Abstract
Depressive disorders are a leading cause of disability worldwide and greatly impact morbidity, health care utilization, and medical costs. Major depression that does not resolve with adequate antidepressant treatment is termed treatment-resistant depression (TRD), There is no universally accepted definition of TRD and several criteria have been suggested to define it. Multiple factors can contribute to treatment resistance, including unrecognized comorbid medical or psychiatric illness, the use of concomitant medications, noncompliance, and psychosocial stressors. TRD is associated with extensive use of depression-related and general medical services, and poses a substantial economic burden. Current approaches to its management include the use of antidepressant strategies, such as increasing the dose of the antidepressant, augmentation strategies, combination strategies, and switching strategies, electroconvulsive therapy, and cognitive behavioral therapy. Although no definite algorithm exists for treating TRD, research in this area has advanced considerably in recent years. One approach to this is a clinical trial called STAR*D (Sequenced Treatment Alternatives to Relieve Depression). This has the potential to increase our understanding about the diagnostic and therapeutic aspects of TRD, to substantially reduce disability, and to enhance the quality of life in individuals with this condition.
Keywords: treatment-resistant depression, management strategy, antidepressant treatment
Abstract
Los trastornos depresivos constituyen una de las principales causas de discapacidad en el mundo y tienen un gran impacto en la morbilidad, en la utilización de servicios de salud yen los costos médicos. La depresión mayor que no se resuelve con un adecuado tratamiento antidepresivo se denomina depresión resistente al tratamiento (DRT), No existe una definición de DRT que sea aceptada universalmente y se han sugerido algunos criterios para definirla. Múltiples factores pueden contribuir a la resistencia al tratamiento, incluyendo la comorbilidad médica o psiquiátrica no reconocida, tratamiento concomitante, la falta de adherencia y los estresores psicosocíales. La DRT se asocia con una amplia utilización de servicios médicos generales y los relacionados con depresión, e implica una significativa carga económica. Las aproximaciones actuales a su manejo incluyen el empleo de estrategias antidepresivas, como el aumento de la dosis de antidepresivos, cambios de tratamiento, estrategias de potenciación, asociaciones de tratamientos, terapia electroconvulsiva y terapia cognitivo conductual. Aunque no existe un algoritmo definitivo para tratarla DRT, la investigación en esta área ha avanzado considerablemente en los años recientes. Una aproximación a esto es el ensayo clínico STAR*D (Sequenced Treatment Alternatives to Relieve Depression). Este proyecto tiene el potencial de aumentar nuestro conocimiento acerca de los aspectos diagnósticos y terapéuticos de la DRT, de reducir significativamente la díscapacidad y de elevar la calidad de vida de los individuos con esta condición.
Abstract
Les troubles dépressifs sont une cause majeure d'incapacité à travers le monde et ont un fort impact sur la morbidité, la consommation des services médicaux et les coûts médicaux. Une dépression majeure qui ne se résout pas avec un traitement antidépresseur adapté est dite «dépression résistant au traitement» (DRT), il n'y a pas de définition de la DRT universellement acceptée et plusieurs critères ont été suggérés pour la définir. De nombreux facteurs peuvent contribuer à la résistance au traitement, tels qu'une pathologie médicale ou psychiatrique associée méconnue, la prise d'autres médicaments, la non-observance du traitement et des facteurs psychosociaux de stress. La DRT est associée à une consommation importante de services tant en médecine générale que spécifiquement liés à la dépression, et représente de ce fait une charge économique importante. La conduite à tenir actuelle devant une DRT fait appel à toute une gamme de stratégies antidépressives: augmentation de la dose de l'antidépresseur; stratégies de potentialisation, d'association et de substitution; électrochocs; et thérapie cognitivocomportementale. Bien qu'aucun algorithme défini n'existe pour traiter la DRT, la recherche dans ce domaine a considérablement progressé ces dernières années. Une des approches est une étude clinique appelée STAR*D (Sequenced Treatment Alternatives to Relieve Depression). Celle-ci devrait permettre d'approfondir notre compréhension des aspects thérapeutiques et diagnostiques de la DRT, réduire considérablement l'incapacité et améliorer la qualité de vie des personnes atteintes.
Depressive disorders are a leading cause of disability worldwide.1,2 By the year 2020, unipolar major depression is projected to be the second leading cause of disability-adjusted life years (DALYS) all over the world.1 Depressive disorders greatly impact morbidity, health care utilization, and medical costs.
Despite advances in psych opharmacology and the reported high rates of treatment success (usually between 50% and 70%), the general rule of thumb is that less than half of patients beginning a course of antidepressant treatment will reach remission with that treatment.3 This implies that a significant proportion of depressed patients either do not respond or continue to have residual symptoms despite treatment with antidepressants. Major depression that does not resolve with adequate antidepressant treatment is termed treatment-resistant depression (TRD).
