Abstract
Treatment-resistance in schizophrenia remains a public health problem: about 20% to 30% of patients do not respond to antipsychotic therapy. Clozapine has been shown to be effective in about one-third of patients, but the medical risks and weekly blood tests limit its broad application. While the heterogeneity of the disease and the duration of untreated psychosis are important, pharmacogenomic aspects must also be considered. Pharmacogenomic investigations offer the opportunity to individualize antipsychotic therapy according to the growing knowledge of the function and effect of the genetic polymorphisms that affect the pharmacokinetics and pharmacodynamics of antipsychotics. On the pharmacokinetic level, polymorphic phase I and II drug-metabolizing enzymes and transport proteins affect drug concentration at the target structure. The cytochrome P450 enzymes, N-acetyltransferase, and multidrug resistance protein (MDR1) particularly influence this parameter. Genetic alterations affecting drug pharmacodynamic properties have an impact on therapeutic outcome that is generally independent of the applied dosage regimen. A combined analysis of genetic polymorphisms in the dopaminergic and serotonergic receptors, neurotransmitter transporters, and other target structures involved in psychiatric disorders is already a powerful predictor of therapeutic outcome. An understanding of other factors influencing gene expression and protein production will facilitate individualized therapy in the future.
Keywords: antipsychotic, pharmacogenomics, pharmacokinetics, nonresponse, schizophrenia
Abstract
La resistencia al tratamiento en la esquizofrenia se mantiene como un problema de salud pública: alrededor del 20% al 30% de los pacientes no responden a la terapia con antipsicóticos. En alrededor de un tercio de los pacientes se ha demostrado la eficacia de la clozapina, pero los riesgos médicos y las pruebas sanguíneas semanales limitan su aplicación a gran escala. Además de considerar la heterogeneidad de la enfermedad y la duración de la psicosis no tratada, también son importantes los aspectos farmacogenómicos. Las investigaciones farmacogenómicas ofrecen la oportunidad de individualizar la terapia antipsicótica de acuerdo con el creciente conocimiento de la función y del efecto del polimorfismo genético que afecta la farmacocinética y la farmacodinámica de los antipsicóticos. A nivel farmacocinético, las enzimas polimorfas que participan en las fases I y II del metabolismo de los medicamentos y las proteínas transportadoras influyen en la concentración de los fármacos en las estructuras bianco. Las enzimas del citoctromo P450, la N-acetiltransferasa y la proteína de resistencia a la acción de diversos medicamentos (“multidrug resistence protein-1”, MDR1) afectan particularmente este parámetro. Las alteraciones genéticas que afectan las propiedades farmacodinámicas de los medicamentos tienen un impacto en la evolución terapéutica que generalmente es independiente de las dosis utilizadas. Un análisis combinado de los polimorfismos genéticos de los receptores dopaminérgicos y serotoninérgicos de los transportadores de neurotransmisores y de otras estructuras blanco involucradas en los trastornos psiquiátricos constituyen desde ya un poderoso predictor de los resultados terapéuticos. A futuro, la comprensión de otros factores que influyan en la expresión génica y la producción de proteínas facilitará los tratamientos individualizados.
Abstract
La résistance au traitement dans la schizophrénie demeure un problème de santé publique ; environ 20 % à 30 % de patients ne répondent pas au traitement antipsychotique. La clozapine s'est montrée efficace pour à peu près un tiers des patients mais les risques médicaux et les contrôles sanguins hebdomadaires limitent son application à grande échelle. Si l'hétérogénéité de la maladie et la durée de cette psychose non traitée sont importantes, les aspects pharmacogénomiques doivent aussi être considérés. Les recherches en pharmacogénomie offrent l'opportunité d'individualiser les traitements antipsychotiques d'après les connaissances croissantes sur la fonction et les effets des polymorphismes génétiques qui influent sur la pharmacocinétique et la pharmacodynamie des molécules antipsychotiques. Sur le plan pharmacocinétique, les enzymes polymorphes qui interviennent sur les phases I et II du métabolisme des médicaments et les protéines de transport influent sur la concentration des médicaments au niveau de la structure cible. Les enzymes cytochrome P450, la N-acétyltransférase et la protéine de résistance à l'action de plusieurs médicaments (« multidrug resistance protein-1 », MDR1) jouent particulièrement sur ce paramètre. Les altérations génétiques agissant sur les propriétés pharmacodynamiques des médicaments ont un impact sur les résultats thérapeutiques généralement indépendant du schéma posologique. L'analyse combinée des polymorphismes génétiques des récepteurs dopaminergiques et sérotoninergiques, des transporteurs des neurotransmetteurs et d'autres structures cibles impliquées dans les troubles psychiatriques constitue déjà un prédicteur puissant des résultats thérapeutiques. Comprendre comment d'autres facteurs influent sur l'expression des gènes et la production des protéines facilitera les traitements individualisés dans l'avenir.
