Abstract
Stress is known to activate distinct neuronal circuits in the brain and induce multiple changes on the cellular level, including alterations in neuronal structures. On the basis of clinical observations that stress often precipitates a depressive disease, chronic psychosocial stress serves as an experimental model to evaluate the cellular and molecular alterations associated with the consequences of major depression. Antidepressants are presently believed to exert their primary biochemical effects by readjusting aberrant intrasynaptic concentrations of neurotransmitters, such as serotonin or noradrenaline, suggesting that imbalances viihin the monoaminergic systems contribute to the disorder (monoaminergic hypothesis of depression). Here, we reviev the results that comprise our understanding of stressful experience on cellular processes, with particular focus on the monoaminergic systems and structural changes within brain target areas of monoaminergic neurons.
Keywords: noradrenaline, adrenaline, serotonin, dopamine, histamine, neuronal remodeling, α2-adrenoceptor, 5-HT1A receptor, dopamine transporter, tree shrew
Abstract
Se sabe que el estrés activa disiintos circuitos neuronales en el cerebroc e induce multiples cambios a nivel celular incluyendo alteraciones en las estructuras neuronales, A partir de las observaciones clínicas, que indican que a menudo el estrés précipita una enfermedad depresiva, el estrés psicosocial crónico sirve como modelo experimental para evaluar las alteracíones celulares y moleculares asociadas con las consecuencias de la depresión mayor, Actualmente se crée que los antidepresivos ejercen sus efectos bioquímicos primaríos mediante un réajuste de las concentraciones inirasinápticas aberrantes de neurotransmisores, como la serotonina y la noradrenalina, lo que sugiere que el desbalance dentro del sistema monoaminérgico contribuye al trastorno (hipótesis monoaminérgica de la depresión), Aqui se revisan los resultados que contribuyen a nuestra comprensión acerca de las consecuencias del estrés sobre los procesos celulares, con particular atención a los sistemas monoaminérgicos y los cambios estructurales de las áreas cérébrales bianco de las neuronas monoamínérgicas.
Abstract
Il est reconnu que le stress met en jeu des circuits neuronaux distincts dans le cerveau et induit de nombreux changements au niveau cellulaire, y compris des altérations des structures neuronales. Sur la base d'observations cliniques ayant montré que le stress déclenchait souvent une maladie dépressive, le stress psychosocial chronique peut être pris comme modèle expérimental pour évaluer les altérations moléculaires et cellulaires associées aux conséquences d'une dépression majeure. Selon les conceptions actuelles, les antidépresseurs exercent leur effet biochimique primaire en réajustant les concentrations intrasynaptiques aberrantes des neurotransmetteurs, telles la sérotonine et la noradrenaline. Ceci suggère que les déséquilibres des systèmes monoaminergiques sont impliqués dans la genèse de la dépression (hypothèse monoaminergique). Cet article passe en revue les résultais qui contribuent à notre compréhension des conséquences du stress sur les processus cellulaires, avec une attention particulière sur les systèmes monoaminergiques et les changements structurels des régions cérébrales cibles des neurones monoaminergiques.
Stressful life events are among the most potent factors that trigger or induce depressive episodes in humans. The brain responds to stress experiences in a complex manner related to the activation and inhibition of neurons that are involved in sensory, motor, autonomic, cognitive, and emotional processes. Chronic stress, which is known to be accompanied by hyperactivity in central nervous neurotransmitter systems, induces cellular changes that can be regarded as a form of plasticity. This causes mood alterations in the affected individual and has the potential to reverse the psychopathological processes, thus alleviating the symptoms of depression. Since social stress in animals evokes symptoms that resemble those found in depressed patients, chronic social stress can serve as an experimental paradigm to investigate the neuronal processes that may also occur during depressive disease in humans. Research over past years has led to considerable advances in the understanding of the neural causes of depression and the cellular mechanisms that underlie the beneficial effects of currently available antidepressants. More importantly, such research forms the basis for the future development of more effective antidepressant drugs.
Stress changes the activity of noradrenergic and adrenergic neurons
Stress is known to activate neurohormonal systems, such as the hypothalamo-pituitary-adrenal (HPA) axis, to release the central nervous “stress peptide” corticotropin-releasing factor,1 and to secrete glucocorticoids from the adrenal gland.2. These corticosteroids have been identified as prominent factors that modify metabolic processes in both the body and the brain during stress as well as depression.3 However, the other group of essential substances in basic and accelerated metabolism includes the monoamines, noradrenaline, adrenaline, dopamine, serotonin (5-hydroxytryptamine [5-HT]), and histamine. The present survey focuses on processes related to stress-mediated activation of monoaminergic neurons in the brain.
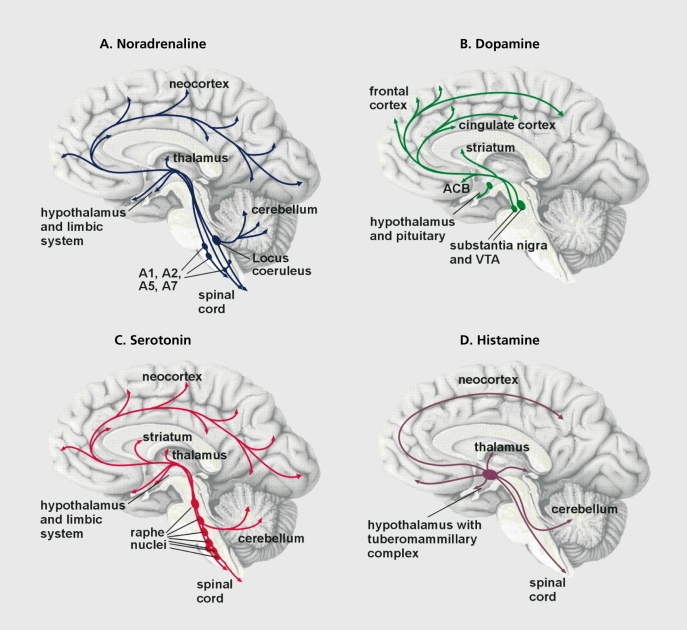
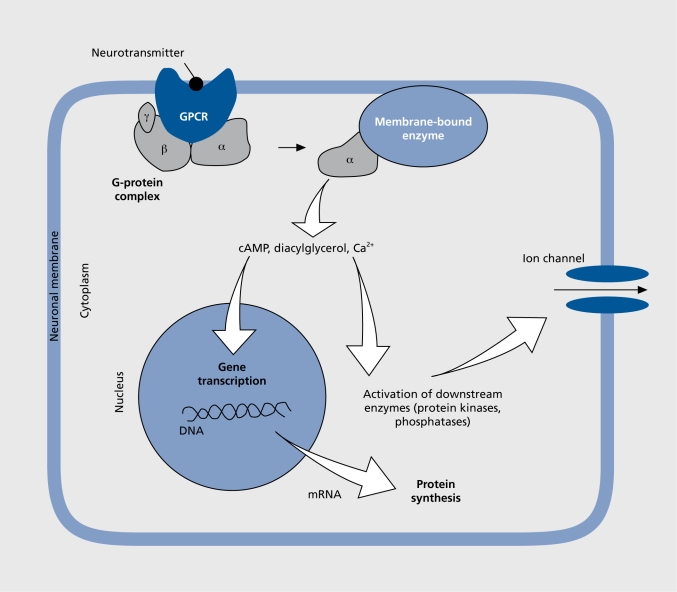
The noradrenergic and adrenergic neurons are located in the brain stem, where they form groups of cells that project axons to many parts of the brain. The beststudied group of noradrenergic neurons, located in the pontine locus ceruleus (LC), innervate several brain regions including the neocortex and the limbic system. The limbic system is a collection of regions that appear to regulate emotional processes (Figure 1). The noradrenergic LC neurons play an important role in the regulation of mood and emotions as well as of attention span. When stimulated through stressful challenge, for example, noradrenaline is released from the nerve terminals in the target brain region and is bound to adrenergic receptors belonging to the group of G protein-coupled receptors (GPCRs). These membrane-bound proteins convey signals from the extracellular to the intracellular compartment of a cell (Figure 2). GPCR signaling requires several steps for transmission of the signal, lasting from milliseconds to many minutes. The binding of a natural agonist such as noradrenaline or adrenaline to the receptor initiates a cascade of intracellular events that drive the activity of the cell and involve effectors such as enzymes (eg, adenylyl cyclase, phospholipasc, kinases, and phosphatases), second messengers (eg, cyclic adenosine monophosphate f[cAMP], cyclic guanosine monophosphate [cGMP], calcium ions, and arachidonic acid), as well as ion channels, which modulate the electrical activity of the neuron. A long-term effect occurring minutes after binding GPCR is the regulation of gene transcription and subsequent protein synthesis (Figure 2).5 There are different types of adrenergic receptors in the brain whose activation either stimulates or inhibits the respective target neurons. Noradrenaline and adrenaline bind to the same types of adrenergic receptors, although with slightly different affinities.6
Figure 1. Monoaminergic neurons innervate almost all brain areas. A. Noradrenaline. The noradrenergic neurons of the locus ceruleus project to the limbic and cortical regions, and to the thalamus, cerebellum, and spinal cord. They play an important role in the regulation of mood and attention. The noradrenergic neurons of cell groups A1 , A2, A5, and A7 project to more restricted regions.4 They are important for autonomic function. B. Dopamine. The dopaminergic neurons of the substantia nigra and the adjacent ventral tegmenta! area (VTA) project to the striatum and to regions in the neocortex. They are important in the initiation of movements and for emotional processes. Furthermore, there is a dopaminergic cell group in the hypothalamus that regulates neuroendocrine processes. C. Serotonin. The serotonergic neurons located in the raphe nuclei project to almost all parts of the brain and are involved in many functions including the regulation of emotional processes. D. Histamine. Histaminergic neurons are located in the tuberomammillary complex of the hypothalamus. They project to all parts of the brain and are important for arousal (the excited brain state). Modulation of neuronal activity by these monoamines is an important factor of well-balanced central nervous activity. Stress leads to hyperactivity of the monoamine neurons and thus to a dysregulation of neuronal activity. Currently available antidepressants are thought to adjust the balance between the different neurotransmitter systems.
