Abstract
Early diagnosis of Alzheimer s disease (AD) is relevant in order to initiate symptomatic treatment with antidementia drugs. This will be of greater significance if the drugs aimed at slowing down the degenerative process (secondary prevention) prove to affect AD pathology and are clinically effective, such as γ-secretase inhibitors. However, there is currently no clinical assessment to differentiate the patients with mild cognitive impairment (MCI) who will progress to AD from those with a benign form of memory impairment that is part of the normal aging process. Thus, there is great clinical need for diagnostic and predictive biomarkers, as well as biomarkers for classification purposes, to identify incipient AD in MCI subjects. The most promising cerebrospinal fluid (CSF) biomarker candidates are total tau protein (T-tau), phosphorylated tau protein (P-tau), and the 42-andno acid form offi-amyloid (Aβ42), which may, if used in the right clinical context, prove to have sufficient diagnostic accuracy and predictive power to resolve this diagnostic challenge.
Keywords: Alzheimer's disease, β-amyloid, tau, phosphorylated tau, biochemical marker, cerebrospinal fluid, diagnosis
Abstract
El diagnóstico precoz de la enfermedad de Alzheimer (EA) es primordial para iniciar un tratandento sintomático con fármacos antidemencia. Esto será de mayor significación si los fármacos orientados a retrasar el proceso degenerativo (prevención secundaria) demuestran que afectan la patología de la EA y son clínicamente eficaces, como los inhibidores de la γ-secretasa. Sin embargo, actualmente no hay evaluaciones clínicas que permitan diferenciar los pacientes con deterioro cognitivo leve (DCL) que evolucionarán hacia una EA de aquéllos con una forma benigna de deterioro de memoria que es parte del proceso de envejecimiento normal. Por lo tanto, existe una gran necesidad clínica de contar con biomarcadores diagnósticos y predictores, como también bio-marcadores con fines de clasificación, para identificar una EA incipiente en sujetos con DCL. Los biomarcadores candidatos del líquido céfalo-raquí-deo más prometedores son la proteína tau total (T-tau), la proteína tau fosforilada (P-tau) y la forma del [3-andloide de 42 andnoácidos (A/342), los cuales pueden probar que tienen una suficiente precisión diagnóstica y un alto poder predictivo para resolver este desafío diagnóstico si se utilizan en un adecuado contexto clínico.
Abstract
Un diagnostic précoce de la maladie d'Alzheimer (MA) est pertinent pour débuter un traitement symptomatique avec des médicaments contre la démence. Cette démarche sera d'autant plus intéressante que ces médicaments destines a ralentir le processus dégénératif (prévention secondaire) prouvent qu'ils agissent sur la MA et sont clinique-ment efficaces, tels les inhibiteurs de la γ-secretase. Cependant, il n'y a actuellement aucune évaluation clinique pour différencier les patients présentant un déficit cognitif léger (Mild Cognitive Impairment, MCI) qui développeront une MA de ceux présentant une forme bénigne de troubles de la mémoire en rapport avec le processus de vieillissement normal. II existe done un grand besoin clinique au niveau des marqueurs biologiques diagnostiques et prévisionnels, ainsi que des marqueurs biologiques pour des besoins de classification et pour identifier une MA débutante chez des sujets présentant un MCI. Les candidats les plus prometteurs par mi les marqueurs biologiques du liquide cerebrospinal sont la protéine tau totale (T-tau), la protéine tau phosphorylee (P-tau) et la forme de la protéine β -amyloïde comportant 42 acides andnes (Aβ 42), qui pourraient, utilises dans un contexte clinique adapte, montrer une exactitude diagnostique et un pouvoir prévisionnel suffisants pour résoudre ce defi.
AIzheimer's disease (AD) Is the most common form of dementia. Although there are rare cases with fandlial (autosomal dominant) forms of AD, the majority of patients have the sporadic form of the disease.1 Neuropathologically, AD is characterized by degeneration of neurons and their synapses, and the presence of extensive amounts of senile plaques and neurofibrillary tangles.2
Due to the Increase In longevity, the prevalence of AD will rise dramatically within the next few decades so that an estimated 20 to 30 million people In the USA will be living with AD by the year 2030.3 The degenerative process probably starts 20 to 30 years before the clinical onset of AD.4 After the preclinical phase of the disease, the first symptoms generally affect episodic memory This first clinical phase of AD without overt dementia Is referred to as the mild cognitive Impairment (MCI) phase of AD.5
A diagnosis of MCI is based on memory disturbances measures adjusted for age and education.6 However, MCI Is an etiologlcally heterogeneous disorder. Although many patients with MCI have Incipient AD, others have a benign form of MCI as part of the normal aging process. The conversion rate of MCI to AD with dementia has been reported to be as high as 15% per year.5 Moreover, other types of pathology, such as cerebrovascular disease, may contribute to the memory impairment In MCI cases. Even if cerebrovascular comorbidity can be suspected by means of medical history (eg, presence of risk factors such as hypertension), clinical exandnation (eg, focal neurological symptoms), or brain Imaging (eg, findings of Infarcts or white-matter lesions on computed tomography [CT] or magnetic resonance tomography [MRT]), It may be difficult to correctly diagnose the underlying cause of MCI. Therefore, research efforts have focused on methods to identify incipient AD In MCI subjects.
In this review, we present the rationale for the development of cerebrospinal fluid (CSF) biomarkers of AD and we discuss the potential of CSF biomarkers for the diagnosis of MCI.
Criteria and evaluation of biomarkers
Criteria for a useful biomarker have been proposed by an International consensus group on molecular and biochemical markers of AD.7 According to these guidelines, a biomarker for AD should detect a manifestation of the fundamental neuropathology and be validated in neuropathologically confirmed cases. Its sensitivity for detecting AD should exceed 85% and Its specificity In differentiating between AD and other dementias should be at least 75%. Ideally, a biomarker test should also be reliable, reproducible, noninvasive, simple to perform, and Inexpensive. One aspect of the test of particular Interest to patients and clinicians Is Its ability to detect the disease at the earliest possible stage. To date, this has been the weakness of neuropsychological techniques in patients In the earliest clinical and even In the presymptomatic phase of AD. Theoretically, an Ideal diagnostic biomarker of AD might be expected to show limited correlation with cognitive performance, as the test should be abnormal In patients who have few or no signs of cognitive deterioration. Conversely, an Ideal prognostic biomarker might be expected to show a significant correlation with cognitive performance (or future cognitive performance), as the test should be excessively abnormal in patients who have a rapidly deteriorating course. Thus, It Is possible that different types of biomarkers will be useful In different clinical situations.
A number of steps are required before a biomarker becomes an asset to clinicians who treat patients with AD. First, the technical feasibility of the new marker has to be established, Including the availability of a validated assay with high precision and reliability of measurement and well-descrlbed reagents and standards. A large range of potential markers have successfully passed this first step. Second, the possible marker has to be evaluated In a relatively pure sample of diseased and comparison groups. This is akin to the phase 2 trial In therapeutics, but the goal here Is to make an initial assessment of Its maximum sensitivity and specificity Few potential markers have passed this step so far. Next, the new marker has to be studied In a more representative population-based sample, providing an assessment of its true diagnostic properties and hence demonstrating Its clinical usefulness. This step also serves as a basis for cost-effectiveness analyses, which are Important because every new marker has to be evaluated in the context of the already available set of diagnostic and therapeutic options and the socioeconomic resources of the health system. At present, there are several multicenter Initiatives with the scope to evaluate new biomarkers In a population-based design; one important example Is the Working Group on Biological Measures as part of the National Institute on Aging (NIA) Alzheimer's Neuroimaging Initiative.8
Diagnostic biomarkers for AD and MCI
The availability of effective symptomatic treatment of AD with cholinesterase Inhibitors has highlighted the Importance of early and accurate diagnosis of AD among clinicians. The awareness In the population of the possibilities for drug treatment has also made patients seek medical advice very early In the course of the disease. In the MCI phase, the characteristic clinical picture of AD has not yet developed, and there Is no clinical method to determine which MCI subjects will progress to AD except with follow-up visits. Thus, there is a great need for diagnostic Instruments to Identify Incipient AD In MCI cases. This need will grow as new disease-modifying drugs become available, such as β-and γ-secretase Inhibitors or pamyloid (Aβ) vaccination. Such compounds will probably be most effective in the earlier stages of the disease before neurodegeneration is too severe and widespread.