There is no universally accepted definition for TRD. Several criteria have been suggested, including failure of at least, one adequate antidepressant trial, two adequate antidepressant, trials, a trial with a monoamine oxidase inhibitor, lithium, or the newer heterocyclics, or at least one trial of electroconvulsive therapy (ECT).4 However, failure of at least, one adequate antidepressant trial appears to be emerging as the consensus operational criterion for TRD.5 Long-term studies indicate that 20% of patients with major depression remain unwell 2 years after the onset, of the illness.6
TRD is associated with extensive use of depression related and general medical services, and poses a substantial economic burden. The psychiatrist, managing these patients is faced with therapeutic challenges and dilemmas. Several management strategies have been suggested for patients with resistant depression, but there is no definite algorithm for treatment. However, research in this area has advanced considerably and has the potential to enhance our understanding about the diagnostic and therapeutic aspects of resistant depression.
We review some of the terms used to define TRD, its prevalence, etiology and impact on the patient and society, and current approaches in management, including antidepressant treatment strategies.
Review of TRD terminology
Absolute and relative treatment resistance. Absolute treatment resistance is defined as failure to respond to one adequate antidepressant trial (ie, 20-40 mg fluoxetine or its equivalent, or 4 weeks of 150 mg imipramine or its equivalent) and relative treatment resistance is defined as nonresponse to an inadequate treatment.5,7
Treatment-refractory depression. This is defined as failure to respond to two drugs of different, pharmacological classes, each used in an adequate dose for an adequate duration.8
Adequate dose . This is defined as the standard recommended dose of the antidepressant (significantly superior to placebo in double-blind trials).5
Adequate duration of treatment. Adequate duration of treatment is defined as at least four consecutive weeks of treatment, during which the patient has had an adequate dose for at least 3 weeks.5
Response. Response is defined as a ≥50% reduction in the Hamilton Rating Scale for Depression (HAM-D) score, a posttreatment HAM-D score of ≤7 or a score of ≤2 (ie, much improved) on the Clinical Global Impressions (CGI) scale.8
Remission, recovery, relapse, and recurrence. Remission is defined as a period during which the patient is asymptomatic (with a 17-item HAM-D [HAM-D-17] score ≤7), lasting >2 weeks, but <6 months. Recovery is a period of remission >6 months. Return of depressive symptoms meeting criteria for major depression during the period of remission is termed relapse; if it occurs during the recovery period, it is termed recurrence.9-11
Factors contributing to TRD
TRD is likely to be due to multiple factors. These can broadly be divided into factors related to the illness, factors related to treatment, and patient and environmental factors. However, usually, a combination of these factors are involved in treatment, resistance.
Factors related to the illness
Unrecognized comorbid medical illness either causing or exacerbating psychiatric syndromes can occur in up to 46% of psychiatric inpatients12 and can contribute to TRD. These include hypercholesterolemia, endocrine disorders, such as hypothyroidism, subclinical hypothyroidism, diabetes, and Cushing's syndrome, Parkinson's disease, Huntington's disease, dementia, cerebrovascular disease, and seizure disorder.13,14 Comorbid psychiatric disorders, such as bipolar disorder, substance abuse or dependence, eating disorders, obsessive-compulsive disorder, and panic disorder, may also contribute to treatment resistance.5,15
An association between treatment resistance and specific depressive subtypes has been reported by researchers.5 Atypical depression with panic may be relatively resistant, to tricyclic antidepressants (TCAs),but responsive to monoamine oxidase inhibitors (MAOIs).16 Psychotic and melancholic depression may also be resistant to treatment, requiring the use of additional treatment strategies. Factors such as greater number of somatic symptoms and reported history of childhood emotional abuse and sequelae of that abuse may be associated with treatment resistance in depressed outpatients.17,18
Factors related to antidepressant treatment
Up to 20% of patients who are termed treatment, resistant may actually be intolerant to the medication.19 The use of concomitant medications may interfere with the absorption and metabolism of antidepressants, and interfere with response. Inadequate treatment of earlier episodes may lead to treatment resistance possibly due to kindling and sensitization at the receptor and synaptic levels.20
Factors related to the patient and environment
These include partial compliance or noncompliance, rapid metabolism, and the presence of severe psychosocial stressors. Partial compliance or noncompliance are important causes of treatment resistance, as up to 50% of patients do not take the medication as prescribed, and tend to stop treatment when symptoms remit.7 Individual differences in drug metabolism may result, in suboptimal blood levels in patients who are rapid metabolizers and contribute to treatment resistance.7 Nutritional status of the patient must be assessed, as deficiencies in folate, thiamine, vitamin B6, vitamin B12, copper, and zinc may contribute to treatment resistance.21,22. The presence of psychosocial stressors and the relative absence of family support may also predict poor outcome for depressed patients.23
Brain imaging studies
