Abstract
During the past two decades, in vivo neuroimaging studies have permitted significant insights into the general location of dysfunctional brain regions in depression. In parallel and often intersecting ways, neuroanatomical, pharmacological, and biochemical studies of postmortem brain tissue are permitting new insights into the pathophysiology of depression. In addition to long-recognized neurochemical abnormalities in depression, novel studies at the microscopic level support the contention that mood disorders are associated with abnormalities in cell morphology and distribution. In the past 6 years, cell-counting studies have identified changes in the density and size of both neurons and glia in a number of frontolimbic brain regions, including dorsolateral prefrontal, orbitofrontal, and anterior cingulate cortex, and the amygdala and hippocampus. Convergence of cellular changes at the microscopic level with neuroimaging changes detected in vivo provides a compelling integration of clinical and basic research for disentangling the pathophysiology of depression. The ultimate integration of these two research approaches will occur with premortem longitudinal clinical studies on well-characterized patients linked to postmortem studies of the same subjects.
Keywords: major depression, bipolar disorder, postmortem brain, glia, neuron, neuropathology
Abstract
Durante los últimos veinte años, los estudios de neuroimágenes in vivo han permitido un importante conocimiento acerca de la ubicación general de regiones cerebrales disfuncionales en la depresión. En paralelo y a menudo intersectándose, los estudios neuroanatómicos, farmacológicos y bioquímicos del tejido cerebral postmortem están facilitando nuevos conocimientos sobre la fisiopatología de la depresión. Además de las alteraciones neuroquímicas reconocidas desde hace bastante tiempo, nuevos estudios a nivel microscópico permiten sostener que los trastornos afectivos están asociados con alteraciones en la morfología y en la distribución celular. En los últimos séis años, estudios de recuento celular han identificado cambios en la densidad y el tamaño tanto de las neuronas como de la glía en varias regiones cerebrales frontolímbicas que incluyen las cortezas dorso-lateral prefrontal, órbita-frontal y cingulada anterior, y la amígdala y el hipocampo. La convergencia de cambios celulares a nivel microscópico con cambios en las neuroimágenes detectados in vivo provee una integración forzada de la investigación clínica y básica para desentrañar la fisiopatología de la depresión. La integración definitiva de estas dos aproximaciones de investigación ocurrirá cuando se puedan relacionar los estudios clínicos longitudinales premortem en pacientes bien caracterizados con los estudios postmortem de los mismos sujetos.
Abstract
Ces 20 dernières années, les études de neuro-imagerie in vivo ont permis des avancées significatives dans la compréhension de la localisation générale des régions cérébrales dysfonctionnelles au cours de la dépression. Les études neuroanatomiques, pharmacologiques et biochimiques des tissus cérébraux post mortem offrent ainsi, en parallèle et souvent de façon croisée, un aperçu renouvelé de la physiopathologie de la dépression. De nouvelles études au niveau microscopique ont conforté l'hypothèse selon laquelle les troubles de l'humeur sont associés à des anomalies de la distribution et de la morphologie cellulaires, tout en confirmant l'existence d'anomalies neurochimiques connues depuis longtemps dans la dépression. Ces 6 dernières années, des études de comptage cellulaire ont identifié des modifications dans la densité et la taille des neurones et de la névroglie au niveau d'un certain nombre de régions cérébrales frontolimbiques comprenant les cortex dorsolateral préfrontal, orbitofrontal et angulaire antérieur, ainsi qu'au niveau de l'amygdale et de l'hippocampe. La convergence entre les modifications cellulaires observées au niveau microscopique et les changements in vivo détectés par neuro-imagerie témoigne avec éloquence de l'utilité d'associer la recherche clinique et fondamentale en vue d'élucider la physiopathologie de la dépression. L'ultime intégration de ces deux approches de recherche consistera à rapprocher les données issues d'études cliniques longitudinales pre mortem sur des patients bien définis avec celles provenant d'études post mortem sur ces mêmes patients.
During the past two decades, anatomical substrates associated with the neuropathology of mood disorders have been revealed through both in vivo neuroimaging studies and morphological and neurochemical studies on postmortem brain tissue. While neuroimaging studies have given significant, insight into the gross morphological location of dysfunctional brain regions in depression, the neurochemical, cellular, and molecular features of depression are being unlocked by studies in postmortem brain tissue.
Novel studies at the microscopic level are establishing that the mood disorders arc associated with abnormalities in cell morphology and distribution, in addition to the long-recognized neurochemical abnormalities. Major depressive disorder (MDD) and bipolar disorder (BPD) have been examined in postmortem brain tissue by several laboratories in the past 6 years. Cell-counting studies report changes in the density and size of both neurons and glia in a number of frontolimbic brain regions, including dorsolateral prefrontal, orbitofrontal, and anterior cingulate cortex, and the amygdala and hippocampus. These studies in postmortem brain tissue confirm and extend structural and functional neuroimaging studies that reveal volumetric and metabolic changes in the same frontolimbic brain regions in the same disorders. Convergence of cellular changes at the microscopic level with neuroimaging changes detected in vivo provides a compelling integration of clinical and basic research for disentangling the pathophysiology of depression. Regionally localized and cell type-specific changes in neuronal and glial cytoarchitecture recently identified in mood disorders complement and expand hypotheses of dysfunction within the monoaminergic, glutamatergic, and γ-aminobutyric acid (GABA) neurotransmitter systems in these disorders.
While MDD and BPD are clearly not neurodegenerative disorders, impaired neuroplasticity is associated with these mood disorders.
The etiology of histopathological changes observed in postmortem brain tissue is unknown. It is not clear how factors such as genetic risk factors, neurodevelopmental abnormalities, the progression of the disease, or exposure to antidepressant or mood-stabilizing medications contribute to the abnormal neuronal and glial observations in mood disorders. It remains to be determined whether the chronic administration of clinically effective therapeutic medications can reverse or even staunch histopathological changes in the mood disorders.
Alterations in neurons and glia in cerebral cortex
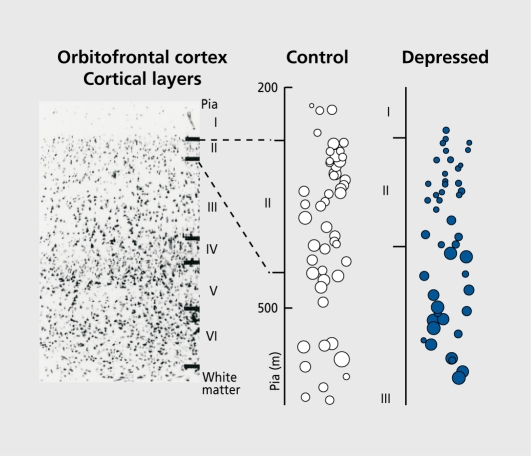
In MDD and BPD, reductions in neuronal density and size in some populations of cortical neurons have been independently reported.1-12 These abnormalities have been described in association cortices such as dorsolateral prefrontal, orbitofrontal, and anterior cingulate cortex, but not in the primary sensory cortical regions such as somatosensory1 or visual cortex.2 Thus, neuronal abnormalities at the microscopic level in mood disorders appear to be specific to frontolimbic cortical regions - observations in postmortem tissue that arc consistent with in vivo neuroimaging studies of volumetric and metabolic alterations in the same frontocortical regions. Neuronal abnormalities in mood disorders are not immediately evident, inasmuch as there is no significant reduction in the density of Nissl-stained neurons measured across all cortical laminae.1,3,4 However, when neurons are assessed within individual cortical layers or in subgroups determined by size or immunohistochemistry, marked reductions in neuron density are found in both MDD and BPD. For example, the density of large-sized neuronal cell bodies is reduced in cortical layers II to VI in the dorsolateral prefrontal and rostral orbitofrontal cortex in MDD.5 These reductions in density of large-sized neuronal cell bodies are accompanied by increases in the density of neurons with smaller-sized cell bodies (Figure 1). The concomitant decrease in the density of large neuronal cell bodies and increase in the density of small neuronal cell bodies suggests that neuronal shrinkage/enlargement or perhaps altered neuronal development, rather than outright neuronal loss, is responsible for neuronal abnormalities in mood disorders.