The neurochemistry of AD
Aβ and senile plaques
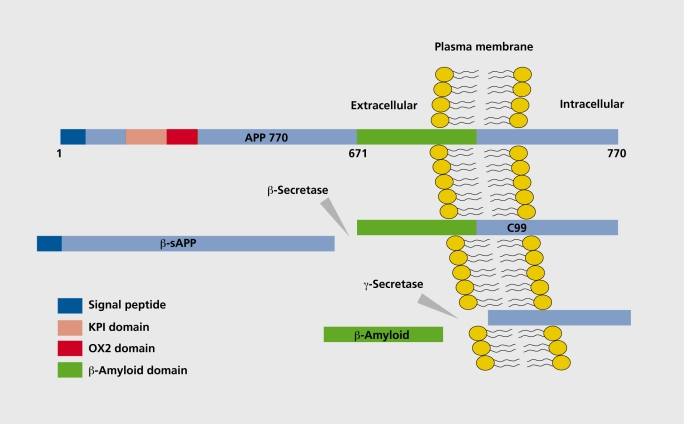
A major breakthrough In AD research was the identification of Aβ as the main protein constituent of plaques.9 Aβ Is generated by proteolytic cleavage of Its precursor, the amyloid precursor protein (APP).10 APP is a single membrane-spanning protein with a large ectodomaln and a smaller cytoplasmic tail,11 a schematic drawing of APP is given in Figure 1
Figure 1. Schematic drawing of the generation of β-amyloid (Aβ) from its precursor, amyloid precursor protein (APP). APP is a transmembrane protein with a large extracellular N-terminal domain and a smaller intracellular tail. The Aβ domain is partly embedded in the plasma membrane. In the amyloidogenic pathway, APP is first cleaved by β-secretase, resulting in the release of β-secretase-cleaved soluble APP (β-sAPP). In the second step, the 99-amino acid C-terminal fragment (CTF, C99) of APP is cleaved by the γ-secretase-complex-releasing free Aβ. KPI, Kunitz protease inhibitor; 0X2, 0X2 antigen.
In the first step, Aβ is produced by cleavage of APP after position 671 by a protease referred to as psecretase (Figure I), which has been identified as p-site APP-cleaving enzyme (BACE).12ThIs cleavage results In the release of a large N-termlnal derivative called β-secretase-cleaved soluble APP (psAPP) (Figure 1). In the second step, the 99-andno acid C-terminal fragment (CTF) of APP (C99) Is cleaved by the γ-secretase-complex-releasing free Aβ (Figure I). Recent studies have shown that the presenillns constitute the catalytic subunit of the γ-secretase.13 The membrane proteins nicastrln, APHla, APHlb, and PEN2 regulate the γ-secretase cleavage,14-16 and together with presenllin form a functional complex, the γ-secretase complex, responsible for cleavage of the APP CTF Another breakthrough was the discovery that there are several C-terminal forms of Aβ, and that the longer form ending at Ala-42 (Aβ42) was found to aggregate more rapidly than the shorter Aβ40 form, and to be the Initial and predominant Aβ form deposited in plaques.17-20
Tau and neurofibrillary tangles
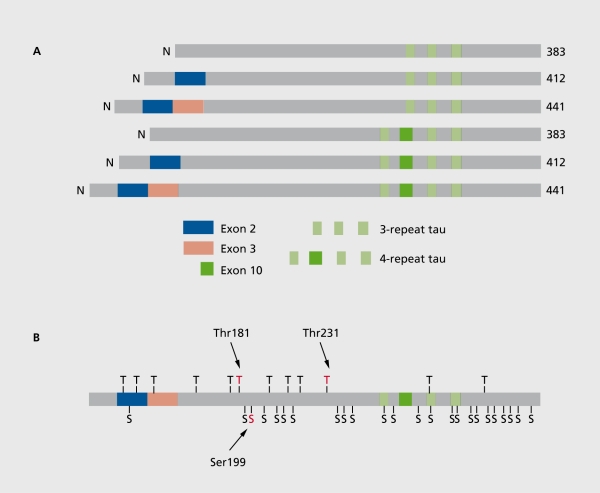
Tau protein is a microtubule-associated protein located In the neuronal axons. Due to alternative splicing of tau mRNA, there are 6 isoforms ranging in size from 352 to 441 andno acids, with molecular weights ranging from 50 to 65 kDa (Figure 2).21-24 Tau binds to tubulin In the axonal micro-tubules, thereby promoting microtubule assembly and stabillty21 Tau protein has more than 30 phosphorylation sites,21 either threonine or serine (Figure 2b), In AD, an abnormally hyperphosphorylated form of tau Is the principal component of the paired helical filaments (PHFs), which make up the neurofibrillary tangles, neuropil threads, and senile plaque neuritis.25 Due to the hyperphosphorylatlon, tau loses Its ability to bind to the microtubules and to stimulate their assembly, and also gets a tendency to aggregate.26
Figure 2. A. Schematic drawing of the six isoforms of tau protein. Alternatively spliced exons are marked. At the top, the smallest tau isoforms containing 352 andno acids, with three repeat (microtubule-binding) domains. Below the other two three-repeat tau isoforms with exon 2 and exons 2 and 3 spliced in. The lower three tau isoforms contain four repeat domains. B. Schematic drawing of the largest tau isoform (tau 441), with phosphorylation sites, either threonine (T) or serine (S), marked. Phosphorylated epitopes used in the ELISA (enzyme-linked immunosorbent assay) methods for quantification of phosphorylated tau (P-tau) in cerebrospinal fluid (CSF) are marked, including threonine 181 ,22 serine 199,23 and threonine 231. 24 .
Aβ and tau in CSF as biomarkers for AD
The biochemical changes In the brain are reflected In CSF, and so CSF is an obvious source In the search for biomarkers for AD. There are two methods to search for CSF biomarkers: the candidate biomarker approach and the proteomlc approach.
The candidate biomarker approach is based on the neurochemlstry of the central pathogenic processes In AD. Candidate biomarkers relate to proteins reflecting the neuronal degeneration, the metabolism and aggregation of Aβ, as well as the hyperphosphorylatlon of tau protein.
-
The proteomic approach Is based on the identification of biomarkers that can differentiate AD from controls and other brain disorders, regardless of whether they are directly linked to the primary steps in AD pathogenesis. Proteomic methods Include two-dimensIonal electrophoresis, protein chips, or liquid chromatography combined with mass spectrometry.27
Using the candidate biomarker approach, the three CSF biomarkers, total tau protein (T-tau), Aβ42, and various phosphorylated tau protein (P-tau) epitopes have been exandned in numerous studies, and have been found to have high diagnostic potential.
Aβ42 isoform
The first studies on CSF total Aβ used ELISA (enzyme-linked Immunosorbent assay) methods that did not discriminate between different Aβ Isoforms. Although some studies found a slight decrease in total CSF Aβ in AD,28-30 other studies found no change.31-33
These negative results provided the conceptual basis for the development of ELISA methods specific for Aβ42.31,34 A large number of studies have evaluated the diagnostic potential for the most commonly used method for Aβ42,34,35 finding a sensitivity >85% and a specificity of 90% for discriminating between AD and normal aging.36
The CSF level of Aβ42 Is decreased In AD to about 40% to 50% of control levels.36 The reason for this decrease Is not clear. One explanation Is that Aβ42 is deposited In plaques, with lower amounts of Aβ being free to diffuse into CSF32 This explanation Is supported by the finding of a strong correlation between low Aβ42 In ventricular CSF and higher numbers of plaques In the neocortex and hippocampus.37 Subsequent studies also found, however, a marked reduction in CSF Aβ42 In disorders without pA plaques, such as Creutzfeldt-Jakob disease (CJD),38 amyotrophic lateral sclerosis,39 and multiple systern atrophy.40 These findings question the notion of a direct reflection of senile plaque formation by Aβ1-42.
