Abstract
Modern drug discovery demands an integrative approach, using many different technologies, but ultimately based on an understanding of the pathophysiology of the disease state to be treated. Targeting drugs at the main pathophysiological process is the key to success. This issue needs to be addressed with the multiple screening systems available, which can be used to find new leads.
Keywords: drug discovery, pathophysiology, drug testing, screening
Abstract
El descubrimiento moderno de fármacos requiere de una aproximación integradora en que se utilizan diversas tecnologías, pero en último término se basa en la comprensión de la fisiopatología del curso clínico de la enfermedad para que así pueda ser tratada. La clave del éxito está en conseguir fármacos que apunten al proceso fisiopatológico central. Este tema necesita ser abordado con los múltiples sistemas de exploración disponibles, los que pueden utilizarse para encontrar nuevas posibles moléculas.
Abstract
La découverte moderne de médicaments demande une approche intégrée, utilisant des technologies variées; elle est fondée sur la compréhension de la physiopathologie de la maladie à traiter. La clé du succès est de cibler les médicaments qui agissent sur le principal processus physiopathologique. Cette question doit être abordée à l'aide des nombreux systèmes de sélection des molécules existants afin de trouver de nouvelles pistes.
The discovery and development of one new drug costs around 800 million (taking failures into account) and takes an average of 10 to 12 years. This degree of investment, with such a late return on this investment, is unparalleled in human activity.
Despite this investment, some areas of great therapeutic need do not. have optimal treatments - acute stroke and Alzheimer's disease, as well as other central nervous system (CNS) disorders. There are no drugs registered for the treatment of acute stroke, which is an area of great therapeutic need, being the third-highest cause of mortality and the second-highest cause of morbidity. Nevertheless, there are distinct methodological reasons in the clinical trials which can preclude demonstrating efficacy in stroke under many circumstances.1 Another area in which the pharmaceutical industry has failed to revolutionize therapy has been in the treatment of Alzheimer's disease. However, preventive therapy by addressing hypertension using angiotensin-converting enzyme inhibitors (perindopril, in the PROGRESS study) has shown marked reduction in the incidence of stroke, and also of dementia and cognitive decline.2,3 Antidepressant drugs with higher efficacy and fewer side effects are much needed.
Effective drug discovery requires drug targets for therapeutic intervention which are pivotal points for the disease process, and up until now these have not been clearly identified for stroke (with the possible exception of tissue plasminogen activator for very early intervention) or Alzheimer's disease.
Background
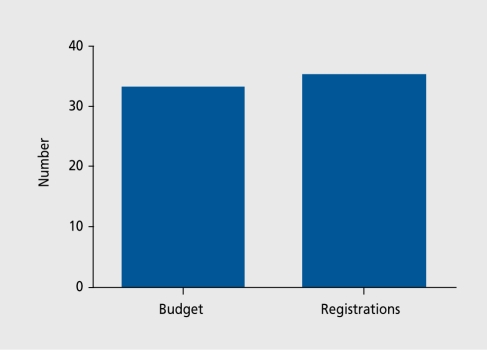
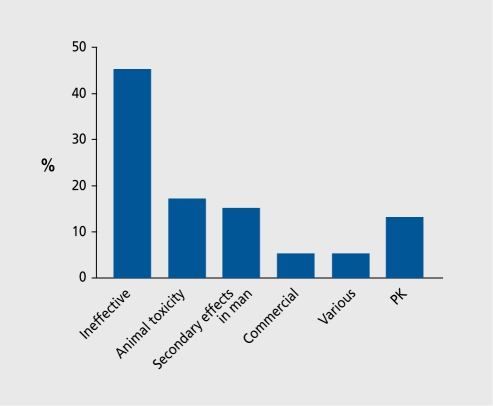
Only 35 new compounds were registered with the Food and Drug Administration (FDA) in 2003 despite a research expenditure by the major pharmaceutical firms of 33 billion dollars (Figure 1). Part of these costs are due to the costs of failure. Figure 2 shows the fate of a sample of 121 drugs put into phase 1 clinical trials by British pharmaceutical companies. The results are edifying. Although some drugs failed because of toxicological problems or metabolic issues, or were even stopped for commercial reasons, the major reason for failure was lack of efficacy. The drugs were stopped because they did not work. This may occur for several reasons.