Nonresponse is a frequent phenomenon in both somatic and psychiatric pharmacotherapy. A considerable number of drugs have become available for the treatment, of various psychiatric diseases since the discovery of chlorpromazine 50 years ago.1 Despite all the progress in this field, there are still many patients who do not, respond to treatment, so-called nonresponders. Around 20% to 30% of the patients with schizophrenia are thought, to be nonresponders to one or more psychotropic drugs.
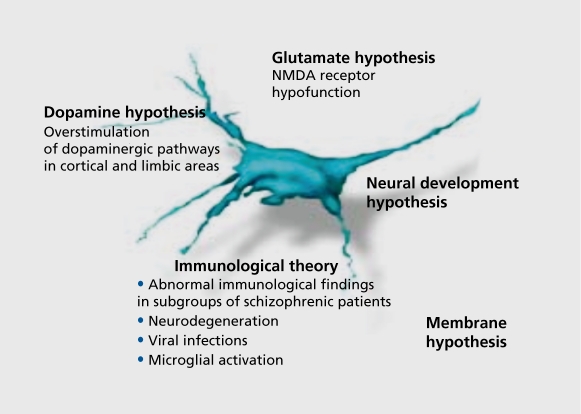
Several reasons for nonresponse to treatment, have been considered. The main issue is the heterogeneity of schizophrenic disorders, which can result, in different, clinical pictures. The existing theories discussed for schizophrenia (Figure 1) may be only a small part of the existing mechanisms causing schizophrenia. Consequently, the research strategy is to focus on the effects of alterations in drug target proteins. In addition, the duration of the untreated psychosis seems to adversely affect, acute treatment, response and short-term outcome.2 Here, we will focus on the pharmacokinetic and pharmacodynamic aspects of nonresponse to treatment, in schizophrenia. The atypical antipsychotic clozapine, which was licensed in the USA especially for the treatment of so-called treatment-resistant schizophrenia3 (after having been available in Europe since 19684), is not, effective in all patients and 20% to 30% are known to be nonresponders. In a naturalistic study in the psychiatric clinic of the University of Munich, responders with no relapse were compared with responders with partial relapse. The response rate was only partially dependent on dose and plasma levels.
Figure 1. Theories for schizophrenia, NMDA, N-methyl-D-aspartate.
Responders received an average dosage of 225 mg/day clozapine and had a plasma level of 205 ng/mL clozapine on average and 120 ng/ml , desmethylclozapine per day.
Responders with partial relapse received lower dosages (125 mg/day) and also had lower plasma levels (100 ng/mL clozapine, 55 ng/mL desmethylclozapine, respectively).
Nonresponders received higher dosages (250 mg/day) and generally also showed considerably higher plasma levels (290 ng/mL clozapine and 225 ng/mL desmethylclozapine, respectively).
Therefore the nonresponders did not respond to therapy even though they received higher dosages and had higher plasma levels (Messer T, personal communication), and that additional factors are involved.
Pharmacogenomics
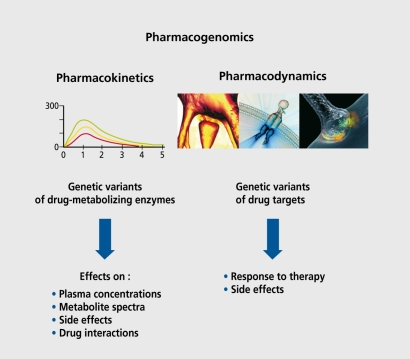
Pharmacogenomics is the study of how an individual's genetic inheritance affects the body's response to drugs (Figure 2). This includes both the interindividual sideeffect profile and the therapeutic outcome. Single nucleotide polymorphisms (SNPs) occur every 100 to 300 bases in the human genome, leading to a large number of possible associations with response to pharmacotherapy. Keeping this in mind, current, research activities mainly focus on pharmacokinetic and pharmacodynamic alterations. Most, notably, genes coding for the metabolizing and transport proteins and drug target, structures (receptors, transporters, and signal transduction pathways) are being investigated.
