 :
They have a genetic origin.
They are controlled by biological clocks (or oscillators or circadian pacemakers).
The biological clocks are reset (Φ) and calibrated (τ=24 h) by environmental signals that also have τ=24 h, such as dawn/dusk (photic signals), activity /rest, or noi.se/silcncc (nonphotic signals). These periodic environmental factors are called synchronizers,9 zeitgebers,10 or entraining agents.7 The range of period cntrainment of circadian rhythms by the zeitgebers may vary between τ=20 h and τ=28 h.
There is a general ubiquity 7, 8 of the properties of the biological rhythms quoted above, from unicellular eukaryotes8-11-12 to humans.2, 5, 13 However, some variability exists and some differences can be observed among plants,12 animals,13 strains of the same species,14 and even different human individuals.5, 13, 15, 16
The master clock versus temporal organization
In recent years, a large amount of information has accumulated about the genetic, molecular, physiological, and environmental induction of biological rhythms and about how they function in various genera and species. Due to the variety and variability of this vast literature, it is no longer an easy task to review concepts in human biological rhythms. We will first try to present the reasons for this difficulty.
Two schools of thoughts coexist in chronobiology. One considers that the study of biological rhythms must involve an analytical approach to phenomena and confine itself to reductionism.17 A relatively simple molecular genetic model is proposed,18-20 as is the existence of one domineering master clock (the suprachiasmatic nucleus [SCN] in mammals and certain species of birds) that controls almost all rhythmic functions.21, 22 Consequently, most studies of the circadian system focused on the recording of one overt rhythm (eg, activity/rest), especially in rodent animal models, such as hamsters, rats, and mice.18, 19 Although this school of thought has recently recognized the existence of peripheral pacemakers and oscillators, they are placed in a lower hierarchical level than the master clock.
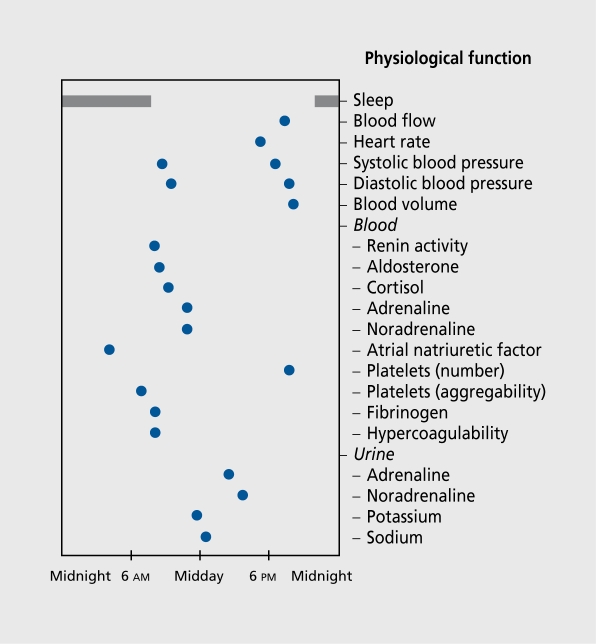
The other school of thought favors a holistic perspective and considers that the studied subject (ie, man) as a whole is engulfed by normal habitat and time cues.4, 5, 23-26 Both the living organism and the rhythmic and nonrhythmic changes in its environmental factors arc taken into account. Thus, a whole range of biological clocks - and not just one - play a role, as well as a rather large set of genes, many with pleiotropic effects,16, 27 rather than just a few.18-20 Another important point about this approach is the emphasis on temporal organization,4-7, 23-26, 28 rather than the study of one or two rhythms. For an organisms synchronized with τ=24 h, the study will document a set of biological variables each characterized by its specific Φ (Figure 1). 26 A review of the literature shows that even unicellular eukaryote organisms such as Acetabularia (an algae) and Euglena (a protist), which possess no nervous or endocrine systems, contain a population of oscillators and a temporal structure can be demonstrated.8-11
Figure 1. Aspects of the human temporal organization: physiological functions of the cardiovascular system. The acrophase (Φ) location (blue circles) of any of the considered rhythms is not randomly distributed over 24 h. On the contrary, acrophases represent physiologically validated temporal relationships. The healthy young human adults' synchronization was approximately 16 h of diurnal activity and 8 h of nocturnal rest. Reproduced from reference 26: Reinberg A. Chronobiologie Médicale, Chronothérapeutique. Paris, France: Flammarion Médecine-Sciences: 2003. Copyright © 2003, Flammarion Médecine-Sciences.
Terms such as temporal organization, temporal structure, temporal order, and time structure are synonymous. Various models have been proposed to better understand the “hierarchy” and the “coupling” between oscillators and/or biological clock systems.13, 22, 23
We propose that these two schools of thoughts are complementary rather than exclusive, but it is clear that an accurate and objective definition is far from easy to make. Another difficulty resides in the fact that some authors recommend avoiding investigations on human subjects, since they believe that humans can only produce “sloppy” rhythms.29 It should be noted that this statement was made without providing a definition of human rhythm sloppiness.This appears to come from the idea that many of the studies carried out 20 years ago were investigations on mammalian rhythms conducted on laboratory hamsters, rats, and mice, for which the prominent synchronizer is light/dark (L:D) alternation. In these species, a photic signal of few lux is powerful enough to synchronize rhythms, which should be compared with the 2500 lux (bright light) needed to synchronize human rhythms.13, 30, 31 Recent studies show that even human rhythms can be entrained by low intensity light.32
Another example that illustrates the confusion in defining a concept due to a focus on the rhythm of one variable rather than on the temporal order is the following. In the 1970s, most sleep studies were extensively carried on cats, using electroencephalography (EEG). It was shown that most individuals of this species are frequent sleepers, with a polyphasic rhythmicity. According to Jouvet,33 no more than 30% of cats exhibit a sleep/wake rhythm with τ=24 h. As a result, it was believed by some authors that cat is a species that does not possess a circadian organization - an idea that was a source of conflict between sleep and biological rhythm specialists. However, cats exhibit circadian rhythms in their feeding behavior and activity/rest rhythm.34, 35 It proved difficult to bridge the gap between those involved in sleep research in cats and those studying circadian rhythms in laboratory rodents.33
The final source of misunderstanding in concept definition relates to the fact that the meaning of a given term evolves as time passes. Let us take the term chronobiolic as an example.25, 26, 36-38 Simpson et al36 hypothesized that a drug might be able to phase shift all circadian rhythms by resetting their respective Os. In fact, there is still no such wonderdrug.37, 38 Thereafter, the meaning of the term chronobiotic was restricted to a drug able to phase shift or reset one39 or a limited number25-26 of rhythms. The latter demonstrates once again the importance of studying systems or temporal order rather than just one rhythm. Considering the above examples, the definitions and concepts presented in this paper have been updated with reference to the recent state of art.
Temporal organization
Temporal organization refers to the sequential array of rhythms of various variables, each with a specific phase on the time axis. An examination of the array provides information about the phase relationship between the rhythms, but does not show whether there is a causal interaction between them. One example is body temperature rhythm and paradoxical sleep (PS) or rapid eye movement (REM) rhythm. In humans, a physiological trough in temperature coincides in time with the longest episodes of PS.40, 41 Animal experiments have demonstrated that hypothermia influences PS.42 Thus, while a phase relationship between the two rhythms docs not in itself imply a causal relationship, the physiological interaction between the two variables raises the strong possibility that they are coupled to the same oscillator or that there is a causal interaction between the two.
A simplified circadian map of the temporal cardiovascular organization of healthy young adults provides another example (Figure 1). It presents the acrophases (ΦS) of a limited number of physiological functions. The peak time locations arc not randomly distributed over 24 h, but correspond to the human needs related to diurnal activity and nocturnal rest. Here, there is a causal phase relationship between the Φ of blood pressure and that of variables known to be involved in its control. The Φs of renin activity, aldosterone, Cortisol, and catecholamines precede in phase the blood pressure Φ. Likewise, the Φs of aldosterone and Cortisol precede the Φs of the urinary excretion of sodium and potassium.
A similar temporal organization can be observed in the rat (Sprague-Dawley [SD]), with a phase shift of 12 h with regard to humans (these rodents are nocturnally active). Lemmcr et al43 used transgenic SD rats, in which the mouse renin gene REN-2 had been inserted into the SD rat genome (TGR (mREN-2)).The transgenic rats developed hypertension and their blood pressure, renin, and aldosterone rhythms were phase shifted with regard to the heart rate rhythm, in comparison to the normal temporal organization control of SD rats.43
This indicates that a physiological function, eg, cardiovascular function, involves a set of rhythms, some of which are independent of each other and some of which exhibit strong interactions (or coupling). Consequently, temporal organization should generally be regarded as a multifactor rhythm system.
