Abstract
The brain interprets experiences and translates them into behavioral and physiological responses. Stressful events are those which are threatening or, at the very least, unexpected and surprising, and the physiological and behavioral responses are intended to promote adaptation via a process called “allostasis. ” Chemical mediators of allostasis include cortisol and adrenalin from the adrenal glands, other hormones, and neurotransmitters, the parasympathetic and sympathetic nervous systems, and cytokines and chemokines from the immune system. Two brain structures, the amygdala and hippocampus, play key roles in interpreting what is stressful and determining appropriate responses. The hippocampus, a key structure for memories of events and contexts, expresses receptors that enable it to respond to glucocorticoid hormones in the blood, it undergoes atrophy in a number of psychiatric disorders; it also responds to stressors with changes in excitability, decreased dendritic branching, and reduction in number of neurons in the dentate gyrus. The amygdala, which is important for “emotional memories, ” becomes hyperactive in posttraumatic stress disorder and depressive illness, in animal models of stress, there is evidence for growth and hypertrophy of nerve cells in the amygdala. Changes in the brain after acute and chronic stressors mirror the pattern seen in the metabolic, cardiovascular, and immune systems, that is, short-term adaptation (allostasis) followed by long-term damage (allostatic load), eg, atherosclerosis, fat deposition obesity, bone demineralization, and impaired immune function. Allostatic load of this kind is seen in major depressive illness and may also be expressed in other chronic anxiety and mood disorders.
Keywords: structural plasticity, brain, allostasis, allostatic load, stress, depression, anxiety
Abstract
El cerebro interpreta experiencias y las traduce en respuestas conductuales y fisiologicas. Los acontecimientos estresantes son aquellas situaciones amenazantes, o al menos, inesperadas y sorpresivas; y las respuestas fisiológicas y conductuales intentan promover una adaptación a través de un proceso llamado “alostasis. ” Los mediadores químicos de la alosiasis incluyen el cortisol y la adrenal ina de las glándulas adrenales, otras hormonas y neurotransmisores, el sistema nervioso parasimpático y simpático, y citoquinas y quimioquinas del sistema inmune. Dos estructuras cerebrales, la amígdala y el hipocampo, juegan papeles clave en la interpretación de lo que es estresante y en la determinación de respuestas apropiadas. El hipocampo, una estructura clave para las memorias de los acontecimientos y del contexte, expresa receptores que lo capacitan para responder a hormonas glucocorticoídeas de la sangre. El hipocampo se atrofia en numerosos trastornos psiquiátricos y también responde a estresores con cambios en la excitabilidad, disminución de las ramificaciones dendríticas y reducción del número de neuronas del giro dentado. La amígdala, que es importante para las “memorias emocionales,” aumenta su actividad en el trasiorno por estrés postraumático y en la enfermedad depresiva. En modelos animales de estrés existen evidencias del crecimiento e hipertrofia de células nerviosas en la amígdala. Los cambios en el cerebro después de situaciones de estrés agudo y crónico reflejan el patrón observado en los sistemas metabólico, cardiovascular e inmune; esto es, una adaptación a corto plazo (alosiasis) seguida de un daño a largo plazo (carga alostática), como por ejemplo, ateroesclerosis, obesidad localizada, desmineralización del hueso y deterioro de la función inmune. La carga alostática de este tipo se observa en la depresión mayor y también se puede expresar en otros trastornos ansiosos y afectivos crónicos.
Abstract
Le cerveau interprète les expériences et les traduit en réponses comportementales et physiologiques. Les événements stressants sont ceux qui sont menaçants ou tout au moins inattendus et surprenants et les réponses physiologiques et comportementales ont pour but de promouvoir l'adaptation via tin processus appelé « allostasie ». Les médiateurs chimiques de l'allostasie incluent le cortisol et l'adrénaline sécrétés par les glandes surrénales, d'autres hormones et des neurotransmetteurs, les systèmes nerveux sympathique et parasympathique, et les cytokines et chimiokines produites par le système immunitaire. Deux structures cérébrales, l'amygdale et l'hippocampe, jouent un rôle-clé dans l'identification des événements stressants et l'élaboration de réponses appropriées. L'hippocampe, une structure-clé pour les souvenirs des événements et contextes, exprime des récepteurs qui lui permettent de répondre aux hormones glucocorticoïdes du sang, il subit une atrophie au cours de nombreux troubles psychiatriques et réagit également aux facteurs de stress par des changements de l'excitabilité, une diminution de la ramification dendritique et une baisse du nombre de neurones dans le gyrus denté. L'amygdale, qui joue en rôle important dans les « souvenirs émotionnels », devient hyperactive dans l'état de stress posttraumatique et la dépression. Les modèles animaux de stress montrent l'existence d'une croissance et d'une hypertrophie des cellules nerveuses dans l'amygdale. La chronologie des modifications du cerveau à la suite de stress aigus ou chroniques (adaptation à court terme [allostasie] suivie d'une altération à long terme [charge allostatique]), reflète celle observée au cours des affections touchant, par ex,, les systèmes métabolique, cardiovasculaire et immunitaire, où la phase d'adaptation se complique, respectivement, d'athérosclérose et obésité localisée, de déminéralisation osseuse et d'altérations de la fonction immunitaire. Une telle charge allostatique se rencontre dans la dépression majeure et peut aussi s'exprimer dans l'anxiété chronique et d'autres troubles de l'humeur.
When we experience a stressful event, the initial response of the brain, body, and behavior is a protective one, and hormones, cytokines, and other mediators, such as the neurotransmitters, are used to survive and adapt to the challenge. However, repeated stressful experiences have deleterious effects, in part because the very same mechanisms that help protect in the short term are now either mismanaged and/or overused.1 And, over weeks, months, and years, the dysregulation and overactivity of these systems can promote changes that appear to be deleterious, and stressful experiences have been reported to be a major risk factor in the occurrence of depressive disorders. For example, in the brain, the overactivity of stress hormones in the blood and endogenous excitatory amino acid neurotransmitters in the brain suppress neurogenesis in dentate gyrus (DG) and causes debranching of dendrites in hippocampus and medial prefrontal cortex, whereas chronic stress causes neurons in amygdala to show dendritic growth.2-5 The hippocampus contains receptors for adrenal steroids, which regulate excitability and morphological changes (Figure 1). Along with many other brain regions, the amygdala also contains adrenal steroid receptors, which influence function in this structure as well (Table I).
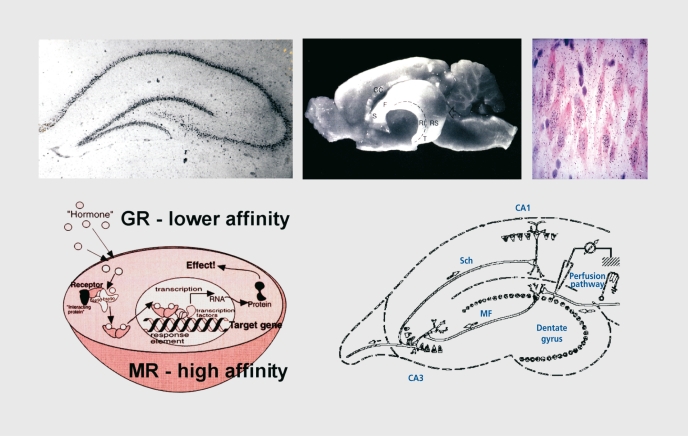
Figure 1. The hippocampus is a target for adrenal steroids. GR, glucocorticoid receptor; MR, mineralocorticoid receptor; Sch, Schaffer colateral; MF, mossy fiber; CC, corpus cailosum.
Table I. Distribution of adrenal steroid receptors in brain regions. GR, glucocorticoid receptor; MR, mineralocorticoid receptor.
| Hippocampus | MR and GR |
| Amygdala | GR and some MR |
| Septum | GR and some MR |
| Hypothatamus | GR mostly; low levels of MR |
| Cerebral cortex | GR mostly; low levels of MR |
| Midbrain | GR mostly; low levels of MR |
| Brain stem | GR mostly; patches of MR |
| Cerebellum | GR mostly |
Acute stress induces formation of spine synapses in CA1 region of hippocampus6 and chronic stress also increases spine synapse formation in hippocampus and amygdala.7 The contrasting changes of dendrites in amygdala and hippocampus after chronic restraint stress (CRS) offers an unprecedented opportunity for understanding underlying mechanisms, as will be discussed below.
CRS for 21 days or longer impairs hippocampal-dependent cognitive function8,9 and enhances amygdala -dependent unlearned fear and fear conditioning,10 which are consistent with the opposite effects of stress on hippocampal and amygdala structure. CRS also increases aggression between animals living in the same cage (Table II).11 Psychosocial stress suppresses neurogenesis and causes dendritic shrinkage,12-15 and one of these stress models, the tree shrew, is considered to be a model of human depressive illness.16
Table II. Cumulative effects of restraint stress on behavior.
| • Cognitive impairment, spatial recognition memory (hippocampus) |
| • Increased anxiety and enhanced fear conditioning (amygdala) |
| • Increased aggression (amygdala) |
Indeed, in major depression and a number of other mood and anxiety disorders, there are reports of hippocampal volume loss and enlargement of the amygdala.17,18 Studies in the tree shrew have shown that treatment with anti-depressant, antiseizure, and mood-stabilizing drugs prevents stress-induced hippocampal structural changes.14,15,19
Besides reduced neurogenesis in DG, there is also evidence for reduced size of principal neuron cell bodies in hippocampus, which is consistent with reduced size of the dendritic tree.20 Synaptic reorganization is also a likely consequence of these rather drastic structural changes, and the animal models cited above provide evidence that synapses can be rapidly formed as a result of stress. Taken together, such structural changes seem likely to play a major role in the volume loss in the human hippocampus and the related effects on cognitive function and affect.18
This article will review underlying mechanisms and consider their applicability to furthering our understanding of the pathophysiology of mood and anxiety disorders.
