Abstract
Mild cognitive impairment (MCI) refers to cognitive impairment that is assumed to be due to pathological central nervous system processes, but which interacts with normal aging-related changes. Epidemiological studies conducted in the general population have been able to examine more heterogeneous forms of this disorder than clinical studies, and have also been able to provide early estimations of population incidence and prevalence. Large differences in case identification procedures and sampling methods have led to considerable divergence in the rates of prevalence reported, which ranged from 1% to 29%. Suggested improvements in the definition of MCI have led to an upward adjustment of prevalence rates in most studies, giving between 5% and 29%. Incidence is estimated as 8 to 58 new cases per thousand persons per year, and the probability of conversion from MCI to dementia is estimated at around 15%. The principal risk factors that have been identified so far for MCI using regression models applied to general population data are age, education, race, medicated hypertension, infarcts, white matter lesions, depression, and apolipoprotein E4 (AP0E-4J allele. An etiological model derived from these studies indicates possible intervention points for future therapeutic strategies at the level of both clinical intervention and environmental exposure. There is, however, a clear need for epidemiological studies that take into account a broader range of risk factors than those studied to date, which have focused principally on known risk factors for dementia.
Keywords: cognition, relevance, incidence, risk, etiology
Abstract
El deterioro cognitivo leve (DCL) se refiere a un deterioro que se asume debido a procesos patológicos del sistema nervioso central, pero que interactúa con cambios relacionados al envejecimiento normal. Estudios epidemiológicos realizados en población general han permitido examinar formas más heterogéneas de este trastorno que los estudios clínicos y también han proporcionado las primeras estimaciones de incidencia y prevalencia en la población. Amplias diferencias en los procedimientos de identificación de casos y métodos de muestreo han conducido a divergencias considerables en las cifras de prevalencia informadas, las cuales van desde el 1% al 29%. Se han propuesto mejorías en la definición del DCL que han conducido a un ajuste hacia arriba de las cifras de prevalencia en la mayoría de los estudios, llegando a valores entre 5% y 29%. La incidencia se estima en 8 a 58 nuevos casos por mil personas por año, y la probabilidad de conversión desde el DCL a la demencia es estimada en alrededor del 15%. Los principales factores de riesgo que se han identificado hasta la fecha para el DCL utilizando modelos de regresión aplicados a los datos de población general son edad, educación, raza, hipertensión en tratamiento farmacológico, infartos, lesiones de la sustancia blanca, depresión y presencia del alelo de apolipoproteína E4 (APOE-4). Un modelo etiológico derivado de estos estudios indica posibles puntos de intervención para futuras estrategias terapéuticas tanto para intervenciones clínicas como ambientales. Existe, sin embargo, una clara necesidad de estudios epidemiológicos que consideren un rango mayor de factores de riesgo que los estudiados hasta la fecha, los cuales se han centrado principalmente en los factores de riesgo conocidos para la demencia.
Abstract
Le déficit cognitif léger (Mild Cognitive Impairment MCI) se rapporte à un déficit cognitif présumé dû à des processus pathologiques du système nerveux central, mais qui interagit avec les changements liés au vieillissement normal. Des études épidémiologiques effectuées dans la population générale ont permis d'examiner des formes plus hétérogènes de ce trouble que les études cliniques et de donner aussi des estimations précoces de la prévalence et de l'incidence dans la population. De grandes différences dans les procédures d'identification des cas et les méthodes d'échantillonnage ont conduit à de grandes divergences entre les taux de prévalence rapportés, qui allaient de 1 % à 29%. Des améliorations proposées pour définir le MCI ont abouti à un ajustement à la hausse des taux de prévalence dans la plupart des études, entre 5% et 29%. L'incidence est estimée à8à 58 nouveaux cas pour 1 000 personnes par an et la probabilité d'évolution du MCI vers la démence autour de 15%. Les principaux facteurs de risque identifiés jusqu'ici pour le MCI en utilisant des modèles de régression appliqués aux données de la population générale sont l'âge, l'éducation, la race, l'hypertension traitée, l'infarctus, les lésions de la substance blanche, la dépression et l'allèle de l'apolipoprotéine E4 (APOE-4). Un modèle étiologique issu de ces études permet d'identifier les points d'intervention possibles pour des stratégies thérapeutiques futures, à la fois au niveau de l'intervention clinique et de l'exposition environnementale. Des études épidémiologiques prenant en compte un plus large échantillon de facteurs de risque que ceux étudiés à ce jour, qui se sont principalement centrés sur les facteurs de risque connus de la démence, sont néanmoins clairement nécessaires.