Although neuroimaging is a useful tool to assess brain function in depression, few published brain imaging studies have compared brain function in TRD and treatmentresponsive or non-treatment-resistant depression (non-TRD). Shah and coworkers found that patients with chronic TRD had reduced gray matter density in the left temporal cortex including the hippocampus, with a trend toward reduction in the right hippocampus.24 These authors also reported right frontostriatal atrophy and subtle magnetic resonance imaging (MRI) changes in the left hippocampus among patients with resistant depression.25 A single photon emission computed tomography (SPECT) found a significant increase in hippocampusamygdala activity in TRD patients compared with non-TRD patients and healthy controls.26 A positron emission tomography (PET) study of depressed patients with TRD, non-TRD, and control subjects found that rostral anterior cingulate metabolism uniquely differentiated eventual treatment responders from nonresponders; nonrcsponders showed hypometabolism and responders showed hypermetabolism compared with controls.27 The authors reported that, cingulate hypometabolism may represent an important adaptive response to depression and failure of this response may underlie poor outcome.27
Impact of TRD
TRD is associated with extensive use of depression-related and general medical services and poses a substantial economic burden. A naturalistic, retrospective analysis of medical claims data by Crown and colleagues found that treatment-resistant patients were at least twice as likely to be hospitalized (general medical and depression related) and had at least 12% more outpatient visits.15 Treatment resistance was also associated with use of 1.4 to 3 times more psychotropic medications (including antidepressants). Patients in the hospitalized TRD group had over 6 times the mean total medical costs of non-TRD patients (US$ 42 344 versus US$ 6512) and their total depression-related costs were 19 times greater than those of patients in the comparison group (US$ 28 001 versus US$ 1455).15 These findings emphasize the need for early identification and effective long-term maintenance strategies for TRD.
Approaches in the management of TRD
General principles
The general principles of the management of TRD are outlined in Table I.
Table I. General principles for the management of treatment-resistant depression.
| Ensure accurate diagnosis, including subtype of depression |
| Assess comorbid psychiatric and medical conditions |
| Evaluate psychosocial stressors and enhance social support |
| Ensure adequate dose and duration of treatment |
| Monitor and treat adverse events |
| Educate the patient regarding depression and antidepressants |
| Ensure compliance |
| Aim for remission |
Antidepressant treatment strategies
Increasing the dose of antidepressant
Increasing the dose of antidepressant is a common strategy for patients who have not responded to an adequate trial with a selective serotonin reuptake inhibitor (SSRI).28 In patients who had not responded to fluoxetine 20 mg/day, Fava and colleagues showed that raising the dose of fluoxetine to 40 to 60 mg/day was significantly more effective than adding desipramine 25 to 50 mg/day or lithium 300 to 600 mg/day29 No guidelines exist regarding the adequate duration of higher-dose antidepressant treatment, but 6 weeks is likely to be sufficient.30 Following response, treatment at the same dose can be maintained for 6 to 9 months, followed by tapering of the dose; if the patient, has a history of recurrent or chronic depression, then a longer duration of treatment must be considered.30
Augmentation strategies
Augmentation involves adding another agent to an ongoing antidepressant treatment that, has failed. Lithium augmentation is a commonly used strategy to treat resistant depression, with a long history of small controlled trials and anecdotal reports on benefits of lithium augmentation.31,32
Thyroid hormone augmentation of antidepressants has been reported since the late 1960s. Altshuler and colleagues summarized the early literature on triiodothyronine (T3), mainly small studies carried out many years ago, demonstrating an acceleration of time to response to antidepressants.33 In a more recent trial, Lasser and Baldessarini also showed an accelerated response to the combination of TCA and T3. This study showed a benefit of T3 used to augment partial or incomplete response to TCA monotherapy.34
Stimulants such as amphetamine, methylphenidate, and pemoline, if used judiciously, and taken responsibly, can be effective in achieving a quicker response in patients with resistant, depression. These should be avoided in patients with a history of substance abuse and patients should be informed about the potential, though minimal, risk of developing tolerence.35-37 There has also more recent interest, in exploring modafanil for TRD.38
Evidence indicates that dopamine may have a role in the pathogenesis of depression.39 Dopaminergic agents such as bromocriptine, pergolidc, and pramipexole are reported to be useful adjuncts for patients with TRD.40 The serotonin-dopamine receptor antagonism of atypical antipsychotics has been suggested as a possible mechanism for the antidepressant action of atypical antipsychotics. Recent studies have found that atypical antipsychotics may produce an antidepressant effect in schizophrenia and may be used either as an adjunctive medication or as an alternative to mood stabilizers in patients with affective disorders.41 Treatment-resistant psychotic depression is shown to be successfully treated with clozapine.42 The addition of risperidone to an SSRI among nonpsychotic depressed patients led to a rapid response among patients who had not responded to either fluoxetine or paroxetine treatment.43 A combination of olanzapine and fluoxetine showed superior efficacy to either olanzapine or fluoxetine monotherapy in patients with treatment-resistant depressive disorder without psychotic features.44 The presence of psychotic features, delusional depression, and bipolar depression may be indications for the use of atypical antipsychotics.7 Other augmentation strategies include buspirone, pindolol, venlafaxine, mirtazapine, tianeptine, benzodiazepines, and anticonvulsant augmentation of antidepressants.30
Combination strategies
This involves the addition to an antidepressant of a compound with a well-established efficacy as a single agent in the treatment of depression.30 Combination strategies include TCAs and SSRIs,TCAs and MAOIs, bupropion and SSRIs, anticonvulsants and antidepressants, and benzodiazepines and antidepressants.5 However, SSRIs, venlafaxine, or clomipramine should not be combined with MAOIs and the MAOI and TCA combination should be used with caution.