Allostasis and mechanisms for behavioral adaptation
The amygdala and hippocampus are both involved in contextual fear conditioning and in passive avoidance learning. In fear conditioning, glucocorticoids enhance learned fear21 and they play an important role in forming the memory of context in contextual fear conditioning, but not of the actual effect of footshock in rats that are already familiar with the context where the shock is administered.22,23
This suggests that the hippocampal role in contextual fear conditioning is enhanced by moderate levels of glucocorticoids, but the fear conditioning is either not so dependent on glucocorticoids or is so strong that glucocorticoid influences are hard to demonstrate. Yet there is evidence for an influence of glucocorticoids on the flow of information within the amygdala.
Glucocorticoids potentiate serotonin inhibition of the processing of excitatory input to the lateral amygdala from the thalamus, suggesting that there is a mechanism for containing, or limiting, the sensory input that is important for fear conditioning.24 Thus, adrenal steroids may regulate the nature of the signals that reach the amygdala and allow for greater discrimination of the most salient cues for learning. Moreover, in passive avoidance, both catecholamines and glucocorticoids play a role in facilitating learning.25,26
Catecholamines work outside of the blood–brain barrier and their effects can be blocked by β-adrencrgic–blocking agents, which do not cross the blood–brain barrier.26 Glucocorticoids enter the brain, and local implants of exogenous corticosterone into hippocampus, amygdala, and nucleus tractus solitarii arc all able to enhance passive avoidance learning.25
Adrenal steroids also play a supporting role in the learning of a spatial navigation task in mice.27 Adrenalectomy impairs the acquisition of the memory of hidden platform location in the Morris water maze, and glucocorticoid administration restores the normal learning curve; however, in mice in which the glucocorticoid receptor (GR) is deleted and replaced with a GR that lacks the UNA binding domain, glucocorticoids do not improve task acquisition.27 This finding illustrates a role for GRs acting upon the genome in a task that is known to depend on the hippocampus. Interestingly, other actions of glucocorticoids via GRs are known to involve the protein–protein interactions that are not prevented in mice carrying the GR defective in the DNA binding domain.28
Other evidence for glucocorticoid actions supports an inverted U-shaped dose–response curve in which low to moderate levels of adrenal steroids enhance acquisition of tasks that involve the hippocampus, whereas high levels of glucocorticoids disrupt task acquisition.22,29-31 Adrenal steroids have biphasic effects upon excitability of hippocampal neurons, which may underlie their biphasic actions on memory and recall.30,32,34
Adaptive structural plasticity
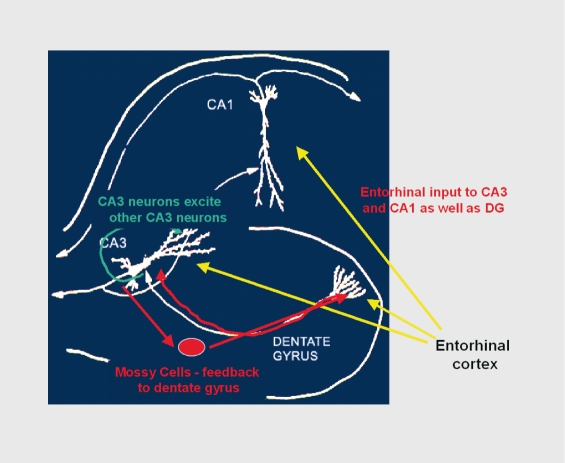
One of the ways that stress hormones modulate function within the brain is by changing the structure of neurons. Within the hippocampus, the input from the entorhinal cortex to the DG is ramified by the connections between the DG and the CA3 pyramidal neurons. One granule neuron innervates, on average, 12 CA3 neurons; and each CA3 neuron innervates, on the average, 50 other CA3 neurons via axon collaterals, as well as 25 inhibitory cells via other axon collaterals (Figure 2).35 The net result is a 600-fold amplification of excitation as well as a 300-fold amplification of inhibition, which provide some degree of control of the system. As to why this system exists, the DG-CA3 system is believed to play a role in the memory of sequences of events, although long-term storage of memory occurs in other brain regions.36,37
Figure 2. Why is the CA3 so vulnerable? Feed-forward excitability serves memory functions but increases vulnerability for excitotoxicity. DG, dentate gyrus.
Neurogenesis in the DG
There is structural plasticity within the DG-CA3 system, in that new neurons continue to be produced in the DG throughout adult life38 and CA3 pyramidal cells undergo remodeling of their dendrites,2 as will be discussed further below.39
The subgranular layer of the DG contains cells that have properties of astrocytes (eg, expression of glial fibrillary acidic protein) and give rise to granule neurons.40 After administration of bromodcoxyuridine (BrdU) to label DNA of dividing cells, these newly born cells appear as clusters in the inner part of the granule cell layer, where a substantial number of them will go on to differentiate into granule neurons within as little as 7 days. The new granule neurons appear to be quite excitable and capable of participating in long-term potentiation. In the adult rat, 9000 new neurons are born per day and survive with a half-life of 28 days.41
There are many hormonal and neurochemical modulators of neurogenesis and cell survival in the DG.15,38,42-44
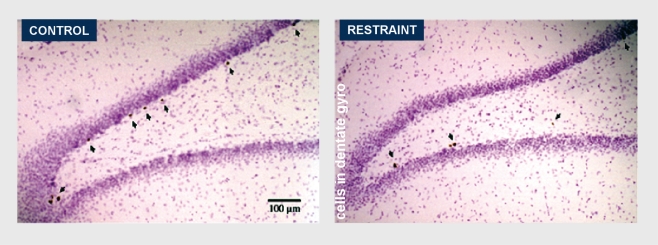
Neurogenesis in the adult DG is enhanced by the hormone insulin-like growth factor–1 (IGF-1) and by serotonin and a number of antidepressant drugs. Estradiol accelerates cell proliferation in female rats. IGF-1 is the mediator of the ability of exercise to increase cell proliferation in the DG. Lack of IGF-1 and insulin in diabetes has the opposite effect and decreases cell proliferation. Neurogenesis and/or survival of newly born cells is increased by putting mice in a complex (”enriched“) environment.45 It is also increased by a form of classical conditioning that activates the hippocampus (”trace conditioning“) prolongs the survival of newly born DG neurons.46,47 On the other hand, certain types of acute stress and many chronic stressors suppress neurogenesis or cell survival in the DG, and the mediators of these inhibitor effects include excitatory amino acids acting via N-methyl-D-aspartate (NMDA) receptors and endogenous opioids.2,48-50 Chronic stress has even more potent effects on neurogenesis and neuronal survival. CRS for 21 days suppressed neurogenesis and CRS for 42 days causes the number of DG neurons to decrease along with total DG volume (Figure 3).51
Figure 3. A single restraint stress does not suppress cell proliferation. Repeated restraint stress for 21 days suppresses cell proliferation. Repeated restraint stress for 42 days reduces volume of the dentate gyrus (DG) and the number of neurons in the DG.
Reproduced from reference 51 with permission: Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17:879-886. Copyright © 2003, Biackwell Publishing, Inc.
Remodeling of dendrites
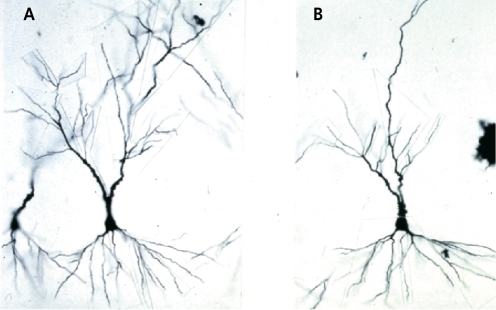
Another form of structural plasticity is the remodeling of dendrites in the hippocampus.39 CRS causes retraction and simplification of dendrites in the CA3 region of the hippocampus (Figure 4). 2 Such dendritic reorganization can also be seen in rats undergoing adaptation of psychosocial stress in the visible burrow system (VBS).The VBS is an apparatus with an open chamber where there is a food and water supply and several tunnels and chambers.52 Rats can be observed from above by a video camera in this apparatus. In the VBS, male rats housed with several females establish a dominance hierarchy within several days. Over the course of the next week, a few subordinate males may die and others (showing scars from bite marks) will show enlarged adrenals, low testosterone, and many changes in brain chemistry. The dominant shows the fewest scars and has the highest level of testosterone, but also has somewhat larger adrenal glands than cage control rats.
Figure 4. Hippocampal CA3 pyramidal neurons are remodeled by 21-d restraint stress. A. Control. B. 21 days′ chronic restraint stress.
Regarding changes in brain structure, it was the dominant rats that had a more extensive pattern of debranching of the apical dendrites of the CA3 pyramidal neurons in the hippocampus, compared with the subordinate rats, which showed reduced branching compared with the cage controls.53 What this result emphasizes is that it is not adrenal size or presumed amount of physiological stress per se that determines dendritic remodeling, but a complex set of other factors that modulate neuronal structure. We refer to the phenomenon as “dendritic remodeling” and we generally find that it is a reversible process. In hibernating hamsters, it occurs in a matter of hours and reverses itself just as quickly when hibernating animals are aroused from torpor (A. M. Magarinos, B. S. McEwen, R Pevet, unpublished data). Below we consider mechanisms involved in structural remodeling.