The functional advantage of human temporal organization
We have seen that the sequential array of rhythms over 24 h constructs temporal organization. The rhythm phase of each variable can be identified by location of its Φ. Another characteristic of rhythm is the ratio A/M, which indicates the strength of the rhythm to shifting signals. Thus, to examine the question of whether temporal organization is structured to endow the organism with a functional advantage, three parameters must be assessed:
Time-dependent distribution of the Φs of the variables' rhythms.
Time distribution of variables' rhythms according to function.
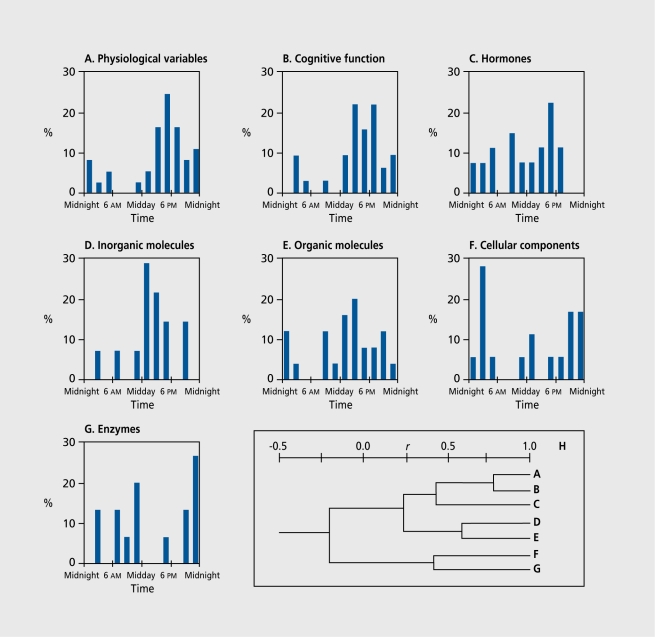
Ticher et al24 conducted such a study by computing these parameters for 168 circadian rhythms of diurnally active (7 am ±30 min to 11 pm ±60 min) young human subjects. The analysis showed that the distribution of the Φs over 24 h exhibits a strong time dependence (Figure 2) . The Φs are unevenly distributed over 24 h and no Φ was detected between 5 am and 7 am. This time zone corresponds to the overall greatest vulnerability of the human organism, eg, the circadian Φ of the human mortality rhythm, including all-cause mortality.25, 26, 41 The number of Φs per hour was then clustered according to function. Seven groups were formed
Figure 2. Distributions of circadian rhythm acrophases (Φ) according to the variables' function. A to G illustrate the 24-h acrophase frequency distribution of 7 groups of variables, and the dendrogram H shows the similarities along the groups by clusters on correlation scale (r). The upper cluster in H presents the greater similarities among the physiological variables (A), cognitive function (B), and hormones (C), where most of the acrophases occur in the late afternoon. In the inorganic molecules (D) and the organic molecules (E) groups (middle cluster H), most acrophases aggregate around midday. The third cluster (lower cluster, H) contained cellular components (F) and enzymes (G), in which most acrophases were distributed around midnight. Reproduced from reference 24: Ticher À, Ashkenazi IE, Reinberg A. Preservation of the functional advantage of human time structure. FASEBJ. 1995;9:269-272. Copyright © 2003, Federation of American Societies for Experimental Biology.
37 physiological rhythms (body temperature, blood pressure, bronchial patency, etc).
32 rhythms for cognitive function.
27 rhythms for endocrine function.
14 rhythms for metabolites.
25 rhythms for organic molecules.
18 rhythms for cellular components.
15 rhythms for enzymatic activity.
A correlation matrix of the pattern of distribution of the ΦS between each of the 7 groups served as a basis for cluster analysis. The greater the coefficient r, the stronger the similarity in the distribution of the ΦS. An dendrogram (H in Figure 2) can be constructed to visualize the similiarities. The level of correlation is shown by the distance between each group. It can be seen that the correlation is very strong between physiological variables and cognitive function rhythms with <l>s clustering in the late afternoon. The correlation between cognitive function and hormone rhythms remains strong. Organic and inorganic substance rhythms exhibit rather strong similarities with <&s clustering around 1 pm. Rhythms in cellular features and enzymatic activities also show a rather strong similarity with a cluster around midnight.
The time distribution of A/M ratio also exhibited a significant time dependence with modes: at preawaking time; postawaking time and morning meal; time of midday meal; time of evening meal; and around midnight (time of falling asleep). This suggests that “stronger” rhythms are clustered around the times where the human is confronted with the domineering exogenous signals.
These types of analyses enable us to explore the possible adaptive value of the human temporal organization, which allows variables of each function to reach their peak time in phase with predictable environmental changes, such as night and day, in alternation with other synchronizing signals.
The synchronization of human circadian rhythms
The major environmental signals that trigger biological clocks in most animals in nature and in laboratory rodents are related to the L:D alternation and photic signals.7, 13, 21 Human circadian rhythms can also be synchronized by photic signals,30, 31, 45, 46 but are mainly determined by social signals, like those involving the senses of sight, sound, smell, or touch (or even other signals like roosters, which signaled the beginning of daily activity in the time before clocks).47
The importance of nonphotic signals can be demonstrated by free-running experiments, in which a group of subjects is isolated from known time clues and cues. When each subject is isolated separately from the others, the circadian rhythm τ differs from 24 h, and it differs also from subject to subject (range 24.3 to 25.4 h).5, 13, 48 In group isolation, the rhythm τ (eg, sleep/wake) differs from 24 h,but is identical for all the subjects in the group (eg, τ=24.8 h),49, 50 ie, social interaction synchronizes the rhythms of subjects living closely in a group. Another nonphotic signal that triggers circadian rhythms, including those of human subjects, is physical activity.51-53 For example, nocturnal activity (15 min/h on ergomet ric bicycle) induced a phase shift of body temperature rhythms in two-thirds of subjects.51 In crewmembers of a transmeridian flight, diurnal outdoor exercise speeds up the resynchronization of the urinary 17-hydroxycorti costeroid circadian rhythm, compared with those without exercise.52
Masking effects
The advantage of a rhythm with the shape of a cosine function was discussed above. However, the patterns of many circadian rhythms deviate from that of an optimal cosine function. In many cases, a secondary peak or shoulder is observed in the 24-h pattern. This shoulder may indicate the presence of additional period component (eg, with τ<20 h), and the rhythm may be defined as a compound rhythm. However, the change may be due to masking effect. Masking is the result of a direct influence of one variable on another, or a direct influence of an external stimulus on a variable, without reference to a rhythmic process.48 In natural settings and habitual life conditions, the body temperature rhythm curve is trapezoidal rather than close to a cosine curve. Mills et al53 and Czeisler and Wright46 proposed a constant routine protocol, where the masking influences of ambient light, temperature, noise, food consumption, and activity level are carefully controlled. Subjects stayed awake in recliners for 24 to 48 h in dim light. In this condition, the unmasked rhythms of, for example, body temperature, exhibited a curve close to a cosine function.
This type of experiment suggests that, in the real world, masking effects may alter the curve of many circadian rhythms. However, it should be noted that the constant routine protocol, which involves sleep deprivation, might alter the circadian period of a set of variables and its adequacy for this study will be discussed in another section of this paper.
Quantification of rhythm parameters with special reference to τ
In circadian rhythm studies, the critical parameter to be quantified is τ. In most investigations, it is assumed that τ=24 h (as a mean) when subjects are synchronized with a diurnal activity and nocturnal rest with stable and regular times (eg, awakening [lights on] at 7 am and retiring [lights off] at 11 pm). Using this procedure, a set of rhythms can be documented in subjects with a sampling interval of, for example, 4 h over a 24- or 48-h period. Using this transverse sampling, other circadian parameters can be computed, such as Φ, A, and 24-h M, provided the parameters exhibit statistically significant rhythms. However, with a transverse sampling of this kind, 24-h rhythm is computed, but not the circadian τ. This can only be obtained by longitudinal sampling over at least 7 days. With these requirements, inter- and intraindividual changes can be taken into account, which is mandatory to document human rhythms in certain circumstances. Prominent τ with the largest A, as well as other periods (with lower As) are quantified from time series by relevant methods including power spectra .5, 6, 16, 26, 28, 44
The precise quantification of τ is critical to problems such as one versus several biological clocks, as well as few versus many clock genes.