CSF Aβ1-42 in tie differential diagnosis of AD and other neurodegenerative disorders
The potential of CSF Aβ1-42 to distinguish AD from other dementias and neurological disorders has been documented In a number of independent studies. Compared with nonAD dementias, a slight decrease has been found In AD.41 Normal levels32 or decreased levels42 were found In Parkinson's disease (PD). In Lewy body dementia (LBD), a disorder also characterized by the presence of senile plaques, low levels have also been detected, similar to AD.43-46 In addition, low CSF Aβi-42 is found in a relatively large percentage of patients with frontotemporal dementia (FTD) and vascular dementia (VD).47,48 In summary, CSF Aβf-42 does not seem to significantly support the differential diagnosis of AD.
Predictive value of CSF Aβ1-42 in MCI for AD
It has been hypothesized that a decrease In CSF Aβ1-42 might Indicate an early stage of AD and be detectable before clinical symptoms of dementia become overt. One study found a significant decrease In CSF Aβ1-42 In MCI subjects compared with controls.43 In another study In MCI patients who eventually developed AD, however, Aβ1-42 levels did not differ significantly from age-matched normal controls.49 We found Aβ1-42 to be an Indicator of early Identification of AD In MCI subjects taking potential confounding factors Into account, such as age, severity of cognitive decline, time of observatlon, apolipoprotein E ε4 (APOE ε4) carrier status, and gender.50
Other Aβ isoforms
In contrast to the reduction in CSF Aβ42, there Is no change in CSF Aβ40 In AD, resulting In a marked decrease in the ratio of CSF Aβ42/Aβ40.51-55 The reduction In the CSF Aβ42/Aβ40 ratio may be more pronounced than the reduction in CSF Aβ42.52-54 Further studies will show whether the CSF Aβ42/Aβ40 ratio has a larger diagnostic potential than CSF Aβ42 alone.
Studies using mass spectrometry,56 urea-based SDSPAGE (sodium dodecylsulfate polyacrylamide gel electrophoresis), Western immunoblot,57 and surface-enhanced laser desorption/ionisation time-of-flight mass spectrometry (SELDITOF)58 have found that there Is a heterogeneous set of Aβ peptides In CSF. Preliminary data show that Increased CSF levels of both Aβ1-40 and Aβ1-38 together with a decrease In Aβ1-42 are found In AD.57 Further studies are needed to examine the diagnostic potential of these Aβ species.
Total tau protein
After the first report on T-tau In CSF using an ELISA method with a polyclonal reporter antibody,59 an ELISA method based on monoclonal antibodies detecting all Isoforms of tau independent of phosphorylation state of tau was developed.60,61 A large number of studies have evaluated the diagnostic potential for the most commonly used method for T-tau,60 finding a sensitivity above 80% and a specificity of 90% discriminate between AD and normal aging.36
T-tau in the differentiation between AD and normal aging
T-tau has been Intensely studied In more than 2000 AD patients and 1000 age-matched elderly controls over the last 5 to 10 years.23,32,41,43,44,47,52,53,59-82 The most consistent finding is a statistically significant increase In CSF T-tau In AD. The mean level of CSF T-tau concentration in AD compared with elderly controls approaches 300%. Across the reviewed studies, sensitivity and specificity levels varied with the differently employed control groups, statistical methods, and reference values. Specificity levels were determined between 65% and 86% and sensitivity between 40% and 86%. 83 In several studies, a significant elevation was even found In patients with early dementia.63,70,81 Therefore, In mild dementia, the potential of CSF T-tau to discriminate between AD and normal aging Is high, with a mean sensitivity of 75% and specificity of 85%.
An age-associated Increase In T-tau has been shown In nondemented subjects.73,84 Therefore, the effect of age should be considered when T-tau levels are diagnostically employed. Age-dependent reference values for normal T-tau have already been established: ages between 21 and 50 years at <300 pg/mL; ages between 51 to 70 years at <450 pg/mL; and ages between 70 and 93 years at <500 pg/mL.85
T-tau in the differentiation between AD and MD
Geriatric major depression (MD) Is an important psychiatric differential diagnosis of AD, as psychopathological symptoms considerably overlap and often only a follow-up assessment allows clear clinical differentiation between both underlying entities. Subgrouping a sample of AD patients, healthy controls (HCs), and patients with MD according to age resulted In a correct classification rate of 94.5% In the “young old” subjects (<70 years of age) compared with only 68.4% In the “old old” (70 years of age). This report supports the notion that elevated CSF T-tau Is highly Indicative of a neurodegenerative process particularly in subjects younger than 70 years of age.73
T-tau in the differential diagnosis of AD and other neurodegenerative disorders
The potential of CSF T-tau, however, Is limited In its ability to discriminate AD from other relevant dementia disorders. At a sensitivity level of 81%, CSF T-tau reached a specificity level of only 57% for distinguishing AD from other dementias.45,47
Due to these inconsistent findings caused by Incomplete or lacking control and comparison groups, and low specificity levels of T-tau In the differentiation of AD from other primary dementias, the value of T-tau In the differential diagnosis was long Inconclusive. Therefore, T-tau has not been suggested as a marker for the differential diagnosis of AD.T-tau rather reflects unspecific processes of axonal damage and neuronal degeneration. This notion is further supported by the Increase in CSF T-tau In disorders with extensive and/or rapid neuronal degeneration, such as CJD.86,87 A highly significant Increase of 580% was documented in CJD compared to AD patients. At a cutoff level of 2130 pg/mL, T-tau yielded a sensitivity of 93% and a specificity of 100% between AD and CJD.88 An elevation of CSF T-tau, correlating with clinical severity, has been shown in normal pressure hydrocephalus.89 Moreover, a marked transient increase In CSF T-tau has been demonstrated after acute stroke. The transient Increase In CSF T-tau correlated with the infarct size measured by cranial OF.90 Elevated levels of CSF T-tau have been found In patients with diffuse axonal damage after traumatic brain Injury, which decrease with clinical Improvement.91 In contrast, In neurological disorders that are mainly linked to more restricted cerebral locations and number of cells, such as alcoholic dementia, PD, progressive supranuclear palsy, and cortlcobasal degeneration, elevated CSF T-tau concentrations have been only occasionally reported48,60,68,76,92,93 or were normal.77
Predictive value of CSF T-tau in MCI for AD
In patients suffering from MCI who converted to AD during follow-up, elevated T-tau levels were found In relatively few samples at baseline.43,66 Memory-Impaired subjects who later progressed to manifest AD could be discriminated by high CSF T-tau from those who did not progress with 90% sensitivity and 100% specificity.66 Longitudinally, elevated CSF levels of T-tau were found In MCI subjects and remained elevated after conversion to clinical AD.49 Another study showed that 88% of patients with MCI had elevated T-tau concentrations and/or low CSF Aβ1-42 levels at baseline.94 Thus, elevated CSF T-tau In MCI may have the potential to predict AD.
Phosphorylated tau protein
In order to Improve specificity of measurement of tau protein as a biomarker of AD, assays have been developed to specifically detect phosphorylated tau protein (P-tau) In CSF. These assays use monoclonal antibodies specific for phosphorylated epitopes of tau: tau protein phosphorylated at serine 199 (P-tau199), threonine 231 and serine 235 (P-tau231-235),23 threonine 231 (P-tau231),24 threonine 181 (P-tau181),22,95 and serine 396 and serine 404 (P-tau396/404).96
A marked Increase In the CSF level of P-tau Is found In AD.83 This Increase probably reflects the phosphorylation state of tau, and thus possibly also the formation of tangles In AD. Indirect evidence for this comes from studies showing no change In CSF P-tau after acute stroke In contrast to T-tau,90 and normal CSF P-tau levels In CJD, despite a very marked increase In T-tau.97 These studies suggest that CSF P-tau Is not simply a marker for neuronal damage.