Figure 1. Changes in neuronal size and size-dependent density in layer II of rostral orbitofrontal cortex in a 73-year-old female with MDD as compared to a 71-year-old psychiatrically normal female control subject. For both subjects, the postmortem delay was less than 17 hours and fixation time was less than 10 months. Photomicrograph depicting cell composition across the six cortical layers in rostral orbitofrontal cortex (upper left). Expanded printouts of cortical layers with neuronal cell bodies represented by equivalent diameter circles with the area measured for the individual neuron in its equatorial plane (right panel). Note that neuronal sizes are smaller in layers II and III in the depressed subject than in the control subject. Note especially dramatic increases in the density of small neurons in layer II associated with significant reductions in the density of the largest neurons of this layer. Reprinted from reference 5: Rajkowska G, Miguel-Hidalgo JJ, Wei J, et al. Morphometry evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085-1098. Copyright © 1999, Elsevier.
In BPD, decreases in laminar neuronal densities have also been reported in the dorsolateral prefrontal cortex4 and anterior cingulate cortex,2,6,7 but not by all studies.1,8 Moreover, in BPD, a decrease in density of pyramidal neurons in cortical layers III and V4 and nonpyramidal neurons in layer II6 has been observed in the same cortical regions. This last observation coincides with reports on reductions in the density of layer II nonpyramidal neurons that are identified with an antibody against the calciumbinding protein, calbindin, in the anterior cingulate cortex7 and dorsolateral prefrontal cortex9 in BPD. Calbindin immunoreactive neurons are known to colocalize GABA. Our recent, measurements of the density and size of calbindin-immunoreactive neurons in layer II and the upper part of layer III of the dorsolateral prefrontal cortex revealed a 43% reduction in the density of these neurons in M'DD as compared to controls.10 The depression-related decrease in calbindin immunoreactive neurons, which colocalize GABA, may be closely related to in vivo clinical evidence suggesting that MDD is associated with decreased levels of GABA in cerebral cortex.11
Another manifestation of neuronal pathology in cerebral cortex in mood disorders is the reduced size of neuronal cell bodies. Smaller soma sizes have been reported in subjects with MDD, as compared to normal controls, in the dorsolateral prefrontal cortex,3,5 orbitofrontal cortex,5 and anterior cingulate cortex.8,12 Two other studies, however, did not report, significant changes in neuronal size in the anterior cingulate cortex.1,2 In a manner more subtle than in MDD, reductions in neuronal soma size have been observed in BPD by some,4,12 but not by all investigators.1,2,8 In another study, a minor increase in the size of small nonpyramidal neurons was noted in the anterior cingulate cortex in BPD subjects.6
Factors leading to a reduction in the size of neuronal soma are not known. Smaller soma size may be related to smaller dendritic trees and/or abnormal morphology of synaptic contacts. However, visualization of neuronal dendritic trees in cerebral cortex using the Golgi silver impregnation method has not yet been conducted in subjects with mood disorders. Studies looking at synaptic proteins in the anterior prefrontal13 and anterior cingulate cortex14 describe reductions14 or no changes13 in synaptic proteins in mood disorders. Systematic studies of dendritic trees and synaptic contacts in prefrontal and cingulate areas are warranted to shed light on the possible etiology of smaller neuronal cell bodies in mood disorders.
The most consistent cell abnormality described in mood disorders has unexpected finding of prominent reductions in the density and number of glial cells. Glial reductions have been reported consistently by independent laboratories in the anterior cingulate cortex, dorsolateral prefrontal cortex, and orbitofrontal cortex in MDD and/or BPD subjects. For example, a 24% to 41 % reduction in the number of a general population of Nissl-stained glial cells is reported in the subgenual region of the anterior cingulate cortex (ventral part of Brodmann's area 24) in a small subgroup of patients with familial MDD and familial BPD, as compared to control subjects.1 However, when data from familial and nonfamilial subgroups of patients were combined, the reductions are not found. The estimation of glial cell number in this study is combined across all six cortical layers, and no information is provided on laminar specificity of glial loss.
Reductions in glial cell density, however, are reported in specific cortical layers of the anterior cingulate and prefrontal cortices in four other studies. These glial reductions are observed in layer VI of the supragenual anterior cingulate cortex,8 layers III and V of the dorsolateral prefrontal cortex3-5 and in layers III, IV, V, and VI of the caudal orbitofrontal cortex,5 in mood disorder patients. Glial cell size and shape, in addition to density, appears to be affected in mood disorders. The size of glial cell bodies (corresponding to glial cell nuclei in Nissl-stained material) has been estimated in several studies. In three of these investigations, glial size is reported as increased,3-5 whereas two other studies find glial size to be unchanged in MDD or BPD.1,15 Significant increases in glial size are observed in the dorsolateral prefrontal cortex in BPD4 and to a smaller degree in MDD,5 comparing these cohorts to psychiatrically normal control subjects. More recently, similar increases in glial size are noted in the anterior cingulate cortex in MDD.12 In addition, changes in the shape of glial nuclei to a less rounded conformation are detected in the dorsolateral prefrontal cortex in BPD.4 Reductions in glial density, paralleled by an increase in the size of glial nuclei, suggest that some compensatory mechanisms may take place in mood disorders. It can be speculated that a decrease in the density of glial cells is indicative of a decrease in the number of normally functioning glial cells. At the same time, glial cells that survive and are not damaged might, be forced to play a larger role in supporting the metabolic needs of the surrounding neurons. As a consequence of increased metabolic demand, the nuclei of these glial cells might enlarge in size and change in shape.
Glutamate-induced swelling of astroglia, reported in animal cell cultures,16 may be another factor in the etiology of enlarged glial cells in depression.