Figure 2. Pharmacogenomics: the study of how genetic inheritance affects the body's response to drugs.
Pharmacokinetics
Various reasons have been discussed for nonresponse (Table I). Among these is the important, role of pharmacokinetic and pharmacodynamic aspects. These are partly based on functional genetic polymorphisms of the responsible genes. Pharmacokinetics, ie, the distribution and metabolism of drugs, is dependent, on genetic variants of the metabolizing enzymes and transport proteins. Some of these are:
The P450 cytochromes, which are members of the phase I metabolizing enzymes responsible for oxidation of functional groups.
The phase II metabolizing enzyme N-acetyltransferase (NAT), which is responsible for the conjugation of substances with hydrophilic molecules for facilitated renal excretion.
The multidrug resistance protein (MDR1), which controls the active transport, through the membranes of cells. These determine the plasma levels and also the spectrum of metabolites, and are thus responsible for the effects and side effects of drugs. In addition, psychotropic drugs interact, at this level and influence each other.
Table I. Reasons for nonresponse to treatment.
| Different etiologies |
| Duration of untreated psychosis (DUP) |
| Pharmacokinetic aspects |
|
| Variations in candidate genes |
Cytochrome P450 enzymes
Various cytochromes are responsible for the metabolism of psychotropic drugs,5 most, notably cytochromes CYP2C19, CYP3 A4, CYP1A2, and CYP2D6. Halopcridol, for example, is metabolized by CYP2D6, CYP3A4, and CYP1A2. Notably, the enzyme CYP2D6 exists in more than 20 different genetic variants, so-called polymorphisms, which show different enzymatic activities.
Next, there are slow metabolizers, extensive metabolizers, and ultrarapid metabolizers. Several allelic variants of the CYP2D6 gene can lead to a nonfunctional enzyme or reduced CYP2D6 activity. The most common mutations in Caucasians associated with a slow metabolizer phenotype are the CYP2D6*3*4*5*6 alleles, or combinations of these, leading to a nonfunctional enzyme or reduced activity when they occur as heterozygous combinations with the wild-type allele. The ultrarapid metabolizer CYP2D6 phenotype is associated with a gene duplication. Up to 13 CYP2D6 copies could be the result of this mutation.6 The extensive metabolizer phenotype could therefore be a result, of a homozygeous CYP2D6 wild-type genotype or a combination of alleles coding for reduced or abundant activity (CYP2D6*3*4*5*6*10*17) and alleles coding for increased activity.
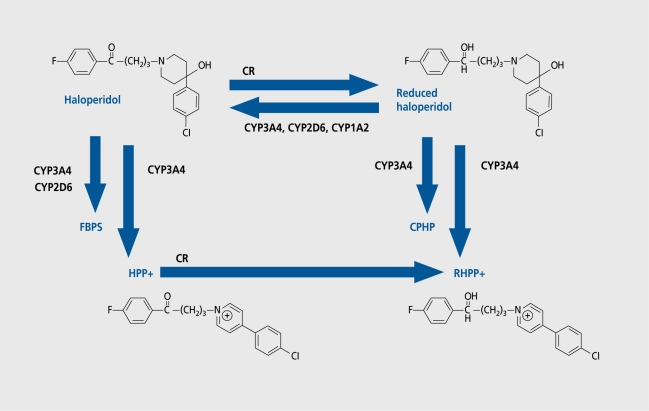
In a study with haloperidol, which is degraded to reduced halopcridol, a pipcridine metobolite, and a presumably neurotoxic substance haloperidol pyridinium (HPP+) (Figure 3), we noted that patients with the allele CYP2D6*4, which exists in around 10% of the Caucasian population and determines a slow metabolizer, show higher plasma levels of haloperidol and reduced haloperidol. At the same time, these patients showed a poorer response to treatment and more side effects. CYP3A4 is responsible for the increased occurrence of the possibly neurotoxic metabolite HPP+,7,8 showing similarities to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridinium (MPTP), which provoked symptoms similar to Parkinson's disease in heroin addicts.9 This may also cause undesired side effects, such as extrapyramidal motor symptoms (EPS).