A clear Increase in CSF P-tau In AD has been found using all these ELISA methods, with a sensitivity of 80% and a specificity above 90% to discriminate between AD and normal aging.36 Interestingly, a normal CSF level of P-tau is not only found In psychiatric disorders such as depression98 and chronic neurological disorders such as PD, but also In other dementia disorders, such as VD, FTD, and LBD.36 Thus, the specificity of CSF P-tau for differentiating AD from other dementias seems to be higher than for T-tau and Aβ42.
P-tau in the differential diagnosis of AD
CSF tau protein phosphorylated at threonine 231 (P-tau231)
Immunohistochemical studies Indicate that phosphorylation of tau protein at threonine 231 (P-tau231) appears early In pathogenesis, even before PHF formation.99 The first study of P-tau231 In CSF showed a discrimination between AD patients and nondemented controls with other neurological disorders with 85% sensitivity and 97% specificity (overall accuracy of 91 %).24 In an Independent sample of 192 subjects, CSF levels of P-tau231 discriminated with a sensitivity of 90.2% and a specificity of 80% between AD and all other non AD subjects. In particular, at a specificity level of 92.3% for P-tau231 and T-tau, sensitivity levels between AD and FTD were raised using P-tau231, in comparison to T-tau, from 57.7% to 90.2%. 10° In summary, P-tau231 may be a valuable biomarker, especially In the differential diagnosis between AD and FTD.
CSF tau protein phosphorylated at threonine 181 (P-tau181)
The discriminative power of CSF P-tau181 has been investlgated in a number of studies with various types of dementia. Results showed a significant Increase In CSF P-tau181 concentrations In AD compared with FTD and controls.22 Focusing on the differentiation between AD and LBD, specificity at a given sensitivity level was Improved by using P-tau181 compared withT-tau.45,95 Comparing receiver operator characteristic (ROC) curves led to a correct classification for cases with AD and LBD of more than 80%.
In a study with 101 subjects comparing P-tau181 and T-tau In various diagnostic subgroups, P-tau181 was Increased In patients with probable and possible AD compared with VD and dementia in PD.84 Compared with FTD, PD, VD, and normal aging, both P-tau181 and T-tau proteins were Increased In probable AD. In possible AD, P-tau181 was Increased compared with FTD and VD. Recently, data on a group of 51 AD patients (25 probable, 18 possible, and 8 incipient AD cases) compared with 16 probable VD cases and 10 HCs became available.101 All AD cases were drug-naive. AD and VD, as well as HCs, were distinguished using P-tau181, whereas VD compared to HCs showed no statistically significant differences In concentration. Among the whole group of AD patients and controls, P-tau181 demonstrated 71% sensitivity and 94% specificity compared with T-tau with 63% sensitivity and 100% specificity. Taken together, diagnostic accuracy was better for P-tau181 (78%) than for T-tau (71%). In summary, P-tau181 may be a valuable biomarker, especially In the differential diagnosis between AD, LBD, and VD.
CSF tau protein phosphorylated at serine 199 (P-tau199)
In one study applying P-tau199, this biomarker was shown to be superior to T-tau protein in separating AD from a patient group of non AD subjects.23 In a large multlcenter sample of 570 subjects,102 P-tau199 protein levels were elevated In the AD group, Independently of age, gender, cognitive status, and APOE e4 carrier status. In the AD group versus the combined groups of demented and non- demented subjects In this study, ROC analysis showed a 85% sensitivity and 85% specificity for P-tau199.102
CSF tau protein phosphorylated at serine 396 and serine 404 (P-tau396/404)
An ultrasensitive bienzyme-substrate-recycle ELISA for Ser 396 and Ser 404 has been developed, which Is slgnifIcantly more sensitive than conventional ELISA In determining the hyperphosphorylated tau protein and T-tau.96 In CSF of 52 AD patients, 56 normal controls, 46 VD patients, and 37 nonAD neurological patients, significantly elevated levels of P-tau396/404 were only found In AD. Using the ratio of hyperphosphorylated tau protein to T-tau of ≥0.33 as a cutoff for AD diagnosis, the clinical diagnosis could be confirmed In 96% of the clinically diagnosed patients. The results of this study suggest that P-tau396/404 Is a promising marker, especially in the differential diagnosis between AD and VD.
Measurement of P-tau epitopes in the differential diagnosis of AD: a comparative CSF study
A recent study directly compared the diagnostic performance of P-tau231, P-tau181, and P-tau199 In the same patient cohort, including a large series of patients with AD, LBD, FTD, VD, and other neurological disorders. The P-tau231 and P-tau181 assays performed nearly equally well In the discrimination of AD from nondemented controls, whereas the P-tau199 assay showed a weaker discriminatlon.103 Interestingly, the separation between AD and FTD was maximized using P-tau231. The separation between AD and DLB was maximized using P-tau181.103 Thus, differences In the phosphorylation of specific tau epitopes between dementia disorders may be reflected In the CSF level of the corresponding P-tau variant.
Predictive value of CSF P-tau in MCI for AD
In a longitudinal study, 77 MCI patients showed elevated levels for P-tau231 In comparison to HCs at baseline.104 High CSF P-tau231, but not T-tau levels at baseline, significantly correlated with subsequent cognitive decline. This study suggests that high P-tau231 may be a predictor for progressive cognitive decline In subjects with MCI.104 One study focused on P-tau231-235 In MCI subjects who converted to AD compared to individuals with subjective memory complaints without cognitive impairment. Results showed significantly higher T-tau and P-tau231-235 levels In the MCI group. P-tau231-235 yielded a specificity level of 100% and a sensitivity level of 65% for differentlating MCI subjects who eventually developed AD.105 In a recent study on 44 MCI subjects who later progressed to AD, P-tau181 was found to differentiate MCI from controls with 70% sensitivity and 90% specificity.106 Although further longitudinal studies are needed, these data suggest that CSF markers are positive very early In the disease process In AD, and may be of clinical value to differentiate MCI subjects with Incipient AD.
Conclusion
In conclusion, the Immunoassays detecting P-tau protein, T-tau protein, and Aβ proteins promise improvements in the early and accurate diagnosis of Incipient AD. Beyond early diagnosis, It Is hoped that markers of prognosis will enable clinicians to monitor whether new candidate treatments of AD are working effectively and Inexpensively. With this In mind, the NIA commissioned a working group on biomarkers as part of Its Alzheimer's Neurolmaging Initiative.8 The Working Group on Biological Measures suggested tau proteins as well as Aβ proteins as feasible core markers suitable for a multiceter, longitudinal study of AD, with special consideration given to MCI. In addition, the NIA also established other working groups, one each for magnetic resonance imaging (MRI, volumetric), positron emission tomography and single photon emission computed tomography (PET and SPECT), and subjects and protocol design. The accuracy of any diagnostic test In AD is likely to be Increased by the cumulative information from clinical and neuropsy-etiological exandnation, and brain Imaging.107,108 Further work Is required here, particularly In relation to CSF P-tau. The proceedings of such working groups in Europe and the USA will greatly assist Individual clinicians and health service providers In deciding which specific diagnostic tests should be standard practice in the assessment of Incipient AD in MCI subjects.