The role of adrenal steroids in the structural remodeling described above reflects may interactions with neurochemical systems in the hippocampus, including serotonin, γ-aminobutyric acid (GABA), and excitatory amino acids (Figure 5). 2 Probably the most important interactions are those with excitatory amino acids such as glutamate. Excitatory amino acids released by the mossy fiber pathway play a key role in the remodeling of the CA3 region of the hippocampus, and regulation of glutamate release by adrenal steroids may play an important role.54-57 We have found that the glutamate transporter, Glt-1, is elevated by CRS in hippocampus, particularly in the CA3 region, providing another indication that elevated glutamate levels arc an important component of structural plasticity. We previously showed that NMDA receptor blockade and the Na/Ca channel blocker, phenytoin, both block CRS- and glucocorticoid-induced remodeling of dendrites in CA3.58-60 Recent evidence indicates that presynaptic receptors containing kainate receptor subunits such as GluR6 are important for the feed-forward actions of glutamate on mossy fiber terminals,61 and one study showed that a number of kainate receptor subunit mRNAs are regulated biphasically by adrenal steroids.57 In particular, preferential mineralocorticoid receptor (MR) occupancy by low corticosterone (CORT) levels enhanced mRNA levels for KAR2, GluR6, and GluR7.57 This agrees with our finding that MR activation by aldosterone in adrenalectomized (ADX) rats restored levels of [3H]kainate binding in the mossy fiber region of CA3.56 However, further studies are needed.
Figure 5. Glucocorticoids increase glutamate levels, N-methyl-D-aspartate (NMDA) receptors, calcium currents, 5-hydroxytryptamine (5-HT) turnover, and 5-HT2 receptors, decrease 5-HT1A receptors, and alter subunit expression of GABAa receptors. A. Cross-section of dorsal hippocampus. B. Blow-up of CA3 region. C. CA3 neurons highlighting stratum lucium (SL), where mossy fiber terminals form synaptic contacts .GABA, γ-aminobutyric acid; DG, dentate gyrus; SR, stratum radiatum; SP, stratum pyramidale; SO, stratum oriens.
Because excitatory amino acids play a key role along with circulating glucocorticoids, the activation of the CREB (cyclic adenosine monophosphate response element–binding protein) system is a likely candidate mediator, and recent evidence indicates that phosphorylation of CREB is chronically activated in rats subjected to CRS. CREB has been linked to regulation of synaptic plasticity and particularly neurogenesis.62 It is possible that CREB is involved in activity-dependent synapse formation, which is evident as a result of long-term potentiation.63,64
However, the role of glucocorticoids in activation of the CREB system has not been thoroughly investigated. Nevertheless, treatment with the mood stabilizer lithium prevented CRS-induced structural remodeling of the stress-induced elevation of Glt-1 and CREB phosphorylation (G. E. Wood, L. T. Young, B. S. McEwen, unpublished data), providing further evidence that CRS-induced structural plasticity and the molecular markers Glt-1 and phosphoCREB arc useful in study of psychiatric illnesses. Structural changes in dendrites and spine synapses are the result of modifications in the microtubule system of the cytoskeleton,65 and new evidence shows that posttranslational modification of tubulin65 and phosphorylation of the microtubule associated protein tau66 take place along with changes in the actin cytoskeleton,67 under conditions in which reorganization of dendrites and synaptic connections occur. Overall, cytoskeletal changes, such as increased paired-helical-like phosphorylation of tau66 and reduced tyrosinated tubulin,65 are consistent with increased cytoskeletal rigidity. However, this needs much careful study.
The Rac/Rho guanosine triphosphatases (GTPases) and related proteins such as the guanosine triphosphate (GTP) exchange factor, kalirin, have been shown to play a key regulatory role in cytoskeletal modifications in developing and adult neurons.67,68 Except for one relevant study on seizures,65 there are no studies thus far of the effects of chronic stress on these pathways or of the modifications of the cytoskeleton itself.
Besides glucocorticoids and excitatory amino acids, neurotrophins and gp130 cytokines are implicated in structural plasticity along with extracellular proteases such as tissue plasminogen activator (tPA) and neuropsin. Brainderived neurotrophic factor (BDNF) plays a major role in activity-dependent synaptic and dendritic remodeling,69-73 and is implicated in hippocampal-dependent memory formation.74 BDNF also regulates tPA release from neurons75 and tPA is released from nerve terminals in hippocampus and other brain areas such as amygdala.76-78 It has been suggested that tPA may play a role in the processing of proBDNF into active forms.79 The activity of tPA is associated with structural plasticity and increased fear,77 motor learning,80 and enhancement of long-term potentiation.81 Activity of tPA is an important mediator of structural plasticity and enhanced fear in the amygdala resulting from acute restraint stress. For example, plasminogen (inactive zymogen) leads to plasmin (active serine protease). Using tPA knockout mice, we have found that in medial and central amygdala77:
tPA is released under stress and initiates neural remodeling.
This release is plasminogen-independent (extracellular signal–regulated kinase [ERK1/2]; guanosine triphosphate–activating protein [GAP-43]).
tPA induces termination of its own action via plasminogen-activator inhibitor–1 (PAI-1).
tPA activity is required for increased anxiety in the elevated plus maze.
We are presently studying the long-term effects of stress. Neuropsin is another protease that is induced in hippocampus by NMDA-mediated excitation in seizures and leads to proteolysis of the presynaptic adhesion molecule, L1.82
The gp130 cytokines are expressed in hippocampus under stimulation by seizures, along with their receptors, which are constitutive]}' expressed.83 Leukemia inhibitor}' factor (LIF) is particularly interesting because it interferes with neurotrophin signaling84 and causes dendritic retraction in cell culture.85 However, it has not yet been determined whether acute or chronic stress increases LIF expression, and it is conceivable that increased expression of LIF might play a role in dendritic shortening.
The ability of neuronal processes to expand or contract, and newly formed neurons to make connections, is dependent on the extracellular environment in which polysialated neural cell adhesion molecule (PSA-NCAM) plays an important role.86 PSA-NCAM is associated with regions of the brain that show structural plasticity such as the inner granule cell layer of the DG and the mossy fiber terminals of CA3.87 CRS for 21 days causes increased PSA-NCAM. expression in the DG proliferative zone even though cell proliferation is suppressed, and these changes have disappeared after CRS for 42 days.51 This raised questions about the role of PSA-NCAM in adaptive structural plasticity, which need to be investigated. Removal of the PSA residue by endoneuraminidase (EndoN)88 is a powerful tool for manipulating this system, since PSA removal abolishes plasticity of suprachiasmatic neurons to environmentally induced phase shifting of the diurnal rhythm.89
We now turn to the important question of whether chronic stress increases or decreases vulnerability of the hippocampus to damage from other insults.
Permanent damage as a result of stress
The remodeling of the hippocampus in response to stress is largely reversible if the CRS is terminated at the end of 3 weeks.10 After 3 weeks of CRS, neurogenesis is reduced in DG and dendrites are shorter and less branched,51,59,60 and there is an increase in PSA-NCAM expression in the DG that is consistent with increased mobility of neuronal processes even in the face of reduced DG neuron production. Continuation of CRS for a total of 6 weeks abolishes the upregulation of PSA-NCAM and results in a significant 6% reduction in DG volume and 13% reduction in granule neuron number.51 We do not yet know whether structural changes occurring after 6 weeks of CRS are reversible or whether they can be accelerated by antidepressant or antiepilcptic drugs that block the effects of stress and glucocorticoids on remodeling. Nor do we know whether the structural changes occurring with CRS increase or decrease the vulnerability of the hippocampus to damage by excitotoxicity.
It is well established that glucocorticoids exacerbate damage to the hippocampus caused by ischemia90 and seizures.91,92 Glucocorticoids exacerbate excitotoxic damage and do so, at least in part, by facilitating trafficking of immune cells to the injury site,93 and, there, cytotoxic T cells are able to produce cytotoxic death of neurons.94 However, the phenomenon of ischemic preconditioning95 reveals that prior stimulation of the hippocampus can induce a protective mechanism that may reduce the damage produced by a full-scale ischemic event. It is not clear whether the same mechanisms might be operative when stress is applied and whether they might affect the response to excitotoxicity in response to seizures, but this possibility needs to be kept in mind if it turns out that prior CRS has a protective effect on subsequent responses to excitotoxic challenge.
Protective agents may also involve substances that are upregulated in the brain in response to damage or threat of damage. One of the prominent features of excitotoxic damage or removal of adrenal steroids is the robust induction of calcitonin gene–related peptide (CGRP) in terminals and cell bodies in hippocampus and in mossy cells. The increased expression of CGRP in mossy cells is especially prominent after bilateral ADX under conditions in which there is apoptosis of granule cells, and the CGRP immunoreactivity is enhanced within the inner third of the molecular layer of the DG. The neuroimmune peptide, CGRP, is one of the most diverse and influential immunoregulators of the periphery. This important neuropeptide has multiple functions including: its actions as a potent vasodilator96 and an immune modulator,97 -102 as well as a neural and immune developmental regulator, a modulator of hormone release involved in growth and development, and a stimulator of sympathetic outflow, which is mediated by CRF and an inducer of apoptosis (reviewed in reference 103). Some of the different functional roles for CGRP may not be independent, but may be part of a cascade of events that constitute the healing response to injury. A number of studies have shown that CGRP is expressed following various kinds of trauma and plays an important role in the acute phase response that may be of particular relevance to the outcome of the regional injury response in the central nervous system (CNS).103,104
In recent studies, the expression of CGRP within the hippocampus increases in four separate models of CNS injury: ADX,105 intrahippocampal colchicine injection,105 trimethyltin ingestion,106 and kainic acid injections. In each case, the expression of this peptide was limited to the specific region of damage and in association with the surviving neuronal population. Although the upregulation of CGRP may be associated with neuronal cell survival,107 other studies have shown that both microglia and astrocytes express CGRP receptors and that exposure to physiological levels of CGRP induces c-fos in microglia and astrocytes and increases plasminogen activators.108
The role of CGRP may then not only protect against immune system damage to neurons, but may also participate in plasticity and healing.