Glial cell pathology in mood disorders does not appear to be universally noted throughout the cerebral cortex. Changes in glial cell density or number are not found in the sensorimotor cortex in either MDD or BPD.1 Recent reports suggest, a lack of marked glial pathology in the supragenual part of the anterior cingulate cortex,12 the entorhinal cortex in BPD and MDD,17 or the most rostral part of the orbitofrontal cortex in MDD (corresponding to the transitional cortex between Brodmann areas 10 and 47). 5
Glial pathology in mood disorders has yet to be systematically studied in subcortical structures. Only one report suggests that glial pathology extends to limbic subcortical regions, with a significant reduction in glial number noted in the amygdala in subjects with MDD and unmedicated subjects with BPD.17
Alterations in neurons and glia in the hippocampus
Preclinical and neuroimaging studies have implicated the hippocampal formation in the pathophysiology of MDD. In addition, plasticity within the hippocampal formation may be involved in neurobiologjcal responses to stress and to antidepressant drug action.18 Evidence for an interaction between the hippocampus and depression comes from magnetic resonance imaging (MRI) studies examining the volume of the hippocampus. Studies using MRI demonstrate reduced volume of the hippocampus in subjects with M'DD or a history of M'DD,19-26 but not in other studies.27-29 It appears that hippocampal atrophy is preferentially seen in older, recurrently depressed subjects or subjects who are refractory to antidepressant medications. Recently, hippocampal volume and function was assessed over the course of illness in younger patients with MDD.26 Recollection memory is diminished in subjects with either a first episode or multiple episodes of depression. However, hippocampal volume is generally considered to be significantly decreased only in older depressed subjects with multiple episodes of depression.25,26
Few studies have structurally examined the postmortem human hippocampus in depression. Cellular integrity and apoptosis have been examined in the hippocampus in subjects with depression, steroid-treated subjects, and normal control subjects.30,31 Using semiquantitative methods, these studies report no significant cell loss in any hippocampal region in any of the subject groups. In most of the dépressives, there was evidence for a slight increase in fragmented DNA associated with apoptosis and necrotic neuron death detected in the dentate gyrus, CA1 and CA4.30 Decreases in astrocytic immunoreactivity for cellular GFAP and the neuron-specific phosphoprotein B50 (or GAP-45) were detected in CA1 and CA2 in depression.31 The authors suggest that apoptosis may only be a minor contributor to volume changes in the hippocampus in depression, while patterns of reactive astrogliosis and synaptic reorganization proteins are significantly altered in only some hippocampal regions in depression. Other reports of hippocampal changes in mood disorders identify a significant decrease in the density of nonpyramidal neurons in the CA2 region and a reduction in reelin-positive cell density in the hilus in subjects with BPD.32,33 Two other studies conducted on the postmortem hippocampal formation in a small sample of subjects with BPD reveal a decrease in the density and size of nonpyramidal neurons in the CA2 region and some disorganization in neuronal clusters in layers II and III of the entorhinal cortex.34,35
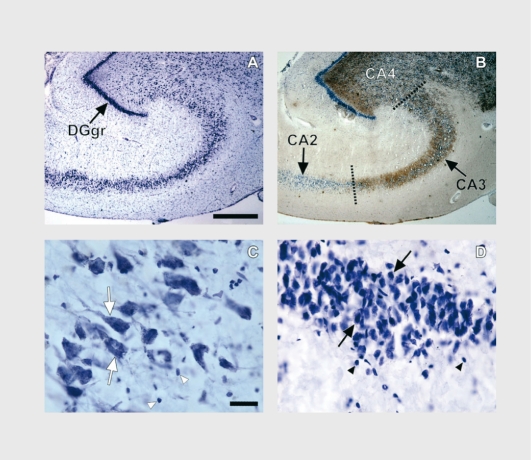
Neuronal and glial cell packing density and soma size were estimated recently in Nissl-stained sections including the hippocampal subfields in 16 subjects with MDD and 16 age-matched normal control subjects.36 Representative photomicrographs are presented in Figure 2. Prominent abnormalities in the CA regions and dentate gyrus are found in subjects with MDD. There is a significant increase in the mean density of pyramidal neurons in depressed subjects, as compared with normal control subjects. In the granule cell layer of the dentate gyrus, cell density is significantly increased in MDD. In addition, there is a significant decrease in the mean soma size of pyramidal neurons in depressed subjects, as compared with normal control subjects. On the basis of covariate analyses, the main findings of increased neuronal density and decreased neuron soma size in depression are not significantly altered when taking into consideration such factors as gender, age, postmortem interval, tissue pH, brain weight, smoking, antidepressant, drug prescription in the last month of life, or suicide. The substantial increases noted in neuronal packing density and decrease in neuronal soma size detected in postmortem tissue may be related to the decrease in hippocampal volume noted by some in MDD.
Figure 2. Brightfield photomicrographs of coronal sections of the postmortem human hippocampal formation. A. Cresyl violet-stained coronal section from a 54-year-old male (23-h postmortem interval). B. An adjacent coronal section processed byTimm staining. Note the intensely stained granule cell layer of the dentate gyrus (DGgr) in A and B, and the clear demarcation in B between hippocampal subfields CA2 and CA3 afforded by the Timm staining. Pyramidal neurons and glial nuclei of CA3 are highlighted in C by the large white arrows and white arrowheads, respectively. Neurons and glial nuclei of the granule cell layer of the dentate gyrus are depicted in D by the large black arrows and black arrowheads, respectively. The scale bars in A and C are 750 urn and 25 urn, respectively.
Glial pathology in depression appears to extend beyond the frontal cortex to the hippocampus. A recent study of the hippocampus in a large number of subjects with MDD and aged-matched normal control subjects reports a significant increase in the density of glial cells in all hippocampal CA subfields and the granule cell layer of the dentate gyrus.36 In MDD, increases in the packing density of glial cells detected in postmortem tissue suggests a potential reduction in surrounding neuropil (see above), and may be related to decreases in hippocampal volume noted by neuroimaging studies in MDD (see above).
The different pattern of density change noted in depression in the hippocampus in contrast to frontal cortical areas may be related to a unique reduction in neuropil in the hippocampus in depression. Neuropil consists of the lattice of glial cells and their processes, dendrites, and proximal axons surrounding neuron cell bodies. The hypothesis of neuropil reduction in the hippocampus in MDD is supported by other postmortem studies revealing a decrease in dendritic spine density on neurons and diminished arborization of apical dendrites in the subiculum in a small group of mixed subjects with bipolar disorder or depression37 and decreased levels of synaptic proteins found in CA4 in BPD.38 Thus, the diminished volume of the hippocampus noted by some in depression may be critically determined by a loss in neuropil including dendritic branching, dendritic spine complexity, and glial processes. The expression of brain-derived neurotrophic factor (BDNF) has been measured in the hippocampus of subjects with depression, and alterations in these factors might be related to changes in cell density and volume in depression. There is preliminary evidence that BDNF in the human hippocampus may be regulated by chronic treatment with antidepressant medications. In an irnmunohistochemical study of subjects with MDD and others with BPD or schizophrenia, the immunoreactivity of BDNF, as measured by optical density, is upregulated in the dentate gyrus and hilus only in subjects taking antidepressant medications (regardless of psychiatric diagnosis).39 Chen et al39 provide the first, evidence beyond rodent studies that chronic antidepressant drugs upregulate the expression of BDNF in the human hippocampus. In a recent study, Dwivedi et al40 observed a significant reduction in mRNA and protein levels of BDNF in hippocampus as well as dorsolateral prefrontal cortex in suicide victims with either MDD or other psychiatric disorders. In the Dwivedi et al40 study, the decrease in expression of BDNF occurred regardless of antidepressant treatment. It remains to be determined whether alterations in BDNF are related to increases in the packing density of neurons in the hippocampal formation or prefrontal cortex.
The different, pattern of neuronal pathology in the frontal cortex (decrease in density) and hippocampus (increase in density) suggests unique involvement of these brain regions in the neuropathology of depression. Other evidence of dissimilarities between prefrontal cortex and hippocampus has been reported in MDD.41-43 Successful clinical treatment (or even the use of placebo) in depression was associated with an increase in metabolism in prefrontal cortex and a decrease in metabolism in hippocampus.
Alterations in neurons and glia in subcortical structures
The search for morphological abnormalities in subjects with mood disorders has been less intense in subcortical structures than in cerebral cortical regions. Only a few studies in postmortem brain tissue on a relatively small number of subjects have attempted to estimate the number of neurons in such subcortical structures as hypothalamus, dorsal raphe nucleus, locus ceruleus, and amygdala.44-52 Results of these subcortical histopathological studies are somewhat inconsistent. Increases, decreases, or no change in the cell number or density are reported in the hypothalamus and brain stem nuclei in depressed subjects.
Stereological investigation of specific types of hypothalamic neurons reveals an increase in the numbers of arginine-vasopressin (AVP)-immunoreactive neurons, oxytocin-expressing neurons, and corticotropin-releasing hormone (CRH) neurons in the paraventricular nucleus in subjects with BPD or MDD, compared to normal controls.44,45 Moreover, increases in CRH mRNA, and in the number of CRH neurons colocalizing AVP are also found in depressed patients.46,47 These findings of increases in specific immunoreactive neurons arc consistent with the evidence of activation of the hypothalamicpituitary-adrenal (HPA) axis in some subsets of depressed patients.48 On the other hand, decreased number and density of nitric oxide synthase-containing neurons in the paraventricular hypothalamic nucleus are described in a small group of subjects with either MDD or BPD.49
Subtle structural abnormalities have been reported in mood disorders in the monoaminergic brain stem nuclei, the major sources of serotonin (dorsal raphe nucleus) and norepinephrine (locus ceruleus) projections to the cerebral cortex. An increased number and density of tryptophan hydroxylase immunoreactive neurons is observed in the dorsal raphe nucleus of suicide victims with MDD compared with controls.50 In suicide victims, Arango et al51 report fewer pigmented neurons within the rostral locus ceruleus. Another study in a larger number of subjects found no differences in the number of pigmented neurons in the locus ceruleus between subjects with MDD (most were suicides) and control subjects.52 Although the number of neurons in the locus ceruleus does not appear altered in MDD, CRH immunoreactivity is increased in the locus ceruleus and pontine dorsal and median raphe nuclei.53,54 No changes in neuronal densities were detected in amygdala in subjects with either M'DD or BPD, as compared to normal controls.17
These postmortem findings suggest that some changes in the morphology of hypothalamic neurons and brain stem neurons may take place in mood disorders. However, future studies employing stereological techniques and a larger number of subjects are required to determine the exact pathology in these regions in depression.