Switching strategies
Switching involves stopping the antidepressant to which the patient is not responding and switching to another antidepressant, usually from a different class.45 Switching to an alternative antidepressant from a different class for SSRI nonresponders may be helpful.5 The options for SSRI nonresponders include using bupropion, nefazodone, venlafaxine, tianeptine, and mirtazapine. MAOIs may be used in TCA- or SSRI-resistant patients. However, dietary restrictions are essential and an appropriate washout period is required after SSRI discontinuation before initiating treatment with MAOIs.
Other treatment modalities
Electroconvulsive therapy
ECT is a potent though underutilized, option for resistant depression. A substantial amount of research has demonstrated the short-term efficacy and safety of ECT in the acute setting. It must be considered in depressed patients who are suicidal, psychotic, or pregnant, or have a medical illness.46,47 However, a complete medical history and a thorough physical examination is required to assess the risks of anesthesia, and cardiovascular and neurological adverse events associated with ECT. It is also important to be aware of the potential drug-ECT interactions, especially in medically ill or elderly patients who are on concomitant medication.48 Common adverse events associated with ECT are headache, temporary confusion, delirium, and transient memory impairment.49-51 Interestingly, nonresponse to pharmacotherapy is highly associated with nonresponse to ECT52
Newer biological approaches
These include repetitive transcranial magnetic stimulation (rTMS)53 and vagus nerve stimulation,54 which have been proposed as alternatives to ECT and are currently under investigation.
Novel psychopharmacological agents
Novel psychopharmacological agents for resistant depression include S-adenosylmethionine (SAMe), second-messenger system modulators (inositol), and neuroendocrine system-modulating agents, eg,dexamcthasone.55
Cognitive behavioral therapy
The purpose of cognitive behavioral therapy (CRT) is to help patients understand the inaccuracy of their cognitive assumptions and learn new ways and strategies of dealing with issues. CBT is a short-term, structured therapy, which involves active collaboration between patient and therapist to achieve therapeutic goals; these are oriented toward current, problems and their resolution. A trial of CBT may be considered in patients with TRD, perhaps with modifications in treatment planning to take into account, its complexity56,57
Evidence-based algorithm
Where does all this leave us with respect to TRD? In general, we have good data on acute treatment, suggestive data, on some second-level strategies, and good data, on ECT as a final step in a treatment strategy. This is far from ideal and not typical of many other areas of medicine, such as cardiology and oncology. A clear priority for our field is the development of an empirically validated treatment algorithm, with clear evidence that guides the choice of approaches at any point in the treatment process.