Protective and damaging effects of the mediators of adaptation
Individual differences in the progression of a number of disorders that accumulate with time can be conceptualized as an accumulation of wear and tear of daily experiences, lifestyle, and major life stressors, which interact with the genetic constitution and predisposing early life experiences.109-111 'Ihc neuroendocrine system, autonomic nervous system, and immune system are mediators of adaptation to the challenges of daily life, referred to as “allostasis,” meaning “maintaining stability through change.”112 Physiological mediators, such as adrenalin from the adrenal medulla, glucocorticoids from the adrenal cortex, and cytokines from cells of the immune system, act upon receptors in various tissues and organs to produce effects that are adaptive in the short term, but can be damaging if the mediators are not shut off when no longer needed. When release of the mediators is not efficiently terminated, their effects on target cells are prolonged, leading to other consequences that may include receptor desensitization and tissue damage. This process has been named “allostatic load,”113-114 which refers to the price the tissue or organ pays for an overactive or inefficiently managed allostatic response. Therefore, allostatic load refers to the “cost” of adaptation.
The brain is the master controller of the three systems noted above and is also a target of these systems, subject to both protection and damage. Allostasis also applies not only to circulating hormones, but also to organs and tissues of the body. In the nervous system, neurotransmitters are released by neuronal activity, and they produce effects locally to cither propagate or inhibit further neural activity. Neurotransmitters and hormones are usually released during a discrete period of activation and then are shut off, and the mediators themselves are removed from the intracellular space by reuptake or metabolism in order not to prolong their effects. When that does not happen, however, there is allostatic load and the brain is at increased risk for damage.115,116
The processes of allostasis and allostatic load have been described and measured for metabolic and cardiovascular changes that are associated with obesity, type 2 diabetes, and cardiovascular disease.117 However, the same type of elevated and prolonged secretion of glucocorticoids during aging has also been associated with impairment of cognitive function in rodents118-120 and humans.121-123 Moreover, the endogenous excitatory amino acid neurotransmitters appear to play a major role in these changes,119 even though they are also an essential part of normal synaptic neurotransmission and plasticity.
Allostatic states in depressive illness
Stress hormones are elevated in major depressive illness. In particular the diurnal rhythm is distorted.124 Normally low evening levels of Cortisol are increased in a subset of depressed patients125,126 and the stress hormone axis in major depression is resistant to suppression by the synthetic glucocorticoid dexamethasone.127 It is also noteworthy that androgen levels are elevated in women with major depression, which undoubtedly reflects adrenal hyperactivity.128 IGF-1 levels are also reported to be elevated in major depression, and this may reflect elevated growth hormone release as a result of the hypercortisolemia.129
Each of these patterns of elevation constitutes an “allostatic state,” and represents a pathway for the development of allostatic load in the brain and in other organs throughout the body. Regarding the brain, we already noted the studies showing that hippocampal volume loss in major depressive illness is related to duration of the depression rather than to age per se of the patients.130-132 Not all studies report such changes (see, for example, references 133 and 134); the reasons for these different results are beyond the scope of this discussion, but they may be explained by differences in the duration of depression, as well as gender and age. It should be noted that hippocampal size in elderly twins shows only 40% genetic contribution, with the predominant influence being environmental.135 This emphasizes the importance of experimental factors and allostatic load in determining hippocampal volume.
Hippocampal atrophy has been found in relation to depression in the elderly,136 with an association detected with presence of the ApoE4 genotype.137 In subjects with a long-term history of depression, Sheline and colleagues described magnetic resonance imaging (MRI) evidence for discontinuities that might represent sites of damage.130
Although some recent postmortem studies on brains from depressed individuals did not show neuron loss in hippocampus,138,139 the duration of the depression and the subtype of depression were not carefully controlled. Thus, the possibility that neural damage may ultimately occur in major depression cannot be disregarded, particularly when depression lasts a long time. However, in a recent study in young depressed subjects, hippocampal volume was not smaller in first-episode depression, but declined rapidly over several years.140 'Ihc key, unanswered question is whether such changes can be prevented or even reversed. It is important to note that other brain regions besides hippocampus are affected in depressive illness and undergo structural changes. One region is the prefrontal cortex, and structural imaging141 showed loss of volume in familial pure depressive disorder, whereas autopsy studies142-144 have shown loss of volume and glial cells, as well as neuronal density in both unipolar and bipolar disorder. There is one animal study showing that chronic glucocorticoid treatment induces loss of dendrites in the rat prefrontal cortex.4 However, much more work needs to be done on this brain region.
Depressive illness is associated with a hyperactivation of the amygdala,145,146 and more recently, with an actual enlargement of the amygdala in the first episode of major depression.147 This is reminiscent of the increased dendritic branching reported in rats after repeated immoblization stress (see above and reference 148). Since the amygdala integrates information related to fear and strong emotions, and also sends outputs via the central nucleus for autonomic arousal and via the basal nucleus for more active aspects of coping,149 the elevation of amygdala activity may be a first step that leads to overactivation of systems involved in physiological and behavioral coping.
The long-term consequences of this may well be a wear and tear on the body that results in a number of pathophysiological consequences, since the amygdala regulates both autonomic nervous system activity and adrenocorticotropic hormone (ACTH) and Cortisol production through outputs of its central nucleus.149,150 It is important to note that there are reports that in recurrent major depression of long duration the amygdala may undergo shrinkage.131,151
It is thus possible that initial hypertrophy gives way to atrophy in this important brain structure. Besides the brain changes in major depression, there are other changes in the body that reflect dysregulatcd hypothalamopituitary axis (HPA) and autonomic activity, and are slow in developing. These constitute allostatic load that produces cumulative pathophysiology, which may also be reversible if caught in time. Such cumulative, long-term effects include bone mineral loss152-154 and abdominal fat deposition.155-157 Moreover, the combination of long-term allostatic load, together with dysregulation of the autonomic nervous system in major depression,158 is associated with increased blood platelet reactivity159-161 and increased risk for cardiovascular disease.162-165
There are parallels between the story for major depression and what is known about psychiatric and somatic features of Cushing’s disease involving melancholia, depression, abdominal obesity, bone mineral loss, and increased risk for cardiovascular disease.166-169 In addition, there is evidence for hippocampal atrophy in Cushing’s disease along with memory impairments.170-172 Interestingly, hippocampal volume loss in Cushing's disease is at least partially reversible over several years after correction of the hypercortisolemia.173-175
Finally, a largely unexplored area concerns the effects of antidepressant medication on the brain and body changes associated with depressive illness. On the one hand, certain antidepressants may contribute to some of the associated pathophysiology, such as cardiovascular instability.176 On the other hand, withdrawal from antidpressant treatment may cause imbalances in neurotransmitter systems, with elevations of excitatory amino acid tone,177 and contribute to the allostatic load that occurs as the depressive state continues.178
Conclusion
Translational studies of brain changes in major psychiatric illnesses such as unipolar and bipolar depression and posttraumatic stress disorder are showing that changes in volume of structures such as hippocampus, prefrontal cortex, and amygdala must be considered as part of the neurobiological consequences of these illnesses.17,18,140,179,180
Structural remodeling in these brain regions is important for human psychiatric disorders because the altered circuitry is likely to contribute to impaired cognitive function and affect regulation. Moreover, stress is widely acknowledged as a predisposing and precipitating factor in psychiatric illness.181,182
Thus, animal models are relevant to human psychiatric disorders in at least four ways:
First, they have led to―and continue to contribute―basic knowledge to the ongoing studies of how the human brain changes structurally in depression and related psychiatric disorders.
Second, the structural changes that occur with chronic stress appear to be reversible as long as the stress is terminated in time. This suggests the hopeful possibility that brain changes in at least some major psychiatricdisorders may be treatable if we can find the right agents or therapies and intervene in time.
Third, reversible or not, the effects of chronic stress may predispose to greater vulnerability to adverse consequences from other insults.
Fourth, the systemic manifestations of the allostatic load generated by chronic psychiatric disorders affects the metabolic, immune, and cardiovascular systems, leading to systemic disorders that add to the costs of healthcare.
Selected abbreviations and acronyms
- CGRP
calcitonin gene–related peptide
- CRS
chronic restraint stress
- DG
dentate gyrus
- GR
glucocorticoid receptor
- IGF-1
insulin-like growth factor-1
- MR
mineralocorticoid receptor
- NMDA
N-methyl-D-aspartate
- PSA-NCAM
polysialated neural cell adhesion molecule
- tPA
tissue plasminogen activator
Research support has come from the National Institute of Mental Health Grants MH 41256 and MH 58911. The author is also indebted to colleagues in the John D. and Catherine T. MacArthur Foundation Health Program and its Network on Socioeconomic Status and Health (Nancy Adler, PhD, Chair).