Figure 2. Neurotransmission via a G protein-coupled receptor (GPCR): binding of the neurotransmitter to the receptor initiates a cascade of intracellular events that drive the activity of the neuron or cell. The G-protein complex, consisting of subunits α, β, and γ, serves as the machinery that transduces the extracellular signal to various effectors at the intracellular side of the plasma membrane, to the enzymes adenylyl cyclase or phospholipase. These enzymes catalyze the synthesis of second messengers, such as cyclic adenosine monophosphate (cAMP) and diacylglycerol, which regulate gene transcription in the nucleus. Transcripts (mRNA) are later translated into protein. Calcium ions released from intracellular stores and other second messengers activate protein kinases and phosphatases. This leads to phosphorylation and/or dephosphorylation of many intracellular proteins as well as ion channels that are located in the plasma membrane of the cell. Phosphorylation/dephosphorylation induces opening and closing of these channels and this modulates the electrical activity of the neuron. These dynamic cellular processes are accelerated during stress when neurotransmitter concentrations are elevated.
Various experiments have shown that during stress, noradrenergic and adrenergic neurons release more noradrenaline and adrenaline, respectively, and that the turnover of these neurotransmitters is accelerated so that their concentrations and/or amounts of their metabolites fluctuate in relation to the intensity and duration of the stressor.7 -10 Acute stress induces only a transient rise in noradrenaline levels, but chronic stress with recurrent environmental challenges can lead to repetitive increases in concentration. As a consequence, adrenoceptors on the surface of the target neurons are bombarded with noradrenaline, leading to a reduction in adrenoceptor numbers (receptor downregulation).11 On the other hand, low concentrations of noradrenaline induce adrenoceptor upregulation.12
Changes in α2-ARS alter the activity of neurons
The most studied adrenergic receptors, with respect to regulation in chronic stress, are the α2-adrenoceptors (α2-ARs), of which three subtypes are known (A, R, and C).13 Because of their widespread distribution in the brain, α2-ARs are diversely involved in mediating the analgesic and sedative effects of agonists such as dexmedetomidine14 and in modulating the baroreceptor reflex.15 The involvement of α2-ARs in the regulation of attention is suggested by the finding that mcthylphcnidate (the nonamphetamine stimulant used to treat children with attention-deficit hyperactivity disorder) affects neuronal activity in the LC.16 Administration of the antagonist yohimbine (a sympatholytic drug that is used to treat impotence) increases firing of the LC neurons, resulting in anxiety-like behavior in rats and monkeys.17 Brain α2-AR changes have been observed in depressed patients (see below).
The α2A-AR autoreccptor in LC noradrenergic neurons, regulates noradrenaline release via a negative feedback loop.14-18 Expression of this autoreceptor is reduced soon after the onset of stress (see below). In addition, α2A-AR is also expressed in neurons that release the excitatory neurotransmitter glutamate.“ In general, α2-AR stimulation leads to a transient inhibition of neuronal firing through hyperpolarization that is related to the modulation of calcium and potassium channels.20,21 There is reduced intracellular formation of the second messenger, cAMP, which itself regulates many cellular functions including gene transcription.22,23
Different forms of stress, such as immobilization or a cold environment, alter α2-AR numbers in distinct brain regions.24,25 We investigated the consequences of chronic psychosocial stress using a stress paradigm in male shrews.26 Our experiments showed that chronic psychosocial stress reduces α2-AR expression in brain regions that regulate autonomic functions and emotional behavior.27 This receptor downregulation is most probably related to the stress-mediated rise in noradrenaline tree concentrations. Regulation of noradrenaline release is impaired soon after the onset of the stress period, as revealed by reduced expression of the α2A-AR in the LC.28 During a stress period lasting several weeks, adrenergic regulation changes, giving an initially high level and then finally a low level of noradrenaline. This is the case in the prefrontal cortex, a brain area important for the regulation of mood and behavior.29 Following a chronic stress period, noradrenaline concentrations are obviously low throughout the whole brain, probably due to a gradually acquired deficit in transmitter synthesis, transport, and/or release from the noradrenergic neurons.30 Interestingly, studies on postmortem material from brains of depressed human patients also revealed the upregulation of oc2-ARs in several brain regions.31-33 These data therefore support the “noradrenaline deficit hypothesis,” which assumes there is a reduced noradrenaline concentration in the brains of depressed patients.34 Antidepressants that interact with α2-ARs such as mirtazapinc probably counteract this deficit.35
β-ARs also change during stress
GPCR P-adrenoceptors (β-ARs) increase cAMP synthesis.36 They are present in neurons and glial cells.37 When stimulated by agonists (adrenaline or noradrenaline), β-ARs are rapidly internalized into the cells. Therefore, high levels of endogenous agonists quickly reduce numbers of β-AR molecules in the plasma membrane of target cells, inducing desensitization.11-38 β-ARs are first internalized into the cell; they undergo intracellular sequestration with subsequent reinsertion into the plasma membrane, thereby restoring the normal receptor pattern in the membrane.
β-AR dysfunction is thought to play a role in psychiatric disorders, and β-AR blockers have been used to treat depression and anxiety.39 The number of β-ARs in the temporal and frontal cortex of suicide victims has been found to be significantly lower than in matched controls.40,41 However, the psychotropic role of β-AR downregulation is still under discussion since the antidepressant desmethylimipramine also downregulates brain β-ARs.42 On the other hand, the treatment of rats with the selective serotonin reuptake inhibitors (SSRIs) citalopram and fluoxetine increased prAR radioligand binding in the frontal cortex and striatum.43
Stress downregulates β-ARs in the brain.44 Our data from the tree shrew chronic stress model reveal that (i) the effects are dependent on the duration of a stressful event; (ii) β1 - and β2-ARs are differentially regulated; and (iii) the effects differ in different brain regions.45 Some of the stress-induced changes are only transient, since normal receptor numbers are restored through the reinsertion of intracellular sequestered receptor molecules into the plasma membrane. Finally, after 4 weeks of psychosocial stress, the number of β1- ARs was decreased in cells of the hippocampus and parietal cortex.