Selected abbreviations and acronyms
- AD
Alzheimer s disease
- APP
amyloid precursor protein
- Aβ
β-amyloid
- CJD
Creutzfeldt-Jakob disease
- FTD
frontotemporal dementia
- HC
healthy control
- LBD
Lewy body dementia
- MCI
mild cognitive impairment
- PD
Parkinson's disease
- PHF
paired helical filament
- P-tau
phosphorylated tail protein
- T-tau
total tau protein
- VD
vascular dementia
REFERENCES
- 1.Blennow K., Skoog I. Genetic testing for Alzheimer's disease: how close is reality? CurrOpin Psychiatry. 1999;12:487–493. [Google Scholar]
- 2.Tomlinson BE., Blessed G., Roth M. Observations on brains of demented old people. J Neurol Sci. 1970;11:205–242. doi: 10.1016/0022-510x(70)90063-8. [DOI] [PubMed] [Google Scholar]
- 3.Fratiglioni L., De Ronchi D., Aguero-Torres H. Worldwide prevalence and incidence of dementia. Drugs Aging. 1999;15:365–375. doi: 10.2165/00002512-199915050-00004. [DOI] [PubMed] [Google Scholar]
- 4.Davïes L., Wolska B., Hilbïch C., et al. A4 amyloid protein deposition and the diagnosis of Alzheimer's disease: prevalence in aged brains determined by immunocytochemistry compared with conventional neuropathologic techniques. Neurology. 1988;38:1688–1693. doi: 10.1212/wnl.38.11.1688. [DOI] [PubMed] [Google Scholar]
- 5.DeCarli C. Mild cognitive impairment: prevalence, prognosis, aetiology and treatment. Lancet Neurol. 2003;2:15–21. doi: 10.1016/s1474-4422(03)00262-x. [DOI] [PubMed] [Google Scholar]
- 6.Petersen RC., Smith GE., Waring SC., Ivnik RJ., Tangalos EG., Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 7.The Ronald and Nancy Reagan Research Institute of the Alzheimer's Association and the National Institute on Aging Working Group. Consensus Report of the Working Group. Molecular and Biochemical Markers of Alzheimer's Disease. Neurobiol Aging. 1998;19:109–116. [PubMed] [Google Scholar]
- 8.Frank RA., Galasko D., Hampel H., et al. Biological markers for therapeutic trials in Alzheimer's disease. Proceedings of the biological markers working group; NIA initiative on neuroimaging in Alzheimer's disease. Review. Neurobiol Aging. 2003;24:521–536. doi: 10.1016/s0197-4580(03)00002-2. [DOI] [PubMed] [Google Scholar]
- 9.Masters CL. Amyloid plaque core protein in Alzheimer's disease and Down syndrome. Proc Natl Acad Sci U S A. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haass C., Selkoe DJ. Cellular processing of β-amyloid precursor protein and the genesis of amyloid p-peptide. Cell. 1993;75:1039–1042. doi: 10.1016/0092-8674(93)90312-e. [DOI] [PubMed] [Google Scholar]
- 11.Kang J., ternaire HG., Unterbeck A., et al. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 12.Vassar R., Bennet BD., Babu-Khan S., et al. β-Secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 13.Wolfe NS., Xia W., Ostaszewski BL., Diehl TS., Kimberly WT., Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- 14.Yu G., Nïshimura M., Arawaka S., et al. Nicastrin modulates presenilin mediated notch/glp-1 signal transduction and PAPP processing. Nature. 2000;407:48–54. doi: 10.1038/35024009. [DOI] [PubMed] [Google Scholar]
- 15.Francis R., McGrath G., Zhang J., et al. Aph-1 and pen-2 are required for Notch pathway signaling, γ-secretase cleavage of PAPP, and presenilin protein accumulation. Dev Cell. 2002;3:85–97. doi: 10.1016/s1534-5807(02)00189-2. [DOI] [PubMed] [Google Scholar]
- 16.Goutte C., Tsunozaki M., Hale VA., Priess JR. APH-1 is a multipass membrane protein essential for the Notch signaling pathway in. Caenorhabditis elegans embryos. Proc Natl Acad Sci U S A. 2002;99:775–779. doi: 10.1073/pnas.022523499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17, Miller DL, Papayannopoulos IA, Styles J, et al.Miller DL., Papayannopoulos IA., Styles J., et al Peptide composition of the cerebrovascular and senile plaque core amyloid deposits of Alzheimer's disease. Arch Biochem Biophys. 1993;301:41–52. doi: 10.1006/abbi.1993.1112. [DOI] [PubMed] [Google Scholar]
- 18.Jarrett JT., Berger EP., Lansbury PT. The carboxy terminus of the β-amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer's disease. Biochemistry. 1993;32:4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- 19.Iwatsubo T., Odaka A., Suzuki N., NMizusawa H., Nukîna N., Ihara Y. Visualization of Aβ42(43) and Aβ40 in senile plaques with end-specific Aβ monoclonals: evidence that an initially deposited species is Aβ42(43). Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 20.Tamaoka A., Kondo T., Odaka A., et al. Biochemical evidence for the long-tail form (Aβ 1-42/43) of amyloid p-protein as a seed molecule cerebral deposits of Alzheimer's disease. Biochem Biophys Res Commun. 1994;205:834–842. doi: 10.1006/bbrc.1994.2740. [DOI] [PubMed] [Google Scholar]
- 21.Buée L., Bussïere T., Buee-Scherrer V., Delacourte A., Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 22.Vanmechelen E., Vanderstichele H., Davidsson P., et al. Quantification of tau phosphorylated at threonine 181 in human cerebrospinal fluid: a sandwich ELISA with a synthetic phosphopeptide for standardization. Neurosci Lett. 2000;285:49–52. doi: 10.1016/s0304-3940(00)01036-3. [DOI] [PubMed] [Google Scholar]
- 23.Ishiguro K., Ohno H., Arai H., et al. Phosphorylated tau in human cerebrospinal fluid is a diagnostic marker for Alzheimer's disease. Neurosci Lett. 1999;270:91–94. doi: 10.1016/s0304-3940(99)00476-0. [DOI] [PubMed] [Google Scholar]
- 24.Kohnken R., Buerger K., Zinkowski R., et al. Detection of tau phosphorylated at threonine 231 in cerebrospinal fluid of Alzheimer's disease patients. Neurosci Lett. 2000;287:187–190. doi: 10.1016/s0304-3940(00)01178-2. [DOI] [PubMed] [Google Scholar]
- 25.Grundke-lqbal I., Iqbal K., Tung YC., Quinland M., Wisniewski HM., Binder LI. Abnormal phosphorylation of the microtubule-associated protein t (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iqbal K., Alonso AD., Gondal JA., et al. Mechanism of neurofibrillary degeneration and pharmacologic therapeutic approach. J Neural Transm. 2000;59(suppl):213–222. doi: 10.1007/978-3-7091-6781-6_22. [DOI] [PubMed] [Google Scholar]
- 27.Heine G., Zucht H-D., Schuhmann MU., et al. High-resolution peptide mapping of cerebrospinal fluid: a novel concept for diagnosis and research in central nervous system diseases. J Chromatogr B. 2002;782:353–361. doi: 10.1016/s1570-0232(02)00571-8. [DOI] [PubMed] [Google Scholar]
- 28.Van Nostrand WE., Wagner SL., Shankle WR., et al. Decreased levels of soluble amyloid p-protein precursor in cerebrospinal fluid of live Alzheimer disease patients. Proc Natl Acad Sci U S A. 1992;89:2551–2555. doi: 10.1073/pnas.89.7.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farlow M., Ghetti B., Benson MD., Farrow JS., van Nostrand WE., Wagner SL. Low cerebrospinal-fluid concentrations of soluble amyloid p-protein precursor in hereditary Alzheimer's disease. Lancet. 1992;340:453–454. doi: 10.1016/0140-6736(92)91771-y. [DOI] [PubMed] [Google Scholar]