One versus several biological clocks in primates
Experiments in rodents yielded a widely accepted model for the control of biological rhythms. According to this model, the SCN functions as a master (central) clock from which slave (peripheral) clocks, or subordinate structures, receive their rhythm characteristics such as the circadian τ, A, and Φ.13, 18, 21, 22, 54 According to Moore and Silver22: “... all of the available data support the view that the SCN is the circadian pacemaker responsible for providing a temporal organization of behavioral, physiological, and endocrine functions. As pacemaker, the SCN sets the phase of oscillators of many physiological and endocrine rhythms in the body.” Transplantation of SCN in hamster τ mutants was associated with a rhythm of activity with the same τ as the donor rather than the host.55 Genetic and molecular studies in rodents support this model. 18, 22, 56, 57
Is this model valid for other mammalian species?
In longitudinal studies, Jouvet et al42 assessed hourly the distribution of PS in cats kept in isolation chambers under continuous light (L:L). Under these conditions, a robust circadian rhythm of PS was detected in all normal cats, and in 4 out of 6 pontine cats (where all neural structures rostral to the pons were removed), as well as in cats without SCN or without hypothalamus. This result is evidence for the presence of a multioscillatory circadian system in this species.
The squirrel monkey, a primate, has a prominent and stable body temperature circadian rhythm.13 After total bilateral SCN lesions, feeding and drinking behaviors lose their circadian rhythms, but the rhythm in body temperature was found to persist when studied over 1 year postlcsion.13 Presumably, in primates, there are other biological clocks outside the SCN, which are responsible for generating a rhythm for temperature, and other variables, such as Cortisol rhythms in the rhesus monkey.58
There is no doubt that the SCN plays an important role because it is the only anatomical structure in which a circadian pacemaker has been identified and it is reset by photic triggers. However, it seems that in cats and primates (and presumably in many other species), other major pacemakers are present.
Desynchronization of human circadian rhythms
Aschoff and Wever recorded rhythms in human subjects individually isolated from known zeitgebers in long-term (>3 weeks) longitudinal experiments.48, 59 They observed that, after a fortnight, 28% of women and 23% of men, exhibited τ =25 h for body temperature rhythm and τ=13 to 36 h for sleep/wake rhythm. Thus, the phase relation between rhythms was distorted compared with the structure of the normal temporal order in the isolated state. On this basis, it was suggested that the two documented rhythms were driven by different biological clocks, a phenomenon called internal desynchronization. 60
External, desynchronization corresponds to a condition in which the phase relation of rhythms are changed by manipulating external synchronizers. This category refers, for example, to a phase shift of at least 5 h due to transmeridian flight or shift work (even if the rhythm τ was not changed) and/or an induced change in τ, becoming longer or shorter than 24 h.
The term desynchronization was used thereafter, to report the experimental fact that, for a set of variables, the (endogenous) circadian τs can differ from one another and from 24 h in the same subject during longitudinal studies, even in the presence of natural zeitgebers. This was documented for circadian rhythms such as activity/rest, body temperature, heart rate, grip strength of both hands, and cognitive performance.48, 61-73
The τ of the circadian rhythm for hand grip strength may even differ between the right and left hands, as well as from 24 h.This was documented in a set of studies involving both Caucasian and Asian shift workers,63, 68, 70 healthy volunteers involved in placebo studies,64 geographers sojourning in the high Arctic summer,65 and saber fencers of the French Olympic team.66 Apart from the night shifts (about 4 nights out of 20) of shift workers,63, 68, 70 all subjects were synchronized with diurnal activity and nocturnal rest. Test times were similar for both hands, eg, 4 to 6 times a day during a 8- to 21 -day span. With regard to the grip strength circadian rhythm, 67 healthy adult males and 24 adult women were investigated. The circadian τ of the dominant hand (DH) differed from 24 h and/or from that of the other hand in 49.2% of male subjects (33/67) and 50% of female subjects (12/24). The circadian period of the nondominant hand (NDH) differed from 24 h and/or from that of the DH in 62.6% of male subjects (33/67) and 62.5% of female subjects (15/24). It should be stressed that the activity/rest rhythmτ, which is presumably controlled by the SCN, was equal to 24 h in 95.6% of the subjects (87/91) involved in the studies.
The finding of a circadian τ that differs among investigated physiological variables has been confirmed by Motohashi,67, 68 in a Japanese population and by Chandrawanshi and Pati,69 in an Indian population.
Thus, generalization of the laboratory rodent model to human beings is inadequate, and the hypothesis has to be modified by stating that: apart from the SCN or in addition to it, circadian rhythms of the human organism may be driven by several clocks, which may differ from each other in their respective τ values.63-69
Functional circadian clocks in the human cortex
One avenue to explore to help understand multibiological clock systems is the difference in the τs for the circadian rhythms of the DH and NDH.The term functional is used here because these clocks do not necessarily have an elective anatomical location, though they are undoubtedly controlled by brain activity. It has been suggested that each of the brain cortex hemispheres has its own biological clock, and differences in the τ of grip strength rhythm may be used to explore the presence of these different clocks. Longitudinal circadian rhythms in reaction time (RT) to light and other signals were documented in two studies, to test the hypothesis that the prominent rhythm τ varies between the DH and the NDH when performing tasks of different complexity. These studies were carried in close cooperation between workers71 at Tel Aviv University and a group investigators72 at the Fondation Adolphe de Rothschild in Paris. The French study72 assessed performance of easy single reaction time (SRT) tests involving a series of 32 yellow light signals following simple and nonvarying instructions; it also assessed the performance of a complex and difficult task, a choice reaction time (CRT) test, involving a series of 96 yellow, red, or green signals following different instructions from test to test, including which hand to use. The Israeli study71 explored DH and NDH RTs of men with an aviation background who were expert in the use of the pilot evaluation system, a flight simulator designed as a modern cockpit with “hands on throttle and stick” instrumentation to test performance under 7 scenarios of varying levels of complexity, from easy to very difficult.
Despite differences in methods, subjects, and data gathering, the two studies yielded similar results. When the task is easy (ie, SRT), the prominent period RT rhythm has τ=24 h for both DH and NDH. When the task is complex and tricky (ie, CRT), the DH maintains a prominent τ=24 h in performance, while the NDH shows a prominent rhythm with τ 24 h, eg, τ=8 h, 6 h, or 12 h. These findings suggest that:
Biological clocks are present in right and left hemispheres of the human cortices.
Functional differences in prominent performance rhythm are task-load-related, and the NDH side is more sensitive than the DH.
The aim of another study73 was to assess the influence of age and gender on the difference in τ for RT of the DH and NDH, in comparison to the grip strength rhythm. Healthy subjects of both genders were involved (9 adolescents [10 to 16 years old] and 15 adults [18 to 67 years old]). They were active between 8 am ±1 h and 11 pm ±1 h; wrist actigraphs were used to assess the activity/rest rhythm, as well as sleep logs. Data were gathered longitudinally at home and work four to seven times daily for 11 to 20 days.
In almost all cases, a 24-h sleep/wake rhythm was detected. For the SRT in adults, a prominentτ=24 h was documented for both DH and NDH, whereas for the CRT a prominent τ=24 h was detected for DH, but τ<24 h for the NDH. This phenomenon was not genderrelated, but was age-related since it was seldom observed in adolescent subjects.
Hand-side differences in grip strength rhythms in the same individuals were detected: τ was ultradian rather than circadian in adolescent subjects, while τ frequently differed from that of the rhythm in CRT in mature subjects.
These findings further support the hypothesis that functional biological clocks with varying periodicities exist in the left and the right hemispheres of the human cortex.
Allochronism versus dyschronism
There is evidence of interest in human biological rhythms and their implications for health and disease in ancient Chinese cultures, since the time of the mythical emperor Chennong (3000 to 4000 years ago). Sickness was related to an alteration of the yin-yang cycles, ie, when they are not in harmony with those of the universe.47 In 1797, Lavoisier and Seguin74 were the first to report a rhythm of “about 24 h” in human body weight. They were so impressed by the regularity of this cyclic phenomenon that they suggested an association of circadian rhythm alterations with states of pain and disease. However, the question of how to handle our biological rhythms to live to a ripe old age and in good health remains unanswered.75
As stated in the introduction, the stable structure of temporal order is highly advantageous for the organism. We have also presented evidence63-73 that desynchonizatlon of a set of human circadian rhythms is rather frequent. Does this mean that a subject with an alteration of temporal organization is a sick (or potentially a sick) person? In the late 1970s, the answer to this question would have been “yes” because the prevailing assumption at that time was that irregularity in a rhythm and/or changes in the temporal organization corresponded to a pathological state, or at least to “... a statistically significant higher (P<0.05) chance of progression toward overt disease.”76 The values of the computed rhythm parameters were averaged from population studies without focusing on interindividual variability. Dyschronism, a term coined by Halberg et al76 was defined as a “time structure (including rhythm) alteration associated with demonstrable physical, physiological, or mental deficit, if not disease.” The definition also states: “Dyschronism is not necessarily a determinant of overt or occult disease.”