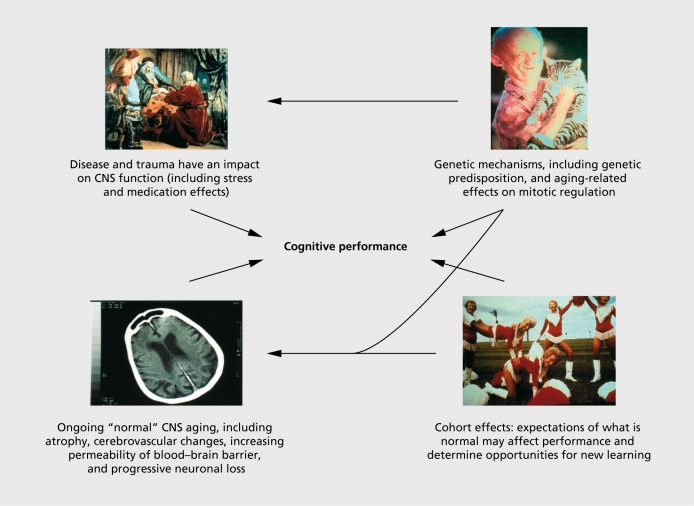
Epidemiology involves the observation of health states within a general population context. In the case of mild cognitive impairment (MCI), this is important in that we are dealing with what is essentially a subclinical state, ie, a health problem that is likely to remain unreported to a health professional for an extended period of time, and for which the most common first point of contact will be the general practitioner. It is also a heterogeneous entity: subclinical cognitive disorder has multiple interacting causes, as illustrated in Figure 1 While the concept of MCI has progressively been narrowed down to a subgroup of persons in the first stages of a probable neurodegenerative process, this group will nonetheless be subject to many of the other causes of cognitive decline, which will interact with the disease process and complicate the definition and screening of MCI. General population studies of a disorder allow us to see the full range of cases within a naturalistic setting, which includes a wide range of potential risk factors. Longitudinal observation of a general population sample with subclinical cognitive deficits has demonstrated multiple patterns of cognitive change with variable clinical outcomes including dementia, depression, cardiovascular disease, and respiratory disorders.1 However, the identification of those cases likely to evolve towards dementia has been given priority, especially given the development of treatments that may delay dementia onset. The potential treatment window for dementia is large, with twin studies indicating that insidious changes in cognitive performance may occur up to 20 years before disease onset.2 Population studies allow us to develop models of disease etiology within this more complex multifactor setting.
Figure 1. Mild cognitive impairment has multiple interacting causes. CNS, central nervous system.
Epidemiology has a triple role in terms of public health:
Descriptive epidemiology: the monitoring of disease prevalence and incidence across time.
Analytical epidemiology: the determination of risk factors and their patterns of interaction, permitting the construction of hypothetical etiological models of disease processes.
Interventional epidemiology: the designation of potential intervention points for the reduction of morbidity and mortality, which may guide more targeted clinical research.
MCI will be discussed here in relation to these three functions.