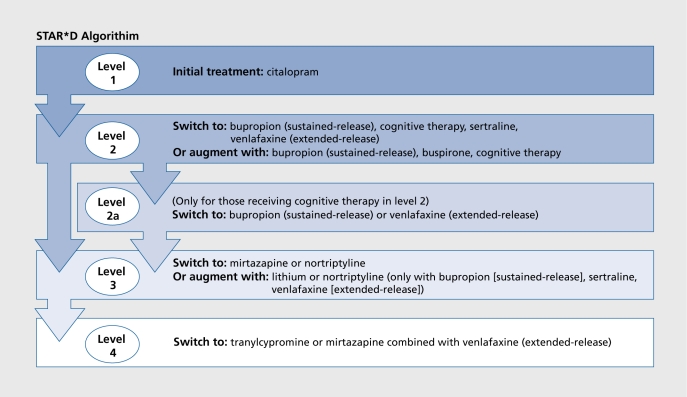
One approach to this is a clinical trial called STAR*D (Sequenced Treatment Alternatives to Relieve Depression) (Figure 1).58,59 STAR*D trial will enroll more than 4000 adults with major depression in the USA. They will be treated openly and aggressively in level 1 for up to 1.2 weeks with SSRI (citalopram) monotherapy. Those who achieve remission will be followed naturalistically for 12 months. Using an innovative statistical procedure called “equipoise randomization,”60 those who do not achieve a satisfactory response will be randomized in level 2 to 1 of 7 treatments: 4 switch options (venlafaxine, sertraline, bupropion, or cognitive therapy) and 3 augment options (cognitive therapy, bupropion, or buspirone added to the citalopram). Thus, in level 2 we will assess the benefit of the different, strategies (switch or augment) and will compare the benefit of individual treatment options within and between these strategies. Those with a satisfactory response will be followed naturalistically for 12 months. Those who do not have a satisfactory response will enter level 3. In level 3, we compare 2 switch options (mirtazapine or nortriptyline) and 2 augment options (T3 and lithium). Those who remit will be followed and those remaining will enter level 4 in which we compare 2 additional switch options: tranylcypromine and the combination of mirtazapine and venlafaxine. The design is summarized in reference 59 and is reproduced in Figure 1.
Figure 1. STAR*D (Sequenced Treatment Alternatives to Relieve Depression) algorithm.59Reproduced from reference 59: Rush AJ, Trivedi M, Fava M. Depression IV: STAR*D treatment trial for depression, [images in Neuroscience]. Am J Psychiatry- 2003;1 60:237. Copyright © 2003, American Psychiatric Association.
Details of the design and rationale have been published elsewhere58; basically the study is being carried out in 12 sites throughout, the USA. Each site serves as the hub for as many as 4 clinics with both primary care practices and specialty practices represented. The first patient enrolled in STAR*D in July 2001 and so the results will not be available for several years.
Conclusions
Treatment-resistance is highly prevalent in depression; it is costly and is associated with extensive use of depression-related and general medical services. It poses unique therapeutic challenges and dilemmas in its management.
Early identification and the use of effective long-term maintenance strategies are important. Decisions regarding treatment, including increase in dosage, antidepressant augmentation, switching to a different class of antidepressants, combination strategies, or other biological treatments and psychotherapeutic treatments should be made appropriately in the course of illness. Although no definite algorithm exists for treating resistant depression, research in this area has advanced considerably in recent years. This has the potential to enhance our understanding about the diagnostic and therapeutic aspects of TRD, to substantially reduce disability in this condition, and to enhance the quality of life of individuals with this condition.
Selected abbreviations and acronyms
- CGI
Clinical Global Impressions (scale)
- ECT
electroconvulsive therapy
- HAM-D
Hamilton Rating Scale for Depression
- MAOI
monoamine oxidase inhibitor
- SSRI
selective serotonin reuptake inhibitor
- T3
triiodothyronine
- TCA
tricyclic antidepressant
- TRD
treatment-resistant depression
Contributor Information
Rajesh M. Parikh, Honarary Consultant Psychiatrist and Neuropsychiatrist, Jaslok Hospital and Research Center, Dr G. Deshmukh Marg, Bombay, India.
Barry D. Lebowitz, National Institute of Mental Health, Bethesda, Md, USA.