Stress and 5-HT neurons
It is generally assumed that changes in serotonergic neurons underlie depressive diseases because the most widely used antidepressants are SSRIs, which raise extracellular levels of 5-HT. Several experimental results have confirmed the “5-HT deficit hypothesis” of depression. In mammals, the majority of 5-HT-producing neurons are located in the brain stem, most of them on or near the midline, and they innervate almost every area of the brain.46 The serotonergic neurons of the dorsal raphe nucleus that project to the forebrain are autoactive and discharge in a stereotyped pattern that changes during the slecp-wake-arousal cycle.47,48 Due to its wide distribution, it. has been suggested that the 5-HT system is involved in almost every brain function, such as the regulation of neuroendocrine secretion, regulation of cardiovascular and respiratory activity, sleep, nociception, analgesia, and motor output.49-51 5-HT definitely regulates mood, since its transporters and receptors are targets for several psychotropic drugs.52 A polymorphism in the promoter region of the 5-HT transporter (5-HTT) gene is thought to contribute to anxiety in humans,53 and an epidemiological study provides evidence that an allele encoding a short DNA sequence in this promoter region increases the risk of developing a depressive disorder.54 Rhesus monkeys with the short-sequence allele have low concentrations of the 5-HT metabolite 5-hydroxyindoleacetic acid in their cerebrospinal fluid.55 This finding agrees with the view that low brain 5-HT levels (“decreased serotonergic activity”) have negative effects on emotionality. However, 5-HT concentration per se is probably not the only trigger for mood changes; humans with a genotype conferring high levels of expression of monoamine oxidase A (MAOA, the enzyme that degrades 5-HT) are less likely to develop antisocial problems than individuals with lower MAOA expression.54
Stress elevates the concentrations of 5-HT and its metabolites in several brain regions, indicating increased turnover rates of the neurotransmitter,8,56 although the serotonergic neurons of the dorsal raphe nucleus do not change their discharge rate during stress.46 Nevertheless, stress induces alterations in those brain regions that are targets of the serotonergic neurons, so that repeated exposure of rats to forced swimming increased 5-HT concentrations in the striatum, whereas they were reduced in the lateral septum.57 Chronic restraint stress in rats accelerated 5-HT turnover in the hippocampus and produced low amounts of the monoamine.58
Many receptors (>14) are known to mediate the effects of 5-HT.59 The present, survey focuses on the 5-HT1A receptor, the best characterized 5-HT receptor. This GPCR inhibits neuronal activity by reducing cAMP formation or phosphoinositide hydrolysis, depending on the type of neuron where it. is expressed, and it modulates potassium and calcium channels.60,61 The somatodendritic 5-HT1A autorcceptors located on the serotonergic neurons in the raphe nuclei regulate 5-HT release. Postsynaptic 5-HT1A receptors regulate the activity of neurons in cortical, limbic, and other regions. .For example, they affect the activity of pyramidal neurons in the hippocampus.62-64
The 5-HT1A receptor has been implicated in many functions. Like other 5-HT receptors, it is involved in the regulation of mood and emotional behavior,65 and there is evidence that. 5-HT1A receptor dysfunction is involved in depressive disorders. The agonists buspirone and gepirone act as anxiolytics and display antidepressant like effects in clinical trials.66 Human brain studies showed that. 5-HT1A receptor binding in depressed patients is lower than in healthy subjects.67,68 However, there are conflicting data on this issue. Brains of nonviolent suicides had increased 5-HT1A receptor binding in the frontal cortex in one report, whereas another report, showed no difference between suicides and controls.69,70 Furthermore, other psychiatric diseases - as well as depression - might cause changes in 5-HT1A receptors of the central nervous system. A variant, of the 5HT1A receptor gene was found in Tourette's patients and, in schizophrenics, 5-HT1A receptor binding sites were increased in the ventral prefrontal cortex.71-73 Schizophrenics also displayed some 5-HT1A receptor binding in the cerebellum, a brain region normally devoid of these receptors.74
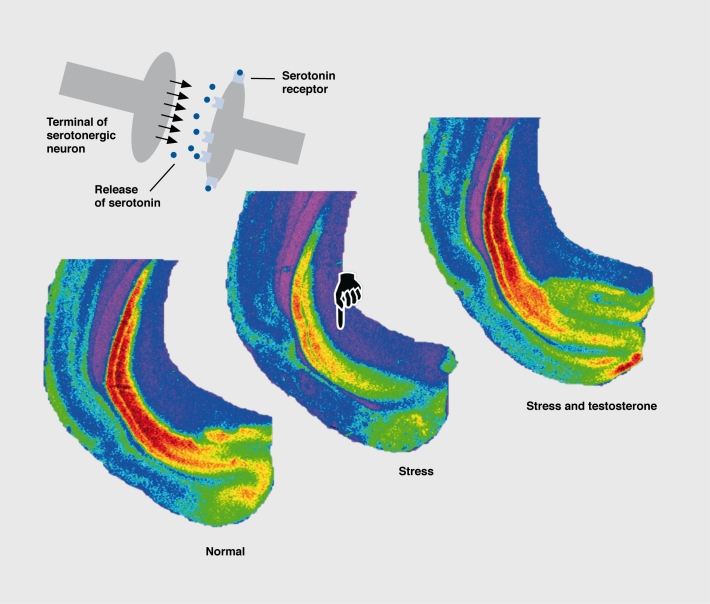
Restraint stress downrcgulated 5-HT1A receptors in the hippocampus of rats, and this effect was attributed to a stress-induced rise in plasma glucocorticoids, the adrenal hormones that regulate the transcription of many genes.75,76 The stress-induced downregulation of postsynaptic 5-HT1A receptors in distinct cortical areas and the hippocampal formation, in tree shrews, could also be attributed to high levels of glucocorticoids.64 However, it is interesting to note in relation to postsynaptic 5-HT1A receptor downregulation that the effect is not exclusively due to high glucocorticoid levels, but also to low testosterone. Social stress in male animals lowers testosterone levels, and normal 5-HT1A receptor numbers can be restored by a testosterone substitution (Figure 3). 77 It is interesting that the number of somatodendritic 5-HT1A autoreceptors in the dorsal raphe nucleus did not change during chronic stress in male tree shrews, with only their affinity being reduced.64 This agrees with electrophysiological data from the rat brain stem, which showed that stress reduces 5-HT1A autoreceptor functioning.78
Figure 3. Serotonergic nerve endings (schematic drawing, upper left) in the hippocampal formation release the neurotransmitter serotonin (gray balls), which binds to its receptors, the serotonin-1 A (5-HT1A) receptors (orange). The three pseudo-color pictures demonstrate receptor binding in normal male tree shrews (left), in tree shrews that were submitted to chronic psychosocial stress (middle), and in stressed tree shrews that had received testosterone as a treatment (right). Colors indicate numbers of receptors in the different regions of the hippocampal formation: orange, high receptor numbers; yellow, moderate numbers; green, low numbers; purple, no receptors. Note that after chronic social stress receptor numbers are decreased, but that the normal receptor number is restored following testosterone treatment.
Stress affects dopaminergic neurons
Responses of the dopamine system to stress received particular attention because of the potential involvement of this catecholamine in human psychopathologies that are known to be exacerbated by stress, such as schizophrenia.