Descriptive epidemiology of MCI
The emergence of MCI as a health problem and the expansion of cognitive morbidity at a population level are clearly related to the general phenomenon of population aging. As Gruenberg3 pointed out in 1977, it is one of the “failures of success” that, while medical research has reduced the mortality of disease, it has concurrently extended life expectancy and increased the proportion of persons in the community with chronic pathologies. Analyses of longitudinal health survey data from the USA by Kramer4 in the early 1980s provided early empirical evidence of the rapid expansion of dependency due to cognitive disorders arising from increases in disease incidence, better management of its physiological consequences, and thus decreased direct mortality This public health dilemma was, in fact, predicted by Jonathan Swift in the early 18th century In Gulliver's Travels,5 he described the cognitive consequences of extended longevity in eternal beings, who, on reaching the age of 80 and in the absence of degenerative disease, continue to perform daily activities but have difficulty in recalling the names of common objects and recently read material, forget the name of friends, and consequently have diminished pleasure in life. It is a description that comes quite close to current definitions of MCI, and distinguishes MCI from normal aging and dementia. Referring to the latter, whom he describes as “those who turn to dotage,” he suggests that they may be more fortunate in that they elicit pity rather than ridicule.
The extent to which MCI may be a disabling process is largely unknown because the usual definition proposed by Petersen and colleagues,6,7 and adopted by most researchers in this area, stipulated that MCI is a state that does not interfere with everyday activities. More recently, this definition has been relaxed to include the possibility that MCI may impede, but not prevent, everyday functioning. On this basis, it has subsequently been shown that MCI may be associated with increasing difficulties in the performance of a wide range of everyday tasks, notably dressing, dental care, and the use of a telephone.8 We do not know, on the other hand, to what extent MCI may indirectly lead to activity restriction due to, for example, withdrawal from a social activity due to fear of being embarrassed by a memory problem. Little is currently known either about the extent to which MCI may influence mortality rates. While dementia has been clearly associated with increased mortality with a life expectancy on average of 8 years from the time of diagnosis, the impact of MCI on survival remains unclear. Cumulative mortality risk in MCI has been estimated by Gussekloo et al9 using a Cox proportional hazards model with a cohort of 891 subjects from the Leiden Aging Study Compared with normal subjects the cumulative risk was found to be 2.5. The study is however, limited by its use of the Mini-Mental-State Examination (MMSE)10 to define MCI. How widespread is MCI in the general population? Establishing the prevalence and incidence of MCI has above all been hindered by the lack of an operational definition of the disorder adapted to general population use, where case selection cannot normally be based on a complete neurological examination. Early conceptualizations of subclinical cognitive deficit were based on the theoretical assumption that such changes are distinct from dementia and other pathologies, being the consequence of inevitable aging-related cerebral changes, such as cortical atrophy, which may be considered a normal feature of the aging process. As parallel research into the causes of dementia and cerebrovascular disease has now led to a clearer understanding of their etiology, it has also been shown that many of the physiological abnormalities seen in these disorders are also present to a lesser extent in normal subjects with cognitive complaints, but these factors cannot currently be incorporated into diagnostic criteria due to difficulties in establishing precise universal cutoff points between MCI and normal subjects. The diagnostic criteria for MCI proposed by Petersen et al6 thus refer to complaints of defective memory and demonstration of abnormal memory functioning for age, which may be more easily quantified by reference to standard deviation from scores obtained by normal elderly subjects. The remaining criteria are principally exclusion criteria: normal general cognitive functioning and conserved ability to perform activities of daily living. MCI is considered above all to be a prodrome of Alzheimer's disease and, variably, of other dementias. MCI criteria refer to poor cognitive functioning as assessed at one point in time, thus precluding an appreciation of decline over time; it is thus difficult to differentiate from cohort effects, low IQ, and education. Later definitions by Petersen et al7 refined the initial concept by referring to memory impairment beyond that expected for both age and education level This has been the working definition adopted by most epidemiological studies.