Figure 1. Research budget (billion $) and total number of US drug registrations in 2003.
Figure 2. Reasons for stopping clinical development of 121 compounds in clinical trials carried out by seven British companies. PK, pharmacokinetics .
First, the original hypothesis may be wrong, and the end result is a useful experiment, albeit a very expensive one. Second, and this is perhaps just as likely, the animal models may not represent the tests used in phase I and phase II clinical trials - it is also possible that the tests used in phase I and II do not represent the true patient response.
Indeed, of the 340 compounds entering phase I per year, four out of five fail, and even when registration is achieved, less than half of the compounds recoup their development costs. The failure of drugs to work in the clinical setting (lack of efficacy due either to the concept not working, or to the animal models or the clinical models not responding to the patients' needs) is a key area for improvement.
Third, increasing safety requirements discourages risk. This is particularly the case for CNS-active drugs which may have cardiovascular side effects (effects on electrocardiographic [ECG] QTc intervals for example). It remains a truism that no drug can be effective without having some measure ofri.sk.
However, it is now possible to have high-throughput screens for safety, and to do a better job of selecting compounds at an early stage.
The difficulties faced by a drug discoverer are shown by the sequence below. First, he or she must find the optimal structure/activity, then exclude structure/activity at other sites:
Definition of structure/activity at site of action
Exclusion of structure/activity at cytochromes
Exclusion of structure/activity - mutagenicity
Exclusion of structure/activity - cardiac QTc
Start of toxicity studies.
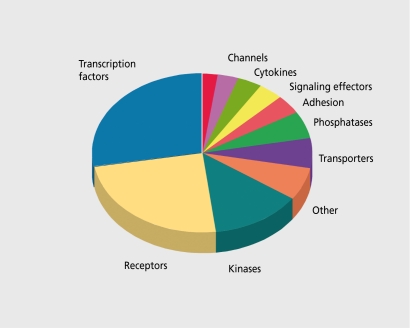
Fourth, there is the realization of the increasing complexity of biological systems. Although there may be only 25 000 to 30 000 genes, many of which are drug targets (Figure 3) , the gene products are much more complex because of alternative splicing, mRNA editing, receptor dimerization, functional trafficking (where drugs acting at the same receptor may have different effects) and the multiple post-translational controls and accessory proteins.
Figure 3. Signaling genes in the human genome.
New technological opportunities
In vitro screening
Screening on recombinant proteins has proven to be immensely powerful, and can provide new leads from high-throughput screening on a scale which would be impossible with other technologies. Now the target proteins may even be crystallized, with the drug, or even with fragments of the drug, and the crystals analyzed to define the conformational changes induced in the target by different drugs. The throughput of this technology is such that entire chemical scries can be analyzed for their direct effects on the protein of interest. Thus, hundreds of thousands of compounds can be tested at the cellular target in a few months, and the “hits” can then be chemically optimized to make new metabolically stable drugs. (Figure 3). Different conformational states during cellular activation, particularly in the presence of accessory proteins, may easily change a singe hydrogen bond or electrostatic attraction, changing affinity. Indeed, it must be pointed out that one additional hydrogen bond between the compound and the target can change the affinity thirty-fold. This complexity may induce inadequate responses to predict therapeutic efficacy. As compound selection is the crucial issue, we have argued that, after preliminary screens in recombinant systems, and following exclusion of inappropriate compounds (for metabolic or safety reasons), the selection of the final compound to proceed onto development should take place in pathophysiological models, and preferably, if breakthrough compounds are looked for, in novel pathophysiological models. However, this means a major investment in screening in animal models.