Groups of dopaminergic neurons arc located in the midbrain, hypothalamus, and other regions.4 The mesocorti cal and mesolimbic dopamine pathways, which arise from the ventral tegmental area, have been implicated in emotional and memory processes. Dopaminergic cells of the substantia nigra and the adjacent midbrain tegmentum project to the telencephalon including the striatum, forming the nigrostriatal pathway, which initiates motor responses. Dopamine transporter (DAT) knockout mice with high extracellular dopamine levels were easily aroused by the mild stress of novelty.79 However, in genetically intact animals, the persistent stress-induced activation demonstrated in the noradrenergic system has not been demonstrated in the dopaminergic system. Under restraint stress, an initial increase in mesolimbic dopamine release was later followed by a decline, suggesting that repeated exposure to the same stressor results in inhibition rather than activation of dopaminergic neurons.80 Other data, suggest that the effects depend on the severity and controllability of the stressor, the genetic background of the animals, and their life history.81 The mesocortical dopaminergic system is obviously more stress-sensitive than the mesolimbic and the nigrostriatal systems.82,83
In the tree shrew model, 4 weeks of psychosocial stress downrcgulated DAT in the striatum. We also found a positive correlation between locomotor activity, which is reduced in stressed animals, and the total number of DAT binding sites.84 Low levels of DAT may indicate low extracellular dopamine concentrations. In agreement with these findings, social defeat in male rats also decreased DAT binding sites in the striatum.85
Dopamine was initially considered to convey its cellular actions via two receptor subtypes, D1 and D2; these exert opposing effects on the adenylate cyclase system. Five distinct dopamine receptors have now been cloned.36 Experiments with various knockouts could not determine where on the neurons these receptor subtypes are located (presynaptic versus postsynaptic location).86 However, there are indications that D1 and D5 receptors arc located postsynaptically, whereas D2, D3, and D4 receptors are located presynaptically and postsynaptically, with the presynaptic receptors acting as inhibitory autoreceptors.87,88 In the tree shrew model, D1 receptors were slightly increased in the striatum after 4 weeks of psychosocial stress (Mijnster et al, unpublished observations), with a reliable increase in the prefrontal cortex, while D2 receptors were upregulated in the hippocampus.89 Taken together, these changes in receptors and DAT indicate impaired dopamine release after stress. Such a deficit in dopamine release might also account for a lack of motivation in depression. Antidepressants that block the D2 receptor (eg, clomipramine and fluvoxam ine) might contribute to an improvement in motivation.
Histaminergic neurons under stress
The central nervous histamine system has been less extensively studied with respect to stress, although it. definitely plays an important role in the stress response. In mammalian brains, histaminergic neurons are found exclusively in the posterior ventral hypothalamus, but send their fibers to all brain regions.36,90 The electrophysiological properties of these cells are similar to those of the other aminergic neurons, with slow spontaneous firing, broad action potentials, and pronounced afterhyper polarization.91,92 Histamine activates three types of receptors whose expression varies between brain regions.36 Histamine modulates glutamatcrgic neurotransmission. H1 and H2 receptors are mainly postsynaptically located with high densities in limbic brain regions, while H3 is a somatodendritic autoreceptor that regulates release of the bioamine.91
The central histamine system is involved in many functions. Activity in histaminergic neurons correlates closely with the sleep-wake cycle, being highest when awake and lowest during rapid-eye movement sleep. Histaminergic neurons are also active in alarm situations and/or during activation of the peripheral sympathetic nervous system.91 H1 and H2 receptors modulate release of the “stress hormones” corticotropin-rclcasing factor and vasopressin from hypothalamic neurons,93 while various stressors such as dehydration or hypoglycemia stimulate histamine release. Even handling of rats raised histamine release in the prefrontal cortex of rats.94 Acute restraint stress stimulates histamine turnover throughout, the diencephalon, whereas during chronic stress histamine turnover in the striatum and nucleus accumbens is affected.95 A relationship between histaminergic neurotransmission and emotional processes is suggested by the fact that Hi receptor antagonists and H3 receptor agonists decrease anxiety, and because of the existence of antidepressants that block the H1 receptor (cg,doxepin and amitriptyline).
Stress-induced neuronal remodeling and plasticity
The stress-induced processes described above include changes in different compartments of cells:
Alterations in membrane-bound proteins that occur within seconds after the stressful stimulus (eg, conformational changes in receptors, enzymes, ion channels via stimulation of GPCRs).
Internalization of receptors and intracellular trafficking as described for α-ARs.
Changes in large enzyme complexes involved in the intracellular signaling cascade.
Changes in gene transcription, which may lead to either increased or decreased synthesis of a given protein (Figure 2).
It is possible that these dynamic processes may even lead to morphological changes in the cells; past research has shown that this is indeed the case.
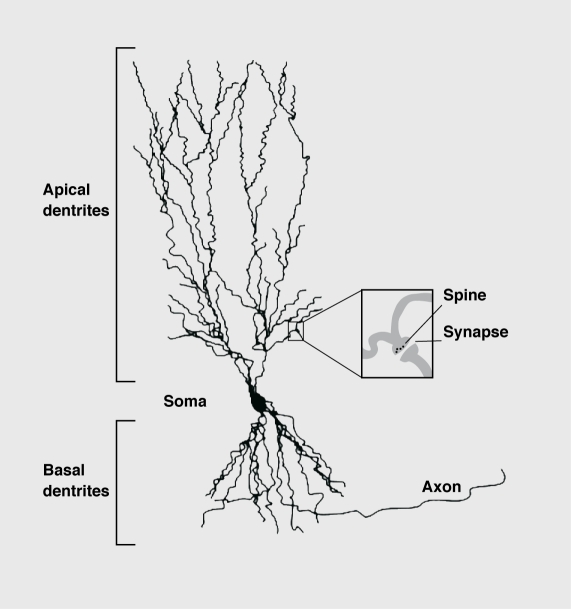
The first proof that chronic stress induces a remodeling of brain cells came from morphological studies on dendrites of pyramidal neurons in area CA3 of the hippocampus. Dendrites arc the major regions of neuronal synaptic contact with other neurons. Neurons with many or highly arborized dendrites potentially have large receptive fields (Figure 4).
Figure 4. Schematic drawing of a CA3 pyramidal neuron plus its dendrites. Note the small soma in comparison to the highly arborized apical and basal dendrites. Inset; dendritic shafts can build up protrusions (spines) that form synapses with axons or dendrites from other neurons. Synapses are sites of signal transmission between neurons. Formation and disappearance of spines are regulated by many factors such as gonadal hormones. Chronic psychosocial stress reduces the arborization of the apical dendrites, thus reducing the surface area of the neuron with the consequence that the neuron receives less information from other neurons (see text for details).
The retraction of the dendrites of these neurons was observed after chronic social stress and this effect was attributed to the stress-induced rise in glucocorticoids.96,97 Similar phenomena occur in pyramidal neurons in the prefrontal cortex, where glucocorticoids also induce alterations in the arborization of dendrites.98 In the C A3 pyramidal neurons of the hippocampus, dendritic retraction could be prevented by the antidepressant tianeptine, but not by the SSRIs fluoxetine and fluvoxamine.99 Also, chronic social defeat in male rats induced a shrinkage of the apical dendrites of the CA3 pyramidal neurons, and electrophysiological measurements revealed that this phenomenon was accompanied by a facilitation of action potentials, with reduced thresholds and higher amplitudes.100 In addition, single experiences of social defeat, on two consecutive days induced similar changes in the apical dendrites, with these changes persisting over 3 weeks. In contrast, to chronic daily social defeat, the arborization of the dendrites at the basal pole of the pyramidal neurons was increased after the double defeat paradigm.100,101 Therefore, two severely stressful experiences had longlasting consequences on the morphology of neurons that. differed from those induced by daily chronic stress. Stress was also shown to prevent long-term potentiation (LTP, a mechanism of synaptic plasticity that is thought to be related to memory formation) of CA neurons in the hippocampus. This inhibition of LTP was observed in male rats after only two exposures to social defeat.101 The antidepressant tiancptinc increases the amplitude of excitatory postsynaptic potentials and this mechanism appears to be related to alterations in the phosphorylation of the N-methyl-D-aspartate (NMDA) receptor, one of the most prominent receptors for the excitatory neurotransmitter glutamate.102
Synapses are often located at the tips of the spine protrusions on the dendritic shafts of neurons (Figure 4). The shape of a spine is related to the arrangement of the actin-containing microfilaments, the cytoskeletal fibers.103 Spines may form rapidly under the influence of synaptic activity.101 Activation of the NMDA receptor initiates changes in the actin cytoskclcton that stabilize the synaptic structure.105 Spine formation in the neurons of the prefrontal cortex can be induced by even minor stimuli, such as handling the experimental animals daily.106 In response to an acute stress, spine density was enhanced in the hippocampus of male rats, whereas, in contrast, female rats showed reduced spine density.107 It therefore appears that spine morphology is modulated by stress, although other factors such as sex hormones may also have an effect, on their formation.