The definition of MCI has been developed within a clinical setting. As such, the definition represents a minimal set of distinguishing criteria, the diagnosis resting largely on the overall clinical picture. Validation of the criteria has been in terms of their capacity to predict conversion to dementia and/or Alzheimer's disease. The two are often used interchangeably, which has led to some confusion in the comparison of results across centers. Table I 7,11-18 shows the predictive value of MCI criteria within a clinical setting. Conversion rates to dementia are also noted for some studies. The conversion rate from MCI to dementia in clinical samples is reported at between 10% and 20%, regardless of age. Together, these studies suggest the predictive validity of the concept within a clinical setting.
These studies are all, however, based on clinical series conducted in specialist centers, so it is not certain to what extent they represent all cases of MCI found in the general population. Clinical signs and symptoms beyond those cited in the official MCI criteria have also been used for diagnosis, so there is likely to be some differences in case identification between centers. While these studies together suggest the high predictive validity of the concept within a clinical setting, they are unable to provide us with information on prevalence and incidence. To date, only a small number of general population studies have been conducted using MCI criteria, giving a range of prevalence estimations from 3% to 19%. There are significant differences in sampling frames, cognitive tests, and drop-out due to mortality and refusal between these studies; nonetheless, the majority of authors report rates of around 3% when MCI criteria are strictly applied (Table II).9-24
Subjects in three of the studies reporting higher rates21-23 have received extensive clinical examinations as well as cognitive testing, which may have led to the inclusion of subjects on the basis of clinical criteria beyond those stipulated in the definition of MCI. In three studies,19-21 the authors conclude in their discussion that the criteria are too strict and a large number of subjects are subsequently excluded who would be considered by clinicians as a high-risk group. The principal problems with existing criteria are reported to be in the areas of “subjective reporting of memory problems” and “intact activities of daily living.” Modifying the criteria to allow for absence of subjective memory problems and permitting changes in ability to perform activities of daily living was found by all three studies to increase MCI prevalence to give rates between 3% and 19%.
Both clinicians and epidemiologists have found the restriction of MCI to an isolated memory deficit difficult to apply in practice. Firstly, isolated memory dysfunction is relatively rare; estimated at about 6% of all cases of subclinical cognitive deficit,25 at a clinical level it is very difficult to define as even specific memory tests involve other cognitive functions, such as language comprehension and attention. A recent working group of clinicians and epidemiologists working in the area of MCI met in Stockholm in 2003 and proposed new working criteria for MCI,26 which take into account the difficulties described above and provide clearer guidelines for clinical research. The new stepwise algorithm, which also defines subtypes of MCI, is based around the following three diagnostic features:
Not normal, not demented (does not meet Diagnostic and Statistical Manual of Mental Disorders Fourth Edition [DSM-IVJ or International Classification of Mental and Behavioral Disorders [ICD-10] criteria for a dementia syndrome).
Cognitive decline indicated by subject and/or informant report and objective cognitive tests.
Preserved basic activities of daily living with some minimal impairment in complex instrumental functions.
It is hoped that the application of these new diagnostic guidelines will increase the comparability of clinical studies and thus produce more accurate estimates of disease prevalence.
Little is currently known about incidence rates. Overall population studies have shown somewhat lower conversion rates from MCI to dementia than clinical studies, which is not surprising given the more heterogeneous nature of the cognitive deficit likely to be seen in this setting. Three studies permit us to make estimates of incidence of 8,19 26,22 and 5821 new cases per thousand subjects annually Yesavage et al27 have attempted to model incidence rates using a first-order Markovian Chain Model to predict transition from normality to MCI based on published prevalence, incidence, and conversion data. They found a new case rate from normality to MCI starting at 10 per 1000 at age 60 and increasing to reach 110 per 1000 at age 85. The proposed model probability estimates are based on recent data on incidence, prevalence, and conversion rates; however, these we have seen to be divergent. The model also relies on age-specific AD prevalence rates derived from a US study, which are lower than those observed in European meta-analyses.