To illustrate this definition, one can regard the clinical intolerance to shift work as dyschronism, from the point of view of medical chronobiology.77, 78 Intolerance to shift work was defined by the following symptoms63, 78, 79:
Sleep alterations, like poor sleep quality, difficulty falling asleep when retiring, frequent awakenings.
Persistent fatigue that does not disappear after sleep, weekends, days off, and vacations.
Changes in behavior, consisting of unusual irritability, tantrums, malaise, and feeling of inadequate performance.
Digestive problems (which seem to be less frequent than 20 years ago).
The regular use of sleeping pills (barbiturates, benzodiazepines, phenothiazines, tranquillizers, antidepressants, etc), especially when sleep cannot be controlled or even improved by these medications or others.
Sleep alterations, persistent fatigue, and regular use of sleeping pills (an almost pathognomonic indicator of intolerance to shift work) are present in any intolerant subject. The intensity and number of symptoms vary from subject to subject. 'ITtie occurrence of intolerance to shift work unrelated to age, duration of shift work, type of industry, or type of rotation, including night work. This battery of symptoms was used to clinically validate intolerance to shift work in a set of prospective studies involving more than 140 shift workers.63, 67, 68, 77-79 A good tolerance amounted to 56% and poor tolerance to 46% of this population.
Dyschronism has been documented in male shift workers (age range: 25-58 years) in various types of industry (oil refinery, steel industry, chemical engineering). Four groups were considered: 9 former nontolerant shift workers with diurnal work resumed for at least 18 months; 14 shift workers with good tolerance; 17 shift workers with poor and very poor tolerance (for the latter, symptoms were so severe that a clinical decision was made to transfer them from shift work). For at least 15 days, including 1 or 2 night shifts, circadian rhythms of sleep/wake, oral temperature, and grip strength of both hands were selfrecorded 4 to 5 times per 24 h during the activity span. Prominent circadianτs were plotted in hours (Figure 3) with regard to both variables and tolerance to shift work.63 The τ of the sleep/wake rhythm (not shown) was 24 h for 38 out of 40 subjects. For the group as a whole, only one variable, oral temperature, yielded statistically significant (P<0.029) probability that desynchronization from 24 h is related to intolerance to shift work.
Figure 3. Prominent circadian period x resulting from power spectra analyses of longitudinal time series for 39 subjects. Prominent is for all of the variables and subjects were plotted for each of the four groups and their tolerance to shift work. Gray circles, oral temperature; blue triangles, right-hand grip strength; gray triangles, left-hand grip strength. Reproduced from reference 63: Reinberg A, Motohashi Y, Bourdeleau P, Andlauer P Levi F, Bicakova-Rocher A. Alteration of period and amplitude of circadian rhythms in shift workers with special reference to temperature, right and left hand grip strength. Eur J Appi Physiol. 1988:57:1 5-25. Copyright © 1988, Springer-Verlag.
With regard to interindividual differences, it is clear that desynchronization is frequent and associated with symptoms quoted above in subjects intolerant to shift work, while desynchronization can be present without clinical complaint in tolerant or former shift workers. In many healthy subjects, one or several desynchronized circadian rhythms can be seen (eg, body temperature, grip strength of both hands, or heart rate) without any decrease in performance or any symptom of shift work intolerance or affective disorder.62, 64-66, 78 With the acquisition of new experimental data, it is becoming clear that time-structure variability (presumably genetically controlled) is very common, as are environmentally induced changes without clinical symptoms.
The general practitioner may be bewildered by the inherited variability, the flexibility of the system, and the changes induced. We should therefore distinguish between a normal variability from abnormal (pathological) changes of the temporal organization. In order to achieve this, at least from a conceptual point of view, the idea of allochronism versus dyschronism was introduced.26
We assigned the term allochronism (alio = different) to designate a variant form of alteration in the human temporal organization with no pathological implications. We restrict the term dyschronism (dys = alteration, perturbation) to changes or alterations in the temporal organization associated with a set of symptoms similar to those observed in subjects intolerant to shift work.
Terms like dyschronsis, dyschrony, jet lag, and jet lag syndrome have been used to name transient subjective phenomena that may follow transmeridian flights,38, 80, 81 in which the primary consequence of these time zone changes is fatigue.82 The major effect of a transmeridian flight (>5 time zones) is a Φ shift (phase shift) for the circadian rhythm of most variables.5, 6, 13, 25, 44, 78, 80
The speed (or duration) of adjustment varies among the variables for a given individual, as well among individuals for a given variable. This phenomenon is named transient desynchronization, since in most subjects the changes in the temporal organization will disappear as the subject becomes adjusted to the new local time. Transient desynchronization occurs in all subjects. However, some passengers - about 50% according to Winget et al80 - suffer from the so-called jet lag symptoms until their adjustment is achieved.
Using shift work and jet lag as our experimental models, we focused on the zeitgeber manipulations mainly involved in allochronism and dyschronism. However, other factors are capable of inducing allochronism with a change in the temporal organization without manipulation of zeitgebers. This is the case for age (eg, newborns or the elderly), work load, complexity of task, unusual environment, odd psychological conditions such as that of placebo effect,64 and intake of certain drugs (eg, lithium, P-blockers, or oral contraceptives) .25, 26, 37, 83
We do not yet have a practical diagnostic tool to distinguish between allochronism and dyschronism. There is no doubt that such a tool would be extremely valuable for assigning people to various work tasks and conditions. Dyschronism cannot be applied to all cases in which there is a change in the temporal order, but to individuals who complain of persisting fatigue, sleep, and mood disorders (and other related clinical symptoms); who take sleeping pills or other medications; in whom no direct clinical cause can be documented; and in whom desynchronization of rhythms can be observed. Furthermore, the critical indicative parameter is a change in τ (changes in other rhythm parameters are secondary).
Clinical conditions that miniick those of dyschronism in shift workers
In many diseases and syndromes, patients may be chronically deprived of night sleep. This may be because the patient's condition prevents sleep, rather than because of a sleep disorder per se.40, 84 As even one or two sleepdeprived nights may deeply alter rhythms in body temperature, heart rate, self-rated vigilance, and mood in healthy young subjects,85 this chronically induced sleep deprivation mimics that which occurs in a night worker. In clinical practice, chronic deprivation of night sleep is a rather frequent condition and, as in the case of nontolerant shift workers, it may lead to dyschronism. Using actigraphic recordings, it is possible to evaluate sleep deprivation related to various conditions, for example, sleep deprivation due to pain.86, 87 Nocturnal exacerbation of pain is rather frequent in rheumatology and there are large interindividual differences.87-89 Following oral or head/neck surgery, changes in temporal organization were also observed associated with restless and/or fragmented sleep.90 Likewise, in cancer patients, Mormont et al91 showed that nocturnal sleep disruption is associated with statistically significant alteration in rhythms of melatonin, Cortisol, and circulating lymphocytes.
Although the conventional explanations for the observed alterations are the effects of factors like tumor type or growth rate, or the toxic effects of chemotherapy, the alteration of temporal order due to deprivation of night sleep should not be excluded in this condition.
Thus, dyschronism may be involved in a rather large variety of circumstances, including chronic pain syndrome, nocturnal asthma, persisting anxiety and stress, prostate adenoma, or fibroma with nocturnal urinary voiding.26
Affective disorders and dyschronism
Possible interference and interactions between psychiatric disorders and biological rhythms have been discussed widely.92-95 Special attention has been paid to affective disorders, for which the occurrence of phase shifts or drifts in some circadian rhythms (though not always linked to changes in the circadian τ) have been reported.
The aim was to clarify to what extent rhythm alteration participates in the psychiatric problem.
It has been hypothesized that depression occurs when circadian oscillators are phase advanced relative to environmental zeitgebers.92-94 If this is correct, depression may occur when certain <I>s are phase shifted with respect to one another, as is the case during shift work. In this approach, emphasis is placed upon Φ shifts or drifts in one or several variables, namely phase instability.