Chronic stress and neuronal death?
There have been reports that social stress leads to cell death in the hippocampal formation.108 However, recent studies using the optical dissector technique, a. reliable method for quantification of neurons within an entire brain region, showed that stress does not affect neuron numbers in the CA1 and CA3 areas of the hippocampus.109 Moreover, experiments using an in situ end-labeling technique to identify apoptotic (dying) cells showed a significant decrease in the number of apoptotic cells when all hippocampal areas were analyzed.110
Although stress-induced death of principal neurons in the hippocampus is questionable, it is clear that stress profoundly affects these neurons. Their nuclear ultrastructure changes as shown in the significant intensification in Nissl staining.111 An electron microscopic analysis indicated that this effect is due to increased heterochromatin formation in the neuronal nuclei.112 The physiological role of these changes is unknown, but one may speculate that they are accompanied by alterations in gene transcription. Recent tree shrew studies showed that chronic psychosocial stress reduced the expression of certain genes that, are related to the shape of neurons and other brain cells.113 In the brains of adult rats that had been prenatally stressed through the stressful treatment of the pregnant dams, expression of genes associated with excitatory neurotransmission and mechanisms ofneurotransmitt.errelea.se were significantly altered.114 Furthermore, a large group of genes in the hippocampus has been shown to be differentially expressed after glucocorticoid treatment.76
Conclusions and further directions
Despite extensive preclinical and clinical investigations, the exact neurobiological processes leading to depression and the mechanisms responsible for the therapeutic effects of antidepressant drugs are still not completely understood. Antidepressants are presently believed to exert their primary biochemical effects by readjusting aberrant intrasynaptic concentrations of neuromodulators such as 5-HT However, the limitations of current antidepressant medications, such as the time delay for a full therapeutic response, the substantial number of nonresponders, and bothersome side effects merit, a full exploration of all plausible agents with novel antidepressant mechanisms of action.
Recent preclinical and clinical studies suggest that major depressive disorders are associated with cellular resilience and an impairment of synaptic and structural plasticity, and that antidepressant medications may act by correcting this dysfunction. Although this concept, is still in its infancy, it has increasingly attracted research efforts that may result in new treatment strategics for the etiopathophysiology of psychiatric disorders, such as major depression.
Selected abbreviations and acronyms
- α2-AR
α2-adrenoceptor
- β-AR
β-adrenoceptor
- DAT
dopamine transporter
- GPCR
G protein-coupled receptor
- 5-HT
5-hydroxytryptamine (serotonin)
- LC
locus ceruleus
The contributions of former and current members of the Clinical Neurobiology Laboratory at the German Primate Center are gratefully acknowledged. The work summarized here was in part supported by the German Science Foundation, the DAAD, and the EC.
Contributor Information
Eberhard Fuchs, Clinical Neurobiology Laboratory, German Primate Center, Göttingen, Germany.
Gabriele Flügge, Clinical Neurobiology Laboratory, German Primate Center, Göttingen, Germany.
REFERENCES
- 1.Koizumi K. The role of the hypothalamus in neuroendocrinology. In: Greger R, Windhorst U, eds. . Comprehensive Human Physiology. Heidelberg, Germany: Springer Verlag; 1996;1:379–401. [Google Scholar]
- 2.Hierholzer K., Bühler H. Metabolism of cortical steroid hormones and their general mode of action. In: Greger R, Windhorst U, eds. . Comprehensive Human Physiology. Heidelberg, Germany: Springer Verlag; 1996;1:403–429. [Google Scholar]
- 3.Duman RS. Neural plasticity: consequences of stress and actions of antidepressant treatment. . Dialogues Clin Neurosci. 2004;6:157–169. doi: 10.31887/DCNS.2004.6.2/rduman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieuwenhuys R. Chemoarchitecture of the Brain. Berlin, Germany: Springer Verlag; 1985 [Google Scholar]
- 5.Squire LR., Bloom FE., McConnell SK., Roberts JL., Spitzer NC., Zigmond MJ. Fundamental Neuroscience. New York, NY: Academic Press; 2003 [Google Scholar]
- 6.Lefkowitz RJ., Hoffman BB., Taylor P. Neurotransmission. The autonomic and somatic motor nervous system. In: Hardman JG, Limbird LE, et al, eds. . Goodman & Gilman s The Pharmacologic Basis of Therapeutics. New York, NY: McGraw-Hill; 1996:105–139. [Google Scholar]
- 7.Bliss EL., Ailion J., Zwanziger J. Metabolism of norepinephrine, serotonin and dopamine in rat brain with stress. . J Pharmacol Exp Ther. 1968;164:122–134. [PubMed] [Google Scholar]
- 8.Thierry AM., Javoy F., Glowinski J., Kety S. Effects of stress on the metabolism of norepinephrine, dopamine and serotonin in the central nervous system of the rat. . J Pharmacol Exp Ther. 1968;163:163–171. [PubMed] [Google Scholar]
- 9.Saavedra JM. Brain epinephrine in hypertension and stress. In: Stolk JM, U'Prichard DC, Fuxe K, eds. . Epinephrine in the Central Nervous System. Oxford, UK: Oxford University Press; 1988:102–116. [Google Scholar]
- 10.Stanford SC. Central noradrenergic neurones. . Pharmacol Ther. 1995;68: 297–342. doi: 10.1016/0163-7258(95)02010-1. [DOI] [PubMed] [Google Scholar]
- 11.von Zastrow M., Kobilka BK. Antagonist-dependent and -independent steps in the mechanism of adrenergic receptor internalization. . J BioiChem. 1994;269:18448–18452. [PubMed] [Google Scholar]
- 12.Ribas C., Miralles A., Busquets X., Garcia-Sevilla JA. Brain α2-adrenoceptors in monoamine-depleted rats: increased receptor density, G-coupling proteins, receptor turnover and receptor mRNA. . Br J Pharmacol. 2001;132:1467–1476. doi: 10.1038/sj.bjp.0703963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacDonald E., Kobilka BK., Scheinin M. Gene targeting-homing in on oçadrenoceptor-subtype function. . Trends Pharmacol Sci. 1997;18:211–219. doi: 10.1016/s0165-6147(97)01063-8. [DOI] [PubMed] [Google Scholar]
- 14.Kable JW., Murrin LC., Bylund DB. In vivo gene modification elucidates subtype-specific functions of α2-adrenergic receptors. . J Pharmacol Exp Ther. 2000;293:1–7. [PubMed] [Google Scholar]
- 15.MacMillan LB., Hein L., Smith MS., Piascik MT., Limbird LE. Central hypotensive effects of the 0C2A-adrenergic receptor subtype. . Science. 1996;273:801–803. doi: 10.1126/science.273.5276.801. [DOI] [PubMed] [Google Scholar]
- 16.Ishimatsu M., Kidani Y., Tsuda A., Akasu T. Effects of methylphenidate on the membrane potential and current in neurons of the rat locus coeruleus. . J Neurophysiol. 2002;87:1206–1212. doi: 10.1152/jn.00463.2001. [DOI] [PubMed] [Google Scholar]
- 17.Redmond AM., Leonard BE. An evaluation of the role of the noradrenergic system in the neurobiology of depression: a review. . Hum Psychopharmacol Clin Exp. 1997;12:407–430. [Google Scholar]