Table I. Rates of conversion to dementia for subjects with mild cognitive impairment (MCI) versus controls (where available).
| Study | n | Time (years) | Rate of conversion of MCI to dementia (%) | Rate of conversion of controls to dementia (%) |
| Petersen7 et al | 76 | 1 | 12 | 2 |
| Johnson11 et al | 68 | 2 | 40 | |
| Tierney12 et al | 123 | 2 | 24 | |
| Flicker13 et al | 32 | 2 | 72 | 12 |
| Grundman14 et al | 687 | 2 | 31 | 2 |
| Bobinski15 et al | 12 | 3 | 50 | 0 |
| Wolf16 et al | 41 | 3 | 20 | |
| McKelvey17 et al | 36 | 3 | 53 | |
| Morris18et al | 277 | 9 | 100 | 7 |
Analytical epidemiology of MCI
Numerous clinical and population studies have examined risk factors for MCI conversion to dementia, but far fewer the risk of transition to MCI from normal cognitive functioning. Clinical case-control studies have provided cross-sectional information on differences between MCI and normal aging with relation to brain structure and function, and cognition. Compared with normal subjects, MCI groups are seen above all to manifest left medial temporal lobe atrophy and smaller medial temporal lobe volumes.16,28 Other studies have suggested that white matter lesions, particularly in periventricular areas, are associated with MCI.29 These findings suggest that the clinical risks for conversion from normal to MCI are principally related to degree of impairment along a continuum from normal aging-related changes to dementia. Clinical cohort studies have provided very little information on other health factors, or psychological, behavioral, and environmental risks for transition to MCI. Two general population epidemiological studies have attempted to isolate clusters of risk factors by regression analysis based on a wide range of clinical and sociodemographic factors.
Tervo et al22 examined a range of demographic, vascular, and genetic factors, and found the most significant risk factors to be age (odds ratio [OR] 1.08), apolipoprotein E4 (APOE-4) allele (OR 2.04), and medicated hypertension (OR 1.86). High educational level was found to be a protective factor (OR 0.79) and the combination given the highest risk was medicated hypertension plus APOE-4 (OR 3.92). Risk factors for MCI were also examined from the multisite longitudinal Cardiovascular Health Study.23,30 In this large study of 3608 subjects, which included neuropsychological and neurological tests, general medical examination, and magnetic resonance imaging (MRI), the principal risk factors for MCI were found to be African-American race, low educational level, Digit Symbol Test score, cortical atrophy, MRI-identified infarcts, and depression. This study also examined MCI subtypes and found risk factors for amnestic MCI to be infarcts, APOE-4 allele, and low MMSE scores, while for multiple domain MCI risk factors were MMSE and Digit Symbol Test scores. It is difficult, however, to consider cognitive scores as a risk factor for MCI, as they are part of the diagnostic algorithm used to select cases. Data from a third study, the Kungsholmen Project in Sweden,31 also suggested that certain psychiatric symptoms may be predictive of MCI, notably anxiety; however, this study did not use the usual MCI criteria to identify cases.
Examining the various risk factors that have been isolated for conversion from normal functioning to MCI, it is possible to construct a hypothetical model of risk. Figure 2 shows theoretical pathways (in black) to MCI incorporating most of the known risk factors, which can be seen to be largely those for dementia. There are, however, insufficient population data at present to permit either a statistical calculation of transition probabilities in relation to individual risk factors or a maximum likelihood calculation to assess the overall predictive value of possible competing hypothetical general models.
Figure 2. Hypothetical etiological model of mild cognitive impairment (MCI) in black, and possible treatment or lifestyle intervention points in blue.