In vivo screening
Animal models are often the limiting factor in research (particularly for cognitive issues), and finding staff skilled in their handling is not easy. Previous drugs have been tested for in the established models, and the way to test, benzodiazepine anxiolytics is to use the classic anxiety screening models, defined by diazepam. However, novel drugs working in new ways may need new models. Thus, compounds should be selected using a model of pathophysiological conditions. However, this needs skilled pharmacologists' with an integrative vision of pathophysiology.
How are new drugs discovered?
New drugs may be discovered in very many ways, but discovery nearly always involves tight collaborations between chemists and pharmacologists, who must identify the cellular and genetic factors important in pathophysiology, produce appropriate hypotheses, and design new test systems. Screening new molecules can be done in a number of ways.
Target identification
Ideally, the target should be the cause of a specific disease which can be targeted on a molecular level. There has been immense progress made in defining the receptor systems in the human genome, by analogy to existing 7-transmcmbrane receptors. This marks a unique moment in science, because many targets are becoming known. Lists of these receptors have been produced (eg, ref 5). Furthermore, new targets remain to be discovered, and the existing targets are known to have many different forms (alternative splicing, messenger ribonucleic acid (mRNA) editing, single-nucleotide polymorphisms, etc) which may allow selective targeting of disease states. The bioinformatics industry provides an immensely powerful tool to scientists, and many of these data are in the public domain.
Target validation
A crucial issue is to validate the target, in animal and preferably in human models. This is critical, because of the high cost, of discovering a new drug for a target and performing the clinical experiment to find out, whether the new drug works in a disease state in man. As there are tens of thousands of potential targets, target validation is a crucial issue. Fortunately, transgenic models may help in this regard, but their predictivity is only relative.
Lead identification
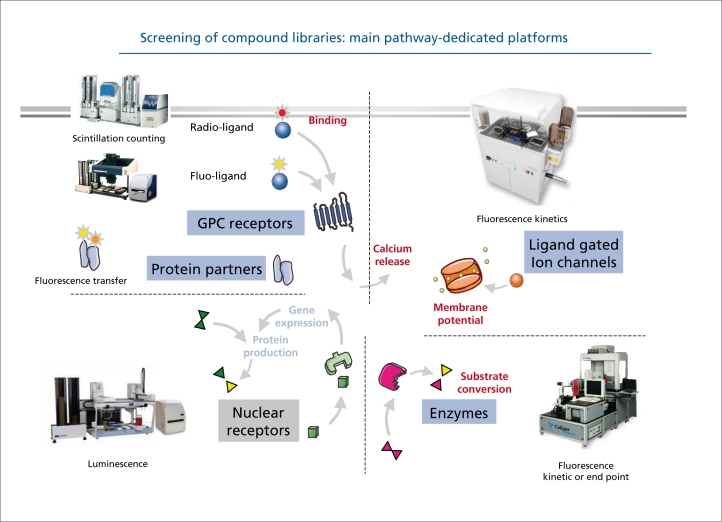
A lead compound is usually selected by high-throughput screening of compound collections, or libraries (Figure 4) These compound libraries may consist of thousands, or hundreds of thousands, of compounds, built, up by the pharmaceutical company over the years. Virtual screens can now be performed by modeling the interactions of the target, with virtual libraries consisting of all the compounds which are commercially available - the best compounds can then be selected for screening. The “hits” or compounds which are active in the first round of screening are then optimized so that, they possess the properties needed in a new drug. Testing is then done on each of these molecules to confirm its effect on the drug target.
Figure 4. Screening of compound libraries: main pathway-dedicated platforms. GPC, G protein-coupled (Figure courtesy of Olivier Nosjean and Jean Boutin, Servier research).
Lead optimization
Lead optimization compares the properties of various lead compounds, allowing selection of the compound or compounds with the greatest potential to be developed into safe and effective medicines. The metabolism is optimized in high-throughput screens to produce compounds which retain their activity at the target of interest, while being metabolically stable and well absorbed.