- 18.Aghajanian GK., Vandermaelen CP. α2-Adrenoceptor-mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. . Science. 1982;215:1394–1396. doi: 10.1126/science.6278591. [DOI] [PubMed] [Google Scholar]
- 19.Meyer H., Palchaudhuri M., Scheinin M., Flügge G. Regulation of α2Aadrenoceptor expression by chronic stress in neurons of the brain stem. . Brain Res. 2000;880:147–158. doi: 10.1016/s0006-8993(00)02787-6. [DOI] [PubMed] [Google Scholar]
- 20.Surprenant A., Horstman DA., Akbarali H., Limbird LE. A point mutation of the α2-adrenoceptor that blocks coupling to potassium but not calcium currents. . Science. 1992;257:977–980. doi: 10.1126/science.1354394. [DOI] [PubMed] [Google Scholar]
- 21.Boehm S., Huck S., Freissmuth M. Involvement of a phorbol ester-insensitive protein kinase C in the (-adrenergic inhibition of voltage-gated calcium current in chick sympathetic neurons. . J Neurosci. 1996;16:4596–4603. doi: 10.1523/JNEUROSCI.16-15-04596.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Limbird LE. Receptors linked to inhibition of adenylate cyclase: additional signaling mechanisms. . FASEBJ. 1988;2:2686–2695. doi: 10.1096/fasebj.2.11.2840317. [DOI] [PubMed] [Google Scholar]
- 23.IMestler EJ., Hyman SE. Regulation of gene expression. In: Davis KL, Charney D, Coyle JT, Nemeroff C, eds. . Neuropsychopharmacology. The Fifth Generation of Progress.Philadelphia, Pa: Lippincott Williams & Wilkins; . 2002:217–228. [Google Scholar]
- 24.U'Prichard D C., Kvetnansky R. Central and peripheral adrenergic receptors in acute and repeated immobilization stress. In: Usdin E, Kvetnansky R, Kopin IJ, eds. . Catecholamine and Stress. Recent Advances, Developments in Neuroscience. Vol 8. Amsterdam, The Netherlands: Elsevier; 1980:299–308. [Google Scholar]
- 25.Nukina I., Glavin GB., La Bella FS. Acute cold-restraint stress affects “2adrenoceptors in specific brain regions of the rat. . Brain Res. 1987;401:30–33. doi: 10.1016/0006-8993(87)91159-0. [DOI] [PubMed] [Google Scholar]
- 26.Fuchs E., Flügge G. Social stress in tree shrews: effects on physiology, brain function, and behavior of subordinate individuals. . Pharmacol Biochem Behav. 2002;73:247–258. doi: 10.1016/s0091-3057(02)00795-5. [DOI] [PubMed] [Google Scholar]
- 27.Flügge G., Jöhren O., Fuchs E. [3H] Rauwolscine binding sites in the brains of male tree shrews are related to social status. . Brain Res. 1992;597:131–137. doi: 10.1016/0006-8993(92)91514-f. [DOI] [PubMed] [Google Scholar]
- 28.Flügge G. Alterations in the central nervous α2-adrenoceptor system under chronic psychosocial stress. . Neuroscience. 1996;75:187–196. doi: 10.1016/0306-4522(96)00292-8. [DOI] [PubMed] [Google Scholar]
- 29.Flügge G., Ahrens O., Fuchs E. Monoamine receptors in the prefrontal cortex of Tupaia belangeri during chronic psychosocial stress. . Cell Tiss Res. 1997;288:1–10. doi: 10.1007/s004410050787. [DOI] [PubMed] [Google Scholar]
- 30.Flügge G., Van Kampen M., Mijnster MJ. Perturbations in brain monoamine systems during stress. . Cell Tiss Res. 2003;315:1–14. doi: 10.1007/s00441-003-0807-0. [DOI] [PubMed] [Google Scholar]
- 31.Meana JJ., Barturen F., Garcia Sevilla JA. α2-Adrenoceptors in the brain of suicide victims: increased receptor density associated with major depression. . Biol Psychiatry. 1992;31:471–490. doi: 10.1016/0006-3223(92)90259-3. [DOI] [PubMed] [Google Scholar]
- 32.Ordway GA., Widdowson PS., Smith KS., Halaris A. Agonist binding to α2-adrenoceptors is elevated in the locus coeruleus from victims of suicide. . J Neurochem. 1994;63:617–624. doi: 10.1046/j.1471-4159.1994.63020617.x. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Sevilla JA., Escriba PV., Ozaita A., et al. Up-regulation of immunolabeled α2A-adrenoceptors, G; coupling proteins, and regulatory receptor kinases in the prefrontal cortex of depressed suicides. . J Neurochem. 1999;72:282–291. doi: 10.1046/j.1471-4159.1999.0720282.x. [DOI] [PubMed] [Google Scholar]
- 34.Holsboer F. Molekulare Mechanismen der Depressionstherapie. In: Ganten D, Ruckpaul K, eds. . Handbuch der moiekularen Medizin. Heidelberg, Germany: Springer Verlag; 1999:273–318. [Google Scholar]
- 35.Schatzberg AF. Pharmacological principles of antidepressant efficacy. . Hum Psychopharmacol. 2002;17:17–22. doi: 10.1002/hup.399. [DOI] [PubMed] [Google Scholar]
- 36.Feldman RS., Meyer JS., Quenzer LF. Principles of Neuropsychopharmacology. Sunderland, Mass: Sinauer Associates Inc. 1997 [Google Scholar]
- 37.Stone EA., John SM. Further evidence for a glial localization of rat cortical (β-adrenoceptors: studies of in vivo cyclic AMP responses to catecholamines. . Brain Res. 1991;549:78–82. doi: 10.1016/0006-8993(91)90601-q. [DOI] [PubMed] [Google Scholar]
- 38.Claing A., Laporte SA., Caron MG., Lefkowitz RJ. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and fiarrestin proteins. . Prog Neurobiol. 2002;66:61–79. doi: 10.1016/s0301-0082(01)00023-5. [DOI] [PubMed] [Google Scholar]
- 39.Bailly D. The role of (β-adrenoceptor blockers in the treatment of psychiatric disorders. . CNS Drugs. 1996;5:115–136. [Google Scholar]
- 40.Depaermentier F., Crompton M R., Katona CLE., Horton RW. β-Adrenoceptors in brain and pineal from depressed suicide victims. . Pharmacol Toxicol. 1993;71:86–95. doi: 10.1111/j.1600-0773.1992.tb01632.x. [DOI] [PubMed] [Google Scholar]
- 41.Little KY., Clark TB., Ranc J., Duncan GE. (β- Adrenergic receptor binding in frontal cortex from suicide victims. . Biol Psychiatry. 1993;34:596–605. doi: 10.1016/0006-3223(93)90151-3. [DOI] [PubMed] [Google Scholar]
- 42.Paetsch PR., Greenshaw AJ. Effects of chronic antidepressant treatment on p-adrenoceptor subtype binding in the rat cerebral cortex and cerebellum. . MolChem Neuropathol. 1993;20:21–31. doi: 10.1007/BF03160067. [DOI] [PubMed] [Google Scholar]
- 43.Palvimaki EP., Laakso A., Kuoppamaki M., Syvalahti E., Hietala J. Upregulation of β1-adrenergic receptors in rat brain after chronic citalopram and fluoxetine treatments. . Psychopharmacology. 1994;115:543–546. doi: 10.1007/BF02245579. [DOI] [PubMed] [Google Scholar]
- 44.Stone EA., Piatt JE. Brain adrenergic receptors and resistance to stress. . Brain Res. 1982;237:405–414. doi: 10.1016/0006-8993(82)90452-8. [DOI] [PubMed] [Google Scholar]
- 45.Flügge G., Ahrens O., Fuchs E. β- Adrenoceptors in the tree shrew brain. II. Time-dependent effects of chronic psychosocial stress on [125]iodocyanopindolol bindings sites. . Cell Mol Neurobiol. 1997;17:417–432. doi: 10.1023/A:1026387311220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacobs BL., Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 47.Aghajanian GK., Vandermaelen CP. Intracellular recordings from serotonergic dorsal raphe neurons: pacemaker potentials and the effect of LSD. . Brain Res. 1982;238:463–469. doi: 10.1016/0006-8993(82)90124-x. [DOI] [PubMed] [Google Scholar]
- 48.Trulson ME., Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. . Brain Res. 1979;163:135–150. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- 49.Whitaker-Azmitia PM., Peroutka SJ. The Neuropharmacology of Serotonin. New York, NY: New York Academy of Sciences; 1990 [PubMed] [Google Scholar]
- 50.Jacobs BL., Fornal CA. Serotonin and behavior. A general hypothesis. In: Bloom FE, Kupfer DJ, eds. . Psychopharmacology. The Fourth Generation of Progress. New York, NY: Raven Press; 1995:461–469. [Google Scholar]