Table II. Prevalence rates for mild cognitive impairment (MCI) (and MCI revised criteria), annual incidence, and dementia conversion rates, where available. AD, Alzheimer's disease.
| Author | Country | n | Prevalence MCI (%) | Modified prevalence of MCI (%) | Rate of conversion to AD (%) years | Incidence |
| Busse19 et al | Germany | 1265 | 3.1 | 5.1 | 33/2.6 | 8/1000 |
| Fisk20 et al | Canada | 1790 | 1.03 | 3.02 | 46.7/5 | |
| Ritchie21 et al | France | 833 | 3.2 | 19.3 | 11/2 | 58/1000 |
| Tervo22 et al | Finland | 806 | 8.8 | 26/1000 | ||
| Lopez23 et al | USA | 3608 | 19 | |||
| Qiu24 et al | China | 3910 | 2.4 |
Interventional epidemiology
Finally, the role of interventional epidemiology is to suggest possible intervention points within a hypothetical etiological model to guide research into therapeutic intervention. It appears increasingly likely that MCI, like dementia, is the result of multiple lifetime insults in combination with genetic vulnerability factors. The different points at which intervention may be likely to reduce risk have been added on to the theoretical model in blue in Figure 2. A more complete clinical discussion of treatment possibilities in MCI has been developed in the paper in this issue by Gauthier.32 At the present time, there is clearly no specific treatment for MCI, but it may be possible to reduce overall risk by a number of simple strategies, which do not in themselves have adverse consequences. These include the management of cardiovascular and cerebrovascular risk factors such as high blood pressure from early adult life onward to reduce the risk of infarcts and white matter lesion accumulation, controlling for depression, and the provision of adequate learning opportunities from childhood. Other more active and contentious intervention therapies for MCI, such as use of statins, anti-inflammatory agents, the anticholinesterase therapies currently used in the treatment of dementia, and hormonal replacement therapy, are being evaluated, but there is currently insufficient evidence for their widespread population use in the prevention of MCI. It has been demonstrated within a longitudinal population study that, by entering MCI risk factors into a regression equation, a probability statistic of developing dementia over a given time period may be produced; this may assist clinicians in the decision to undertake a therapeutic intervention that has adverse side effects.33
An avenue for future research in conjunction with the provision of cholinergic system therapies is to explore to what extent the overall cholinergic burden may be reduced by the readjustment of other medication being taken by an elderly person. A very wide range of drugs have anticholinergic effects, often unknown to the general practitioner, and it is not known to what extent these may be a common risk factor for MCI. The administration of anti-cholinergic agents, such as scopolamine, in healthy young subjects has been shown to produce very similar cognitive deficits to MCI.34 The range of drugs with known anticholinergic effects is very large and includes such commonly prescribed drugs as the antihistamines, bronchodilators, antidepressants, antiulcer medication, preanesthetics, and even some herbal teas. It has been estimated that around a third of nursing home patients in the USA take more than two anticholinergic drugs and 5% more than five.35 It is surprising that this important environmental risk factor has not been taken into account in epidemiological studies of environmental risk in MCI.
Conclusion
MCI rates are likely to increase rapidly in parallel with the extension of life expectancy at higher ages. Current estimates of prevalence are limited by problems related to case identification, but, in the light of several revisions of the original definition, appear to be converging at around 5% of the general population with around 15% per year going on to develop dementia. Mortality risk is doubled in MCI subjects. While the principal value of MCI remains the identification of persons in the first stages of neurodegenerative disease, it also covers other forms of cognitive impairment due to multiple causes, making the construction of meaningful hypothetical etiological models extremely difficult. The few studies of risk that have been carried out have largely focused on known risk factors for dementia and there is a clear need for longitudinal epidemiological studies that examine a wider range of genetic, biological, demographic, and environmental risk factors. Such studies are extremely costly and difficult to justify for a health state that is subclinical, poorly defined, often benign, and for which no specific treatment is currently available. Epidemiologists in this area should explore the possibility of grafting this type of study on to existing longitudinal databases of population aging, which cover a much broader range of risk factors than those included in studies of dementia.