REFERENCES
- 1.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 3.Sousa N., Lukoyanov NV., Madeira MD., Almeida OFX., Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- 4.Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 5.Vyas A., Mitra R., Rao BSS., Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shors TJ., Chua C., Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sunanda MSR., Raju TR. Effect of chronic restraint stress on dendritic spines and excrescences of hippocampal CA3 pyramidal neurons―a quantitative study. Brain Res. 1995;694:312–317. doi: 10.1016/0006-8993(95)00822-8. [DOI] [PubMed] [Google Scholar]
- 8.Luine V., Villegas M., Martinez C., McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- 9.Conrad CD., Galea LAM., Kuroda Y., McEwen BS. Chronic stress impairs rat spatial memory on the Y-maze and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- 10.Conrad CD., Magarinos AM., LeDoux JE., McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- 11.Wood GE., Young LT., Reagan LP., McEwen BS. Acute and chronic restraint stress alters the incidence of social conflict in male rats. Norm Behav. 2003;43:205–213. doi: 10.1016/s0018-506x(02)00026-0. [DOI] [PubMed] [Google Scholar]
- 12.Gould E., Tanapat P., McEwen BS., Flugge G., Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gould E., McEwen BS., Tanapat P., Galea LAM., Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magarinos AM., McEwen BS., Flugge G., Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czeh B., Michaelis T., Watanabe T., et al. Stress-induced changes in cerebral metabolites, hippocampal volume and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Kampen M., Kramer M., Hiemke C., Flugge G., Fuchs E. The chronic psychosocial stress paradigm in male tree shrews: evaluation of a novel animal model for depressive disorders. Stress. 2002;5:37–46. doi: 10.1080/102538902900012396. [DOI] [PubMed] [Google Scholar]
- 17.McEwen BS. Mood disorders and allostatic load. Biol Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 18.Sheline Yl. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- 19.van der Hart MGC., Czeh B., de Biurrun G., et al. Substance P receptor antagonist and clomipramine prevent stress-induced alterations in cerebral metabolites, cytogenesis in the dentate gyrus and hippocampal volume. Mol Psychiatry. 2002;7:933–941. doi: 10.1038/sj.mp.4001130. [DOI] [PubMed] [Google Scholar]
- 20.Stockmeier CA., Mahajan GJ., Konick LC., et al. Preliminary evidence that neuronal and glial density is increased and neuronal size is decreased in hippocampus in major depressive disorder (MDD). Abst Soc Neurosci. 2002;28:497.19. [Google Scholar]
- 21.Corodimas KP., LeDoux JE., Gold PW., Schulkin J. Corticosterone potentiation of learned fear. Ann N Y Acad Sci. 1994;746:392. doi: 10.1111/j.1749-6632.1994.tb39264.x. [DOI] [PubMed] [Google Scholar]
- 22.Pugh CR., Tremblay D., Fleshner M., Rudy JW. A selective role for corticosterone in contextual-fear conditioning. Behav Neurosci. 1997;111:503–511. [PubMed] [Google Scholar]
- 23.Pugh CR., Fleshner M., Rudy JW. Type II glucocorticoid receptor antagonists impair contextual but not auditory-cue fear conditioning in juvenile rats. Neurobiol Learn Memory. 1997;67:75–79. doi: 10.1006/nlme.1996.3741. [DOI] [PubMed] [Google Scholar]
- 24.Stutzmann GE., McEwen BS., LeDoux JE. Serotonin modulation of sensory inputs to the lateral amygdala: dependency on corticosterone. J Neurosci. 1998;18:9529–9538. doi: 10.1523/JNEUROSCI.18-22-09529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roozendaal B. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology. 2000;25:213–238. doi: 10.1016/s0306-4530(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 26.Cahill L., Prins B., Weber M., McGaugh JL. β-Adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- 27.Oitzl MS., Reichardt HM., Joels M., de Kloet ER. Point mutation in the mouse glucocorticoid receptor preventing DNA binding impairs spatial memory. Proc Natl Acad Sci U S A. 2001;98:12790–12795. doi: 10.1073/pnas.231313998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reichardt HM., Schutz G. Glucocorticoid signaling―multiple variations of a common theme. Mol Cell Endocrinol. 1998;146:1–6. doi: 10.1016/s0303-7207(98)00208-1. [DOI] [PubMed] [Google Scholar]
- 29.Conrad CD., Lupien SJ., McEwen BS. Support for a bimodal role for type Il adrenal steroid receptors in spatial memory. Neurobiol Learn Memory. 1999;72:39–46. doi: 10.1006/nlme.1998.3898. [DOI] [PubMed] [Google Scholar]
- 30.Diamond DM., Bennett MC., Fleshner M., Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- 31.Diamond DM., Park CR., Heman KL., Rose GM. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus. 1999;9:542–552. doi: 10.1002/(SICI)1098-1063(1999)9:5<542::AID-HIPO8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 32.Pavlides C., Kimura A., Magarinos AM., McEwen BS. Type I adrenal steroid receptors prolong hippocampal long-term potentiation. Neuroreport. 1994;5:2673–2677. doi: 10.1097/00001756-199412000-00067. [DOI] [PubMed] [Google Scholar]
- 33.Pavlides C., Watanabe Y., Magarinos AM., McEwen BS. Opposing role of adrenal steroid type I and type II receptors in hippocampal long-term potentiation. Neuroscience. 1995;68:387–394. doi: 10.1016/0306-4522(95)00151-8. [DOI] [PubMed] [Google Scholar]
- 34.Pavlides C., McEwen BS. Effects of mineralocorticoid and glucocorticoid receptors on long-term potentiation in the CA3 hippocampal field. Brain Res. 1999;851:204–214. doi: 10.1016/s0006-8993(99)02188-5. [DOI] [PubMed] [Google Scholar]
- 35.Feng R., Rampon C., Tang YP., et al. Deficient neurogenesis in forebrainspecific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron. 2001;32:911–926. doi: 10.1016/s0896-6273(01)00523-2. [DOI] [PubMed] [Google Scholar]
- 36.Eichenbaum H., Harris K. Toying with memory in the hippocampus. Nat Neurosci. 2000;3:205–206. doi: 10.1038/72901. [DOI] [PubMed] [Google Scholar]
- 37.Lisman JE. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron. 1999;22:233–242. doi: 10.1016/s0896-6273(00)81085-5. [DOI] [PubMed] [Google Scholar]
- 38.Gould E., Tanapat P., Rydel T., Hastings N. Regulation of hippocampal neurogenesis in adulthood. Biol Psychiatry. 2000;48:715–720. doi: 10.1016/s0006-3223(00)01021-0. [DOI] [PubMed] [Google Scholar]
- 39.McEwen BS., Magarinos AM. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol. 2001;16:S7–S19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- 40.Seri B., Garcia-Verdugo JM., McEwen BS., Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altman J., Das GD. Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol. 1965;126:337–390. doi: 10.1002/cne.901260302. [DOI] [PubMed] [Google Scholar]
- 42.Malberg JE., Eisch AJ., Nestler EJ., Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aberg MA., Aberg ND., Hedbacker H., Oscarsson J., Eriksson PS. Peripheral infusion of IGF-1 selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trejo JL., Carro E., Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kempermann G., Kuhn HG., Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;586:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 46.Gould E., Beylin A., Tanapat P., Reeves A., Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 47.Shors TJ., Miesegaes G., Beylin A., Zhao M., Rydel T., Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 48.Gould E., Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- 49.Cameron HA., Tanapat P., Gould E. Adrenal steroids and N-methyl-D-aspartate receptor activation regulate neurogenesis in the dentate gyrus of adult rats through a common pathway. Neuroscience. 1998;82:349–354. doi: 10.1016/s0306-4522(97)00303-5. [DOI] [PubMed] [Google Scholar]
- 50.Eisch AJ., Barrot M., Schad CA., Self DW., Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci U S A. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pham K., Nacher J., Hof PR., McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17:879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- 52.Blanchard RJ., McKittrick CR., Blanchard DC. Animal models of social stress: effects on behavior and brain neurochemical systems. Physiol Behav. 2001;73:261–271. doi: 10.1016/s0031-9384(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 53.McKittrick CR., Magarinos AM., Blanchard DC., Blanchard RJ., McEwen BS., Sakai RR. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36:85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 54.Lowy MT., Gault L., Yamamoto BK. Adrenalectomy attenuates stress-induced elevations in extracellular glutamate concentrations in the hippocampus. J Neurochem. 1993;61:1957–1960. doi: 10.1111/j.1471-4159.1993.tb09839.x. [DOI] [PubMed] [Google Scholar]