REFERENCES
- 1.Murray JCL., Lopez AD. The Global Burden of Disease in 1990. Final results and their sensitivity to alternative epidemiological perspectives. Distant rates, age weights, and disability weights. In: Murray JCL, Lopez AD, eds. The Global Burden of Disease. Cambridge, Mass: Harvard University Press; 1996:247–296. [Google Scholar]
- 2.World Health Organization (WHO). World Health Report Mental Health: New Understanding, New Hope. Geneva, Switzerland: WHO; 2002 [Google Scholar]
- 3.Khan A., Khan SR., Walens G., et al. Frequency of positive studies among fixed and flexible dose antidepressant clinical trials: an analysis of the Food and Drug Administration summary basis of approval reports. Neuropsychopharmacology. 2003;28:552–557. doi: 10.1038/sj.npp.1300059. [DOI] [PubMed] [Google Scholar]
- 4.Nelsen MR., Dunner DL. Treatment resistance in unipolar depression and other disorders. Diagnostic concerns and treatment possibilities. Psychiatr Clin N Am. 1993;16:541–566. [PubMed] [Google Scholar]
- 5.Fava M., Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. 1996;19:179–200. doi: 10.1016/s0193-953x(05)70283-5. [DOI] [PubMed] [Google Scholar]
- 6.Paykel ES. Epidemiology of refractory depression. In: Nolen WA, Zohar J, Roose SP, Amsterdam JD, eds. Refractory Depression: Current Strategies and Future Directions. New York, NY: John Wiley and Sons; 1994:3–7. [Google Scholar]
- 7.Sonawalla SB., Fava M. La depression résistante [Resistant depression]. In: Olié JP, Dalery J, Azorin JM, eds. Medicaments antipsychotiques. Paris, France: Pub Acanthe; 2001:459–465. [Google Scholar]
- 8.Thase ME., Rush AJ. Treatment-resistant depression. In: Bloom FE, Kupfer DJ, eds. Psychopharmacology: Fourth Generation of Progress. New York, NY: Raven Press; 1995:1081–1097. [Google Scholar]
- 9.Frank E., Prien RF., Jarrett RB., et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48:851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- 10.Kupfer DJ., Frank E. The interaction of drug and psychotherapy in the long-term treatment of depression. J Affect Disord. 2001;62:131–137. doi: 10.1016/s0165-0327(00)00357-8. [DOI] [PubMed] [Google Scholar]
- 11.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):10–17. [PubMed] [Google Scholar]
- 12.Hall RC., Gardner ER., Popkin MK., Lecann AF., Stickney SK. Unrecognized physical illness prompting psychiatric admission: a prospective study. Am J Psychiatry. 1981;138:629–635. doi: 10.1176/ajp.138.5.629. [DOI] [PubMed] [Google Scholar]
- 13.Ghoge H., Sharma S., Sonawalla S., Parikh R. Cerebrovascular diseases and depression. Curr Psychiatry Rep. 2003;5:231–238. doi: 10.1007/s11920-003-0048-7. [DOI] [PubMed] [Google Scholar]
- 14.Sonawalla SB., Papakostas Gl., Petersen TJ., et al. Elevated cholesterol levels associated with nonresponse to fluoxetine treatment in major depressive disorder. Psychosomatics. 2002;43:310–316. doi: 10.1176/appi.psy.43.4.310. [DOI] [PubMed] [Google Scholar]
- 15.Crown WH., Finkelstein S., Berndt ER., et al. The impact of treatment-resistant depression on health care utilization and costs. J Clin Psychiatry. 2002;63:963–971. doi: 10.4088/jcp.v63n1102. [DOI] [PubMed] [Google Scholar]
- 16.Liebowitz MR., Quitkin FM., Stewart JW., et al. Antidepressant specificity in atypical depression. Arch Gen Psychiatry. 1988;45:129–137. doi: 10.1001/archpsyc.1988.01800260037004. [DOI] [PubMed] [Google Scholar]
- 17.Papakostas Gl., Petersen T., Denninger J., et al. Somatic symptoms in treatment-resistant depression. Psychiatry Res. 2003;118:39–45. doi: 10.1016/s0165-1781(03)00063-5. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan MJ., Klinetob NA. Childhood emotional trauma and chronic posttraumatic stress disorder in adult outpatients with treatment-resistant depression, J Nerv Ment Dis. 2000;188:596–601. doi: 10.1097/00005053-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Schatzberg AF. Controversies in antidepressant therapy. J Clin Psychiatry. 1983;44(9Pt 2):29–30. [PubMed] [Google Scholar]
- 20.Post R., Weiss S. The neurobiology of treatment-resistant mood disorders. In: Bloom FE, Kupfer DJ, eds. Psychopharmacology: The Fourth Generation of Progress. Philadelphia, Pa: Lippincott-Raven; 1995:1115–1170. [Google Scholar]