Figure 3. Phase I metabolism of haloperidol. CPHP, chlorophenyhydroxypiperidine; CR, carbonylreductase; FBPS, fluorobutyrophenon acid; HPP+, haloperidol pyridinium; RHPP+ reduced HPP+.
The new atypical antipsychotic risperidone is also metabolized by cytochrome CYP2D6. Patients who are homozygous to the CYP2D6*4 allele, ie, slow metabolizers, also show higher plasma levels. Furthermore, schizophrenic patients who were heterozygous for cytochrome CYP2C19 and CYP1A2 had fewer side effects. Cytochrome CYP1A2*1F seems to be important too. Using the PANSS (Positive and Negative Symptom Scale), a poorer therapy response regarding the negative symptoms could be seen in homozygous CYP1A2*1F allele patients.
Pharmacokinetic aspects are also responsible for the plasma levels of other drugs, eg, amisulpride. Tenfold differences in the amisulpride plasma level can be measured at the same dosage. We found the same results for the atypical antipsychotic clozapine and also for other antipsychotics, such as risperidone. Too low and too high plasma levels reduce the therapy response and arc the reason for many side effects at high plasma levels.
Multidrug resistance protein
On the pharmacokinetic level, drug nonresponse can also occur via mechanisms affecting the efflux of a diverse range of drugs out of the cells or the permeability of the blood–brain barrier. These mechanisms have evolved during cell evolution in order to protect, against toxic environmental substances or metabolites. The extensively described efflux mechanism is mediated via P-glycoprotein(P-gp)or MDRl.
Multidrug resistance in humans is caused by the overexpression of the multidrug transporter: P-gp/MDR1.This overexpression can be induced by drugs affecting the MDR1 gene transcription or via functional genetic polymorphisms.
P-gp is an adenosine triphosphate (ATP)–dependent transmembrane efflux pump present in the hepatic, renal, and endothelial cells forming the blood-brain barrier. The only common structural feature of MDR1 substrate compounds identified so far is their relatively hydrophobic and amphipathic nature. Risperidone and quetiapine are relatively good substrates to the P-gp, whereas haloperidol, clozapine, and flunitrazepam are hardlytransported or not at all.10
So far, a total of 28 SNPs has been found on the MDR1 gene. Interestingly, the C2435T polymorphism, which causes no amino acid change, has been found to be associated with duodenal expression of MDRl.11,12 The C2435T mutation is located in an noncoding promoter position (exon 26) of the MDR1 gene13 and is therefore unlikely to be the only cause of this effect. Up to now, the results of worldwide clinical studies are still inconsistent regarding the association of the C2535T polymorphism with MDR1 expression, making further investigations necessary.
N-Acetyltransferase
Acetylation by NAT is a phase II conjugation reaction. It is controlled by two autosomal alleles at a single gene locus. Around 60% of Caucasians are poor metabolizers, whereas 20% of Orientals are slow metabolizers, and 90% to 95% of Mongoloid races are fast acetylators. Three NAT loci are found in humans: two expressed genes, NAT! and NAT2, and a pseudogene, N-acetyltransferase pseudogene (NATP).NAT2 plays a role in the metabolism of benzodiazepines, and theoretically also plays a role in the metabolism of some antipsychotics.
Pharmacodynamic effects
The effect of both classical and atypical neuroleptics is mainly due to a blockade of dopaminergic receptors in the nigrostriatal and mesolimbic dopamine system. There are five different dopamine receptors: D1 to D5. D1 and D5 stimulate adenylatecyclase, whereas D2, D3, and D4 inhibit the adenylatecyclase.14 Most, important, for the antipsychotic effect is the inhibition of the D2 receptor, which is more or less markedly caused by all antipsychotics. In comparison to haloperidol, clozapine has a relatively higher effect, on the D4 receptor.15 The O-substituted benzamide amisulpride has a relatively higher effect on the D3 receptor.