REFERENCES
- 1.Ritchie K., Leibovici D., Ledésert B., Touchon J. A typology of subclinical senescent cognitive disorder. Br J Psychiatry. 1996;168:470–476. doi: 10.1192/bjp.168.4.470. [DOI] [PubMed] [Google Scholar]
- 2.La Rue A., Jarvik LF. Cognitive function and prediction of dementia in old age. Int J Aging Hum Dev. 1987;25:79–89. doi: 10.2190/DV3R-PBJQ-E0FT-7W2B. [DOI] [PubMed] [Google Scholar]
- 3.Gruenberg EM. The failures of success. Milbank Memorial Fund Quart. 1977;55:3–24. [PubMed] [Google Scholar]
- 4.Kramer M. The rising pandemic of mental disorders and associated chronic diseases and disabilities. Acta Psychiatr Scand. 1980;62:282–297. [Google Scholar]
- 5.Swift J. Gulliver's Travels. London, UK: Everyman; 1906 [Google Scholar]
- 6.Petersen RC., Smith GE., Waring SC., Ivnik RJ., Kokmen E., Tangelos EG. Aging, memory and mild cognitive impairment. Int Psychogeriatr. 1997;9:65–69. doi: 10.1017/s1041610297004717. [DOI] [PubMed] [Google Scholar]
- 7.Petersen RC., Smith GE., Waring SC., Ivnik RJ., Tangalos EG., Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 8.Artero S., Touchon J., Ritchie K. Disability and mild cognitive impairment: a longitudinal population-based study. Int J Geriatr Psychiatry. 2001;16:1092–1097. doi: 10.1002/gps.477. [DOI] [PubMed] [Google Scholar]
- 9.Gussekloo J., Westendorp RGJ., Remarque EJ., Lagaay AM., Heeren TJ., Knook DL. Impact of mild cognitive impairment on survival in very elderly people: cohort study. BMJ. 1997;315:1053–1054. doi: 10.1136/bmj.315.7115.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folstein MF., Folstein SE., McHugh PR. “Mini-Mental-State. ” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 11.Johnson KA., Jones K., Holman BL. Preclinical prediction of Alzheimer's disease using SPECT. Neurology. 1998;50:1563–1572. doi: 10.1212/wnl.50.6.1563. [DOI] [PubMed] [Google Scholar]
- 12.Tierney MC., Szalai JP., Snow WG., Fisher RH., Mores A., Nadon G. Prediction of probable Alzheimer's disease in memory-impaired patients: a prospective longitudinal study. Neurology. 1996;46:661–665. doi: 10.1212/wnl.46.3.661. [DOI] [PubMed] [Google Scholar]
- 13.Flicker C., Ferris FH., Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology. 1991;41:1006–1009. doi: 10.1212/wnl.41.7.1006. [DOI] [PubMed] [Google Scholar]
- 14.Grundman M., Petersen RC., Ferris SH., et al. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- 15.Bobinski M., De Leon M., Convït A., et al. MRl of entorhinal cortex in mild Alzheimer's disease. Lancet. 1999;353:38–40. doi: 10.1016/s0140-6736(05)74869-8. [DOI] [PubMed] [Google Scholar]
- 16.Wolf H., Grunwald M., Ecke GM., et al. The prognosis of mild cognitive impairment in the elderly. J Neural Transm. 1998;54:31–50. doi: 10.1007/978-3-7091-7508-8_4. [DOI] [PubMed] [Google Scholar]
- 17.McKelvey R., Bergman H., Stern J. Lack of prognostic significance of SPECT abnormalities in elderly subjects with mild memory loss. Can J Neurol Sci. 1999;26:23–28. [PubMed] [Google Scholar]
- 18.Morris JC., Storandt M., Miller JP., et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 19.Busse A., Bischkopf J., Riedel-Heller SG., Angermeyer MC. Mild cognitive impairment: prevalence and incidence according to different diagnostic criteria. Br J Psychiatry. 2003;182:449–454. [PubMed] [Google Scholar]
- 20.Fisk JD., Merry HR., Rockwood K. Variations in case definition affect prevalence, but not outcomes of mild cognitive impairment. Neurology. 2003;61:1179–1184. doi: 10.1212/01.wnl.0000089238.07771.c7. [DOI] [PubMed] [Google Scholar]