Functional implications of pathological changes in neural circuits
Morphological abnormalities detected postmortem in mood disorders are most likely related to dysfunction of neural circuits regulating emotional, cognitive, and somatic symptoms exhibited by subjects with MDD or BPD. In fact, alterations in neuronal density and size have been found in the dorsolateral prefrontal, orbitofrontal, and anterior cingulate cortex, the neurons of which give rise to the frontal circuits critical for higher cognitive and limbic functioning.55 Subtle neuronal alterations are also reported in the hypothalamus and hippocampus, further evidence of dysfunction in limbic circuits in depression.
Some of the cellular abnormalities detected postmortem in cortical and subcortical structures in MDD and BPD may be related to disruption of monoaminergic transmission in depression. Studies in postmortem brain tissue identify alterations in serotonin and norepinephrine receptors and transporters in the dorsolateral prefrontal cortex and ventrolateral/orbitofrontal cortex in brains from suicide victims with or without clinical depression.56 These cortical regions also exhibit, abnormal cell density and size in cell-counting studies of postmortem tissue. For example, cellular changes found in superficial layers of the prefrontal cortex in depressed subjects may be related to alterations in serotonin-1A receptors in superficial layers of cortex in suicide victims.57 In a neuroimaging study, the authors find that radioligand binding to scrotonin-1A receptors is decreased in medication-free subjects with MDD in several cortical regions, including medial temporal cortex, the temporal pole, orbitofrontal cortex, anterior cingulate cortex, insula and dorsolateral prefrontal cortex.58 Expression of another component of serotonin neurotransmission, the serotonin transporter, is also decreased in the dorsolateral prefrontal and ventral/orbitofrontal cortex in postmortem brains from depressed suicide victims.59,60 Detailed laminar analysis of the density of serotonin transporter-immunoreactive axons reveals that this deficit in depression is localized in cortical layer VI of the dorsolateral prefrontal cortex.59 The serotonin-transporter deficit, may be related to the pathology of layer VI neurons reported in the same cortical layer by postmortem cell-counting studies in depression.
Moreover, subtle neuronal abnormalities reported by some studies in the monoaminergic brain stem nuclei suggest dysfunction of monoaminergic projections originating from the brain stem neurons and terminating in frontolimbic cortical regions. It is likely that the functions and morphology of cortical neurons are affected by alterations in the functional state of noradrenergic, serotonergic, and dopaminergic neurons that project axons to prefrontal and anterior cingulate cortex. Postmortem neurochemical studies in MDD report alterations in noradrenergic α2-adrenergic receptors and the norepinephrine transporter, as well as levels of tyrosine hydroxylase in the locus ceruleus,52,61,62 scrotonin-lA receptors in the midbrain dorsal raphe nucleus,63 and dopaminergic receptors and transporters in the amygdala.64
The layer-specific changes in neuronal density and size identified in mood disorders implies that both inhibitory local circuit neurons and excitatory projection types of cortical neurons may be involved in the neuropathology of mood disorders. Nonpyramidal inhibitory neurons using GABA are localized mainly in cortical layer II and establish local cortico-cortical connections within or between adjacent functional columns of cortical cells. In contrast, pyramidal glutamatergic excitatory neurons reside predominantly in cortical layers III, V, and VI and give rise to long projections to other cortical associational regions (layer III), striatum (layer V), and thalamus (layer VI).
Neuronal pathology detected in cortical layers III, V, and VI of the dorsolateral prefrontal cortex and anterior cingulate cortex in MDD may be associated with the pathology of excitatory pyramidal neurons within these laminae that use glutamate as their neurotransmitter. Moreover, the density of pyramidal neurons is selectively reduced in the dorsolateral prefrontal cortex in subjects with BPD,4 further confirming the pathology of glutamatergic neurons in mood disorders. These findings in postmortem brain tissue coincide with an in vivo proton magnetic resonance spectroscopy study in the anterior cingulate cortex revealing a reduction in glutamate levels in depression.65 There is increasing preclinical and clinical evidence that antidepressant drugs directly or indirectly reduce the function of N-methyl-D-aspartate (NMDA) glutamate receptors.66 Depression-related decreases in glutamate levels or the density of glutamatergic pyramidal neurons may alter in cortex and elsewhere the glutamatergic recognition site and its coupling to the NMDA receptor complex. One study of suicide victims, some of whom were diagnosed with MDD, reveals changes in the glutamatergic recognition site and its coupling to the NMDA receptor complex in the anterior prefrontal cortex.67 Interestingly, drugs that reduce glutamatergic activity or glutamate receptor-related signal transduction may also have antimanic effects.66
Reductions in size and density of layer II neurons in the orbitofrontal and dorsolateral prefrontal cortex, as well as reductions in the density of nonpyramidal neurons in layer II of the anterior cingulate cortex suggest deficient GABAergic neurotransmission. Most nonpyramidal neurons in cortical layer II colocalize GABA and recent clinical evidence suggests that MDD is associated with decreased levels of cortical GABA.11
In summary, the localization of morphological abnormalities in the mood disorders occurs in prefrontolimbic circuits that arc likely to regulate emotional, cognitive, and somatic symptoms in depression. The observation in the mood disorders of neuronal pathology in specific cortical layers gives support to the hypotheses that the monoamine, glutamate, and GABA neurotransmitter systems are involved in the pathophysiology of these disorders. It remains to be determined whether the cellular pathology is the reason for, or the consequence of, depression.
Functional implications of glial abnormalities in depression
The glial cells analyzed in the above studies do not represent a homogeneous population of cells. Glial cells are composed of distinct populations of oligodendrocytes, microglia, and astrocytes. The crucial role of glial cell types in brain function is currently being reevaluated. In addition to their traditional roles in neuronal migration (radial glia), myelin formation (oligodendrocytes), and inflammatory processes (astrocytes and microglia), glia (predominantly astrocytes) are now thought to provide trophic support to neurons, neuronal metabolism, and the formation of synapses and neurotransmission.15
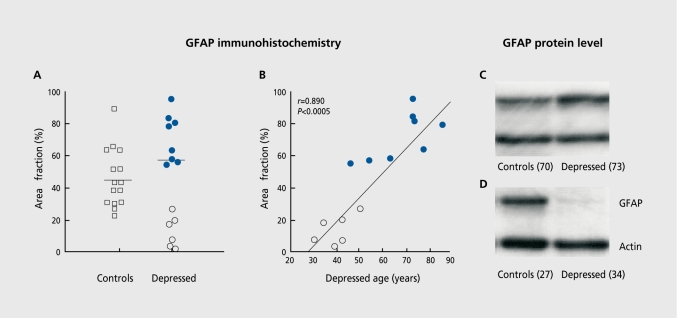
The three distinct glial cell types cannot be identified in the previously mentioned studies as those tissues were stained for Nissl substance and such staining does not distinguish reliably between types of glial cells. Nissl staining only reveals morphological features of glial cell bodies and not glial cell processes. On the other hand, recent irnmunohistochemical examination of glial fibrillary acidic protein (GFAP), a marker of reactive astroglia, in the dorsolateral prefrontal cortex implicates astrocytes in the overall glial pathology in MDD.68 Although no significant group differences in the packing density of GFAP-reactive astrocytes are present in this study, there is a significant correlation between age and GFAP immunoreactivity among subjects with MDD, when the entire group of MDD (young and old) is compared with normal controls. A significant reduction in the population of reactive astroglia is found in a small subgroup of young (30 to 45 years old) subjects with MDD, as compared to young control subjects and older (46 to 86 years old) subjects with MDD (Figures 3A and 3B). This subgroup of younger adults with MDD also had a shorter duration of depression and most of these subjects were suicide victims. Recent observations from our laboratory confirm that the levels of GFAP protein are also reduced in these young adults with MDD as compared to age-matched control subjects (Figures 3C and 3D), and that GFAP levels are positively correlated with age at the time of death and with the age of onset of depression.69 Thus, the involvement of GFAP expression in early- versus late-life depression differs because the underlying pathophysiology in early-life depression is different from that in late-life depression. Clinical evidence confirms that late-onset depression (first depressive episode when older than 50 years) differs from early-onset depression by its etiology, phenomenology, and cerebrovascular pathology.70-72
Figure 3. An illustration of the pathology of glial cells found in the dorsolateral prefrontal cortex in MDD.5,68 Reductions in the glial fibrillary acidic protein (GFAP) immunoreactive astroglia are found in a subgroup of young adults with major depression as compared to aged-matched control subjects and older subjects with major depression (A) and these reductions are correlated with the age of the subjects at the time of death (B). Recent preliminary observations (Si et al, unpublished observation) indicate that the levels of GFAP protein in the same area of the dorsolateral prefrontal cortex are also reduced in these young (D) but not old (C) subjects with major depression as compared to age-matched control subjects. Note that the level of actin, another protein in brain, is unchanged in a depressed subject as compared to the control. Reproduced (A and B) from reference 68: Miguel-Hidalgo JJ, Baucom C, Dilley G, et al. Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biol Psychiatry. 2000;48:861-873. Copyright © 2000, Elsevier.