Drug testing in humans
Testing an investigational new drug requires submission of all the information about the drug for permission to administer to healthy volunteers or patients. Not, only regulatory authorities, but also institutional or independent, review boards (IRB) or ethical advisory boards approve the experimental protocol, well as the informed consent documents signed by the volunteers.
The clinical testing of the drug passes through Phase I, Phase II, and Phase III clinical studies. In each successive phase, increasing numbers of patients are tested, but the success or failure of the drug (see Figure 2) depends not only on its mode of action, but also on the good methodological quality of the testing schedule used in the clinic.
Phase I clinical studies
Phase I studies must verify the safety and tolerability of the new drug in volunteers, showing the maximal tolerated dose, and how it is absorbed, distributed, metabolized, and excreted. This phase takes 6 months to a year. Healthy volunteers are administered the drug acutely and then chronically. TTic hypothesis of action may be tested pharmacologically with indexes of brain penetration, brain imaging, and electroencephalogram (RFG). However, it must be borne in mind that healthy individuals may not react in the same way as patients. Some drugs cannot be tested in healthy volunteers (eg, in oncology).
Phase II clinical studies
Phase IT studies are a critical research area designed to show effectiveness, define dose-response for the critical phase III approval studies, and demonstrate a measure of safety in the patient population. This phase of development, generally takes from 1 to 3 years with several hundred patients. It is here that an appropriate choice of drug effectiveness criteria for drug effectiveness, linked to animal models, yet providing a realistic test, of the drug in the patient population, can make a real difference.
Phase III clinical studies
Phase III studies show effectiveness and safety in randomized and blinded clinical trials involving thousands of patients. This phase can take 2 to 5 years, and is the most, expensive clinical testing phase.
New Drug Application/Marketing Authorization
A New Drug Application (NBA), in the US, or Marketing Authorization (MA), in Europe, documents the safety and efficacy of the proposed drug, and the applications contain all the reports from the drug development process. At, the end of phase III, the evidence proving efficacy and safety are submitted. The approval process can take 1 to 2 years, followed by post-marketing surveillance and extension of the therapeutic indications and patient populations.
Fast-tracking
Several regulatory issues may be seen as opportunities. Fast, tracking for very urgent therapeutic needs, such as treatment for acquired immune deficiency syndrome (AIDS), has been introduced by the FDA. Furthermore, the FDA have issued guidelines on pharmacogenetic subtyping of patient populations (responders, patients at risk for side effects, rapid metabolizers, etc).
Partnership
Modern drug discovery and development, depends on a constant partnership between the actors in the project, in the many disciplines which are involved. The partnership between industry and academia is a critical issue, because basic research can lead to many unexpected breakthroughs, of which the researcher may not appreciate the industrial and medical importance. It is correct, that financial return should be associated with inventiveness. However, the fewer industrial partners there are (as in France), the fewer local industrial partners there are for startup biotechnology companies. There is thus a delicate balance between support, of pharmaceutical companies and small biotechnology companies. As the main industrial experience (to avoid the pitfalls shown in Figure 2 for example) is located in pharmaceutical companies, this pragmatic feedback and review is an essential part, of the health of the local industrial environment. It is also essential that research remains very medically oriented, because the patients“ needs come first. Partnership with clinicians and top medical teams is therefore also a key element, for success. However, all of the stages of drug discovery remain an experiment, and must be designed as such. After the initial selection process which finds the drug, the only thing which does not change in the development process is the molecule; all the others - the scientists, sometimes even the therapeutic area - may change. However, the molecule can do no more or less than on the day when it is chosen, which is why the tests which select, the molecule are so important.
Table I shows the factors influencing success in the drug discovery process.