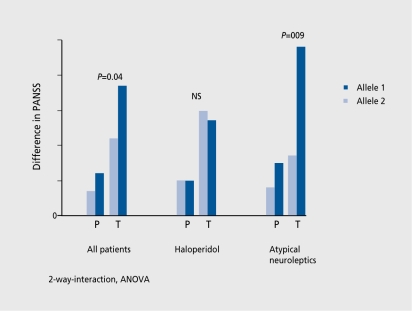
Moreover, other receptors, such as serotonin and norepinephrine, are inhibited and contribute as well to the effect, and side effects of antipsychotics. The different genetic variants of the dopamine receptors seem to be most, important, with regard to the responsiveness to therapy. The D3 receptor exists in two different, variations.16 According to our findings, as well as those of others, atypical antipsychotics act, much better with an allele with a BAL1 polymorphism in exon I. Classical antipsychotics such as haloperidol are less effective with this allele (Figure 4). The effect, of a single allele, however, only partly contributes to the variance. Another study in 2000 by Arranz and Kerwin17 has shown that the serotonin 5-HT2A receptor, the 5-HT2C receptor, the histamine receptor type 2 H2R, and the serotonin transporter 5-HTT2PR are additionally important. The combination of the various polymorphisms made it, possible to predict the success of treatment with a rate of 77%, thus indicating future possibilities in treatment.18 These findings show that the interaction of various genetic variants determines the effect, of a drug and is thus responsible for response or nonresponse.
Figure 4. Dopamine D3, receptor gene: BAL1 polymorphism in exon land response to treatment. Allele 2 of the BAL1 polymorphism is associated with a better response to treatment in patients medicated with atypical antipsychotics. PANSS, Positive and Negative Symptoms Scale; ANOVA, analysis of variance; P, PANSS positive; T, PANSS total.
Future aspects
The future of a medicinal treatment will be based on individualized therapy. In addition to the heterogeneity of diseases with different, pathophysiologies, there is also a difference in the effectiveness of drugs themselves due to different genetic variants. Genetic alterations in cellular ion transporters, such as KCNE2, have impact, on the predisposition of patients to toxic effects of drugs. An 8T>A mutation in the KCNE2 gene can cause death by a drug-induced long-QT syndrome. Since this mutation occurs in 1.6% of the population, KCNE2 genotyping should be considered in patients treated with antipsychotics that are known to prolong the QT time intervals, such as sertindole or ziprasisdone.
New microassay technologies will allow us to genotype different candidate genes simultaneously, and to determine which are responsible for the pharmacodynamic effects, as well to genotype different, cytochromes, making it, possible to predict, the plasma levels at, the equivalent, dosage. Furthermore, the discovery of up to now unknown genes affecting the action of a drug by means of the so-called pharmacogenomics, ie, the recording of the whole genome, will in the future become increasingly important, in both psychiatry and in other diseases. Further factors that, influence gene expression and protein production, measured by proteomics, will improve our knowledge of drug effects.
Selected abbreviations and acronyms
- HPP+
haloperidol pyridinium
- MDR1
multidrug resistance protein
- NAT
N-acetyltransferase
- P-gp
P-glycoprotein
- SNP
single nucleotide polymorphism
Contributor Information
Manfred Ackenheil, Psychiatric Hospital of the University of Munich, Munich, Germany.
Klaus Weber, Psychiatric Hospital of the University of Munich, Munich, Germany.