Alterations in GFAP in both BPD and M.DD arc also suggested by a proteomic study in which different forms of GFAP proteins displayed disease-specific abnormalities.73 Oligodendrocytes may also be involved in the cellular pathology of depression. In both the dorsolateral prefrontal and anterior frontal cortex in subjects with BPD or MDD, there are ultrastructural changes in oligodendrocytes and there is a reduction in the density and immunoreactivity of these cells.74,75 Moreover, key oligodendrocyte-related and myelin-related gene expression is reduced in the dorsolateral prefrontal cortex in BPD.76 While these results are intriguing, further immunohistochemical and molecular studies are needed to definitively determine which specific glial cell types are compromised in BPD and whether the same or different types of glial cells are involved in the pathology reported in MDD. Reductions in glial number and density, in addition to changes in size and shape, might, be related to the dysfunction of monoamine and glutamate systems reported extensively in depression. For example, astrocytes express virtually all of the receptor systems, ion channels, and transporters found in neurons.15 Thus, the postsynaptic monoaminergic receptors distributed on glial cell bodies and processes may play a role in serotonin, norepinephrine, or dopamine neurotransmission. Moreover, astroglia are the primary sites of glutamate uptake by glial transporters and are important in regulating NMDA receptor activity. Astroglia regulate the levels of extracellular glutamate and thereby protect neurons in vitro from cell death and provide energy for neurons. Astrocytic pathology in MDD may indirectly promote glutamate-mediated neuronal excitotoxiclty, with consequences that may be detected by functional neuroimaging.
A mounting body of data suggests that treatment with antidepressant or mood-stabilizing medications regulates neuronal survival and also influences neurogenesis. Pharmacologically induced increases in neurogenesis in adult rodent brain have been reported in two independent studies.77,78 Moreover, there is evidence that treatment with lithium induces an increase in the astrocytic protein GFAP in rodent hippocampus79,80 and the neural lobe of the pituitary.81 However, whether these increases represent a protective or compensatory effect of these medications, and the mechanisms underlying the regulation of neurogenesis and glial proliferation have to be further investigated. Furthermore, a precise link between cell loss and atrophy, observed in the postmortem human brain, and medication-induced production of new cells, observed in the animal brain, has yet to be established.
Limitations in postmortem pathology studies in mood disorders
Postmortem studies cannot yet clearly define whether a true loss of cells underlies prominent reductions in cell density and size detected in mood disorders. For the estimation of a total number of neurons or glia in a particular brain region, it is essential that the total volume of a studied area be calculated. To measure the entire volume, the exact borders of the studied region have to be establish ed,82,83 so that sampling is confined to the region within these borders. Unfortunately, in most studies of mood disorders in postmortem tissue, limited availability of the complete tissue region, as well as limitations in reliably distinguishing cytoarchitectonic borders of a studied region, have prevented the estimation of a total tissue volume and, consequently, total cell number. In one study where the total cell number was estimated in the subgenual cortex, a loss of glial but not neuronal cells has been demonstrated in familial mood disorders.1 Glial reductions reported in this study may in fact reflect a true loss of glial cells since the neuroimaging studies in the same cortical region show a reduction in the volume of gray matter.84
There are unquestionable limitations to the use of postmortem brain tissue in studying the mood disorders.56 Some of the critical issues to be considered when interpreting the studies of postmortem brain tissue include the psychiatric status of the subject at the time of death and the underlying psychiatric disorder, whether “control” subjects were psychiatrically normal, the cause of death of the subjects (suicide or by other means), evolving criteria used to establish psychiatric diagnoses, the possible inclusion of subjects with concurrent psychoactive substance use disorders, the regional and hemispheric localization of the brain regions being studied, and the presence and duration of treatment with a psychotropic medication. Other frequent drawbacks to studies of postmortem brain tissue include low numbers of subjects per cohort, or inadequate expertise in cytoarchitectonic delineation of individual brain regions. Ideally, longitudinal clinical studies on wellcharacterized patients should be linked to subsequent postmortem studies of the same subjects. It is important to seek to control for the potential effects of suicide on postmortem biological observations in depression. In two of our studies,5,36 enough depressed nonsuicide subjects were available to tentatively determine that the main findings of these studies appear to persist regardless of whether the depressed subjects died by suicide or natural causes. While suicide makes tissues available for most postmortem studies of depression, the results obtained with this cohort must be cautiously interpreted since the majority of living individuals with depression do not attempt or commit suicide. In the mood disorders, the alterations in cell density and size are likely to be related to the disorder itself and not to the age of subjects at the time of death, postmortem delay, or the time of fixation of the tissue. Statistical analyses conducted in all of the above morphometric studies yielded no significant correlation between cell density or size and any of these confounding variables. It cannot be ruled out, however, that some of the cellular alterations in mood disorders are related to prior treatment with antidepressants and lithium (for further discussion see reference 85).
The question of whether cell abnormalities can be attributed to the effect of therapeutic medications is open to debate. There have been no systematic studies on the effect of antidepressant and mood-stabilizing medications on cell number and morphology in the postmortem human brain, most likely due to an insufficient number of treated versus untreated subjects.
Conclusion
Cellular abnormalities in mood disorders are observed in the dorsolateral prefrontal cortex, anterior cingulated cortex, orbitofrontal cortex, hippocampus, and amygdala. In these same brain regions, neuroimaging studies reveal volumetric, metabolic, and neurochemical alterations in subjects with mood disorders.
Structural neuroimaging studies in mood disorders provide evidence of modest but intriguing volumetric changes that suggest cell loss and/or atrophy.86 Some studies, but not all, report, enlargement of the lateral and third ventricles in mood disorders87 that may be indicative of atrophy of surrounding cortical and subcortical regions.