Table I. What are the main factors influencing success in drug discovery processes, and how can research output be improved?
| • 1. Resources. Resources are critical, and all programs must have access to major molecular resources for screening for drug discovery. |
| • 2. Access to a rapid drug development program, with clinical tests which are tightly related to the animal tests. Thus, feedback to discovery scientists can be rapid to optimize discovery and to ensure that researchers do not continue to work on hypotheses which do not stand up in the clinic. |
| • 3. Approaches and strategies. This is a critical issue for success, and requires medicalized research, in association with the specialized resources. Reverse engineering animal models from our knowledge of brain systems in disease states will better reflet pathophysilogical situations and allow selection of new drugs. |
| • 4. Quality/creativity. The quality, application, and creativity of the scientists involved, together with the relevance of the approaches and strategies, are key factors for success. However, there arc few measures, other than past records, which can be quantified by objective criteria (other than publications, but these are not always related to drug discovery capacity). |
| • 5. Outside research. Having access to a large network of academic contacts is crucial to icreasing the number of ideas processed and increasing throughput. |
Key points for definition of new way s forward in psychiatric disorders
It is important to define the specific nodes or switchpoints which are modified by disease processes and suitable for therapeutic intervention. These can be at several levels, such as:
Molecular - the multiple intracellular signaling cascades have key nodal points which can be targetted. Cancer drugs are targetted at key points, and now the same situation is being extended into CNS research, where drugs for bipolar disorder, such as lithium, may interact with key signaling molecules such as glycogen synthase kinase 3 (GSK3).
Epi genetic changes where the genes expressed relate to the past, history of the idividual. Furthermore, many gene products are modified by alternative splicing or mRNA editing which can change the function of key proteins in pathophysiological conditions.
Cell plasticity. Neurotrophins and cytokines have major effects on cell plasticity and integrity. .Many genes can interact within the neurotrophic signaling cascades, and these are major points for therapeutic interventions. For example, we have shown that brain-derived neurotrophic factor (BDNF), the key neurotrophin involved in activity-dependent resculpting of neuronal networks, can also change the respirator}' coupling efficiency of mitochondria, indicating a new way forward in the links between cellular activity and coupled metabolism.6
The neurotransmitters involved in modulating brain systems are well defined, and still represent sources of drug discovery (noradrenaline, 5-HT, dopamine, etc). However, the multiple states of receptors and their signaling pathways warn against oversimplification.7
Chronobiological issues are important, in resetting biological rhythms, and may be even more important than previously thought. The finding that agomelatine, a melatonin agonist, and 5-HT2C antagonist, can be an effective antidepressant with a low side-effect potential8,9 reconfirms the interest in chronobiological systems, because their dysregulation is a common feature of ageing and psychiatric disorders.
Cell firing on specific nodal points. The systems in the brain are becoming well defined, and it is now possible to intervene on brain switch-points, which may be deregulated. These can be quantified electrophysiologically, or by microdialysis of the main neurotransmitters, or by brain imaging techniques.
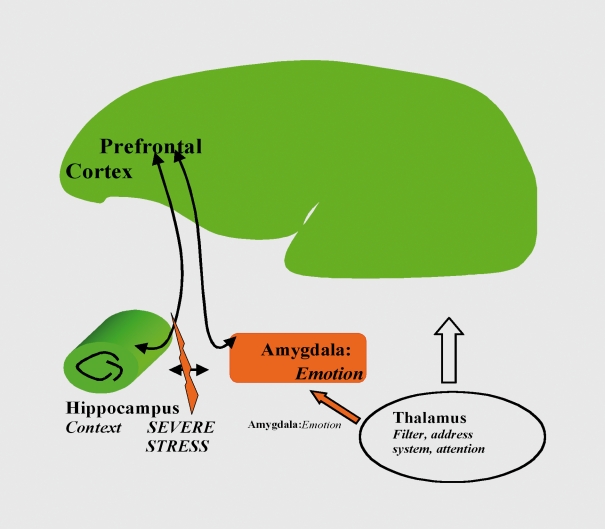
Neuronal networks for brain functions (eg, the main systems involved in cognition, decision, and emotivity and fear (prefrontal cortex, amygdala, hippocampus, (Figure 5). An example of research in this area is the finding that stress blocks long-term potentiation (LT.P, a measure of plasticity) in the hippocampal to ventromedial prefrontal cortex,11 and these effects are reversed acutely by an atypical antidepressant, tianeptine. McEwen's group have shown that these acute effects change into effects on dendritic arborization.1' Furthermore, there is now proof of concept that, this pathway is of critical importance for depression because Mayberg's group13 have implanted electrodes into the white matter behind Cg25 (the equivalent in man of the ventromedial prefrontal cortex in rodents) and found immediate antidepressant effects in patients who had been entirely treatment-resistant. Targctting these brain areas therefore opens up new perspectives in drug discovery for depression. Furthermore, reingineering animal models to study these brain areas will allow the selection of new classes of molecule.