Changes in rhythm τ and period instability have also been considered. Pflug96 documented alteration in τ for body temperature rhythm of depressed patients. likewise, Bicakova-Rocher et al97 recorded the body temperature of patients hospitalized for major affective disorders for several days and found that in half of the cases that the temperature τ was shorter than 24 h, while the sleep/wake rhythm τ remained at 24 h. Moreover, improvement in these patients (treated by antidepressant or electroshock therapy) was associated with the reoccurrence of a body temperature rhythm with τ=24 h.
However, not all cases of affective disorders can be classified as dyschronism, because, unlike the intolerance to shift work, which is always accompanied by changes in rhythm τ, in depression (even major affective disorder), only half of all patients present a change in temperature τ.97 Furthermore, in shift workers, dyschronism disappears (both the symptoms and the desynchronization) when the subject returns to regular lifestyle, and medications are ineffective in the treatment of intolerance to shift work. We can thus conclude that there is a strong link between changes in rhythm τ values and clinical symptoms in dyschronism, whereas such a link is not present or else very weak in depressive states and can be evidenced in only a fraction of cases. Consequently, depression and dyschronism presumably represent two different nosological entities.
Putative mechanisms involved in allochronism and dyschronism
In a discussion on depression, Kripke95, 98 raised the idea that it is the individual sensitivity to desynchronization, rather than the desynchronization itself, that tips the scale between the occurrence and nonoccurrence of clinical symptoms. This idea can be extended to interindividual differences in the occurrence of symptoms resulting from intolerance to jet lag, shift work, and disease-related chronic deprivation of night sleep.
Temporal organization variability has been known for many years. Its association with clinical and pathological conditions has also been documented. However, there has been no attempt to array the temporal organization variants and, consequently, no experimental data are available with regard to the mechanisms that underlie this variability. We will offer here some hypotheses and models for possible putative mechanisms involved in allochronism (temporal organization variants without clinical symptoms) and dyschronism (temporal organization variants with clinical symptoms).
Hypothesis
A rather large variety of environmental factors serve as signals that may affect the human temporal organization. Let us assume that two groups, A and B, are exposed to any of these signals. In group A, no changes in the time structure are detected (nonreactors) , while in group B, changes are detected (reactors). Group B can then divided to two subgroups: group Bl , in whom no clinical symptoms or complaints are encountered; and group B2, in whom clinical symptoms and complaints are found. According to our terminology, group B1 should be categorized as having allochronism and group B2 dyschronism.
The presence of interindividual variability (with genderrelated differences) and variability in the propensity of human subjects to exhibit a change (even temporary, ie, reversible),48, 64 suggests the involvement of genetic factors. However, while the mere presence of variability can be explained by simple models of genetic polymorphism, more complicated control mechanisms are needed to explain why some people are more prone to change their temporal organization than others, even if natural zeitgebers are present, and suggest how these changes can be reversed.
Temporal organization variability: a genetic model for allochronism
While evaluating the effects of external signals, some authors forwarded the idea that certain zeitgebers are strong, while others are weak.13, 48 We propose that reference to the strength or weakness of a zeitgeber will not relate to the environmental signal itself, but to the susceptibility of the subject to that zeitgeber. These differences in the level of susceptibility should be channeled to describe differences among the internal oscillators that govern the biological clocks. Hence, strong (stable) oscillators will be defined as those less prone to be affected by changes in external signals, and weak (fragile) oscillators as those which can more readily be affected by any change in external signals.
Our proposal gauges the strength of an oscillator by its capacity to maintain τ=24 h when exposed to many challenging circumstances. As an example of a strong oscillator, we would like to suggest the sleep/wake oscillator. This suggestion is based on the fact that, in our time series analyses, theτ of this rhythm seldom differed from 24 h. Body temperature rhythm can serve as an example of a weak oscillator since documentation has revealed that its τ frequently differs from 24 h.63, 64, 67, 70, 85, 99, 100
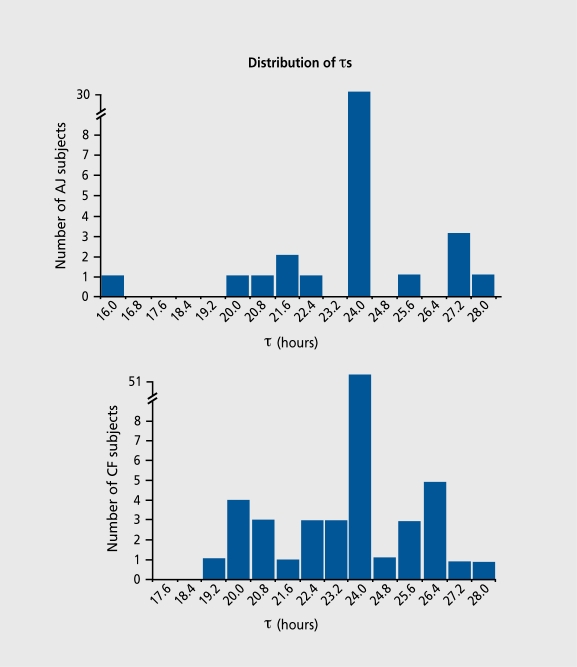
However, within one population, there are interindividual differences with regard to the susceptibility levels of the same oscillator. It seems that the strength or weakness of oscillators does not exhibit a fixed level, but rather a range of levels. To find an explanation for this polymorphic phenomenon, we analyzed individual time series for 69 male Caucasian-French (CF) shift workers16 and 42 male AsianJapanese (AJ) shift workers.67, 68 In 30% of both populations, a change in temporal organization between sleep/wake and oral temperature rhythms was observed. Theτ of the sleep/wake rhythm seldom differed from 24 h (in only 4 subjects of the AJ group and none of the CF group), while in 30% of both populations the τ of the temperature rhythm exhibited deviation from 24 h, which arrayed as a symmetrical distribution around the 24-h value (Figure 4) . In both groups, the interval of the deviations from the predominantly 24-h level clustered in multiples of +0.8 h and -0.8 h (eg, 24+n[0.8 h] yielding τ=24.8 h, 25.6 h, 26.4 h, 27.2 h, 28.0 h, etc; and 24-n[0.8 h], yielding τ=23.2 h, 22.4 h, 21.6 h, 20.8 h, 20.0 h, etc; Figure 4).
Figure 4. Periods of oral temperature rhythm: frequency distribution in Caucasian-French (CF) and Asiatic-Japanese (AJ) subjects. The CF distribution includes theτ frequency distribution of 78 individuals was extracted from data of Ashkenazi et al.16 In both CF and AJ populations, the 0.8 h deviation unit was found to reach a higher degree of statistical significance, with P<0.02 to P<0.Q004 according to tests. Reproduced from reference 68: Motohashi Y, Reinberg AE, Ashkenazi IE, Bikcakova-Rocher A. Genetic aspects of circadian dyschronism: comparison between Asiatic-Japanese and Caucasian-French populations. Chronobiol Int. 1995;12:324-332. Copyright © 1995, Marcel Dekker.
The analyses of these findings resulted in the dian-circadian model, which integrates the function of a constitutive (essential) gene that produces an exact τ=24 h (the dian domain) with a set of polygenes, the alleles of which can add or subtract identical time entities (“[0.8 h]) to the 24h period.16 Such an assembly of genes creates periods ranging from 20 to 28 h in the circadian domain. Further elaboration of this genetic model suggests that these polygenes are usually repressed when natural zeitgebers are present. Induction of these genes will occur under conditions that distort or weaken the perception of the zeitgeber signals. The system will not behave like a “flip-flop” control, but the intensity of its output will depend on the individually related strength of zeitgebers (eg, the time taken for a susceptible individual to exhibit a change in temporal organization in a given situation). This model allows “free running” to be seen as a special case in which the entities of 0.8 h (or multiplications thereof) are always induced. This model differs from conventional models based on attributing changes inτ to the effects of a single mutation. Although the possible presence of a multiple allele system can explain the range of deviation, it will still not be adequate to explain the change and restoration of the period. The polygene system with the inducible-repressible modification seems more appropriate to account for the various changes and dynamics found in rhythm periods.
It is interesting to note that a year after the dian-circadian genetic model was presented, similar thoughts were also presented for rhythm behavior in another species. Emery et al27 were examining a 24-h true-breeding strain of Drosophila melanogaster and reported that “period, phase, definition [the degree to which a rhythmic signal is obscured by noise], and rhythm waveform were all found to vary continuously among the strains, although within each strain the rhythm phenotypc was remarkably consistent.” This continuous variation contrasts with the discrete period of the mutant phenotype reported by Konopka and Benzer.101 This is not cited to compare the results of the two studies in humans16 and Drosophila;27 but to stress that even in Drosophila the oversimplified genetic model does not fit well with the natural genetic variability of the circadian system of this insect species. The advantages of the dian-circadian model reside in:
Providing a better understanding of observed phenomena related to changes in temporal organization and interindividual differences, as well as the effects of jet lag and shift work.