Functional neuroimaging studies in MDD and BPD lend further support to physiological abnormalities in cortical and subcortical frontolimbic regions. Abnormal regulation of glucose metabolism, regional cerebral blood flow, and high-energy phosphate metabolism are observed in the prefrontal and temporal cortex, basal ganglia, and amygdala, in mood disorders.88 Neuroimaging studies that examine neurochemical changes in the living brain provide further support for the hypothesis that mood disorders are associated with changes in cell viability and function. For example, high-resolution magnetic resonance spectroscopy in unmedicated subjects with BPD report decreased N-acetylaspartate (NAA) levels bilaterally in the hippocampus89 and in the dorsolateral prefrontal cortex,90 as compared to healthy controls. In contrast, therapeutic doses of lithium increase levels of NAA in the brain of subjects with BPD.91 Such increases in NAA are found in a number of regions including frontal cortex, and are localized almost exclusively in the gray matter. NAA is regarded as a measure of neuronal viability and function, and therefore the changes in NAA levels seen in BPD strongly implicate alterations in neuronal viability, which may be related to alterations in cell number, cell density, and size, and related volumetric changes. Interestingly, recent magnetic resonance spectroscopic studies of nonhuman primates exposed to early life stressors or repeated stressors also reveal a significant decrease in NAA. The NAA decrease in the animals exposed to repeated stressors was normalized by chronic treatment with the antidepressant tianeptine.92 Increases in glutamate-glutamine-GABA metabolites in the adult anterior cingulate cortex of these animals were also observed 10 years after the stressors. These NAA measures reflect neuronal integrity and metabolism whereas changes in glutamate-glutamine-GABA metabolites may reflect changes in membrane structure, glial functions, and glutamate content. Together, the above data suggest that structural and metabolic alterations observed in vivo may be related to alterations in cell viability, which, itself, may be related to alterations in cell number, density, and size observed in postmortem tissues at the microscopic level. The studies reviewed above undeniably prove the usefulness of postmortem tissue in unraveling the microscopic anatomical substrate of depression. For the first time, postmortem cell-counting studies in mood disorders have established that MDD and BPD are brain diseases with unique pathological alterations in neuronal and glial cells. The precise region- and layer-specific alterations in neuronal and glial architecture observed in mood disorders are consistent with the hypotheses of specific dysfunction in monoamine, glutamate, and GABA neurotransmitter systems in these disorders. Moreover, colocalization of cellular changes detected in postmortem tissues with in vivo neuroimaging findings proves that postmortem studies provide an important interface between clinical and basic research in unraveling the ncuroanatomical substrates of depression.
Postmortem studies in depression also indicate that while MDD and BPD are clearly not neurodegenerative disorders, these disorders are associated with impaired cellular neuroplasticity and resilience. It remains to be fully elucidated to what, extent these findings represent neurodevelopmental abnormalities, progression of the disorder, biochemical changes (in glucocorticoid or trophic factors levels) accompanying repeated disease episodes, or the results of treatment with therapeutic medications. It is unknown whether the cellular changes observed postmortem in mood disorders can be reversed by antidepressant and mood-stabilizing medications. Although molecular and genetic mechanisms associated with depression are yet to be unraveled, preliminary microarray studies of gene expression in postmortem brain tissues from subjects with mood disorders confirm that the dorsolateral prefrontal and anterior cingulate cortex are sites of pathology in mood disorders.93,94
Selected abbreviations and acronyms
- AVP
arginine-vasopressin
- BDNF
brain-derived neurotrophic factor
- BPD
bipolar disorder
- CRH
corticotropin-releasing hormone
- GABA
γ-aminobutyric acid
- GFAP
glial fibrillary acidic protein
- HPA
hypothalamic-pituitary-adrenal (axis)
- MDD
major depressive disorder
- NAA
N-acetylaspartate
- NMDA
N-methyl-D-aspartate
The authors acknowledge the support of the National Alliance for Research on Schizophrenia and Depression, and Public Health Service Grants MH60451, MH61578, MH63187, MH67996, and P20 RR17701.
REFERENCES
- 1.Ongur D., Drevets WC., Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouras C., Kovari E., Hof PR., et al. Anterior cingulate cortex pathology in schizophrenia and bipolar disorder. Acta Neuropathol (Berl). 2001;102:373–379. doi: 10.1007/s004010100392. [DOI] [PubMed] [Google Scholar]
- 3.Cotter D., Mackay D., Chana G., et al. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12:386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- 4.Rajkowska G., Halaris A., Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- 5.Rajkowska G., Miguel-Hidalgo JJ., Wei J., et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 6.Benes FM., Vincent SL., Todtenkopf M. The density of pyramidal and nonpyramidal neurons in anterior cingulate cortex of schizophrenic and bipolar subjects. Biol Psychiatry. 2001;50:395–406. doi: 10.1016/s0006-3223(01)01084-8. [DOI] [PubMed] [Google Scholar]
- 7.Cotter D., Landau S., Beasley C., et al. The density and spatial distribution of GABAergic neurons, labelled using calcium binding proteins, in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia. Biol Psychiatry. 2002;51:377–386. doi: 10.1016/s0006-3223(01)01243-4. [DOI] [PubMed] [Google Scholar]
- 8.Cotter D., Mackay D., Landau S., et al. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- 9.Reynolds GP., Zhang ZJ., Patten I., et al. Selective deficits of frontal cortical GABAergic neuronal subtypes defined by calcium binding proteins in psychotic illness. Schizophr Res. 2000;41:255. [Google Scholar]
- 10.Rajkowska G., O'Dwyer G., Shao Q., et al. Calbindin immunoreactive nonpyramidal neurons are reduced in the dorsolateral prefrontal cortex in major depression and schizophrenia. Society for Neuroscience, 32nd Annual Meeting, Orlando, Fla. Program No. 497.20. 2002 Abstract Viewer/ Itinerary Planner. Washington, DC: Society for Neuroscience; 2002. Available at http://sfn.scholarone.com/itin2002/. Accessed 5 May 2004. [Google Scholar]
- 11.Sanacora G., Mason GF., Krystal JH. Impairment of GABAergic transmission in depression: new insights from neuroimaging studies. Crit RevNeurobiol. 2000;14:23–45. doi: 10.1615/critrevneurobiol.v14.i1.20. [DOI] [PubMed] [Google Scholar]
- 12.Chana G., Landau S., Beasley C., et al. Two-dimensional assessment of cytoarchitecture in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia: evidence for decreased neuronal somal size and increased neuronal density. Biol Psychiatry. 2003;53:1086–1098. doi: 10.1016/s0006-3223(03)00114-8. [DOI] [PubMed] [Google Scholar]
- 13.Honer WG., Falkai P., Chen C., et al. Synaptic and plasticity-associated proteins in anterior frontal cortex in severe mental illness. Neuroscience. 1999;91:1247–1255. doi: 10.1016/s0306-4522(98)00679-4. [DOI] [PubMed] [Google Scholar]
- 14.Eastwood SL., Harrison PJ. Synaptic pathology in the anterior cingulate cortex in schizophrenia and mood disorders. A review and a Western blot study of synaptophysin, GAP-43 and the complexins. Brain Res Bull. 2001;55:569–578. doi: 10.1016/s0361-9230(01)00530-5. [DOI] [PubMed] [Google Scholar]
- 15.Cotter DR., Pariante CM., Rajkowska G. Glial pathology in major psychiatric disorders. In: Agam G, Everall IP, Belmaker RH, eds. The Postmortem Brain in Psychiatric Research. Boston, Mass: Kluwer Academic Publishers; 2002:49–73. [Google Scholar]
- 16.Hansson E., Muyderman H., Leonova J., et al. Astroglia and glutamate in physiology and pathology: aspects on glutamate transport, glutamateinduced cell swelling and gap-junction communication. Neurochem Int. 2000;37:317–329. doi: 10.1016/s0197-0186(00)00033-4. [DOI] [PubMed] [Google Scholar]