Figure 5. The impact of stress on neuroplasticity may be a novel target for drugs in psychiatry, as stress inhibits plasticity in hippocampal and prefrontal cortex circuits while increasing plasticity in the circuits dealing with emotion (amygdala, prefrontal cortex).10 .
REFERENCES
- 1.Spedding M. Reasons why stroke trials underestimate the neuroprotective effects of drugs. Stroke. 2002;33 :324–325. [PubMed] [Google Scholar]
- 2.The PROGRESS Collaborative Group. Effects of blood pressure lowering with perindopril and indapainide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med. 2003;163:1069–1075. doi: 10.1001/archinte.163.9.1069. [DOI] [PubMed] [Google Scholar]
- 3.Dufouil C., Chalmers J., Coskun O., et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke. Circulation. 2005;112:1644–1650. doi: 10.1161/CIRCULATIONAHA.104.501163. [DOI] [PubMed] [Google Scholar]
- 4.Collis MG. Integrative pharmacology and drug discovery - is the tide turning? Nature Drug Discovery. 2006 ;5:377–379. doi: 10.1038/nrd2036. [DOI] [PubMed] [Google Scholar]
- 5.Foord SM., Bonner Tl., Neubig RR., et al. International Union of Pharmacology. XLVI. G protein-coupled receptor list. Pharmacol Rev. 2005;2:279–288. doi: 10.1124/pr.57.2.5. [DOI] [PubMed] [Google Scholar]
- 6.Markham A., Cameron I., Franklin P., Spedding M. BDNF increases mitochondrial respiratory coupling, and glutamate metabolism, at complex I, but not complex II. EurJNeurosci. 2004;20:1189–1196. doi: 10.1111/j.1460-9568.2004.03578.x. [DOI] [PubMed] [Google Scholar]
- 7.Kenakin T. New concepts in drug discovery: collateral efficacy and permissive antagonism. Nature Drug Discovery. 2005;4:919–927. doi: 10.1038/nrd1875. [DOI] [PubMed] [Google Scholar]
- 8.Loo H., Hale A., D'haenen H. Determination of the dose of agomelatine, a melatoninergic agonist and selective 5-HT2c antagonist, in the treatment of major depressive disorder: a placebo-controlled dose range study. Int Clin Psychopharmacol. 2002;17:239–247. doi: 10.1097/00004850-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy SH., Emsley R. Placebo-controlled trial of agomelatine in the treatment of major depressive disorder. Eur Neuropsychopharmacol. 2006;16:93–100. doi: 10.1016/j.euroneuro.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Spedding M., Jay T., Costa e Silva J., Perret L. A pathophysiological paradigm for the therapy of psychiatric disease. Nature Drug Discovery. 2005;4:467–476. doi: 10.1038/nrd1753. [DOI] [PubMed] [Google Scholar]
- 11.Rocher C., Spedding M., Munoz C., Jay T. Acute stress-induced changes in cortical synaptic plasticity: interactions with antidepressants. Cereb Coitex. 2004;14:224–229. doi: 10.1093/cercor/bhg122. [DOI] [PubMed] [Google Scholar]
- 12.cEwen BS., Olié JP. Neurobiology of mood, anxiety and emotions, as revealed by studies of a unique antidepressant: tianeptine. Mol Psych. 2005;10:525–537. doi: 10.1038/sj.mp.4001648. [DOI] [PubMed] [Google Scholar]
- 13.ayberg HS., Lozano A., Voon V., et al. Deep brain stimulation for treatment resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]