Consideration of the fact that the characteristics of circadian rhythms cannot be reduced to the presence of only one phenotype, but instead relate to predictable phenotypic variability (polymorphisms).102
Conclusion
The present review did not attempt to cover all the concepts - established or contradictory - that prevail in chronobiology. Its aim was to present phenomena that are mainly characteristic and unique to human chronobiology and which cannot be fully explained by concepts and model drawn from laboratory experiments with plants, insects, and rodents. Attention was given to nonphotic signals that play a major role in affecting human biological rhythms, and the range of interindividual variability (with an attempt to offer a genetic model). Special emphasis was placed on distinguishing between states of human health and disease that are connected to changes in temporal organization, and a conceptual classification was suggested for these situations.
Selected abbreviations and acronyms
- A
amplitude
- CRT
choice reaction time
- DH
dominant hand
- L:D
Iight/dark
- M
mean
- NDH
nondominant hand
- Φ
acrophase (peak time)
- PS
paradoxical sleep
- REM
rapid eye movement
- RT
reaction time
- SCN
suprachiasmatic nucleus
- SD
Sprague-Dawley (rat)
- SRT
single reaction time
- τ
period
Contributor Information
Alain Reinberg, Unité de Chronobiologie, Fondation Adolphe de Rothschild, Paris, France; Department of Human Genetics and Molecular Medicine, School of Medicine, Tel Aviv University, Ramat Aviv, Israel.
Israel Ashkenazi, Department of Human Genetics and Molecular Medicine, School of Medicine, Tel Aviv University, Ramat Aviv, Israel.
REFERENCES
- 1.Arendt J., Minors DJ., Waterhouse JM. Basic concepts and implications. In: Arendt J, Minors DJ, Waterhouse JM, eels. Biological Rhythms in Clinical Practice. London, UK: Wright, Butterworth & Co; 1989:3–7. [Google Scholar]
- 2.Haus E., Touitou Y. Principles of clinical chronobiology. In: Touitou Y, Haus E, eds. Biological Rhythms in Clinical and Laboratory Medicine. Berlin, Germany: Springer-Verlag; 1992:6–33. [Google Scholar]
- 3.Aschoff J. Circadian Clocks. Circadian vocabulary. Amsterdam, The Netherlands: North-Holland Publishing Company; 1965 [Google Scholar]
- 4.Halberg F., Tong YL., Johnson EA. Circadian system phase. An aspect of temporal morphology. In: von Mayersbach H, ed. The Cellular Aspect of Biorhythms. Berlin, Germany: Springer-Verlag; 1967:20–48. [Google Scholar]
- 5.Halberg F., Reinberg A. Rythmes circadiens et rythmes de basses fréquences en physiologie humaine, J Physiol (Paris). 1967;59:117–200. [PubMed] [Google Scholar]
- 6.Touitou Y., Haus E. eds. Biological Rhythms in Clinical and Laboratory Medicine. Berlin, Germany: Springer-Verlag; 1992 [Google Scholar]
- 7.Pittendrigh CS. Circadian rhythms and the circadian organization of living systems. Cold Spring Harb Syrnp Quant Biol. 1960;25:159–182. doi: 10.1101/sqb.1960.025.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Edmunds LN Jr. Cellular and Molecular Basis of Biological Clocks. Berlin, Germany: Springer-Verlag; 1987 [Google Scholar]
- 9.Halberg F., Visscher MG., Bittner JJ. Eosinophil rhythm in mice: range of occurrence; effects of illumination, feeding and adrenalectomy. Am J Physiol. 1953;174:313–315. doi: 10.1152/ajplegacy.1953.174.1.109. [DOI] [PubMed] [Google Scholar]
- 10.Aschoff J. Zeitgeber der tierischen Tagesperiodik. Naturwissenschaften . 1954;41:49–56. [Google Scholar]
- 11.Vanden Driessche TH. Les rythmes circadiens, mécanismes de la régulation cellulaire. La Recherche. 1971;2:255–261. [Google Scholar]
- 12.Kerkhof GA. Interindividual differences in the human. In: Sweeney BM, ed. Rhythmic Phenomena in Plants. 2nd ed. San Diego Calif: Academic Press; 1987 [Google Scholar]
- 13.Moore-Ede MC., Sulzman FM., Fuller CA. The Clocks That Time Us. Cambridge, Mass: Harvard University Press; 1992 [Google Scholar]
- 14.Scheving L., Pauly JE. Circadian rhythms of physiological variables as reflected in rat bioassay. In: Altman R, Dittner DS, ed. Biology Data Book, Bethesda, Md: FASEB; 1973;2:1039–1047. [Google Scholar]
- 15.Kerkhof GA. Interindividual differences in the circadian system. Biol Psychol. 1985;20:83–112. doi: 10.1016/0301-0511(85)90019-5. [DOI] [PubMed] [Google Scholar]
- 16.Ashkenazi IE., Reinberg A., Bicakova-Rocher A., Ticher A. The genetic background of individual variations of circadian rhythm period in healthy human adults. Am J Hum Genet. 1993;52:1250–1259. [PMC free article] [PubMed] [Google Scholar]
- 17.Manaker M. Special topic: circadian rhythms. Annu Rev Physiol. 1993;55:657–659. [Google Scholar]
- 18.King DP., Takahashi JS. Forward genetic approach to circadian clocks in mice. Cold Spring Harb Syrnp Quant Biol. 1996;61:295–302. [PubMed] [Google Scholar]
- 19.Young MW. ed. Molecular Genetics of Biological Rhythms. New York, NY: Marcel Dekker; 1993 [Google Scholar]
- 20.Dunlap JC. The genetic analysis of circadian rhythms. Annu Rev Physiol. 1993;55:683–728. doi: 10.1146/annurev.ph.55.030193.003343. [DOI] [PubMed] [Google Scholar]
- 21.Moore RY. Organisation and function of a central nervous system circadian oscillator: the suprachiasmatic hypothalamic nucleus. Fed Proc. 1983;42:2783–2789. [PubMed] [Google Scholar]
- 22.Moore RY., Silver R. Suprachiasmatic nucleus organization. Chronobiol int. 1998;15:475–487. doi: 10.3109/07420529808998703. [DOI] [PubMed] [Google Scholar]
- 23.Pittendrigh CS. Temporal organization: reflections of a Darwinian clockwatcher. Annu Rev Physiol. 1993;55:17–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- 24.Ticher A., Ashkenazi IE., Reinberg A. Preservation of the functional advantage of human time structure. FASEB J. 1995;9:269–272. doi: 10.1096/fasebj.9.2.7781929. [DOI] [PubMed] [Google Scholar]
- 25.Smolensky MH., Lamberg L. The Body Clock Guide to a Better Health. New York, NY: Henry Holt; 2000 [Google Scholar]
- 26.Reinberg A. Chronobiologie Médicale, Chronothérapeutique. Paris, France: Flammarion Médecine-Sciences; 2003 [Google Scholar]
- 27.Emery PTJ., Morgan E., Birly AJ. An investigation of natural genetic variation in the circadian system of Drosophila melanogaster rhythm characteristics and methods of quantification. Chronobiol Int. 1994;11:72–84. doi: 10.3109/07420529409055893. [DOI] [PubMed] [Google Scholar]
- 28.Ashkenazi IE., Reinberg AE., Motohashi Y. Interindividual difference in the flexibility of human temporal organization: pertinence to jet lag and shiftwork. Chronobiol Int. 1997;14:99–113. doi: 10.3109/07420529709001148. [DOI] [PubMed] [Google Scholar]
- 29.Murphy PJ., Campbell SS. Physiology of the circadian system in animals and humans. J Clin Neurophysiol. 1996;13:2–16. doi: 10.1097/00004691-199601000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Turek F. Neurobiology of circadian rhythms in mammals. Bioscience. 1983;33:439–444. [Google Scholar]
- 31.Czeisler CA., Allan JS., Strogatz SH., et al. Bright light resets the human circadian pacemaker independent of the timing of sleep wake cycle. Science. 1986;233:667–671. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- 32.Danilenko KV., Wirz-Justice A., Krauchi K., Weber JM., Terman M. The human circadian pacemaker can see the dawn's early light, J Biol Rhythms. 2000;15:437–446. doi: 10.1177/074873000129001521. [DOI] [PubMed] [Google Scholar]