- 21.Ritchie K., Artero S., Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology. 2001;56:37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- 22.Tervo S., Kivïpelto M., Hannïnen T., et al. Incidence and risk factors for mild cognitive impairment: a population-based 3-year follow-up study of cognitively healthy elderly subjects. Dement Geriatr Cogn Disord. 2004;17:196–203. doi: 10.1159/000076356. [DOI] [PubMed] [Google Scholar]
- 23.Lopez OL., Jagust WJ., DeKosky ST., et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study. Part 1. Arch Neurol. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 24.Qiu CJ., Tang MN., Zhang W., et al. The prevalence of mild cognitive impairment among residents aged 55 or over in Chengdu area. Zhonghua Liu Xing Bing Xue Za Zhi. 2003;12:1104–1107. [PubMed] [Google Scholar]
- 25.Richards M., Touchon J., Ledésert B., Ritchie K. Cognitive decline in ageing: are AAMI and AACD distinct entities? Int J Geriatr Psychiatry. 1999;14:534–540. doi: 10.1002/(sici)1099-1166(199907)14:7<534::aid-gps963>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 26.Winblad B., Palmer K., Kivïpelto M., et al. Mild cognitive impairment: beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 27.Yesavage JA., O'Hara R., Kraemer H., et al. Modeling the prevalence and incidence of Alzheimer's disease and mild cognitive impairment. J Psychiatr Res. 2002;36:281–286. doi: 10.1016/s0022-3956(02)00020-1. [DOI] [PubMed] [Google Scholar]
- 28.Krasuki JS., Alexander GE., Horwitz B., et al. Volumes of medial temporal lobe structures in patients with Alzheimer's disease and mild cognitive impairment (and in healthy controls). Biol Psychiatry. 1998;43:60–68. doi: 10.1016/s0006-3223(97)00013-9. [DOI] [PubMed] [Google Scholar]
- 29.Artero S., Tiemeier H., Prins ND., Sabatier R., Breteler MMB., Ritchie K. Neuroanatomical localization and clinical correlates of white matter lesions in the elderly. J Neurol Neurosurg Psychiatry. 2004;75:1304–1308. doi: 10.1136/jnnp.2003.023713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez OL., Jagust WJ., Dulberg C., et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study. Part 2. Arch Neurol. 2003;60:1394–1399. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- 31.Forsell Y., Palmer K., Fratiglioni L. Psychiatric symptoms/syndromes in elderly persons with mild cognitive impairment. Data from a cross-sectional study. Acta Neurol Scand Suppl. 2003;179:25–28. doi: 10.1034/j.1600-0404.107.s179.4.x. [DOI] [PubMed] [Google Scholar]
- 32.Gauthier S. Pharmacotherapy of mild cognitive impairment. Dialogues Clin Neurosci. 2004;6:391–395. doi: 10.31887/DCNS.2004.6.4/sgauthier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Artero S., Tierney MC., Touchon J., Ritchie K. Prediction of transition from cognitive impairment to senile dementia: a prospective longitudinal study. Acta Psychiatr Scand. 2003;107:390–393. doi: 10.1034/j.1600-0447.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 34.Flicker G., Ferris SH., Serby M. Hypersensitivity to scopolamine in the elderly. Psychopharmacology. 1992;107:437–441. doi: 10.1007/BF02245172. [DOI] [PubMed] [Google Scholar]
- 35.Feinberg M. The problems of anticholinergic adverse effects in older patients. Drugs Aging. 1993;3:335–348. doi: 10.2165/00002512-199303040-00004. [DOI] [PubMed] [Google Scholar]