- 30.Tabaton M., Nunzi MG., Xue R., Usiak M., Autilio-Gambetti L., Gambetti P. Soluble amyloid p-protein is a marker of Alzheimer amyloid in brain but not in cerebrospinal fluid. Biochem Biophys Res Commun. 1994;200:1598–1603. doi: 10.1006/bbrc.1994.1634. [DOI] [PubMed] [Google Scholar]
- 31.van Gool WA., Kuiper MA., Walstra GJ., Wolters EC., Bolhuis PA. Concentrations of amyloid p protein in cerebrospinal fluid of patients with Alzheimer's disease. Ann Neurol. 1995;37:277–279. doi: 10.1002/ana.410370221. [DOI] [PubMed] [Google Scholar]
- 32.Motter R., Vigo-Pelfrey C., Kholodenko D., et al. Reduction of β-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer's disease. Ann Neurol. 1995;38:643–648. doi: 10.1002/ana.410380413. [DOI] [PubMed] [Google Scholar]
- 33.Southwick PC., Yamagata SK., Echols CLJ., et al. Assessment of amyloid p protein in cerebrospinal fluid as an aid in the diagnosis of Alzheimer's disease. J Neurochem. 1996;66:259–265. doi: 10.1046/j.1471-4159.1996.66010259.x. [DOI] [PubMed] [Google Scholar]
- 34.Vanderstichele H., Blennow K., D'Heuvaert ND., et al. Development of a specific diagnostic test for measurement of β-amyloid(1-42) in CSF. In: Fisher A, Hanin I, Yoshida M, eds. Progress in Alzheimer's and Parkinson's Diseases. New York, NY: Plenum Press; 1998:773–778. [Google Scholar]
- 35.Andreasen N., Hesse C., Davidsson P., et al. Cerebrospinal fluid β-amyloid(1-42) in Alzheimer disease: differences between early- and late-onset Alzheimer disease and stability during the course of disease. Arch Neurol. 1999;56:673–680. doi: 10.1001/archneur.56.6.673. [DOI] [PubMed] [Google Scholar]
- 36.Blennow K., Hampel H. CSF markers for incipient Alzheimer's disease. Review. Lancet Neurol. 2003;2:605–613. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 37.Strozyk D., Blennow K., White LR., Launer LJ. CSF AP42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003;60:652–656. doi: 10.1212/01.wnl.0000046581.81650.d0. [DOI] [PubMed] [Google Scholar]
- 38.Otto M., Esselmann H., Schulz-Shaeffer W., et al. Decreased β-amyloid 1-42 in cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. Neurology. 2000;54:1099–1102. doi: 10.1212/wnl.54.5.1099. [DOI] [PubMed] [Google Scholar]
- 39.Sjoegren M., Davidsson P., Wallin A., et al. Decreased CSF p-amyloid42 in Alzheimer's disease and amyotrophic lateral sclerosis may reflect mismetabolism of β-amyloid induced by separate mechanisms. Dem Geriatr Cogn Disord. 2002;13:112–118. doi: 10.1159/000048642. [DOI] [PubMed] [Google Scholar]
- 40.Holmberg B., Johnels B., Blennow K., Rosengren L. Cerebrospinal fluid Aβ42 is reduced in multiple system atrophy but normal in Parkinson's disease and progressive supranuclear palsy. Mov Disord. 2003;18:186–190. doi: 10.1002/mds.10321. [DOI] [PubMed] [Google Scholar]
- 41.Galasko D., Chang L., Motter R., et al. High cerebrospinal fluid tau and low amyIoid-p-42 levels in the clinical diagnosis of Alzheimer disease and relation to apolipoprotein E genotype. Arch Neurol. 1998;55:937–945. doi: 10.1001/archneur.55.7.937. [DOI] [PubMed] [Google Scholar]
- 42.Ida N., Hartmann T., Pantel J., et al. Analysis of heterogeneous A4 peptides in human cerebrospinal fluid and blood by a newly developed sensitive Western blot assay. J Biol Chem. 1996;271:22908–22914. doi: 10.1074/jbc.271.37.22908. [DOI] [PubMed] [Google Scholar]
- 43.Andreasen N., Minthon L., Vanmechelen E., et al. Cerebrospinal fluid tau and A-p42 as predictors of development of Alzheimer's disease in patients with mild cognitive impairment. Neurosci Lett. 1999;273:5–8. doi: 10.1016/s0304-3940(99)00617-5. [DOI] [PubMed] [Google Scholar]
- 44.Kanemaru K., Kameda N., Yamanouchi H. Decreased CSF amyloid P42 and normal tau levels in dementia with Lewy bodies. Neurology. 2000;54:1875–1876. doi: 10.1212/wnl.54.9.1875. [DOI] [PubMed] [Google Scholar]
- 45.Parnetti L., Lanari A., Amid S., Gallai V., Vanmechelen E., Hulstaert F. CSF phosphorylated tau is a possible marker for discriminating Alzheimer's disease from dementia with Lewy bodies. Neurol Sci. 2001;22:77–78. doi: 10.1007/s100720170055. [DOI] [PubMed] [Google Scholar]
- 46.Vanmechelen E., Vanderstichele H., Hulstaert F., et al. Cerebrospinal fluid tau and β-amyloid (1-42) in dementia disorders. Mech Ageing Dev. 2001;122:2005–2011. doi: 10.1016/s0047-6374(01)00304-9. [DOI] [PubMed] [Google Scholar]
- 47.Hulstaert F., Blennow K., Ivanoiu A., et al. Improved discrimination of AD patients using β-amyloid (1-42) and tau levels in CSF. Neurology. 1999;52:1555–1562. doi: 10.1212/wnl.52.8.1555. [DOI] [PubMed] [Google Scholar]
- 48.Sjoegren M., Minthon L., Davidsson P., et al. CSF levels of tau, β-amyloid(142) and GAP-43 in frontotemporal dementia, other types of dementia and normal aging. J Neural Transm. 2000;107:563–579. doi: 10.1007/s007020070079. [DOI] [PubMed] [Google Scholar]
- 49.Maruyama M., Arai H., Sugita M., et al. Cerebrospinal fluid amyloid P(142) levels in the mild cognitive impairment stage of Alzheimer's disease. Exp Neurol. 2001;172:433–436. doi: 10.1006/exnr.2001.7814. [DOI] [PubMed] [Google Scholar]
- 50.Hampel H., Teïpel SJ., Fuchsberger T., et al. Value of CSF p-Amyloid1-42 and tau as predictors of Alzheimer's disease in patients with mild cognitive impairment. Mol Psychiatry. 2004;9:705–710. doi: 10.1038/sj.mp.4001473. [DOI] [PubMed] [Google Scholar]
- 51.Tamaoka A., Sawamura N., Fukushima T., et al. Amyloid p protein 42(43) in cerebrospinal fluid of patients with Alzheimer's disease. J Neurol Sci. 1997;148:41–45. doi: 10.1016/s0022-510x(96)00314-0. [DOI] [PubMed] [Google Scholar]
- 52.Kanai M., Matsubara E., Isoe K., et al. Longitudinal study of cerebrospinal fluid levels of tau, A-pi-40, and A-pi -42(43) in Alzheimer's disease: a study in Japan. Ann Neurol. 1998;44:17–26. doi: 10.1002/ana.410440108. [DOI] [PubMed] [Google Scholar]
- 53.Shoji M., Matsubara E., Kanai M., et al. Combination assay of CSF Tau, AP1-40, A-pi -42(43) as a biochemical marker of Alzheimer's disease. J Neurol Sci. 1998;158:134–140. doi: 10.1016/s0022-510x(98)00122-1. [DOI] [PubMed] [Google Scholar]
- 54.Fukuyama R., Mizuno T., Mori S., Nakajima K., Fushiki S., Yanagisawa K. Age-dependent change in the levels of AP40 and Aβ42 in cerebrospinal fluid from control subjects, and a decrease in the ratio of Aβ42 to AP40 level in cerebrospinal fluid from Alzheimer's disease patients. Eur Neurol. 2000:155–160. doi: 10.1159/000008156. [DOI] [PubMed] [Google Scholar]