- 33.Jouvet M. Les fiançailles difficiles de la chronobiologie et de l'hypnologie. Presented at the meeting of La Société Francophone de Chronobiologie. Lyon, France. May. 1998 [Google Scholar]
- 34.Randall W., Johnson RF., Randall S., Cunningham JT. Circadian rhythms in food intake and activity in domestic cats. Behav Neurosci. 1985;99:1162–1174. doi: 10.1037//0735-7044.99.6.1162. [DOI] [PubMed] [Google Scholar]
- 35.Randall W., Cunningham JT., Randall S., Luttschwager J., Johnson RF. A two-peak circadian system in body temperature and activity in the domestic cat Felis catus L. J Therm Biol. 1987;12:27–37. [Google Scholar]
- 36.Simpson HW., Bellamy N., Bohlen J., Halberg F. Double-blind trial of a possible chronobiotic: quidon. Intj Chronobiol. 1973;1:287–311. [PubMed] [Google Scholar]
- 37.Reinberg A., Smolensky MH., Labrecque G. he hunting of the wonder pill for resetting all biological clocks. Ann Rev Chronopharmacol. 1988;4:171–200. [Google Scholar]
- 38.Dawson D., Armstrong SM. Chronobiotics - drugs that shift rhythms. Pharmacol Ther. 1996;69:15–36. doi: 10.1016/0163-7258(95)02020-9. [DOI] [PubMed] [Google Scholar]
- 39.Lewy A., Saeeduddin A., Lathan Jackson JM., Sack RL. Melatonin shifts human circadian rhythms according to a phase response curve. Chronobiol int. 1992;5:380–392. doi: 10.3109/07420529209064550. [DOI] [PubMed] [Google Scholar]
- 40.Benoit O., Foret J. ie. Sommeil Humain. Paris, France: Masson; 1992 [Google Scholar]
- 41.Czeisler CA., Zimmerman JC., Ronda JM., Moore-Ede MC., Weitzman ED. Timing of REM sleep is coupled to the circadian rhythm of body temperature in man. Sleep. 1980;2:329–346. [PubMed] [Google Scholar]
- 42.Jouvet M., Buda C., Sastre JP. Hypothermia induces a quasi permanent paradoxical sleep state in pontine cats. In: Malan A, Canguilhem B, eds. Living in the Cold It Colloque INSERM. Paris, France: John Libbey Eurotext; 1989:487–497. [Google Scholar]
- 43.Lemmer B., Witte K., Schànzer A., Findeisen A. Circadian rhythms in the renin-angiotensin system and adrenal steroids may contribute to the inverse blood pressure rhythm in hypertensive TGR(mREN-2)27 rats. Chronobiol Int. 2000;17:645–658. doi: 10.1081/cbi-100101071. [DOI] [PubMed] [Google Scholar]
- 44.Reinberg AE., Smolensky MH. Biological Rhythms in Medicine. New York, NY: Springer-Verlag; 1983 [Google Scholar]
- 45.Lewy AJ., Wehr TA., Goodwin FK., et al. Light suppress melatonin secretion in humans. Science. 1980;210:1267–1269. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- 46.Czeisler CA., Wright KP. Influence of light on circadian rhythms in humans. In: Turek FW, Zee PC, eds. Regulation of Sleep and Orcadian Rhythms. New York, NY: Marcel Dekker; 1999:149–180. [Google Scholar]
- 47.Reinberg A. Le Temps Humain et les Rythmes Biologiques. Paris, France: Éditions du Rocher; 1998 [Google Scholar]
- 48.Wever RA. The Circadian System of Man. New York, NY: Springer-Verlag. 1979 [Google Scholar]
- 49.Apfelbaum M., Reinberg A., Nillus P., et al. Rythmes circadiens de l'alternance veille-sommeil pendant l'isolement souterrain de sept jeunes femmes. Presse Méd. 1969;77:879–882. [PubMed] [Google Scholar]
- 50.Aschoff J., Fatranska M., Giedke H., et al. Human circadian rhythms in continuous darkness: entrainment by social cues. Science. 1971;171:213–215. doi: 10.1126/science.171.3967.213. [DOI] [PubMed] [Google Scholar]
- 51.Eastman C., Hoese EK., Yougstedt SD., Liu L. Phase shifting human circadian rhythms with exercise during the night shift. Physiol Behav. 1995;58:1287–1291. doi: 10.1016/0031-9384(95)02031-4. [DOI] [PubMed] [Google Scholar]
- 52.Shiota M., Sudou M., Ohshima M. Using outdoor exercise to decrease jet lag in airline crewmembers. Aviat Space Environ Med. 1996;67:1155–1160. [PubMed] [Google Scholar]
- 53.Mills JN., Minors DS., Waterhouse JM. Adaptation to abrupt time shift of the oscillator(s) controlling human circadian rhythms. J Physiol (Lond). 1978;285:455–470. doi: 10.1113/jphysiol.1978.sp012582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rietveld WJ. The suprachiasmatic nucleus and other pacemakers. In: Touitou Y, Haus E, eds. Biological Rhythms in Clinical and Laboratory Medicine. Berlin, Germany: Springer-Verlag; 1992:55–64. [Google Scholar]
- 55.Ralph MR., Foster RG., Davis FC., Manaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 56.Hastings M. The brain circadian rhythms and clock genes. J Mol Biol. 1998;317:1704–1707. doi: 10.1136/bmj.317.7174.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abe H., Honma S., Namihira M., et al. Clock gene expression in the suprachiasmatic nucleus and other areas of the brain during rhythm splitting in CS mice. Mol Brain Res. 2001;87:92–99. doi: 10.1016/s0169-328x(00)00295-3. [DOI] [PubMed] [Google Scholar]
- 58.Reppert SM., Perlow MJ., Underleider LG., et al. Effects of damage to the suprachiasmatic area of the anterior hypothalamus on the daily melatonin and Cortisol rhythms in the rhesus monkey. J Neurosci. 1981;1:1414–1425. doi: 10.1523/JNEUROSCI.01-12-01414.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aschoff J., Wever RA. The circadian system of man. In: Aschoff J, ed. Handbook of Behavioral Neurobiology. Biological Rhythms. London, UK: Plenum; 1981:311–331. [Google Scholar]
- 60.Zulley J., Campbell SS. Napping behavior during “spontaneous internal desynchronization” sleep remains in synchrony with body temperature. Human Neurobiol. 1985;4:123–126. [PubMed] [Google Scholar]
- 61.Folkard S., Wever RA., Wildgruber CH. Multioscillatory control of circadian rhythms in human performances. Nature. 1983;305:223–226. doi: 10.1038/305223a0. [DOI] [PubMed] [Google Scholar]
- 62.Monk T., Weitzman ED., Fookson JE., Moline ML. Circadian rhythms in human performance efficiency under free running conditions. Chronobiologia. 1984;11:343–354. [PubMed] [Google Scholar]
- 63.Reinberg A., Motohashi Y., Bourdeleau P., Andlauer P., Levi F., BicakovaRocher A. Alteration of period and amplitude of circadian rhythms in shift workers with special reference to temperature, right and left hand grip strength. Eur J Appl Physiol. 1988;57:15–25. doi: 10.1007/BF00691232. [DOI] [PubMed] [Google Scholar]
- 64.Reinberg A., Bicakova-Rocher A., Gorceix A., Ashkenazi IE., Smolensky MH. Placebo effect on the circadian rhythm period t of temperature and hand-grip rhythms: interindividual and gender-related differences. Chonobiol Int. 1994;11:54–61. doi: 10.3109/07420529409057230. [DOI] [PubMed] [Google Scholar]
- 65.Reinberg A., Brossard T., André MF., et al. Interindividual differences in a set of biological rhythms documented during the high Arctic summer (79°N) in 3 healthy subjects. Chronobiol Int. 1984;1:127–138. doi: 10.3109/07420528409059130. [DOI] [PubMed] [Google Scholar]
- 66.Reinberg A., Proux S., Bartal JP., Levi F., Bicakova-Rocher A. Circadian rhythms in competitive saber fencers: internal desynchronization and performance. Chronobiol Int. 1985;2:195–201. doi: 10.1080/07420528509055559. [DOI] [PubMed] [Google Scholar]