- 51.Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. . J Neuroendocrine! . 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- 52.Nutt DJ. The neuropharmacology of serotonin and noradrenaline in depression. . Int Clin Psychopharmacol. 2002;17:1–12. doi: 10.1097/00004850-200206001-00002. [DOI] [PubMed] [Google Scholar]
- 53.Lesch KP., Bengel D., Heils A., et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. . Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 54.Caspi A., Sugden K., Moffitt TE., et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. . Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 55.Bennett AJ., Lesch KP., Heils A., et al. Early experience and serotonin transporter gene variation interact to influence primate CNS function. . Mol Psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- 56.Chaouloff F. Physiopharmacological interactions between stress hormones and central serotonergic systems. . Brain Res Rev. 1993;18:1–32. doi: 10.1016/0165-0173(93)90005-k. [DOI] [PubMed] [Google Scholar]
- 57.Kirby LG., Lucki I. The effect of repeated exposure to forced swimming on extracellular levels of 5-hydroxytryptamine in the rat. . Stress. 1998;2:251–263. doi: 10.3109/10253899809167289. [DOI] [PubMed] [Google Scholar]
- 58.Torres IL., Gamaro GD., Vasconcellos AP., Silveira R., Dalmaz C. Effects of chronic restraint stress on feeding behavior and on monoamine levels in different brain structures in rats. . Neurochem Res. 2002;27:519–25. doi: 10.1023/a:1019856821430. [DOI] [PubMed] [Google Scholar]
- 59.Aghajanian GK., Sanders-Bush E. Serotonin. In: Davis KL, Charney D, Coyle JT, Nemeroff C, eds. . Neuropsychopharmacology The Fifth Generation of Progress. Philadelphia, Pa: Lippincott Williams & Wilkins; 2002:15–34. [Google Scholar]
- 60.Sanders-Bush E., Canton H. Serotonin receptors. Signal transduction pathways. In: Bloom FE, Kupfer DJ, eds. . Psychopharmacology The Fourth Generation of Progress. New York, NY: Raven Press; 1995:431–441. [Google Scholar]
- 61.Johnson RG., Fiorella D., Winter JC., Rabin RA. [3H]8-OH-DPAT labels a 5HT site coupled to inhibition of phosphoinositide hydrolysis in the dorsal raphe. . Eur J Pharmacol. 1997;329:99–106. doi: 10.1016/s0014-2999(97)10113-3. [DOI] [PubMed] [Google Scholar]
- 62.Vergé D., Daval G., Marcinkiewicz M., et al. Quantitative autoradiography of multiple 5-HT1 receptor subtypes in the brain of control or 5,7-dihydroxytryptamine-treated rats. . J Neurosci. 1986;6:3474–3482. doi: 10.1523/JNEUROSCI.06-12-03474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Joëls M., Hesen W., De Kloet ER. Mineralocorticoid hormones suppress serotonin-induced hyperpolarization of rat hippocampal CA1 neurons. . J Neurosci. 1991;11:2288–2294. doi: 10.1523/JNEUROSCI.11-08-02288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flügge G. Dynamics of central nervous 5-HTia receptors under psychosocial stress. . J Neurosci. 1995;15:7132–7140. doi: 10.1523/JNEUROSCI.15-11-07132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lucki I. 5-HT1 receptors and behavior. . Neurosci Biobehav Rev. 1992;16:83–93. doi: 10.1016/s0149-7634(05)80055-7. [DOI] [PubMed] [Google Scholar]
- 66.Blier P., de Montigny C. A decade of serotonin research: antidepressant mechanisms and therapeutics. Possible serotonergic mechanisms underlying the antidepressant and anti-obsessive-compulsive disorder responses. . Biol Psychiatry. 1998;44:313–323. doi: 10.1016/s0006-3223(98)00114-0. [DOI] [PubMed] [Google Scholar]
- 67.Lopez JF., Chalmers DT., Little KY., Watson SJ. A. E. Bennett Research Award Regulation of serotoniniA, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. . Biol Psychiatry. 1998;43:547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- 68.Drevets WC., Frank E., Price JC., et al. PET imaging of serotonin 1 A receptor binding in depression. . Biol Psychiatry. 1999;46:1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- 69.Matsubara S., Arora RC., Meltzer HY. Serotonergic measures in suicide brain: 5-HT1A binding sites in frontal cortex of suicide victims. . J Neural Transm Gen Sect. 1991;85:181–194. doi: 10.1007/BF01244944. [DOI] [PubMed] [Google Scholar]
- 70.Lowther S., De Paermentier F., Cheetham SC., Crompton MR., Katona CL., Norton RW. 5-HT1A receptor binding sites in post-mortem brain samples from depressed suicides and controls. . J Affect Disord. 1997;42:199–207. doi: 10.1016/s0165-0327(96)01413-9. [DOI] [PubMed] [Google Scholar]
- 71.Lam S., Shen Y., Nguyen T., et al. A serotonin receptor gene (5HT1A) variant found in a Tourette's syndrome patient. . Biochem Biophys Res Commun. 1996;219:853–858. doi: 10.1006/bbrc.1996.0322. [DOI] [PubMed] [Google Scholar]
- 72.Simpson MD., Lubman Dl., Slater P., Deakin JF. Autoradiography with [3H]8-OH-DPAT reveals increases in 5-HT1A receptors in ventral prefrontal cortex in schizophrenia. . Biol Psychiatry. 1996;39:919–928. doi: 10.1016/0006-3223(95)00026-7. [DOI] [PubMed] [Google Scholar]
- 73.Sumiyoshi T., Stockmeier CA., Overholser JC., Dilley GE., Meltzer HY. SerotoniniA receptors are increased in postmortem prefrontal cortex in schizophrenia. . Brain Res. 1996;708:209–214. doi: 10.1016/0006-8993(95)01361-x. [DOI] [PubMed] [Google Scholar]
- 74.Slater P., Doyle CA., Deakin JF. Abnormal persistence of cerebellar serotonin-1A receptors in schizophrenia suggests failure to regress in neonates. . J Neural Transm. 1998;105:305–315. doi: 10.1007/s007020050060. [DOI] [PubMed] [Google Scholar]
- 75.McKittrick CR., Blanchard DC., Blanchard RJ., McEwen BS., Sakai RR. Serotonin receptor binding in a colony model of chronic social stress. . Biol Psychiatry. 1995;37:383–393. doi: 10.1016/0006-3223(94)00152-s. [DOI] [PubMed] [Google Scholar]
- 76.Datson NA., van der Perk J., de Kloet ER., Vreugdenhil E. Identification of corticosteroid-responsive genes in rat hippocampus using serial analysis of gene expression. . Eur J Neurosci. 2001;14:675–689. doi: 10.1046/j.0953-816x.2001.01685.x. [DOI] [PubMed] [Google Scholar]
- 77.Flügge G., Kramer M., Rensing S., Fuchs E. 5-HT1A receptors and behaviour under chronic stress: selective counteraction by testosterone. . Eur J Neurosci. 1998;10:2685–2693. [PubMed] [Google Scholar]
- 78.Laaris N., Le Poul E., Hamon M., Lanfumey L. Stress- induced alterations of somatodendritic 5-HT1A autoreceptor sensitivity in the rat dorsal raphe nucleus - in vitro electrophysiological evidence. . Fund Clin Pharmacol. 1997;11:206–214. doi: 10.1111/j.1472-8206.1997.tb00187.x. [DOI] [PubMed] [Google Scholar]
- 79.Spielewoy C., Roubert C., Hamon M., Nosten-Bertrand M., Bncur C., Giros B. Behavioural disturbances associated with hyperdopaminergia in dopamine-transporter knockout mice. . Behav Pharmacol. 2000;11:279–290. doi: 10.1097/00008877-200006000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Imperato A., Cabib S., Puglisi-Allegra S. Repeated stressful experiences differently affect the time-dependent responses of the mesolimbic dopamine system to the stressor. . Brain Res. 1993;60:333–336. doi: 10.1016/0006-8993(93)91732-8. [DOI] [PubMed] [Google Scholar]