- 55.Mehta PD., Pirttilà T., Mehta SP., Sersen EA., Aisen PS., Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid p proteins 1-40 and 1-42 in Alzheimer's disease. Arch Neurol. 2000;57:100–105. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- 56.Vigo-Pelfrey C., Lee D., Keim P., Lieberburg I., Schenk DB. Characterization of β-amyloid peptide from human cerebrospinal fluid. J Neurochem. 1993;61:1965–1968. doi: 10.1111/j.1471-4159.1993.tb09841.x. [DOI] [PubMed] [Google Scholar]
- 57.Wiltfang J., Esselmann H., Bibl M., et al. Highly conserved and disease-specific patterns of carboxyterminally truncated Aβ-peptides 1-37/38/39 in addition to 1-40/42 in Alzheimer's disease and in patients with chronic neuroïnflammatïon. J Neuroem. 2002;81:481–496. doi: 10.1046/j.1471-4159.2002.00818.x. [DOI] [PubMed] [Google Scholar]
- 58.Lewczuk P., Esselmann H., Meyer M., et al. The amyloid-p (AP) peptide pattern in cerebrospinal fluid in Alzheimer's disease: evidence of a novel carboxyterminally elongated Aβ-peptide. Rapid Commun Mass Spectrom. 2003;17:1291–1296. doi: 10.1002/rcm.1048. [DOI] [PubMed] [Google Scholar]
- 59.Vandermeeren M., Mercken M., Vanmechelen E., et al. Detection of tau proteins in normal and Alzheimer's disease cerebrospinal fluid with a sensitive sandwich enzyme-linked immunosorbent assay. J Neurochem. 1993;61:1828–1834. doi: 10.1111/j.1471-4159.1993.tb09823.x. [DOI] [PubMed] [Google Scholar]
- 60.Blennow K., Wallin A., Agren H., Spenger C., Siegfried J., Vanmechelen E. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol. 1995;26:231–245. doi: 10.1007/BF02815140. [DOI] [PubMed] [Google Scholar]
- 61.Vigo-Pelfrey C., Seubert P., Barbour R., et al. Elevation of microtubule associated protein tau in the cerebrospinal fluid of patients with Alzheimer's disease. Neurology. 1995;45:788–793. doi: 10.1212/wnl.45.4.788. [DOI] [PubMed] [Google Scholar]
- 62.Jensen M., Basun H., Lannfeldt L. Increased cerebrospinal fluid tau in patients with Alzheimer's disease. Neurosci Lett. 1995;186:189–191. doi: 10.1016/0304-3940(95)11297-a. [DOI] [PubMed] [Google Scholar]
- 63.Riemenschneïder M., Buch K., Schmolke M., Kurz A., Guder WG. Cerebrospinal protein tau is elevated in early Alzheimer's disease. Neurosci Lett. 1996;212:209–211. doi: 10.1016/0304-3940(96)12810-x. [DOI] [PubMed] [Google Scholar]
- 64.Roesler N., Wichart I., Jellinger KA. Total tau protein immunoreactivity in lumbar cerebrospinal fluid of patients with Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1996;60:237–238. doi: 10.1136/jnnp.60.2.237-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arai H., Morïkawa Y., Higuchi M., et al. Cerebrospinal fluid tau levels in neurodegenerative diseases with distinct tau-related pathology. Biochem Biophys Res Commun. 1997;236:261–264. doi: 10.1006/bbrc.1997.6908. [DOI] [PubMed] [Google Scholar]
- 66.Arai H., Nakagawa T., Kosaka Y., et al. Elevated cerebrospinal fluid tau protein level as a predictor of dementia in memory-impaired individuals. Alzheimer's Res. 1997;3:211–213. [Google Scholar]
- 67.Arai H., Terajima M., Miura M., et al. Tau in cerebrospinal fluid: a potential diagnostic marker in Alzheimer's disease. Ann Neurol. 1995;38:649–652. doi: 10.1002/ana.410380414. [DOI] [PubMed] [Google Scholar]
- 68.Arai H., Higuchi S., Sasaki H. Aβolipoprotein E genotyping and cerebrospinal fluid tau protein: implications for the clinical diagnosis of Alzheimer's disease. Gerontology. 1997;43:2–10. doi: 10.1159/000213879. [DOI] [PubMed] [Google Scholar]
- 69.Andreasen N., Vanmechelen E., Van de Voorde A., et al. Cerebrospinal fluid tau protein as a biochemical marker for Alzheimer's disease: a community-based follow-up study [see comments]. J Neurol Neurosurg Psychiatry. 1998;64:298–305. doi: 10.1136/jnnp.64.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kurz A., Riemenschneïder M., Buch K., et al. Tau protein in cerebrospinal fluid is significantly increased at the earliest clinical stage of Alzheimer disease. Alzheimer Dis Assoc Disord. 1998;12:372–377. doi: 10.1097/00002093-199812000-00020. [DOI] [PubMed] [Google Scholar]
- 71.Nishimura T., Takeda M., Nakamura Y., et al. Basic and clinical studies on the measurement of tau protein in cerebrospinal fluid as a biological marker for Alzheimer's disease and related disorders: multicenter study in Japan. Methods Find Exp Clin Pharmacol. 1998;20:227–235. [PubMed] [Google Scholar]
- 72.Tapiola T., Lehtovirta M., Ramberg J., et al. CSF tau is related to apolipoprotein E genotype in early Alzheimer's disease. Neurology. 1998;50:169–174. doi: 10.1212/wnl.50.1.169. [DOI] [PubMed] [Google Scholar]
- 73.Buerger K., Padberg F., Nolde T., et al. CSF tau protein shows a better discrimination in young old (<70 years) than in old old patients with Alzheimer's disease, compared with controls. Neurosci Lett. 1999;277:21–24. doi: 10.1016/s0304-3940(99)00845-9. [DOI] [PubMed] [Google Scholar]
- 74.Green AJ., Harvey RJ., Thompson EJ., Rossor MN. Increased tau in the cerebrospinal fluid of patients with frontotemporal dementia and Alzheimer's disease. Neurosci Lett. 1999;259:133–135. doi: 10.1016/s0304-3940(98)00904-5. [DOI] [PubMed] [Google Scholar]
- 75.Hampel H., Teipel SJ., Padberg F., et al. Discriminant power of combined CSF tau and soluble gp1 30 in the diagnosis of Alzheimer's disease. Brain Res. 1999;823:104–112. doi: 10.1016/s0006-8993(99)01146-4. [DOI] [PubMed] [Google Scholar]
- 76.Molina L., Touchon J., Herpé M., et al. Tau and apo E in CSF: potential aid for discriminating Alzheimer's disease from other dementias. Neuroreport. 1999;10:3491–3495. doi: 10.1097/00001756-199911260-00005. [DOI] [PubMed] [Google Scholar]
- 77.Morikawa Y., Arai H., Matsushita S., et al. Cerebrospinal fluid tau protein levels in demented an nondemented alcoholics. Alcohol Clin Exp Res. 1999;23:575–577. [PubMed] [Google Scholar]
- 78.Kahle PJ., Jakowec M., Teipel SJ., et al. Combined assessment of tau and neuronal thread protein in Alzheimer's disease CSF. Neurology. 2000;54:1498–1504. doi: 10.1212/wnl.54.7.1498. [DOI] [PubMed] [Google Scholar]
- 79.Sjoegren M., Rosengren L., Minthon L., Davidsson P., Blennow K., Wallin A. Cytoskeleton proteins in CSF distinguish frontotemporal dementia from AD. Neurology. 2000;54:1960–1964. doi: 10.1212/wnl.54.10.1960. [DOI] [PubMed] [Google Scholar]
- 80.Munroe WA., Southwick PC., Chang L., et al. Tau protein in cerebrospinal fluid as an aid in the diagnosis of Alzheimer's disease. Ann Clin Lab Sci. 1995;25:207–217. [PubMed] [Google Scholar]
- 81.Galasko D., Clark C., Chang L., et al. Assessment of CSF levels of tau protein in mildly demented patients with Alzheimer's disease. Neurology. 1997;48:632–635. doi: 10.1212/wnl.48.3.632. [DOI] [PubMed] [Google Scholar]
- 82.Mori H., Hosoda K., Matsubara E., et al. Tau in cerebrospinal fluids: establishment of the sandwich ELISA with antibody specific to the repeat sequence in tau. Neurosci Lett. 1995;186:181–183. doi: 10.1016/0304-3940(95)11291-4. [DOI] [PubMed] [Google Scholar]