- 55.Lowy MT., Wittenberg L., Novotney S. Adrenalectomy attenuates kainic acid–induced spectrin proteolysis and heat shock protein 70 induction in hippocampus and cortex. J Neurochem. 1994;63:886–894. doi: 10.1046/j.1471-4159.1994.63030886.x. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe Y., Weiland NG., McEwen BS. Effects of adrenal steroid manipulations and repeated restraint stress on dynorphin mRNA levels and excitatory amino acid receptor binding in hippocampus. Brain Res. 1995;680:217–225. doi: 10.1016/0006-8993(95)00235-i. [DOI] [PubMed] [Google Scholar]
- 57.Joels M., Bosnia A., Hendriksen H., Diegenbach P., Kamphuis W. Corticosteroid actions on the expression of kainate receptor subunit mRNAs in rat hippocampus. Mol Brain Res. 1996;37:15–20. doi: 10.1016/0169-328x(95)00267-v. [DOI] [PubMed] [Google Scholar]
- 58.Watanabe Y., Gould E., Cameron HA., Daniels DC., McEwen BS. Phenytoin prevents stress- and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992;2:431–436. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- 59.Magarinos AM., McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 1995;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- 60.Magarinos AM., McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- 61.Contractor A., Swanson G., Heinemann SF. Kainate receptors are involved in short- and long-term plasticity at mossy fiber synapses in the hippocampus. Neuron. 2001;29:209–216. doi: 10.1016/s0896-6273(01)00191-x. [DOI] [PubMed] [Google Scholar]
- 62.Nakagawa S., Kim JE., Lee R., et al. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci. 2002;22:3673–3682. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andersen P., Soleng AF. Long-term potentiation and spatial training are both associated with the generation of new excitatory synapses. Brain Res Rev. 1998;26:353–359. doi: 10.1016/s0165-0173(97)00042-8. [DOI] [PubMed] [Google Scholar]
- 64.Toni N., Buchs PA., Nikonenko I., Bron CR., Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- 65.Bianchi M., Heidbreder C., Crespi F. Cytoskeletal changes in the hippocampus following restraint stress: role of serotonin and microtubules. Synapse. 2003;49:188–194. doi: 10.1002/syn.10230. [DOI] [PubMed] [Google Scholar]
- 66.Arendt T., Stieler J., Strijkstra AM., et al. Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J Neurosci. 2003;23:6972–6981. doi: 10.1523/JNEUROSCI.23-18-06972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luo L. Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci. 2000;1:173–180. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- 68.Ma XM., Mains RE., Eipper BA. Plasticity in hippocampal peptidergic systems induced by repeated electroconvulsive shock. Neuropsychopharmacology. 2002;27:55–71. doi: 10.1016/S0893-133X(02)00284-1. [DOI] [PubMed] [Google Scholar]
- 69.Bonhoeffer T. Neurotrophins and activity-dependent development of the neocortex. Curr Opin Neurobiol. 1996;6:119–126. doi: 10.1016/s0959-4388(96)80017-1. [DOI] [PubMed] [Google Scholar]
- 70.McAllister AK., Katz LC., Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- 71.Poo M. Neurotrophins as synaptic modulators. Nature. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 72.Lee FS., Kim AH., Khursigara G., Chao MV. The uniqueness of being a neurotrophin receptor. Curr Opin Neurobiol. 2001;11:281–286. doi: 10.1016/s0959-4388(00)00209-9. [DOI] [PubMed] [Google Scholar]
- 73.Gorski JA., Zeiler SR., Tamowski S., Jones KR. Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J Neurosci. 2003;23:6856–6865. doi: 10.1523/JNEUROSCI.23-17-06856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alonso M., Vianna MRM., Depino AM., et al. BDNF-triggered events in the rat hippocampus are required for both short- and long-term memory formation. Hippocampus. 2002;12:551–560. doi: 10.1002/hipo.10035. [DOI] [PubMed] [Google Scholar]
- 75.Fiumelli H., Jabaudon D., Magistretti PJ., Martin JL. BDNF stimulates expression, activity and release of tissue-type plasminogen activator in mouse cortical neurons. Eur J Neurosci. 1999;11:1639–1646. doi: 10.1046/j.1460-9568.1999.00580.x. [DOI] [PubMed] [Google Scholar]
- 76.Salles FJ., Strickland S. Localization and regulation of the tissue plasminogen activator-plasmin system in the hippocampus. J Neurosci. 2002;22:2125–2134. doi: 10.1523/JNEUROSCI.22-06-02125.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pawlak R., Magarinos AM., Melchor J., McEwen B., Strickland S. Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nat Neurosci. 2003;6:168–174. doi: 10.1038/nn998. [DOI] [PubMed] [Google Scholar]
- 78.Sappino A-P., Madani R., Huarte J., et al. Extracellular proteolysis in the adult murine brain. J Clin Invest. 1993;92:679–685. doi: 10.1172/JCI116637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu B. Pro-region of neurotrophins: role in synaptic modulation. Neuron. 2003;39:735–738. doi: 10.1016/s0896-6273(03)00538-5. [DOI] [PubMed] [Google Scholar]
- 80.Seeds NW., Basham ME., Ferguson JE. Absence of tissue plasminogen activator gene or activity impairs mouse cerebellar motor learning. J Neurosci. 2003;23:7368–7375. doi: 10.1523/JNEUROSCI.23-19-07368.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhuo M., Holtzman DM., Li Y., et al. Role of tissue plasminogen activator receptor LRP in hippocampal long-term potentiation. J Neurosci. 2000;20:542–549. doi: 10.1523/JNEUROSCI.20-02-00542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matsumoto-Miyai K., Ninomiya A., Yamasaki H., Tarnura H., Nakamura Y., Shiosaka S. NMDA-dependent proteolysis of presynaptic adhesion molecule L1 in the hippocampus by neuropsin. J Neurosci. 2003;23:7727–7736. doi: 10.1523/JNEUROSCI.23-21-07727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosell DR., Akama KT., Nacher J., McEwen BS. Differential expression of suppressors of cytokine signaling-1, -2, and -3 in the rat hippocampus after seizure: implications for neuromodulation by gp130 cytokines. Neuroscience. 2003;122:349–358. doi: 10.1016/s0306-4522(03)00594-3. [DOI] [PubMed] [Google Scholar]
- 84.Ng YP., He W., Ip NY. Leukemia inhibitory factor receptor signaling negatively modulates nerve growth factor-induced neurite outgrowth in PC12 cells and sympathetic neurons. J Biol Chem. 2003;278:38731–38739. doi: 10.1074/jbc.M304623200. [DOI] [PubMed] [Google Scholar]
- 85.Guo X., Chandrasekaran V., Lein P., Kaplan PL., Higgins D. Leukemia inhibitory factor and ciliary neurotrophic factor cause dendritic retraction in cultured rat sympathetic neurons. J Neurosci. 1999;19:2113–2121. doi: 10.1523/JNEUROSCI.19-06-02113.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rutishauser U., Landmesser L. Polysialic acid in the vertebrate nervous system: a promoter of plasticity in cell-cell interactions. Trends Neurosci. 1996;19:422–427. doi: 10.1016/0166-2236(96)10041-2. [DOI] [PubMed] [Google Scholar]
- 87.Seki T., Arai Y. The persistent expression of a highly polysialylated NCAM in the dentate gyrus of the adult rat. Neurosci Res. 1991;12:503–513. doi: 10.1016/s0168-0102(09)80003-5. [DOI] [PubMed] [Google Scholar]
- 88.Seki T., Rutishauser U. Removal of polysialic acid-neural cell adhesion molecule induces aberrant mossy fiber innervation and ectopic synaptogenesis in the hippocampus. J Neurosci. 1998;18:3757–3766. doi: 10.1523/JNEUROSCI.18-10-03757.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Prosser RA., Rutishauser U., Ungers G., Fedorkova L., Glass D. Intrinsic role of polysialylated neural cell adhesion molecule in photic phase resetting of the mammalian circadian clock. J Neurosci. 2003;23:652–658. doi: 10.1523/JNEUROSCI.23-02-00652.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sapolsky R., Pulsinelli W. Glucocorticoids potentiate ischemic injury to neurons: therapeutic implications. Science. 1985;229:1397–1399. doi: 10.1126/science.4035356. [DOI] [PubMed] [Google Scholar]
- 91.Stein B., Sapolsky R. Chemical adrenalectomy reduces hippocampal damage induced by kainic acid. Brain Res. 1988;473:175–180. doi: 10.1016/0006-8993(88)90332-0. [DOI] [PubMed] [Google Scholar]
- 92.Roozendaal B., Phillips RG., Power AE., Brooke SM., Sapolsky RM., McGaugh JL. Memory retrieval impairment induced by hippocampal CA3 lesions is blocked by adrenocortical suppression. Nat Neurosci. 2001;4:1169–1171. doi: 10.1038/nn766. [DOI] [PubMed] [Google Scholar]
- 93.Dinkel K., MacPherson A., Sapolsky RM. Novel glucocorticoid effects on acute inflammation in the CNS. J Neurochem. 2003;84:705–716. doi: 10.1046/j.1471-4159.2003.01604.x. [DOI] [PubMed] [Google Scholar]
- 94.Dinkel K., Dhabhar FS., Sapolsky RM. Neurotoxic effects of polymorphonuclear granulocytes on hippocampal primary cultures. Proc Natl Acad Sci U S A. 2004;101:331–336. doi: 10.1073/pnas.0303510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dirnag U., Simon RP., Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- 96.Drossman DA. Irritable bowel syndrome. Gastroenterologist. 1994;2:315–326. [PubMed] [Google Scholar]