- 21.Fava M., Borus JS., Alpert JE., Nierenberg AA., Rosenbaum JF., Bottiglieri T. Folate, vitamin B12, and homocysteine in major depressive disorder. Am J Psychiatry. 1997;154:426–428. doi: 10.1176/ajp.154.3.426. [DOI] [PubMed] [Google Scholar]
- 22.Alpert JE., Fava M. Nutrition and depression: the role of folate. Nutr Rev. 1997;55:145–149. doi: 10.1111/j.1753-4887.1997.tb06468.x. [DOI] [PubMed] [Google Scholar]
- 23.Keitner Gl., Ryan CE., Miller IW., et al. Recovery and major depression: factors associated with 1 2-month outcome. Am J Psychiatry. 1992;149:93–99. doi: 10.1176/ajp.149.1.93. [DOI] [PubMed] [Google Scholar]
- 24.Shah PJ., Ebmeier KP., Glabus MF., Goodwin GM. Cortical grey matter reductions associated with treatment-resistant chronic unipolar depression. Controlled magnetic resonance imaging study. Br J Psychiatry. 1998;172:527–532. doi: 10.1192/bjp.172.6.527. [DOI] [PubMed] [Google Scholar]
- 25.Shah PJ., Glabus MF., Goodwin GM., Ebmeier KP. Chronic, treatment-resistant depression and right fronto-striatal atrophy. Br J Psychiatry. 2002;180:434–440. doi: 10.1192/bjp.180.5.434. [DOI] [PubMed] [Google Scholar]
- 26.Hornig M., Mozley PD., Amsterdam JD. HMPAO SPECT brain imaging in treatment-resistant depression. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1097–1114. doi: 10.1016/s0278-5846(97)00100-0. [DOI] [PubMed] [Google Scholar]
- 27.Mayberg HS., Brannan SK., Mahurin RK., et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–1061 . doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 28.Fredman SJ., Fava M., Kienke AS., et al. Partial response, nonresponse and relapse with SSRIs in major depression: a survey of current “next-step” practices. J Clin Psychiatry. 2000;61:403–408. doi: 10.4088/jcp.v61n0602. [DOI] [PubMed] [Google Scholar]
- 29.Fava M., Rosenbaum JF., McGrath PJ., et al. Lithium and tricyclic augmentation of fluoxetine treatment for resistant major depression: a double-blind, controlled study. Am J Psychiatry. 1994;15:1372–1374. doi: 10.1176/ajp.151.9.1372. [DOI] [PubMed] [Google Scholar]
- 30.Rosenbaum JF., Fava M., Nierenberg AA., Sachs GS. Treatment-resistant mood disorders. In: Gabbard GO, ed. Treatment of Psychiatric Disorders. 3rd ed. Washington, DC: American Psychiatric Press. 2001;2:1307–1383. [Google Scholar]
- 31.Shelton RC. The use of antidepressants in novel combination therapies. J Clin Psychiatry. 2003;64(suppl 2):14–18. [PubMed] [Google Scholar]
- 32.Bauer M., Dopfmer S. Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacol. 1999;19:427–434. doi: 10.1097/00004714-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Altshuler LL., Bauer M., Frye MA., et al. Does thyroid supplementation accelerate tricyclic antidepressant response? A review and meta-analysis of the literature. Am J Psychiatry. 2001;158:1617–1622. doi: 10.1176/appi.ajp.158.10.1617. [DOI] [PubMed] [Google Scholar]
- 34.Lasser RA., Baldessarini RJ. Thyroid hormones in depressive disorders: a reappraisal of clinical utility. Harv Rev Psychiatry. 1997;4:291–305. doi: 10.3109/10673229709030557. [DOI] [PubMed] [Google Scholar]
- 35.Blier P., Bergeron R. Effectiveness of pindolol with selected antidepressant drugs in the treatment of major depression. J Clin Psychopharmacol. 1995;15:217–222. doi: 10.1097/00004714-199506000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Chiarello RJ., Cole JO. The use of psychostimulants in general psychiatry: a reconsideration. Arch Gen Psychiatry. 1987;44:286–295. doi: 10.1001/archpsyc.1987.01800150110013. [DOI] [PubMed] [Google Scholar]
- 37.Lavretsky H., Kumar A. methylphenidate augmentation of citalopram in elderly depressed patients. Am J Geriatr Psychiatry. 2001;9:298–303. [PubMed] [Google Scholar]
- 38.Menza MA., Kaufman KR., Castellanos A. Modafanil augmentation of antidepressant treatment in depression. J Clin Psychiatry. 2000;61:378–381 . doi: 10.4088/jcp.v61n0510. [DOI] [PubMed] [Google Scholar]
- 39.Kapur S., Mann JJ. Role of the dopaminergic system in depression. Biol Psychiatry. 1992;32:1–17. doi: 10.1016/0006-3223(92)90137-o. [DOI] [PubMed] [Google Scholar]
- 40.Sporn J., Ghaemi SN., Sambur MR., et al. Pramipexole augmentation in the treatment of unipolar and bipolar depression: a retrospective chart review. Ann Clin Psychiatry. 2000;12:137–140. doi: 10.1023/a:1009060800999. [DOI] [PubMed] [Google Scholar]