- 83.Blennow K., Vanmechelen E., Hampel H. CSF total tau, A-p42 and phosphorylated tau protein as biomarkers for Alzheimer's disease. Mol Neurobiol. 2001;24:87–97. doi: 10.1385/MN:24:1-3:087. [DOI] [PubMed] [Google Scholar]
- 84.Sjoegren M., Davidsson P., Tullberg M., et al. Both total and phosphorylated tau are increased in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2001;70:624–630. doi: 10.1136/jnnp.70.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sjoegren M., Vanderstichele H., Agren H., et al. Tau and Aβ42 in cerebrospinal fluid from healthy adults 21-93 years of age: establishment of reference values. Clin Chem. 2001;47:1776–1781. [PubMed] [Google Scholar]
- 86.Otto M., Wiltfang J., Tumani H., et al. Elevated levels of tau-protein in cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. Neurosci Lett. 1997;225:210–212. doi: 10.1016/s0304-3940(97)00215-2. [DOI] [PubMed] [Google Scholar]
- 87.Otto M., Wiltfang J., Cepek L., et al. Tau protein and 14-3-3 protein in the differential diagnosis of Creutzfeldt-Jakob disease. Neurology. 2002;58:192–197. doi: 10.1212/wnl.58.2.192. [DOI] [PubMed] [Google Scholar]
- 88.Kapaki E., Kilidireas K., Paraskevas GP., Michalopoulou M., Patsouris E. Highly increased CSF tau protein and decreased β-amyloid (1-42) in sporadic CJD: a discrimination from Alzheimer's disease? J Neurol Neurosurg Psychiatry. 2001;71:401–403. doi: 10.1136/jnnp.71.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kudo T., Mima T., Hashimoto R., et al. Tau protein is a potential biological marker for normal pressure hydrocephalus. Psychiatry Clin Neurosci. 2000;54:199–202. doi: 10.1046/j.1440-1819.2000.00658.x. [DOI] [PubMed] [Google Scholar]
- 90.Hesse C., Rosengren L., Andreasen N., et al. Transient increase in total but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci Lett. 2001;297:187–190. doi: 10.1016/s0304-3940(00)01697-9. [DOI] [PubMed] [Google Scholar]
- 91.Zemlan FP., Rosenberg WS., Luebbe PA., et al. Quantification of axonal damage in traumatic brain injury: affinity purification and characterization of cerebrospinal fluid tau proteins. J Neurochem. 1999;72:741–750. doi: 10.1046/j.1471-4159.1999.0720741.x. [DOI] [PubMed] [Google Scholar]
- 92.Mitani K., Furiya Y., Uchihara T., et al. Increased CSF tau protein in corticobasal degeneration. J Neurol. 1998;245:44–46. doi: 10.1007/s004150050173. [DOI] [PubMed] [Google Scholar]
- 93.Urakand K., Mori M., Wada K., et al. A comparison of tau protein in cerebrospinal fluid between corticobasal degeneration and progressive supranuclear palsy. Neurosci Lett. 1999;259:127–129. doi: 10.1016/s0304-3940(98)00923-9. [DOI] [PubMed] [Google Scholar]
- 94.Andreasen N., Minthon L., Davidsson P., et al. Evaluation of CSF-tau and CSF-A-p-42 as diagnostic markers for Alzheimer disease in clinical practice. Arch Neurol. 2001;58:373–379. doi: 10.1001/archneur.58.3.373. [DOI] [PubMed] [Google Scholar]
- 95.Vanmechelen E., Van Kerschaver E., Blennow K., et al. CSF-Phospho tau (181 P) as a promising marker for discriminating Alzheimer's disease from dementia with Lewy bodies. In: Iqbal K, Disodia S, Winblad B, eds. Alzheimer's Disease: Advances in Etiology Pathogenesis and Therapeutics. Chichester, UK: John Wiley & Sons; 2001 [Google Scholar]
- 96.Hu YY., He SS., Wang, et al. Levels of nonphosphorylated and phosphorylated tau in cerebrospinal fluid of Alzheimer's disease patients: an ultrasensitive bienzyme-substrate-recycle enzyme-linked immunosorbent assay. Am J Pathol. 2002;160:1269–1278. doi: 10.1016/S0002-9440(10)62554-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Riemenschneïder M., Wagenpfeïl S., Vanderstichele H., et al. Phosphotau/total tau ratio in cerebrospinal fluid discriminates Creutzfeldt-Jakob disease from other dementias. Mol Psychiatry. 2003;8:343–347. doi: 10.1038/sj.mp.4001220. [DOI] [PubMed] [Google Scholar]
- 98.Buerger K., Zinkowski R., Teipel SJ., et al. Differentiation of geriatric major depression from Alzheimer's disease with CSF tau protein phosphorylated at threonine 231 . Am J Psychiatry. 2003;160:376–379. doi: 10.1176/appi.ajp.160.2.376. [DOI] [PubMed] [Google Scholar]
- 99.Augustinack JC., Schneider A., Mandelkow EM., Hyman BT. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer's disease. Acta Neuropathol. 2002;103:26–35. doi: 10.1007/s004010100423. [DOI] [PubMed] [Google Scholar]
- 100.Buerger K., Zinkowski R., Teipel SJ., et al. Differential diagnosis of Alzheimer's disease with CSF tau protein phosphorylated at threonine 231 . Arch Neurol. 2002;59:1267–1272. doi: 10.1001/archneur.59.8.1267. [DOI] [PubMed] [Google Scholar]
- 101.Schoenknecht P., Pantel J., Hunt A., et al. Levels of total tau and tau protein phosphorylated at threonine 181 in patients with incipient and manifest Alzheimer's diesase. Neurosci Lett. 2003;339:172–174. doi: 10.1016/s0304-3940(02)01481-7. [DOI] [PubMed] [Google Scholar]
- 102.Itoh N., Arai H., Urakamï K., et al. Large-scale, multicenter study of cerebrospinal fluid tau protein phosphorylated at serine 199 for the antemortem diagnosis of Alzheimer's disease. Ann Neurol. 2001;50:150–156. doi: 10.1002/ana.1054. [DOI] [PubMed] [Google Scholar]
- 103.Hampel H., Buerger K., Zinkowski R., et al. Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease: a comparative cerebrospinal fluid study. Arch Gen Psychiatry. 2004;61:95–102. doi: 10.1001/archpsyc.61.1.95. [DOI] [PubMed] [Google Scholar]
- 104.Buerger K., Teipel SJ., Zinkowski R., et al. CSF tau protein phosphorylated at threonine 231 correlates with cognitive decline in MCI subjects. Neurology. 2002;59:627–629. doi: 10.1212/wnl.59.4.627. [DOI] [PubMed] [Google Scholar]
- 105.Arai H., Ishiguro K., Ohno H., et al. CSF Phosphorylated tau protein and mild cognitive impairment: a prospective study. Exp Neurol. 2000;166:201–203. doi: 10.1006/exnr.2000.7501. [DOI] [PubMed] [Google Scholar]
- 106.Andreasen N., Vanmechelen E., Vanderstichele H., Davidsson P., Blennow K. Cerebrospinal fluid levels of total-tau, phospho-tau and Aβ42 predicts development of Alzheimer's disease in patients with mild cognitive impairment. Acta Neurol Scand. 2003;107:47–51. doi: 10.1034/j.1600-0404.107.s179.9.x. [DOI] [PubMed] [Google Scholar]
- 107.Okamura N., Arai H., Maruyama M., et al. Combined analysis of CSF tau levels and [(123)1] iodoamphetandne SPECT in mild cognitive impairment: implications for a novel predictor of Alzheimer's disease. Am J Psychiatry. 2002;159:474–476. doi: 10.1176/appi.ajp.159.3.474. [DOI] [PubMed] [Google Scholar]
- 108.de Leon MJ., Segal S., Tarshish CY., et al. Longitudinal cerebrospinal fluid tau load increases in mild cognitive impairment. Neurosci Lett. 2002;333:183–186. doi: 10.1016/s0304-3940(02)01038-8. [DOI] [PubMed] [Google Scholar]