- 97.Umeda Y., Arisawa M. Inhibition of natural killer activity by calcitonin gene-related peptide. Immunopharmacol Immunotoxicol. 1989;11:309. doi: 10.3109/08923978909005372. [DOI] [PubMed] [Google Scholar]
- 98.Sirinek LP., O'Dorisio MS. Modulation of immune function by intestinal neuropeptides. Acta Oncol. 1991;30:509–517. doi: 10.3109/02841869109092410. [DOI] [PubMed] [Google Scholar]
- 99.Lombardi VR., Garcia M., Cacabelos R. Microglial activation induced by factor(s) contained in sera from Alzheimer-related ApoE genotypes. J Neurosci Res. 1998;54:539–553. doi: 10.1002/(SICI)1097-4547(19981115)54:4<539::AID-JNR11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 100.McGillis JP., Humphreys S., Reid S. Characterization of functional calcitonin gene-related peptide receptors on rat lymphocytes. J Immunol. 1991;147:3482–3489. [PubMed] [Google Scholar]
- 101.Nong Y., Titus RG., Ribeiro JMC., Remold HG. Peptides encoded by the calcitonin gene inhibit macrophage function. J Immunol. 1989;143:45–49. [PubMed] [Google Scholar]
- 102.Foremen J. Substance P and calcitonin gene-related peptide: effects on mast cells in human skin. Int Arch Allergy Appl Immunol. 1987;82:366. doi: 10.1159/000234229. [DOI] [PubMed] [Google Scholar]
- 103.Bulloch K. Regional neural regulation of immunity: anatomy and function. In: McEwen BS, ed. Handbook of Physiology. Coping with the Environment; Neural and Endocrine Mechanisms. New York, NY: Oxford University Press; 2000:353–379. [Google Scholar]
- 104.Berczi I., Chalmers IM., Nagy E., Warrington RJ. The immune effects of neuropeptides. Bailiieres Clin Rheumatol. 1996;10:227–257. doi: 10.1016/s0950-3579(96)80016-1. [DOI] [PubMed] [Google Scholar]
- 105.Bulloch K., Prasad A., Conrad CD., McEwen BS., Milner TA. Calcitonin gene-related peptide level in the rat dentate gyrus increases after damage. Neuroreport. 1996;7:1036–1040. doi: 10.1097/00001756-199604100-00016. [DOI] [PubMed] [Google Scholar]
- 106.Bulloch K., Sadamatsu M., Patel A., McEwen BS. Calcitonin gene-related peptide immunoreactivity in the hippocampus and its relationship to cellular changes following exposure to trimethyltin. J Neurosci Res. 1999;55:441–457. doi: 10.1002/(SICI)1097-4547(19990215)55:4<441::AID-JNR5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 107.Wang FZ., Feng CH., Liu ZP. The role of Ca in changes of membrane function and the protection of CGRP in hippocampal slice during hypoxia. Abstr Soc Neurosci. 1993;16:479.93. [Google Scholar]
- 108.Reddington M., Priller J., Treichel J., Haas C., Kreutzberg GW. Astrocyte and microglia as potential targets for calcitonin gene related peptide in the CNS. Can J Physiol Pharm. 1995;73:1047–1049. doi: 10.1139/y95-148. [DOI] [PubMed] [Google Scholar]
- 109.Rowe JW., Kahn RL. Successful Aging. New York, NY: Pantheon Books; 1998 [Google Scholar]
- 110.Singer BH., Ryff CDE. New Horizons in Health. An integrative Approach. Washington, DC: National Research Council, National Academy Press; 2001 [PubMed] [Google Scholar]
- 111.Geronimus AT. The weathering hypothesis and the health of AfricanAmerican women and infants: evidence and speculations. Ethnic Dis. 1992;2:207–221. [PubMed] [Google Scholar]
- 112.Sterling P., Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, Reason J, eds. Handbook of Life Stress, Cognition and Health. New York, NY: John Wiley & Sons; 1988:629–649. [Google Scholar]
- 113.McEwen BS., Stellar E. Stress and the individual: mechanisms leading to disease. Arch intern Med. 1993;153:2093–101. [PubMed] [Google Scholar]
- 114.McEwen B. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 115.Lowy MT., Wittenberg L., Yamamoto BK. Effect of acute stress on hippocampal glutamate levels and spectrin proteolysis in young and aged rats. J Neurochem. 1995;65:268–274. doi: 10.1046/j.1471-4159.1995.65010268.x. [DOI] [PubMed] [Google Scholar]
- 116.Moghaddam B., Boliano ML., Stein-Behrens B., Sapolsky R. Glucocorticoids mediate the stress-induced extracellular accumulation of glutamate. Brain Res. 1994;655:251–254. doi: 10.1016/0006-8993(94)91622-5. [DOI] [PubMed] [Google Scholar]
- 117.Seeman TE., Singer BH., Rowe JW., Horwitz Rl., McEwen BS. Price of adaptation―allostatic load and its health consequences: MacArthur studies of successful aging. Arch Intern Med. 1997;157:2259–2268. [PubMed] [Google Scholar]
- 118.Landfield P. Modulation of brain aging correlates by long-term alterations of adrenal steroids and neurally active peptides. Prog Brain Res. 1987;72:279–300. doi: 10.1016/s0079-6123(08)60215-0. [DOI] [PubMed] [Google Scholar]
- 119.Sapolsky R. Stress, the Aging Brain and the Mechanisms of Neuron Death. Cambridge, Mass: MIT Press; 1992;1:423. [Google Scholar]
- 120.Nishimura E., Billestrup N., Perrin M., Vale W. Identification and characterization of a pituitary corticotropin-releasing factor binding protein by chemical cross-linking. J Biol Chem. 1987;262:12893–12896. [PubMed] [Google Scholar]
- 121.Lupien S., Lecours AR., Lussier I., Schwartz G., Nair NPV., Meaney MJ. Basal Cortisol levels and cognitive deficits in human aging. J Neurosci. 1994;14:2893–2903. doi: 10.1523/JNEUROSCI.14-05-02893.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Seeman TE., McEwen BS., Singer BH., Albert MS., Rowe JW. Increase in urinary Cortisol excretion and memory declines: MacArthur studies of successful aging. J Clin Endocrinol Metab. 1997;82:2458–2465. doi: 10.1210/jcem.82.8.4173. [DOI] [PubMed] [Google Scholar]
- 123.Lupien SJ., de Leon M., de Santi S., et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- 124.Sachar BJ., Hellman J., Fukushima DK., Gallagher TF. Cortisol production in depressive illness. Arch Gen Psychiatry. 1970;23:289–298. doi: 10.1001/archpsyc.1970.01750040001001. [DOI] [PubMed] [Google Scholar]
- 125.Young EA., Haskett RF., Grunhaus L., et al. Increased evening activation of the hypothalamic-pituitary-adrenal axis in depressed patients. Arch Gen Psychiatry. 1994;51:701–707. doi: 10.1001/archpsyc.1994.03950090033005. [DOI] [PubMed] [Google Scholar]
- 126.Deuschle M., Weber B., Colla M., Depner M., Heuser I. Effects of major depression, aging and gender upon calculated diurnal free plasma Cortisol concentrations: a re-evaluation study. Stress. 1998;2:281–287. doi: 10.3109/10253899809167292. [DOI] [PubMed] [Google Scholar]
- 127.Carroll B., Martin F., Davies B. Resistance to suppression by dexamethasone of plasma 11-OHCS levels in severe depressive illness. BMJ. 1968;3:285–287. doi: 10.1136/bmj.3.5613.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Weber B., Lewicka S., Deuschle M., Colla M., Heuser I. Testosterone, androstenedione and dihydrotestosterone concentrations are elevated in female patients with major depression. Psychoneuroendocrinology. 2000;25:765–771. doi: 10.1016/s0306-4530(00)00023-8. [DOI] [PubMed] [Google Scholar]
- 129.Deuschle M., Blum WF., Strasburger CJ., et al. Insulin-like growth factorI (IGF-I) plasma concentrations are increased in depressed patients. Psychoneuroendocrinology. 1997;22:493–503. doi: 10.1016/s0306-4530(97)00046-2. [DOI] [PubMed] [Google Scholar]
- 130.Sheline Yl., Wang PW., Gado MH., Csernansky JC., Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sheline Yl., Sanghavi M., Mintun MA., Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bremner JD., Narayan M., Anderson ER., Staib LH., Miller HL., Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–117. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 133.Vakili K., Pillay SS., Later B., et al. Hippocampal volume in primary unipolar major depression. A magnetic resonance imaging study. Biol Psychiatry. 2000;47:1087–1090. doi: 10.1016/s0006-3223(99)00296-6. [DOI] [PubMed] [Google Scholar]
- 134.Rusch BD., Abercrombie HC., Oakes TR., Schaefer SM., Davidson RJ. Hippocampal morphometry in depressed patients and control subjects: relations to anxiety symptoms. Biol Psychiatry. 2001;50:960–964. doi: 10.1016/s0006-3223(01)01248-3. [DOI] [PubMed] [Google Scholar]
- 135.Sullivan EV., Pfefferbaum A., Swan GE., Carmelli D. Heritability of hippocampal size in elderly twin men: equivalent influence from genes and environment. Hippocampus. 2001;11:754–762. doi: 10.1002/hipo.1091. [DOI] [PubMed] [Google Scholar]
- 136.Steffens DC., Byrurn CE., McQuoid DR., et al. Hippocampal volume in geriatric depression. Biol Psychiatry. 2000;48:301–309. doi: 10.1016/s0006-3223(00)00829-5. [DOI] [PubMed] [Google Scholar]
- 137.Kim DH., Payne ME., Levy RM., MacFall JR., Steffens DC. APOE genotype and hippocampal volume change in geriatric depression. Biol Psychiatry. 2002;51:426–429. doi: 10.1016/s0006-3223(01)01272-0. [DOI] [PubMed] [Google Scholar]