- 17.Bowley MP., Drevets WC., Ongur D., et al. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002;52:404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- 18.Durnan RS., Malberg J., Thome J. Neural plasticity to stress and antidepressant treatment. Biol Psychiatry. 1999;46:1181–1191. doi: 10.1016/s0006-3223(99)00177-8. [DOI] [PubMed] [Google Scholar]
- 19.Sheline Yl., Wang PW., Gado MH., Csernansky JG., Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah PJ., Ebmeier KP., Glabus MF., Goodwin GM. Cortical grey matter reductions associated with treatment-resistant chronic unipolar depression. Controlled magnetic resonance imaging study. Br J Psychiatry. 1998;172:527–532. doi: 10.1192/bjp.172.6.527. [DOI] [PubMed] [Google Scholar]
- 21.Sheline Yl., Sanghavi M., Mintun MA., et al. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bremner JD., Narayan M., Anderson ER., et al. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 23.Mervaala E., Fohr J., Kononen M., et al. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med. 2000;30:117–125. doi: 10.1017/s0033291799001567. [DOI] [PubMed] [Google Scholar]
- 24.Steffens DC., Byrum CE., McQuoid DR., et al. Hippocampal volume in geriatric depression. Biol Psychiatry. 2000;48:301–309. doi: 10.1016/s0006-3223(00)00829-5. [DOI] [PubMed] [Google Scholar]
- 25.Frodl T., Meisenzahl EM., Zetzsche T., et al. Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry. 2002;159:1112–1118. doi: 10.1176/appi.ajp.159.7.1112. [DOI] [PubMed] [Google Scholar]
- 26.MacQueen GM., Campbell S., McEwen BS., et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci USA. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vakili K., Pillay SS., Lafer B., et al. Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biol Psychiatry. 2000;47:1087–1090. doi: 10.1016/s0006-3223(99)00296-6. [DOI] [PubMed] [Google Scholar]
- 28.Rusch BD., Abercrombie HC., Oakes TR., Schaefer SM., Davidson RJ. Hippocampal morphometry in depressed patients and control subjects: relations to anxiety symptoms. Biol Psychiatry. 2001;50:960–964. doi: 10.1016/s0006-3223(01)01248-3. [DOI] [PubMed] [Google Scholar]
- 29.Posener JA., Wang L., Price JL., et al. High-dimensional mapping of the hippocampus in depression. Am J Psychiatry. 2003;160:83–89. doi: 10.1176/appi.ajp.160.1.83. [DOI] [PubMed] [Google Scholar]
- 30.Lucassen PJ., Muller MB., Holsboer F., et al. Hippocampal apoptosis in major depression is a minor event and absent from subareas at risk for glucocorticoid overexposure. Am J Pathol. 2001;158:453–468. doi: 10.1016/S0002-9440(10)63988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millier MB., Lucassen PJ., Yassouridis A., et al. Neither major depression nor glucocorticoid treatment affects the cellular integrity of the human hippocampus. Eur J Neurosci. 2001;14:1603–1612. doi: 10.1046/j.0953-816x.2001.01784.x. [DOI] [PubMed] [Google Scholar]
- 32.Benes FM., Kwok EW., Vincent SL., et al. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44:88–97. doi: 10.1016/s0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- 33.Fatemi SH., Earle JA., McMenomy T. Reduction in reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol Psychiatry. 2000;56:654–663. doi: 10.1038/sj.mp.4000783. [DOI] [PubMed] [Google Scholar]
- 34.Beckmann H., Jakob H. Prenatal disturbances of nerve cell migration in the entorhinal region: a common vulnerability factor in functional psychoses? J Neural Transm. 1991;84:155–164. doi: 10.1007/BF01249120. [DOI] [PubMed] [Google Scholar]
- 35.Bernstein HG., Krell D., Baumann B., et al. Morphometric studies of the entorhinal cortex in neuropsychiatrie patients and controls: clusters of heterotopically displaced lamina II neurons are not indicative of schizophrenia. Schizophr Res. 1998;33:125–132. doi: 10.1016/s0920-9964(98)00071-1. [DOI] [PubMed] [Google Scholar]
- 36.Stockmeier CA., Mahajan GJ., Konick L., et al. Neural and glial density is increased and neural soma size is decreased in hippocampus in major depressive disorder. Biol Psychiatry. 2003;53(suppl 8):198. [Google Scholar]
- 37.Rosoklija G., Toomayan G., Ellis SP., et al. Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch Gen Psychiatry. 2000;57:349–356. doi: 10.1001/archpsyc.57.4.349. [DOI] [PubMed] [Google Scholar]
- 38.Harrison PJ., Eastwood SL. Neuropathological studies of synaptic connectivity in the hippocampal formation in schizophrenia. Hippocampus. 2001;11:508–519. doi: 10.1002/hipo.1067. [DOI] [PubMed] [Google Scholar]
- 39.Chen B., Dowlatshahi D., MacQueen GM., et al. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. BiolPsychia try. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- 40.Dwivedi Y., Rizavi HS., Conley RR., Roberts RC., Tammlnga CA., Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 41.Mayberg HS., Brannan SK., Tekell JL., et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 42.Kennedy SH., Evans KR., Kruger S., et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- 43.Mayberg HS., Silva JA., Brannan SK., et al. The functional neuroanatomy of the placebo effect. Ami J Psychiatry. 2002;159:728–737. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- 44.Purba JS., Hoogendijk WJ., Hofman MA., et al. Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch Gen Psychiatry. 1996;53:137–143. doi: 10.1001/archpsyc.1996.01830020055007. [DOI] [PubMed] [Google Scholar]
- 45.Raadsheer FC., Hoogendijk WJ., Stam FC., et al. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60:436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- 46.Raadsheer FC., van Heerikhuize JJ., Lucassen PJ., et al. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer's disease and depression. Am J Psychiatry. 1995;152:1372–1376. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- 47.Swaab DF., Hofman MA., Lucassen PJ., et al. Functional neuroanatomy and neuropathology of the human hypothalamus. Anat Embryol (Berl). 1993;187:317–330. doi: 10.1007/BF00185889. [DOI] [PubMed] [Google Scholar]
- 48.Holsboer F., Spengler D., Heuser I. The role of corticotropin-releasing hormone in the pathogenesis of Cushing's disease, anorexia nervosa, alcoholism, affective disorders and dementia. Prog Brain Res. 1992;93:385–417. doi: 10.1016/s0079-6123(08)64586-0. [DOI] [PubMed] [Google Scholar]
- 49.Bernstein HG., Stanarius A., Baumann B., et al. Nitric oxide synthase-containing neurons in the human hypothalamus: reduced number of immunoreactive cells in the paraventricular nucleus of depressive patients and schizophrenics. Neuroscience. 1998;83:867–875. doi: 10.1016/s0306-4522(97)00461-2. [DOI] [PubMed] [Google Scholar]
- 50.Underwood MD., Khaibulina AA., Ellis SP., et al. Morphometry of the dorsal raphe nucleus serotonergic neurons in suicide victims. Biol Psychiatry. 1999;46:473–483. doi: 10.1016/s0006-3223(99)00043-8. [DOI] [PubMed] [Google Scholar]
- 51.Arango V., Underwood MD., Mann JJ. Fewer pigmented locus coeruleus neurons in suicide victims: preliminary results. Biol Psychiatry. 1996;39:112–120. doi: 10.1016/0006-3223(95)00107-7. [DOI] [PubMed] [Google Scholar]
- 52.Klimek V., Stockmeier C., Overholser J., et al. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J Neurosci. 1997;17:8451–8458. doi: 10.1523/JNEUROSCI.17-21-08451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Austin MC., Janosky JE., Murphy HA. Increased corticotropin-releasing hormone immunoreactivity in monoamine-containing pontine nuclei of depressed suicide men. Mol Psychiatry; 2003;8:324–332. doi: 10.1038/sj.mp.4001250. [DOI] [PubMed] [Google Scholar]
- 54.Bissette G., Klimek V., Pan J., Stockmeier C., Ordway G. Elevated concentrations of CRF in the locus coeruleus of depressed subjects. Neuropsychopharmacology. 2003;28:1328–1335. doi: 10.1038/sj.npp.1300191. [DOI] [PubMed] [Google Scholar]
- 55.Alexander GE., Crutcher MD., DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- 56.Stockmeier CA., Jurjus G. Monoamine receptors in postmortem brain: do postmortem brain studies cloud or clarify our understanding of the affective disorders. In: Agam G, Everall IP, Belmaker RH, eds. The Postmortem Brain in Psychiatric Research. Boston, Mass: Kluwer Academic Publishers; 2002:363. [Google Scholar]