REFERENCES
- 1.Stip E. Happy birthday neuroleptics! 50 years later: la folie du doute. Eur Psychiatry. 2002;17:115–119. doi: 10.1016/s0924-9338(02)00639-9. [DOI] [PubMed] [Google Scholar]
- 2.Bottlender R., Sato T., Jager M., et al. The impact of the duration of untreated psychosis prior to first psychiatric admission on the 15-year outcome in schizophrenia. Schizophr Res. 2003;62:37–44. doi: 10.1016/s0920-9964(02)00348-1. [DOI] [PubMed] [Google Scholar]
- 3.Kane J M., Honigfeld G., Singer J., Meltzer H. Clozapine in treatment-resistant schizophrenics. Psychopharmacol Bull. 1988;24:62–67. [PubMed] [Google Scholar]
- 4.Hippius H. A historical perspective of clozapine. J Clin Psychiatry. 1999;60(suppl 12):22–23. [PubMed] [Google Scholar]
- 5.Flockhart DA., Oesterheld JR. Cytochrome P450–mediated drug interactions. Child Adolesc Psychiatr Clin N Am. 2000;9:43–76. [PubMed] [Google Scholar]
- 6.Kroemer HK., Eichelbaum M. “It's the genes, stupid.” Molecular bases and clinical consequences of genetic cytochrome P450 2D6 polymorphism. Life Sci. 1995;56:2285–2298. doi: 10.1016/0024-3205(95)00223-s. [DOI] [PubMed] [Google Scholar]
- 7.Usuki E., Pearce R., Parkinson A., Castagnol N Jr. Studies on the conversion of haloperidol and its tetrahydropyridine dehydration product to potentially neurotoxic pyridinium metabolites by human liver microsomes. Chem Res Toxicol. 1996;9:800–806. doi: 10.1021/tx960001y. [DOI] [PubMed] [Google Scholar]
- 8.Kalgutkar AS., Taylor TJ., Venkatakrishnan K., Isin EM. Assessment of the contributions of CYP3A4 and CYP3A5 in the metabolism of the antipsychotic agent haloperidol to its potentially neurotoxic pyridinium metabolite and effect of antidepressants on the bioactivation pathway. Drug Metab Dispos. 2003;31:243–249. doi: 10.1124/dmd.31.3.243. [DOI] [PubMed] [Google Scholar]
- 9.Halliday GM., Pond SM., Cartwright H., McRitchie DA., Castagnoli N Jr., Van der Schyf CJ. Clinical and neuropathological abnormalities in baboons treated with HPTP, the tetrahydropyridine analog of haloperidol. Exp Neurol. 1999;158:155–163. doi: 10.1006/exnr.1999.7090. [DOI] [PubMed] [Google Scholar]
- 10.Schinkel AH., Wagenaar E., Mol CA., van Deemter L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97:2517–2524. doi: 10.1172/JCI118699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balram C., Sharma A., Sivathasan C., Lee EJ. Frequency of C34357T single nucleotide MDR1 genetic polymorphism in an Asian population: phenotypic-genotypic correlates. Br J Clin Pharmacol. 2003;56:78–83. doi: 10.1046/j.1365-2125.2003.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmeyer S., Burk O., von Richter O., et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A. 2000;97:3473–3478. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kroetz DL., Pauli-Magnus C., Hodges LM., et al. Sequence diversity and haplotype structure in the human ABCB1 (MDR1, multidrug resistance transporter) gene. Pharmacogenetics. 2003;13:481–494. doi: 10.1097/00008571-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Seeman P., Van Tol HH. Dopamine receptor pharmacology. Curr Opin Neurol Neurosurg. 1993;6:602–608. [PubMed] [Google Scholar]
- 15.McRitchie DA., Cartwright H., Pond SM., et al. The midbrain dopaminergic cell groups in the baboon Papio ursinus. . Brain Res Bull. 1998;47:611–623. doi: 10.1016/s0361-9230(98)00128-2. [DOI] [PubMed] [Google Scholar]
- 16.Griffon N., Crocq MA., Pilon C., et al. Dopamine D3 receptor gene: organization, transcript variants, and polymorphism associated with schizophrenia. Am J Med Genet. 1996;67:63–70. doi: 10.1002/(SICI)1096-8628(19960216)67:1<63::AID-AJMG11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 17.Arranz MJ., Kerwin RW. Neurotransmitter-related genes and antipsychotic response: pharmacogenetics meets psychiatric treatment. Ann Med. 2000;32:128–133. doi: 10.3109/07853890009011762. [DOI] [PubMed] [Google Scholar]
- 18.Arranz MJ., Munro J., Birkett J., et al. Pharmacogenetic prediction of clozapine response. Lancet. 2000;355:1615–1616. doi: 10.1016/s0140-6736(00)02221-2. [DOI] [PubMed] [Google Scholar]