- 138.Lucassen PJ., Muller MB., Holsboer F., et al. Hippocampal apoptosis in major depression is a minor event and absent from subareas at risk for glucocorticoid overexposure. Am J Pathol. 2001;158:453–468. doi: 10.1016/S0002-9440(10)63988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Muller MB., Lucassen PJ., Yassouridis A., Hoogendijk WJG., Holsboer F., Swaab DF. Neither major depression nor glucocorticoid treatment affects the cellular integrity of the human hippocampus. Eur J Neurosci. 2001;14:1603–1612. doi: 10.1046/j.0953-816x.2001.01784.x. [DOI] [PubMed] [Google Scholar]
- 140.MacQueen GM., Campbell S., McEwen BS., et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Drevets WC., Price JL., Simpson Jr JR., et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 142.Rajkowska G., Miguel-Hidalgo JJ., Wei J., et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 143.Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- 144.Rajkowska G., Halaris A., Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- 145.Drevets WC., Videen TO., Price JL., Preskorn SH., Carmichael ST., Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sheline Yl., Barch DM., Donnelly JM., Ollinger JM., Snyder AZ., Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 147.Frodl T., Meisenzahl E., Zetzsche T., et al. Enlargement of the amygdala in patients with a first episode of major depression. Biol Psychiatry. 2002;51:708–714. doi: 10.1016/s0006-3223(01)01359-2. [DOI] [PubMed] [Google Scholar]
- 148.Chattarji S., Vyas A., Mitra R., Rao BSS. Effects of chronic unpredictable and immobilization stress on neuronal plasticity in the rat amygdala and hippocampus. Soc Neurosci Abs. 2000;26:#571.9, p. 1533. [Google Scholar]
- 149.LeDoux JE. The Emotional Brain. New York, NY: Simon and Schuster; 1996 [Google Scholar]
- 150.Schulkin J., McEwen BS., Gold PW. Allostasis, amygdala, and anticipatory angst. Neurosci Biobehav Rev. 1994;18:385–396. doi: 10.1016/0149-7634(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 151.Sheline Yl., Gado MH., Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- 152.Michelson D., Stratakis C., Hill L., et al. Bone mineral density in women with depression. N Engl J Med. 1996;335:1176–81. doi: 10.1056/NEJM199610173351602. [DOI] [PubMed] [Google Scholar]
- 153.Cizza G., Ravn P., Chrousos GP., Gold PW. Depression: a major, unrecognized risk factor for osteoporosis? Trends Endocrinol Metab. 2001;12:198–203. doi: 10.1016/s1043-2760(01)00407-6. [DOI] [PubMed] [Google Scholar]
- 154.Schweiger U., Weber B., Deuschle M., Heuser I. Lumbar bone mineral density in patients with major depression: evidence of increased bone loss at follow-up. Am J Psychiatry. 2000;157:118–120. doi: 10.1176/ajp.157.1.118. [DOI] [PubMed] [Google Scholar]
- 155.Thakore JH., Richards PJ., Reznek RH., Martin A., Dinan TG. Increased intra-abdominal fat deposition in patients with major depressive illness as measured by computed tomography. Biol Psychiatry . 1997;41:1140–1142. doi: 10.1016/S0006-3223(97)85394-2. [DOI] [PubMed] [Google Scholar]
- 156.Mann JN., Thakore JH. Melancholic depression and abdominal fat distribution: a mini-review. Stress. 1999;3:1–15. doi: 10.3109/10253899909001108. [DOI] [PubMed] [Google Scholar]
- 157.Weber-Hamann B., Hentschel F., Kniest A., et al. Hypercortisolemic depression is associated with increased intra-abdominal fat. Psychosom Med. 2002;64:274–277. doi: 10.1097/00006842-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 158.Thayer JF., Smith M., Rossy LA., Sellers JJ., Friedman BH. Heart period variability and depressive symptoms: gender differences. Biol Psychiatry. 1998;44:304–306. doi: 10.1016/s0006-3223(98)00008-0. [DOI] [PubMed] [Google Scholar]
- 159.Musselman DL., Tomer A., Manatunga AK., et al. Exaggerated platelet reactivity in major depression. Am J Psychiatry. 1996;153:1313–1317. doi: 10.1176/ajp.153.10.1313. [DOI] [PubMed] [Google Scholar]
- 160.Lederbogen F., Gilles M., Maras A., et al. Increased platelet aggregability in major depression. Psychiatry Res. 2001;102:255–261. doi: 10.1016/s0165-1781(01)00259-1. [DOI] [PubMed] [Google Scholar]
- 161.Walsh M-T., Dinan TG., Condren RM., Ryan M., Kenny D. Depression is associated with an increase in the expression of the platelet adhesion receptor glycoprotein lb. Life Sci. 2002;70:3155–3165. doi: 10.1016/s0024-3205(02)01569-2. [DOI] [PubMed] [Google Scholar]
- 162.Musselman DL., Evans DL., Nemeroff CB. The relationship of depression to cardiovascular disease. Arch Gen Psychiatry. 1998;55:580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- 163.Heuser I. Depression, endocrinological a syndrome of premature aging? Maturitas. 2002;41(suppl 1):S19–S23. doi: 10.1016/s0378-5122(02)00012-9. [DOI] [PubMed] [Google Scholar]
- 164.Ballenger JC., Davidson JRT., Lecrubier Y., et al. Consensus statement on depression, anxiety, and cardiovascular disease. J Clin Psychiatry. 2001;62:24–27. [PubMed] [Google Scholar]
- 165.Perlmutter JB., Frishman WH., Feinstein RE. Major depression as a risk factor for cardiovascular disease. Therapeutic implications. Heart Dis. 2000;2:75–82. [PubMed] [Google Scholar]
- 166.Starkman MN., Schteingart DE. Neuropsychiatrie manifestations of patients with Cushing's syndrome. Arch Intern Med. 1981;141:215–219. [PubMed] [Google Scholar]
- 167.Starkman MN., Schteingart DE., Schork MA. Depressed mood and other psychiatric manifestations of Cushing's syndrome: relationship to hormone levels. Psychosom Med. 1981;43:3–18. doi: 10.1097/00006842-198102000-00002. [DOI] [PubMed] [Google Scholar]
- 168.Condren RM., Thakore JH. Cushing's disease and melancholia. Stress. 2001;4:91–119. doi: 10.3109/10253890109115725. [DOI] [PubMed] [Google Scholar]
- 169.Gold PW., Loriaux DL., Roy A., et al. Responses to corticotropin-releasing hormone in the hypercortisolism of depression and Cushing's disease. N Engl J Med. 1986;314:1329–1335. doi: 10.1056/NEJM198605223142101. [DOI] [PubMed] [Google Scholar]
- 170.Starkman MN., Gebarski SS., Berent S., Schteingart DE. Hippocampal formation volume, memory dysfunction, and Cortisol levels in patients with Cushing's syndrome. Biol Psychiatry. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- 171.Mauri M., Sinforiani E., Bono G., et al. Memory impairment in Cushing's disease. Acta Neurol Scand. 1993;87:52–55. doi: 10.1111/j.1600-0404.1993.tb04075.x. [DOI] [PubMed] [Google Scholar]
- 172.Forget H., Lacroix A., Somma M., Cohen H. Cognitive decline in patients with Cushing's syndrome. J Int Neuropsychol Soc. 2000;6:20–29. doi: 10.1017/s1355617700611037. [DOI] [PubMed] [Google Scholar]
- 173.Heinz RE., Martinez J., Haenggeli A. Reversibility of cerebral atrophy in anorexia nervosa and Cushing's syndrome. J Comput Assisted Tomogr. 1977;1:415–418. doi: 10.1097/00004728-197710000-00006. [DOI] [PubMed] [Google Scholar]
- 174.Starkman MN., Giordani B., Gebrski SS., Berent S., Schork MA., Schteingart DE. Decrease in Cortisol reverses human hippocampal atrophy following treatment of Cushing's disease. Biol Psychiatry. 1999;46:1595–1602. doi: 10.1016/s0006-3223(99)00203-6. [DOI] [PubMed] [Google Scholar]
- 175.Bourdeau I., Bard C., Noel B., et al. Loss of brain volume in endogenous Cushing's syndrome and its reversibility after correction of hypercortisolism. J Clin Endocrinol Metab. 2002;87:1949–1954. doi: 10.1210/jcem.87.5.8493. [DOI] [PubMed] [Google Scholar]
- 176.Lederbogen F., Weber B., Colla M., Heuser I., Dreuschle M., Dempfle CE. Antidepressant treatment and global tests of coagulation and fibrinolysis. J Clin Psychiatry. 2001;62:130. doi: 10.4088/jcp.v62n0210g. [DOI] [PubMed] [Google Scholar]
- 177.Harvey BH., Jonker LP., Brand L., Heenop M., Stein DJ. NMDA receptor involvement in imipramine withdrawal-associated effects on swim stress, GABA levels and NMDA receptor binding in rat hippocampus. Life Sci. 2002;71:43–54. doi: 10.1016/s0024-3205(02)01561-8. [DOI] [PubMed] [Google Scholar]
- 178.Harvey B., McEwen BS., Stein D. Neurobiology of antidepressant withdrawal: implications for the longitudinal outcome of depression. Biol Psychiatry. 2003;54:1105–1117. doi: 10.1016/s0006-3223(03)00528-6. [DOI] [PubMed] [Google Scholar]
- 179.Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- 180.Bremner JD. Does stress damage the brain? Biol Psychiatry. 1999;45:797–805. doi: 10.1016/s0006-3223(99)00009-8. [DOI] [PubMed] [Google Scholar]
- 181.Caspi A., Sugden K., Moffitt TE., et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 182.Kendler KS., Karkowski-Shuman L. Stressful life events and genetic liability to major depression: genetic control of exposure to the environment? Psychol Med. 1997;27:539–547. doi: 10.1017/s0033291797004716. [DOI] [PubMed] [Google Scholar]