- 57.Arango V., Underwoood MD., Gubbi AV., et al. Localized alterations in preand postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res. 1995;688:121–133. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- 58.Sargent PA., Kjaer KH., Bench CJ., et al. Brain serotoninl A receptor binding measured by positron emission tomography with [nC]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- 59.Austin M., Whitehead R., Edgar C., et al. Localized decrease in serotonin transporter-immunoreactive axons in the prefrontal cortex of depressed subjects committing suicide. Neuroscience. 2002;114:807. doi: 10.1016/s0306-4522(02)00289-0. [DOI] [PubMed] [Google Scholar]
- 60.Mann JJ., Huang YY., Underwood MD., et al. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry. 2000;57:729–738. doi: 10.1001/archpsyc.57.8.729. [DOI] [PubMed] [Google Scholar]
- 61.Ordway GA., Widdowson PS., Smith KS., et al. Agonist binding to r/2-adrenoceptors is elevated in the locus coeruleus from victims of suicide. J Neurochem. 1994;63:617–624. doi: 10.1046/j.1471-4159.1994.63020617.x. [DOI] [PubMed] [Google Scholar]
- 62.Zhu M-Y., Klimek V., Dilley GE., et al. Elevated levels of tyrosine hydroxylase in the locus coerleus in major depression. Biol Psychiatry. 1999;46:1275–1286. doi: 10.1016/s0006-3223(99)00135-3. [DOI] [PubMed] [Google Scholar]
- 63.Stockmeier CA., Shapiro LA., Dilley GE., et al. Increase in serotonin-1 A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci. 1998;18:7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klimek V., Schenck JE., Han H., et al. Dopaminergic abnormalities in amygdaloid nuclei in major depression: a postmortem study. Biol Psychiatry. 2002;52:740–748. doi: 10.1016/s0006-3223(02)01383-5. [DOI] [PubMed] [Google Scholar]
- 65.Auer DP., Putz B., Kraft E., et al. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000;47:305–313. doi: 10.1016/s0006-3223(99)00159-6. [DOI] [PubMed] [Google Scholar]
- 66.Krystal JH., Sanacora G., Blumberg H., et al. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry. 2002;7:S71–S80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- 67.Nowak G., Ordway GA., Paul IA. Alterations in the “-methyl-D-aspartate (NMDA) receptor complex in the frontal cortex of suicide victims. Brain Res. 1995;675:157–164. doi: 10.1016/0006-8993(95)00057-w. [DOI] [PubMed] [Google Scholar]
- 68.Miguel-Hidalgo JJ., Baucom C., Dilley G., et al. Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biol Psychiatry. 2000;48:861–873. doi: 10.1016/s0006-3223(00)00999-9. [DOI] [PubMed] [Google Scholar]
- 69.Si X., Miguel-Hidalgo JJ., Rajkowska G. GFAP expression is reduced in the dorsolateral prefrontal cortex in depression. Society for Neuroscience, 33rd Annual Meeting, New Orleans, La. Program No. 640.8. 2003 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience, 2003. Online. Available at http://sfn.scholarone.com/itin2003/. Accessed 5 May 2004. [Google Scholar]
- 70.Heun R., Kockler M., Papassotiropoulos A. Distinction of early-and late-onset depression in the elderly by their lifetime symptomatology. Int J Geriatr Psychiatry. 2000;15:1138–1142. doi: 10.1002/1099-1166(200012)15:12<1138::aid-gps266>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 71.Krishnan K., Hays J., Tupler L., et al. Clinical and phenomenological comparisons of late-onset and early-onset depression. Am J Psychiatry. 1995;152:785–788. doi: 10.1176/ajp.152.5.785. [DOI] [PubMed] [Google Scholar]
- 72.Lavretsky H., Lesser IM., Wohl M., et al. Relationship of age, age at onset, and sex to depression in older adults. Am J Geriatr Psychiatry. 1998;6:248–256. [PubMed] [Google Scholar]
- 73.Johnston-Wilson NL., Sims CD., Hofmann JP., et al. Disease-specific alterations in frontal cortex brain proteins in schizophrenia, bipolar disorder, and major depressive disorder. The Stanley Neuropathology Consortium. Mol Psychiatry. 2000;5:142–149. doi: 10.1038/sj.mp.4000696. [DOI] [PubMed] [Google Scholar]
- 74.Orlovskaya DD., Vostrikov VM., Rachmanova NA., et al. Decreased numerical density of oligodendroglial cells in postmortem prefrontal cortex in schizophrenia, bipolar affective disorder and major depression. Schizophr Res. 2000;41:105. [Google Scholar]
- 75.Uranova N., Orlovskaya D., Vikhreva Q., et al. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull. 2001;55:597–610. doi: 10.1016/s0361-9230(01)00528-7. [DOI] [PubMed] [Google Scholar]
- 76.Tkachev D., Mimmack ML., Ryan MM., et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- 77.Chen G., Rajkowska G., Du F., et al. Enhancement of hippocampal neurogenesis by lithium. J Neurochem. 2000;75:1729–1734. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- 78.Malberg JE., Eisch AJ., Nestler EJ., et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rocha E., Achaval M., Santos P., et al. Lithium treatment causes gliosis and modifies the morphology of hippocampal astrocytes in rats. Neuroreport. 1998;9:3971–3974. doi: 10.1097/00001756-199812010-00037. [DOI] [PubMed] [Google Scholar]
- 80.Rocha E., Rodnight R. Chronic administration of lithium chloride increases immunodetectable glial fibrillary acidic protein in the rat hippocampus. J Neurochem. 1994;63:1582–1584. doi: 10.1046/j.1471-4159.1994.63041582.x. [DOI] [PubMed] [Google Scholar]
- 81.Levine S., Saltzman A., Klein AW. Proliferation of glial cells in vivo induced in the neural lobe of the rat pituitary by lithium. Cell Prolif. 2000;33:203–207. doi: 10.1046/j.1365-2184.2000.00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rajkowska G., Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46. Cereb Cortex. 1995;4:323–327. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- 83.Uylings HB., Sanz-Arigita E., de Vos K., et al. The importance of a human 3D database and atlas for studies of prefrontal and thalamic functions. Prog Brain Res. 2000;126:357–368. doi: 10.1016/S0079-6123(00)26024-X. [DOI] [PubMed] [Google Scholar]
- 84.Drevets W., Price J., Simpson JR Jr. et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 85.Miguel-Hidalgo JJ., Rajkowska G. Morphological brain changes in depression: can antidepressants reverse them? CN S Drugs. 2002;16:361–372. doi: 10.2165/00023210-200216060-00001. [DOI] [PubMed] [Google Scholar]
- 86.Soares J., Mann J. The anatomy of mood disorders - review of structural neuroimaging studies. Biol Psychiatry. 1997;41:86–106. doi: 10.1016/s0006-3223(96)00006-6. [DOI] [PubMed] [Google Scholar]
- 87.Elkis H., Friedman L., Wise A., et al. Meta-analyses of studies of ventricular enlargement and cortical sulcal prominence in mood disorders. Arch Gen Psychiatry. 1995;52:735–746. doi: 10.1001/archpsyc.1995.03950210029008. [DOI] [PubMed] [Google Scholar]
- 88.Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- 89.Bertolino A., Frye M., Callicott JH., et al. Neuronal pathology in the hippocampal area of patients with bipolar disorder. Biol Psychiatry. 1999;45:135S. doi: 10.1016/s0006-3223(02)01911-x. [DOI] [PubMed] [Google Scholar]
- 90.Winsberg ME., Sachs N., Tate DL., et al. Decreased dorsolateral prefrontal N-acetyl aspartate in bipolar disorder. Biol Psychiatry. 2000;47:475–481. doi: 10.1016/s0006-3223(99)00183-3. [DOI] [PubMed] [Google Scholar]
- 91.Moore GJ., Bebchuk JM., Hasanat K., et al. Lithium increases N-acetylaspartate in the human brain: in vivo evidence in support of bcl-2's neurotrophic effects? Biol Psychiatry. 2000;48:1–8. doi: 10.1016/s0006-3223(00)00252-3. [DOI] [PubMed] [Google Scholar]
- 92.Czeh B., Michaelis T., Watanabe T., et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Evans S., Akil H., Choudary P., et al. Microarray studies in mood disorders: distinct patterns seen between major depression and bipolar disorder in two frontal cortical regions. ACNP 41st Annual Meeting. December 8-12, 2002. San Juan, Puerto. Scientific Abstract 36; 2002 [Google Scholar]
- 94.Tomita H., Vawter M., Evans S., et al. Gene expression profiles in postmortem brains of mood disorder patients. Society for Neuroscience, 33rd Annual Meeting, New Orleans, La. Program No. 640.19.2003 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience, 2003. Online, 2003. Available at http://sfn.scholarone.com/itin2003/. Accessed 5 May 2004. [Google Scholar]