- 41.Collaborative Working Group on Clinical Trial Evaluations. Atypical antipsychotics for treatment of depression in schizophrenia and affective disorders. J Clin Psychiatry. 1998;59(suppl 12):41–45. [PubMed] [Google Scholar]
- 42.Ranjan R., Meltzer HY. Acute and long-term effectiveness of clozapine in treatment-resistant psychotic depression. Biol Psychiatry. 1996;40:253–258. doi: 10.1016/0006-3223(95)00305-3. [DOI] [PubMed] [Google Scholar]
- 43.Ostroff RB., Nelson JC. Risperidone augmentation of selective serotonin reuptake inhibitors in major depression. J Clin Psychiatry. 1999;60:256–259. doi: 10.4088/jcp.v60n0410. [DOI] [PubMed] [Google Scholar]
- 44.Shelton R., Tollefson G., Tohen M., et al. The study of olanzapine plus fluoxetine in treatment-resistant major depressive disorder without psychotic features. New Clinical Drug Evaluation Unit Program. Presented at 38th Annual Meeting, Boca Raton, Fla, June. 1998 [Google Scholar]
- 45.Rosenbaum JF., Fava M., Nierenberg A. The Pharmacologic Treatment of Mood Disorders. Psychiatric Clinics of North America: Annual of Drug Therapy. Philadelphia, Pa: WB Saunders; 1994;1:17–50. [Google Scholar]
- 46.Nelson JC. Overcoming treatment resistance in depression. J Clin Psychiatry.Discussion. 1998;59(suppl 16):13-40–19-42. [PubMed] [Google Scholar]
- 47.Rosenbaum JF., Fava M., Nierenberg A., Sachs G. Treatment-resistant mood disorders. In: Gabbard GO, ed. Treatments of Psychiatric Disorders. 2nd ed. Washington, DC: APPI; 1995;1:1276–1328. [Google Scholar]
- 48.Klapheke M. Potential drug-ECT interactions. Biol Ther Psychiatry. 1991;14:34–37. [Google Scholar]
- 49.Devanand DP., Fitzsimons L., Prudic J., et al. Subjective side-effects during electro-convulsive therapy. Convulsive Ther. 1995;11:232–240. [PubMed] [Google Scholar]
- 50.Janicak PG., Sharma RP., Israni TH., et al. Effects of unilateral-nondominant vs bilateral ECT on memory and depression: a preliminary report. Psychopharmacol Bull. 1991;79:353–357. [PubMed] [Google Scholar]
- 51.Weiner SJ., Ward TN., Ravaris CL. Headache and electro-convulsive therapy. Headache. 1994;34:155–159. doi: 10.1111/j.1526-4610.1994.hed3403155.x. [DOI] [PubMed] [Google Scholar]
- 52.O'Connor MK., Knapp R., Husain M., et al. The influence of age on the response of major depression to electroconvulsive therapy: a CORE report. Am J Geriatr Psychiatry. 2001;9:382–390. [PubMed] [Google Scholar]
- 53.Burt T., Lisanby SH., Sackeim HA. Neuropsychiatrie applications of transcranial magnetic stimulation: a meta analysis. IntJ Neuropsychopharmacol. 2002;5:73–103. doi: 10.1017/S1461145702002791. [DOI] [PubMed] [Google Scholar]
- 54.Marangell LB., Rush AJ., George MS., et al. Vagus nerve stimulation (VNS) for major depressive episodes: 1-year outcomes. Biol Psychiatry. 2002;51:280–287. doi: 10.1016/s0006-3223(01)01343-9. [DOI] [PubMed] [Google Scholar]
- 55.Hornig-Rohan M., Wolkowitz OM., Amsterdam JD. Novel strategies for treatment-resistant depression. Psychiatr Clin N Am. 1996;19:387–405. doi: 10.1016/s0193-953x(05)70294-x. [DOI] [PubMed] [Google Scholar]
- 56.Thase ME., Friedman ES., Howland RH. Management of treatment-resistant depression: psychotherapeutic perspectives. J Clin Psychiatry. 2001;62(suppl 18):18–24. [PubMed] [Google Scholar]
- 57.Sonawalla SB., Fava M. Severe depression: is there a best approach? CNS Drugs. 2001;15:765–776. doi: 10.2165/00023210-200115100-00003. [DOI] [PubMed] [Google Scholar]
- 58.Rush AJ., Fava M., Wisniewski SR., et al. Sequenced Treatment Alternatives to Relieve Depression (STAR*D): rational and design. Controlled Clinical Trials. In press. 2004 doi: 10.1016/s0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 59.Rush AJ., Trivedi M., Fava M. Depression IV: STAR*D treatment trial for depression. [Images in Neuroscience]. Am J Psychiatry. 2003;160:237. doi: 10.1176/appi.ajp.160.2.237. [DOI] [PubMed] [Google Scholar]
- 60.Lavori PW., Rush AJ., Wisniewski SR., et al. Strengthening clinical effectiveness trials: equipoise-stratified randomization. Biol Psychiatry. 2001;50:792–801. doi: 10.1016/s0006-3223(01)01223-9. [DOI] [PubMed] [Google Scholar]