- 81.Cabib S., Puglisi-Allegra S. Stress, depression and the mesolimbic dopamine system. . Psychopharmacol. 1996;128:331–342. doi: 10.1007/s002130050142. [DOI] [PubMed] [Google Scholar]
- 82.Abercrombie ED., Keefe KA., DiFrischia DS., Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens and medial frontal cortex. . J Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- 83.Tidey JW., Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. . Brain Res. 1996;721:140–114. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- 84.Isovich E., Mijnster MJ., Flùgge G., Fuchs E. Chronic psychosocial stress reduces the density of dopamine transporters. . Eur J Neurosci. 2000;12:1071–1078. doi: 10.1046/j.1460-9568.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 85.Isovich E., Engelmann M., Landgraf R., Fuchs E. Social isolation after a single defeat reduces striatal dopamine transporter binding in rats. . Eur J Neurosci. 2001;13:1254–1256. doi: 10.1046/j.0953-816x.2001.01492.x. [DOI] [PubMed] [Google Scholar]
- 86.Picciotto MR. Knock-out mouse models used to study neurobiological systems. . Crit Rev Neurobiol. 1999;13:103–149. doi: 10.1615/critrevneurobiol.v13.i2.10. [DOI] [PubMed] [Google Scholar]
- 87.Meador-Woodruff JH. Update on dopamine receptors. . Ann Clin Psychiatry. 1994;6:79–90. doi: 10.3109/10401239409148986. [DOI] [PubMed] [Google Scholar]
- 88.Vallone D., Picetti R., Borrelli E. Structure and function of dopamine receptors. . Neurosci Biobehav Rev. 2000;24:125–132. doi: 10.1016/s0149-7634(99)00063-9. [DOI] [PubMed] [Google Scholar]
- 89.Mijnster MJ., Isovich E., Fuchs E. Chronic psychosocial stress alters the density of dopamine D2-like binding sites. Soc . Neurosci Abstr. 1998;24:277. [Google Scholar]
- 90.Airaksinen MS., Flügge G., Fuchs E., Panula P. Histaminergic system in the tree shrew brain. . J Cornp Neurol. 1989;286:289–310. doi: 10.1002/cne.902860302. [DOI] [PubMed] [Google Scholar]
- 91.Brown RE., Stevens DR., Haas HL. The physiology of brain histamine. . Prog Neurobiol. 2001;63:637–672. doi: 10.1016/s0301-0082(00)00039-3. [DOI] [PubMed] [Google Scholar]
- 92.Watanabe T., Yanai K. Studies on functional roles of the histaminergic neuron system by using pharmacological agents, knockout mice and positron emission tomography. . Tohoku J Exp Med. 2001;195:197–217. doi: 10.1620/tjem.195.197. [DOI] [PubMed] [Google Scholar]
- 93.Knigge U., Warberg J. The role of histamine in the neuroendocrine regulation of pituitary hormone secretion. . Acta Endocrinol (Copenh). 1991;124:609–619. doi: 10.1530/acta.0.1240609. [DOI] [PubMed] [Google Scholar]
- 94.Westerink BH., Cremers Tl., De Vries JB., Liefers H., Tran N., De Boer P. Evidence for activation of histamine H3 autoreceptors during handling stress in the prefrontal cortex of the rat. . Synapse. 2002;43:238–243. doi: 10.1002/syn.10043. [DOI] [PubMed] [Google Scholar]
- 95.Ito C. The role of brain histamine in acute and chronic stresses. . Biomed Pharmacother. 2000;54:263–267. doi: 10.1016/S0753-3322(00)80069-4. [DOI] [PubMed] [Google Scholar]
- 96.Woolley CS., Gould E., Frankfurt M., McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. . J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Magarifïos AM., McEwen BS., Flügge G., Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. . J Neurosci. 1996;5:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. . J Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 99.Magarinos AM., Deslandes A., McEwen BS. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. . Eur J Pharmacol. 1999;371:113–122. doi: 10.1016/s0014-2999(99)00163-6. [DOI] [PubMed] [Google Scholar]
- 100.Kole MHP., Czéh B., Fuchs E. Homeostatic maintenance in tree shrew hippocampal CA3 neuron excitability after chronic stress. . Hippocampus. 2004. In press. xx doi: 10.1002/hipo.10212. [DOI] [PubMed] [Google Scholar]
- 101.Kole MHP. CA3 pyramidal neuron correlates of the stress response analyses of form and function. University of Groningen, The Netherlands. PhD Thesis; 2003 [Google Scholar]
- 102.Kole MH., Swan L., Fuchs E. The antidepressant tianeptine persistently modulates glutamate receptor currents of the hippocampal CA3 commissural associational synapse in chronically stressed rats. . Eur J Neurosci. 2002;16:807–816. doi: 10.1046/j.1460-9568.2002.02136.x. [DOI] [PubMed] [Google Scholar]
- 103.Oliver CJ., Terry-Lorenzo RT., Elliott E., et al. Targeting protein phosphatase 1 (PP1) to the actin cytoskeleton: the neurabin I/PP1 complex regulates cell morphology. Mol Cell Biol. 2002;22:4690–4701. doi: 10.1128/MCB.22.13.4690-4701.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Muller D., Toni N., Buchs PA. Spine changes associated with long-term potentiation. . Hippocampus. 2000;10:596–604. doi: 10.1002/1098-1063(2000)10:5<596::AID-HIPO10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 105.Ackermann M., Matus A. Activity-induced targeting of profilin and stabilization of dendritic spine morphology. . Nat Neurosci. 2003;6:1194–1200. doi: 10.1038/nn1135. [DOI] [PubMed] [Google Scholar]
- 106.Seib LM., Wellman CL. Daily injections alter spine density in rat medial prefrontal cortex. . Neurosci Lett. 2003;337:29–32. doi: 10.1016/s0304-3940(02)01287-9. [DOI] [PubMed] [Google Scholar]
- 107.Shors TJ., Chua C., Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. . J Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sapolsky RM., Krey LC., McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. . Endocr Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- 109.Keuker J., Vollmann-Honsdorf GK., Fuchs E. How to use the optical fractionator: an example based on the estimation of neurons in the hippocampal CA1 and CA3 regions of tree shrews. . Brain Res Protocols. 2001;7:211–221. doi: 10.1016/s1385-299x(01)00064-2. [DOI] [PubMed] [Google Scholar]
- 110.Lucassen PJ., Vollmann-Honsdorf GK., Gleisberg M., Czeh B., De Kloet ER., Fuchs E. Chronic psychosocial stress differentially affects apoptosis in hippocampal subregions and cortex of the adult tree shrew. . Eur J Neurosci. 2001;14:161–166. doi: 10.1046/j.0953-816x.2001.01629.x. [DOI] [PubMed] [Google Scholar]
- 111.Fuchs E., Uno H., Flügge G. Chronic psychosocial stress induces morphological alterations in hippocampal pyramidal neurons of the tree shrew. . Brain Res. 1995;673:275–282. doi: 10.1016/0006-8993(94)01424-g. [DOI] [PubMed] [Google Scholar]
- 112.Vollmann-Honsdorf GK., Flügge G., Fuchs E. Cortisol treatment and psychosocial stress differentially alter the nuclear ultrastructure of hippocampal pyramidal neurons. In: Eisner N, Eysel U, eds. . Gôttingen Neurobiology Report 1999. Stuttgart, Germany: Georg Thieme Verlag; 1999;524 [Google Scholar]
- 113.Alfonso J., Pollevick GD., van der Hart MG., Flügge G., Fuchs E., Frasch ACC. Identification of genes regulated by chronic psychosocial stress and antidepressant treatment in the hippocampus. . Eur J Neurosci. 2004;19:659–666. doi: 10.1111/j.1460-9568.2004.03178.x. [DOI] [PubMed] [Google Scholar]
- 114.Kinnunen AK., Koenig Jl., Bilbe G. Repeated variable prenatal stress alters pre- and postsynaptic gene expression in the rat frontal pole. . J Neurochem. 2003;86:736–748. doi: 10.1046/j.1471-4159.2003.01873.x. [DOI] [PubMed] [Google Scholar]