- 67.Motohashi Y. Desynchronization of oral temperature and grip strength circadian rhythms in healthy subjects with irregular sleep-wake behavior. In: Hayes DK, Pauly JE, Reiter RJ, eds. Chronobiology in Clinical Medicine, General Biology and Agriculture. New York, NY: Wiley-Liss; 1990:57–63. [PubMed] [Google Scholar]
- 68.Motohashi Y., Reinberg AE., Ashkenazi IE., Bikcakova-Rocher A. Genetic aspects of circadian dyschronism: comparison between Asiatic-Japanese and Caucasian-French populations. Chronobiol Int. 1995;12:324–332. [Google Scholar]
- 69.Chandrawanshi A., Pati AK. Could externally desynchronized circadian rhythms be resynchronized in shift workers. Biol Rhythm Res. 2000;31:160–176. [Google Scholar]
- 70.Pati AK., Chandrawanshi A., Reinberg A. Shift work. Consequences and management. Current Sci. 2001;81:32–52. [Google Scholar]
- 71.Shub Y., Ashkenazi IE., Reinberg A. Difference between left- and righthand reaction time rhythms: indications of shifts in strategies of human brain activities. Cogn Brain Res. 1997;6:141–146. doi: 10.1016/s0926-6410(97)00025-6. [DOI] [PubMed] [Google Scholar]
- 72.Reinberg A., Bicakova-Rocher A., Nouguier J., et al. Circadian rhythm period in reaction time to light signal: difference between right- and lefthand side. Cogn Brain Res. 1997;6:135–140. doi: 10.1016/s0926-6410(97)00024-4. [DOI] [PubMed] [Google Scholar]
- 73.Reinberg A., Bicakova-Rocher A., Mechkouri M., Ashkenazi I. Right- and left-brain hemisphere. Rhythms in reaction time to light signals is task load dependant: age, gender, and hand grip strength rhythm comparison. Chronobiol Int. 2002;19:1087–1106. doi: 10.1081/cbi-120015959. [DOI] [PubMed] [Google Scholar]
- 74.Lavoisier A., Seguin A. Sur la transpiration des animaux. Mémoires de l'Académie des Sciences Paris. 1797:601–612. [Google Scholar]
- 75.Monk TH., Reynolds FR III., Kupfer DJ., Hoch CC., Carrier J., Houyck PR. Differences over the life span in daily life-style regularity. Chronobiol Int. 1997;14:295–306. doi: 10.3109/07420529709001421. [DOI] [PubMed] [Google Scholar]
- 76.Halberg F., Carandente F., Cornelissen G., Katinas GS. Glossary of chronobiology. Chronobiologia. 1977;4 (suppl 1):1–189. [PubMed] [Google Scholar]
- 77.Reinberg A. ed. Chronobiological field studies of oil refinery shift workers. Chronobiologia. 1979;6(suppl1):1–122. [Google Scholar]
- 78.Reinberg AE., Smolensky MH. Night and shift work and transmeridian flights. In: Touitou Y, Haus E, eds. Biological Rhythms in Clinical and Laboratory Medicine. Berlin, Germany: Springer-Verlag; 1992:243–255. [Google Scholar]
- 79.Andlauer P., Reinberg A., Fourré L., Battle W., Duverneuil G. Amplitude of the oral temperature circadian rhythm and tolerance to shift work, J Physiol (Paris). 1979;75:507–512. [PubMed] [Google Scholar]
- 80.Winget CM., Deroshia CW., Markley CL., Holley DC. A review of human physiological and performance changes associated with desynchronosis and biological rhythms. Aviat Space Environ Med. 1994;55:1085–1096. [PubMed] [Google Scholar]
- 81.Monk T. Traffic accident increases as a possible indicant of dyschronosis. Chronobiologia. 1980;7:527–529. [PubMed] [Google Scholar]
- 82.Reinberg A. Le rythme circadien de la fatigue. In: Serratrice G, Vildé JL, eds. Chronic Fatigue Syndrome. Paris, France: Springer-Verlag; 1991:61–82. [Google Scholar]
- 83.Labrecque G., Sirois-Labrecque M. eds. Chronopharmacologie. Montreal, Canada: Presse de L'Université de Montréal; 2003 [Google Scholar]
- 84.Turek FW., Zee PC. eds. Regulation of Sleep and Circadian Rhythms. New York, NY: Marcel Dekker; 1999 [Google Scholar]
- 85.Benoit O., Foret J., Merle B., Reinberg A. Circadian rhythms (temperature, heart rate, vigilance, mood) of short and long sleepers: effects of sleep deprivation. Chronobiologia. 1981;8:341–350. [PubMed] [Google Scholar]
- 86.Sadeh A., Hauri PJ., Kripke F., Lavie P. The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995;18:288–302. doi: 10.1093/sleep/18.4.288. [DOI] [PubMed] [Google Scholar]
- 87.Labrecque G., Karzazi M., Vanier MC. Biological rhythms in pain and analgesia. In: Redfern PH, Lemmer B, eds. Physiology and Pharmacology of Biological Rhythms. Berlin, Germany: Springer-Verlag; 1997:619–650. [Google Scholar]
- 88.Hermann D., Chan W., Smolensky MH. Twenty-four-hour wrist actigraphy for evaluation of nocturnal sleep of pain patients. 6th International Conference on Chronopharmacology. Amelia Island, Fla. 1994. Abstract XIII-2 [Google Scholar]
- 89.Lavie P., Lorber M., Tzischinsky O., Epstein R., Sharf Y. Wrist actigraphic measurements in patients with rheumatoid arthritis: a novel method to assess drug efficacy. Drug Invest. 1992;(suppl2):15–21. [Google Scholar]
- 90.Farr LA., Todero CM., Boen LM. Advancement of circadian rhythms reduces disruption and improves recovery from surgery. 6th International Conference on Chronopharmacology. Amelia Island, Fla. 1994. Abstract XV-4 [Google Scholar]
- 91.Mormont MC., Claustrât B., Waterhouse JM., et al. Clinical relevance of circadian rhythm assessment in cancer patients. In: Touitou Y, ed. Biological Clocks: Mechanisms and Applications. Amsterdam, The Netherlands: Elsevier; 1998:497–505. [Google Scholar]
- 92.Wehr TA., Goodwin FK. eds. Biological Rhythms in Psychiatry. Pacific Grove, Calif: Boxwood Press; 1983 [Google Scholar]
- 93.Halaris A. ed. Chronobiology and Psychiatric Disorders. New York, NY: Elsevier Science; 1987 [Google Scholar]
- 94.Taillard J., Lemoine P., Boule P., Drogue M., Mouret J. Sleep and heart rate circadian rhythm in depression: the necessity to separate. Chronobiol Int. 1993;10:63–72. doi: 10.3109/07420529309064483. [DOI] [PubMed] [Google Scholar]
- 95.Kripke DF. Phase-advance theory for affective illnesses. In: Wehr TA, Goodwin FK, eds. Biological Rhythms in Psychiatry. Pacific Grove, Calif: Boxwood Press; 1983:41–69. [Google Scholar]
- 96.Pflug B. Circadian rhythms in depression. In: Rensing L, der Heiden U, Mackey MC, eds. Temporal Disorders in Human Oscillatory System. Berlin, Germany: Springer-Verlag; 1987:194–201. [Google Scholar]
- 97.Bicakova-Rocher A., Gorceix A., Reinberg A., Ashkenazi IA., Ticher A. Temperature rhythms of patients with major affective disorders: reduced circadian period length. Chronobiol Int. 1996;13:47–57. doi: 10.3109/07420529609040841. [DOI] [PubMed] [Google Scholar]
- 98.Kripke DF., Mullaney DJ., Savides TJ., Gillin JC. Phototherapy for nonseasonal major depressive disorders. In: Rosenthal NE, Blehar MC, eds. Seasonal Affective Disorders and Phototherapy. New York, NY: Gilford Press; 1989:342–356. [Google Scholar]
- 99.Johnson A., Engelman W., Pflug B., Klemke W. Influence of lithium ions on human circadian rhythms. Z Naturforsch (C). 1980;35:503–507. doi: 10.1515/znc-1980-5-623. [DOI] [PubMed] [Google Scholar]
- 100.Reinberg A., Smolensky M. Chronobiology and thermoregulation. In: Schonbaurn E, Lomax P, eds. Thermoregulation Physiology and Biochemistry. New York, NY: Pergamon Press; 1990:61–100. [Google Scholar]
- 101.Konopka RJ., Benzer R. Clock mutant of. Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971;58:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brok-Simoni F., Ashkenazi IE., Ramot B., Holtzman F. The diurnal rhythm of enzymes in human red blood cells: in vivo studies. Br J Haematol. 1976;32:601–605. doi: 10.1111/j.1365-2141.1976.tb00964.x. [DOI] [PubMed] [Google Scholar]